Abstract
Introduction
Reproductive scientists have postulated various risk factors for lower birthweight following conventional gonadotropin‐stimulated in vitro fertilization compared with spontaneously conceived children: parental factors (age, health, duration of subfertility and smoking habits); ovarian stimulation; laboratory procedures; the number of oocytes retrieved and the number of embryos transferred. Our aim was to investigate the impact of gonadotropin stimulation and serum estradiol level on the risk of a newborn being small‐for‐gestational‐age.
Material and methods
We conducted a cohort study (2010‐2016) of singletons (n = 155) born either after conventional gonadotropin‐stimulated in vitro fertilization (using ≥150 IU/d human gonadotropin for stimulation) or after natural cycle in vitro fertilization without any stimulation. We analyzed perinatal outcomes using birthweight percentiles, adjusted for gestational age and sex.
Results
The proportion of small‐for‐gestational‐age was 11.8% following conventional gonadotropin‐stimulated in vitro fertilization and 2.9% after natural cycle in vitro fertilization (P = 0.058). The odds of small‐for‐gestational‐age were significantly higher with supraphysiological estradiol levels in maternal serum on ovulation trigger day (unadjusted odds ratio 4.58; 95% confidence interval 1.35‐15.55; P = 0.015). It remained significant after adjusting for maternal height, age and body mass index (adjusted odds ratio 3.83; 95% confidence interval 1.06‐13.82; P = 0.041).
Conclusions
We found an associated risk of children being born small‐for‐gestational‐age after conventional gonadotropin‐stimulated in vitro fertilization compared with natural cycle in vitro fertilization. This higher risk is significantly associated with supraphysiological estradiol levels. We propose a reduction in the dosage of gonadotropin to minimize the risk of small‐for‐gestational‐age and future health consequences.
Keywords: gonadotropin, high‐risk pregnancy, in vitro fertilization, infertility, pregnancy, reproductive endocrinology
Abbreviations
- BMI
body mass index
- CI
confidence interval
- c‐IVF
conventional gonadotropin‐stimulated IVF
- E2
estradiol
- FA
follicle aspiration
- IVF
in vitro fertilization
- LBW
low birthweight
- NC‐IVF
natural cycle in vitro fertilization
- OR
odds ratio
- SGA
small‐for‐gestational‐age
Key message.
Supraphysiological serum estradiol levels under gonadotropin stimulation in in vitro fertilization are associated with a higher incidence of children born small‐for‐gestational‐age. We propose a reduction in gonadotropin dosage to minimize potential negative health consequences.
1. INTRODUCTION
Birthweights in children born following conventional gonadotropin‐stimulated in vitro fertilization (c‐IVF) and fresh embryo transfer are shown in several systematic reviews and meta‐analyses1, 2 to be lower than birthweights of spontaneously conceived singletons. Various risk factors were postulated:3 parental factors (mainly preexisting diseases, duration of subfertility4 and parental smoking); in vitro fertilization (IVF) laboratory procedures;5 the number of oocytes retrieved;6 and the number of embryos transferred. After the transfer of more than one embryo, fetal intrauterine growth restriction may be a consequence of vanishing twins.2 Two studies addressing E2 levels in maternal serum at ovulation trigger day postulated a dosage‐dependent impact of gonadotropins on birthweight and birthweight percentile.7, 8
Comparing c‐IVF with natural cycle IVF (NC‐IVF) with natural follicle selection and without the use of gonadotropins offers an excellent way to study the influence of hormones on obstetric and perinatal outcomes. A recent systematic review found a significantly higher risk of low birthweight (LBW) after c‐IVF (risk ratio 1.95; 95% confidence interval [CI] 1.03‐3.67) compared with modified IVF/NC‐IVF therapy.9 Studies included in the review focused on birthweight as the main outcome and defined LBW as <2500 g, without taking into consideration exact gestational age.6, 10, 11 Using birthweight percentiles adjusts for gestational age and sex of the neonate.
Our study compares birthweight and birthweight percentiles of c‐IVF with those of NC‐IVF newborns.
2. MATERIAL AND METHODS
2.1. Study population
The cohort of this study includes women treated for infertility at the Bern University Hospital, Division of Gynecological Endocrinology and Reproductive Medicine, Switzerland, from 2010 to 2016. We retrieved prospectively collected data and prepared it for analysis using REDCap electronic data (REDCap 8.5.19 Vaderbilt University, Nashville, USA) capture tools hosted at the Clinical Trials Unit, University of Bern.
We included data on all women with a singleton live birth after a fresh cleavage‐stage embryo transfer who underwent either c‐IVF or NC‐IVF. Women with a regular menstrual cycle chose the treatment (NC‐IVF or c‐IVF) as per their preference. We did not include women receiving thawing cycles and cycles with modified IVF treatment (eg, the use of clomiphene citrate [CC]). At our clinic, we offer treatments with modifying drugs such as CC and low‐dose human menopausal gonadotropin only as second‐line options after several unsuccessful NC‐IVF treatments. Women receiving those treatments had previously several unsuccessful treatment cycles. Our aim was to compare two clearly distinct approaches, namely, NC‐IVF without the use of any stimulating drugs with c‐IVF, both used as first‐line treatments.
2.2. Data sources
We collected data on patient characteristics and reproductive treatment from medical records. Chronic disease included asthma, Hashimoto's thyroiditis and arterial hypertension. From the treating gynecologist or the maternity hospital we received results of later pregnancies, delivery reports and perinatal information on mother and child. We considered the following conditions to be pregnancy complications: gestational hypertension, gestational diabetes, placental pathologies (eg, abnormal placentation such as placenta previa or placenta accreta/increta), antepartum hemorrhage, preterm contractions, preterm rupture of membranes and fetal growth restriction. We calculated fetal weight during pregnancy using the Hadlock formula. From the available fetal growth charts, we evaluated utero‐placental dysfunction (decrease in growth rate) and characteristic fetal perfusion changes if Doppler ultrasound was available. We defined according to the TRUFFLE study for fetal growth restriction12 an abnormal umbilical artery Doppler with a pulsatility index above the 95th percentile of the Doppler reference chart13 with or without reversed or absent end‐diastolic flow as pathologic. We calculated gestational age as the time from fertilization at the day of follicular aspiration (FA) to delivery, plus 14 days. We used growths charts from Voigt & Fenton14 to determine the birthweight percentile. We defined a priori small‐for‐gestational‐age (SGA) as a birthweight ≤5th percentile. This reflects a degree of smallness that is more likely to be pathologic rather than constitutional.15, 16
2.3. Conventional gonadotropin‐stimulated IVF
At our center, we perform c‐IVF as either a short agonist protocol or an antagonist protocol. For the short agonist protocol, we use triptoreline (gonadotropin‐releasing hormone agonist, eg, Decapeptyl®) and 150‐300 units of follicle‐stimulating hormone (eg, Fostimon®) or human menopausal gonadotropin (Menopur® or Merional®) for follicular stimulation, depending on age, Anti‐Müllerian hormone level and antral follicle count. For the antagonist protocol, we use human menopausal gonadotropin (150‐300 units a day) for follicular stimulation and 0.25 mg cetrorelix (gonadotropin‐releasing hormone antagonist, eg, Cetrotide®) once a day, beginning at stimulation day 6 or 7. We monitor cycles using ultrasound, check serum E2 levels, and trigger ovulation with human choriogonadotropin (eg, Ovitrelle®) when the size of the leading follicles is >17 mm. We perform FA 36 hours later under conscious sedation.
2.4. Natural cycle IVF
We perform NC‐IVF in accordance with best practice.17 We perform follicle monitoring by ultrasound and analysis of E2 and luteinizing hormone (LH) concentrations, once preovulatory. When the follicular diameter reaches at least 16 mm and the E2 concentration is expected to be ≥800 pmol/L, we administer a single dose of 5000 IU human choriogonadotropin as a trigger and FA is performed 36 hours later without anesthesia using a 19G single lumen needle. In the event of luteinizing hormone‐surge (>10 IU/L) on the day of human choriogonadotropin administration, we give 400 mg ibuprofen every 8 hours starting a maximum of 48 hours before FA until the day of FA. If information on the E2 concentration on ovulation trigger day is not available, we calculate it based on follicular phase measurements.
For both types of treatment, we fertilize the mature oocytes by intracytoplasmic sperm injection, and we culture a cleavage stage embryo. For the assessment of embryo quality, we use ASEBIR classification18 based on the number of cells, the heterogeneity of the cells, and the fragmentation rate of the embryo. We transfer the embryo at cleavage stage with a soft transfer catheter under ultrasound surveillance. Women receive luteal phase support twice a day after c‐IVF with intravaginal micronized progesterone (eg, Utrogestan® 200 mg or Crinone®), beginning the evening of the day of FA until the 12th week of pregnancy.
2.5. Statistical analyses
We performed statistical analysis using Fisher's exact test (SGA and LBW) or chi‐square test and Mantel‐Haenszel statistics for categorical outcomes and linear regression for continuous outcomes. As seven women with two IVF children each were included in the dataset, we used robust standard errors controlling for clusters in the regressions. For E2 and birth percentile, we performed multivariate linear regression, and for binary outcomes, multivariate logistic regression. We adjusted for the known risk factors: maternal age, maternal height and body mass index (BMI) according the large US multicenter study from Gardosi & Francis19 and controlled for multicollinearity between BMI and height. For parity (nulliparous/parous), vanishing twins and smoking during pregnancy (yes/no), we conducted a sensitivity analysis using exact logistic regression. We also used supraphysiological E2 levels (>10 000 pmol/L) as a binary exposure. The E2 cut‐off levels in previous studies ranged between 9178 and 12 665 pmol/L.7, 8, 20 We considered E2 >10 000 pmol/L to be supraphysiological, “because this E2 level is higher than that of 10 mature follicles and lower than that of high‐risk ovarian hyperstimulation syndrome”8 and it comprises median and mean E2 levels of our c‐IVF patients (9590 pmol/L; 10 459 pmol/L). We present odds ratios (OR) and 95% CIs; a P value <0.05 is considered statistically significant.
For statistical analysis, we used STATA statistical software Version 15 (Stata Corporation, College Station, Texas, USA).
2.6. Ethics approval
The cantonal ethical committee of Bern (KEK Bern, 397/15), Switzerland, approved the study on 26 January 2016.
3. RESULTS
We identified 643 pregnancies from the 2010‐2016 period. We excluded 159 births after frozen embryo transfer. Of 484 women who underwent a fresh embryo transfer, one woman was lost to follow up, 188 had a miscarriage and 26 had a multiple pregnancy. We excluded 114 singleton deliveries because of modifications (see Study population 2.1.). These resulted in 155 singleton deliveries in the analysis: 85 conceived after c‐IVF and 70 after a NC‐IVF treatment. Twelve women did not consent to further data collection associated with their child.
3.1. Study population characteristics
Within the c‐IVF group, 21 had an agonist protocol and 64 had an antagonist protocol. See Table 1 for patient and treatment characteristics. We could not find a significant difference in age, BMI, parity or smoking between the two groups. Prevalence of chronic diseases was comparable between c‐IVF and NC‐IVF (22.40% vs 17.14%; P = 0.420).
Table 1.
Patient characteristics
| NC‐IVF | %/SD | c‐IVF | %/SD | P value | |
|---|---|---|---|---|---|
| n = 70 | n = 85 | ||||
| Maternal age (y) | 34.23 | 3.76 | 34.57 | 4.15 | 0.598a |
| Maternal height (cm) | 166.99 | 6.04 | 167.01 | 7.98 | 0.982a |
| Maternal weight (kg) | 62.18 | 10.57 | 62.28 | 11.17 | 0.956a |
| Maternal BMI (kg/m2) | 22.32 | 3.76 | 22.33 | 3.72 | 0.982a |
| Parity (nulliparous) | 52 | 74.29 | 72 | 84.71 | 0.107b |
| Smoking before pregnancy (y/n) | 7 | 10 | 15 | 17.65 | 0.248c |
| Smoking during pregnancy (y/n) | 0 | 0 | 4 | 4.71 | 0.141c |
| Chronic disease mother (y/n) | 12 | 17.14 | 19 | 22.40 | 0.420b |
| AMH mother | 21.52 | 22.30 | 24.79 | 21.32 | 0.358a |
| Indication for IVF | |||||
| Male factor | 45 | 64.30 | 42 | 49.41 | 0.381c |
| Endometriosis | 11 | 15.70 | 18 | 21.18 | |
| Tube factor | 3 | 4.30 | 5 | 5.88 | |
| PCO‐S | 0 | 0.00 | 3 | 3.53 | |
| Idiopathic | 10 | 14.29 | 15 | 17.65 | |
| Other | 1 | 1.43 | 2 | 2.35 | |
AMH, Anti‐Müllerian hormone; BMI, body mass index; c‐IVF, conventional IVF; cm, centimeter; IVF, in vitro fertilization; kg, kilogram; m2, square meter NC‐IVF, natural cycle IVF; PCO‐S, polycystic ovary syndrome; SD, standard deviation; y, years; y/n, yes/no.
Linear regression.
Chi‐square test.
Fisher's exact test.
We found that stimulation characteristics were significantly higher in c‐IVF but the number of previous embryo transfers was similar (Table 2).
Table 2.
Stimulation and pregnancy outcome characteristics
| NC‐IVF | %/SD | c‐IVF | %/SD | P value | |
|---|---|---|---|---|---|
| N = 70 | N = 85 | ||||
| Stimulation | |||||
| Nb previous transfers (n) | 1.94 | 1.74 | 1.62 | 0.88 | 0.138a |
| Day of retrieval (d) | 12.65 | 1.87 | 12.19 | 2.09 | 0.207a |
| Nb oocytes retrieved (n) | 1.01 | 0.12 | 8.20 | 4.73 | <0.001 a |
| Nb of embryos transferred (n) | 1.01 | 0.12 | 2.01 | 0.36 | <0.001 a |
| Day of embryo transfer (d) | 2.69 | 0.71 | 2.61 | 0.74 | 0.531a |
| Total gonadotropin dosage (IU) | — | — | 2322.77 | 758.41 | — |
| Estradiol at trigger day (pmol/L) | 1028.94 | 330.39 | 10 459.78 | 4552.12 | <0.001 a |
| Estradiol at trigger day (>10 000 pmol/L) | 0 | 0 | 40 | 48.19 | <0.001 b |
| Endometrium thickness (mm) | 8.59 | 1.75 | 9.86 | 2.37 | <0.001 a |
| Pregnancy | |||||
| Duration of pregnancy (wk.d) | 39.2 | 1.52 | 38.94 | 2.51 | 0.451a |
| Pregnancy hypertension (n) | 1 | 1.43 | 1 | 1.18 | 0.890c |
| Pregnancy complication (n) | 16 | 22.56 | 26 | 30.59 | 0.309b |
| Induction of labor (n) | 22 | 35.48 | 28 | 34.57 | 0.909b |
| Very preterm birth (<31 wk) | 0 | 0 | 2 | 2.35 | 0.418c |
| Preterm birth (31‐36 wk) | 5 | 7.14 | 5 | 5.88 | |
| Term birth (≥37 wk) | 65 | 92.85 | 78 | 91.76 | |
| Infant gender female | 36 | 51.43 | 34 | 40 | 0.155b |
| Infant gender male | 34 | 48.57 | 51 | 60 | |
| Birthweight (g) | 3310.34 | 475.08 | 3218.25 | 704.38 | 0.352a |
| VLBW (<1500 g) | 0 | 0 | 3 | 3.53 | 0.231b |
| LBW (1500‐2500 g) | 2 | 2.86 | 4 | 4.71 | |
| Birthweight ≥2500 g (n) | 68 | 65.9 | 78 | 91.76 | |
| Birthweight ≥4000 g (n) | 6 | 8.57 | 9 | 10.59 | 0.673b |
| Percentile (mean) | 43.14 | 26.74 | 38.58 | 27.91 | 0.304a |
| SGA (≤5th percentile) | 2 | 2.86 | 10 | 11.76 | 0.039 b |
| Birthweight ≤10th percentile (n) | 10 | 14.49 | 18 | 21.18 | 0.285b |
| Vanishing twin (n) | 1 | 1.43 | 6 | 7.32 | 0.088c |
Values are presented as means with standard deviations or n with proportions (%).
c‐IVF, conventional IVF; d, day; g, gram; IU, international units; IVF, in vitro fertilization; LBW, low birthweight; mm, millimeter; NC‐IVF, natural cycle IVF; n, number; pmol/L, pico mol per liter; SD, standard deviation; SGA, small‐for‐gestational‐age; VLBW, very low birthweight; wk, week of gestation; wk.d, week.day.
Linear regression.
Chi‐square test.
Fisher's exact test.
Bold values are statistically significant.
3.2. Pregnancy characteristics
We note that there were no differences in pregnancy complications, eg, gestational hypertension, between the two groups (Table 2). The rate of preterm births (<37 gestational weeks) was 8.24% (n = 7) in the c‐IVF group and 7.14% (n = 5) in the NC‐IVF (P = 1.000) group.
The overall mean birthweight and percentile were 3218 g (±704 g), 38.6th percentile for c‐IVF and 3310 g (±475), 43.1st percentile for NC‐IVF, respectively, P = 0.352. The proportion of LBW was 8.24% (n = 7) in c‐IVF and 2.90% (n = 2) in NC‐IVF, P = 0.188, whereas the incidence of birthweight >4000 g was 10.59% (n = 9) in the c‐IVF group and 8.57% (n = 6) in the NC‐IVF group, P = 0.673.
3.3. Small‐for‐gestational‐age
More children were born as SGA following c‐IVF (11.76%, n = 10; 4 male, 6 female) than following NC‐IVF (2.86%, n = 2; both male), with an odds ratio of 4.53 for an SGA child in a logistic regression (95% CI 0.95‐21.61, P = 0.058, Figure 1). After we adjusted for confounding factors such as maternal age, height and BMI, the odds ratio was somewhat attenuated (OR 4.23; 95% CI 0.87‐20.41; P = 0.073, Model I in Table 3). If we additionally adjust for the E2 level, the odds ratio is further reduced to 1.01 (95% CI 0.87‐1.19, P = 0.971, Model III in Table 3).
Figure 1.
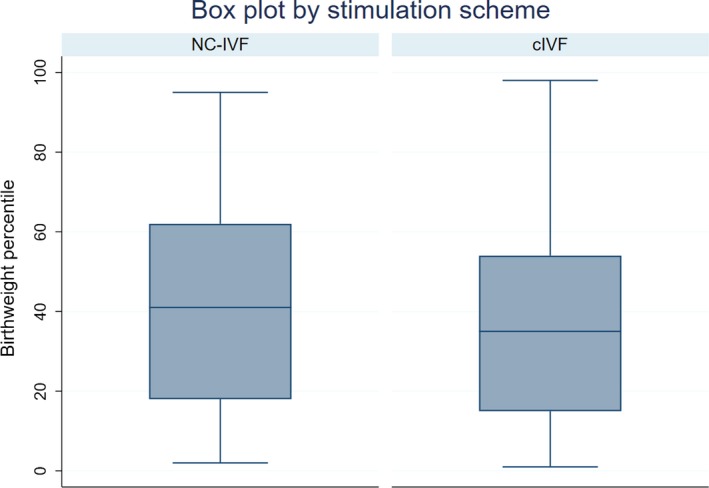
Boxplot of birthweight percentiles of conventional gonadotropin‐stimulated in vitro fertilization (c‐IVF) vs natural cycle in vitro fertilization (NC‐IVF) [Color figure can be viewed at http://www.wileyonlinelibrary.com]
Table 3.
Multilevel logistic regression for small‐for‐gestational‐age
| N = 155 | Unadjusted OR (95% CI) (for each determinant individually) | P value | Model I | P value | Model II | P value | Model III | P value |
|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||||||
| Stimulation (c‐IVF) | 4.53 (0.95‐21.61) | 0.058 | 4.23 (0.87‐20.41) | 0.073 | 1.05 (0.87‐1.17) | 0.971 | ||
| Mother age (continuous) | 1.01 (0.89‐1.17) | 0.820 | 0.99 (0.88‐1.13) | 0.952 | 1.01 (0.87‐1.19) | 0.811 | 1.01 (0.87‐1.17) | 0.901 |
| Mother age (>36 y) | 0.98 (0.28‐3.48) | 0.987 | ||||||
| Mother height (continuous) | 0.88 (0.79‐0.99) | 0.026 | 0.89 (0.81‐0.98) | 0.019 | 0.90 (0.81‐0.99) | 0.042 | 0.90 (0.81‐.99) | 0.032 |
| Mother height (<160 cm) | 5.46 (1.42‐20.92) | 0.013 | ||||||
| Mother BMI (continuous) | 0.99 (0.83‐1.18) | 0.944 | 0.96 (0.78‐1.17) | 0.668 | 0.96 (0.74‐1.24) | 0.753 | 0.98 (0.77‐1.24) | 0.847 |
| Mother BMI (<20 m2) | 1.24 (0.35‐4.40) | 0.735 | ||||||
| E2 level (continuous) | 1 (1.00‐1.00) | 0.003 | 1.00 (1.00‐1.00) | 0.127 | ||||
| E2 level on trigger day (<10 000 vs ≥10 000) | 4.58 (1.35‐15.55) | 0.015 | 3.83 (1.06‐13.82) | 0.041 | ||||
| Nulliparous (0 vs ≥1) | 3.81a , b | 0.188 | ||||||
| Smoking during pregnancy (y/n) | 0.99 (0‐1.04) b | 0.984 | ||||||
| Vanishing twin (y/n) | 2.25 (0.24‐20.73) | 0.474 | ||||||
| Pregnancy complication (y/n) | 2.88 (0.87‐9.6) | 0.083 | ||||||
| Pregnancy hypertension (y/n) | 12.90 (0.75‐222.94) | 0.078 | ||||||
| Induction of labor (y/n) | 0.92 (0.26‐3.26) | 0.902 | ||||||
| LBW (<2500 vs ≥2500 g) | 24.64 (5.4‐113.98) | <0.001 |
Model I: adjusted multivariate logistic regressions with SGA as outcome and stimulation as exposure adjusted for age, height and BMI as continuous variables.
Model II: adjusted multivariate logistic regression with SGA as outcome and supraphysiological E2 level as exposure adjusted for age, height and BMI as continuous variables.
Model III: adjusted multivariate logistic regression with SGA as outcome and stimulation as exposure adjusted for E2, age, height and BMI as continuous variables.
c‐IVF, conventional IVF; cm, centimeters; g, gram; IVF, in vitro fertilization; NC‐IVF, natural cycle IVF; y, years; n, number; m2, square meter; OR, odds ratio; pmol/L, picomol per liter; y/n, yes/no; 95% CI, 95% confidence interval.
Median unbiased estimate.
Exact logistic regression.
3.4. Supraphysiological estradiol level
The influence on birthweight percentile of an E2 level of >10 000 pmol/L on ovulation trigger day was significant in the crude analysis (unadjusted OR 4.58; 95% CI 1.35‐15.55; P = 0.015) and remained significant when adjusted (adjusted OR 3.83; 95% CI 1.06‐13.82; P = 0.041) (Model II in Table 3; Figure 2). The sensitivity analysis regarding parity, smoking and vanishing twins showed very similar odds ratios for stimulation schemes in crude and adjusted analyses (OR 1.45 vs 1.41, 1.48 and 1.41, respectively). In the linear regression, the association between E2 level and birthweight and birthweight percentile is modest. Birthweights and birthweight percentiles are lower with higher E2 levels on ovulation trigger day. The adjusted linear regression of NC‐IVF children shows a significant decrease of three percentiles by E2 increase of 100 pmol/L at ovulation trigger day (CI −4.61 to −1.39; P <0.001, Figure 3). For c‐IVF this is a decrease of 0.05 percentiles with an E2 increase of 100 pmol/L (CI −0.02 to 0.18; P = 0.451, Figure 4). The effect of the stimulation scheme was completely leveled out when we controlled for the E2 level.
Figure 2.
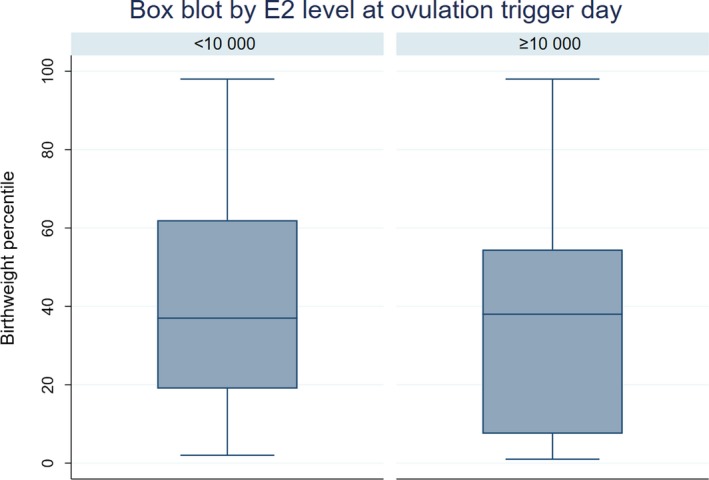
Boxplot of birthweight percentiles estradiol (E2) level <10 000 pmol/L vs ≥10 000 pmol/L E2 level [Color figure can be viewed at http://www.wileyonlinelibrary.com]
Figure 3.
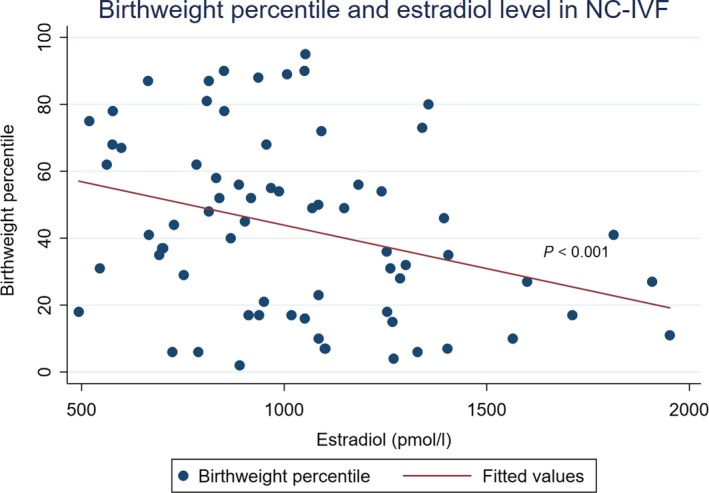
Birthweight percentile with higher estradiol (E2) level (in maternal serum, pmol/L) on ovulation trigger day in natural cycle in vitro fertilization [Color figure can be viewed at http://www.wileyonlinelibrary.com]
Figure 4.
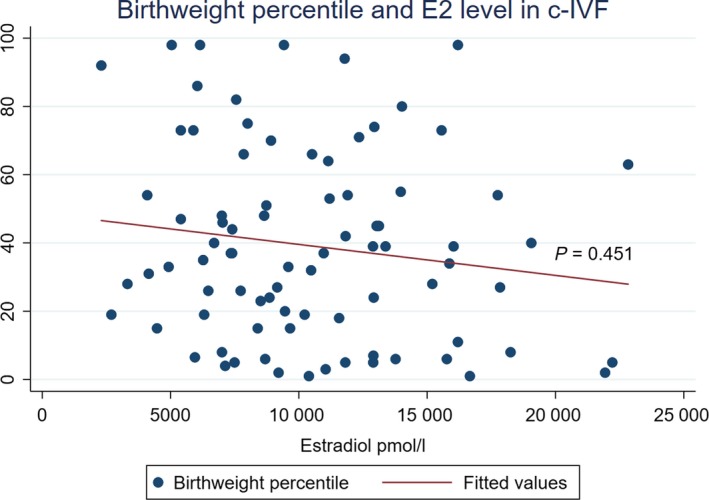
Birthweight percentile with higher estradiol (E2) level (in maternal serum, pmol/L) on ovulation trigger day in conventional gonadotropin‐stimulated in vitro fertilization [Color figure can be viewed at http://www.wileyonlinelibrary.com]
3.5. Doppler analysis
We obtained complete pregnancy records for 8 of 12 children born as SGA. The evaluation of their fetal growth charts showed four (42%; 1 NC‐IVF, 3 c‐IVF) cases with utero‐placental dysfunction (late flattening growth, pathological Doppler analysis) and one case with a placental infarction (c‐IVF) and pathological Doppler analysis. We did not have comparable information for the other four cases because the treating gynecologist did not perform a Doppler measurement.
4. DISCUSSION
This cohort study of singletons conceived after fresh IVF therapy focuses on the effect of ovarian stimulation on birth outcomes. Overall, gonadotropins seem to reduce birthweight and birthweight percentiles, especially when used for ovarian stimulation reaching supraphysiological E2 levels on ovulation trigger day. The two main findings of our study are: (1) the risk associated of children being born SGA after c‐IVF compared with NC‐IVF; and (2) the association of SGA children with supraphysiological E2 levels on ovulation trigger day. After adjusting for established risk factors, the supraphysiological E2 level in particular was determined to be a relevant risk factor for SGA; this even levels out completely the effect of the stimulation scheme.
These results contribute to the important debate on whether ovarian stimulation poses a risk for SGA or LBW and to the health of the IVF children later in life and what could be the determining factors.
For children conceived by NC‐IVF, the incidence of SGA was analyzed only once, by Mak et al.10 They compared 190 singletons after NC‐IVF with 174 after c‐IVF. In both groups, the percentage of preterm births was exceptionally high (31.5% vs 42%) but the difference in birthweights was significant (NC‐IVF 3426 ± 420 g vs c‐IVF 3273 ± 574 g, P = 0.01). Because of the high rate of preterm neonates (36.3%), this population is not completely comparable to ours (7.74% preterm only).
Our results suggest that the stimulation is detrimental if a supraphysiological estradiol level is reached. Measures of intensive ovarian hyperstimulation, such as supraphysiological E2 levels on ovulation trigger day7, 20 and a high number of oocytes retrieved,6 have been identified previously as independent risk factors for lower birthweight in IVF therapy. Supraphysiological E2 levels are also associated with a higher risk of preeclampsia (18). Our results even suggest that the effect of E2 level on ovulation trigger day outweighs the choice of stimulation scheme and the amount of gonadotropins used. It would be necessary to predict more accurately individual stimulation responses and the E2 levels.
The strength of our study is the equal access of all couples to both treatments in our center, which reduces (but does not eliminate) selection bias. The only difference between the groups is the use of gonadotropins for stimulation, resulting in higher serum E2 levels, a higher number of oocytes retrieved, and a higher number of embryos transferred within the c‐IVF group, also resulting in more vanishing twins. All other factors, such as laboratory conditions and the staff providing the treatment, were similar for the two groups. With regard to parental health and underlying subfertility as well as manipulation and culture of the embryos, we would not expect any differences between the two groups. Additionally, the use of birthweight percentiles increases the prognostic value of this particular parameter compared with birthweight alone.
A limitation of our study is the small sample size. We recruited within our center to increase comparability between both groups and we were able to have access to complete treatment data for all cases. A prospective design with random allocation of fertility treatment is not possible in Switzerland because the couple completely pays for fertility treatment themselves.
Further factors may affect our results; when two or more embryos are transferred and one gestation vanishes, the remaining fetus is physiologically seen as a twin. Pinborg showed its association with LBW and SGA (2). In our population, we had six vanishing twins in the c‐IVF group and one in the NC‐IVF group; this did not affect the incidence of SGA.
Animal models showed that high serum E2 levels suppress the extra villous trophoblast vascular endothelial growth factor and hinder uterine spiral artery invasion into the placenta.21 A supraphysiological E2 environment, such as in gonadotropin‐stimulated cycles, may result in an edematous endometrium impairing trophoblast differentiation and abnormal placentation, compared with the physiologic endometrium conditions in NC‐IVF.22 We hypothesize that these mechanisms might be one reason for the increased incidence of SGA children after gonadotropin‐stimulated IVF therapies compared with spontaneously conceived children.
In 1998, Barker linked maternal nutrition during the preimplantation period to intrauterine growth; his work showed that intrauterine‐affected individuals are at greater risk of developing coronary heart disease, hypertension and diabetes; this is known as the “Barker's hypothesis” or the “developmental origins of health and disease theory”.23
IVF offspring have not yet reached late adult life. Multiple studies suggest that c‐IVF children, especially with an associated fetal growth restriction,24 may face health issues later in life, such as reduced insulin sensitivity, cardiovascular dysfunction and higher blood pressure at school age.25, 26 Larger prospective cohort studies should investigate further the effects of gonadotropins and supraphysiological E2 levels on intrauterine growth and the health of IVF children.
The extent to which gonadotropin stimulation is associated with LBW and SGA, and consequently their possible negative health conditions, is still not clear. However, while we cannot alter many factors in IVF therapy to achieve acceptable pregnancy outcomes, we can reduce the use of gonadotropin and the dosage when it is used. Furthermore, the risk of SGA can be reduced by frozen embryo transfer cycles, but there instead a higher risk of large‐for‐gestational‐age (LGA),27 and preeclampsia28 has been described recently. In NC‐IVF, the serum E2 milieu remains within physiologic limits,17, 29 whereas gonadotropin stimulation in c‐IVF alters it. NC‐IVF as well as low‐dose stimulation‐IVF may be options to reduce the risk of supraphysiological E2 levels and consequently LBW and SGA. Several studies that looked at mild stimulation found similar pregnancy and live birth rates, better quality oocytes and less adverse effects of the stimulation. Although Baart et al.30 found fewer aneuploidy and mosaic embryos following mild stimulation, there is still not much known about perinatal outcome including the effect on LBW and SGA.
5. CONCLUSION
Lower stimulation dosages are associated with lower E2 levels at ovulation trigger day. There is consecutively a lower risk of ovarian hyperstimulation syndrome and we assume lower associated perinatal health risks. Even if the effect of gonadotropins on the risk for SGA is not yet fully proven, we advise reproductive specialists to consider natural cycle or low‐dose ovarian stimulation.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGMENTS
We thank Andreas Limacher for statistical consulting and Anja Fink, Livia Purtschert, Daniela Steiner and Marlene Berchtold for support with data collection.
Kohl Schwartz AS, Mitter VR, Amylidi‐Mohr S, Fasel P, Minger MA, Limoni C, Zwahlen M, von Wolff M. The greater incidence of small‐for‐gestational‐age newborns after gonadotropin‐stimulated in vitro fertilization with a supraphysiological estradiol level on ovulation trigger day. Acta Obstet Gynecol Scand. 2019; 98:1575–1584. 10.1111/aogs.13691
Funding information
The data evaluation for this study was supported by a grant from Merck (Switzerland) AG, Zug, Switzerland.
Alexandra S. Kohl Schwartz and Vera R. Mitter are joint first authors.
REFERENCES
- 1. Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta‐analysis. Hum Reprod Update. 2012;18:485‐503. [DOI] [PubMed] [Google Scholar]
- 2. Pinborg A, Wennerholm UB, Romundstad LB, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta‐analysis. Hum Reprod Update. 2013;19:87‐104. [DOI] [PubMed] [Google Scholar]
- 3. Berntsen S, Söderström‐Anttila V, Wennerholm U‐B, et al. The health of children conceived by ART: “the chicken or the egg?”. Hum Reprod Update. 2019;25:137‐158. [DOI] [PubMed] [Google Scholar]
- 4. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803‐1813. [DOI] [PubMed] [Google Scholar]
- 5. Kleijkers SHM, Van Montfoort APA, Smits LJM, et al. IVF culture medium affects post‐natal weight in humans during the first 2 years of life. Hum Reprod. 2014;29:661‐669. [DOI] [PubMed] [Google Scholar]
- 6. Sunkara SK, La Marca A, Seed PT, Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod. 2015;30:1473‐1480. [DOI] [PubMed] [Google Scholar]
- 7. Pereira N, Elias RT, Christos PJ, et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full‐term singletons born after fresh embryo transfer. Hum Reprod. 2017;32:1410‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu XL, Feng C, Lin XH, et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small‐for‐gestational‐age birth. J Clin Endocrinol Metab. 2014;99:2217‐2224. [DOI] [PubMed] [Google Scholar]
- 9. Kamath MS, Kirubakaran R, Mascarenhas M, Sunkara SK. Perinatal outcomes after stimulated versus natural cycle IVF: a systematic review and meta‐analysis. Reprod Biomed Online. 2018;36:94‐101. [DOI] [PubMed] [Google Scholar]
- 10. Mak W, Kondapalli LA, Celia G, Gordon J, Dimattina M, Payson M. Natural cycle IVF reduces the risk of low birthweight infants compared with conventional stimulated IVF. Hum Reprod. 2016;31:789‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pelinck MJ, Hadders‐Algra M, Haadsma ML, et al. Is the birthweight of singletons born after IVF reduced by ovarian stimulation or by IVF laboratory procedures? Reprod Biomed Online. 2010;21:245‐251. [DOI] [PubMed] [Google Scholar]
- 12. Lees CC, Marlow N, van Wassenaer‐Leemhuis A, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet. 2015;385:2162‐2172. [DOI] [PubMed] [Google Scholar]
- 13. Harrington K, Carpenter RG, Nguyen M, Campbell S. Changes observed in Doppler studies of the fetal circulation in pregnancies complicated by pre‐eclampsia or the delivery of a small‐for‐gestational‐age baby. I. Cross‐sectional analysis. Ultrasound Obstet Gynecol. 1995;6:19‐28. [DOI] [PubMed] [Google Scholar]
- 14. Fenton TR, Kim JH, Secker D, et al. A systematic review and meta‐analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Mikolajczyk R, Grewal J, Neta G, Klebanoff M. Prenatal application of the individualized fetal growth reference. Am J Epidemiol. 2011;173:539‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234‐1238. [DOI] [PubMed] [Google Scholar]
- 17. von Wolff M. The role of natural cycle IVF in assisted reproduction. Best Pract Res Clin Endocrinol Metab. 2019;33:35‐45. [DOI] [PubMed] [Google Scholar]
- 18. Jos de los Santos M, Arroyo G, Busquet A, et al. A multicenter prospective study to assess the effect of early cleavage on embryo quality, implantation, and live‐birth rate. Fertil Steril. 2014;101:981‐987. [DOI] [PubMed] [Google Scholar]
- 19. Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population‐based percentiles. Am J Obstet Gynecol. 2009;201:28.e1‐28.e8. [DOI] [PubMed] [Google Scholar]
- 20. Imudia AN, Awonuga AO, Doyle JO, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374‐1379. [DOI] [PubMed] [Google Scholar]
- 21. Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology. 2008;149:5078‐5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devroey P, Bourgain C, Macklon NS, Fauser BCJM. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84‐90. [DOI] [PubMed] [Google Scholar]
- 23. Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412‐417. [DOI] [PubMed] [Google Scholar]
- 24. Crispi F, Miranda J, Gratacós E. Long‐term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218:S869‐S879. [DOI] [PubMed] [Google Scholar]
- 25. Kuiper D, Hoek A, la Bastide‐van Gemert S, et al. Cardiovascular health of 9‐year‐old IVF offspring: no association with ovarian hyperstimulation and the in vitro procedure. Hum Reprod. 2017;32:2540‐2548. [DOI] [PubMed] [Google Scholar]
- 26. Ceelen M, Van Weissenbruch MM, Vermeiden JPW, Van Leeuwen FE, Delemarre‐Van De Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow‐up study. J Clin Endocrinol Metab. 2008;93:1682‐1688. [DOI] [PubMed] [Google Scholar]
- 27. Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29:618‐627. [DOI] [PubMed] [Google Scholar]
- 28. Wei D, Liu J‐Y, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310‐1318. [DOI] [PubMed] [Google Scholar]
- 29. DiMattina M, Gordon JD, Botes A, Celia G, Payson M, Graves‐Herring J. Follicular and estradiol parameters that improve success with natural cycle in vitro fertilization. J Reprod Med. 2014;59:267‐273. [PubMed] [Google Scholar]
- 30. Baart EB, Martini E, Eijkemans MJ, et al. Milder ovarian stimulation for in‐vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980‐988. [DOI] [PubMed] [Google Scholar]


