Summary
Background
Atopic dermatitis (AD) is a heterogeneous disease with a multifactorial aetiology and complex pathophysiology. This heterogeneity translates into different trajectories of disease progression with respect to severity, persistence and risk of development of atopic comorbidities. Determining which possible disease trajectories or comorbidities any individual child might develop is challenging in clinical practice. Tools that help identify paediatric patients at higher risk of disease progression would greatly aid clinicians.
Methods
We reviewed recent cohort studies to synthesize and simplify the epidemiological data to try to identify shared clinically relevant characteristics that may help physicians estimate the risk of disease progression in paediatric patients with AD.
Results
Despite the variability in data collection and methods of analysis and their limitations, there are common patterns of early‐childhood AD that may aid in the estimation of risk for disease progression. Factors associated with risk of AD progression include younger age of onset, family history of atopy, greater AD severity, filaggrin mutations, urban environment and polysensitization and/or allergic multimorbidity. Based on these factors, we provide a practitioner's guide for identifying, counselling and/or referring infants and children with AD at potentially higher risk of developing persistent AD and atopic comorbidities. We also present clinical scenarios to illustrate how these data relate to real‐life situations.
Conclusions
Useful insights are provided for physicians and patients to inform them better about the risk of AD progression and to help guide care pathways for the paediatric population with AD.
What's already known about this topic?
The complex pathophysiology of atopic dermatitis (AD) translates into a heterogeneous clinical presentation and trajectories of disease progression.
Although the consensus is that most paediatric patients with AD will eventually ‘outgrow’ the disease or follow the longitudinal trajectory known as the ‘atopic march’, a significant proportion will develop persistent AD and/or other atopic conditions.
No known factors conclusively predict the risk of progression or development of comorbidities.
What does this study add?
Recent analyses of data from large cohorts of paediatric patients with AD have suggested the existence of potentially discrete clusters of patients who present with relatively common AD phenotypes.
These studies have shed some light onto the factors associated with risk of progression, which we review in this article.
A practitioner's guide with clinical scenarios is provided to help identify patients at high risk of progression to determine whether a patient should be monitored and/or would require specialist referral.
Short abstract
Linked Comment: https://doi.org/10.1111/bjd.18440.
https://doi.org/10.1111/bjd.18488 available online
Atopic dermatitis (AD), the most common skin disorder in infants and children,1, 2 is a complex disease with multifactorial aetiology, involving immune system dysregulation and epidermal barrier dysfunction, which are both influenced by genetic and environmental factors.3, 4, 5, 6 This complex pathophysiology translates into a heterogeneous clinical presentation (phenotype) with differences in age of onset, lesion localization, severity, sensitization profiles, disease persistence, presence of comorbid atopic and nonatopic conditions and longitudinal trajectories of disease progression (Fig. 1).7, 8
Figure 1.
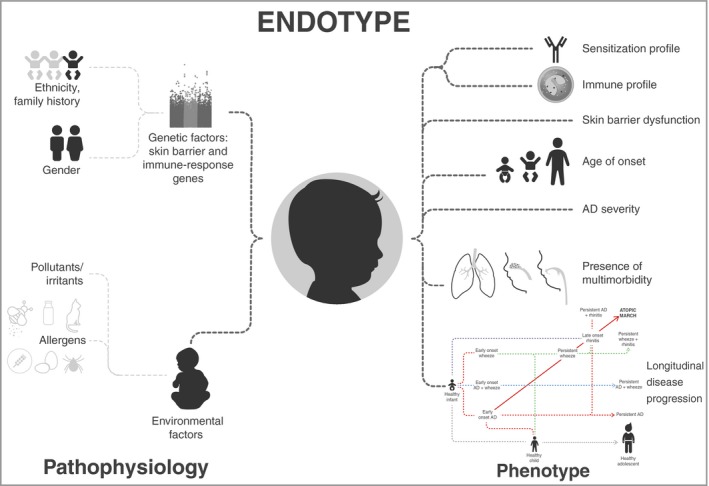
Atopic dermatitis (AD) has a multifactorial pathophysiology involving genetic and environmental factors leading to immune dysregulation and skin barrier dysfunction. This complexity translates into heterogeneous clinical presentations (phenotypes). The term ‘endotype’ encompasses all these clinical, pathological and aetiological aspects of a disease and has been applied to asthma and other complex diseases to refer to the molecular mechanisms underlying observable disease characteristics (phenotypes).
While the consensus in the population at large and among many practitioners is that most paediatric patients with AD will eventually ‘outgrow’ the disease, a significant proportion of paediatric cases of AD will develop persistent AD and/or other atopic conditions such as asthma or allergic rhinitis. In an effort to shed some light on the trajectories of disease progression of allergic conditions in the paediatric population, numerous cohort studies have been conducted in the last two decades (Fig. S1; see Supporting Information).9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 More recently, data‐driven approaches to analyse complex epidemiological data have led to the identification of potentially discrete clusters of patients who present with relatively common phenotypes.19, 44, 45
Although there are no known factors that conclusively predict the risk of disease progression for each of the known AD phenotypes, understanding the characteristics of these patient clusters and their distinct disease trajectories could help clinicians make an early determination of whether a paediatric patient with AD should be closely monitored for their potential to develop comorbidities and/or be referred to a specialist for evaluation and follow‐up.
Methods
This is a narrative review that synthesizes and simplifies the epidemiological data from various large cohort studies (Fig. S1; see Supporting Information). This review will help to identify a common pattern among childhood AD characteristics (subphenotypes) and to increase awareness about the complexity of AD among clinicians, as well as the multiple potential trajectories of AD progression. Studies were selected based on the following criteria: (i) cohorts with sample sizes larger than 500 patients; (ii) studies focusing on the patient population of interest (paediatric patients with AD; cohorts studying paediatric patients with asthma or allergic rhinitis were mostly excluded, even if some AD‐related outcomes were included – with the exception of a few insightful studies); (iii) longitudinal studies or long‐term studies with more than one cross‐sectional data analysis to determine development of comorbidities or persistence over time and (iv) studies focusing on key questions such as influence of environmental factors or parental history of atopy on the risk of development of comorbid conditions. We complement this information by providing a practitioner's guide with some representative clinical scenarios for identifying infants and children with AD who are at risk of disease progression and developing atopic comorbidities.
The challenge of analysing epidemiological data
The clustering of atopic comorbidities has long been recognized.46 Cross‐sectional and longitudinal analyses of epidemiological studies have suggested a phenotype known as the ‘atopic march’, described as a natural progression of atopic manifestations from AD to asthma and allergic rhinitis (Fig. 2).47, 48, 49, 50 This has created a common perception that AD is the ‘entry point’ for the development of other allergic diseases and that most paediatric patients with AD either follow the atopic march trajectory or outgrow the disease. However, the concept of the atopic march as the most common phenotype in paediatric patients with AD has been challenged by newer longitudinal studies applying more sophisticated statistical techniques.51
Figure 2.
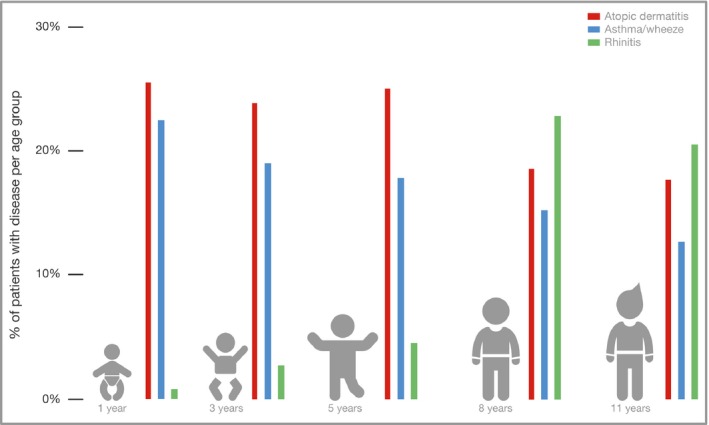
The atopic march has been defined as the sequential progression from atopic dermatitis to asthma and allergic rhinitis. The common perception is that patients either follow this trajectory or outgrow the disease. This graph shows the percentage of patients with each of the indicated allergic conditions (y‐axis) at different age groups (x‐axis) in the subset of patients who follow what is traditionally known as the atopic march trajectory. This corresponds to the cross‐sectional analysis of the data described by Belgrave et al.41
One of the most problematic aspects of analysing epidemiological data in AD is the difficulty of identifying incident phenotypes and establishing a causal relationship between AD and other concurrent atopic comorbidities. Many allergic diseases share common pathophysiology and genetic risk factors with AD, possibly explaining their co‐occurrence.52, 53 The Mechanisms of the Development of Allergy (MeDALL) project has proposed the term ‘multimorbidity’ to describe the co‐occurrence of allergic diseases in patients with AD, as the primary allergic disease is often not easily established. Accordingly, a variety of phenotypes and disease trajectories have been described, with the classic atopic march not applicable to every patient.54
Reporting bias can occur when clinicians are requested to score the presence or absence of concomitant comorbid conditions.55 This is due to the fact that allergic comorbidities, such as allergic rhinitis and asthma, can appear at the point in the disease trajectory at which AD severity has already decreased significantly, meaning recall bias may lead to under‐reporting of AD and other atopic comorbid conditions.56 For example, in an Italian cohort (205 children, age 6–36 months, average 16·9 years of follow‐up) diagnosed with AD by clinical examination (48·3% moderate, 19% severe), 60·5% of patients showed remission of AD by the end of follow‐up.57 However, when the investigators assessed the presence of other allergic conditions, such as asthma or rhinoconjunctivitis, only 20·5% of participants were reported as free of AD symptoms.57
Other issues encountered in the interpretation of epidemiological data include: (i) heterogeneity in data collection methods, (ii) use of different AD diagnostic criteria across cohorts, (iii) lack of consistency in the use of AD severity scales and (iv) absence of studies that stratify AD persistence by baseline AD severity.58, 59, 60, 61, 62, 63 All these factors make harmonizing datasets and defining and analysing disease severity and persistence across studies difficult.58, 59, 60, 61, 62, 63 Additionally, patients are frequently assigned to a phenotype based on the presence of individual symptoms listed in a questionnaire, when ideally syndromes of coexisting symptoms would be used, as they may represent patient subsets more accurately.64
Novel approaches to analysing longitudinal data disclose clear and reproducible atopic dermatitis phenotypes
In an attempt to address these confounding factors and biases, advanced analytical techniques frequently used in other fields, such as oncology, are being applied in atopic disease to elucidate complex disease phenotypes through pattern identification in large datasets. One example is machine‐learning methods, a data‐driven approach (not without limitations) that can capture the heterogeneity in longitudinal patterns of disease within individual patients, where conventional methods might overemphasize the prevalence of discrete subsets such as the atopic march phenotype.41, 45, 65, 66 Latent‐class analysis helps to overcome investigator bias when evaluating temporal patterns of age of onset and sensitization, as well as distinguishing overlapping categories of allergen sensitization.10, 44, 45
Interestingly, despite the variety of analytical methods applied, several common themes have emerged from multiple cohorts, suggesting that some of these phenotypes are highly reproducible and are likely to be clinically relevant. Figure 3 lists some of the phenotypes that have been identified using a few large, high‐quality studies, and highlights their commonalities. These more recent analyses show that while the atopic march is supported by cross‐sectional analyses of longitudinal studies, most atopic individuals do not follow this classical longitudinal pattern, suggesting that multiple trajectories of longitudinal disease progression exist (Fig. 4).19, 25, 41, 42, 44, 45, 67, 68, 69, 70, 71, 72, 73
Figure 3.
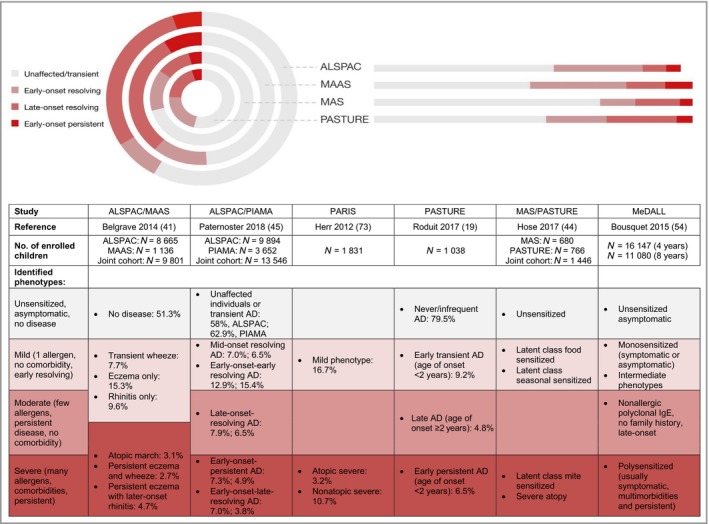
Despite the different methodologies used in recent data‐driven analyses of cohort data, the characteristics of the patient clusters representing distinct trajectories of disease progression are remarkably similar among studies. We have consolidated the information from the table into four more or less overlapping subsets represented in different shades of red in the concentric circles. AD, atopic dermatitis; ALSPAC, Avon Longitudinal Study of Parents and Children; MAAS, Manchester Asthma and Allergy Study; MAS, Multicenter Allergy Study; MeDALL, Mechanisms of the Development of Allergy; PARIS, Pollution and Asthma Risk: an Infant Study; PASTURE, Protection Against Allergy: Study in Rural Environments; PIAMA, Prevention and Incidence of Asthma and Mite Allergy.
Figure 4.
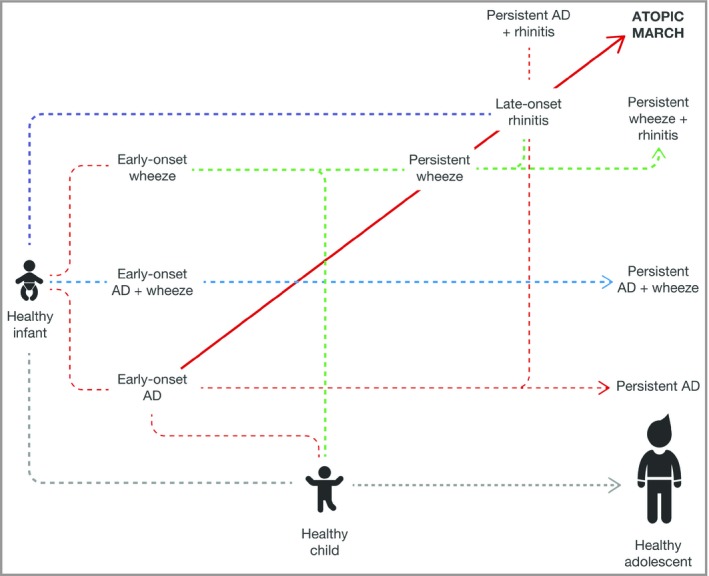
Paediatric patients with atopic dermatitis (AD) can follow multiple trajectories of disease progression. The atopic march represents one of multiple such possible trajectories. Analysing data cross‐sectionally at a population level at any of the indicated time points could result in either under‐ or overestimation of the co‐occurrence of any number of atopic comorbidities studied. The figure is based on the findings described by Belgrave et al.41
The challenge of identifying patients who will ‘outgrow’ atopic dermatitis and those who will develop persistent disease and/or comorbid conditions
Not only is it difficult to predict whether a patient with AD will develop other atopic conditions, it is also challenging to ascertain whether the disease will persist over time, for various reasons. Firstly, there is a need for a standardized definition of ‘persistence’ and for a consensus on how to define inactive disease and long periods of latency.74 The proportion of patients who ‘outgrow’ the disease varies per study, ranging from 20% to 80%.7, 29, 33, 75, 76, 77, 78 Secondly, cross‐sectional studies do not consider the time course of symptom development and may not account for the disease trajectory of individual patients. The episodic nature of AD complicates and confounds measurement of disease activity over time because data may be captured during a period of low disease activity or apparent remission or not captured frequently enough to detect changes. Individuals have periods of apparent remission followed by recurrence, highlighting the importance of longitudinal follow‐up of individual patients in the determination of persistence.74 Thirdly, recall bias is another significant problem in relation to ‘outgrown’ AD, with many adult patients not remembering that they had AD in their childhood or not recognizing the diagnosis due to the specific terminology used in questionnaires, such as ‘eczema’, ‘itchy rash’ or ‘atopic dermatitis’.55, 58, 79
A meta‐analysis (n = 110 651) showed that 20% of childhood AD persisted beyond 8 years of age and that < 5% of individuals had persistent disease 20 years after diagnosis.60 Persistence was defined as the presence of active AD on consecutive study visits, and thus the issue of low disease activity at the time of the visit could be a limitation.60 Another systematic review and meta‐analysis of seven longitudinal birth cohort studies comprising over 13 000 individuals with AD, which included repeated measurements in the same cohort during and after childhood, showed a similar prevalence of AD in childhood and early adulthood.74
Identifying factors to estimate risk of development of atopic comorbities
Currently, there is no known definitive set of predictors for persistent AD. However, data including those from the cohorts included in this review suggest associations between the following factors and risk of progression: younger age of onset, family history of atopy, greater AD severity, filaggrin gene (FLG) mutations, urban environment and polysensitization and/or allergic multimorbidity (Fig. 5).80, 81, 82
Figure 5.
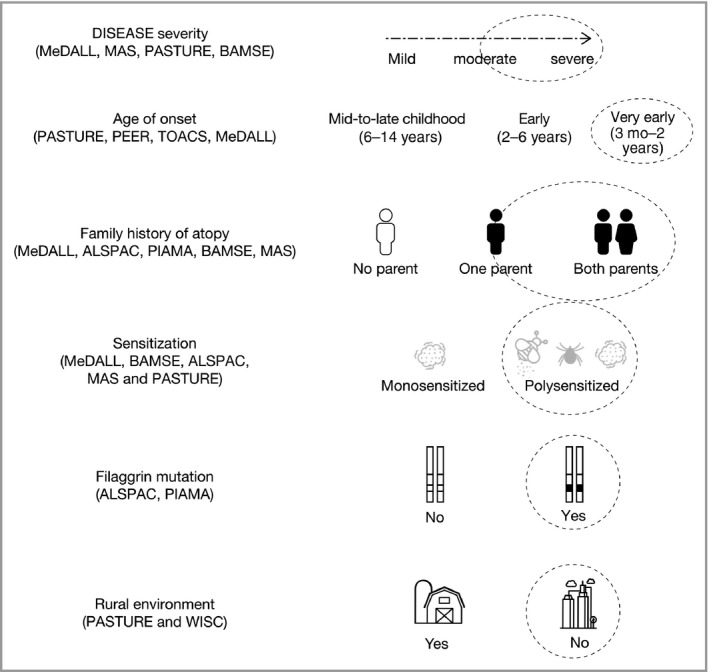
A practitioner's guide for identifying paediatric patients with atopic dermatitis who are at risk of disease progression and developing atopic comorbidities. This figure presents the relevant questions to ask in the clinic, which may aid in family counselling and the decision‐making process for the management and/or early referral of paediatric patients with atopic dermatitis. Based on data from the cohorts listed on the left, circled responses to these questions represent the highest risk. Referral to a specialist is recommended for patients with any of these characteristics. ALSPAC, Avon Longitudinal Study of Parents and Children; BAMSE, Children Allergy Milieu Stockholm Epidemiology; MAS, Multicenter Allergy Study; MeDALL, Mechanisms of the Development of Allergy; PASTURE, Protection Against Allergy: Study in Rural Environments; PEER, Pediatric Eczema Elective Registry; PIAMA, Prevention and Incidence of Asthma and Mite Allergy; TOACS, The Odense Adolescents Cohort Study; WISC, Wisconsin Birth Cohort Study.
Age of onset
Among the risk factors for AD persistence, early onset of disease and severity in infancy are among the most frequently identified.75, 80, 83 Very early‐onset AD appears to be associated with the development of atopic comorbidities.7, 11, 49, 78, 84, 85 In the Protection Against Allergy: Study in Rural Environments (PASTURE) cohort, both early transient and early persistent phenotypes, in particular the latter, showed a tendency towards an increased risk of developing asthma, with an asthma prevalence of 17·5% among children with an early persistent phenotype vs. 7·5% among those without AD or with infrequent symptoms.19 No association was observed with the late AD phenotype. The Tucson Children's Respiratory Study found that the presence of AD during the first year of life is an independent risk factor for persistent wheezing, and 18% of patients with wheezing at 6 years of age had AD before 2 years of age.32
In the Pediatric Eczema Elective Registry (PEER) cohort (median follow‐up 7·5 years), 73% of patients reported AD onset before the age of 2 years, and the risk of new‐onset seasonal allergies (but not asthma) was significantly higher in the early‐onset AD category (56·1% vs. 30·6% in the late‐onset group).11 Baseline asthma prevalence was also higher in the early‐onset group, but in patients without asthma at baseline, the incidence of new‐onset asthma was not significant after adjusting for demographic variables.11 In The Odense Adolescents Cohort Study cohort, patients with disease persistence had early‐onset AD (< 2 years), childhood hand eczema and allergic rhinitis.26 MeDALL identified a phenotype with AD and a high rate of multimorbidities and disease persistence over time characterized by family history and early disease onset.18 Finally, in the Multicenter Allergy Study (MAS) cohort, the presence of eczema before the age of 3 years was one of the independent predictors of allergic rhinitis up to age 20 years.34
Family history
A history of atopic disease in the immediate family appears to be more highly correlated with a diagnosis of AD than a history in any family member,86 and the significance of family history to the development of atopic disease appears to decrease with the age of patients with AD.54 However, the majority of studies have found a high frequency of parents with allergy among patients with a confirmed AD diagnosis.87 Family history is also a strong predictor for persistence of other atopic conditions such as asthma.56, 88, 89 In the Pollution and Asthma Risk: an Infant Study (PARIS) cohort, parental history of asthma and/or allergic rhinitis and/or eczema was associated with a severe atopic phenotype.73 Similarly, in the PASTURE study, parental allergy status was strongly associated with the early persistent phenotype, and participants with both parents having a history of allergy had an almost sixfold higher risk of the early persistent AD trajectory than those with no family history.19 MeDALL identified a phenotype of children with a family history of allergy who develop polysensitization and multimorbidities in early childhood,81 while in the MAS cohort, a strong atopic family history (defined as two or more atopic family members) was a significant predictor of poor prognosis.78 Parental history has been shown to have sex‐specific effects on the risk of allergy and asthma.87, 90, 91
Atopic dermatitis severity
There is general agreement among clinicians that patients with more severe AD tend to present with multimorbidity and more persistent disease. Unfortunately, most birth cohort studies do not include measures of disease severity or have insufficient patients with severe disease; therefore, the rate of persistence may be diluted by the inclusion of patients with mild disease.19, 70 However, studies in which severity was evaluated show a clear association between severity and persistence, demonstrating a need for longitudinal studies in patients with moderate‐to‐severe disease. Accordingly, in the MAS cohort, the strongest risk factor for poor prognosis of early AD was disease severity.78 In the PASTURE cohort, with disease severity measured using Scoring Atopic Dermatitis, children with the early persistent phenotype had greater AD severity.19 Similar results were reported in the PEER study, using self‐reported disease activity at baseline.29
In the BAMSE cohort (Swedish acronym for Children, Allergy, Milieu, Stockholm, Epidemiology), more participants with moderate‐to‐severe AD had more persistent disease and a higher prevalence of rhinitis and asthma than participants with mild AD,43 while in the aforementioned Italian cohort,57 in which patients with AD were followed for an average of 16·9 years, AD severity was significantly related to the appearance of asthma. The risk of asthma onset increases twofold in patients with moderate AD vs. mild AD and fourfold in patients with severe AD vs. moderate AD.57 Similar findings have been reported in prospective and retrospective cohort studies of patients with severe AD.7, 75
Genetic factors
Complex combinations of genetic factors can increase the direct risk of developing AD,82, 92 while other genetic factors influence disease severity and persistence and the risk of developing comorbidities such as asthma and hay fever. Of these, the best known disease modifiers are FLG loss‐of‐function mutations. Patients with AD with FLG mutations are more likely to have severe, persistent and multimorbid disease trejectories, a finding that has been replicated in many studies over the past decade.82, 93, 94, 95 In a meta‐analysis, FLG null alleles were found to increase the risk of asthma development approximately 1·5‐fold.96 Importantly, this association with asthma was only observed in the context of eczema, suggesting once again the existence of a distinct phenotype and disease trajectory in which FLG‐related eczema plus asthma corresponds to a distinct endophenotype. The EMSY (previously C11orf30) single‐nucleotide polymorphism confers a risk not only of AD but also of asthma and allergic rhinitis,97, 98 probably due to variants in LRRC32, encoding glycoprotein A repetitions predominant.99, 100
Type 1 sensitization
Development of IgE antibodies from childhood until adolescence is a dynamic process.101, 102 Many of the cohorts in this review have demonstrated an association between type 1 sensitization and the risk of disease progression to atopic multimorbidity. For instance, polysensitization and/or allergic multimorbidity was assessed as a predictor in a prospective cohort of 16 147 4‐year‐old children and 11 080 8‐year‐old children from 12 ongoing European birth studies.70 MeDALL found a rare but severe phenotype in which patients who are both polysensitized and multimorbid had a very high frequency of more severe and persistent AD symptoms over time, as well as higher total and specific IgE levels compared with other phenotypes.70 In the BAMSE cohort, 62% of children sensitized to airborne allergens between the age of 4 and 16 years had co‐occurrence of asthma, rhinitis or eczema.101 In a German cohort, the highest levels were detected in patients with early‐onset persistent phenotypes.7 Similar findings have been reported in the PARIS cohort.35
In the Canadian Healthy Infant Longitudinal Development birth cohort, AD without allergic sensitization was not associated with an increased risk of asthma at 3 years of age after adjusting for common confounders, while AD with sensitization increased the risk of asthma more than sevenfold.25 In the Isle of Wight birth cohort, both allergic sensitization with FLG variants and AD with FLG variants increased the risk of subsequent asthma, by 4·93‐ and 3·33‐fold, respectively, in the first 18 years of life.103 In the Swedish cohort, the risk of developing asthma was higher in children with eczema, and early onset of eczema was associated with an increased risk of sensitization to inhalant allergens.56 Compared with nonsensitized children, children sensitized to a variety of allergens are more likely to have asthma.66 In the Danish Allergy Research Center cohort, with 14‐year follow‐up, the highest frequency of sensitization was observed among those with multimorbidity.33
Clinical scenarios: translating epidemiological data into clinical practice
Taking into account the phenotypes identified by cluster analysis from the epidemiological studies shown in Figures 3 and Figure S1 (see Supporting Information) and the epidemiological studies reviewed, we present some common clinical scenarios in paediatric patients (Tables 1, 2, 3, 4, 5). These summarize their clinical presentation, estimated risk of persistence of AD and management recommendations to advise clinicians on key questions to ask during a visit. These cases reflect those commonly seen in clinical practice. We summarize the data informing the responses to these questions in Figure 5.
Table 1.
Clinical scenario 1
| Clinical presentation and pathophysiology |
| Age of AD onset: 10‐month‐old boy with very early onset (4 months) |
AD severity
|
| Prior treatment: emollients and low‐potency topical corticosteroids |
| Family history: childhood AD (both parents) and asthma (mother) |
| Genetic factors: very dry skin typical of ichthyosis vulgaris and hyperlinear (additional linear markings) palms are highly suggestive of a filaggrin mutation |
| Risk of disease progression and recommendations |
| Risk of progression: high risk of persistent AD and development of additional atopic comorbidities |
| Referral to a specialist comfortable and experienced in treating AD in young children is recommended |
| Assessment of peanut allergy should be performed; if absent, early introduction of peanut may prevent peanut allergy109 |
| Treatment: topical moisturizers and anti‐inflammatory treatment, and adequate quantities of topical corticosteroids of appropriate strength and suitable duration, as detailed in AD management guidelines110, 111 |
| Family counselling: the family or caregivers should be counselled that the condition is likely to be persistent and will require prospective maintenance therapy |
AD, atopic dermatitis.
Table 2.
Clinical scenario 2
| Clinical presentation and pathophysiology |
| Age of AD onset: 8‐month‐old boy with very early onset (3 months) |
| AD severity: mild AD |
Indications of potential multimorbidity
|
| Family history: both parents have a history of atopy (asthma or allergic rhinitis) |
| Risk of disease progression and recommendations |
| Risk of progression: significant risk of persistent asthma |
| Referral to a specialist comfortable with and experienced in treating childhood asthma is recommended |
| Treatment: as AD severity is mild, main recommendation is gaining good long‐term control of AD with active management as per guideline recommendations110, 111 |
AD, atopic dermatitis; RSV, respiratory syncytial virus.
Table 3.
Clinical scenario 3
| Clinical presentation and pathophysiology |
| Age of AD onset: 7‐year‐old girl with early onset (2 years) |
AD severity
|
Signs of multimorbidity
|
| Family history: no family history of atopy |
| Risk of disease progression and recommendations |
| Risk of progression: low risk of AD severity progression and development of comorbid atopic diseases |
Treatment: management with topical therapy, according to AD management guidelines110
|
AD, atopic dermatitis.
Table 4.
Clinical scenario 4
| Clinical presentation and pathophysiology |
| Age of AD onset: 7‐year‐old girl with early onset at 18 months and one episode at 30 months |
AD severity
|
| Signs of multimorbidity: no atopic comorbidities |
| Risk of disease progression and recommendations |
| Risk of progression: no significant risk of persistent AD |
AD, atopic dermatitis.
Table 5.
Clinical scenario 5
| Clinical presentation and pathophysiology |
| A pregnant mother with a 3‐year‐old child who has well‐controlled AD |
| Family history of atopy |
| Risk of disease progression and recommendations |
| Risk of AD development: increased risk for unborn child |
Treatment
|
AD, atopic dermatitis.
Limitations and strengths
The studies presented in this review used multiple different analytical methods, which influence cross‐study comparability. However, this review highlights that no matter the method employed for analysis, the same key findings are consistently shown across the original studies, suggesting the clinical relevance of the conclusions of the studies.
Conclusions
Associating specific observable AD phenotypes with a risk of disease progression or the development of comorbidities is relevant to clinical practice, as it has a profound impact on disease management. For instance, there is growing evidence that early restoration of skin barrier function could have an impact on the long‐term prevention of AD,104, 105 and early referral or multidisciplinary management of patients at high risk of developing comorbidities would have a positive impact on patient care.106
Although clustering of atopic comorbidities is well recognized, the typical longitudinal atopic march occurs only in a small proportion of patients. Despite the absence of conclusive data to allow for precise prediction of the risk of AD progression, we have collated data on disease trajectories from various cohort studies to provide some guidance to practitioners for identifying infants and children with AD who may be at risk of developing other allergic diseases. In addition, we have presented a practitioner's guide, based on current data, to estimate the risk of patients with AD developing subsequent comorbid asthma and rhinitis.
Importantly, one of the most frequently identified risk factors for disease persistence and development of comorbidities, regardless of the methodology, is early‐onset severe disease in patients with a family history of allergy.
Because patients with severe disease are typically under‐represented in birth cohorts, trajectories of disease progression cannot be derived from birth cohorts alone. Longitudinal studies of patients with severe AD are needed,7, 57, 75 especially those including identification of biomarkers of disease progression that could help clinicians more reliably identify patients at high risk.107, 108 For example, recent advances in AD research, including the use of systems biology, have led to the identification of a ‘double switch’, which provides mechanistic insights into common AD phenotypes, possibly explaining disease severity and the frequency of flares.72
Supporting information
Fig S1. Multiple birth cohort studies on asthma and allergic diseases have been conducted over the last few decades. This figure lists the key characteristics of some of the studies included in this review.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Acknowledgments
We would like to thank Linda Williams of Regeneron Pharmaceuticals, Inc. and El‐Bdaoui Haddad of Sanofi Genzyme for their contributions, and Ravi Subramanian, PhD and Jamie Lim, PhD of Excerpta Medica for medical writing, editorial and submission support.
Funding sources Editorial assistance was provided by Ravi Subramanian, PhD and Jamie Lim, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflicts of interest A.D.I. is a consultant for AbbVie, Chugai Pharma, Genentech, Janssen, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme and Pfizer. P.M.‐O. is an employee and shareholder of Regeneron Pharmaceuticals, Inc. The opinions expressed in this article are those of the authors and do not necessarily represent the views of the company.
https://doi.org/10.1111/bjd.18488 available online
References
- 1. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol 2015; 33:281–8. [DOI] [PubMed] [Google Scholar]
- 2. Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am 2010; 30:269–80. [DOI] [PubMed] [Google Scholar]
- 3. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 4. Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol 2016; 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guttman‐Yassky E, Waldman A, Ahluwalia J et al Atopic dermatitis: pathogenesis. Semin Cutan Med Surg 2017; 36:100–3. [DOI] [PubMed] [Google Scholar]
- 6. Weidinger S, Beck LA, Bieber T et al Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. [DOI] [PubMed] [Google Scholar]
- 7. Garmhaussen D, Hagemann T, Bieber T et al Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 2013; 68:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bieber T, D'Erme AM, Akdis CA et al Clinical phenotypes and endophenotypes of atopic dermatitis: where are we, and where should we go? J Allergy Clin Immunol 2017; 139(4 Suppl.):S58–64. [DOI] [PubMed] [Google Scholar]
- 9. Nissen SP, Kjær HF, Høst A et al The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol 2013; 24:549–55. [DOI] [PubMed] [Google Scholar]
- 10. Bousquet J, Anto J, Auffray C et al MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy 2011; 66:596–604. [DOI] [PubMed] [Google Scholar]
- 11. Wan J, Mitra N, Hoffstad OJ et al Variations in risk of asthma and seasonal allergies between early‐ and late‐onset pediatric atopic dermatitis: a cohort study. J Am Acad Dermatol 2017; 77:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Margolis DJ, Gupta J, Apter AJ et al Filaggrin‐2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol 2014; 133:784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergmann RL, Bergmann KE, Lau‐Schadensdorf S et al Atopic diseases in infancy. The German multicenter atopy study (MAS‐90). Pediatr Allergy Immunol 1994; 5 (6 Suppl.):19–25. [DOI] [PubMed] [Google Scholar]
- 14. Bisgaard H. The Copenhagen Prospective Study on Asthma in Childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol 2004; 93:381–9. [DOI] [PubMed] [Google Scholar]
- 15. Böhme M, Svensson A, Kull I et al Clinical features of atopic dermatitis at two years of age: a prospective, population‐based case–control study. Acta Derm Venereol 2001; 81:193–7. [DOI] [PubMed] [Google Scholar]
- 16. Boyd A, Golding J, Macleod J et al Cohort profile: the ‘children of the 90s’ – the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013; 42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halkjaer LB, Loland L, Buchvald FF et al Development of atopic dermatitis during the first 3 years of life: the Copenhagen prospective study on asthma in childhood cohort study in high‐risk children. Arch Dermatol 2006; 142:561–6. [DOI] [PubMed] [Google Scholar]
- 18. Pinart M, Benet M, Annesi‐Maesano I et al Comorbidity of eczema, rhinitis, and asthma in IgE‐sensitised and non‐IgE‐sensitised children in MeDALL: a population‐based cohort study. Lancet Respir Med 2014; 2:131–40. [DOI] [PubMed] [Google Scholar]
- 19. Roduit C, Frei R, Depner M et al Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr 2017; 171:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson BM, Hallam CL, Marolia H et al Manchester Asthma and Allergy Study (MAAS): recruitment of infant cohort. J Allergy Clin Immunol 2000; 105 (1 Part 2):S4. [Google Scholar]
- 21. Simpson BM, Custovic A, Simpson A et al NAC Manchester Asthma and Allergy Study (NACMAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy 2001; 31:391–9. [DOI] [PubMed] [Google Scholar]
- 22. von Mutius E, Schmid S. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy 2006; 61:407–13. [DOI] [PubMed] [Google Scholar]
- 23. Wahidi M, Vrtis RF, Fujimura KE et al Evaluation of indoor microbiota in Wisconsin farm vs. non‐farm homes using electrostatic dust collectors. J Allergy Clin Immunol 2017; 139 (2 Suppl.):AB116 (Abstr. 364). [Google Scholar]
- 24. Wijga AH, Kerkhof M, Gehring U et al Cohort profile: the prevention and incidence of asthma and mite allergy (PIAMA) birth cohort. Int J Epidemiol 2014; 43:527–35. [DOI] [PubMed] [Google Scholar]
- 25. Tran MM, Lefebvre DL, Dharma C et al Predicting the atopic march: results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol 2018; 141:601–7. [DOI] [PubMed] [Google Scholar]
- 26. Mortz CG, Andersen KE, Dellgren C et al Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015; 70:836–45. [DOI] [PubMed] [Google Scholar]
- 27. Strachan DP, Aït‐Khaled N, Foliaki S et al Siblings, asthma, rhinoconjunctivitis and eczema: a worldwide perspective from the International Study of Asthma and Allergies in Childhood. Clin Exp Allergy 2015; 45:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004–2012. J Invest Dermatol 2014; 134:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Margolis JS, Abuabara K, Bilker W et al Persistence of mild to moderate atopic dermatitis. JAMA Dermatol 2014; 150:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clarisse B, Nikasinovic L, Poinsard R et al The Paris prospective birth cohort study: which design and who participates? Eur J Epidemiol 2007; 22:203–10. [DOI] [PubMed] [Google Scholar]
- 31. Ziyab AH, Raza A, Karmaus W et al Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy 2010; 40:1776–84. [DOI] [PubMed] [Google Scholar]
- 32. Martinez FD, Wright AL, Taussig LM et al Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995; 332:133–8. [DOI] [PubMed] [Google Scholar]
- 33. Christiansen ES, Kjaer HF, Eller E et al The prevalence of atopic diseases and the patterns of sensitization in adolescence. Pediatr Allergy Immunol 2016; 27:847–53. [DOI] [PubMed] [Google Scholar]
- 34. Grabenhenrich LB, Keil T, Reich A et al Prediction and prevention of allergic rhinitis: a birth cohort study of 20 years. J Allergy Clin Immunol 2015; 136:932–40. [DOI] [PubMed] [Google Scholar]
- 35. Gabet S, Just J, Couderc R et al Early polysensitization is associated with allergic multimorbidity in PARIS birth cohort infants. Pediatr Allergy Immunol 2016; 27:831–7. [DOI] [PubMed] [Google Scholar]
- 36. University of Bristol . Avon Longitudinal Study of Parents and Children. Cohort profile. Available at http://www.bristol.ac.uk/alspac/researchers/cohort-profile (last accessed 10 April 2019).
- 37. Custovic A, Simpson A, Woodcock A. Manchester cohort. Pediatr Pulmonol Suppl 2004; 26:12–13. [DOI] [PubMed] [Google Scholar]
- 38. Garcia‐Aymerich J, Benet M, Saeys Y et al Phenotyping asthma, rhinitis and eczema in MeDALL population‐based birth cohorts: an allergic comorbidity cluster. Allergy 2015; 70:973–84. [DOI] [PubMed] [Google Scholar]
- 39. Schoos AM, Chawes BL, Melén E et al Sensitization trajectories in childhood revealed by using a cluster analysis. J Allergy Clin Immunol 2017; 140:1693–9. [DOI] [PubMed] [Google Scholar]
- 40. Lau S, Matricardi PM, Wahn U et al Allergy and atopy from infancy to adulthood: messages from the German birth cohort MAS. Ann Allergy Asthma Immunol 2019; 122:25–32. [DOI] [PubMed] [Google Scholar]
- 41. Belgrave DCM, Granell R, Simpson A et al Developmental profiles of eczema, wheeze, and rhinitis: two population‐based birth cohort studies. PLOS Med 2014; 11:e1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vrbova M, Dorociakova P, Vyskovsky R et al Dynamics of allergy development during the first 5 years of life. Eur J Pediatr 2018; 177:1317–25. [DOI] [PubMed] [Google Scholar]
- 43. Ballardini N, Kull I, Söderhäll C et al Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol 2013; 168:588–94. [DOI] [PubMed] [Google Scholar]
- 44. Hose AJ, Depner M, Illi S et al Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol 2017; 139:1935–45. [DOI] [PubMed] [Google Scholar]
- 45. Paternoster L, Savenije OEM, Heron J et al Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol 2018; 141:964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coca AF, Cooke RA. On the classification of the phenomenon of hypersensitiveness. J Immunol 1923; 8:163–82. [Google Scholar]
- 47. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003; 112:S118–27. [DOI] [PubMed] [Google Scholar]
- 48. Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am 2005; 25:231–46. [DOI] [PubMed] [Google Scholar]
- 49. Burgess JA, Lowe AJ, Matheson MC et al Does eczema lead to asthma? J Asthma 2009; 46:429–36. [DOI] [PubMed] [Google Scholar]
- 50. Hill DA, Spergel JM. The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol 2018; 120:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Busse WW. The atopic march: fact or folklore? Ann Allergy Asthma Immunol 2018; 120:115–19. [DOI] [PubMed] [Google Scholar]
- 52. Ferreira MA, Vonk JM, Baurecht H et al Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet 2017; 49:1752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khan SJ, Dharmage SC, Matheson MC, Gurrin LC. The atopic march related to confounding by genetics and early‐life environment? A systematic review of sibship and twin data. Allergy 2018; 73:17–28. [DOI] [PubMed] [Google Scholar]
- 54. Bousquet J, Anto JM, Wickman M et al Are allergic multimorbidities and IgE polysensitization associated with the persistence or re‐occurrence of foetal type 2 signalling? The MeDALL hypothesis. Allergy 2015; 70:1062–78. [DOI] [PubMed] [Google Scholar]
- 55. Mortz CG, Andersen KE, Bindslev‐Jensen C. Recall bias in childhood atopic diseases among adults in the Odense Adolescence Cohort Study. Acta Derm Venereol 2015; 95:968–72. [DOI] [PubMed] [Google Scholar]
- 56. Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis – a prospective follow‐up to 7 years of age. Allergy 2000; 55:240–5. [DOI] [PubMed] [Google Scholar]
- 57. Ricci G, Patrizi A, Baldi E et al Long‐term follow‐up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol 2006; 55:765–71. [DOI] [PubMed] [Google Scholar]
- 58. Pinart M, Albang R, Maier D et al Systematic review on the definition of allergic diseases in children: the MeDALL study. Int Arch Allergy Immunol 2015; 168:110–21. [DOI] [PubMed] [Google Scholar]
- 59. Dharmage SC, Lowe AJ, Matheson MC et al Atopic dermatitis and the atopic march revisited. Allergy 2014; 69:17–27. [DOI] [PubMed] [Google Scholar]
- 60. Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta‐analysis. J Am Acad Dermatol 2016; 75:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abuabara K, Langan SM, Yu AM. Conclusions about atopic dermatitis persistence might be premature. J Am Acad Dermatol 2017; 76:e177–8. [DOI] [PubMed] [Google Scholar]
- 62. Silverberg JI, Simpson EL. Reply to: ‘Conclusions about atopic dermatitis persistence might be premature’. J Am Acad Dermatol 2017; 76:e179. [DOI] [PubMed] [Google Scholar]
- 63. Williams HC, Wüthrich W. The natural history of atopic dermatitis In: Atopic Dermatitis: the Epidemiology, Causes and Prevention of Atopic Eczema. (Williams HC, ed), Cambridge: Cambridge University Press, 2000; 41–59. [Google Scholar]
- 64. Smith JA, Drake R, Simpson A et al Dimensions of respiratory symptoms in preschool children: population‐based birth cohort study. Am J Respir Crit Care Med 2008; 177:1358–63. [DOI] [PubMed] [Google Scholar]
- 65. Simpson A, Tan VY, Winn J et al Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med 2010; 181:1200–6. [DOI] [PubMed] [Google Scholar]
- 66. Lazic N, Roberts G, Custovic A et al Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy 2013; 68:764–70. [DOI] [PubMed] [Google Scholar]
- 67. Burgess JA, Walters EH, Byrnes GB et al Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol 2007; 120:863–9. [DOI] [PubMed] [Google Scholar]
- 68. Leynaert B, Neukirch C, Kony S et al Association between asthma and rhinitis according to atopic sensitization in a population‐based study. J Allergy Clin Immunol 2004; 113:86–93. [DOI] [PubMed] [Google Scholar]
- 69. Shaaban R, Zureik M, Soussan D et al Rhinitis and onset of asthma: a longitudinal population‐based study. Lancet 2008; 372:1049–57. [DOI] [PubMed] [Google Scholar]
- 70. Anto JM, Bousquet J, Akdis M et al Mechanisms of the Development of Allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol 2017; 139:388–99. [DOI] [PubMed] [Google Scholar]
- 71. van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol 2007; 120:565–9. [DOI] [PubMed] [Google Scholar]
- 72. Domínguez‐Hüttinger E, Christodoulides P, Miyauchi K et al Mathematical modeling of atopic dermatitis reveals ‘double‐switch’ mechanisms underlying 4 common disease phenotypes. J Allergy Clin Immunol 2017; 139:1861–72. [DOI] [PubMed] [Google Scholar]
- 73. Herr M, Just J, Nikasinovic L et al Risk factors and characteristics of respiratory and allergic phenotypes in early childhood. J Allergy Immunol 2012; 130:389–96. [DOI] [PubMed] [Google Scholar]
- 74. Abuabara K, Yu AM, Okhovat JP et al The prevalence of atopic dermatitis beyond childhood: a systematic review and meta‐analysis of longitudinal studies. Allergy 2018; 73:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rystedt I. Prognostic factors in atopic dermatitis. Acta Derm Venereol 1985; 65:206–13. [PubMed] [Google Scholar]
- 76. Lammintausta K, Kalimo K, Raitala R, Forsten Y. Prognosis of atopic dermatitis. A prospective study in early adulthood. Int J Dermatol 1991; 30:563–8. [PubMed] [Google Scholar]
- 77. Sandström MH, Faergemann J. Prognosis and prognostic factors in adult patients with atopic dermatitis: a long‐term follow‐up questionnaire study. Br J Dermatol 2004; 150:103–10. [DOI] [PubMed] [Google Scholar]
- 78. Illi S, von Mutius E, Lau S et al The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 2004; 113:925–31. [DOI] [PubMed] [Google Scholar]
- 79. Kulig M, Bergmann R, Edenharter G, Wahn U. Does allergy in parents depend on allergy in their children? Recall bias in parental questioning of atopic diseases. Multicenter Allergy Study Group. J Allergy Clin Immunol 2000; 105:274–8. [DOI] [PubMed] [Google Scholar]
- 80. Abuabara K, Hoffstad O, Troxel AB et al Patterns and predictors of atopic dermatitis disease control past childhood: an observational cohort study. J Allergy Clin Immunol 2018; 141:778–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bousquet J, Anto JM, Akdis M et al Paving the way of systems biology and precision medicine in allergic diseases: the MeDALL success story: Mechanisms of the Development of ALLergy; EU FP7‐CP‐IP; project no: 261357; 2010–2015. Allergy 2016; 71:1513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brown SJ, Kroboth K, Sandilands A et al Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose‐dependent effect. J Invest Dermatol 2012; 132:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rystedt I. Long term follow‐up in atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1985; 114:117–20. [DOI] [PubMed] [Google Scholar]
- 84. Somanunt S, Chinratanapisit S, Pacharn P et al The natural history of atopic dermatitis and its association with atopic march. Asian Pac J Allergy Immunol 2017; 35:137–43. [DOI] [PubMed] [Google Scholar]
- 85. Wüthrich B. Clinical aspects, epidemiology, and prognosis of atopic dermatitis. Ann Allergy Asthma Immunol 1999; 83:464–70. [DOI] [PubMed] [Google Scholar]
- 86. Williams HC, Burney PG, Hay RJ et al The U.K. Working Party's Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994; 131:383–96. [DOI] [PubMed] [Google Scholar]
- 87. Vaughn AR, Sivamani RK, Lio PA, Shi VY. Paternal vs. maternal factors in childhood atopic dermatitis. Dermatitis 2017; 28:241–5. [DOI] [PubMed] [Google Scholar]
- 88. Depner M, Fuchs O, Genuneit J et al Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med 2014; 189:129–38. [DOI] [PubMed] [Google Scholar]
- 89. Grabenhenrich LB, Gough H, Reich A et al Early‐life determinants of asthma from birth to age 20 years: a German birth cohort study. J Allergy Clin Immunol 2014; 133:979–88. [DOI] [PubMed] [Google Scholar]
- 90. Alford SH, Zoratti E, Peterson EL et al Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J Allergy Clin Immunol 2004; 114:1046–50. [DOI] [PubMed] [Google Scholar]
- 91. Wahn U, von Mutius E. Childhood risk factors for atopy and the importance of early intervention. J Allergy Clin Immunol 2001; 107:567–74. [DOI] [PubMed] [Google Scholar]
- 92. Paternoster L, Standl M, Waage J et al Multi‐ancestry genome‐wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet 2015; 47:1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Henderson J, Northstone K, Lee SP et al The burden of disease associated with filaggrin mutations: a population‐based, longitudinal birth cohort study. J Allergy Clin Immunol 2008; 121:872–7. [DOI] [PubMed] [Google Scholar]
- 94. Irvine A. Fleshing out filaggrin phenotypes. J Invest Dermatol 2007; 127:504–7. [DOI] [PubMed] [Google Scholar]
- 95. Weidinger S, O'Sullivan M, Illig T et al Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol 2008; 121:1203–9. [DOI] [PubMed] [Google Scholar]
- 96. Rodríguez E, Baurecht H, Herberich E et al Meta‐analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol 2009; 123:1361–70. [DOI] [PubMed] [Google Scholar]
- 97. Li X, Ampleford EJ, Howard TD et al The C11orf30‐LRRC32 region is associated with total serum IgE levels in asthmatic patients. J Allergy Clin Immunol 2011; 129:575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ramasamy A, Curjuric I, Coin LJ et al A genome‐wide meta‐analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol 2011; 128:996–1005. [DOI] [PubMed] [Google Scholar]
- 99. Manz J, Rodríguez E, ElSharawy A et al Targeted resequencing and functional testing identifies low‐frequency missense variants in the gene encoding GARP as significant contributors to atopic dermatitis risk. J Invest Dermatol 2016; 136:2380–6. [DOI] [PubMed] [Google Scholar]
- 100. Nousbeck J, Irvine AD. Atopic dermatitis according to GARP: new mechanistic insights in disease pathogenesis. J Invest Dermatol 2016; 136:2340–1. [DOI] [PubMed] [Google Scholar]
- 101. Wickman M, Asarnoj A, Tillander H et al Childhood‐to‐adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol 2014; 133:580–2. [DOI] [PubMed] [Google Scholar]
- 102. Bieber T. How to define atopic dermatitis? Dermatol Clin 2017; 35:275–81. [DOI] [PubMed] [Google Scholar]
- 103. Ziyab AH, Karmaus W, Zhang H et al Association of filaggrin variants with asthma and rhinitis: is eczema or allergic sensitization status an effect modifier? Int Arch Allergy Immunol 2014; 164:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Simpson EL, Chalmers JR, Hanifin JM et al Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014; 134:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Horimukai K, Morita K, Narita M et al Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014; 134:824–30. [DOI] [PubMed] [Google Scholar]
- 106. Boguniewicz M, Nicol N, Kelsay K, Leung DY. A multidisciplinary approach to evaluation and treatment of atopic dermatitis. Semin Cutan Med Surg 2008; 27:115–27. [DOI] [PubMed] [Google Scholar]
- 107. Bieber T, Cork M, Reitamo S. Atopic dermatitis: a candidate for disease‐modifying strategy. Allergy 2012; 67:969–75. [DOI] [PubMed] [Google Scholar]
- 108. Esparza‐Gordillo J, Schaarschmidt H, Liang L et al A functional IL‐6 receptor (IL6R) variant is a risk factor for persistent atopic dermatitis. J Allergy Clin Immunol 2013; 132:371–7. [DOI] [PubMed] [Google Scholar]
- 109. du Toit G, Sayre PH, Roberts G et al Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol 2018; 141:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Eichenfield LF, Tom WL, Berger TG et al Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71:116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Eichenfield LF, Boguniewicz M, Simpson EL et al Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics 2015; 136:554–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Multiple birth cohort studies on asthma and allergic diseases have been conducted over the last few decades. This figure lists the key characteristics of some of the studies included in this review.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


