Abstract
Aim
The ability of patients with poor pudendal nerve function to voluntarily contract their external anal sphincter is limited. However, it is not known whether the condition of the pudendal nerve influences voluntary puborectal muscle contraction. Recently, we described the puborectal continence reflex that maintains faecal continence by involuntary contractions of the puborectal muscle. We aim to investigate whether both voluntary and involuntary contractions of the puborectal muscle are influenced by the condition of the pudendal nerve.
Method
We retrospectively analysed 129 adult patients who underwent anorectal function tests at the Anorectal Physiology Laboratory. Anal electrosensitivity was used as a measurement of the pudendal nerve function. Voluntary and involuntary contractions of the puborectal muscle were defined as maximum puborectal muscle contractility and maximum pressure at the level of the puborectal muscle during the balloon retention test.
Results
Voluntary contraction of the puborectal muscle was significantly decreased in patients with pudendal nerve damage (P = 0.002). Involuntary contractions, however, were not associated with the condition of the pudendal nerve (P = 0.63). Multiple linear regression analysis showed that the condition of the pudendal nerve and patients’ sex significantly predicted voluntary contraction but not involuntary contraction.
Conclusion
Voluntary contractions of the puborectal muscle are significantly decreased in patients with pudendal nerve damage, while involuntary contractions of the puborectal muscle are comparable to those of patients without nerve damage. We conclude that the puborectal continence reflex, which controls involuntary contractions of the puborectal muscle, is not regulated by the pudendal nerve.
Keywords: Fecal incontinence, puborectal continence reflex, pudendal nerve, fecal continence, puborectal muscle, anorectal manometry
What does this paper add to the literature?
Faecal incontinence is a devastating condition. This study shows that the puborectal continence reflex, a faecal continence mechanism regarding involuntary contractions of the puborectal muscle, is not regulated by the pudendal nerve. Further determination of the exact nerve pathway might help to avoid accidental damage of the faecal continence mechanism.
Introduction
Faecal continence is regulated by different mechanisms including voluntary and involuntary contractions of certain muscles of the pelvic floor 1. The internal anal sphincter closes the anorectum by tonic involuntary contraction, while the external anal sphincter can contract both voluntarily and involuntarily 1, 2. The puborectal muscle can also contract voluntarily, which results in a sharper anorectal angle to maintain continence 1. Recently, we have shown that the puborectal muscle can also contract involuntarily and that these contractions are regulated by the puborectal continence reflex 3.
The pudendal nerve is one of the major nerves of the anorectum 1. It is known that patients with pudendal nerve damage have a limited ability to voluntarily contract their external anal sphincter 1, 4, 5, 6. On the other hand, involuntary contractions of the external anal sphincter are not regulated by the pudendal nerve 7. There is no consensus in the literature as to whether the puborectal muscle is innervated by the pudendal nerve and, consequently, whether pudendal nerve damage results in diminished voluntary puborectal muscle contraction 4, 8, 9, 10, 11. Furthermore, the nerve pathway responsible for involuntary contractions of the puborectal muscle has thus far not been investigated. In addition, the influence of sex and age on both voluntary and involuntary contractions of the puborectal muscle is still unknown.
In this study, we aimed to investigate whether both voluntary and involuntary contractions of the puborectal muscle are regulated by the pudendal nerve. Additionally, we aimed to investigate the influence of age and sex on voluntary and involuntary puborectal muscle contractions.
Method
Patients
Retrospectively, we reviewed the medical records of all patients older than 17 years (n = 425) who had undergone anorectal function tests at the Anorectal Physiology Laboratory in the University Medical Center Groningen from January 2010 to June 2018 because of defaecatory problems.
As any nerve damage could influence anorectal measurements, we excluded patients who had undergone previous pelvic floor surgery, those who had experienced any trauma in that area, who were diagnosed with polyneuropathy or who suffered from any other condition that could influence innervation. We excluded 296 patients for the following reasons: generalized neurological disorders [e.g. multiple sclerosis, spinal cord injury, spina bifida or polyneuropathy (n = 43)], anal sphincter rupture during childbirth, episiotomy or sphincterotomy (n = 51), surgery for prolapse or perianal fistula (n = 30), hysterectomy (n = 43), surgery for anorectal malformation or Hirschsprung's disease (n = 34), recto‐sigmoid resection (n = 12), sacral nerve stimulation therapy (n = 6), other [e.g. prostatectomy, ileo‐anal pouch, sphincter repair, surgery for haemorrhoids, anal or prostate cancer, pelvic floor trauma, radiation injury, recent botox injection or mental retardation (n = 41)] or a combination of the reasons above (n = 27). Further, as a result of technical problems during measurement, we had to exclude another nine patients. A total of 129 patients were eligible for analysis. The indications for having to undergo anorectal function tests in these patients were as follows: incontinence (n = 59), constipation (n = 44), anal pain (n = 8), anal fissures (n = 8), a combination of incontinence and constipation (n = 4) and other reasons (n = 6).
The study was conducted at the University Medical Center Groningen, The Netherlands, in compliance with the requirements of our local medical ethics review board.
Measuring equipment and anorectal function tests
Anorectal function tests were performed using solar, gastrointestinal, high‐resolution manometry equipment, version 9.3 (Laborie/Medical Measurements Systems, Enschede, The Netherlands). As was described by us previously, we used three different types of catheters to perform the measurements 3, 7. Here, we provide a description of the three tests we performed.
Anal electrosensitivity test
By applying superficial anal electrical stimulation, the anal electrosensitivity test measures the sensitivity of the anal canal and thus discloses the sensory condition of the pudendal nerve 12. To administer this test, we used a Laborie/Unisensor catheter that has an outer diameter of 8F and two circularly located electrodes of 2 mm. The distance between the two electrodes is 8 mm.
We inserted the catheter into the anal canal of the patient, who was lying in the left lateral position, and set the generator to produce a 0.1 ms square wave, at a constant frequency of 5 Hz, with a train duration of 1.0 s. Starting proximally, we stimulated every centimetre of the anal canal from 1 to 20 mA, with steps of 1 mA. We recorded the lowest threshold out of three, as reported by the patient. For our analysis, we measured anal electrosensitivity at 2 cm from the anal verge into the anal canal. By choosing 2 cm, and thus taking into account the considerable inter‐individual variability in the length of the anal canal, we could be sure that we were measuring inside the anal canal.
Anorectal pressure test
For the anorectal pressure test, we used a Laborie/Unisensor K12981 solid state (Boston type), circumferential catheter, with an outer diameter of 12F. While the patient was lying in the left lateral position, we inserted the catheter into the patient's anal canal. The catheter measured anorectal pressure every 8 mm over a total length of 6.8 cm into the lower rectum and the anal canal. To prevent the catheter from slipping out of the anal canal, we fixed it onto the patient's buttocks with adhesive tape. Measurement started by registering basal pressures. Subsequently, we asked the patient to squeeze. We registered maximum puborectal pressure during squeeze, and thus this test reflects voluntary contraction of the puborectal muscle. To ensure that we analysed voluntary contraction of the puborectal muscle and not, inadvertently, partial contractions of the anal sphincter, we defined a zone located proximal to the anal canal, i.e. at the level of the puborectal muscle, where the basal pressure was lower than the basal pressure of the anal sphincter.
Balloon retention test
We have previously described the balloon retention test in detail 3. For the test, we used two catheters: the aforementioned Laborie/Unisensor K12981 catheter, with an outer diameter of 12F, and the Laborie/Unisensor K14204 with an outer diameter of 14F. The Laborie/Unisensor K14204 catheter is connected to the rectal balloon, which is inflated, and the pressure inside the balloon is registered with two microtip sensors. The solar, gastrointestinal, high‐resolution manometry automatically corrects the pressure measured for the balloon resistance pressure, so that only the real pressure in the rectal wall is given.
We did not use the balloon retention test for its standard purpose, namely measuring filling sensations and volumes corresponding to certain rectal sensations. Instead, as described by us previously, we used the balloon retention test to investigate the presence of the puborectal continence reflex by measuring changes of pressure at the level of the puborectal muscle 3.
We stopped testing when the patient had reached the maximum tolerable sensation. If the patient was unable to retain the balloon until the maximum tolerable sensation was reached, the test was stopped as soon as the balloon was involuntarily lost, and this was recorded as the maximum retainable sensation.
Normal values of the anorectal function tests
For the anorectal sensitivity test during which anal stimuli ranging between 1 and 20 mA are administered, the normal values for healthy subjects are ≥ 3 and ≤ 4 mA 13, 14. However, because borderline poor electrosensitivity is better than electrosensitivity of 20 mA, we used continuous data of anal electrosensitivity and did not use the threshold of 4 mA for grouping the pudendal nerve function.
In our previous study, we presented the normal values for the maximum voluntary contraction of the puborectal muscle as measured during the anorectal pressure test in young, healthy subjects 3. For these subjects, the median maximum pressure observed during the voluntary puborectal muscle contraction was 70 (25–245) mmHg and took a median of 1.5 min. The median value of the maximal pressure during involuntary contractions of the puborectal muscle was 150 (70–260) mmHg and the median duration of the contractions was 5.8 min 3.
Statistical analysis
The data were analysed with spss statistics version 23.0 for Windows (ibm spss statistics, IBM Corporation, Armonk, New York, USA). We displayed values as number (percentage) or as median (minimum – maximum). When distribution was not normal, a natural log‐transformation was performed. Simple regression analysis was used to determine predictors of puborectal muscle contractions. We used a separate P < 0.15 as statistical significance for simple linear regression analysis 15. After this, the parameters were used in multiple linear regression analysis. The level of statistical significance was defined as P < 0.05.
Figures were generated using graphpad prism 7.02 (GraphPad Software Inc., La Jolla, California, USA).
Results
The group of 129 patients included for analysis consisted of 38 (29%) men and 91 (71%) women (Table 1). The median age of the patients was 57 years (18–81). In these patients, we observed a median anal electrosensitivity of 6 mA (2–20) at 2 cm into the anal canal. The median basal pressure of the puborectal muscle was 10 mmHg (5–50) and the median of maximum voluntary contractions was 40 mmHg (5–215). The median pressure of the puborectal muscle at the start of the involuntary contraction was 25 mmHg (5–185) and the mean pressure of the maximum involuntary contraction was 130 ± 59 mmHg.
Table 1.
Demographics
| Number (n = 129) | |
|---|---|
| Patients’ characteristics | |
| Female patients | 91 (71%) |
| Age (years) | 57 (18–81) |
| Anal electrosensitivity | |
| At 2 cm (mA) | 6 (2–20) |
| Anorectal pressure test | |
| Basal puborectal pressure (mmHg) | 10 (5–50) |
| Voluntary contraction (mmHg) | 40 (5–185) |
| Balloon retention test | |
| Pressure at start (mmHg) | 25 (5–185) |
| Involuntary contraction (mmHg) | 130 ± 59 |
Voluntary contraction is the pressure at the level of the puborectal muscle contraction during maximum squeeze. Involuntary contraction is the pressure at the level of the puborectal muscle during maximal tolerable volume or maximal retainable volume.
While pudendal nerve damage diminishes voluntary contraction, it does not affect involuntary contraction
To investigate whether the sensory condition of the pudendal nerve was associated with the maximum voluntary contraction of the puborectal muscle, we analysed the relationship between anal electrosensitivity and the maximum voluntary contraction (Fig. 1a). We found that patients with an increased threshold for anal electrosensitivity, i.e. patients in whom the condition of the pudendal nerve was impaired, had significantly diminished voluntary contractions of the puborectal muscle compared to patients with normal anal sensation (P = 0.002, Table 2).
Figure 1.
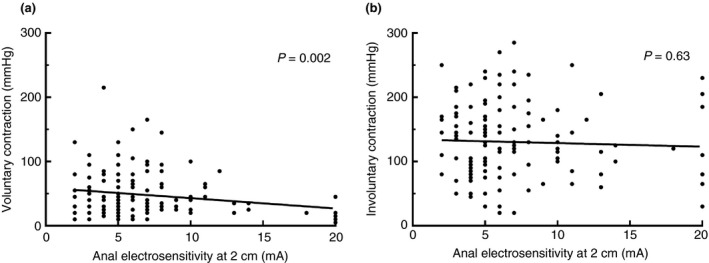
Linear regression between anal electrosensitivity at 2 cm into the anal canal and (a) voluntary and (b) involuntary contractions of the puborectal muscle.
Table 2.
Simple and multiple regression analysis
| B | Beta | 95% CI | P value | |
|---|---|---|---|---|
| Simple regression | ||||
| Voluntary contraction* | ||||
| Anal electrosensitivity | −0.043 | −0.267 | −0.071 to −0.016 | 0.002 |
| Age | −0.006 | −0.133 | −0.013 to 0.002 | 0.13 |
| Sex | 0.730 | 0.459 | 0.482–0.978 | < 0.001 |
| Involuntary contraction | ||||
| Anal electrosensitivity | −0.558 | −0.043 | −2.862 to 1.747 | 0.63 |
| Age | 0.431 | 0.128 | −0.157 to 1.019 | 0.15 |
| Sex | −0.050 | 0.000 | −22.607 to 22.508 | 0.997 |
| Multiple regression | ||||
| Voluntary contraction | ||||
| Anal electrosensitivity | −0.038 | −2.36 | −0.063 to −0.014 | 0.003 |
| Sex | 0.703 | 0.442 | 0.462–0.944 | < 0.001 |
*Natural log transformation. Model fitness multiple regression: adjusted R 2 = 0.254.
Furthermore, we investigated whether the sensory condition of the pudendal nerve influenced the involuntary contraction of the puborectal muscle (Fig. 1b). We found no relationship with the anal electrosensitivity and the involuntary contractions of the puborectal muscle, regulated through the puborectal continence reflex (P = 0.63, Table 2).
Voluntary and involuntary puborectal muscle contractions function independently of each other
We investigated whether voluntary and involuntary contractions of the puborectal muscle are functionally associated. We found no correlation between the maximum voluntary and the maximum involuntary contraction (P = 0.34, Fig. 2).
Figure 2.
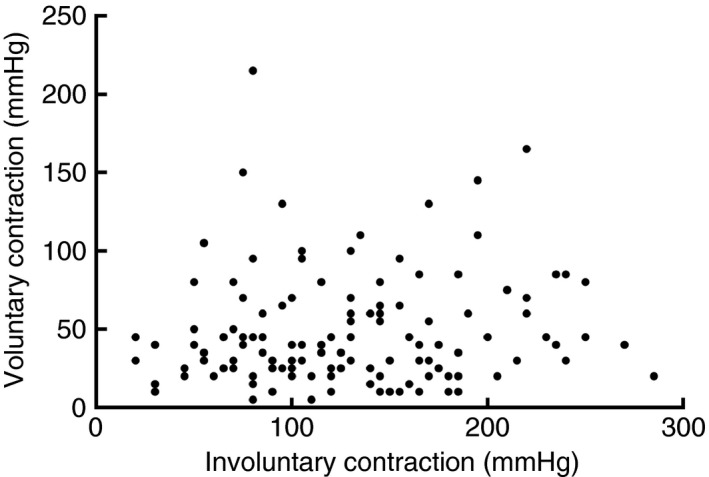
There is no association between voluntary and involuntary contractions of the puborectal muscle (P = 0.34).
Influence of age and sex on voluntary and involuntary puborectal muscle contractions
We investigated whether age exerted an influence on voluntary and involuntary contractions of the puborectal muscle. Simple linear regression analysis showed that there was no significant correlation between age and either voluntary or involuntary contractions (P = 0.13 and P = 0.15, respectively, Table 2).
Additionally, we investigated whether sex influenced voluntary and involuntary contractions of the puborectal muscle. Simple linear regression analysis revealed that there was a significant relationship between voluntary contractions and sex (P < 0.001, Table 2). Voluntary contractions of the puborectal muscle were significantly stronger in men than in women. By contrast, we did not observe any significant correlation between involuntary contractions and sex (P = 0.997, Table 2).
Multiple regression analysis showed that anal electrosensitivity and sex together were significantly correlated with voluntary contractions of the puborectal muscle (P = 0.003 and P < 0.001, respectively, Table 2).
Discussion
In this study, we investigated whether voluntary and involuntary contractions of the puborectal muscle are regulated by the same nerve pathway. We found that voluntary contractions are regulated by the pudendal nerve, because malfunctioning of the pudendal nerve was significantly associated with impaired voluntary contractions of the puborectal muscle. In contrast, our results suggest that involuntary contractions of the puborectal muscle, i.e. the puborectal continence reflex, function independently of the condition of the pudendal nerve (Fig. 3). This finding implies that the puborectal continence reflex is not regulated by the pudendal nerve. Additionally, this finding is supported by the fact that there is no functional correlation between voluntary and involuntary contractions of the puborectal muscle. Therefore, our conclusion is that these contractions function independently of one another.
Figure 3.
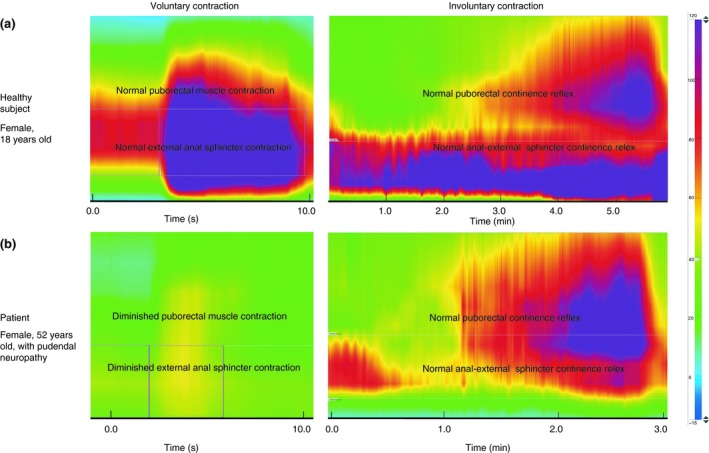
(a) Voluntary and involuntary contraction of a healthy subject (woman 18 years old). There is a normal voluntary contraction of the external anal sphincter and puborectal muscle. In addition, there is activation of the anal external sphincter continence reflex and the puborectal continence reflex which regulate the involuntary contractions of the external anal sphincter and the puborectal muscle, respectively 2, 3. (b) Voluntary and involuntary contraction of a patient with pudendal neuropathy (woman 52 years old). The voluntary contractions of both the external anal sphincter and the puborectal muscle are diminished, due to the pudendal nerve damage. However, there is still activation of the anal external sphincter continence reflex and the puborectal continence reflex, which results in normal involuntary contractions.
The critical question is therefore what the innervation responsible for involuntary contractions of the puborectalis muscle is. It is known that the levator ani nerve innervates the puborectal muscle 10. This could be the possible nerve pathway of the puborectal continence reflex. Barber, however, discussed that the puborectal muscle is innervated directly by a different nerve independently of the levator ani nerve 16. This might be the nerve that is responsible for involuntary contractions of the puborectal muscle. Further research is needed to identify the exact nerve pathway responsible for the puborectal continence reflex.
Age did not significantly influence either voluntary or involuntary contractions. Nevertheless, popular belief has it that faecal incontinence is a natural process associated with ageing, because voluntary contractions of the external anal sphincter, whose function it is to delay the defaecation process, are indeed negatively influenced by age 13, 17. On the contrary, the majority of elderly people are continent, and our research group showed that for the Dutch population the prevalence of faecal incontinence does not seem to increase with age 18. Our finding that age does not influence either involuntary contractions or voluntary contractions of the puborectal muscle explains this phenomenon. Apparently, the puborectal muscle plays such an important role in faecal continence that its condition is preserved and therefore not influenced by age. This is in a like manner to our earlier research which showed that the involuntary contractions of the external anal sphincter are also not influenced by age 7.
It has been reported that voluntary contractions of the external anal sphincter are stronger in men 19. Therefore, we also investigated the influence of sex on both voluntary and involuntary contractions of the puborectal muscle. In the case of voluntary contractions, men had significantly stronger voluntary contractions compared to women. Interestingly, we found that involuntary contractions of the puborectal muscle were not influenced by sex. This suggests once again that voluntary and involuntary contractions of the puborectal muscle are regulated through different nerve pathways.
Clinical implications
Pudendal neuropathy leads to impaired voluntary contraction of the external anal sphincter and puborectal muscle, but this does not necessarily lead to complete faecal incontinence because involuntary contractions of the external anal sphincter and puborectal muscle are not regulated via the pudendal nerve and can preserve faecal continence 7. Further research with nerve blockages might be necessary to identify the exact nerve pathway responsible for involuntary contractions of the puborectal muscle. This knowledge would help prevent accidental surgical damage which currently leads to full faecal incontinence in all age groups.
Limitations
In this study, anal electrosensitivity was used to assess the condition of the pudendal nerve 12, 20. Indirectly, this sensory parameter gives an indication of the status of the motor pathway of the pudendal nerve. It might be argued that no relationship between involuntary contractions and the condition of the pudendal nerve was identified because the sensory pathway only was assessed. Nevertheless, this study demonstrated that voluntary contractions of the puborectal muscle were diminished in patients with a high anal electrosensitivity threshold. Thus, in the case of voluntary contractions, damage to the sensory part of the pudendal nerve negatively influences the function of the motor part. It is not our routine practice to perform pudendal motor nerve terminal latency and electromyography tests because they are very unpleasant and painful for patients. Further, at least two previous studies have reported that these tests do not correspond with responses to treatment for faecal incontinence 21, 22. One possible explanation for this, as suggested by the results of this study, is that the puborectal continence reflex is not regulated by the pudendal nerve.
The results of the current study require further investigation of the possible nerve pathways, including the anorectal receptors belonging to such pathways. This requires additional research using other physiological tests such as rapid balloon distension of the rectum and anal canal as described by Haas et al. 23.
This study showed that involuntary contractions of the puborectal muscle were not associated with the condition of the pudendal nerve. Nevertheless, we cannot exclude the possibility that these contractions might be regulated by an early branch of the pudendal nerve as a consequence of which no association with pudendal neuropathy was found. However, we also showed that the voluntary and involuntary contractions of the puborectal muscle were functionally independent of each other, which makes the probability of an early branch of the pudendal nerve unlikely.
The puborectal continence reflex is not the only involuntary mechanism of the pelvic floor regulating faecal continence. The anal external sphincter continence reflex additionally regulates faecal continence, via involuntary contractions of the external anal sphincter 2. Previous research has shown that this anal external sphincter continence reflex is also not regulated by the pudendal nerve 7. In the current study, we did not analyse the anal external sphincter continence reflex; however, further research about the characteristics, collaboration and possible dual innervation is needed to describe both faecal continence reflexes.
Conclusions
The results of this study confirm that the pudendal nerve mediates voluntary contractions of the puborectal muscle but is not responsible for involuntary contractions of the puborectal muscle, i.e. the puborectal continence reflex. In addition, these voluntary and involuntary contractions are functionally independent of each other. Further studies are required to determine the exact nerve pathway that regulates involuntary contraction of the puborectal muscle. This finding might help avoid accidental damage to the continence mechanism during surgical interventions in the region of the pelvic floor.
Conflicts of interest
The authors declare they have no conflicts of interest.
Acknowledgements
The authors thank Mrs O.J. Pras, Mrs T. de Groot, Mrs S. Gerritsen and Mrs B. Brongers‐Posthuma for their invaluable assistance in the Anorectal Physiology Laboratory and T. van Wulfften Palthe, PhD, for correcting the English manuscript.
There was no grant support.
References
- 1. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004; 126(1 Suppl 1): S14–22. [DOI] [PubMed] [Google Scholar]
- 2. Broens PM, Penninckx FM, Ochoa JB. Fecal continence revisited: the anal external sphincter continence reflex. Dis Colon Rectum 2013; 56: 1273–81. [DOI] [PubMed] [Google Scholar]
- 3. Broens PMA, Jonker JE, Trzpis M. The puborectal continence reflex: a new regulatory mechanism controlling fecal continence. Int J Colorectal Dis 2018; 33: 627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shafik A, el‐Sherif M, Youssef A, Olfat ES. Surgical anatomy of the pudendal nerve and its clinical implications. Clin Anat 1995; 8: 110–5. [DOI] [PubMed] [Google Scholar]
- 5. Vasudevan SP, Scott SM, Gladman MA, Lunniss PJ. Rectal hyposensitivity: evaluation of anal sensation in female patients with refractory constipation with and without faecal incontinence. Neurogastroenterol Motil 2007; 19: 660–7. [DOI] [PubMed] [Google Scholar]
- 6. Parks AG, Swash M, Urich H. Sphincter denervation in anorectal incontinence and rectal prolapse. Gut 1977; 18: 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Meegdenburg MM, Heineman E, Broens PM. Pudendal neuropathy alone results in urge incontinence rather than in complete fecal incontinence. Dis Colon Rectum 2015; 58: 1186–93. [DOI] [PubMed] [Google Scholar]
- 8. Wallner C, van Wissen J, Maas CP, Dabhoiwala NF, DeRuiter MC, Lamers WH. The contribution of the levator ani nerve and the pudendal nerve to the innervation of the levator ani muscles; a study in human fetuses. Eur Urol 2008; 54: 1136–42. [DOI] [PubMed] [Google Scholar]
- 9. Schraffordt SE, Tjandra JJ, Eizenberg N, Dwyer PL. Anatomy of the pudendal nerve and its terminal branches: a cadaver study. ANZ J Surg 2004; 74: 23–6. [DOI] [PubMed] [Google Scholar]
- 10. Barber MD, Bremer RE, Thor KB, Dolber PC, Kuehl TJ, Coates KW. Innervation of the female levator ani muscles. Am J Obstet Gynecol 2002; 187: 64–71. [DOI] [PubMed] [Google Scholar]
- 11. Guaderrama NM, Liu J, Nager CW et al Evidence for the innervation of pelvic floor muscles by the pudendal nerve. Obstet Gynecol 2005; 106: 774–81. [DOI] [PubMed] [Google Scholar]
- 12. Roe AM, Bartolo DC, Mortensen NJ. New method for assessment of anal sensation in various anorectal disorders. Br J Surg 1986; 73: 310–2. [DOI] [PubMed] [Google Scholar]
- 13. Broens PM, Penninckx FM. Relation between anal electrosensitivity and rectal filling sensation and the influence of age. Dis Colon Rectum 2005; 48: 127–33. [DOI] [PubMed] [Google Scholar]
- 14. Speakman CT, Kamm MA. Abnormal visceral autonomic innervation in neurogenic faecal incontinence. Gut 1993; 34: 215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009; 31: b604. [DOI] [PubMed] [Google Scholar]
- 16. Barber MD. Contemporary views on female pelvic anatomy. Cleve Clin J Med 2005; 72(Suppl 4): S3–11. [DOI] [PubMed] [Google Scholar]
- 17. Laurberg S, Swash M. Effects of aging on the anorectal sphincters and their innervation. Dis Colon Rectum 1989; 32: 737–42. [DOI] [PubMed] [Google Scholar]
- 18. Meinds RJ, van Meegdenburg MM, Trzpis M, Broens PM. On the prevalence of constipation and fecal incontinence, and their co‐occurrence, in the Netherlands. Int J Colorectal Dis 2017; 32: 475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda Y, Vaizey CJ, Hollington P, Stern J, Kamm MA. Physiological, psychological and behavioural characteristics of men and women with faecal incontinence. Colorectal Dis 2009; 11: 927–32. [DOI] [PubMed] [Google Scholar]
- 20. Rogers J, Laurberg S, Misiewicz JJ, Henry MM, Swash M. Anorectal physiology validated: a repeatability study of the motor and sensory tests of anorectal function. Br J Surg 1989; 76: 607–9. [DOI] [PubMed] [Google Scholar]
- 21. Brouwer R, Duthie G. Sacral nerve neuromodulation is effective treatment for fecal incontinence in the presence of a sphincter defect, pudendal neuropathy, or previous sphincter repair. Dis Colon Rectum 2010; 53: 273–8. [DOI] [PubMed] [Google Scholar]
- 22. Lacima G, Pera M, Gonzalez‐Argente X, Torrents A, Valls‐Sole J, Espuna‐Pons M. Is electromyography a predictive test of patient response to biofeedback in the treatment of fecal incontinence? Neurourol Urodyn 2016; 35: 390–4. [DOI] [PubMed] [Google Scholar]
- 23. Haas S, Brock C, Krogh K et al Cortical evoked potentials in response to rapid balloon distension of the rectum and anal canal. Neurogastroenterol Motil 2014; 26: 862–73. [DOI] [PubMed] [Google Scholar]


