Abstract
Purpose
The outer stent lumen can be located either deeper (in or under Tenon's layer) or more superficially in the conjunctival stroma after the transscleral XEN Glaucoma Gel Microstent (XEN‐GGM; Allergan Plc., USA) implantation. The present study aimed to investigate the effect of the postoperative conjunctival implant position on surgical success and intraocular pressure (IOP) after XEN‐GGM.
Methods
Prospective data from 66 consecutive open‐angle glaucoma eyes of 54 patients were collected preoperatively and 1 and 2 weeks, and 1, 6 and 12 months postoperatively. The layer of implantation was determined in the first month postoperatively as intra‐ and subtenon or intraconjunctival depending on the location of the outer lumen of the stent in OCT (Visante OCT; Zeiss, Germany). Primary outcome measures were differences in relative IOP reduction at 12 months between the two groups. Further, complete and qualified surgical success, number of secondary needlings and number of IOP‐lowering medications and absolute IOP were assessed.
Results
Relative IOP reduction was higher in intra‐ and subtenon group (n = 37/66, 56%) at week 1 (−54% versus −19%, p < 0.001), week 2 (−39% versus −21%, p = 0.02), month 1 (−42% versus −28%, p = 0.035) and month 12 (−39% versus −24%, p = 0.024). The mean absolute IOP was lower in intra‐ and subtenon group at week 1 (10.8 [95%CI, 8.8–14.1] versus 16.6 [95%CI, 14.1–19.0] mmHg, p < 0.001) and months 12 (13.9 [95%CI, 12.4–15.4] versus 16.7 [95%CI, 14.6–18.8] mmHg, p = 0.041). At month 6, a lower burden for IOP‐lowering medication was shown for the intra‐ and subtenon group (0.2 ± 0.5 versus 1.0 ± 1.1, p = 0.034). The mean number of secondary needlings, which were done in 47/66 (71%) of the eyes, was lower in the intra‐ and subtenon group in the first year (1.9 ± 1.7 versus 1.2 ± 1.2, p = 0.03). Qualified surgical success was higher in the intra‐ and subtenon group (90% versus 61%, p = 0.01) after 1 year.
Conclusion
The present study demonstrates a higher efficacy achieved with lower secondary needling rates in deeper implant positions in conjunctiva after XEN‐GGM.
Keywords: conjunctiva, implant position, intraconjunctival, MIGS, subtenon, XEN
Introduction
Since glaucoma is known to be one of the leading causes of irreversible blindness in the world, much effort has been spent in the development of surgical techniques for glaucoma treatment (Cairns 1968; Leske 1983; Klaver et al. 1998; Hennis et al. 2009; Leske et al. 2010). In the last years, several minimally invasive surgical procedures have been introduced for glaucoma therapy (Saheb & Ahmed 2012; Brandao & Grieshaber 2013; Saheb et al. 2013). These new surgical techniques are subsumed under the term minimally invasive glaucoma surgery (MIGS). Procedures within the MIGS group are requested to reduce intraocular pressure (IOP), decrease the need for additional glaucoma medication and determine a high safety profile (Saheb & Ahmed 2012).
The XEN Glaucoma Gel Microstent (XEN‐GGM; Allergan Plc, USA) is a member in the MIGS group (Sheybani et al. 2015a,b). The length of the XEN‐GGM implant is 6 mm, with an inner lumen width of 45 μm. It bypasses aqueous humour from the anterior chamber to the subconjunctival space. The route of implantation starts through a clear corneal incision, follows the opposite angle in the anterior chamber, through the sclera, and ends in the subconjunctival space. The XEN‐GGM creates comparable outflow paths like the classic trabeculectomy while minimizing conjunctival trauma during the process of implantation (Sheybani et al. 2015a,b). During XEN‐GGM implantation, classic dissection of the conjunctiva, as is done during trabeculectomy, is not necessary. At the end of the procedure, the inner lumen of the XEN‐GGM is localized in the angle of the anterior chamber with a length of approximately 1 mm. To connect the XEN‐GGM to the subconjunctival space, the outer lumen of the stent is situated approximately 5 mm posterior to the limbus suprascleral.
The bulbar conjunctiva consists of a superficial epithelial layer and a stromal layer. It borders the sclera with Tenon's layer (Zhang et al. 2011; Howlett et al. 2014). Postoperatively, the final position of the outer lumen of the XEN‐GGM can be either near the sclera (intra‐ and subtenon) or more superficially (intraconjunctival).
In a previous study, the authors could show a correlation of intrableb morphology with IOP and surgical long‐term success (Lenzhofer et al. 2018). Not only the bleb morphology after XEN‐GGM implantation, but also the positioning of the outer lumen of the stent (whether a deeper intra‐ and subtenon or superficial intraconjunctival positioning is present) may influence surgical outcomes. Therefore, the present study aimed to investigate the effect of the outer implant position on surgical success, IOP and IOP‐lowering medication in the first year after the XEN‐GGM implantation.
Methods
The study and data accumulation were carried out with prospective approval from the local ethics committee. Informed consents were obtained, and the study was in adherence with the tenets of the Declaration of Helsinki. In this prospective, single‐armed, single‐centre, longitudinal clinical study, patients with open‐angle glaucoma (OAG) and insufficiently controlled IOP or intolerance to topical glaucoma therapy were treated with the XEN‐GGM (45 μm) with or without combined cataract surgery. Exclusion criteria were a foregoing glaucoma surgery, a lack of free and mobile conjunctiva in the quadrant of implantation, congenital glaucoma, neovascular glaucoma or secondary glaucoma related to uveitis, as well as another previous intraocular surgery, except selective laser trabeculoplasty or uncomplicated phacoemulsification with intracapsular lens implantation. Sixty‐six consecutive eyes of 54 patients were enrolled and received one XEN‐GGM, as described previously (Schlenker et al. 2017). Twenty minutes prior to XEN‐GGM implantation, a balanced salt solution (BSS) plus mitomycin C (MMC) was injected subtenon using a 30‐gauge needle (0.05–0.1 ml, 4–8 μg MMC total). The concentration was determined by the discretion of the surgeon. If the injected MMC distributed distinct, a higher volume (0.1 ml) was injected compared to when the injected MMC distributed diffuse over several clock hours, then less amount of MMC was injected (0.05 ml). Primary needlings during the XEN‐GGM implantation were done to ensure that the outer lumen of the stent was completely free and mobile in its layer of implantation in every patient. The primary needling technique can be seen in Video Clip S1.
The postoperative care regimen included a stop of IOP‐lowering drops postoperatively. Topical steroid eye drops (TID) were applied for a minimum of 6 weeks postoperatively and slowly tapered out after 6 weeks. TID were additionally applied in the first postoperative week. If the investigator decided to return to IOP‐lowering medications postoperatively, subjects were prescribed the same IOP‐lowering medications that were used at preoperatively in a stepwise fashion by introducing one drug class at a time.
Visits were scheduled at baseline (BL), weeks 1 (W1) and 2 (W2), and months 1 (M1), 6 (M6) and 12 (M12) postoperatively. BL demographic data included type of glaucoma, IOP and number of glaucoma medications. Postoperative visits included IOP, number of glaucoma medications and secondary IOP‐lowering procedures. The layer of implantation was determined by anterior segment optical coherence tomography (OCT; Visante OCT, Carl Zeiss Meditec AG, Germany) in the first month, in particular before any secondary needling procedure. The conjunctiva was imaged tangentially to the XEN‐GGM at the outer stent lumen and rectangular to the first section through the outer lumen (Fig. 1). The two scans were used to classify the layer of implantation. As shown by Howlett et al. (2014), Tenon's layer in the OCT appears as a hyperreflective section as opposed to the hyporeflective section underlying the sclera. The conjunctival stroma in OCT consists of irregular fibres, perfused blood vessels and cystic spaces and therefore appears more irregular (Howlett et al. 2014). The assignment to a group was done blinded without the knowledge of the actual IOPs or number of medication. The layer of implantation was categorized as intra‐ and subtenon or intraconjunctival according to the following criteria by the same observer (ML; Fig. 2):
Figure 1.
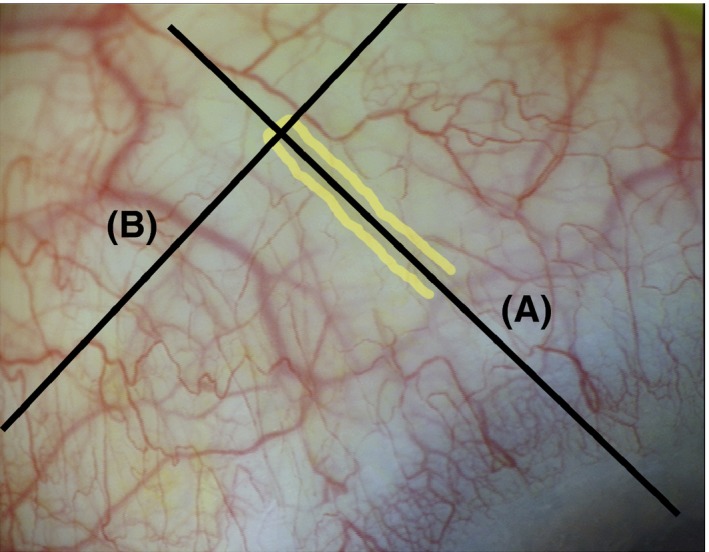
OCT sections of the XEN Glaucoma Gel Microstent (XEN‐GGM) in the conjunctiva to determine the layer of implantation. The XEN‐GGM is highlighted in yellow. The conjunctiva was imaged tangentially to the XEN‐GGM at the outer stent lumen (A) and rectangular to the first section through the outer lumen (B) to determine the layer of implantation.
Figure 2.
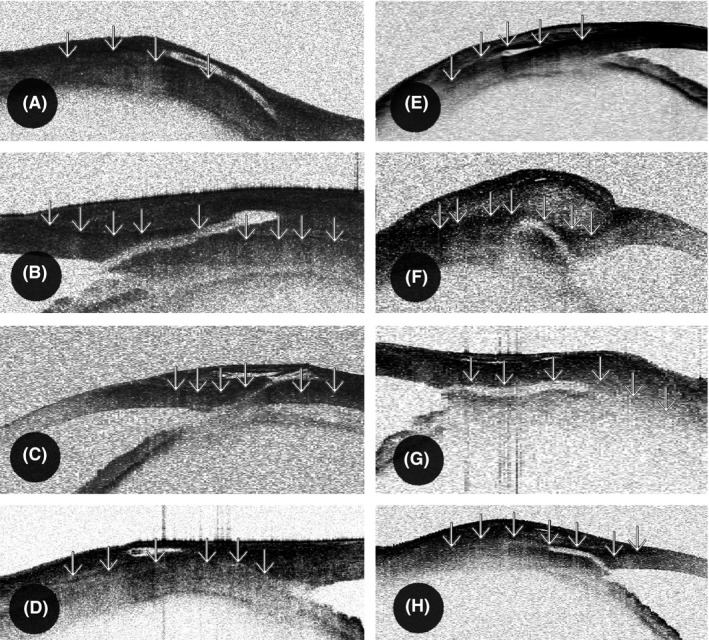
Position of the outer XEN Glaucoma Gel Microstent (XEN‐GGM) lumen in the conjunctiva visualized by OCT. As shown by Howlett et al., Tenon's layer (‘↓’) in the OCT appears as a hyperreflective section as opposed to the hyporeflective section underlying the sclera. The conjunctival stroma in OCT consists of irregular fibres, perfused blood vessels and cystic spaces and therefore appears more irregular. A–D were classified to the intraconjunctival group (Tenon's layer below the outer stent lumen in OCT) and E–H to intra‐ and subtenon group (Tenon's layer above the outer stent lumen in OCT).
Intraconjunctival implantation: (i) Tenon's layer below the outer stent lumen in OCT (Howlett et al. 2014), (ii) obvious superficial implant position in OCT due to a very superficial stent position without tenting of Tenon's layer, and (iii) fluid‐filled spaces underneath the outer lumen of the stent in OCT without fluid on top of the implant indicating a superficial implant position.
Intra‐ and subtenon implantation: (i) none of the above criteria fulfilled, (ii) Tenon's layer on top of the outer stent lumen in OCT (Howlett et al. 2014), and (iii) lack of fluid‐filled spaces underneath but fluid on top of the outer lumen of the stent in OCT indicating a deep implant position.
Primary end‐points were differences in relative IOP reduction at M12 between the two groups. Secondary end‐points were differences in relative IOP reduction at the remaining postoperative visits, in absolute IOP and in IOP‐lowering medication at all postoperative visits, in number of secondary needlings in the first year and in surgical success at M12 between the two groups.
According to the definition by the World Glaucoma Association (Shaarawy et al. 2009), all patients were classified in two groups based on their IOP at M12 (relative IOP reduction ≥ 20% compared with BL was defined as surgical success, relative IOP reduction < 20% compared with BL was defined as surgical failure). In the case of an increase in the number of medications compared with BL and secondary surgical procedure (except for secondary needling procedures or standard cataract operation), loss to follow‐up, loss of light perception acuity or worse or a postoperative IOP of < 6 mmHg, the patient was also classified in the surgical failure group. Surgical success was further characterized according to whether it was achieved without (complete success) or with ocular hypotensive medication (qualified success).
Statistical methods
Data were checked for consistency in terms of typing errors, and ranges were inspected for validity. Because data did not follow normal distributions, generalized estimation equation models were used to analyse the IOP (mmHg) and change in IOP in percentages. Both variables were modelled using Gamma distributions. The identity function was used as a link function. Group, time and the interaction factor were used to set up the model. Preoperative IOP was used as a covariate to model IOP (mmHg). The robust estimator for the covariance matrix was used. Least significant difference tests were used for pairwise comparisons of means. Means with 95% confidence intervals (CIs) were computed. The Kruskal–Wallis test for singly ordered tables based on Monte Carlo methods was used to analyse cross‐tabulations. All tests were two‐sided, and p < 0.05 was considered statistically significant. All statistical analyses in this report were performed by Mathematica 7 (Wolfram Research, Inc., Mathematica, version 7.0, Champaign, IL), STATISTICA 12 (Hill, T., & Lewicki, P., Statistics: Methods and Applications. StatSoft, Tulsa, OK), PASW 23 (IBM SPSS Statistics for Windows, version 21.0., Armonk, NY) and StatXact 10 (Cytel Software 2013, Cambridge, MA, USA).
Results
Twenty‐nine right eyes (44%) and 37 left eyes (56%) of 38 female (58%) and 28 male (42%) patients were enrolled. In total, 66 eyes from 54 patients were assessed. Thirty‐four eyes (52%) had combined XEN‐GGM plus cataract surgery, and 32 eyes (48%) underwent a solo XEN‐GGM procedure (Table 1). Twenty‐two eyes (33%) had pseudoexfoliation glaucoma, 1 eye (2%) had pigment dispersion glaucoma, and 41 eyes (65%) had primary OAG. The mean age of the patients was 72.2 ± 12.5 years (95% CI, 69.7–74.8) at the time of operation. The mean BL IOP was 23.5 ± 5.6 mmHg (95% CI, 22.0–25.1), and the number of preoperative IOP‐lowering medications was 3.3 ± 0.6 (95% CI, 3.2–3.5). The mean central corneal thickness was 529.2 ± 36.0 μm (95% CI, 520.1–538.3). In 47 eyes (71%), one or more secondary needlings were performed in the first year. In total, 98 secondary needling procedures were performed in the first year with a maximum of five secondary needling procedures at one single eye. The mean time to first secondary needling after surgery was 143 ± 132 days (95% CI, 105–182). None of the patients had a second XEN‐GGM implantation.
Table 1.
Intra‐ and subtenon versus intraconjunctival implantation of XEN Glaucoma Gel Microstent.
| Intra‐ and subtenon group ± SD, n = 29 | Intraconjunctival group ± SD, n = 37 | Both groups together ± SD, n = 66 | p‐Value | |
|---|---|---|---|---|
| Patients | ||||
| Combined XEN‐GGM plus cataract surgery (n) | 18 (62%) | 16 (43%) | 34 (52%) | 0.15 |
| XEN‐GGM solo procedure (n) | 13 (45%) | 19 (51%) | 32 (48%) | 0.63 |
| Pseudoexfoliation present | 14 (36%) | 8 (22%) | 22 (33%) | 0.21 |
| Mean deviation in visual field (dB) | −10.5 ± 7.3 | −10.9 ± 8.5 | −10.7 ± 7.9 | 0.83 |
| IOP | ||||
| IOP baseline (mmHg) | 24.2 ± 5.9 | 21.9 ± 5.7 | 23.5 ± 5.6 | 0.11 |
| IOP 1 week (mmHg) | 10.8 ± 5.3 | 16.6 ± 7.3 | 13.9 ± 7.0 | <0.001a |
| IOP 2 weeks(mmHg) | 14.1 ± 6.2 | 16.9 ± 5.6 | 15.6 ± 6.3 | 0.054 |
| IOP 1 month (mmHg) | 13.4 ± 5.2 | 15.8 ± 5.6 | 14.5 ± 6.1 | 0.077 |
| IOP 6 months (mmHg) | 15.6 ± 6.7 | 15.7 ± 6.4 | 15.6 ± 6.4 | 0.92 |
| IOP 12 months (mmHg) | 13.9 ± 4.1 | 16.7 ± 6.1 | 15.1 ± 5.6 | 0.041a |
| IOP Reduction (%) from baseline to | ||||
| 1 week | −54 ± 23 | −19 ± 48 | −35 ± 43 | <0.001a |
| 2 weeks | −39 ± 28 | −21 ± 32 | −29 ± 31 | 0.02a |
| 1 month | −42 ± 25 | −28 ± 27 | −34 ± 27 | 0.035a |
| 6 months | −31 ± 30 | −27 ± 32 | −29 ± 31 | 0.53 |
| 12 months | −39 ± 24 | −24 ± 29 | −31 ± 28 | 0.024a |
| Medications | ||||
| Medications baseline | 3.3 ± 0.7 | 3.4 ± 0.6 | 3.3 ± 0.6 | 0.83 |
| Medications 1 week | 0.1 ± 0.8 | 0.0 ± 0.0 | 0.1 ± 0.5 | 0.89 |
| Medications 2 weeks | 0.0 ± 0.2 | 0.3 ± 0.8 | 0.2 ± 0.6 | 0.10 |
| Medications 1 month | 0.4 ± 1.0 | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.76 |
| Medications 6 months | 0.2 ± 0.5 | 1.1 ± 1.1 | 0.7 ± 1.0 | 0.034a |
| Medications 12 months | 1.0 ± 1.1 | 1.0 ± 1.2 | 1.0 ± 1.1 | 0.93 |
indicates statistical significance p < 0.05, standard deviation (SD), XEN Glaucoma Gel Microstent (XEN‐GGM), and p‐values refer to the comparison of the intra‐ and subtenon group versus intraconjunctival group.
At each visit, a significant reduction in mean IOP and reduction in mean IOP‐lowering medication could be achieved after XEN‐GGM implantation (each p < 0.001). The mean postoperative IOP ranged from 13.9 ± 7.0 mmHg (95% CI, 12.1–15.7) at W1 to 15.6 ± 6.3 mmHg (95% CI, 14.0–17.2) at W2 within the first year postoperatively at different postoperative visits. The mean IOP at M12 was 15.1 ± 5.6 mmHg.
Twenty‐nine of 66 (44%) eyes were graded into the intra‐ and subtenon group, and 37 of 66 (56%) eyes were classified into the intraconjunctival group. There was no difference in depth of insertion between the left and right eye (p = 1.0). At BL or M12, there were no differences in IOP between combined cataract versus solo procedure (combined: 23.8 ± 6.1 mmHg, solo: 22.2 ± 4.6 mmHg, p = 0.27; combined: 15.3 ± 5.0 mmHg, solo: 15.0 ± 6.2 mmHg, p = 0.44), presence of pseudoexfoliation (no: 22.2 ± 4.9 mmHg, yes: 24.7 ± 6.2 mmHg, p = 0.25; no: 15.8 ± 5.9 mmHg, yes: 13.9 ± 5.0 mmHg, p = 0.23), pigmentary dispersion (no: 22.9 ± 5.4 mmHg, yes: 31.0 ± n/a mmHg, p = 0.15; no: 15.0 ± 5.6 mmHg, yes: 19.5 ± n/a mmHg, p = 0.50), position of the eye (right: 23.9 ± 6.6 mmHg, left: 22.3 ± 4.3 mmHg, p = 0.54; right: 14.5 ± 3.9 mmHg, left: 15.5 ± 6.6 mmHg, p = 0.59) or sex (female: 21.8 ± 4.4 mmHg, male: 24.6 ± 6.3 mmHg, p = 0.13; female: 15.8 ± 6.7 mmHg, male: 13.7 ± 5.4 mmHg, p = 0.1).
Mean numbers of secondary needlings were lower in the intra‐ and subtenon group compared with the intraconjunctival group in the first year (1.2 ± 1.2 [95% CI, 0.7–1.6] versus 1.9 ± 1.7 [95% CI, 1.3–2.5], p = 0.03), although no statistical difference in the percentage of patients receiving one or more secondary needlings (intra‐ and subtenon group: 68%, intraconjunctival group: 80%, p = 0.70) could be revealed.
No patient had loss to light perception acuity or worse or a postoperative IOP of < 6 mmHg. In one eye, a stent explanation had to be done due to persisting bleb erosion. This eye was graded into the intraconjunctival group due to OCT data and further classified into surgical failure group. The bleb erosion did not happen spontaneously but rather accidentally during a secondary needling procedure.
IOP and number of medications
Preoperative IOP and preoperative number of IOP‐lowering medications (intra‐ and subtenon: 3.3 ± 0.7, intraconjunctival: 3.4 ± 0.6, p = 0.83) showed no statistical difference under the groups, although there was a trend towards a lower mean IOP in the intraconjunctival group compared with the intra‐ and subtenon group preoperatively (21.9 ± 4.5 [95% CI, 20.4–23.4] mmHg versus 24.3 ± 6.6 [95% CI, 21.8–26.8] mmHg, p = 0.11; Fig. 3).
Figure 3.
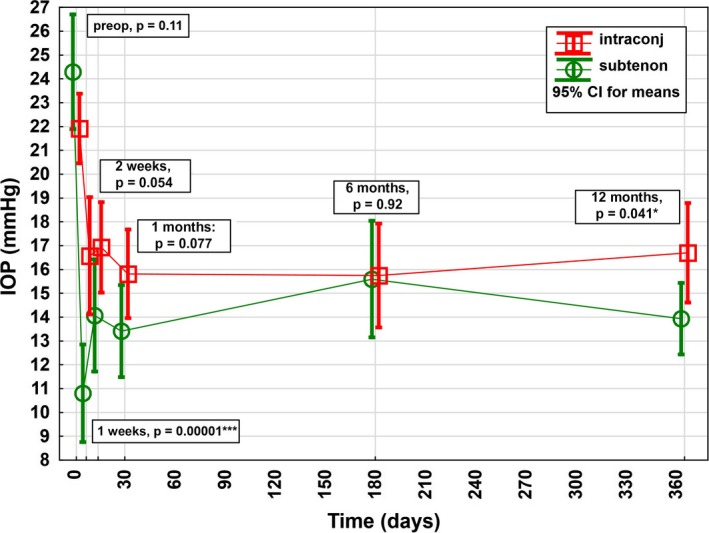
Intraocular pressure (IOP) course in the first postoperative year after XEN Glaucoma Gel Stent implantation stratified by layer of implantation of the outer lumen of the stent. Preoperatively, there was no significant difference in the mean baseline IOPs between the 2 groups, although there was a trend towards higher IOP in the intra‐ and subtenon group compared with the intraconjunctival group preoperatively. Postoperatively, a significantly lower mean IOP was shown in the intra‐ and subtenon group compared with the intraconjunctival group at 1 week and 12 months.
The mean relative reduction in IOP was higher in the intra‐ and subtenon group compared with the intraconjunctival group at W1 (–54% [95% CI, –46% to –63%] versus –19% [95% CI, –3% to –35%], p < 0.001), W2 (–39% [95% CI, –29% to –50%] versus –21% [95% CI, –11% to –32%], p = 0.02), M1 (–42% [95% CI, –33% to –51%] versus –28% [95% CI, –19% to –37%], p = 0.035) and M12 (–39% [95% CI, –30% to –47%] versus –24% [95% CI, –14% to –33%], p = 0.024; Fig. 4). At M6, there was no statistically significance but rather only a trend was visible in higher IOP reduction in the intra‐ and subtenon group (–31% [95% CI, –21% to –41%] versus –27% [95% CI, –16% to –37%], p = 0.53).
Figure 4.
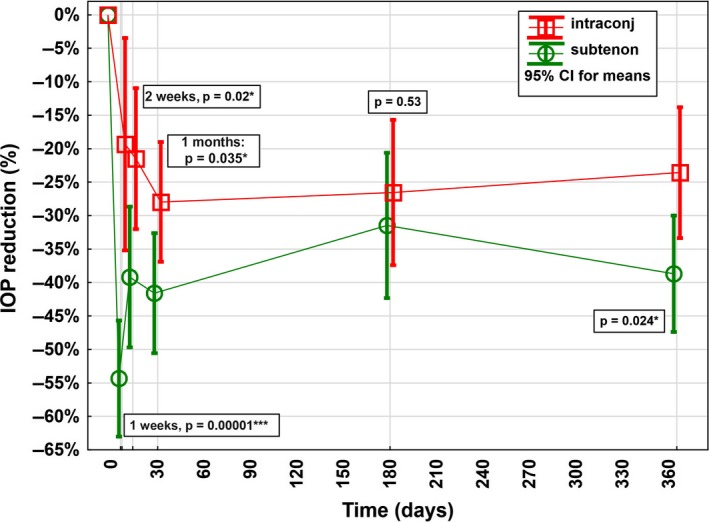
Relative intraocular pressure (IOP) reduction in the first postoperative year after XEN Glaucoma Gel Stent implantation stratified by layer of implantation of the outer lumen of the stent. Postoperatively, a statistically significant higher mean relative IOP reduction could be detected in the intra‐ and subtenon group compared with the intraconjunctival group at 1 and 2 weeks, as well as at 1 and 12 months.
Further, the postoperative mean absolute IOP was lower in the intra‐ and subtenon group compared with the intraconjunctival group at W1 (10.8 [95% CI, 8.8–14.1] versus 16.6 [95% CI, 14.1–19.0] mmHg, p < 0.001) and M12 (13.9 [95% CI, 12.4–15.4] versus 16.7 [95% CI, 14.6–18.8] mmHg, p = 0.041). During the remaining postoperative visits, no statistical difference was shown, although a trend towards a lower IOP in the intra‐ and subtenon group was recorded postoperatively throughout.
At M6, a lower burden for IOP‐lowering medication was shown for the intra‐ and subtenon group compared with the intraconjunctival group (0.2 ± 0.5 versus 1.0 ± 1.1, p = 0.034), while the other postoperative visits showed no statistically significant difference between the two groups in number of IOP‐lowering medications (Fig. 5).
Figure 5.
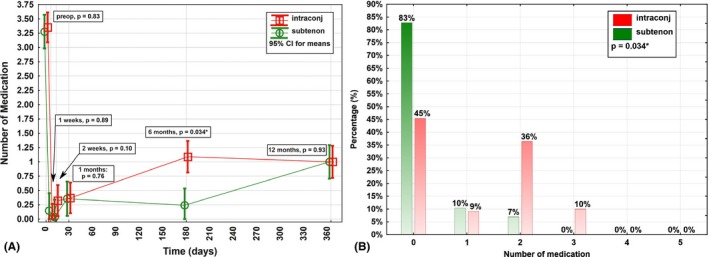
Course for number of intraocular pressure (IOP)‐lowering medications in the first postoperative year after XEN Glaucoma Gel Stent implantation stratified by layer of implantation of the outer lumen of the stent. (A) One has to point out that at all remaining postoperative visits, at which no significant difference in number of medications could be shown, a statistically significant higher mean IOP reduction was shown for the intra‐ and subtenon group (see Fig. 2). (B) Postoperatively, a statistically significant lower number of medications could be detected in the intra‐ and subtenon group compared with the intraconjunctival group at 6 months.
Surgical success
The intra‐ and subtenon group showed higher qualified surgical success (90%; 95% CI, 73%–98%) compared with the intraconjunctival group (61%; 95% CI, 44%–77%) at M12 (p = 0.01). The odds ratio was 5.5 (95% CI, 1.3–33; p = 0.018), and the ratio of proportions was 1.47 (95% CI, 1.1–2.0, p = 0.009). Full surgical success was not statistically significantly different between both groups at M12 (39% versus 44%, p = 0.81).
Discussion
Under the new surgical treatment options, which equipoise in the surgical glaucoma treatment algorithm, the XEN‐GGM shows promising first efficacy data (Saheb & Ahmed 2012; Brandao & Grieshaber 2013; Saheb et al. 2013; Galal et al. 2017; Schlenker et al. 2017; Lenzhofer et al. 2018, 2019). During the procedure, a defined shunt between the anterior chamber and the subconjunctival space is created with a needle‐based injector ab interno, as it is created during the trabeculectomy manually ab externo. The final implant of the outer lumen of the XEN‐GGM can be positioned either superficially in the conjunctival stroma (intraconjunctival) or deeper near the sclera (intra‐ or subtenon).
Our patient population is comparable to already published results investigating XEN‐GGM efficacy (Saheb & Ahmed 2012; Brandao & Grieshaber 2013; Saheb et al. 2013; Galal et al. 2017; Schlenker et al. 2017; Lenzhofer et al. 2018, 2019). Overall success rates and efficacy (IOP and number of medications) results are comparable to the recently published APEX study (Reitsamer et al. 2019). There are no data yet published establishing whether a deep or superficial implantation in the conjunctiva is advantageous for an efficacious patient outcome after XEN‐GGM implantation. Therefore, the layer of implantation in the conjunctiva was measured via OCT during the first postoperative month in this prospective study. The OCT is capable of distinguishing Tenon's layer from the stromal layer of the conjunctiva and therefore shows the position of the stent after the implantation (Howlett et al. 2014).
Primary outcome measures in this study were long‐term efficacy parameters, while a detailed safety analysis was not intended and not feasible because of the number of included patients. However, one bleb erosion was reported during the study period – this eye was graded into the intraconjunctival group due to OCT data. This erosion was associated with a foregoing secondary needling procedure. Although a deeper (intra‐ or subtenon) position of the outer XEN‐GGM lumen is not able to ensure prevention of bleb erosions, a more superficial position of the outer XEN‐GGM lumen could bare a higher risk of bleb erosions, bleb infections and consecutive endophthalmitis.
The present study clearly shows higher efficacy in the deep conjunctiva (the intra‐ and subtenon group) after XEN‐GGM implantation, while this could be achieved with a lower number of secondary needlings in this group. Although not in every postoperative visit a difference in mean absolute IOP between the two groups could be detected postoperatively, the results may be clinically relevant. Comparing the proportion of patients receiving one or more secondary needling in both groups, no statistical difference could be shown.
The OCT grading was done in the first postoperative month and before any secondary needling procedure. Primary needlings during the XEN‐GGM implantation were done cautiously and always parallel to the conjunctival surface. Although primary needlings might connect different layers, different layers in the conjunctiva are connected anyway. We have to point out that better efficacy was present in deeper XEN‐GGM implantation (intra‐ and subtenon) in combination with primary needlings, although some results were only slightly significant. A further limitation of the study is, that a single observer did the grading; on the other hand, we here have to point out that the grading was done blinded and independently to the knowledge of the actual IOP and medications of the patients. The lack of randomization of the two groups, the not standardized re‐introduction of the medication and various concentrations of MMC applied during the XEN‐GGM surgery could confound results. Therefore further prospective randomized investigations on implant efficacy and safety with larger sampling sizes are suggested.
During the XEN‐GGM implantation, it is difficult to distinguish exactly Tenon's, subtenon's or stromal layers with the help of the operation microscope without OCT function, especially when a foregoing BSS (+MMC) injection has altered the physiological thickness of the conjunctiva. However, aiming for a deep or a superficial outer stent position can be done during the operation with the operation microscope.
How to better achieve the deeper stent position?
During trabeculectomy, which is still the gold standard in filtering glaucoma surgery, some surgeons use a foregoing subtenon anaesthesia (Jones et al. 2005). Beside its anaesthetic effects, it also separates Tenon's layer from sclera. This allows the mobility of the conjunctiva to be checked for selection of the surgical site and for easier bleb preparation (Jones et al. 2005). It also gives the surgeon the possibility of simultaneously applying MMC. Based on this principle, foregoing hydrodissection with BSS (+MMC) also helps the surgeon to reach the aimed layer of implantation with the XEN‐GGM. The BSS (+MMC) injection can be placed superficially (in the conjunctival stroma) or deeper (in or under Tenon's layer). Therefore, whether the final implant position of the outer lumen of the stent is deeper or more superficial at least partially depends on the location of the foregoing BSS injection. To form a fluid‐filled space under Tenon's layer, a 30‐gauge needle with bevel up is advanced parallel to the sclera, while a smooth pressure perpendicular to the eyes surface adjusts the depth of the needle. Avoiding vessels, the needle is advanced until the full bevel is under Tenon's layer. BSS (+MMC) should be injected carefully (Video Clip S2).
A deep position of the outer lumen of the stent is not trivial to be approached during XEN‐GGM implantation, since the aimed layer is very thin. A too‐deep stent position is associated with a higher risk of functional failure due to a malconnection of the stent to the conjunctival area with a locked stent in the sclera. Therefore, advancing the injector until the complete bevel of the injector needle is visible outside of the sclera is mandatory before deploying the implant. At this point, the surgeon can choose to advance the injector parallel to the sclera or in the direction of the conjunctival surface. The implant can then be deployed.
Aiming for a deeper layer of implantation might make a captured implant more likely, since Tenon's layer is firmer compared with the stromal conjunctiva. In the authors’ eyes, it is very important that the outer lumen of the implant be completely free and mobile after implantation. A primary needling should be done carefully if the implant is not free and mobile in the conjunctiva after implantation (Video Clip S1).
This is the first study to systematically describe efficacy outcomes after XEN‐GGM implantation based on the layer of implantation of the outer stent lumen. The present study demonstrates that a deeper outer lumen implant position in the conjunctiva (intra‐ and subtenon group) after XEN‐GGM surgery results in better reduction in IOP and higher success rates achieved with lower secondary needling rates, while keeping in mind that a primary needling was done in each patient in this study. We want to indicate that a deep implant position might further reduce the risk of endophthalmitis because of the potentially lower risk for bleb erosions.
Supporting information
Video Clip S1. Primary Needling.
Video Clip S2. Hydrodissection.
Acknowledgments/disclosure: This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. HR received financial support from Allergan Plc as a consultant; all other authors declare no conflict of interest.
References
- Brandao LM & Grieshaber MC (2013): Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol 2013: 705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns JE (1968): Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 66: 673–679. [PubMed] [Google Scholar]
- Galal A, Bilgic A, Eltanamly R & Osman A (2017): XEN glaucoma implant with mitomycin C 1‐year follow‐up: result and complications. J Ophthalmol 2017: 5457246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennis AJ, Wu SY, Nemesure B, Hyman L, Schachat AP & Leske MC (2009): Nine‐year incidence of visual impairment in the Barbados Eye Studies. Ophthalmology 116: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett J, Vahdani K & Rossiter J (2014): Bulbar conjunctival and Tenon's layer thickness measurement using optical coherence tomography. J Curr Glaucoma Pract 8: 63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Clarke J & Khaw PT (2005): Recent advances in trabeculectomy technique. Curr Opin Ophthalmol 16: 107–113. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Wolfs RC, Vingerling JR, Hofman A & de Jong PT (1998): Age‐specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol 116: 653–658. [DOI] [PubMed] [Google Scholar]
- Lenzhofer M, Hohensinn M, Strohmaier C & Reitsamer HA (2018): [Subconjunctival minimally invasive glaucoma surgery: methods and clinical results]. Ophthalmologe 115: 381–387. [DOI] [PubMed] [Google Scholar]
- Lenzhofer M, Strohmaier C, Hohensinn M et al. (2019): Longitudinal bleb morphology in anterior segment OCT after minimally invasive transscleral ab interno Glaucoma Gel Microstent implantation. Acta Ophthalmol 97: e231–e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC (1983): The epidemiology of open‐angle glaucoma: a review. Am J Epidemiol 118: 166–191. [DOI] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Nemesure B & Hennis A (2010): Causes of visual loss and their risk factors: an incidence summary from the Barbados Eye Studies. Rev Panam Salud Publica 27: 259–267. [DOI] [PubMed] [Google Scholar]
- Reitsamer H, Sng C, Vera V, Lenzhofer M, Barton K & Stalmans I (2019): Two‐year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open‐angle glaucoma. Graefes Arch Clin Exp Ophthalmol 257: 983–996. [DOI] [PubMed] [Google Scholar]
- Saheb H, Ahmed II (2012): Micro‐invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 23: 96–104. [DOI] [PubMed] [Google Scholar]
- Saheb H, Ianchulev T & Ahmed II (2013): Optical coherence tomography of the suprachoroid after CyPass Micro‐Stent implantation for the treatment of open‐angle glaucoma. Br J Ophthalmol 98: 19–23. [DOI] [PubMed] [Google Scholar]
- Schlenker MB, Gulamhusein H, Conrad‐Hengerer I et al. (2017): Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology 124: 1579–1588. [DOI] [PubMed] [Google Scholar]
- Shaarawy T, Grehn F & Sherwood M; World Glaucoma Association (2009): Guidelines on design and reporting of glaucoma surgical trials.
- Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II (2015a): Phacoemulsification combined with a new ab interno gel stent to treat open‐angle glaucoma: pilot study. J Cataract Refract Surg 41: 1905–1909. [DOI] [PubMed] [Google Scholar]
- Sheybani A, Reitsamer H, Ahmed II (2015b): Fluid dynamics of a novel micro‐fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci 56: 4789–4795. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li Q, Liu B et al. (2011): In vivo cross‐sectional observation and thickness measurement of bulbar conjunctiva using optical coherence tomography. Invest Ophthalmol Vis Sci 52: 7787–7791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Clip S1. Primary Needling.
Video Clip S2. Hydrodissection.


