Abstract
Aim
Recurrent pain of unknown origin is a major problem in children. The aim of the present review was to examine the hypothesis of negative stress as an aetiology of recurrent pain from different aspects.
Methods and Results
Epidemiological studies, clinical experience and hormonal data give support for such a hypothesis. Negative stress as a tentative aetiology for recurrent pain is reviewed. Stress, muscular tension, the startle reaction and its tentative relation to pain is illuminated. Deviations of hormonal secretion supporting a stress aetiology are mentioned. The role of central sensitisation for recurrent pain is discussed. Possible aetiological implications of recurrent pain as a local symptom or a general disorder are presented. Brain changes due to stress are shortly reviewed. Stress and pain in the clinic are highlighted. The importance of biological, psychological and social factors, as well as genetic elements, is discussed.
Conclusion
Stress elicits neurobiological mechanisms. They may lead to many neurophysiological deviances. Increase of muscle tension and neuromuscular excitability and enhanced startle reaction may be of importance for recurring pain. The identification of stress as a primary cause of recurrent pain can have huge implications for understanding signs and treatment in clinical practice.
Keywords: Electromyography, Muscle, Pain, Startle, Stress
Abbreviations
- CAPS
Centrally mediated disorders of gastrointestinal pain
- EMG
Electromyography
- FGID
Functional gastrointestinal disorder
- PTSD
Post‐traumatic stress disorder
Key notes.
The possible importance of negative stress in the aetiology of recurrent pain is explored, and epidemiological studies, clinical experience and hormonal data give support for such a hypothesis.
How negative stress affects central nervous system is also explored, with focus on increased muscle tone and startle reflexes.
The identification of stress as a primary cause of pain can have huge implications for clinical understanding and treatment.



Introduction
Recurrent pain of unknown origin is a major medical problem in children 1, 2, 3, 4 affecting their daily activity 5. The starting point for the present review is a recent electromyographical study showing that there is an elevated resting muscle activity in children with recurrent pain related to negative stress. In addition, the study showed enhanced startle reaction with a decreased latency, increased response and duration in different muscles 6.
The main aim of this review is to examine the hypothesis that negative stress can lead to recurrent pain. The importance of central sensitisation in recurrent pain and the possible aetiological implications of recurrent pain as a local or of a general disorder are discussed. Muscular tension, brain changes, and pain and its relation to the startle reaction are highlighted. The biopsychosocial concept viewed as the cause of recurrent pain will also be discussed.
To cover the concept of recurrent pain of negative stress aetiology, the psychosomatic pain concept will be used. Psychosomatic is according to Dorland Illustrated Medical Dictionary pertaining to the mind‐body, relationship having body symptoms of psychic, emotional or mental origin 7.
The hypothesis that negative stress causes recurrent pain
Negative stress was defined by Hans Seyle 8. According to him, it is the sum of the biological reactions to any adverse stimulus, physical, mental, or emotional, internal or external, compensating reactions be inadequate or inappropriate, they may lead to disorders. However, this definition has not been generally accepted. Today, we know that the stress reaction has the amygdala as an important centre 9, that the right anterior cingulate cortex plays a pivotal regulating role (Fig. 1) and that the anterior insula cortex is a centre for the feeling of stress (Craig).
Figure 1.
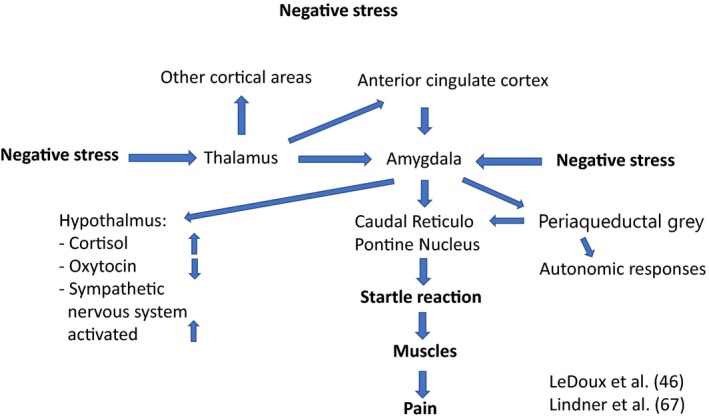
A schematic picture of how negative stress may activate brain and body.
The discussion in the literature of how negative stress can be a possible aetiological agent for recurrent pain can be broken down into the three following sections below. In the first, stress was as a tentative hypothesis. In the second, stress as a primary aetiology was denied. During recent years, epidemiological and clinical studies strengthen the hypothesis of negative stress as a cause of recurrent pain.
Stress as a tentative hypothesis
As early as in 1913, the German physician Ernst Moro published a paediatric study of 18 cases of recurrent abdominal pain with co‐morbidity of pains in leg, head and back 10. He claimed that they were suffering from essentially psychogenic conditioned states. This hypothesis was strengthened some decades later by several studies indicating that stress, anxiety and psychosocial strain might cause recurrent pain 11, 12, 13, 14. In a review concerning recurrent abdominal pain of possible psychogenic factors, 21 out of 23 studies gave support for a psychosomatic origin, but provided no clear‐cut answers 15.
In the 1990s, Alfvén 16, 17 suggested that negative stress can increase muscular tension and muscular pain sensitivity, causing psychosomatic pain. A potentiated startle reflex was suggested as a possible mechanism with an increase of muscular tension and neuromuscular excitability 16, 17. This was recently corroborated in physiological experiments 6.
Rejection of stress as a primary aetiology
Although epidemiological and clinical studies supported the hypothesis that negative stress causes recurrent pain in the absence of well‐documented evidence with measures verifying the suggested pathophysiological mechanisms, distrust of this hypothesis increased. The nonorganic pain concept, meaning that no mechanism had been established, was re‐named to functional pain 18 another obscure term. In the influential Rome II Diagnostic Criteria for Childhood Functional Gastrointestinal Disorder, the paediatric working team announced that ‘Despite the fact that evidence for visceral hyperalgesia in children with functional abdominal pain remains preliminary, we are currently using this theory to explain the pathogenesis of pain, in order to alleviate parents’ feelings of guilt and concern that the problem is psychological’ 18. This denial of stress as a possible cause also influenced attitudes to the aetiology of other forms of recurrent pain.
During the following more than two decades, the possibility of stress as an aetiology has had little support from leading scientists 18, 19, and stress has been mentioned as only associated with pain. The vague and all‐inclusive biopsychosocial concept became the main aetiological explanation for recurrent pain 18, 19.
In Rome IV, the biopsychosocial aetiology of functional pain, including dyspepsia and irritable colon, is defined. They describe that although cultural background, educational level and sex are factors which can contribute to explanatory models, they also identify that local biologics including genetics, microbiome/postinfectious irritable bowel syndrome, environmental hygiene, cytokines and the effects of CNS can influence on symptom generation, manifestation and interpretation. Negative stress as a possible primary cause is not mentioned here 19.
The current view among many paediatricians is that stress can be associated with pain, but the real cause of pain is still not yet fully defined. This is expressed in the review by Zeiter 2017 who states that FGIDs [Functional Gastrointestinal Disorder] are a consequence of a complex interaction of factors that affect the individual and combine to produce disease. Further, he states that this paradigm is the biopsychosocial conceptual model and stress seems to be one trigger for children with FGIDs 20.
The hypothesis that negative stress is a cause of recurrent pain is strengthened
Lien et al 2011 found in a cross‐sectional study highly significant odds ratios for children 15–16 years old with mental distress, measured with 10 items during the previous week, and pain complaints in various body regions. The odds ratio is a measure of association between an exposure – here mental distress – and an outcome – here pain locations. For girls and boys, respectively, the odds ratios for pain region were headache 2.7 and 3, neck/shoulder 3.4 and 3.6, arm/leg 2.2 and 3.4, stomach 3.1 and 2.9 and back 3.0 and 4.0. For 3–5 pain localisations, the odds ratios were 7.5 and 14.8 for girls and boys, respectively 21.
Østerås et al 2015 found severe pain as reported from head, neck, shoulder, back, arms and legs in a sample comprising 422 adolescents (218 females) aged 15 and 16 years. In both genders, there were significant correlations (p < 0.01) between all forms of continuous pain and stress variables. Stress was measured with the Perceived Stress Questionnaire, a validated instrument for measuring perceived stress in an adolescent population 22.
Huguet et al 2016 found in a meta‐analysis combining seven studies that children with higher levels of negative emotional states were more likely to develop musculoskeletal pain than those with lower levels. The cohort included 10 922 children, odds ratio 1.54; 95% CI 1.06–2.24 23.
Teodorescu et al 2015 found a very high occurrence of stomach pain, chest pain, arms/limb pains, back pain, headache in multi‐traumatised outpatients with a refugee background. Of the 61 outpatients included, all but one reported at least one chronic pain location, with a mean of 4.6 locations per patient. An amount of 70% had post‐traumatic stress disorder. The most prevalent chronic pain locations were head (80%), chest (74%), arms/legs (66%), back (62%) and stomach (57,3%). Women had significantly more chronic pain locations than men 24.
Stensland et al 2018, in a matched control study of adolescent survivors from the Utøya terror attack in Norway, with 213 (59%) respondents, found the odds ratio for migraine to be 4.27 (95% CI 2.54–7.17) and for tension headache 3.39 (95% CI 2.22–5.18) largely related to an increase in weekly and daily headaches 25.
Being exposed to bullying was associated with an increased risk of perceived stress (OR = 3.06, 95% CI 1.55, 6.03), as well as recurrent pain (OR = 3.39, 95% CI 1.62, 7.09) 26.
In a cross‐sectional study based on data linked to national household surveys in Sweden during 2002–2003, information concerning harassment, perceived stress, headache and abdominal pain was gathered from a questionnaire. The study population consisted of 2597 children aged 10–18 years. Perceived stress and recurrent pain were measured using the item ‘felt stressed and pain’ at least once a week during the previous six months. For bullying, almost every day, the odds ratios for perceived stress were 3.60 (2.34–5.55), for headache 1.45 (0.88–2.39), abdominal pain 1.98 (1.07–3–68) and co‐occurrence of pain 4.62 (2.26–5.11). Pain intensity and number of pain locations increases with more stress 27 (Fig. 2).
Figure 2.
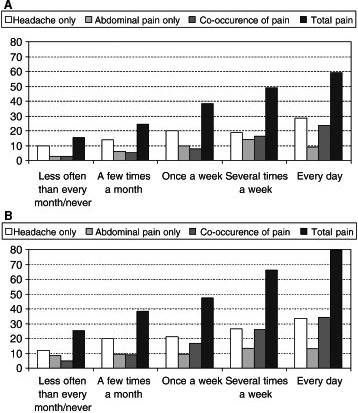
(A) Proportion of boys (%) experiencing recurrent pain (at least every week) by frequency of perceived stress. Sweden 2002–2003, n = 1303 (Alfvén et al 2008, 23. (B) Proportion of girls (%) experiencing recurrent pain (at least every week) by frequency of perceived stress. Sweden 2002–2003, n = 1294.
The cross‐sectional studies show that stress correlates to recurrent pain. Although it cannot be judged from cross‐sectional studies whether stress is the cause or the effect, the three latter studies mentioned above 25, 26, 27 show that the stress, that is bullying and harassment, can cause pain; as the opposite relation is not probable.
Three of the above‐mentioned studies had perceived stress as a stress parameter. Perceived stress is the conscious feeling of stress, for which the anterior insula cortex is involved 28. The last above‐mentioned study also shows that an increased level of stress leads to an increased number of pain locations 27. This relation is discussed below.
Based on Karl Popper’s hypothetical‐deductive method 29, Alfvén has developed criteria for psychosomatic pain. In a clinical study, 48 out of 100 cases of abdominal pain – 65 per cent of them having headache – fulfilled the criteria and the diagnosis 30. The diagnosis of psychosomatic pain is based on seven criteria: (i) Onset or aggravation of chronic negative stress at the time of onset of recurrent pain. (ii) Pain in parallel with chronic negative stress. (iii) Feeling better or pain‐free during periods of no or lessened chronic negative stress. (iv) Stress‐induced acute pain. (v) Most pain attacks are related to acute stress. (vi) The child is followed up for at least 1 year. (vii) Parents and child and doctor agree on diagnosis. At least six of the seven criteria have to be met for diagnosis and other disorders must be excluded.
These criteria have been applied in several studies, and the diagnosis psychosomatic pain has been established 6, 30, 31, 32, 33.
In children with psychosomatic pain, hormone cortisol is higher 32 and oxytocin lower 33 than in controls according to studies. This is signs of a right anterior cingulate cortex activated by stress according to Craig 28.
In conclusion, epidemiological data, case studies meeting criteria for psychosomatic pain and hormonal deviation, suggest that negative stress is a primary aetiological agent of recurrent pain.
Neuromuscular mechanisms for recurrent pain of negative stress origin
In a case–control study, 21 matched healthy controls were compared to 19 children 6 fulfilling the criteria for psychosomatic pain. These children had a pain duration in mean of 37 months. All reported recurrent headache, 18 abdominal pain, five backache and three shoulder pain.
The acoustic startle reaction was elicited by a short signal of white noise of 105 dB via ear‐phones on several occasions, and the response measured with electromyography (EMG). The muscles studied were the orbicularis oculi, temporal, trapezius, great pectoral near shoulder, abdominal wall near the umbilicus and the lumbar erector spinae, that is sites for recurrent pain and stress tender points (Fig. 3 A). All but one of the children had nine tender points, the exception having seven 6.
Figure 3.
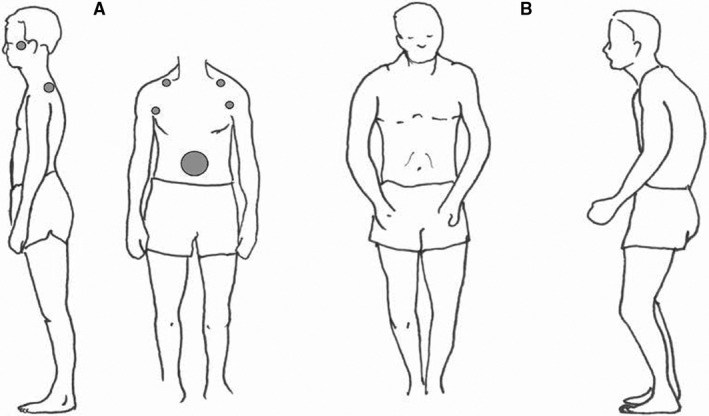
Stress tender points and typical startle reaction position. (A) From a photograph of an adolescent with long‐standing recurrent psychosomatic pain. Typical stress tender point pattern shown as grey dots, cf Figure B. (B) Hunt and Landis classical picture from 1936 of a person in startle after unexpected pistol shot near the ear with a typical crouch 68, cf figure A.
In the psychosomatic pain group, compared to controls, EMG in these muscles in different parts of the body showed increased resting activity and thus a continuous barrage throughout each day. The startle burst was initiated with a reduced latency, and the amplitude was larger as was the duration of the burst in children with recurrent psychosomatic pain compared to a control group (Fig. 4). In conclusion, in muscles in which recurrent psychosomatic pain is common and stress tender points occur, the startle reaction is potentiated and more easily elicited in psychosomatic pain children, as is the resting activity. These findings suggest that this reaction may be of importance for stress‐induced recurrent pain in children, showing evidence for increased neuromuscular excitability both with higher resting activity in several muscles and potentiated startle reactions with earlier, higher and longer muscle answers, as compared to controls.
Figure 4.
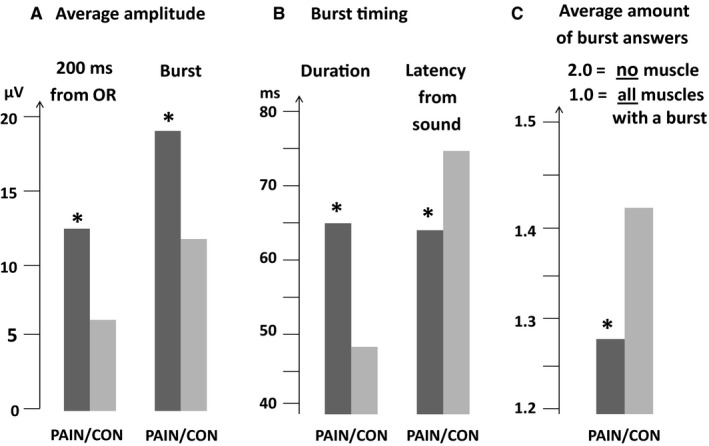
Average for all eight startle sound events together (no other stress provocation), including all six muscles (Alfvén et al 2017 6, for: (A) Mean amplitude (µV) during 200 ms from OR‐activity‐start and during burst, (B) Burst duration and latency (ms) from sound to muscle‐activity‐start (≥10 µV), (C) Average amount of bursts:–mean of 1.0 = all muscles burst (peak ≥ 10 µV) and 2.0 = no muscle had burst (peak ≥ 10 µV) in any of the eight stimuli. PAIN versus CON showed significant differences (star). (OR = orbicularis oculi).
Central sensitisation and recurrent pain
Woolf in his review of central sensitisation conclude that the induction of activity‐dependent increases in synaptic function in these circuits, triggered and maintained by dynamic nociceptor inputs, shifts the sensitivity of the pain system such that normally innocuous inputs can activate it and the perceptual responses to noxious inputs are exaggerated, prolonged and spread widely 34. Thus, central sensitisation is a mechanism to consider for the understanding of recurrent pain.
The possibility that central pain sensitisation is a primary cause of recurrent pain has been raised 35, but so far, no clear evidence has been presented. It has been demonstrated in several studies that peripheral pain comes first and the central sensitisation later. Gunnarsson et al 2016 found an increased deep pain sensitivity in patients with persistent musculoskeletal pain, but not in regularly recurrent pain patients or in acute pain 36. In a headache study, signs of sensitisation were secondary to the primary pain 37.
In a study of 47 children (6‐ to 17‐year‐old) with abdominal pain of a mean duration 29 months 31, five children reported only abdominal pain, 25 had two locations of pain, seven children three, six children four and two children five different locations. The number of pain locations correlated significantly with the duration of the pain (R = 0.35, p < 0.05). Of 44 children, 27 had sign of allodynia in the abdominal pain area tested with the Nipple test. The test is done by nipping a layer of a small area of the cutis ⁄ subcutis between thumb and index finger, with short fingernails, with a pressure calibrated to below approximately 125 kPa/cm2. This does not normally elicit pain. A positive pain response is considered a sign of allodynia and of central sensitisation 31. This study indicates that pain sensitisation may be common in this group of children having more pain locations and being a secondary phenomenon to longer pain.
Aetiological implications of the occurrence of one or several pains
Whether the pain has primarily one location or several is a question of importance in the search for the aetiology of recurrent pain. If only one pain location is identified – and others actively dismissed – a local disorder is more probable. Several pain locations favour a general condition such as a negative stress origin under a prolonged time.
In many studies, only one location is in focus, as for instance with headache 38, 39, abdominal pain 18, 19 and back pain 40, 41. The question of recurrent pains in more than one site is often not raised, although this is common according to many studies. The co‐occurrence of pain was demonstrated statistically for the first time in children 1993 42 and corroborated by Petersen et al in 2006 43.
Not long ago, the chronic pain concept musculoskeletal pain excluded abdominal pain and headache 44. However, a change of attitude is underway, and it is now stated that ‘Chronic pain affects the entire nervous system’ according to the American Pain Society 45. This also affects abdominal pain and headache.
Multiple pain at different locations often occurs in psychosomatic pain 6, 30, 31. In the search for the origin of pain, the finding of multiple pain locations, related to muscles activated by the startle reflex, may be a support for a negative stress aetiology. The startle reaction affects muscles in the head temporal region, neck, shoulder, abdomen and back.
Multiple recurrent pain has many different aetiologies and trauma with pain spread, rheumatic, endocrine or metabolic diseases should always be considered.
Stress, muscular tension, brain regions, pain and its relation to the startle reaction
In the review Psychosomatic pain in children: a psychomuscular tension reaction? 16, the author suggested that muscular tension has a central role for the origin of recurrent pain of stress origin, a hypothesis corroborated in the controlled startle study measuring several muscles at different locations with EMG 6. The amygdala, an important centre of stress 9, can be activated by mental stress from the anterior cingulate cortex, a neurobiological centre for motivation and behaviour 28. The amygdala may influence the muscular system via the basal ganglia and nuclei in the brain stem such as the periaqueductal grey 46 and the caudal reticulopontine nucleus, regulating the startle reflex (Fig. 1).
Thus, chronic stress may cause an increased neuromuscular excitability (both under resting conditions and during provocation tests such as the startle reflex) via altered descending neural activity from higher brain centres, such as the anterior cingulate cortex and the amygdala 6, 28, 46.
Increased muscular tension might be further potentiated by various muscle metabolites (such as bradykinin, prostaglandins, arachidonic acid, potassium and lactic acid) contributing to eliciting a pain response 47, 48, 49, 50. An alternative possibility for muscular psychosomatic pain is a lowered pain threshold via hyperalgesic priming elicited by stress 51.
The increased neuromuscular tension and excitability in children exposed to prolonged stress with recurrent pain may be due to a change of activity in a variety of brain regions. Prolonged stress has been reported to make amygdala overactive and decrease the prefrontal cortex control over amygdala 52. Stress‐induced increased levels of cortisol decreases the volume of hippocampus, which impairs memory 53. Children with post‐traumatic stress disorder (PSTD) can have a smaller brain volume and frontal lobe changes 54, and imbalance between salience and default‐mode network 55. PTSD individuals show brain hyperconnectivity in emotional processing, with a heightened response to threatening stimuli in thalamus and several cortex areas 56. These facts are of interest regarding the potentiated startle reaction found among children with recurrent pain due to prolonged stress 6.
Early life stress in childhood predicts an increased thalamic global connectivity, which can explain observations of multiple network disruption 57. Fear‐related phobia‐specific pictures increases activity not only in amygdala and thalamus, but also in the anterior cingulate cortex, insular cortex and supplementary motor cortex 58. Adopted children with early life stress show, during peer rejection, altered neural response in various brain regions, including thalamus, and more intense feelings of exclusion and frustration than controls 59. Thus, the thalamo‐amygdala and cortical regions can be involved in a dynamic interplay, which can be disturbed, also seen in various neuropsychiatric syndromes 60.
Acoustic, visual and other sensory input occurring in stress/fear reactions (such as the startle) can be transmitted directly from thalamus to amygdala without passing frontal cortex 61. In situations requiring a rapid organisation of defensive or other emotional responses, thalamic sensory inputs to the amygdala can therefore offer transmission with a minimal delay 61. However, different repetitive and prolonged stressful events have been reported to be associated with changes of activity, connectivity and size in various brain regions. Several of these proven changes may be related to the stress‐induced recurrent pain and the associated increased neuromuscular excitability and startle reactions. Further research is here needed to study various closer connections between different neurophysiological findings and signs in medical practice.
Muscle stress in clinical practice
Clinical practice is facilitated if negative stress signs in the body are actively searched for. An activated startle reflex results in a typical pattern of tense muscles and crouching (Fig. 4A). In long‐standing stress, it will often appear a pattern of tender points (Fig. 4A), 30, 31, 32, 33 and recurrent pain in a similar pattern such as headache, abdominal pain, neck and backache and limb pain. Other expressions of negative stress such as symptoms of disturbed appetite, disturbed intestinal activity with constipation and/or diarrhoea, nausea and dizziness are also often found. A positive nipping test, 31, easy to apply, a sign of central sensitisation, can increase the understanding of pain in clinical practice.
The biopsychosocial concept
The denial of negative stress as a possible aetiological factor cleared the way for the all‐inclusive aetiological term biopsychosocial, a term that can be applied to all diseases from allergy to cancer, from Crohn’s disease to post‐traumatic stress disorder. This aetiological truism has hampered the search for the cause of recurrent pain, a search that should try to respond to the questions: What was the primary factor starting the disorder? What made it continue? and, if this factor is alleviated, does the symptom disappear? Questions that more specifically address provoking and exacerbating factors may help elucidate concrete and varied causes of recurrent pain such as allergy, infection and stress. This approach was fundamental for the choice of the criteria for psychosomatic pain.
A new sub‐diagnosis for functional abdominal pain was established in Rome IV and termed Centrally Mediated Disorders of Gastrointestinal Pain (CAPS) 35. The structural brain deviations found in CAPS are similar as in toxic stress 62, 63. Toxic stress response can occur when a child experiences strong, frequent and/or prolonged adversity –such as physical or emotional abuse and chronic neglect from caregiver, substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support 62.
Children with a history of toxic stress have an exaggerated stress response due to a hyperactive amygdala and as a result of hypotrophy of the prefrontal cortex diminished top‐down control. As a consequence of hippocampal reduction, they have impaired memory and mood control. Children can as a consequence of severe stress develop multiple pains, secondary central pain sensitisation, pain spread and in the long run fibromyalgia 31, 44.
This indicates that a trajectory of toxic negative stress with multiple pains and the development of brain damage could be a cause for the children suffering from CAPS. Seven of the 47 children in the recurrent pain study mentioned above had a history of severe/toxic stress and fulfilled the criteria for fibromyalgia 31. Fibromyalgia is diagnosed on the basis of the presence of at least 11 of 18 well‐defined tender points (TP) in musculoskeletal structures near the tendons, together with spread pain 64.
Genetic aspects regarding pain
Pain is an important biological warning signal that can be triggered by stress and a multitude of other factors. There is a genetic and epigenetic variation of pain sensitivity 65, 66 as for all types of interoceptive signal. Hunger and pain to well‐being and pleasure vary with the genetic set‐up and epigenetic factors. Interoceptive signals are influenced by past and present biological, psychological and social circumstances. Interoceptive signals influence homoeostasis and in the long‐term survival. These different factors explain susceptibility or resistance to pain, but not the pain per se. ‘…pain is the perceptual correlate of a behavioural motivation that results from integrated homeostatic sensory activity indicating an imbalanced physiological condition that the automatic homeostatic response mechanisms cannot rectify’, writes Craig 28. The sensitivity to such a warning signal may differ, but not knowing the cause of pain is not an indication of a pain without a cause, only of a lack of knowledge.
Conclusion
This review further strengthens the evidence that negative stress can cause recurrent pain. Based on growing neurobiological, epidemiological, clinical and experimental data, we propose that negative stress affects the different parts of central nervous system including the anterior cingulate cortex and the amygdala and affects perception, behaviour, the autonomic nervous system, hormones, cytokines and the central and peripheral neuromuscular system. Of special interest are the increased muscle tone and neuromuscular excitability and the potentiated startle reactions occurring in several muscles, which may contribute to pain, as well as underlying neurobiological mechanisms. Pain is an interoceptive warning signal of a homoeostatic disturbance and increases with increased stress. Central pain sensitisation is a next warning step that can influence spread of pain and in the end lead to various diseases, for example fibromyalgia. The identification of stress as a primary cause of pain has huge implications for the clinical practice, regarding understanding of specific diagnostic signs as well as developing relevant treatment. Thus, the understanding of stress and its consequences for body and brain are a prerequisite for preventing progressions of negative health effects, and for developing effective therapeutic means.
Conflict of interest
No conflict of interest declared.
Funding
University of Gothenburg Centre for Person‐Centered Care, GPCC and the Swedish School of Sport and Health Sciences have supported the work.
Acknowledgement
We wish to thank Tim Crosfield for language revisions.
References
- 1. Roth Isigkeit A, Thyen U, Raspe H, Stoven H, Schmucker P. Reports of pain among German children and adolescents: An epidemiological study. Acta Paediatr 2004; 93: 258–263. [PubMed] [Google Scholar]
- 2. Perquin CW, Hazebroek‐Kampschreur A, Hunfeld J, Bohnen A, van Suijlekom‐Smit L, Passchier J, et al. Pain in children and adolescents: a common experience. Pain 2000; 87: 51–8. [DOI] [PubMed] [Google Scholar]
- 3. Bakoula C, Kapi A, Veltsista A, Kavadias G, Kolaitis G. Prevalence of recurrent complaints of pain among Greek schoolchildren and associated factors: a population‐based study. Acta Paediatr 2006; 95: 947–51. [DOI] [PubMed] [Google Scholar]
- 4. King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, et al. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011; 152: 2729–38. [DOI] [PubMed] [Google Scholar]
- 5. Petersen S, Hägglöf BL, Bergström EI. Impaired health‐related quality of life in children with recurrent pain. Pediatrics 2009; 124: 759–67. [DOI] [PubMed] [Google Scholar]
- 6. Alfvén G, Grillner S, Andersson E. Children with chronic stress‐induced recurrent muscle pain have enhanced startle reaction. Eur J Pain 2017; 21: 1561–70. [DOI] [PubMed] [Google Scholar]
- 7. Dictionary Dorland Illustrated Medical. Philadelphia, London. Toronto: W B Saunders Company,1988.
- 8. Seyle H. The Stress of Life. New York, NY: McGraw‐Hill, 1956. [Google Scholar]
- 9. McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci 2010; 1186: 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moro E. Über rezidivierende Nabelkoliken bei älteren Kindern. Münch Medizin Wochen 1913; 60: 2827–29. [Google Scholar]
- 11. McKeith R, O´Neil D. Recurrent abdominal pain in children. The Lancet 1951; 258(6677): 278–82. [DOI] [PubMed] [Google Scholar]
- 12. Nylander I. Children of alcoholic fathers. Acta Paediatr 1960; 49(Suppl. 121): 313–14. [PubMed] [Google Scholar]
- 13. Apley J. The Child with Abdominal Pains. Oxford, UK: Blackwell, 1975. [Google Scholar]
- 14. Öster J. Recurrent Abdominal Pain, Headache and Limb Pains in Children. Pediatrics 1972; 50: 429–36. [PubMed] [Google Scholar]
- 15. Alfven G. Recurrent abdominal pain of non‐organic origin in childhood. Clinical, muscular, epidemiological, endocrinological and aetiological aspects. Thesis Karolinska Institute Stockholm1993; page 21.
- 16. Alfven G. Psychosomatic pain in children: A psychomuscular tension reaction? Eur J Pain 1997; 1: 5–14. [DOI] [PubMed] [Google Scholar]
- 17. Alfven G. Psychosomatic pain. Eur J Pain 1998; 2: 189–90. [DOI] [PubMed] [Google Scholar]
- 18. Rasquin‐Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut 1999; 45(Supplement 2): ii60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmulson M, Drossman A. What Is New in Rome IV. J Neurogastroenterol Motil 2017; 23: 151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeiter DK. Abdominal pain in children. From the eternal city to the examination room. Pediatr Clin N Am 2017; 64: 525–41. [DOI] [PubMed] [Google Scholar]
- 21. Lien L, Green K, Thoresen M, Bjertness E. Pain complaints as risk factor for mental distress: a three‐year follow‐up study. Eur Child Adolesc Psychiatry 2011; 20: 509–16. [DOI] [PubMed] [Google Scholar]
- 22. Østerås B, Sigmundsson H, Haga M. Perceived stress and musculoskeletal pain are prevalent and significantly associated in adolescents: An epidemiological cross‐sectional study. BMC Public Health 2015; 15: 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huguet A, Tougas ME, Hayden J, McGrath PS, Stinson JN, Chambers CT. Systematic review with meta‐analysis of childhood and adolescent risk and prognostic factors for musculoskeletal pain. Pain 2016; 157: 2640–56. [DOI] [PubMed] [Google Scholar]
- 24. Teodorescu D‐S, Heir T, Siqveland J, Hauff E, Wentzel‐Larsen T, Lars Lien L. Chronic pain in multi‐traumatized outpatients with a refugee background resettled in Norway: a cross‐sectional study. BMC Psychology 2015; 3: 7 10.1186/s40359-015-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stensland SØ, Zwart J‐A, Wentzel‐LarsenT Dyb, G.. The headache of terror: A matched cohort study of adolescents from the Utøya and the HUNT Study. Neurology 2018; 90(09): e111–18. [DOI] [PubMed] [Google Scholar]
- 26. Östberg V, Låftman SB, Modin B, Lindfors P. Bullying as a stressor in mid‐adolescent girls and boys‐associations with perceived stress, recurrent pain, and salivary cortisol. Int J Environ Res Public Health 2018; 15: E364 10.3390/ijerph15020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alfven G, Östberg V, Hjern A. Stressor, perceived stress and recurrent pain in Swedish schoolchildren. J Psychosom Res 2008; 65: 381–87. [DOI] [PubMed] [Google Scholar]
- 28. Craig AD. How do you feel. An interoceptive moment with your neurobiological self. Princeton, NJ: Princeton University: Press Princeton and Oxford, 2015. [Google Scholar]
- 29. Popper K.The logic of scientific discovery. Rothledge Classics: London and New York; 2002 (First published in German 1935).
- 30. Alfven G. One hundred cases of recurrent abdominal pain inchildren: Diagnostic procedures and criteria for a psychosomatic diagnosis. Acta Paediatr. 2003; 92: 43–9. [DOI] [PubMed] [Google Scholar]
- 31. Alfven G. Recurrent pain, stress, tender points and fibromyalgia in childhood: an exploratory descriptive clinical study. Acta Paediatr 2012; 101: 283–91. [DOI] [PubMed] [Google Scholar]
- 32. Törnhage CJ, Alfvén G. Children with recurrent psychosomatic abdominal pain display increased morning salivary cortisol and high serum cortisol concentrations. Acta Paediatr 2015; 104: 577–80. [DOI] [PubMed] [Google Scholar]
- 33. Alfvén G. Plasma oxytocin in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr 2004; 38: 513–17. [DOI] [PubMed] [Google Scholar]
- 34. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011; 152: 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keefer L, Drossman DA, Guthrie E, Simrén M, Tillisch K, Olden K. Whorwell. Centrally Mediated Disorders of Gastrointestinal Pain. Gastroenterology 2016; 150: 1408–09. [DOI] [PubMed] [Google Scholar]
- 36. Gunnarsson HE, Grahn B, Agerström J. Increased deep pain sensitivity in persistent musculoskeletal pain but not in other musculoskeletal pain states. Scandinavian Journal of Pain 2016; 13: 1–5. [DOI] [PubMed] [Google Scholar]
- 37. Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Frequency of headache is related to sensitization: a population study. Pain 2006; 123: 19–27. [DOI] [PubMed] [Google Scholar]
- 38. Larsson BO. Recurrent headache in adolescents. A study of background factors and effects of psychological treatment. Uppsala, Sweden: Uppsala University; 1988. [Google Scholar]
- 39. Huguet A, Tougas Michelle E, ME, Hayden J, McGrath PJ, Chambers CT, Stinson JN, Wozney L.. Systematic Review of Childhood and Adolescent Risk and Prognostic Factors for Recurrent Headaches. The Journal of Pain. 2016; 17: 855–73. [DOI] [PubMed] [Google Scholar]
- 40. Michaleff ZA, Kamper SJ, Maher CG, Evans R, Broderick C, Henschke N. Low back pain in children and adolescents: a systematic review and meta‐analysis evaluating the effectiveness of conservative interventions. Systematic review. European Spine Journal 2014; 23: 2046–58. [DOI] [PubMed] [Google Scholar]
- 41. Koes BW, van Tulder M, Lin CW, Macedo LG, McAuley J. Maher CAn updated overview of clinical guidelines for the management of non‐specific low back pain in primary care. Eur Spine J 2010; 19: 2075–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alfven G. The covariation of common psychosomatic symptoms among children from socio‐economically differing residential areas. An epidemiological study. Acta Paediatr. 1993; 82: 484–87. [DOI] [PubMed] [Google Scholar]
- 43. Petersen S, Brulin C, Bergström E. Recurrent pain in young schoolchildren are often multiple. Pain 2006; 121: 145–50. [DOI] [PubMed] [Google Scholar]
- 44. Mikkelsson M. Musculoskeletal pain and fibromyalgia in preadolescents. Prospective 1‐yer. Turun Yliopoista. Turko1998.
- 45. Management of Children with Chronic Pain . A Position Statement from the American Pain Society (accessed on 7 December 2016)]. Available online: http://americanpainsociety.org/uploads/get-involved/pediatric-chronic-pain-statement.pdf.
- 46. LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdala nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8: 2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johansson H, Sojka P. Pathophysiological mechanism involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: A hypotheses. Med Hypoth 1991; 35: 196–203. [DOI] [PubMed] [Google Scholar]
- 48. Djupsjöbacka M, Johansson H, Bergenheim M. Influences on the gamma‐muscle‐spindle system from muscle afferents stimulated by increased intramuscular concentrations of arachidonic acid. Brain Res. 1994; 663: 293–302. [DOI] [PubMed] [Google Scholar]
- 49. Knutson GA. The role of the gamma‐motor system in increasing muscle tone and muscle pain syndromes: A review of the Johansson/Sojka hypothesis. J Manipulative Physiol Ther 2000; 23: 564–72. [DOI] [PubMed] [Google Scholar]
- 50. Djupsjöbacka M, Johansson H, Bergenheim M, Wenngren BI. Influences on the gamma‐muscle spindle system from muscle afferents stimulated by increased intramuscular concentrations of bradykinin and 5‐HT. Neurosci Res 1995; 22: 325–33. [DOI] [PubMed] [Google Scholar]
- 51. Reichling DB, Green PG, Levine JD. The fundamental unit of pain is the cell. Pain 2013; 154: S2–9. 10.1016/j.pain.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 2015; 11: 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015; 22: 411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001; 50: 943–51. [DOI] [PubMed] [Google Scholar]
- 55. Lei D, Li L, Li L, Suo X, Huang X, Lui S, et al. Microstructural abnormalities in children with post-traumatic stress disorder: a diffusion tensor imaging study at 3.0T. Sci Rep. 2015; 5: 8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dunkley BT, Wong SM, Jetly R, Wong JK, Taylor MJ. Post‐traumatic stress disorder and chronic hyperconnectivity in emotional processing. Neuroimage Clin. 2018; 20: 197–204. 10.1016/j.nicl.2018.07.007. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Philip NS, Tyrka AR, Albright SE, Sweet LH, Almeida J, Price LH, et al. Early life stress predicts thalamic hyperconnectivity: A transdiagnostic study of global connectivity. J Psychiatr Res. 2016; 79: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goossens L, Schruers K, Peeters R, Griez E, Sunaert S. Visual presentation of phobic stimuli: amygdala activation via an extrageniculostriate pathway? Psychiatry Res. 2007; 155: 113–20. [DOI] [PubMed] [Google Scholar]
- 59. Puetz VB, Kohn N, Dahmen B, Zvyagintsev M, Schüppen A, Schultz RT, et al. Neural response to social rejection in children with early separation experiences. J Am Acad Child Adolesc Psychiatry. 2014; 53(12): 1328–37.e8. [DOI] [PubMed] [Google Scholar]
- 60. Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, et al. Pathways for fear perception: modulation of amygdala activity by thalamo‐cortical systems. NeuroImage 2005; 26: 141–8. [DOI] [PubMed] [Google Scholar]
- 61. LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990; 10: 1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, Garner AS, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012; 129(1): e232–46. [DOI] [PubMed] [Google Scholar]
- 63. McEwen BS, Gianaros PJ. Stress‐ and Allostasis‐Induced Brain Plasticity. Annu Rev Med 2011; 62(1): 431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. Diagnostic and Statistical Manual of Mental Disorder: DSM‐IV. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
- 65. Kerr JK, Burri A. Genetic and epigenetic epidemiology of chronic widespread pain. J Pain Res. 2017; 10: 2021–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Williams FMK, Spector TD, MacGregor AJ. Pain reporting at different body sites is explained by a single underlying genetic factor. Rheumatology 2010; 49: 1753–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lindner K, Neubert J, Pfannmöller J, Lotze M, Hamm AO, Wendt J. Fear‐potentiated startle processing in humans: Parallel fMRI and orbicularis EMG assessment during cue conditioning and extinction. Int J Psychophysiol 2015; 98(3): 535–45. 10.1016/j.ijpsycho.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 68. Hunt WA, Landis C. The Overt Behavior Pattern in Startle. Journal of Experimental Psychology 1936; 14: 309–31. [Google Scholar]


