Abstract
Changes in the frequency, duration, and intensity of rainfall events are among the abiotic effects predicted under anthropogenic global warming. Heavy downpours may profoundly affect the development and survival of small organisms such as insects. Here, we examined direct (physically on the insects) and indirect (plant‐mediated) effects of simulated downpours on the performance of caterpillars of two lepidopteran herbivores (Plutella xylostella and Pieris brassicae) feeding on black mustard (Brassica nigra) plants. Host plants were exposed to different rainfall regimes both before and while caterpillars were feeding on the plants in an attempt to separate direct and indirect (plant‐mediated) effects of rainfall on insect survival and development. In two independent experiments, downpours were simulated as a single long (20 min) or as three short (5 min) daily events. Downpours had a strong negative direct effect on the survival of P. xylostella, but not on that of P. brassicae. Direct effects of downpours consistently increased development time of both herbivore species, whereas effects on body mass depended on herbivore species and downpour frequency. Caterpillar disturbance by rain and recorded microclimatic cooling by 5°C may explain extended immature development. Indirect, plant‐mediated effects of downpours on the herbivores were generally small, despite the fact that sugar concentrations were reduced and herbivore induction of secondary metabolites (glucosinolates) was enhanced in plants exposed to rain. Changes in the frequency of precipitation events due to climate change may impact the survival and development of insect herbivores differentially. Broader effects of downpours on insects and other arthropods up the food chain could seriously impair and disrupt trophic interactions, ultimately destabilizing communities.
Keywords: climate change, development, global warming, glucosinolates, insect herbivores, phytochemistry, plant–insect interactions, rain, secondary plant metabolites
Introduction
Species interactions, such as those occurring between plants and insects, are not only affected by the biotic environment (e.g., presence of competitors and predators, quality and quantity of resources), but also by the abiotic environment (Schoonhoven et al. 2005). Abiotic factors such as temperature, wind, and rain can directly or indirectly affect insect herbivores feeding on their host plants (Schoonhoven et al. 2005). For instance, heavy rainfall can dislodge insects from the plants (Kobori and Amano 2003) and can also affect microclimatic conditions (through cooling; Dobkin et al. 1987, Kamata and Igarashi 1994), which, like temperature in general, strongly affects the developmental rate of ectotherms. Indirectly, insect herbivores can be influenced through effects of abiotic factors on survival, fitness, and foraging behavior of their natural enemies and herbivorous competitors. Indirect plant‐mediated effects of abiotic factors on insect herbivores may occur if these factors influence plant traits determining food plant quality for insect herbivores. Variation in temperature and precipitation may change levels of primary and secondary metabolites in plant tissues that are consumed by the herbivores, affecting their growth and development (Jamieson et al. 2017). It has also been reported that the emission of volatile plant metabolites is affected by rainfall, relative humidity, and temperature (Vallat et al. 2005, Loreto and Schnitzler 2010). Changes in the emissions of plant volatiles could potentially affect the ability of insect herbivores to find host plants when these changes concern specific cues important for foraging behavior. In addition, it may affect the foraging behavior of higher trophic‐level arthropods such as predators and parasitoids that is mediated by the volatiles emitted in response to herbivory (Dicke 2016). Thus, changes in abiotic factors may strongly affect species interactions.
Small‐scale heterogeneity in microclimatic patterns, as well as weather and large‐scale climatic patterns, has long been recognized as major factors influencing population dynamics of small ectothermic arthropods (Uvarov 1931, Bale et al. 2002). Studies of the impact of anthropogenic global warming on ecological processes have largely focused on the effects of elevated mean temperature and/or atmospheric concentrations of CO2 (Christidis et al. 2011, Norby and Zak 2011, Zavala et al. 2013). However, global and regional patterns of precipitation are also expected to change with rising temperatures (IPCC 2014). Intense heat generates convection that leads to severe thunderstorms and attendant downpours (Brooks 2013). Short‐duration extreme weather events are predicted to increase in frequency by approximately 14% with every 1°C increase in temperature (Lenderink and van Meijgaard 2008). Heavy rain can seriously affect the behavior and development of small organisms such as insects and other arthropods (Moran et al. 1987, Kamata and Igarashi 1994, Fink and Völkl 1995). Moreover, seasonal change in rainfall across years can cause a reduction in herbivore abundances and lead to shifts (i.e., simplification) in trophic structure, thus, modifying community‐level interactions (Suttle et al. 2007, Zhu et al. 2014).
With the exception of temperature, abiotic factors, such as rainfall and wind, are rarely considered in laboratory and greenhouse experiments investigating insect–plant interactions. However, in the field, organisms have to deal with both the biotic and abiotic environment. By greatly reducing, eliminating, or making abiotic factors static in lab experiments, some of the results may have limited value. Therefore, it is important to determine the relative importance of abiotic and biotic factors when studying plant–herbivore (multitrophic) interactions. In a previous study (Chen et al. 2018), we showed that wind exposure can extend larval development time of two lepidopteran herbivores, Plutella xylostella (L.) (Plutellidae) and Pieris brassicae (L.) (Pieridae), respectively. Interestingly, wind exposure also reduced adult biomass of P. xylostella, whereas butterflies of P. brassicae were heavier on wind‐exposed plants. Plant‐mediated effects were relatively small (Chen et al. 2018).
Here, we examined the effects of simulated heavy short‐term rainfall exposure on the survival and development of the same two herbivore species P. xylostella and P. brassicae, on one of their important natural food plants, Brassica nigra (L.) (Brassicales: Brassicaceae). In a first experiment performed in a greenhouse, we simulated short (20 min) daily downpours like those that accompany thunderstorms. In a second experiment, we increased the frequency (three) and at the same time reduced the length (5 min) of the daily downpours to determine whether the effects on the herbivore differ when exposed to these two different downpour conditions. We hypothesize that more frequent disturbance by triple daily downpour events would have a stronger impact on the plants and the herbivores than a single long downpour event. In an attempt to separate direct and indirect, i.e., plant‐mediated, effects of downpours on insect development and survival, rainfall regimes were applied during growth of the plants before (phase 1) and after insects were introduced and were feeding on the plants (phase 2). Plants in the two experiments were exposed to one of four rainfall regimes: (1) no rain, (2) only rain during phase 1, (3) only rain during phase 2, and (4) rain during both phases. Whereas the effects of rain during phase 1 on caterpillars feeding during phase 2 clearly represent indirect, plant‐mediated effects, effects of rain during phase 2 will mainly represent direct effects on caterpillars, but could include some plant‐mediated effects as well. Given the relative short duration of the second phase, for simplicity, we will refer to phase‐2 effects as direct effects.
We hypothesize that (1) the direct effects of intensive rainfall such as physical disturbance and changes in microclimate negatively affect herbivore performance and (2) the direct effects of downpours are stronger than the indirect plant‐mediated effects. To determine how chemical traits in the plants are affected by downpours we measured glucosinolate and sugar concentrations, representatives of primary and secondary metabolites, respectively. Both groups of plant metabolites are considered important for insect performance (Scriber and Slansky 1981, Awmack and Leather 2002, Hopkins et al. 2009). We also measured temperature close to the leaf surface before and after the simulated downpour events. Our results are discussed in relation to the effects of predicted changes in extreme weather events on insect–plant interactions in Europe under Anthropogenic Global Warming projected by the IPCC (2014).
Methods
Study system
Black mustard, B. nigra, grows in a range of climatic regions in Europe (Prakash and Hinata 1980). Like other members of the Brassicaceae family, it produces secondary compounds known as glucosinolates (GS; Fahey et al. 2001). GS can act as feeding deterrents or exhibit negative effects on the growth and development of non‐adapted phytophages and their natural enemies, while they can be used as feeding and oviposition cues by specialist enemies (Gols and Harvey 2009, Hopkins et al. 2009). Concentrations of these compounds are also known to change in response to herbivory (Textor and Gershenzon 2009). We measured GS, as well as sugar concentrations, in leaf tissues in plants exposed to the various downpour and herbivore treatments described below. Sugars are powerful feeding stimulants for many insects and, in addition to nitrogen, are important determinants of the nutritional quality of host plants (Awmack and Leather 2002, Schoonhoven et al. 2005).
Caterpillars of both the microlepidopteran P. xylostella and the macrolepidopteran P. brassicae primarily feed on brassicaceous plant species and are important pests of cruciferous crops (Feltwell 1982, Furlong et al. 2013). Caterpillars of both species were obtained from the laboratory of Entomology, Wageningen University and were originally collected in cabbage fields near Wageningen. They were maintained on cabbage plants (Brassica oleracea, var. gemmifera, cv. Cyrus) in climate rooms at 22° ± 2°C, 50–70% relative humidity and a photoperiod of 16 h.
Experimental protocol
Plants were grown from seeds in a greenhouse (22° ± 3°C, 50–70% relative humidity and a 16‐h photoperiod. One‐week‐old seedlings were transferred to 2‐L pots (one plant per pot) filled with potting soil (Lentse potgrond no. 4: Lentse, Lent, The Netherlands). Extra soil was added to each pot, including those that were not exposed to downpours, creating a mount around the stem that was covered with tin foil to accommodate runoff of water during the downpours and to avoid that nutrients would leach from the soil. Pots were placed in saucers for manual watering. All plants were well‐watered. Downpour‐exposure treatments were divided into two phases: growth phase of the plants before introduction of the insects (phase 1) and insect exposure phase (phase 2). Plants were allocated to one of the following four downpour treatments (Fig. 1): (1) control (no exposure to downpours), (2) exposure to downpour only during phase 1, (3) only during phase 2, and (4) during both phases. We refer to these treatments as R00, R10, R01, and R11, respectively, where the two digits refer to the two phases and 0 and 1 to downpour treatments (0, no; 1, yes). The four precipitation regimes allowed us to separate the indirect, plant‐mediated effects of downpours on the herbivores (R10, R11 vs. R00, R01) from predominantly direct effects (R01, R11 vs. R00, R10). Rainfall was simulated using nozzles (full cone Hardi nozzles, type 1553; Homburg Holland, Stiens, The Netherlands) with an intensity of ~20.9 mm water/h. The nozzles were attached to a frame approximately 2 m above movable tables (80 × 80 cm, one nozzle per table). In total, there were 12 tables with simulated rainfall and 12 tables without simulated rainfall, which were each placed in rows of six tables (Fig. 1).
Figure 1.
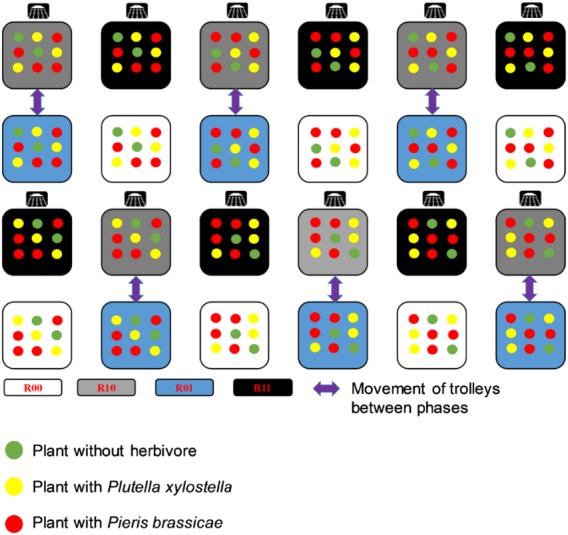
Schematic diagram of the design of the simulated downpour experiment. Downpour‐exposure treatments were applied during growth of the plants (phase 1) and while insects were feeding on the plant (phase 2). There were four treatments: (1) no downpours (R00, the two digits refer to phase 1 and 2, respectively, 0, no rain, 1, rain), (2) downpours during phase 1 (R10), (3) during phase 2 (R01), and (4) during both phases (R11). These four precipitation regimes allowed us to separate the indirect, plant‐mediated effects of downpours on the herbivores (R10, R11 vs. R00, R01) from predominantly direct effects (R01, R11 vs. R00, R10). The positions of the downpour treatments in the greenhouse, denoted by shower symbols, were set but tables were moved as indicated by the arrows at the transition from phase 1 to phase 2. Plants were placed on tables (replicate) with nine plants of which three were infested with L2 larvae of Plutella xylostella, four were infested with L1 larvae of Pieris brassicae, and two served as control (no herbivores). Assignment of treatments to plants within a table was randomized.
Two independent experiments were performed each with newly grown plants. In the first experiment, the frequency of rain was set at once a day for 20 min at 09:00. In the second experiment, the frequency of rain was increased to three times a day (09:00, 14:00, and 19:00) and the duration of each downpour was reduced to 5 min. Tables were moved within the greenhouse according to their assigned downpour treatment (Fig. 1). In the single downpour experiment, phase 1 was initiated 3 weeks after seedlings were transplanted and lasted 7 d whereas in the triple downpour experiment phase 1 was initiated 1 week after seedlings were transplanted and lasted 21 d. In both experiments, caterpillars were introduced when the plants were 4 weeks old. Phase 2, in which plants were exposed to herbivory, lasted approximately 7 d for P. xylostella and 14 d for P. brassicae due to differences in larval development time (see paragraph below).
The experiment was set up according to a block design comprising 24 blocks (tables) with six replicates per rainfall treatment, each containing nine plants of which two were not exposed to herbivory (control) and seven were exposed to one of the two herbivore species. On each of three plants, 10 early L2 larvae of P. xylostella were introduced (L1 larvae of P. xylostella are difficult to handle as they are mining in the leaves), and on each of four plants, 10 early L1 larvae of P. brassicae were introduced. The 216 plants in total were randomly allocated to blocks and treatments within blocks. The insect herbivores were allowed to move and feed freely on the plants. Plants were not touching preventing dispersal of insects to adjacent plants. When caterpillars of P. brassicae reached the third instar, their numbers were reduced to three per plant to ensure sufficient resources for caterpillar development. When larvae reached their final instar (fifth for P. brassicae and fourth for P. xylostella), plants with the same herbivore species within blocks were placed together in a netted cage (40 × 40 × 60 cm; Vermandel, Hulst, The Netherlands). At this point, exposure to downpours ended. This was done at day 7 on plants with P. xylostella and at day 14 on plants with P. brassicae after phase 2 had started. Pupae of P. xylostella and P. brassicae were collected per cage and transferred to Petri dishes. Development time until eclosion and offspring sex were recorded. Newly emerged adults were frozen immediately and weighed individually on a Mettler‐Toledo (Columbus, Ohio, USA) microbalance. The number of caterpillars that survived to the pupal (P. xylostella) and the adult stage (P. brassicae) was counted. Development time from L1 (P. brassicae) or L2 (P. xylostella) to adult eclosion was recorded in days.
Simulated downpours were expected to affect the microclimate. Therefore, we measured the temperature of the leaf surface of plants in the middle of each block using a thermo‐detector (Bosch PTD 1, Leinfelden‐Echterdingen, Germany) during the second experiment. Temperatures were measured three times; before, immediately after, and 1 h after the second downpour on four consecutive days during phase 2.
Before the plants were placed in cages, leaf tissues were collected from all plants for chemical analyses (GS and sugars). Five leaf discs (12 mm diameter) were collected from the youngest fully expanded leaf of each plant. Leaf discs were taken from the tips of leaves (Agrawal and Fishbein 2006). In each block, all leaf discs sampled from plants belonging to the same treatment were pooled, resulting in three samples (control, exposure to P. xylostella, and exposure to P. brassicae) per block and six samples per treatment. Samples were immediately submerged into liquid nitrogen and stored at −20°C until chemical analyses were conducted, which are described in detail in the Appendix S1.
Statistical analyses
Data (insect performance, chemistry) from the two experiments in which different frequencies of downpours were applied were analyzed separately. We used downpours during phase 1 (yes/no) and during phase 2 (yes/no) and their interaction terms as factors in the analyses to separate direct and indirect effects of rainfall. Effects of rainfall treatments on survival of P. xylostella larvae from L2 to pupation and for P. brassicae from L1 to the adult eclosion were analyzed using generalized linear models with a binomial distribution and logit link function, with downpour treatments and their interactions as fixed factors. Development time from L2 (P. xylostella) or L1 (P. brassicae) to adulthood and adult body mass of the herbivores were analyzed using general linear mixed models with the same fixed factors as for the analysis of insect survival and blocks as a random factor. Data on sinigrin (the dominant GS in B. nigra, comprising >99% of the GS content) and total sugar concentrations in leaf tissues were also analyzed using general linear mixed models. In addition to terms included in the models above, herbivory treatment was included as well (none, P. xylostella, or P. brassicae). Temperatures measured three times in each block during the second experiment were analyzed using a general linear mixed model with downpour regime, time and their interactions as fixed factors. Blocks and date were entered as random factors. Multiple comparisons were conducted when any of the model terms were significant (Tukey‐adjusted comparison). All analyses were performed in R version 3.4.0 (R Core Team 2017). The results of the statistical analysis are summarized in the Results section and fully listed in Appendix S1: Tables S1–S5.
Results
Direct effects of downpours on insect herbivore survival and development
Direct exposure of P. xylostella caterpillars to downpours (R00, R10 vs. R01, R11) significantly reduced their survival to pupation ( = 58.5, P < 0.001, Fig. 2a) by approximately 36%. This effect was even stronger (64% reduction) when caterpillars were exposed to three short 5‐min downpours each day ( = 179, P < 0.001, Fig. 2c). In contrast, survival of P. brassicae was much higher (>75%), irrespective of the rainfall regime (Fig. 2b, d). Survival of rain‐exposed P. brassicae caterpillars was only marginally affected in the single daily long downpour experiment ( = 5.28, P = 0.02, Fig. 2c) and it was not affected when the frequency was increased and the duration reduced ( = 0.91, P = 0.34, Fig. 2d).
Figure 2.
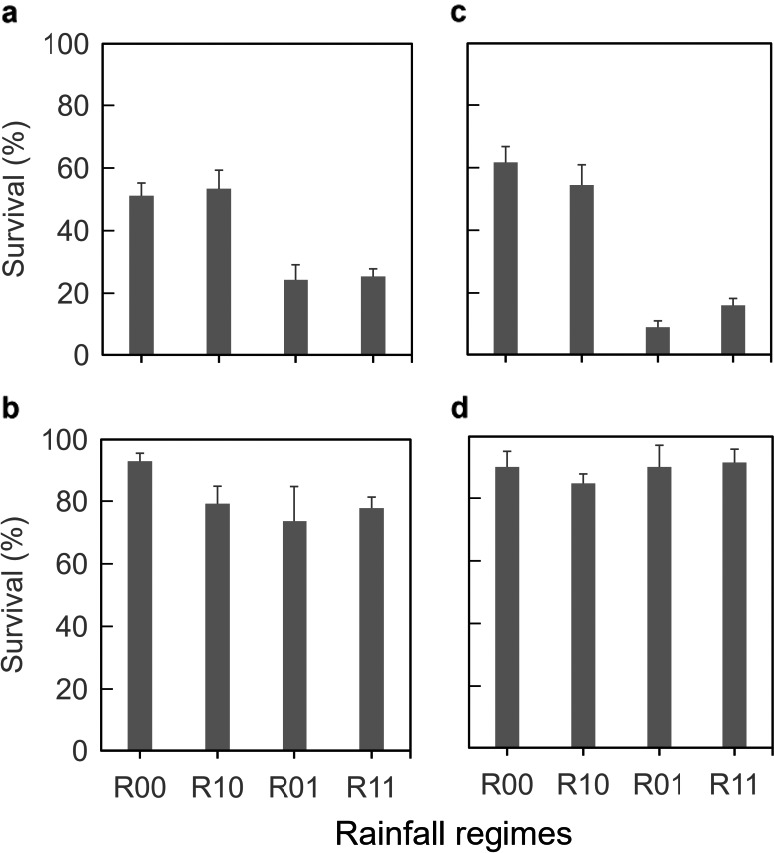
Survival (mean and SE) of (a, c) Plutella xylostella from L2 to pupation and (b, d) of Pieris brassicae from L1 to adult eclosion when developing on Brassica nigra plants exposed to different rainfall regimes: none (R00), only before caterpillars were introduced (R10), only after caterpillars were introduced (R01), or during both phases (R11). Insect herbivores were exposed to different frequencies of rainfall: (a, b) once (20 min) per day or (c, d) three times (5 min each) per day.
In both experiments, the two herbivores species took longer to develop when directly exposed to downpours (for P. xylostella: Exp. 1, F 1,37 = 8.95, P = 0.005, Fig. 3a; Exp. 2, F 1,31 = 31.7, P < 0.001, Fig. 3c; for P. brassicae: Exp. 1, F 1,20 = 34.9, P < 0.001, Fig. 4a; Exp. 2, F 1,20 = 59.3, P < 0.001, Fig. 4c). Interestingly, butterflies of P. brassicae directly exposed to downpours were lighter in the single downpour experiment (F 1,20 = 10.7, P = 0.003; Fig. 4b), but heavier in the triple downpour experiment (F 1,20 = 7.38, P = 0.01, Fig. 4d). The biomass of adult P. xylostella moths was also higher in the triple downpour experiment (F 1,31 = 13.8, P < 0.001, Fig. 3d), whereas there was no effect on biomass of a single daily downpour exposure (F 1,37 = 0.36, P = 0.55; Fig. 3b).
Figure 3.
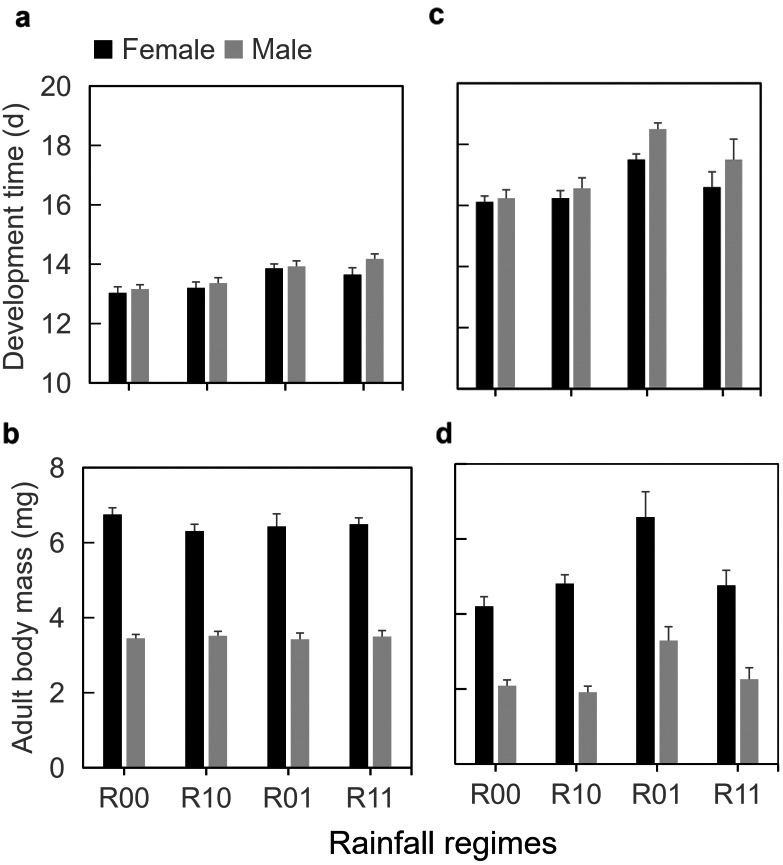
(a, c) Development time from L2 to adult eclosion and (b, d) adult body mass of Plutella xylostella on Brassica nigra plants exposed to different rainfall regimes: none (R00), only before caterpillars were introduced (R10), only after caterpillars were introduced (R01), or during both phases (R11). Note that axes of development time do not start at zero. Values are mean and SE. Insect herbivores were exposed to different frequencies of rainfall: (a, b) once (20 min) per day or (c, d) three times (5 min each) per day.
Figure 4.
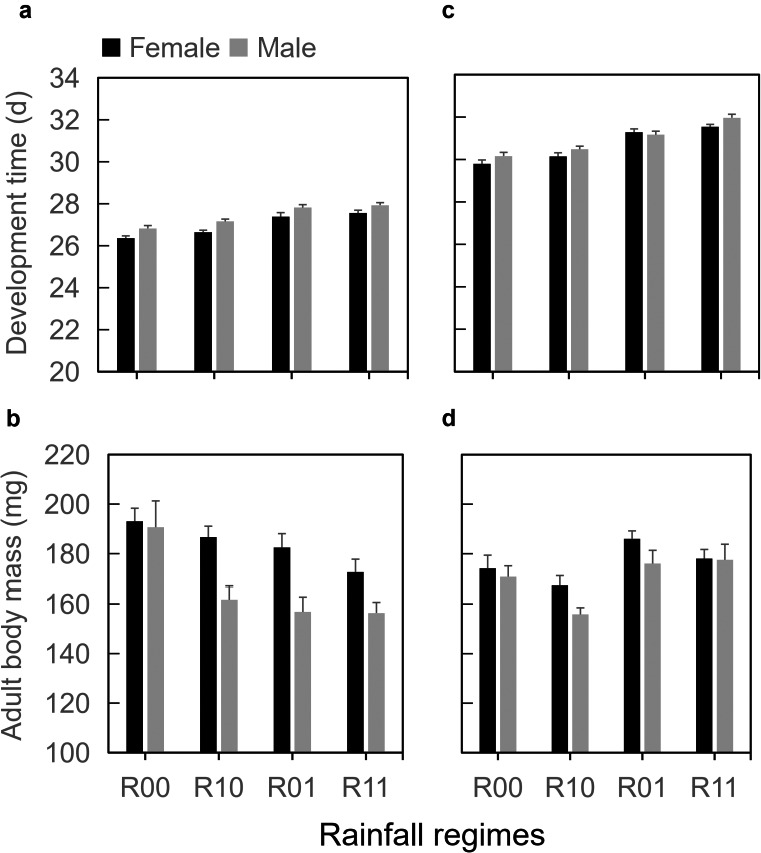
(a, c) Development time from L1 to adult eclosion and (b, d) adult body mass of Pieris brassicae on Brassica nigra plants exposed to different rainfall regimes: none (R00), only before caterpillars were introduced (R10), only after caterpillars were introduced (R01), or during both phases (R11). Note that axes do not start at zero. Values are mean and SE. Insect herbivores were exposed to different frequencies of rainfall: (a, b) once (20 min) per day or (c, d) three times (5 min each) per day.
Indirect effects of downpours on insect herbivore survival and development
Indirect, plant‐mediated effects of downpours (R00, R01 vs. R10, R11) on insect survival were relatively small and depended on whether plants were exposed to downpours while the caterpillars were feeding on the plants (interaction between direct and indirect effects, Appendix S1: Table S1). There were no indirect effects of downpours on immature development times and adult biomass of P. xylostella in the single downpour experiment (development time, F 1,37 = 0.02, P = 0.89; biomass, F 1,37 = 0.81, P = 0.89, Fig. 3a, b). Only in the experiment with triple downpours, both development time and biomass were affected by indirect effects of rainfall regime, but this depended on whether the larvae were exposed to rain or not (interaction between direct and indirect effects; development time, F 1,31 = 8.65, P = 0.006; biomass, F 1,31 = 13.36, P < 0.001). Caterpillars developed faster and moths were larger predominantly on plants that were only exposed to rain during phase 2 and not during phase 1 (Fig. 3c, d). For P. brassicae, negative indirect effects were relatively small. On plants exposed to rain during growth (phase 1), development times were extended, but this was only significant in the experiment with the triple downpours (single downpour, F 1,20 = 2.16, P = 0.16, Fig. 4c; triple downpour, F 1,20 = 7.32, P = 0.01, Fig. 4d). In the single downpour experiment, P. brassicae butterflies were larger on host plants that had not been exposed to rain during phase 1 (F 1,20 = 8.98, P = 0.007, Fig. 4b).
Irrespective of the rain exposure treatments, P. xylostella females were approximately twice as heavy as males (single rain downpour, F 1,37 = 161, P < 0.001; three downpours, F 1,31 = 113, P < 0.001; Fig. 3). Females also developed faster, but this was only significant in the experiment with three short daily downpours (single downpour, F 1,37 = 1.00, P = 0.32; three downpours, F 1,31 = 9.52, P = 0.004, Fig. 3). Also in P. brassicae, females developed faster (both experiments; F 1,20 = 42.6, P < 0.001; F 1,20 = 15.2, P < 0.001) and were heavier (only significant in single downpour experiment: F 1,20 = 22.4, P < 0.001) than males (Fig. 4). For both species, immature development was slower and adults were lighter in the second than in the first experiment.
Effects of downpours on microclimate
Downpours had a significant effect on the temperature of the leaf surface (F 3,20 = 10.5, P < 0.001) and temperature depended on the time point that it was measured (F 6, 253 = 36.2, P < 0.001). In response to downpours, the temperature immediately dropped by approximately 5°C and it took at least an hour to return to the original temperature (Appendix S1: Fig. S1).
Direct and indirect effects of downpours on chemical traits of Brassica nigra plants
Sinigrin
In the single downpour experiment, leaf concentrations of sinigrin, the dominant GS produced by B. nigra, were not affected by downpours during phase 1 (F 1,20 = 3.02, P = 0.10), but increased significantly in response to downpours during phase 2 (F 1,20 = 18.5, P < 0.001, Fig. 5a). Overall, sinigrin concentrations were higher in leaf tissues of plants exposed to herbivory than on plants without herbivores, and this effect was stronger when plants were also exposed to downpours during the insect feeding phase (F 2,40 = 6.82, P = 0.003; both herbivores: Tukey HSD tests, P < 0.05). In the second experiment in which plants were exposed to three brief downpours per day, sinigrin concentrations tended to be high irrespective of the rainfall regime and herbivore treatment (compare Fig. 5a and c). In the absence of downpours during the insect feeding phase (R00, R10), sinigrin levels were not affected by P. xylostella and actually decreased in the presence of P. brassicae (Fig. 5c). By contrast, in the presence of downpours during the insect feeding phase (R10, R11), sinigrin levels were slightly increased by caterpillar feeding, resulting in a significant interaction between herbivory and downpour regime during the insect feeding phase (F 2,40 = 6.82, P = 0.003, Fig. 5c).
Figure 5.
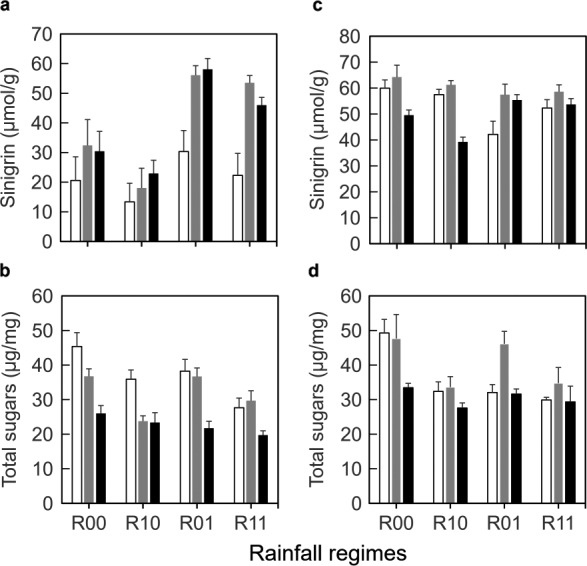
(a, c) Concentrations of sinigrin (a, c) and (b, d) total sugars of Brassica nigra plants exposed to different herbivory treatments (none [white bars[, Plutella xylostella [gray bars], Pieris brassicae [black bars]) and rainfall regimes: none (R00), only before caterpillars were introduced (R10), only after caterpillars were introduced (R01), or during both phases (R11). Values are mean and SE. Insect herbivores were exposed to different frequencies of rainfall: (a, b) once (20 min) per day or (c, d) three times (5 min each) per day.
Sugars
When downpours occurred once a day, total sugar concentration in leaf tissues was affected by downpour regime and herbivory (Fig. 5c). Sugar concentrations were lower in plants exposed to downpours during phase 1 than in plants not exposed to downpours during this phase (F 1,20 = 18.3, P < 0.001), whereas downpours during phase 2 did not significantly reduce sugar concentrations further (F 1,20 = 2.82, P = 0.11). Feeding by the herbivores reduced foliar sugar and this effect was stronger for P. brassicae than for P. xylostella, irrespective of downpour regime (Tukey HSD, P < 0.05). In the second experiment with three downpours per day, the effects of downpour regime and herbivory were similar but less significant than in the first experiment (Fig. 5d).
Discussion
Simulated rainfall, which mimicked downpours occurring during thunderstorms, had a profound direct negative effect on the survival of P. xylostella, and this effect was stronger when the frequency of downpour events increased from one to three daily. By contrast, the survival of P. brassicae was marginally affected, but only in the single downpour experiment. Direct exposure of insects on plants to downpours also increased development time of the two herbivores, whereas the effects of downpours on adult body mass were more idiosyncratic. In spite of significant changes in leaf chemistry, indirect, plant‐mediated effects of downpours on the performance, i.e., development time and adult biomass, of the two specialist insects were generally small.
Feeding in P. xylostella is characterized by bouts of tissue intake interspersed with periods of resting away from the feeding site. When disturbed, they often drop from the food plant along a silk thread, which is later used by the larvae to climb back onto the plant. However, heavy rainfall may prevent this from occurring (Kobori and Amano 2003). By contrast, larvae of P. brassicae feed gregariously during the first three instars. Moreover, P. brassicae larvae tend to move much less between feeding bouts and produce silk beds with which they are tightly attached to the leaf. Therefore, P. brassicae larvae are in general less affected by physical disturbance. In a previous study, we reported that caterpillars of P. xylostella are also more strongly disturbed by wind than the caterpillars of P. brassicae (Chen et al. 2018). The higher mortality of P. xylostella in response to rainfall, especially when occurring at higher frequencies (80% reduction), is even stronger than mortality caused by wind exposure (10%; this study, Chen et al. 2018). Eggs and (early) first‐instar larvae of Lepidoptera are the most vulnerable life stages and mortality during each of these stages can be significant (>40%; Zalucki et al. 2002). First instar caterpillars of P. xylostella feed and live inside leaf tissues and are, therefore, less exposed to environmental conditions. Though females of both species lay their eggs predominantly on the underside off the leaves, it cannot be excluded that downpours affects survival of eggs, thus underestimating the effect on survival in this study.
The development time of both herbivores was extended by both direct and indirect (plant‐mediated) effects of exposure to rainfall whereas the effects on biomass were not very consistent as they differed depending on the frequency of rainfall as well as between the two herbivore species. The direct effect of downpours on development time was possibly caused by the fact that during downpours caterpillars do not feed. Even after the downpour ended, feeding was not immediately resumed. We also found that the temperature near the leaf surface dropped dramatically in response to the downpours, and it took more than 1 h before the temperature returned to its original level. Metabolism and growth rate in ectotherms such as insects are strongly determined by ambient temperature. In addition to disturbance, microclimatic cooling caused by rain may explain the extended immature development times of the two herbivores.
In both species, extended larval development time coincided with adults being heavier, but only in the experiment in which the caterpillars were exposed to three short downpour events. This effect was more prominent for P. brassicae than for P. xylostella. In contrast, although development time of P. brassicae was also extended when exposed to a single rain event per day, adults in this treatment group were lighter. In a previous study (Chen et al. 2018), P. brassicae exposed to wind developed slower but attained more biomass. Predation of caterpillars by birds (Great Tits) was considerably reduced when reared on plants exposed to wind. We speculated that this phenotypic shift to a prolonged developmental program might be an adaptive response to reduced predation risk that is perceived by the feeding caterpillars that benefit significantly from larger size in terms of fecundity (Chen et al. 2018). Whether a similar scenario could hold for increased exposure to rain remains to be investigated. Heavy downpours are not only likely to have direct effects on insect herbivores, but on their natural enemies as well. Studies with birds (Robbins 1981, Radford et al. 2001) and parasitoids (Fink and Völkl 1995) showed a significant decrease in foraging activity of these natural enemies during rainy periods. If rainfall is also perceived by herbivores as reducing predation risk, as it does with wind, then this might explain trade‐offs between traits such as development time and body size. However, the result of this study suggests that abiotic factors such as rainfall need to be prolonged or more frequent in order to induce phenotypic shifts, as this phenomenon was only observed in the experiment in which rainfall frequency was increased to three times per day.
Indirect effects of rainfall may affect food plant quality through both chemical and morphological changes. The two groups of chemicals measured in this study, glucosinolates and sugars, thus, represent only a very small subset of the full arsenal of traits in plants that can affect the performance of insect herbivores and of which the expression may also change in response to rain. In plants exposed to both rainfall (single daily events) and herbivory, the induction of foliar levels of sinigrin, the dominant GS in B. nigra, was strongest (2 fold increase), whereas the induction of sinigrin by either herbivory or rainfall alone was not statistically significant. This result suggests that, in B. nigra, rainfall dramatically increased the sensitivity of GS metabolism to herbivory. However, specialist herbivores such as P. brassicae and P. xylostella, which both have efficient mechanisms to prevent exposure to the toxic GS breakdown products (Winde and Wittstock 2011), are generally less affected by changes in GS and potentially other defensive traits specific for brassicaceous plants. This could be the reason that, despite these considerable changes in sinigrin concentrations in response to rainfall, indirect (plant‐mediated) effects of rainfall on these insects were small. We therefore speculate that generalist insect herbivores that are more strongly affected by changes in GS (Gols et al. 2008, Jeschke et al. 2017) may be more sensitive to such indirect, plant‐mediated effects of rainfall. In the second experiment, in which the frequency (but not duration) of downpours was increased, sinigrin concentrations were high in all plants including those that were not exposed to rain and or herbivory. Sinigrin concentrations reached the same levels as those found in plants with the highest concentrations (>50 μmol/g dry mass) in the first experiment. These concentrations are high in comparison with results previously reported for B. nigra (van Dam et al. 2004, Gols et al. 2009). It is not clear why the concentrations were higher in the second than in the first experiment, but it seemed that the conditions were less favorable for both the plant and the herbivores (on control plants, caterpillars developed faster and were heavier in the first than in the second experiment). The second experiment was conducted in a greenhouse in winter, whereas the first experiment was conducted in a greenhouse in autumn. Even in climate‐controlled greenhouses, conditions, such as the quality and intensity of the artificial light for plant and insect growth, may vary seasonally (Gols et al. 2007). It should also be noted that we used a short‐lived annual plant. Such species may be less likely to exhibit strong indirect effects of rainfall or other climate‐related effects than perennial plants, as these events may occur in multiple years causing accumulative effects on host plant quality in perennials.
Sugar concentrations declined significantly in response to rain, but only during early development of plants before the insects were feeding on them. Sugar concentrations were also lower in leaf tissues that had been exposed to herbivory, especially feeding by P. brassicae reduced foliar sugar concentrations. Previous studies have shown that plant nutrients can be leached from leaf tissues by rainfall (Tukey 1970, Schreiber 1999). Moreover, damaged leaves of plants are much more susceptible to leaching than undamaged leaves (Tukey 1970). Feeding damage by P. brassicae caterpillars is more severe than feeding by P. xylostella caterpillars and this could explain the lower sugar concentration in leaf tissues damaged by the former herbivore species. Sugars such as sucrose and glucose are important feeding stimulants (Schoonhoven et al. 2005). To what extent the reduced sugar concentrations and leaching of nutrients in general explain extended development and changes in other fitness correlates of insect herbivores feeding on rain‐exposed plants merits further investigation. In addition, changes in nitrogen, a very important nutrient that is usually very low in plant tissues, and changes in morphological traits should be considered to better understand how abiotic factors influence the nutritional quality for and resistance to insect herbivores.
At the other end of extreme weather events, periods of extended drought are also predicted to increase in frequency under conditions of climate warming. In particular, heat waves and droughts generate conditions that are very different from heavy downpours. Prolonged lack of water and exposure to extreme heat can inflict huge physiological stresses both on plants and higher trophic level organisms. For instance, drought can lead to changes in primary and secondary metabolism and the overall physiological status of the plant (Jamieson et al. 2012), which, in turn, can affect the behavior and development of insect herbivores (Huberty and Denno 2004), as well as the interaction with their natural enemies (Karban et al. 2017). In extreme cases, prolonged drought can lead to early plant estivation or even death (McDowell et al. 2011), and this, in turn, will clearly affect and entire community of organisms associated with the plant. In addition to the biotic environment, the microclimatic environment plays an important role in determining the population dynamics of insect herbivores (Dobkin et al. 1987, Walsh 2017). For instance, Walsh (2017) found that, after a serious drought event, factors influencing the microclimate better explained the population decline of an endangered butterfly species, the Karner blue butterfly (Lycaeides melissa samuelis) than the biotic factors. These results demonstrate the relative importance of abiotic factors on the survival of small ectothermic organisms. Moreover, it is important to note that heat waves, droughts, and intense, sudden downpours can occur in close proximity, e.g., intensive heat waves that are often broken by severe thunderstorms. These contrasting abiotic conditions may induce a range of different, and even opposite, stresses on plants and insects associated with them.
More extreme weather events, including short, intense downpours, are predicted by the IPCC (2014) under Anthropogenic Global Warming. Asymmetric effects of rainfall on population dynamics of species within and across trophic levels have consequences for the structure of the whole community. Climate change has far reaching effects on ecological processes ranging from effects on individual species to entire ecosystems (Harrington et al. 1999, McLaughlin et al. 2002, Walther et al. 2002, Knapp et al. 2008). Our study emphasizes the importance of rainfall in understanding the effects of climate change on ecological responses in trophic chains and broader ecological communities. Future experiments should investigate combined effects of climate‐related factors on plant–insect as well as multitrophic interactions.
Supporting information
Acknowledgments
We thank Unifarm at Wageningen University for the assistance in the greenhouse and Laboratory of Entomology (WUR) for providing caterpillars. We also thank Ciska Raaijmakers for helping with chemical analyses. Funding for this study was provided by China Scholarship Council (CSC) and Department of Terrestrial Ecology at Netherlands Institute of Ecology. We declare that there are no conflicts of interest.
Chen, C. , Harvey J. A., Biere A., and Gols R.. 2019. Rain downpours affect survival and development of insect herbivores: the specter of climate change? Ecology 100(11):e02819 10.1002/ecy.2819
Corresponding Editor: James T. Cronin.
Literature Cited
- Agrawal, A. A. , and Fishbein M.. 2006. Plant defense syndromes. Ecology 87:S132–S149. [DOI] [PubMed] [Google Scholar]
- Awmack, C. S. , and Leather S. R.. 2002. Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology 47:817–844. [DOI] [PubMed] [Google Scholar]
- Bale, J. S. , Masters G. J., Hodkinson I. D., Awmack C., Bezemer T. M., Brown V. K., Butterfield J., Buse A., Coulson J. C., and Farrar J.. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology 8:1–16. [Google Scholar]
- Brooks, H. E. 2013. Severe thunderstorms and climate change. Atmospheric Research 123:129–138. [Google Scholar]
- Chen, C. , Biere A., Gols R., Halfwerk W., van Oers K., and Harvey J. A.. 2018. Responses of insect herbivores and their food plants to wind exposure and the importance of predation risk. Journal of Animal Ecology 87:1046–1057. [DOI] [PubMed] [Google Scholar]
- Christidis, N. , Stott P. A., and Brown S. J.. 2011. The role of human activity in the recent warming of extremely warm daytime temperatures. Journal of Climate 24:1922–1930. [Google Scholar]
- Dicke, M. 2016. Plant phenotypic plasticity in the phytobiome: a volatile issue. Current Opinion in Plant Biology 32:17–23. [DOI] [PubMed] [Google Scholar]
- Dobkin, D. S. , Olivieri I., and Ehrlich P. R.. 1987. Rainfall and the interaction of microclimate with larval resources in the population dynamics of checkerspot butterflies (Euphydryas editha) inhabiting serpentine grassland. Oecologia 71:161–166. [DOI] [PubMed] [Google Scholar]
- Fahey, J. W. , Zalcmann A. T., and Talalay P.. 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51. [DOI] [PubMed] [Google Scholar]
- Feltwell, J. 1982. Large white butterfly, the biology, biochemistry and physiology of Pieris brassicae (Linaeus). Dr W. Junk Publishers, The Hague, The Netherlands. [Google Scholar]
- Fink, U. , and Völkl W.. 1995. The effect of abiotic factors on foraging and oviposition success of the aphid parasitoid, Aphidius rosae . Oecologia 103:371–378. [DOI] [PubMed] [Google Scholar]
- Furlong, M. J. , Wright D. J., and Dosdall L. M.. 2013. Diamondback moth ecology and management: problems, progress, and prospects. Annual Review of Entomology 58:517–541. [DOI] [PubMed] [Google Scholar]
- Gols, R. , Bukovinszky T., van Dam N. M., Dicke M., Bullock J. M., and Harvey J. A.. 2008. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. Journal of Chemical Ecology 34:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gols, R. , and Harvey J. A.. 2009. Plant‐mediated effects in the Brassicaceae on the performance and behaviour of parasitoids. Phytochemistry Reviews 8:187–206. [Google Scholar]
- Gols, R. , Raaijmakers C. E., van Dam N. M., Dicke M., Bukovinszky T., and Harvey J. A.. 2007. Temporal changes affect plant chemistry and tritrophic interactions. Basic and Applied Ecology 8:421–433. [Google Scholar]
- Gols, R. , van Dam N. M., Raaijmakers C. E., Dicke M., and Harvey J. A.. 2009. Are population differences in plant quality reflected in the preference and performance of two endoparasitoid wasps? Oikos 118:733–743. [Google Scholar]
- Harrington, R. , Woiwod I., and Sparks T.. 1999. Climate change and trophic interactions. Trends in Ecology & Evolution 14:146–150. [DOI] [PubMed] [Google Scholar]
- Hopkins, R. J. , van Dam N. M., and van Loon J. J. A.. 2009. Role of glucosinolates in insect‐plant relationships and multitrophic interactions. Annual Review of Entomology 54:57–83. [DOI] [PubMed] [Google Scholar]
- Huberty, A. F. , and Denno R. F.. 2004. Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85:1383–1398. [Google Scholar]
- IPCC . 2014. Climate change 2014: synthesis report in Core Writing Team, R. K. Pachauri and L. A. Meyer editors. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland, 151 pp. [Google Scholar]
- Jamieson, M. A. , Burkle L. A., Manson J. S., Runyon J. B., Trowbridge A. M., and Zientek J.. 2017. Global change effects on plant–insect interactions: the role of phytochemistry. Current Opinion in Insect Science 23:70–80. [DOI] [PubMed] [Google Scholar]
- Jamieson, M. , Trowbridge A. M., Raffa K. F., and Lindroth R. L.. 2012. Consequences of climate warming and altered precipitation patterns for plant‐insect and multitrophic interactions. Plant Physiology 160:1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke, V. , Kearney E. E., Schramm K., Kunert G., Shekhov A., Gershenzon J., and Vassao D. G.. 2017. How glucosinolates affect generalist lepidopteran larvae: growth, development and glucosinolate metabolism. Frontiers in Plant Science 8:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata, N. , and Igarashi Y.. 1994. Influence of rainfall on feeding behavior, growth, and mortality of larvae of the beech caterpillar, Quadricalcarifera punctatella (Motschulsky) (Lep, Notodontidae). Journal of Applied Entomology 118:347–353. [Google Scholar]
- Karban, R. , Grof‐Tisza P., and Holyoak M.. 2017. Wet years have more caterpillars: interacting roles of plant litter and predation by ants. Ecology 98:2370–2378. [DOI] [PubMed] [Google Scholar]
- Knapp, A. K. , Beier C., Briske D. D., Classen A. T., Luo Y., Reichstein M., Smith M. D., Smith S. D., Bell J. E., and Fay P. A.. 2008. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience 58:811–821. [Google Scholar]
- Kobori, Y. , and Amano H.. 2003. Effect of rainfall on a population of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Applied Entomology and Zoology 38:249–253. [Google Scholar]
- Lenderink, G. , and van Meijgaard E.. 2008. Increase in hourly precipitation extremes beyond expectations from temperature changes. Nature Geoscience 1:511. [Google Scholar]
- Loreto, F. , and Schnitzler J.‐P.. 2010. Abiotic stresses and induced BVOCs. Trends in Plant Science 15:154–166. [DOI] [PubMed] [Google Scholar]
- McDowell, N. G. , Beerling D. J., Breshears D. D., Fisher R. A., Raffa K. F., and Stitt M.. 2011. The interdependence of mechanisms underlying climate‐driven vegetation mortality. Trends in Ecology & Evolution 26:523–532. [DOI] [PubMed] [Google Scholar]
- McLaughlin, J. F. , Hellmann J. J., Boggs C. L., and Ehrlich P. R.. 2002. Climate change hastens population extinctions. Proceedings of the National Academy of Sciences USA 99:6070–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, V. C. , Hoffmann J. H., and Basson N. C. J.. 1987. The effects of simulated rainfall on cochineal insects (Homoptera, Dactylopiidae): colony composition and survival on cactus cladodes. Ecological Entomology 12:51–60. [Google Scholar]
- Norby, R. J. , and Zak D. R.. 2011. Ecological lessons from Free‐Air CO2 Enrichment (FACE) experiments. Annual Review of Ecology, Evolution, and Systematics 42:181–203. [Google Scholar]
- Prakash, S. , and Hinata K.. 1980. Taxonomy, cytogenetics and origin of crop Brassicas, a review. Opera Botanica 55:1–57. [Google Scholar]
- R Core Team . 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org [Google Scholar]
- Radford, A. N. , McCleery R. H., Woodburn R. J. W., and Morecroft M. D.. 2001. Activity patterns of parent great tits Parus major feeding their young during rainfall. Bird Study 48:214–220. [Google Scholar]
- Robbins, C. S. 1981. Bird activity levels related to weather. Studies in Avian Biology 6:301–310. [Google Scholar]
- Schoonhoven, L. M. , van Loon J. J. A., and Dicke M.. 2005. Insect‐plant biology. Second edition Oxford University Press, Oxford, UK. [Google Scholar]
- Schreiber, J. D. 1999. Nutrient leaching from corn residues under simulated rainfall. Journal of Environmental Quality 28:1864–1870. [Google Scholar]
- Scriber, J. M. , and Slansky F.. 1981. The nutritional ecology of immature insects. Annual Review of Entomology 26:183–211. [Google Scholar]
- Suttle, K. B. , Thomsen M. A., and Power M. E.. 2007. Species interactions reverse grassland responses to changing climate. Science 315:640–642. [DOI] [PubMed] [Google Scholar]
- Textor, S. , and Gershenzon J.. 2009. Herbivore induction of the glucosinolate–myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochemistry Reviews 8:149–170. [Google Scholar]
- Tukey, H. 1970. The leaching of substances from plants. Annual Review of Plant Physiology 21:305–324. [Google Scholar]
- Uvarov, B. P. 1931. Insects and climate. Transactions of the Royal Entomological Society of London 79:1–232. [Google Scholar]
- Vallat, A. , Gu H., and Dorn S.. 2005. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 66:1540–1550. [DOI] [PubMed] [Google Scholar]
- van Dam, N. M. , Witjes L., and Svatos A.. 2004. Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytologist 161:801–810. [DOI] [PubMed] [Google Scholar]
- Walsh, R. P. 2017. Microclimate and biotic interactions affect Karner blue butterfly occupancy and persistence in managed oak savanna habitats. Journal of Insect Conservation 21:219–230. [Google Scholar]
- Walther, G.‐R. , Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.‐M., Hoegh‐Guldberg O., and Bairlein F.. 2002. Ecological responses to recent climate change. Nature 416:389–395. [DOI] [PubMed] [Google Scholar]
- Winde, I. , and Wittstock U.. 2011. Insect herbivore counteradaptations to the plant glucosinolate‐myrosinase system. Phytochemistry 72:1566–1575. [DOI] [PubMed] [Google Scholar]
- Zalucki, M. P. , Clarke A. R., and Malcolm S. B.. 2002. Ecology and behavior of first instar larval lepidoptera. Annual Review of Entomology 47:361–393. [DOI] [PubMed] [Google Scholar]
- Zavala, J. A. , Nabity P. D., and DeLucia E. H.. 2013. An emerging understanding of mechanisms governing insect herbivory under elevated CO2 . Annual Review of Entomology 58:79–97. [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Wang D., Wang L., Fang J., Sun W., and Ren B.. 2014. Effects of altered precipitation on insect community composition and structure in a meadow steppe. Ecological Entomology 39:453–461. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


