Abstract
Inferior frontal regions in the left and right hemisphere support different aspects of language processing. In the canonical model, left inferior frontal regions are mostly involved in processing based on phonological, syntactic and semantic features of language, whereas the right inferior frontal regions process paralinguistic aspects like affective prosody. Using diffusion tensor imaging (DTI)‐based probabilistic fibre tracking in 20 healthy volunteers, we identify a callosal fibre system connecting left and right inferior frontal regions that are involved in linguistic processing of varying complexity. Anatomically, we show that the interhemispheric fibres are highly aligned and distributed along a rostral to caudal gradient in the body and genu of the corpus callosum to connect homotopic inferior frontal regions. In the light of converging data, taking previous DTI‐based tracking studies and clinical case studies into account, our findings suggest that the right inferior frontal cortex not only processes paralinguistic aspects of language (such as affective prosody), as purported by the canonical model, but also supports the computation of linguistic aspects of varying complexity in the human brain. Our model may explain patterns of right‐hemispheric contribution to stroke recovery as well as disorders of prosodic processing. Beyond language‐related brain function, we discuss how inter‐species differences in interhemispheric connectivity and fibre density, including the system we described here may also explain differences in transcallosal information transfer and cognitive abilities across different mammalian species.
Keywords: corpus callosum, diffusion tensor imaging, homotopic, language processing, linguistic complexity
The integration of linguistic and paralinguistic information in speech processing requires the close interaction and coordination of left and right‐hemispheric perisylvian brain regions. This study uses probabilistic fibre tracking in diffusion tensor imaging data from healthy participants to map transcallosal homotopic fibre pathways between left and right inferior frontal cortex that are involved in speech processing of varying complexity.
Abbreviations
- BA
Brodmann Area
- CC
corpus callosum
- DTI
diffusion tensor imaging
- FFX
fixed effect [in analysing fMRI data]
- fMRI
functional magnetic resonance imaging
- HARDI
high angular resolution diffusion imaging [a DTI measurement technique]
- IBM
International Business Machines
- IFC
inferior frontal cortex
- IFG
inferior frontal gyrus
- IFGpo
inferior frontal gyrus pars opercularis
- IFGpt
inferior frontal gyrus pars triangularis
- L
left
- MCSRW
Monte Carlo simulation of random walks [a statistical method]
- mm
millimetre
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- ms
milliseconds
- PIBI
probability index forming part of the bundle of interest
- PICo
Probabilistic Index of Connectivity
- RFX
random effects [in analysing fMRI data]
- R
right
- rTMS
repetitive transcranial magnetic stimulation
- SPM
statistical parametric mapping
- s
seconds
- TE
echo time [a specific MRI pulse sequence parameter]
- TR
repetition time [a specific MRI pulse sequence parameter]
1. INTRODUCTION
Cumulative and converging evidence from clinical neurology, neuroanatomy, neuropsychology and neuroimaging shows that the left and the right hemispheres support common as well as differing aspects of language processing. In this canonical model, the left perisylvian cortex mainly supports linguistic aspects of language processing like the analysis of phonological, syntactic or semantic features (Hillis, 2007; Price, 2012; Shalom & Poeppel, 2008; Vigneau et al., 2006) and the right perisylvian cortex computes predominantly paralinguistic features of language like rhythm or prosody (Blonder, Bowers, & Heilman, 1991; Frühholz, Gschwind, & Grandjean, 2015; Ross, Thompson, & Yenkosky, 1997; Sammler, Grosbras, Anwander, Bestelmeyer, & Belin, 2015).
Macroanatomically, the left and right inferior frontal cortex (IFC) both consist of three parts: a superior dorsal part (pars opercularis and parts of ventral premotor cortex, IFCpo/PMv), a middle part (anterior pars opercularis and posterior pars triangularis, LIFCpo/pt) and an anterior inferior part (pars triangularis, IFCpt). For cytoarchitecture, anatomists have found differences in volume and cell‐packing density between left and right inferior frontal cortex for BA 44 and to a lesser extent for BA 45 (Amunts et al., 1999). This suggests that BA 44 may be more left lateralized than BA 45 cytoarchitectonically.
For the role of left inferior frontal cortex in speech processing, aggregated evidence from 25 years of neuroimaging studies suggests a distinct function‐anatomical organization in which the anatomical tripartition reflects a functional processing gradient (Bookheimer, 2002; Kellmeyer et al., 2013; Poeppel, Emmorey, Hickok, & Pylkkänen, 2012; Price, 2010; Vigneau et al., 2006). In this model, the LIFCpo/PMv part is preferentially involved in phonological, the LIFCpo/pt in syntactic and the LIFGpt part in semantic processing (Dapretto & Bookheimer, 1999; Shalom & Poeppel, 2008). Other neurolinguists have argued for a more supramodal view of left inferior frontal cortex in integrating syntactic and semantic features not exclusive to speech processing (Bornkessel‐Schlesewsky & Schlesewsky, 2013).
The role of the homotopic right inferior frontal cortex in speech processing, however, is much less clear. In the canonical model described above, the right IFC mostly supports the analysis of paralinguistic, (specifically affective) features of prosody (Heilman, Bowers, Speedie, & Coslett, 1984; Ross, 1993). Prosody was originally proposed by the Norwegian neurologist Monrad‐Krohn—the founder of the modern patholinguistic study of disorders of prosody—to be the “third element” of speech, the other elements being “grammar” (syntax) and “vocabulary” (semantics; Monrad‐Krohn, 1947, 1957). In this model, prosodic information is also conveyed by so‐called intrinsic features like stress, rhythm and pitch and these features are processed by right and left inferior frontal cortex (Frühholz et al., 2015; Vigneau et al., 2006, 2011). Thus, the dynamic interaction between left and right inferior frontal cortex seems to be a necessary condition for successful language use in real time. Psycholinguistic research with patients after corpus callosotomy (usually for intractable epilepsy) in the 1970s has described processing deficits at the phonetic and semantic level, supporting this model of transcallosal integration (Levy & Trevarthen, 1977). These early findings, however, were not systematically explored and henceforth largely forgotten. Furthermore, it is difficult to ascertain to which degree the large‐scale organization of the language system in patients with severe epilepsy differs from the healthy brain.
The left and right homotopic inferior frontal regions are connected by fibres of the corpus callosum (CC; Hewitt, 1962). White matter fibres of the CC are among the most aligned fibres in the brain, connecting homotopic and heterotopic regions, and show high reliability in autoradiographic or MRI‐based fibrr tracking procedures (Kim, Park, Kim, Lee, & Kim, 2008; Naets et al., 2017; Park et al., 2008). In terms of language development, the CC plays an important role for facilitating language lateralization (Hinkley et al., 2016) as well the interplay between left and right inferior frontal areas for linguistic and paralinguistic aspects of speech processing as discussed above. Pathological changes in CC structure or development, such as congenital agenesis of the CC negatively affect speech and language abilities in later life (Adibpour, Dubois, Moutard, & Dehaene‐Lambertz, 2018; Siffredi et al., 2018). Given this importance of the CC as a structure for facilitating language development and speech, comparatively little in vivo studies on interhemispheric language‐related white matter pathways have been performed.
Much of the available tracking studies with diffusion tensor imaging (DTI) investigating interhemispheric connectivity, however, are not grounded in or related to neurolinguistics research but look at more general patterns of CC connections often based on deterministic tractography. A further methodological limitation of these most commonly used DTI methods is that the presence of crossing fibres often prevents a detailed mapping of lateral projections, such as the corpus callosum (Tuch et al., 2002). The aim of our DTI study here, using a probabilistic fibre tracking method that is particularly robust against crossing fibres, is to map the interhemispheric white matter fibre network that facilitates the interaction between left and right inferior frontal cortex in the healthy brain.
To this end, we use results from an fMRI language experiment, in which the paradigm involved linguistic computations of different complexity, for mapping the interhemispheric transcallosal fibre network between left and right inferior frontal regions with probabilistic DTI‐based fibre tracking. Because the seed points for the probabilistic tractography derive from a well‐controlled neurolinguistic fMRI experiment, we can relate the tracking results to specific aspects of interhemispheric callosal interaction based on linguistic complexity.
We show that highly aligned transcallosal fibres connect both left and right anterior inferior IFC (BA45, ventral BA 44) and left and right posterior superior IFC (dorsal BA 44) to facilitate the rapid and dynamic interplay between linguistic and paralinguistic features in processing language.
2. METHODS
2.1. Selection of seed coordinates for fibre tracking
The seed coordinates for the DTI‐based tractography experiment performed for the study presented here were derived from an fMRI study on phonological transformation by Peschke, Ziegler, Eisenberger, and Baumgaertner (2012); see their paper for the full description of the study (and the fMRI paradigm). Briefly, subjects in this experiment had to overtly REPEAT or TRANSFORM particular pseudowords or pseudo‐noun phrases (NPs) in the MR scanner. Peschke et al. modelled the pseudowords after names of countries (e.g., “Doga” [engl. “Doga”] in analogy to “Kuba” [engl. “Cuba”]), and the pseudo‐NPs were modelled after monosyllabic German NPs (e.g., “der Mall” [engl. e.g., “the goll”] in analogy to “der Ball” [engl. “the ball”]).
In the REPEAT condition, subjects had to just repeat the pseudoword or pseudo‐NP and in the TRANSFORM condition, the pseudo‐countries had to be transformed into the corresponding pseudo‐language (e.g., “Doga” → “Doganisch” [engl. “Doga” → “Dogan”]) and the pseudo‐NP into their corresponding diminutive form (e.g., “der Mall” → “das Mällchen” [engl. e.g., “the goll” → “the little goll”]). Linguistically, the transformation of the pseudowords entails mostly prosodic changes (PROSODIC), that is, stress (“Dóga” → “Dogánisch”), and transforming the pseudo‐NP requires more complex, segmental and morphosyntactic (SEGMENTAL), changes (e.g., in “der Ball” → “das Bällchen” the segment “‐all” is substituted with “‐äll” and the pronoun changes from “der” to “das”).
The procedure for defining the seed points was the same as described in Kellmeyer et al. (2013). For our tracking experiment, we used the suprathreshold coordinates from the random‐effects group‐level fMRI analysis of the contrast TRANSFORM > REPEAT in the SEGMENTAL TRANSFORMATION. We did not use the random‐effects analysis of REPEAT alone for tracking because previous experiments have already demonstrated the structural connectivity patterns in the context of repetition of pseudowords via dorsal and ventral temporo‐frontal pathways (Saur et al., 2008, 2010).
In the contrast of interest, we identified the peak voxel coordinate in MNI space and then transformed it to the native space of each subjects’ DTI data and enlarged the seed coordinate to a seed sphere with a radius of 4 mm (containing 33 voxels), see Table 1 for a list of the seeds and Figure 1 for the SPM visualization. For better demarcation in defining the seed regions, we chose a different threshold (p < 0.001, uncorrected) for the t‐maps in SPM8 on the original fMRI data from the fMRI study by Peschke et al. (2012). Therefore, peak coordinates, cluster size and t‐values partly differ from Peschke et al. (2012), which used a threshold of p < 0.05, FDR‐corrected for the whole brain and a cluster level of >10 voxels. We should point out, that the sphere (containing 33 voxels) from which each tracking started encompassed the coordinate voxels from the published version of the study by Peschke et al. (2012) in each case. Thus, slight differences in the peak coordinates from our tracking experiment and the published version of Peschke et al. (2012) should not be a major concern as the sphere (containing 33 voxels) from which the tracking was started encompasses both the original coordinates and the coordinates used here.
Table 1.
Seed regions for the DTI‐based fibre tracking experiment
| Condition/task in fMRI experiment | Region | Hemisphere | Cluster size (voxels) | Peak MNI coordinates | t‐value* | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Segmental manipulation/transform > repeat | IFG, pars opercularis | L | 1,778 | −48 | 12 | 27 | 9.38 |
| IFG, pars triangularis | L | 1,778 | −45 | 39 | 9 | 8.26 | |
| IFG, pars opercularis | R | 613 | 45 | 12 | 24 | 5.12 | |
| IFG, pars triangularis | R | 613 | 45 | 36 | 12 | 6.01 | |
Abbreviations: IFG, inferior frontal gyrus; L, left; MNI, Montreal Neurological Institute (atlas of brain coordinates); R, right.
*p < 0.001, uncorrected.
Figure 1.
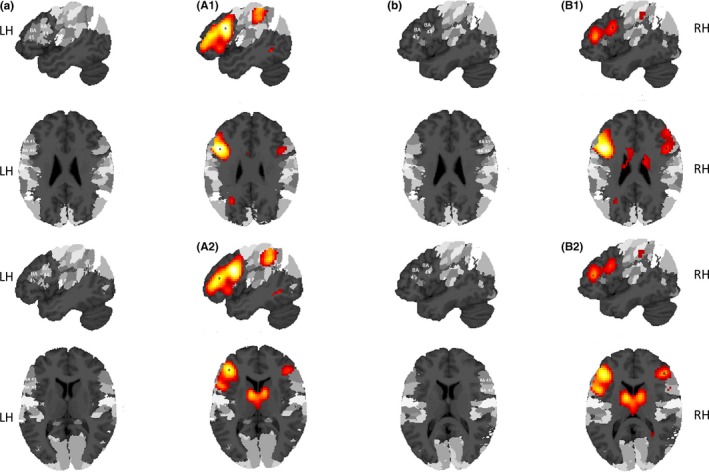
Suprathreshold peak coordinates (at p < 0.001, uncorrected) in inferior frontal cortex that were used as seed coordinates for the interhemispheric probabilistic DTI‐based fiber tracking are marked with an *. A1 shows the peak coordinate in LIFGpo, A2 in LIFGpt, B1 in RIFGpo, and B2 in RIFGpt. All clusters are superimposed on the cytoarchitectonic probability atlas by Eickhoff et al. (2005) in SPM8
In the left hemisphere, we identified the two peak coordinates in the inferior frontal cortex (left inferior frontal gyrus [LIFG], pars opercularis [po], LIFGpo; LIFG, pars triangularis [pt], LIFGpt) as seed coordinates. In the right hemisphere, we identified two frontal peaks (RIFGpo; RIFGpt) as seed coordinates. Table 1 and Figure 1 provide an overview of the fMRI results showing the seed coordinates (A1, A2, B1, B2).
2.2. Participants, group matching and methodological aspects of inter‐group data analysis
The participants and the procedure for matching participants from the DTI group to the fMRI group (Peschke et al., 2012) were the same as described in Kellmeyer et al. (2013).
In the study by Peschke et al. (2012), the researchers did not obtain DTI sequences from the participants in the fMRI study. For the DTI study presented here, we therefore matched 20 subjects in age, gender and handedness to the fMRI group. As in the fMRI study, all subjects were also native German speakers without any history of serious medical, neurological or psychiatric illness. All participants in this study were students recruited from the University of Freiburg and had therefore at least a diploma from German secondary school qualifying for university admission or matriculation as a basic level of education. We did not record information on socio‐economic status or employment record. Informed consent was obtained from all individual participants included in the study, and all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The DTI study was approved by the Ethics Committee of the Medical Center—University Medical Center of Freiburg.
The mean age in the DTI group was 24 years, and the age range was 20–38 years, and eight females and 12 males participated. Hand preference was tested with the 10‐item version of the Edinburgh Handedness Inventory (Oldfield, 1971), and subjects were identified as having predominantly right hand preference with an average laterality quotient of 0.8 (range 0.55–1.0). This group of participants did not differ significantly from the fMRI group in Peschke et al. (2012) in terms of age, gender or handedness.
The matching of the groups should account for the majority of gross anatomical differences between two groups of healthy individuals, see also Kellmeyer et al. (2013) and Suchan et al. (2014). Furthermore, all subjects’ anatomical scans (T1) were checked for gross anatomical anomalies (if necessary with expertise from a qualified neuroradiologist).
2.2.1. Methodological aspects of inter‐group and inter‐method data collection and analysis
We use functional coordinates from one group of participants, the fMRI experiment by Peschke et al. (2012), for a fibre tracking experiment in another group of participants—in contrast to the widespread practice to perform functional and tracking experiments on the same group. We would however argue that our approach is not only legitimate but even preferable, considering the basic tenets of group‐level statistical MRI‐based data analysis. Generally, group‐level neuroimaging data can be analysed using either fixed‐effects (FFX) or random‐effects (RFX) analysis. While FFX analysis can be used for reporting (collections of multiple) case studies, the aim of RFX is to make statistical inferences about the population from which the group is drawn (Penny, Friston, Ashburner, Kiebel, & Nichols, 2011). Today, RFX is widely used as standard approach in functional and structural neuroimaging data analysis, including the fMRI data set of the present study and had also been typically used in the functional data sets informing fibre tracking in other published reports. If, however, both (RFX) functional analysis and fibre tracking are performed in the same group of subjects, it is our view that it cannot be expected anymore that the resulting connectivity findings have any significance beyond the specific group that was investigated—that is, inferences about the population from which the group was drawn become impossible, similar to FFX. Hence, we used the functional localizers in a second group from the same population.
Given the group‐level focus of our study here, we would argue that measuring and analysing salient MRI data in two (carefully matched) groups of healthy participants should, if nothing else, increase the robustness and validity of our group‐level findings and inferences. These aspect are commensurate, in our view, with recent discussions in the neuroimaging community around establishing best practices and promoting open and reproducible measurement and analyses protocols in neuroimaging research (Nichols et al., 2017; Poldrack et al., 2017; Smith & Nichols, 2018).
2.2.2. Limitations of study design and methodology
We did not replicate the fMRI experiment from Peschke et al. (2012) in the subjects of the DTI study here. While this would have been generally useful for validation of the fMRI results, we do not believe that it would have further improved the quality and validity of our tracking experiment. By transforming peak voxel coordinates into 33 voxel spheres as seed areas for the fibre tracking algorithm, we account for possible small inter‐individual and inter‐group differences in peak voxel localization, thereby avoiding overspecification of individual peak voxels. Given limited resources on scanning time, we therefore elected to solely perform the DTI measurements.
The absence of fMRI data and behavioural data on linguistic performance from the participants, however, limits the interpretation of the results to questions regarding anatomical connectivity. The within‐subject combination of fMRI and DTI measurements would have allowed us to perform also anatomically constrained functional connectivity analyses (e.g., as in Saur et al., 2010). This would have provided a fuller picture of the functional role of the anatomical interhemispheric white matter network that we have identified with DTI. The additional measurement of behavioural data—such as response time, response duration and accuracy on the linguistic transformation tasks—could have provided valuable data for parametric variation analyses of the fMRI data and/or correlational analyses with DTI parameters across subjects.
2.3. DTI image acquisition
We acquired high angular resolution diffusion imaging (HARDI) data with a 3 Tesla Siemens MAGNETOM Trio TIM scanner using a diffusion‐sensitive spin‐echo echo planar imaging sequence with suppression of the cerebrospinal fluid signal. In total, we acquired 70 scans (with 69 slices) with 61 diffusion‐encoding gradient directions (b‐factor = 1,000 s/mm) and nine scans without diffusion weighting (b‐factor = 0). The sequence parameters were as follows: voxel size = 2 × 2×2 mm3, matrix size = 104 × 104 pixel2, TR = 11.8 s, TE = 96 ms and TI = 2.3 s. We corrected all scans for motion and distortion artefacts based on a reference measurement during reconstruction (Zaitsev, Hennig, & Speck, 2004). Finally, we obtained a high‐resolution T1 anatomical scan (160 slices, voxel size = 1 × 1×1 mm3, matrix = 240 × 240 pixel2, TR = 2.2 s, TE = 2.6 ms) for spatial processing of the DTI data.
2.4. DTI‐based probabilistic fibre tracking
We analysed the DTI data by using the method of pathway extraction introduced by Kreher et al. (2008) which is part of the MATLAB‐based “DTI&Fiber toolbox” (Kreher et al., 2008). This toolbox is available online for download (http://www.uniklinik-freiburg.de/mr/live/arbeitsgruppen/diffusion_en.html). Previously, this method has been used to identify white matter connections involved in language processing (Kellmeyer et al., 2013; Saur et al., 2008, 2010), attention (Umarova et al., 2010) and motor cognition (Hamzei et al., 2015; Vry et al., 2012).
For this procedure, we first computed the effective self‐diffusion tensor (sDT) from the HARDI data set (Basser, Mattiello, & LeBihan, 1994), which was corrected for movement and distortion artefacts.
Then, we performed a Monte Carlo simulation of “random walks” to calculate the probabilistic maps for each seed region separately. This procedure is similar to the probabilistic index of connectivity (PICo) method (Parker, Haroon, & Wheeler‐Kingshott, 2003). In extension to the PICo method, our probabilistic MCRW experiment preserves the information about the main traversing directions of the propagated fibre trajectories for each voxel. We then used this information for combining the probability maps. We extracted the orientation density function empirically from the effective diffusion tensor. The number of propagated trajectories was set to 10, and maximal fibre length was set to 150 voxels in accordance with our experience from the previous tracking studies mentioned above. We restricted the tracking area in each individual by a white matter mask to avoid tracking across anatomical borders. This mask included a small rim of grey matter to ensure that the cortical seed regions had indeed contact with the underlying white matter tracts.
To compute region‐to‐region anatomical connectivity between two seed spheres, we used a pairwise combination of two probability maps of interest (Kreher et al., 2008). Computationally, this combination is a multiplication, which takes the main traversing trajectory of the random walk into account. Random walks starting from seed regions may face in either opposing directions (connecting fibres) or merge and face in the same direction (merging fibres). In a pathway connecting two seed regions, the proportion of connecting fibres should exceed the proportion of merging fibres. Using this directional information during the multiplication, merging fibres are suppressed and connecting fibres are preserved by the tracking algorithm (Kreher et al., 2008). This procedure allows for extracting the most probable connecting pathway between two seed spheres without relying on a priori knowledge about the putative course of the white matter fibres. The resulting values represent a voxel‐wise estimation of the probability that a particular voxel is part of the connecting fibre bundle of interest (represented by a “probability index forming part of the bundle of interest” [PIBI]). In order to extract the most probable fibre tracts connecting left and right inferior frontal regions, all left inferior frontal maps were combined permutatively with all right inferior frontal maps based on the respective linguistic context (prosodic or segmental manipulation).
2.5. Post‐processing of the individual probability maps
We scaled the individual probability maps to a range between 0 and 1. Then, we spatially normalized the maps into standard Montreal Neurological Institute (MNI) space and subsequently smoothed them with an isotropic Gaussian kernel (3 mm) using SPM8. We computed group maps for each connection between seed regions by averaging the combined probability maps from all subjects. This resulted in one mean group map for each connection. Thus, any voxel in these group maps represents the arithmetic mean of the PIBI across subjects. To remove random artefacts, only voxels with PIBI values of >0.0145 were displayed, which excludes 95% of the voxels with PIBI >10−6. This cut‐off value was empirically derived from the distribution observed in a large collection of preprocessed combined probability maps (Saur et al., 2008). At the group level (n = 20), we used a non‐parametric statistic because PIBI values are not normally distributed (Saur et al., 2010).
2.6. Visualization and rendering of white matter fibre pathways
We visualized the resulting combined probability maps with the MATLAB‐based “DTI&Fiber Toolbox,” MricroN (http://www.sph.sc.edu/comd/rorden/mricron/) for 2D sections and rendered the fibre tracks with OpenDX by International Business Machines (IBM) (http://www.research.ibm.com/dx/).
3. RESULTS
The transcallosal fibre pathways between different subregions of left and right inferior frontal cortex show a homotopic region‐to‐region pattern of connectivity, and the fibre systems are clearly segregated and aligned from a ventral anterior inferior (left↔right IFG, pars triangularis) to dorsal posterior superior (left↔right IFG, pars opercularis) gradient in the body and genu of the corpus callosum (Figure 2). Importantly, the probabilistic tracking of the non‐homotopic seed regions (i.e., A1–B2 and A2–B1 in Figure 2) did not yield significant group‐level anatomical connections.
Figure 2.
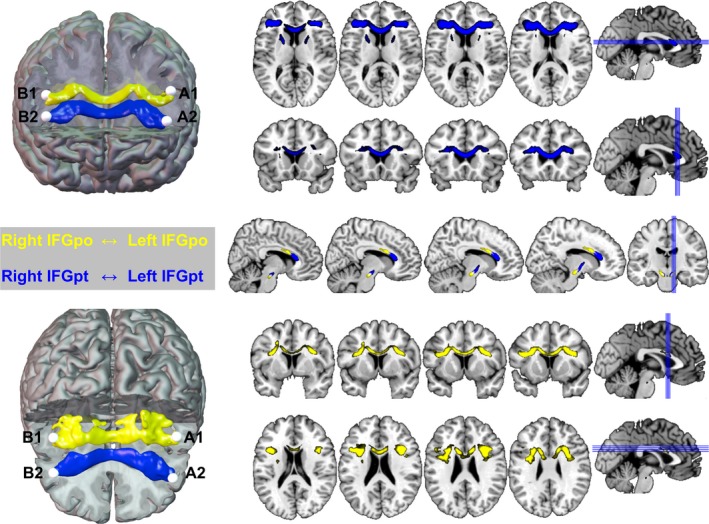
Mapping and rendering of the transcallosal white matter fiber tracts from left to right inferior frontal gyrus (pars opercularis, IFGpo, BA 44, in yellow) and left to right inferior frontal gyrus (pars triangularis, IFGpt, B45 and ventral BA44, in blue). A1, A2, B1, B2 = seed coordinates from Figure 1
The observed pattern of clearly segregated and homotopic transcallosal pathways between L/R IFGop and L/R IFGpt was found in each individual participant of the studied group. The crossover tracking from left IFGpo to right IFGpt and left IFGpt to right IFGpo, respectively, did not yield suprathreshold group‐level probability maps and are thus not visualized here.
4. DISCUSSION
The highly aligned transcallosal white matter pathways described here demonstrate a direct interhemispheric pathway for interaction between left and right homotopic inferior frontal cortex for language processing. The results are in agreement with previous anatomical ex vivo studies in humans on the topography of interhemispheric callosal fibres (Hewitt, 1962; Tomasch, 1954), as well as in vivo DTI‐based parcellation studies (Chao et al., 2009; Fabri, Pierpaoli, Barbaresi, & Polonara, 2014; Hofer & Frahm, 2006; Huang et al., 2005; Park et al., 2008; Teipel et al., 2009). The results also show a close cross‐species correspondence to homotopic patterns of interhemispheric fibres in non‐human primates (Makris et al., 2007; Pandya, Karol, & Heilbronn, 1971; Phillips & Hopkins, 2012). Next, we first discuss the putative functional role of this transcallosal pathway connecting homotopic left and inferior frontal cortex for language processing in the healthy brain in the context of language development. Then, we analyse the potential role of this interhemispheric network for language reorganization and close with some remarks on comparative inter‐species anatomy of the CC and its possible role for cognition.
4.1. Function of interhemispheric inferior frontal connections for language development and function
In terms of functional significance, this transcallosal route may generally facilitate rapid neural processing for a variety of cognitive functions that involve homotopic inferior frontal regions in both hemispheres. In our own area of expertise—language function in the healthy and injured brain—it has long been recognized, that right and left inferior frontal cortex contribute substantially to paralinguistic aspects of language processing like prosody (Belyk & Brown, 2014; George et al., 1996; Hoekert, Vingerhoets, & Aleman, 2010) and/or speech rhythm (Geiser, Zaehle, Jancke, & Meyer, 2007; Jungblut, Huber, Pustelniak, & Schnitker, 2012; Riecker, Wildgruber, Dogil, Grodd, & Ackermann, 2002). More specifically, dysfunction of intrinsic (also called linguistic) features of prosody (such as stress, rhythm and pitch) seems to result from damage to left inferior frontal regions, whereas lesions to right inferior cortex often results in impaired processing of extrinsic features of speech like affective prosody—the tonal variation conveying emotions (Belyk & Brown, 2014; Heilman et al., 1984; Ross, 1993; Ross et al., 1997; Speedie, Coslett, & Heilman, 1984).
Relating this model of prosodic processing to the functional and structural interhemispheric inferior frontal network identified here, we propose the following interpretation: the transformation of the pseudo‐country into the respective pseudo‐language most prominently entails a shift of stress (“Dóga” → “Dogánisch”)—a clear feature of linguistic prosody—but also the addition of segments and a change in grammatical category. This PROSODIC transformation condition (not shown in Figure 1, see Peschke et al., 2012) resulted in purely left fronto‐parietal suprathreshold clusters, which is consistent with the putative role of LIFG in intrinsic/linguistic prosodic features of language in the models mentioned above. In the SEGMENTAL transformation task of the pseudo‐noun phrases (“Der Mall” → “Das Mällchen”)—the basis of our DTI‐tracking experiment here—more complex linguistic operations occur like morphosyntactic changes and a change in the pronoun. At the level of extrinsic/affective prosody, the features most often associated with the right IFC in the classic models, it would be difficult to construe different emotional valences of the pseudo‐stimuli in the prosodic vs. the segmental transformation condition. Therefore, we interpret the involvement of right inferior frontal areas to reflect the greater linguistic complexity of the stimuli in the SEGMENTAL transformation condition. This is in accordance with previous fMRI studies and meta‐analyses that have shown a right inferior frontal involvement in the context of complex linguistic processing independent of emotional content (Price, 2010; Vigneau et al., 2006, 2011).
From a developmental perspective, the callosal transfer capacity is also an important factor for the successful integration of linguistic and paralinguistic information in speech development. One DTI‐based study, for example, showed that the macroanatomical thickness of the CC can be related to interhemispheric information transfer in a cohort of children between the age of six and eight (Westerhausen et al., 2011) and another study in 55 children between the age of five and 12 showed that the diffusivity of callosal fibres—as a putative measure of fewer but larger callosal axons—is correlated with phonological skills (Dougherty et al., 2007).
One important open question—which we cannot answer in the context of the study here—is whether the functional role of interhemispheric callosal fibres in language processing is predominantly excitatory or inhibitory (Bloom & Hynd, 2005; Reggia, Goodall, Shkuro, & Glezer, 2001; van der Knaap & van der Ham, 2011). The clinical literature allows only for very limited and preliminary conclusions with regard to this question. Only very few clinical reports, let alone systematic studies, on the consequences for language processing of direct damage to callosal fibres connecting left and right inferior frontal cortex are available. Mostly, callosal dysfunction in relation to language was investigated in the context of congenital callosal disorders like agenesis or dysgenesis of the CC (Brown, Symingtion, VanLancker‐Sidtis, Dietrich, & Paul, 2005; Genç, Ocklenburg, Singer, & Güntürkün, 2015; Jeeves & Temple, 1987; Paul, Van Lancker‐Sidtis, Schieffer, Dietrich, & Brown, 2003; Sanders, 1989). These studies have identified deficits in phonological and syntactic processing in relation to congenital CC dysfunction (Jeeves & Temple, 1987; Paul et al., 2003; Sanders, 1989; Temple, Jeeves, & Vilarroya, 1989). Direct non‐developmental callosal damage is a rare clinical event, mostly as a result of ischaemic stroke or cerebral vasculitis (Mahale et al., 2016). With respect to subsequent language dysfunction, we found only one report that describes a patient with a haemorrhagic lesion of the anterior part of the corpus callosum without damage to cortical projection areas (Klouda, Robin, Graff‐Radford, & Cooper, 1988). The patient initially showed a complete aprosody, that is a lack of tonal variation and reduced speed of speech, which recovered substantially throughout the follow‐up period of 1 year.
From this body of clinical studies too, it is difficult to infer whether callosal fibres predominantly have an inhibitory or excitatory role in the transfer of linguistic computations in the brain. Next, we therefore highlight evidence from neuroimaging and non‐invasive neurostimulation studies and research on language reorganization to shed light on the putative role of callosal fibres.
Other limitations for interpreting the results are as follows: (a) due to the relatively small sample size, analysing the data for differences in sex‐related interhemispheric white matter connectivity was not feasible in this study but would certainly be worthwhile in a larger sample of DTI data; (b) the interpretation of the findings here is also limited by the homogeneity of the subjects in terms of age (young) and education (university students), given that other demographic factors, such as socio‐economic status (especially during infancy/adolescence), may influence language development and skills (and the underlying neural network architecture); and (c) given the young average age of the subjects investigated here, the patterns of structural connectivity at a smaller scale might be different in the ageing brain, for example, reflecting age‐related reorganization of large‐scale interhemispheric networks that support language processing.
Bearing these limitations in mind, the inferior frontal interhemispheric network described here may have important implications for language reorganization in the ageing brain and following injury, for example, stroke.
4.2. The role of inferior frontal callosal connections for language reorganization
FMRI studies on the dynamics of language reorganization have shown increased activity of right‐hemispheric inferior frontal region following left hemispheric stroke in homologous inferior frontal cortex with post‐stroke aphasia which has been interpreted as a sign of adaptive plasticity (Saur et al., 2006; Winhuisen et al., 2007). Importantly, the early up‐regulation in the right hemisphere occurs in homotopic regions to the left hemispheric injured region which supports an important role of callosal fibres connecting homotopic regions (Staudt et al., 2002). Further evidence corroborating the concept of right‐hemispheric involvement in aphasia recovery comes from research applying rTMS‐based continuous theta burst stimulation (cTBS) over the left inferior frontal gyrus (Hartwigsen et al., 2013). In this study, the virtual lesion of left inferior frontal cortex with cTBS resulted in up‐regulation of the homotopic right inferior frontal cortex in reaction to the perturbation (Hartwigsen, 2015). The integrity of the interhemispheric white matter fibre network may therefore be critical to allow for adaptive recovery based on cross‐hemispheric transfer. These studies on actual aphasia recovery and virtual lesion modelling support the concept of interhemispheric inhibition as the main role of homotopic inferior frontal callosal fibres (Bloom & Hynd, 2005; Kano, Kobayashi, Ohira, & Yoshida, 2012). This concept opens avenues for further exploring new concepts for stroke recovery with non‐invasive and (invasive) neurostimulation as an emerging therapeutic approach (Balossier, Etard, Descat, Vivien, & Emery, 2015; Borich, Wheaton, Brodie, Lakhani, & Boyd, 2016; Cherney, 2015; Hamilton, Chrysikou, & Coslett, 2011; Otal, Olma, Flöel, & Wellwood, 2015).
To summarize, we interpret the homotopic interhemispheric inferior frontal white matter pathways, which we found here, as follows: the callosal connections of homotopic inferior frontal regions most likely supports integration of different levels of linguistic complexity. In this model, left inferior frontal regions would be sufficient to support linguistic transformations based on basic prosodic changes (like a shift of stress), whereas more complex linguistic operations, like segmental changes, additionally tap right inferior frontal regions for complementary computations. However, as real‐time language comprehension and production requires fast information transfer for integration, it may be that this model of cooperative hierarchical processing of left and right IFC prefers homotopic regions. Processing of paralinguistic features like emotional prosody, which is less time‐sensitive than computing linguistic features, in turn, may not depend upon strict homotopic connections. This model accounts for the hemispheric adaptive patterns in stroke recovery discussed above, as well as the neurotypology of disorders of prosodic perception and production as a result of right‐hemispheric inferior frontal injury (Belyk & Brown, 2014; Blonder et al., 1991; Hoekert et al., 2010). Finally, as this study is an in vivo anatomical study based on DTI, we want to highlight briefly some interesting inter‐species features of the anatomy and function of the CC.
4.3. Some remarks on inter‐species anatomy and function of the corpus callosum
From an evolutionary and mammalian inter‐species perspective, the CC is a highly conserved macroanatomic structure, indicating that it is important for supporting a variety of interhemispheric computations, independent of (but in humans including) language processing (Aboitiz & Montiel, 2003; Olivares, Michalland, & Aboitiz, 2000; Olivares, Montiel, & Aboitiz, 2001). A MRI tractography study in chimpanzees, the closest species related to humans which has been studied with DTI to date, shows a very similar topical pattern of interhemispheric connections and fibre alignment in the CC as human tractography studies and our results here (Phillips & Hopkins, 2012). If we move further away back on the eutherian clade of our evolutionary ancestry, however, fine differences in CC microstructure and connectivity emerge. In the largest cross‐species study to date, Olivares et al. (2001) demonstrated that the proportional numeric composition of fibres of the CC is preserved across six different species (the rat, the rabbit, the cat, the dog, the horse and the cow). Whereas the number of callosal fibres does not scale with increased brain size, the fibre diameter (and hence conduction velocity) does. This indicates that the type of fibre and quite likely also the pattern of connectivity might determine interhemispheric information transfer capabilities and that callosal transmission time may not be constant across species. These fine differences in interhemispheric network architecture and conduction properties, in turn, may relate to differences in the cognitive abilities of different mammalian species through processing constraints (Aboitiz, López, & Montiel, 2003). For humans, this structurally and functionally honed interplay and division of labour between the left and right hemisphere may indeed be the prerequisite for complex cognition and, ultimately, “the human condition” (Gazzaniga, 2000).
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Philipp Kellmeyer involved in data collection, experimental design, data analysis and writing of the article; Magnus‐Sebastian Vry involved in experimental design, data analysis and writing of the article; Tonio Ball involved in experimental design and reviewing of the article.
ACKNOWLEDGEMENTS
The authors thank the two reviewers and the section editor of the journal for the thorough review of the article and valuable comments. We also thank Professor Dorothee Saur (University of Leipzig—Medical Center) for valuable discussions of the work presented here.
Kellmeyer P, Vry M‐S, Ball T. A transcallosal fibre system between homotopic inferior frontal regions supports complex linguistic processing. Eur J Neurosci. 2019;50:3544–3556. 10.1111/ejn.14486
Funding information
This work was (partly) supported by the German Ministry of Education and Research (BMBF; grant number 13GW0053D) to the Medical Center—University of Freiburg and the German Research Foundation (DFG), grant number EXC1086 BrainLinks‐BrainTools, to the University of Freiburg, Germany.
The peer review history for this article is available at https://publons.com/publon/10.1111/EJN.14486
Parts of this study were used for the dissertation in medicine of author P.K. (University of Freiburg, Faculty of Medicine).
Kellmeyer and Vry equally contributed to this article.
Edited by Susan Rossell.
DATA ACCESSIBILITY
The data sets analysed for this study are available from the corresponding author on reasonable request.
REFERENCES
- Aboitiz, F. , López, J. , & Montiel, J. (2003). Long distance communication in the human brain: Timing constraints for inter‐hemispheric synchrony and the origin of brain lateralization. Biological Research, 36, 89–99. [DOI] [PubMed] [Google Scholar]
- Aboitiz, F. , & Montiel, J. (2003). One hundred million years of interhemispheric communication: The history of the corpus callosum. Brazilian Journal of Medical and Biological Research, 36, 409–420. 10.1590/S0100-879X2003000400002 [DOI] [PubMed] [Google Scholar]
- Adibpour, P. , Dubois, J. , Moutard, M.‐L. , & Dehaene‐Lambertz, G. (2018). Early asymmetric inter‐hemispheric transfer in the auditory network: Insights from infants with corpus callosum agenesis. Brain Structure and Function, 223, 2893–2905. 10.1007/s00429-018-1667-4 [DOI] [PubMed] [Google Scholar]
- Amunts, K. , Schleicher, A. , Bürgel, U. , Mohlberg, H. , Uylings, H. B. M. , & Zilles, K. (1999). Broca's region revisited: Cytoarchitecture and intersubject variability. The Journal of Comparative Neurology, 412, 319–341. 10.1002/(ISSN)1096-9861 [DOI] [PubMed] [Google Scholar]
- Balossier, A. , Etard, O. , Descat, C. , Vivien, D. , & Emery, E. (2015). Epidural cortical stimulation as a treatment for poststroke aphasia a systematic review of the literature and underlying neurophysiological mechanisms. Neurorehabilitation and Neural Repair, 30, 120–130. [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , Mattiello, J. , & LeBihan, D. (1994). Estimation of the effective self‐diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B, 103, 247–254. 10.1006/jmrb.1994.1037 [DOI] [PubMed] [Google Scholar]
- Belyk, M. , & Brown, S. (2014). Perception of affective and linguistic prosody: An ALE meta‐analysis of neuroimaging studies. Social Cognitive and Affective Neuroscience, 9, 1395–1403. 10.1093/scan/nst124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder, L. X. , Bowers, D. , & Heilman, K. M. (1991). The role of the right hemisphere in emotional communication. Brain, 114, 1115–1127. 10.1093/brain/114.3.1115 [DOI] [PubMed] [Google Scholar]
- Bloom, J. S. , & Hynd, G. W. (2005). The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychology Review, 15, 59–71. 10.1007/s11065-005-6252-y [DOI] [PubMed] [Google Scholar]
- Bookheimer, S. (2002). Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience, 25, 151–188. 10.1146/annurev.neuro.25.112701.142946 [DOI] [PubMed] [Google Scholar]
- Borich, M. R. , Wheaton, L. A. , Brodie, S. M. , Lakhani, B. , & Boyd, L. A. (2016). Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: A TMS‐EEG investigation. Neuroscience Letters, 618, 25–30. 10.1016/j.neulet.2016.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkessel‐Schlesewsky, I. , & Schlesewsky, M. (2013). Reconciling time, space and function: A new dorsal–ventral stream model of sentence comprehension. Brain and Language, 125, 60–76. 10.1016/j.bandl.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Brown, W. S. , Symingtion, M. , VanLancker‐Sidtis, D. , Dietrich, R. , & Paul, L. K. (2005). Paralinguistic processing in children with callosal agenesis: Emergence of neurolinguistic deficits. Brain and Language, 93, 135–139. 10.1016/j.bandl.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Chao, Y.‐P. , Cho, K.‐H. , Yeh, C.‐H. , Chou, K.‐H. , Chen, J.‐H. , & Lin, C.‐P. (2009). Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Human Brain Mapping, 30, 3172–3187. 10.1002/hbm.20739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney, L. R. (2015). Epidural cortical stimulation as adjunctive treatment for nonfluent aphasia phase 1 clinical trial follow‐up findings. Neurorehabilitation and Neural Repair, 30, 131–142. [DOI] [PubMed] [Google Scholar]
- Dapretto, M. , & Bookheimer, S. Y. (1999). Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron, 24, 427–432. 10.1016/S0896-6273(00)80855-7 [DOI] [PubMed] [Google Scholar]
- Dougherty, R. F. , Ben‐Shachar, M. , Deutsch, G. K. , Hernandez, A. , Fox, G. R. , & Wandell, B. A. (2007). Temporal‐callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academy of Sciences of the United States of America, 104, 8556–8561. 10.1073/pnas.0608961104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fabri, M. , Pierpaoli, C. , Barbaresi, P. , & Polonara, G. (2014). Functional topography of the corpus callosum investigated by DTI and fMRI. World Journal of Radiology, 6, 895–906. 10.4329/wjr.v6.i12.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühholz, S. , Gschwind, M. , & Grandjean, D. (2015). Bilateral dorsal and ventral fiber pathways for the processing of affective prosody identified by probabilistic fiber tracking. NeuroImage, 109, 27–34. 10.1016/j.neuroimage.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication. Brain, 123, 1293–1326. 10.1093/brain/123.7.1293 [DOI] [PubMed] [Google Scholar]
- Geiser, E. , Zaehle, T. , Jancke, L. , & Meyer, M. (2007). The neural correlate of speech rhythm as evidenced by metrical speech processing. Journal of Cognitive Neuroscience, 20, 541–552. [DOI] [PubMed] [Google Scholar]
- Genç, E. , Ocklenburg, S. , Singer, W. , & Güntürkün, O. (2015). Abnormal interhemispheric motor interactions in patients with callosal agenesis. Behavioral Brain Research, 293, 1–9. [DOI] [PubMed] [Google Scholar]
- George, M. S. , Parekh, P. I. , Rosinsky, N. , Ketter, T. A. , Kimbrell, T. A. , Heilman, K. M. , … Post, R. M. (1996). Understanding emotional prosody activates right hemisphere regions. Archives of Neurology, 53, 665–670. 10.1001/archneur.1996.00550070103017 [DOI] [PubMed] [Google Scholar]
- Hamilton, R. H. , Chrysikou, E. G. , & Coslett, B. (2011). Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and Language, 118, 40–50. 10.1016/j.bandl.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei, F. , Vry, M.‐S. , Saur, D. , Glauche, V. , Hoeren, M. , Mader, I. , … Rijntjes, M. (2015). The dual‐loop model and the human mirror neuron system: An exploratory combined fMRI and DTI Study of the inferior frontal gyrus. Cerebral Cortex, 26, 2215–2224. [DOI] [PubMed] [Google Scholar]
- Hartwigsen, G. (2015). The neurophysiology of language: Insights from non‐invasive brain stimulation in the healthy human brain. Brain and Language, 148, 81–94. [DOI] [PubMed] [Google Scholar]
- Hartwigsen, G. , Saur, D. , Price, C. J. , Ulmer, S. , Baumgaertner, A. , & Siebner, H. R. (2013). Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proceedings of the National Academy of Sciences of the United States of America, 110, 16402–16407. 10.1073/pnas.1310190110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman, K. M. , Bowers, D. , Speedie, L. , & Coslett, H. B. (1984). Comprehension of affective and nonaffective prosody. Neurology, 34, 917–921. 10.1212/WNL.34.7.917 [DOI] [PubMed] [Google Scholar]
- Hewitt, W. (1962). The development of the human corpus callosum. Journal of Anatomy, 96, 355–358. [PMC free article] [PubMed] [Google Scholar]
- Hillis, A. E. (2007). Aphasia progress in the last quarter of a century. Neurology, 69, 200–213. 10.1212/01.wnl.0000265600.69385.6f [DOI] [PubMed] [Google Scholar]
- Hinkley, L. B. N. , Marco, E. J. , Brown, E. G. , Bukshpun, P. , Gold, J. , Hill, S. , … Nagarajan, S. S. (2016). The contribution of the corpus callosum to language lateralization. Journal of Neuroscience, 36, 4522–4533. 10.1523/JNEUROSCI.3850-14.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekert, M. , Vingerhoets, G. , & Aleman, A. (2010). Results of a pilot study on the involvement of bilateral inferior frontal gyri in emotional prosody perception: An rTMS study. BMC Neuroscience, 11, 93 10.1186/1471-2202-11-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer, S. , & Frahm, J. (2006). Topography of the human corpus callosum revisited—Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage, 32, 989–994. 10.1016/j.neuroimage.2006.05.044 [DOI] [PubMed] [Google Scholar]
- Huang, H. , Zhang, J. , Jiang, H. , Wakana, S. , Poetscher, L. , Miller, M. I. , … Mori, S. (2005). DTI tractography based parcellation of white matter: Application to the mid‐sagittal morphology of corpus callosum. NeuroImage, 26, 195–205. 10.1016/j.neuroimage.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Jeeves, M. A. , & Temple, C. M. (1987). A further study of language function in callosal agenesis. Brain and Language, 32, 325–335. 10.1016/0093-934X(87)90131-3 [DOI] [PubMed] [Google Scholar]
- Jungblut, M. , Huber, W. , Pustelniak, M. , & Schnitker, R. (2012). The impact of rhythm complexity on brain activation during simple singing: An event‐related fMRI study. Restorative Neurology and Neuroscience, 30, 39–53. [DOI] [PubMed] [Google Scholar]
- Kano, T. , Kobayashi, M. , Ohira, T. , & Yoshida, K. (2012). Speech‐induced modulation of interhemispheric inhibition. Neuroscience Letters, 531, 86–90. 10.1016/j.neulet.2012.10.027 [DOI] [PubMed] [Google Scholar]
- Kellmeyer, P. , Ziegler, W. , Peschke, C. , Juliane, E. , Schnell, S. , Baumgaertner, A. , … Saur, D. (2013). Fronto‐parietal dorsal and ventral pathways in the context of different linguistic manipulations. Brain and Language, 127, 241–250. 10.1016/j.bandl.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Kim, E. Y. , Park, H.‐J. , Kim, D.‐H. , Lee, S.‐K. , & Kim, J. (2008). Measuring fractional anisotropy of the corpus callosum using diffusion tensor imaging: Mid‐sagittal versus axial imaging planes. Korean Journal of Radiology, 9, 391–395. 10.3348/kjr.2008.9.5.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouda, G. V. , Robin, D. A. , Graff‐Radford, N. R. , & Cooper, W. E. (1988). The role of callosal connections in speech prosody. Brain and Language, 35, 154–171. 10.1016/0093-934X(88)90106-X [DOI] [PubMed] [Google Scholar]
- Kreher, B. W. , Schnell, S. , Mader, I. , Il'yasov, K. A. , Hennig, J. , Kiselev, V. G. , & Saur, D. (2008). Connecting and merging fibres: Pathway extraction by combining probability maps. NeuroImage, 43, 81–89. 10.1016/j.neuroimage.2008.06.023 [DOI] [PubMed] [Google Scholar]
- Levy, J. , & Trevarthen, C. (1977). Perceptual, semantic and phonetic aspects of elementary language processes in split‐brain patients. Brain, 100(Pt 1), 105–118. 10.1093/brain/100.1.105 [DOI] [PubMed] [Google Scholar]
- Mahale, R. , Mehta, A. , Buddaraju, K. , John, A. A. , Javali, M. , & Srinivasa, R. (2016). Diffuse corpus callosum infarction—Rare vascular entity with differing etiology. Journal of the Neurological Sciences, 360, 45–48. 10.1016/j.jns.2015.11.045 [DOI] [PubMed] [Google Scholar]
- Makris, N. , Papadimitriou, G. M. , van der Kouwe, A. , Kennedy, D. N. , Hodge, S. M. , Dale, A. M. , … Rosene, D. L. (2007). Frontal connections and cognitive changes in normal aging rhesus monkeys: A DTI study. Neurobiology of Aging, 28, 1556–1567. 10.1016/j.neurobiolaging.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Monrad‐Krohn, G. H. (1947). The prosodic quality of speech and its disorders: (A brief survey from a neurologist's point of view). Acta Psychiatrica Scandinavica, 22, 255–269. 10.1111/j.1600-0447.1947.tb08246.x [DOI] [Google Scholar]
- Monrad‐Krohn, G. H. (1957). The third element of speech: Prosody in the neuro‐psychiatric clinic. British Journal of Psychiatry, 103, 326–331. [DOI] [PubMed] [Google Scholar]
- Naets, W. , Loon, J. V. , Paglioli, E. , Paesschen, W. V. , Palmini, A. , & Theys, T. (2017). Callosotopy: Leg motor connections illustrated by fiber dissection. Brain Structure and Function, 222, 661–667. 10.1007/s00429-015-1167-8 [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , Das, S. , Eickhoff, S. B. , Evans, A. C. , Glatard, T. , Hanke, M. , … Yeo, B. T. T. (2017). Best practices in data analysis and sharing in neuroimaging using MRI. Nature Neuroscience, 20, 299–303. 10.1038/nn.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Olivares, R. , Michalland, S. , & Aboitiz, F. (2000). Cross‐species and intraspecies morphometric analysis of the corpus callosum. Brain, Behavior and Evolution, 55, 37–43. 10.1159/000006640 [DOI] [PubMed] [Google Scholar]
- Olivares, R. , Montiel, J. , & Aboitiz, F. (2001). Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain, Behavior and Evolution, 57, 98–105. 10.1159/000047229 [DOI] [PubMed] [Google Scholar]
- Otal, B. , Olma, M. C. , Flöel, A. , & Wellwood, I. (2015). Inhibitory non‐invasive brain stimulation to homologous language regions as an adjunct to speech and language therapy in post‐stroke aphasia: A meta‐analysis. Frontiers in Human Neuroscience, 9, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya, D. N. , Karol, E. A. , & Heilbronn, D. (1971). The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Research, 32, 31–43. 10.1016/0006-8993(71)90153-3 [DOI] [PubMed] [Google Scholar]
- Park, H.‐J. , Kim, J. J. , Lee, S.‐K. , Seok, J. H. , Chun, J. , Kim, D. I. , & Lee, J. D. (2008). Corpus callosal connection mapping using cortical gray matter parcellation and DT‐MRI. Human Brain Mapping, 29, 503–516. 10.1002/(ISSN)1097-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, G. J. M. , Haroon, H. A. , & Wheeler‐Kingshott, C. A. M. (2003). A framework for a streamline‐based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. Journal of Magnetic Resonance Imaging, 18, 242–254. 10.1002/(ISSN)1522-2586 [DOI] [PubMed] [Google Scholar]
- Paul, L. K. , Van Lancker‐Sidtis, D. , Schieffer, B. , Dietrich, R. , & Brown, W. S. (2003). Communicative deficits in agenesis of the corpus callosum: Nonliteral language and affective prosody. Brain and Language, 85, 313–324. 10.1016/S0093-934X(03)00062-2 [DOI] [PubMed] [Google Scholar]
- Penny, W. D. , Friston, K. J. , Ashburner, J. T. , Kiebel, S. J. , & Nichols, T. E. (2011). Statistical parametric mapping: The analysis of functional brain images. London, UK; Burlington, MA: Elsevier. [Google Scholar]
- Peschke, C. , Ziegler, W. , Eisenberger, J. , & Baumgaertner, A. (2012). Phonological manipulation between speech perception and production activates a parieto‐frontal circuit. NeuroImage, 59, 788–799. 10.1016/j.neuroimage.2011.07.025 [DOI] [PubMed] [Google Scholar]
- Phillips, K. A. , & Hopkins, W. D. (2012). Topography of the chimpanzee corpus callosum. PLoS One, 7, e31941 10.1371/journal.pone.0031941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel, D. , Emmorey, K. , Hickok, G. , & Pylkkänen, L. (2012). Towards a new neurobiology of language. Journal of Neuroscience, 32, 14125–14131. 10.1523/JNEUROSCI.3244-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack, R. A. , Baker, C. I. , Durnez, J. , Gorgolewski, K. J. , Matthews, P. M. , Munafò, M. R. , … Yarkoni, T. (2017). Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. (2010). The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences, 1191, 62–88. 10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62, 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggia, J. A. , Goodall, S. M. , Shkuro, Y. , & Glezer, M. (2001). The callosal dilemma: Explaining diaschisis in the context of hemispheric rivalry via a neural network model. Neurological Research, 23, 465–471. 10.1179/016164101101198857 [DOI] [PubMed] [Google Scholar]
- Riecker, A. , Wildgruber, D. , Dogil, G. , Grodd, W. , & Ackermann, H. (2002). Hemispheric lateralization effects of rhythm implementation during syllable repetitions: An fMRI Study. NeuroImage, 16, 169–176. 10.1006/nimg.2002.1068 [DOI] [PubMed] [Google Scholar]
- Ross, E. D. (1993). Nonverbal aspects of language. Neurologic Clinics, 11, 9–23. 10.1016/S0733-8619(18)30168-3 [DOI] [PubMed] [Google Scholar]
- Ross, E. D. , Thompson, R. D. , & Yenkosky, J. (1997). Lateralization of affective prosody in brain and the callosal integration of hemispheric language functions. Brain and Language, 56, 27–54. 10.1006/brln.1997.1731 [DOI] [PubMed] [Google Scholar]
- Sammler, D. , Grosbras, M.‐H. , Anwander, A. , Bestelmeyer, P. E. G. , & Belin, P. (2015). Dorsal and ventral pathways for prosody. Current Biology, 25, 3079–3085. 10.1016/j.cub.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Sanders, R. J. (1989). Sentence comprehension following agenesis of the corpus callosum. Brain and Language, 37, 59–72. 10.1016/0093-934X(89)90101-6 [DOI] [PubMed] [Google Scholar]
- Saur, D. , Kreher, B. W. , Schnell, S. , Kümmerer, D. , Kellmeyer, P. , Vry, M.‐S. , … Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America, 105, 18035–18040. 10.1073/pnas.0805234105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur, D. , Lange, R. , Baumgaertner, A. , Schraknepper, V. , Willmes, K. , Rijntjes, M. , & Weiller, C. (2006). Dynamics of language reorganization after stroke. Brain, 129, 1371–1384. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Saur, D. , Schelter, B. , Schnell, S. , Kratochvil, D. , Küpper, H. , Kellmeyer, P. , … Weiller, C. (2010). Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. NeuroImage, 49, 3187–3197. 10.1016/j.neuroimage.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Shalom, D. B. , & Poeppel, D. (2008). Functional anatomic models of language: Assembling the pieces. The Neuroscientist, 14, 119–127. 10.1177/1073858407305726 [DOI] [PubMed] [Google Scholar]
- Siffredi, V. , Anderson, V. , McIlroy, A. , Wood, A. G. , Leventer, R. J. , & Spencer‐Smith, M. M. (2018). A neuropsychological profile for agenesis of the corpus callosum? Cognitive, academic, executive, social, and behavioral functioning in school‐age children. Journal of the International Neuropsychological Society, 24, 445–455. 10.1017/S1355617717001357 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2018). Statistical challenges in “big data” human neuroimaging. Neuron, 97, 263–268. 10.1016/j.neuron.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Speedie, L. J. , Coslett, H. B. , & Heilman, K. M. (1984). Repetition of affective prosody in mixed transcortical aphasia. Archives of Neurology, 41, 268–270. 10.1001/archneur.1984.04050150046014 [DOI] [PubMed] [Google Scholar]
- Staudt, M. , Lidzba, K. , Grodd, W. , Wildgruber, D. , Erb, M. , & Krägeloh‐Mann, I. (2002). Right‐hemispheric organization of language following early left‐sided brain lesions: Functional MRI topography. NeuroImage, 16, 954–967. 10.1006/nimg.2002.1108 [DOI] [PubMed] [Google Scholar]
- Suchan, J. , Umarova, R. , Schnell, S. , Himmelbach, M. , Weiller, C. , Karnath, H.‐O. , & Saur, D. (2014). Fiber pathways connecting cortical areas relevant for spatial orienting and exploration. Human Brain Mapping, 35, 1031–1043. 10.1002/hbm.22232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel, S. J. , Pogarell, O. , Meindl, T. , Dietrich, O. , Sydykova, D. , Hunklinger, U. , … Hampel, H. (2009). Regional networks underlying interhemispheric connectivity: An EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Human Brain Mapping, 30, 2098–2119. 10.1002/hbm.20652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple, C. M. , Jeeves, M. A. , & Vilarroya, O. (1989). Ten pen men: Rhyming skills in two children with callosal agenesis. Brain and Language, 37, 548–564. 10.1016/0093-934X(89)90111-9 [DOI] [PubMed] [Google Scholar]
- Tomasch, J. (1954). Size, distribution, and number of fibres in the human corpus callosum. Anatomical Record, 119, 119–135. 10.1002/(ISSN)1097-0185 [DOI] [PubMed] [Google Scholar]
- Tuch, D. S. , Reese, T. G. , Wiegell, M. R. , Makris, N. , Belliveau, J. W. , & Wedeen, V. J. (2002). High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine, 48, 577–582. 10.1002/(ISSN)1522-2594 [DOI] [PubMed] [Google Scholar]
- Umarova, R. M. , Saur, D. , Schnell, S. , Kaller, C. P. , Vry, M.‐S. , Glauche, V. , … Weiller, C. (2010). Structural connectivity for visuospatial attention: Significance of ventral pathways. Cerebral Cortex, 20, 121–129. 10.1093/cercor/bhp086 [DOI] [PubMed] [Google Scholar]
- van der Knaap, L. J. , & van der Ham, I. J. M. (2011). How does the corpus callosum mediate interhemispheric transfer? A review Behavioural Brain Research, 223, 211–221. 10.1016/j.bbr.2011.04.018 [DOI] [PubMed] [Google Scholar]
- Vigneau, M. , Beaucousin, V. , Hervé, P. Y. , Duffau, H. , Crivello, F. , Houdé, O. , … Tzourio‐Mazoyer, N. (2006). Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage, 30, 1414–1432. 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Vigneau, M. , Beaucousin, V. , Hervé, P.‐Y. , Jobard, G. , Petit, L. , Crivello, F. , … Tzourio‐Mazoyer, N. (2011). What is right‐hemisphere contribution to phonological, lexico‐semantic, and sentence processing?: Insights from a meta‐analysis. NeuroImage, 54, 577–593. 10.1016/j.neuroimage.2010.07.036 [DOI] [PubMed] [Google Scholar]
- Vry, M.‐S. , Saur, D. , Rijntjes, M. , Umarova, R. , Kellmeyer, P. , Schnell, S. , … Weiller, C. (2012). Ventral and dorsal fiber systems for imagined and executed movement. Experimental Brain Research, 219, 203–216. 10.1007/s00221-012-3079-7 [DOI] [PubMed] [Google Scholar]
- Westerhausen, R. , Luders, E. , Specht, K. , Ofte, S. H. , Toga, A. W. , Thompson, P. M. , … Hugdahl, K. (2011). Structural and functional reorganization of the corpus callosum between the age of 6 and 8 years. Cerebral Cortex, 21, 1012–1017. 10.1093/cercor/bhq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen, L. , Thiel, A. , Schumacher, B. , Kessler, J. , Rudolf, J. , Haupt, W. F. , & Heiss, W. D. (2007). The right inferior frontal gyrus and poststroke aphasia a follow‐up investigation. Stroke, 38, 1286–1292. 10.1161/01.STR.0000259632.04324.6c [DOI] [PubMed] [Google Scholar]
- Zaitsev, M. , Hennig, J. , & Speck, O. (2004). Point spread function mapping with parallel imaging techniques and high acceleration factors: Fast, robust, and flexible method for echo‐planar imaging distortion correction. Magnetic Resonance in Medicine, 52, 1156–1166. 10.1002/(ISSN)1522-2594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets analysed for this study are available from the corresponding author on reasonable request.


