Abstract
Objective
This analysis assessed migraine‐related burden and treatment decisions in Chronic Migraine Epidemiology and Outcomes (CaMEO) Study survey respondents who stopped taking acute prescription medications for migraine.
Background
Migraine is a common yet underdiagnosed and undertreated neurological disease often associated with significant disability. Acute prescription medications are underused, in part because patients discontinue treatment. Rates and reasons for discontinuing acute prescription medications require exploration.
Methods
The CaMEO Study is a longitudinal, Internet‐based survey that identified and followed people who met modified ICHD‐3 migraine criteria. For this analysis, eligible respondents had used acute prescription medication for migraine in the past but no longer used or kept these treatments on hand (discontinued users). Respondents who reported discontinuing acute prescription treatment answered questions about length of time since last use and reasons for stopping. Reasons for discontinuing were thematically summarized. Monthly headache day frequency, Migraine Disability Assessment (MIDAS), Patient Health Questionnaire 9‐item depression screener, Generalized Anxiety Disorder 7‐item screener, and the 12‐item Allodynia Symptom Checklist were also assessed.
Results
Of 13,624 respondents with migraine, 4840 (35.5%) had ever used acute prescription medications and 1719 (35.5%) of those were discontinued users. Discontinued users had a mean (SD) age of 42.1 (14) years, and 1348/1719 (78.4%) were female. Monthly headache frequency of 0‐4 days was reported by 1073/1719 (62.4%) of respondents, 5‐9 days by 322/1719 (18.7%), 10‐14 days by 135/1719 (7.9%), and ≥15 days by 189/1719 (11.0%). Two‐thirds (1160/1719 [67.5%]) of discontinued users reported a receiving migraine (or chronic migraine) diagnosis from a doctor or other health professional in the past. Although all had spoken to a doctor about their headaches, 1504/1719 (87.5%) had stopped having their headaches managed or treated by a doctor for at least 12 months. Only 1 in 5 discontinued users reported being able to work or function normally with a headache, and 717/1719 (41.7%) had moderate to severe disability (MIDAS). Among the most commonly reported reasons for prescription medication discontinuation were switching to non‐prescription pain medication (782/1719 [45.5%]), as well as concerns about prescription medication efficacy (484/1719 [28.2%]) and tolerability (428/1719 [24.9%]). Nearly half of respondents who reported either efficacy or tolerability concerns had moderate to severe disability.
Conclusions
People with migraine who discontinue acute prescription medication have a high level of unmet treatment need. The majority cannot work or function normally with headaches, with 646/1719 (37.6%) of discontinued users reporting 5 or more headache days per month.
Keywords: Chronic Migraine Epidemiology and Outcomes, migraine, disability, triptans, acute treatment
Abbreviations
- AMPP
American Migraine Prevalence and Prevention
- AMS
American Migraine Study
- ASC‐12
12‐item Allodynia Symptom Checklist
- CaMEO
Chronic Migraine Epidemiology and Outcomes
- GAD‐7
Generalized Anxiety Disorder 7‐item screener
- ICHD‐3
International Classification of Headache Disorders, 3rd edition
- MAST
Migraine in America Symptoms and Treatment
- MIDAS
Migraine Disability Assessment
- NSAID
nonsteroidal anti‐inflammatory drug
- PHQ‐9
Patient Health Questionnaire 9‐item depression screener
Introduction
Migraine is a chronic disease with episodic attacks that affects approximately 1 in 7 individuals worldwide.1 Migraine attacks are characterized by moderate to severe headache pain that is often throbbing and frequently unilateral, and often accompanied by nausea, vomiting, and sensitivity to light, sound, or movement.2 According to the World Health Organization and the Global Burden of Disease Study (2017), migraine is the second‐highest cause of years lived with disability.1
The primary goals for the acute treatment of migraine attacks include the rapid relief of pain and associated symptoms, restoration of function, minimal recurrence, reducing subsequent resource use, being cost‐effective, and minimizing the occurrence of adverse events.3 Widely used options for the acute pharmacologic treatment of migraine attacks include medications that are not specific for migraine, such as nonsteroidal anti‐inflammatory drugs (NSAIDs), opioids, and barbiturates, as well as migraine‐specific medications, such as triptans and ergots.4 However, for many individuals, these acute treatments are associated with limited effectiveness, poor tolerability, or contraindications.5 For example, because of potential complications, NSAIDs should be used with caution in individuals with cardiovascular disease, and avoided in those with ulcers or renal disease, or those taking anticoagulants.6 Triptans are contraindicated in individuals with cardiovascular disease and use is restricted in patients with multiple cardiovascular risk factors.7, 8 Furthermore, opioids and barbiturates are not recommended for the acute treatment of migraine attacks because of limited efficacy and the risk of tolerance, dependence, and migraine progression.9, 10
Acute treatment options for migraine attacks are often poorly optimized for many people and may fail to meet treatment goals.11, 12, 13, 14 Suboptimal acute treatment of migraine attacks can be associated with an increased likelihood of disability and a substantially increased risk of migraine disease progression.14, 15 Poorly controlled or uncontrolled attacks may lead to overuse of acute headache medications. Acute medication overuse is associated with greater headache severity and increased pain intensity.16 Excessive use of medication is also associated with a greater likelihood of progression from episodic migraine to chronic migraine.17, 18
In the prior American Migraine Prevalence and Prevention (AMPP) study, fewer than 1 in 5 people with migraine reported the use of migraine‐specific prescription medications for the acute treatment of migraine.4 Many individuals with migraine report dissatisfaction with their current acute treatment, with a high number of individuals reporting discontinuing or switching to alternative treatment options.19, 20, 21, 22 Lack of efficacy and/or side effects often lead to discontinuation of acute treatment. Understanding the primary reasons for treatment dissatisfaction and subsequent discontinuation of acute treatment of migraine attacks could help to identify unmet treatment needs. Identifying and addressing these needs should lead to improved treatment and better outcomes.
The goals of this paper are to quantify rates of acute treatment discontinuation, to characterize people with migraine who discontinue acute treatment use, to understand the reasons for discontinuation, and to assess the burden of migraine among those who discontinue acute prescription medications for migraine. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study provides an ideal setting for addressing these issues.
Methods
Study Design
The complete CaMEO study design and baseline data have been previously reported.23 Although a power analysis was not implemented for the initial data collection effort, the sampling plan was intended to provide data on sufficient numbers of high‐frequency and chronic migraine respondents to characterize this subset of the population. The study was approved by the institutional review board of the Albert Einstein College of Medicine, which waived written informed consent for study volunteers.
The study consisted of a series of Internet‐based longitudinal survey modules issued at 3‐month intervals and several cross‐sectional modules administered once each over the course of a year between September 2012 and November 2013. Migraine respondents were identified based on modified International Classification of Headache Disorders, 3rd edition (ICHD‐3) migraine criteria, as assessed by the American Migraine Study (AMS)/AMPP study diagnostic module.24 Invitations to participate were sent via e‐mail to 489,537 members of an Internet research panel using quota sampling to produce a sample that represented the US population, yielding 80,783 respondents; 58,418 of the returns were usable (ie, provided data sufficient to determine migraine status), and 16,789 of these met study inclusion criteria. The present cross‐sectional, descriptive analysis used answers from the CaMEO baseline assessment that included sociodemographic and headache features (screening module), burden measures (core module), and prescription acute medication use (barriers to care module). Baseline survey data were obtained between September 2012 and October 2012. A subsequent survey was administered in October 2012 and November 2012 to assess migraine endophenotypes and allodynia. The authors had full access to all study data.
Study Population and Assessments
For this analysis, only respondents who had discontinued the use of acute prescription medications for migraine were evaluated. To be included in the analysis, respondents must have selected that they “had ever used” a “prescription pain medication that you take when you get a headache (or feel one coming on)” in response to the question, “Which of the following types of headache treatments have you ever used to treat or manage your headache pain?” All available responses are shown in Supplementary Table S1. To qualify as a “discontinued user,” respondents had to indicate that they had used prescription pain medication in the past but were “not currently using” “prescription pain medication that you take when you get a headache (or feel one coming on).” The analysis population was further refined to eliminate individuals with contradictory responses. Respondents who indicated that they had discontinued acute prescription medication for migraine were presented with a list of potential reasons (Supplementary Table S2). Focus group discussions among persons with migraine and expert clinician input guided development of the initial “reasons” item set. Cognitive debriefing before fielding the survey eliminated redundant items and confirmed readability and interpretation. For the analysis, similar items from the list were grouped into 4 domains: lack of efficacy, safety/tolerability concerns, switched from prescription medication, and financial concerns. The following items were reported separately: switched to preventive medication, headaches improved, and doctor’s orders.
Additional patient‐reported outcome measures included the Migraine Disability Assessment (MIDAS), Patient Health Questionnaire 9‐item depression screener (PHQ‐9), and Generalized Anxiety Disorder 7‐item screener (GAD‐7). The MIDAS questionnaire comprises 5 headache‐related disability questions; the score is derived by summing the number of lost days recorded for each question.25 MIDAS scores are descriptively analyzed based on severity category: grade I (minimal or infrequent disability, score 0‐5), grade II (mild or infrequent disability, score 6‐10), grade III (moderate disability, score 11‐20), and grade IV (severe disability, score ≥21). The ability to function with respect to headache was also assessed in the Barriers to Care module with a single question (“How are you usually affected by your headaches?”). The PHQ‐9 is composed of 9‐items pertaining to depression.26 Each item is rated on a 4‐point scale, ranging from 0 (never) to 3 (nearly every day) for the preceding 2 weeks; overall scores range from 0 to 27. PHQ‐9 scores are descriptively analyzed based on severity category, with the scores of 5‐9, 10‐14, 15‐19, and 20‐27, corresponding to mild, moderate, moderately severe, and severe depression, respectively. The GAD‐7 consists of 7‐items that describe symptoms of generalized anxiety disorder for the preceding 2 weeks that are rated on a 4‐point scale that ranges from 0 (not at all) to 3 (nearly every day), for a total score range of 0 to 21.27 GAD‐7 scores are descriptively analyzed based on severity category, with scores of 5‐9, 10‐14, and 15‐21 corresponding to mild, moderate, and severe anxiety, respectively. The presence of allodynia was assessed with the 12‐item Allodynia Symptom Checklist (ASC‐12). Responses for this scale were obtained from the endophenotypes module that was administered during a subsequent assessment. The ASC‐12 includes questions about the frequency of allodynia symptoms associated with migraine attacks; items are scored on a 3‐point scale, ranging from 0 (never, rarely, or does not apply to me) to 2 (half of the time or more), for a total score that ranges from 0 to 24.28 The ASC‐12 is descriptively analyzed based on severity category: none (score 0‐2), mild (score 3‐5), moderate (score 6‐8), and severe (9 or more). Monthly headache day frequency was assessed in the screening and core modules, and the latter also encompassed an accounting of headache treatments used within the previous 30 days.
Statistical Analyses
Sociodemographic features, headache characteristics, and reasons for discontinuation of acute prescription medication were summarized descriptively as the percentage of respondents endorsing each variable. Study population age was characterized with mean and standard deviation. Monthly headache days within subgroups were summarized by median and 25th/75th percentile. Some respondents were missing data for sociodemographics and headache characteristics and are identified in the respective tables. MIDAS, PHQ‐9, and GAD‐7 scores are descriptively analyzed; the number of respondents meeting criteria for each severity category is reported. No formal statistical testing for differences between groups was performed. All analyses were conducted with IBM SPSS Statistics, version 20.0 (IBM, Armonk, NY, USA).
Results
CaMEO Analysis Sample
In total, 13,624 total respondents were included in these analyses. Only 4840 (35.5%) respondents reported ever using acute prescription medication for migraine, and 1719 (35.5%) of that subgroup reported discontinuing the use of acute prescription medication for migraine. Among those who discontinued treatment, the mean (SD) age was 42.1 (14.0) years, and 78.4% were female. Full demographics stratified by time since discontinuation are shown in Table 1. Nearly two‐thirds (65.6%) of those who discontinued treatment had a body mass index in the category of overweight or obese. While the majority of respondents who discontinued treatment reported active health insurance coverage (84.6%), most were not currently consulting with a healthcare professional about their headaches (87.5%). Demographic characteristics across the time since discontinuation groups were generally similar, with the highest mean age in the >12‐month subgroup (43.6 years).
Table 1.
Sociodemographics of Respondents Who Discontinued Acute Prescription Medication for Migraine
| Characteristic | Total (n = 1719)† | Time Since Last Prescription Medication for Migraine | ||
|---|---|---|---|---|
| <7 Months (n = 419)† | 7‐12 Months (n = 158)† | >12 Months (n = 1142)† | ||
| Age, mean (SD), y | 42.1 (14.0) | 38.8 (14.0) | 39.8 (15.1) | 43.6 (13.6) |
| Female, n (%) | 1384 (78.4) | 296 (70.6) | 122 (77.2) | 930 (81.4) |
| Caucasian, n (%) | 1508 (88.0)‡ | 344 (82.5)§ | 137 (86.7) | 1027 (90.2)¶ |
| BMI category, n (%) | ||||
| Underweight | 35 (2.0) | 12 (2.9) | 2 (1.3) | 21 (1.8) |
| Normal | 556 (32.3) | 136 (32.5) | 53 (33.5) | 367 (32.1) |
| Overweight | 472 (27.5) | 109 (26.0) | 45 (28.5) | 318 (27.8) |
| Obese | 656 (38.2) | 162 (38.7) | 58 (36.7) | 436 (38.2) |
| Household income, n (%) | (n = 1707) | (n = 418) | (n = 157) | (n = 1132) |
| <$30,000 | 392 (23.0) | 97 (23.2) | 45 (28.7) | 250 (22.1) |
| $30,000‐$49,999 | 307 (18.0) | 79 (18.9) | 28 (17.8) | 200 (17.7) |
| $50,000‐$74,999 | 364 (21.3) | 104 (24.9) | 28 (17.8) | 232 (20.5) |
| ≥$75,000 | 644 (37.7) | 138 (33.0) | 56 (35.7) | 450 (39.8) |
| Currently consulting with an HCP/doctor, n (%) | 215 (12.5) | 112 (26.7) | 22 (13.9) | 81 (7.1) |
| Insurance coverage, n (%) | 1429 (84.6)†† | 351 (85.2)‡‡ | 129 (84.3)§§ | 949 (84.4)¶¶ |
| Employed, n (%) | 1182 (68.8) | 285 (68.0) | 104 (65.8) | 793 (69.4) |
Except where noted.
n = 1714.
n = 417.
n = 1139.
n = 1689.
n = 412.
n = 153.
n = 1124.
BMI = body mass index; HCP = healthcare provider.
Respondents’ headache characteristics stratified by the time since discontinuation are shown in Table 2. Nearly 50% of those who discontinued had allodynia, and 37.6% reported 5 or more headache days per month, with a median (25th/75th percentile) of 3.3 (1.3, 6.7) days. Two‐thirds (67.5%) of discontinued users reported receiving a migraine (or chronic migraine) diagnosis from a doctor or other health professional in the past. Those who had discontinued treatment within the previous year were more likely to have experienced at least 15 headache days per month than those who discontinued 12 months before.
Table 2.
Headache Characteristics of Respondents Who Discontinued Acute Prescription Medication for Migraine
| Headache Characteristic | Total (n = 1719) | Time Since Last Prescription Medication for Migraine | ||
|---|---|---|---|---|
| <7 Months (n = 419) | 7‐12 Months (n = 158) | >12 Months (n = 1142) | ||
| Monthly headache frequency, median (25th/75th percentile), d | 3.3 (1.3, 6.7) | 3.3 (1.7, 8.7) | 3.5 (1.3, 8.3) | 3.0 (1.3, 6.7) |
| Monthly headache frequency categories, n (%) | ||||
| 0‐4 days | 1073 (62.4) | 253 (60.4) | 92 (58.2) | 728 (63.7) |
| 5‐9 days | 322 (18.7) | 70 (16.7) | 34 (21.5) | 218 (19.1) |
| 10‐14 days | 135 (7.9) | 40 (9.6) | 11 (7.0) | 84 (7.4) |
| ≥15 days | 189 (11.0) | 56 (13.4) | 21 (13.3) | 112 (9.8) |
| Self‐reported diagnosis of migraine or CM, n (%) | 1160 (67.5) | 224 (53.5) | 82 (51.9) | 854 (74.8) |
| Presence of allodynia, n (%)† | 673 (50.4) | 170 (53.1) | 72 (57.1) | 431 (48.5) |
Allodynia question included in endophenotype module only, included 1335 discontinued users.
CM = chronic migraine.
Migraine Disability, Depression, and Anxiety
Only 19.1% of respondents who discontinued treatment reported being able to work or function normally with headache; 59.5% reported their working ability or activity was impaired to some degree; 10.8% reported their working ability or activity was severely impaired, but required no bed rest; and 10.6% reported that bed rest was required. Assessment of migraine‐related disability as measured by MIDAS score showed 41.7% of respondents who discontinued treatment scored within the moderate to severe disability range at baseline (Fig. 1). Furthermore, approximately one‐third of respondents who discontinued treatment exhibited moderate to severe depression (33.0%) and/or moderate to severe anxiety (30.0%), as assessed by the PHQ‐9 and GAD‐7, respectively.
Figure 1.
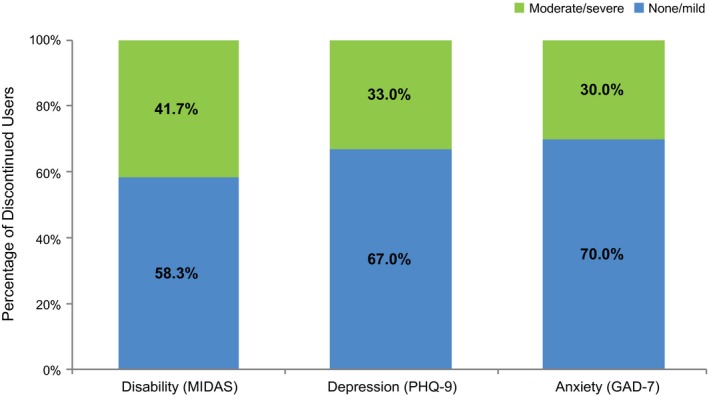
Severity of disability, depression, and anxiety in respondents who discontinued acute prescription medication for migraine.a aSeverity levels as determined by the individual scales. GAD‐7 = Generalized Anxiety Disorder 7‐item screener; MIDAS = Migraine Disability Assessment; PHQ‐9 = Patient Health Questionnaire 9‐item depression screener.
Reasons Reported as Contributing to Acute Prescription Medication Discontinuation
The most common reason associated with acute prescription medication discontinuation was the decision to switch away from prescription medication (45.5%) to over‐the‐counter or alternative treatment options, followed by concerns about lack of efficacy (28.2%) and safety/tolerability (24.9%) (Table 3). Additionally, 21.3% of respondents who discontinued treatment reported doing so because their headaches had improved and 19.4% reported financial concerns. Reasons for discontinuing treatment were further evaluated to assess the overlap of efficacy and concerns about safety and tolerability in this subpopulation. Among those who reported discontinuing medication due to a lack of efficacy, 33.1% (160/484) also reported safety and tolerability concerns. Among those who reported safety and tolerability concerns, 37.4% (160/428) of respondents also reported a lack of efficacy as a reason contributing to treatment discontinuation.
Table 3.
Reasons for Discontinuation of Acute Prescription Medication for Migraine
| Reason, n (%)† | All Discontinued Users (n = 1719) |
|---|---|
| Lack of efficacy | 484 (28.2) |
| The medication did not provide enough pain relief | 298 (17.3) |
| I could not count on the medication working on every headache attack | 203 (11.8) |
| My headache pain went away but came back later | 148 (8.6) |
| The medication worked OK for a while but then stopped working | 95 (5.5) |
| Safety/tolerability concerns | 428 (24.9) |
| I had side effects when I took the medication | 232 (13.5) |
| I worried about side effects | 235 (13.7) |
| I was concerned about interactions with other medication I take | 74 (4.3) |
| I was concerned about interactions with food or alcohol | 36 (2.1) |
| Switched from prescription medication | 782 (45.5) |
| I switched to over‐the‐counter pain medication | 579 (33.7) |
| I switched to using natural remedies | 70 (4.1) |
| I just didn’t want to take prescription medication | 322 (18.7) |
| Financial concerns | 333 (19.4) |
| No health insurance | 189 (11.0) |
| Out‐of‐pocket cost too high/insurance would not cover enough of cost | 217 (12.6) |
| Other reasons | |
| Started using preventive medication | 43 (2.5) |
| My headaches got better | 367 (21.3) |
| My doctor told me to stop taking it | 58 (3.4) |
Percentages do not sum to 100% because respondents were able to endorse multiple reasons for discontinuation.
The degree of migraine‐related disability was lowest in those whose headaches improved and those who reportedly switched away from prescription medications (Fig. 2). The highest rates of severe migraine‐related disability were among those who discontinued acute treatment because they started preventive treatment (41.9%), discontinued due to doctor’s instruction (37.9%), or reported financial concerns (33.9%). Among those respondents who discontinued treatment due to lack of efficacy and/or safety/tolerability concerns (n = 752), higher rates of moderate or severe disability were found among those who discontinued for both efficacy and safety/tolerability reasons (64.4%) than because of efficacy or safety/tolerability alone (Fig. 3).
Figure 2.
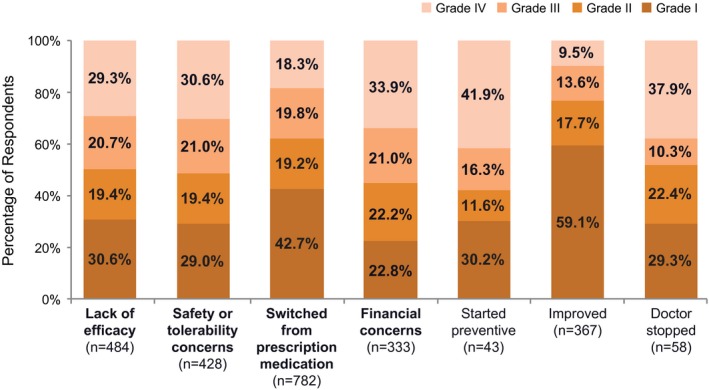
Migraine‐related disabilitya among users who discontinued medication use by domains associated with discontinuation. aAs determined by the Migraine Disability Assessment (MIDAS), where score 0‐5 = grade I (little or no disability), 6‐10 = grade II (mild disability), 11‐20 = grade III (moderate disability), ≥21 = grade IV (severe disability).
Figure 3.
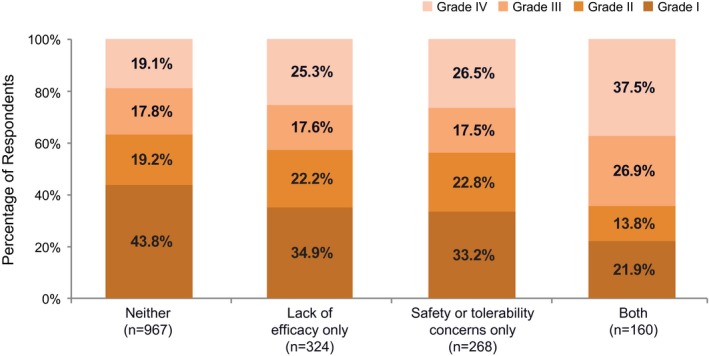
Migraine‐related disabilitya among respondents endorsing efficacy and/or safety domains. aAs determined using the Migraine Disability Assessment (MIDAS), where score 0‐5 = grade I (little or no disability), 6‐10 = grade II (mild disability), 11‐20 = grade III (moderate disability), and ≥21 = grade IV (severe disability).
Discussion
In this analysis of CaMEO study respondents, 35.5% of those who had ever tried an acute prescription medication for migraine discontinued use. A full characterization of respondents who reported never using or continued use of acute prescription medications for migraine has been presented separately.29 Common reasons for discontinuing treatment included switching to over‐the‐counter treatments, lack of efficacy, and concerns about medication safety and tolerability. Reasons related to financial concerns were also prevalent, with 11.0% of respondents indicating that they discontinued due to lack of health insurance and 12.6% endorsing the idea that out of pocket costs were too high or medication was inadequately covered by insurance.
Among discontinued users, many (37%) reported still having more than 4 headache days per month. Additionally, more than 40% of respondents who discontinued acute prescription medication for migraine experienced moderate to severe migraine disability on the MIDAS questionnaire, 33% reported moderate to severe depression, and 30% reported moderate to severe anxiety. These data indicate a substantial unmet need among those who discontinue acute prescription medications. When evaluating the relationship between migraine disability and reasons for discontinuation, the highest levels of disability were found in respondents who discontinued because of concerns regarding both efficacy and tolerability, indicating a potential need for medications with improved efficacy and tolerability profiles. Notably, 87.5% of the overall respondents were not currently consulting with a healthcare provider, which likely limited their treatment options.
These results parallel those from other studies that have identified efficacy and safety concerns as influencing a patient’s decision to discontinue his or her acute medication.11, 13, 30, 31, 32, 33 In an analysis of the AMPP study data, investigators found that the most commonly reported reasons for discontinuation of triptans and opioids were related to efficacy and safety.11 Specific reasons included failure to relieve pain (29.6%), recurrence of pain after initial relief (24.1%), concern about medication affecting stomach (20.8%), concern about side effects in general (18.8%), and medication stopped working (17.5%). In a multicenter cross‐sectional survey of US headache clinics, individuals with migraine reported a lack of efficacy (44%) and concerns with side effects (29%) as the most common reasons for discontinuation of triptan use.13 Additionally, those who discontinued triptans in this study had higher depression and higher disability scores, which is consistent with our data showing a sizable headache‐related burden in the form of depression and disability among respondents who discontinued medication. In agreement with these results, Cady et al found that satisfaction with treatment and belief in medication were the most significant predictors of continued and sustained triptan use.31 In an evaluation of patients who switched between triptans, the investigators found that “incomplete or no relief” and inadequate “time to relief” were the most common reasons contributing to the decision to switch.30 Results from the Migraine in America Symptoms and Treatment (MAST) Study also included high rates of discontinuation analyzed by route of drug administration, including injectable triptans (81.5%), nasal sprays (66.5%), and oral triptans (55.2%).33 A reported lack of efficacy and concerns over side effects were the most common reasons for discontinuing triptans among these respondents. For oral triptans, the most commonly reported side effects associated with treatment discontinuation were dizziness (37.4%) and nausea (30.7%).
The limitations of the CaMEO Study have been discussed previously.23 All data were self‐reported and not confirmed by medical claims or medical records, and were subject to the recall bias that is inherent with any survey‐based methodology. Reasons for discontinuing acute prescription medication for migraine were self‐reported and, for some respondents, based on action taken many months before this assessment. Additionally, different rates of treatment dissatisfaction and discontinuation between acute medications have been reported.11 As our analysis was not subcategorized by acute treatment type, our results do not address differences across medications. Strengths of the CaMEO study include the fact that it is a large, nationwide, diverse sample of people with migraine, and relies on validated assessment tools where possible. Non‐validated measures were derived from migraine patient interviews and expert clinician guidance.
Conclusions
These results further confirm a high level of disability and unmet treatment need among those with migraine who discontinue acute prescription medication for migraine. Switching away from prescription treatments, a lack of efficacy, and concerns over safety and tolerability were among the most commonly reported reasons contributing to discontinuation. Addressing efficacy and safety concerns commonly associated with these medications may help to improve treatment adherence, improve outcomes for patients, and mitigate the societal burden of migraine.
Statement of Authorship
Category 1
(a) Conception and Design
Richard B. Lipton, Michael L. Reed, Aubrey Manack Adams, Dawn C. Buse
(b) Acquisition of Data
Michael L. Reed
(c) Analysis and Interpretation of Data
Richard B. Lipton, Susan Hutchinson, Jessica Ailani, Michael L. Reed, Kristina M. Fanning, Aubrey Manack Adams, Dawn C. Buse
Category 2
(a) Drafting the Manuscript
Richard B. Lipton, Kristina M. Fanning
(b) Revising It for Intellectual Content
Richard B. Lipton, Susan Hutchinson, Jessica Ailani, Michael L. Reed, Kristina M. Fanning, Aubrey Manack Adams, Dawn C. Buse
Category 3
(a) Final Approval of the Completed Manuscript
Richard B. Lipton, Susan Hutchinson, Jessica Ailani, Michael L. Reed, Kristina M. Fanning, Aubrey Manack Adams, Dawn C. Buse
Supporting information
Acknowledgments
The authors would like to thank Valerie Marske of Vedanta Research for support for survey development and management of data collection.
Conflict of Interest: Richard B. Lipton, MD, serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache. He has received research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He has reviewed for the NIA and NINDS; he serves as consultant or advisory board member or has received honoraria from Alder, Allergan, Amgen, Autonomic Technologies, Avanir, Boston Scientific, Dr. Reddy’s, ElectroCore, Eli Lilly, eNeura, GlaxoSmithKline, Merck, Novartis, Teva, and Vedanta. He receives royalties from Wolff’s Headache (8th Edition, Oxford University Press) and Informa. He holds stock options in eNeura and Biohaven. Susan Hutchinson, MD, has served on advisory boards for Alder, Allergan, Amgen, Avanir, Biohaven, ElectroCore, Eli Lilly, Supernus, and Teva. She is on the speakers bureau for Allergan, Amgen, Avanir, ElectroCore, Eli Lilly, Promius, Supernus, and Teva. Jessica Ailani, MD, has served as a consultant for Alder, Allergan, Alpha Sites Consulting, Amgen, Electrocore, Eli Lilly and Company, Impel, Promius, Satsuma, Teva; has been involved with CME programming with Avent (CME content and speaker’s fee); Miller Communications (CME content and speaker fee), and Peer View (CME speaker fee). She has been a speaker for Allergan, Avanir, Amgen, Electrocore, Eli Lilly and Company, Promius, and Teva. She has received grant support from the American Migraine Foundation, Allergan, and Biohaven and provided editorial services to Current Pain and Headache Reports. Michael L. Reed, PhD, is Managing Director of Vedanta Research, which has received research funding from Allergan, Amgen, Dr. Reddy’s Laboratories, Eli Lilly, GlaxoSmithKline, Merck & Co., Inc., and Novartis, via grants to the National Headache Foundation. Vedanta Research has received funding directly from Allergan for work on the CaMEO Study. Kristina M. Fanning, PhD, is an employee of Vedanta Research, which has received research funding from Allergan, Amgen, Dr. Reddy’s Laboratories, Eli Lilly, GlaxoSmithKline, Merck & Co., Inc., and Novartis, via grants to the National Headache Foundation. Vedanta has received funding directly from Allergan for work on the CaMEO Study. Aubrey Manack Adams, PhD, is an employee of Allergan, plc. Dawn C. Buse, PhD, has received grant support and honoraria from Allergan, Avanir, Amgen, Biohaven, Eli Lilly and Company, and Promius and for work on the editorial board of Current Pain and Headache Reports.
Funding Disclosures: This study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and was funded by Allergan plc. Neither honoraria nor other form of payments was made for authorship.
References
- 1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goadsby PJ, Lipton RB, Ferrari MD. Migraine – Current understanding and treatment. N Engl J Med. 2002;346:257‐270. [DOI] [PubMed] [Google Scholar]
- 3. Silberstein SD. Practice parameter: Evidence‐based guidelines for migraine headache (an evidence‐based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754‐762. [DOI] [PubMed] [Google Scholar]
- 4. Bigal ME, Borucho S, Serrano D, Lipton RB. The acute treatment of episodic and chronic migraine in the USA. Cephalalgia. 2009;29:891‐897. [DOI] [PubMed] [Google Scholar]
- 5. Buse DC, Pearlman SH, Reed ML, Serrano D, Ng‐Mak DS, Lipton RB. Opioid use and dependence among persons with migraine: Results of the AMPP study. Headache. 2012;52:18‐36. [DOI] [PubMed] [Google Scholar]
- 6. Food and Drug Administration . Drug Safety Communication: FDA Strengthens Warning that Non‐Aspirin Nonsteroidal Anti‐Inflammatory Drugs (NSAIDs) Can Cause Heart Attacks or Strokes. Silver Spring, MD: Food and Drug Administration; 2015. [Google Scholar]
- 7. Imitrex [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. [Google Scholar]
- 8. Tajti J, Majlath Z, Szok D, Csati A, Vecsei L. Drug safety in acute migraine treatment. Expert Opin Drug Saf. 2015;14:891‐909. [DOI] [PubMed] [Google Scholar]
- 9. Langer‐Gould AM, Anderson WE, Armstrong MJ, et al. The American Academy of Neurology's top five choosing wisely recommendations. Neurology. 2013;81:1004‐1011. [DOI] [PubMed] [Google Scholar]
- 10. Loder E, Weizenbaum E, Frishberg B, Silberstein S. Choosing wisely in headache medicine: The American Headache Society's list of five things physicians and patients should question. Headache. 2013;53:1651‐1659. [DOI] [PubMed] [Google Scholar]
- 11. Holland S, Fanning KM, Serrano D, Buse DC, Reed ML, Lipton RB. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci. 2013;326:10‐17. [DOI] [PubMed] [Google Scholar]
- 12. Messali AJ, Yang M, Gillard P, et al. Treatment persistence and switching in triptan users: A systematic literature review. Headache. 2014;54:1120‐1130. [DOI] [PubMed] [Google Scholar]
- 13. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache. 2014;54:278‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new‐onset chronic migraine. Neurology. 2015;84:688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serrano D, Kori S, Papapetropoulos S, et al. Does adding acute treatment improve migraine outcomes in patients on triptans?: Results of the America Migraine Prevalence & Prevention (AMPP) Study [abstract]. Presented at the Annual Scientific Meeting of the American Headache Society, Los Angeles, CA; 2012. [Google Scholar]
- 16. Schwedt TJ, Alam A, Reed ML, et al. Factors associated with acute medication overuse in people with migraine: Results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2018;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population‐based study. Headache. 2008;48:1157‐1168. [DOI] [PubMed] [Google Scholar]
- 18. Lipton RB, Serrano D, Nicholson RA, Buse DC, Runken MC, Reed ML. Impact of NSAID and triptan use on developing chronic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:1548‐1563. [DOI] [PubMed] [Google Scholar]
- 19. Rahimtoola H, Buurma H, Tijssen CC, Leufkens HG, Egberts AC. Single use of sumatriptan: A patient interview study. Headache. 2003;43:109‐116. [DOI] [PubMed] [Google Scholar]
- 20. Gendolla A. Part I: What do patients really need and want from migraine treatment? Curr Med Res Opin. 2005;21(Suppl. 3):S3‐S7. [DOI] [PubMed] [Google Scholar]
- 21. Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: Experience from 3 centers in US and Sweden. Headache. 2007;47:475‐479. [DOI] [PubMed] [Google Scholar]
- 22. Katic BJ, Rajagopalan S, Ho TW, Chen YT, Hu XH. Triptan persistency among newly initiated users in a pharmacy claims database. Cephalalgia. 2011;31:488‐500. [DOI] [PubMed] [Google Scholar]
- 23. Adams AM, Serrano D, Buse DC, et al. The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35:563‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition . Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 25. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache‐related disability. Neurology. 2001;56:S20‐S28. [DOI] [PubMed] [Google Scholar]
- 26. Seo JG, Park SP. Validation of the Patient Health Questionnaire‐9 (PHQ‐9) and PHQ‐2 in patients with migraine. J Headache Pain. 2015;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch Intern Med. 2006;166:1092‐1097. [DOI] [PubMed] [Google Scholar]
- 28. Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutchinson S, Lipton RB, Ailani J, et al. Patterns and characterization of acute prescription migraine medication use: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study [abstract]. Neurology. 2019;92(Suppl. 15):S59.008. [Google Scholar]
- 30. Sheftell FD, Feleppa M, Tepper SJ, Volcy M, Rapoport AM, Bigal ME. Patterns of use of triptans and reasons for switching them in a tertiary care migraine population. Headache. 2004;44:661‐668. [DOI] [PubMed] [Google Scholar]
- 31. Cady RK, Maizels M, Reeves DL, Levinson DM, Evans JK. Predictors of adherence to triptans: Factors of sustained vs lapsed users. Headache. 2009;49:386‐394. [DOI] [PubMed] [Google Scholar]
- 32. Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: Results from the second international burden of migraine study (IBMS‐II). Headache. 2013;53:644‐655. [DOI] [PubMed] [Google Scholar]
- 33. Alam A, Munjal S, Reed ML, et al. Triptan use and discontinuation in a representative sample of persons with migraine: Results from Migraine in America Symptoms and Treatment (MAST) study [abstract OR11]. Headache. 2018;58:68‐69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


