Abstract
Background
The pathophysiological mechanism(s) of gastroesophageal reflux disease (GERD)‐related chronic cough (CC) is unclear. We aimed to determine the mechanism of reflux‐induced cough by synchronous monitoring of reflux episodes, esophageal motility, and cough.
Methods
Patients with GERD were prospectively enrolled and classified into GERD with CC (GERD‐CC) and without CC (GERD) groups. Twenty‐four‐hour ambulatory pH‐impedance‐pressure monitoring was performed; the reflux patterns, esophageal motility during prolonged exposure to acid and characteristics of reflux episodes that induced coughing paroxysms were analyzed.
Key Results
Thirty‐one patients with GERD‐CC and 47 with GERD were enrolled; all of whose monitoring results fulfilled the criteria for diagnosis of GERD. Patients with GERD‐CC had higher reflux symptom scores, longer exposure to acid, higher DeMeester scores, and more frequent reflux episodes, proximal extent reflux detected by impedance, and higher percentage of strongly acidic reflux than patients in the GERD group (all P < .05). Of 63 reflux‐cough episodes identified in the GERD‐CC group, 74.6% of distal reflux and 67.0% of proximal reflux episodes were acidic. More patients had low pan‐esophageal pressure in primary peristalsis (48.5% vs 11.8%, P = .000) and synchronous contraction in secondary peristalsis during prolonged exposure to acid in the GERD‐CC than in the GERD group (63.9% vs 9.1%, P = .000).
Conclusions & Inferences
Proximal acidic reflux and distal reflux‐reflex are jointly associated with reflux‐induced cough in patients with GERD. Low pan‐esophageal pressure in primary peristalsis and synchronous contraction in secondary peristalsis may play important roles in GERD‐associated chronic cough.
Keywords: ambulatory pH‐impedance‐pressure monitoring, chronic cough, esophageal motility, exposure to acid, gastroesophageal reflux disease
Proximal acidic reflux and distal reflux‐reflex, as well as ineffective esophageal motility during prolonged acid exposure, were associated with GERD‐associated chronic cough. Optimization of proton pump inhibitor and/or addition of prokinetic therapy might enhance the treatment in GERD patients with chronic cough.
Abbreviations
- ACEI
angiotensin‐converting enzyme inhibitors
- AET
acid exposure time
- BAL
bronchoalveolar lavage
- CC
chronic cough
- EE
erosive esophagitis
- GER
gastroesophageal reflux
- GERD
gastroesophageal reflux disease
- HRM
high‐resolution manometry
- IEM
ineffective esophageal motility
- IQRs
interquartile ranges
- LA
Los Angeles
- LES
lower esophageal sphincter
- NERD
non‐erosive reflux disease
- SAP
symptom association probability
- SD
standard deviation
Key Points.
The hypotheses of reflux and reflex have been proposed for explaining chronic cough with gastroesophageal reflux disease (GERD).
Patients with GERD and chronic cough have more severe reflux episodes and proximal extent reflux than those without chronic cough; thus, both proximal acidic reflux and distal reflux‐reflex are associated with reflux‐associated cough.
Low pan‐esophageal pressure in primary peristalsis and synchronous contraction in secondary peristalsis during prolonged exposure to acid may play an important role in GERD‐associated chronic cough.
1. INTRODUCTION
According to the Montreal definition and classification, gastroesophageal reflux disease (GERD) causes esophageal and extra‐esophageal symptoms.1 Chronic cough (CC), defined as cough for more than 8 weeks, is accepted as a definite extra‐esophageal symptom and affects estimated 9%‐33% of European and US individuals with GERD.2 GERD, asthma, and postnasal drip are considered as the most important factors contributing to chronic cough. Two possible pathophysiological mechanisms, namely “reflux theory” and “reflex theory,” may cause reflux‐induced cough episodes.3
Acid reflux has been identified as one of the most important causes of chronic cough and some patients with CC benefit from anti‐acid therapy.4, 5 However, many patients experience significant adverse effects of anti‐acid therapy without benefit. Studies have also shown that weakly acidic reflux plays a role in reflux‐induced cough.6, 7 Increased esophageal exposure to acid associated with esophageal dysmotility has been identified in patients with extra‐esophageal symptoms.8, 9, 10 Most studies using traditional manometric techniques have identified associations between ineffective esophageal motility (IEM) and both long exposure time and poor clearance of reflux events. The current Chicago Classification11 and Lyon Consensus12 listed more details regarding esophageal dysmotility as assessed by esophageal high‐resolution manometry (HRM); however, HRM has infrequently been used to assess GERD with CC.
An esophageal‐tracheobronchial reflex mediated by afferent nerves in the distal esophagus, defined as reflex theory, is another possible explanation for the relationship between GERD and CC. Enhanced cough reflex sensitivity and neurogenic airway inflammation are associated with reflux‐induced cough.13, 14, 15 However, few ambulatory data have been published.
Accurate diagnosis of reflux‐associated CC is challenging, one problem being identification of the refluxate's properties. Esophageal pH monitoring does not detect all gastroesophageal events, particularly when the refluxate is weakly acidic or non‐acidic. Combined esophageal pH‐impedance monitoring is a new means of detecting and classifying reflux events into acidic, weakly acidic, and non‐acidic reflux.16 Another unresolved problem is establishment of a causal relation between reflux and cough. The most frequently used indicator is symptom association probability (SAP),17 which indicates whether or not the relationship between reflux and perceived symptoms is random.18 However, the recorded time of cough may not coincide exactly with when it occurred and symptoms occurring during sleep may be omitted. Therefore, a new objective method for recording cough is needed. Ambulatory pH‐impedance‐pressure monitoring has been used in a few studies to record occurrence of cough, a 2‐minute time window being used to assess the temporal association between reflux and cough in patients with unexplained CC.19, 20, 21, 22 However, there are still several unanswered questions, such as why some patients with GERD have chronic cough while others do not and whether esophageal dysmotility plays a crucial role in inducing reflux‐associated cough.
Therefore, the aims of our study were to (a) compare the reflux characteristics of patients of GERD with and without CC; (b) identify ambulatory esophageal motility changes around reflux and coughing paroxysms; and (c) identify a subset of patients with GERD and CC who might benefit from anti‐acids and/or prokinetics.
2. MATERIALS AND METHODS
2.1. Participants
Consecutive patients (18‐65 years old) with typical reflux symptoms (heartburn and/or regurgitation) for more than 3 months were enrolled in a tertiary gastroenterology clinic in Peking Union Medical College Hospital, China, from October 2014 to October 2015. All patients had experienced mild reflux symptoms for at least 2 days per week or moderate/severe reflux symptoms for more than 1 day per week during the previous month. The frequency and degree of reflux symptoms were recorded and reflux symptom scores were used to assess severity of reflux symptom. Patients with peptic ulcer, malignancy, severe systemic disease, history of upper gastrointestinal surgery, or pregnancy were excluded from the study.
The typical GERD patients were then divided into two subgroups according to whether they had CC during the course of GERD: a GERD with CC (GERD‐CC group) and a GERD without CC (GERD group). CC was defined as cough persisting for more than 8 weeks, excluding asthma, postnasal drip, or use of angiotensin‐converting enzyme inhibitors (ACEIs).
After enrollment, all participants underwent gastroscopy unless they had undergone gastroscopy within the previous month. They were diagnosed as having either erosive esophagitis (EE), which was classified according to the Los Angeles (LA) classification or non‐erosive reflux disease (NERD), that is, without esophageal mucosal erosions.
All participants provided written informed consent. This study was approved by the Ethics Committee of Peking Union Medical College Hospital (No. JS‐829).
2.2. Ambulatory pH‐impedance‐pressure monitoring
All participants underwent 24‐hour ambulatory pH‐impedance‐pressure monitoring, which was achieved through two catheters, a combined pH‐impedance catheter and an intra‐esophageal pressure catheter. The pH‐impedance catheter (6.9 French, MMS‐Z2L‐A‐LES; MMS, An Enschede, the Netherlands) has six impedance channels and two pH antimony electrodes (positioned at 5 cm [pH1] and 27 cm [pH2] above the lower esophageal sphincter [LES]). The intra‐esophageal pressure catheter (GIM6000; MMS) has four pressure sensors, which are located 5 cm (P1), 10 cm (P2), 15 cm (P3), and 20 cm (P4) proximal to the upper border of the LES. The positions of pH‐impedance‐pressure electrodes were shown in Figure 1. Both catheters were introduced simultaneously via the same nostril and taped to the face. The pH, impedance, and pressure signals were stored on a digital data logger (Omega; MMC). Monitoring was performed for 24 hours.
Figure 1.
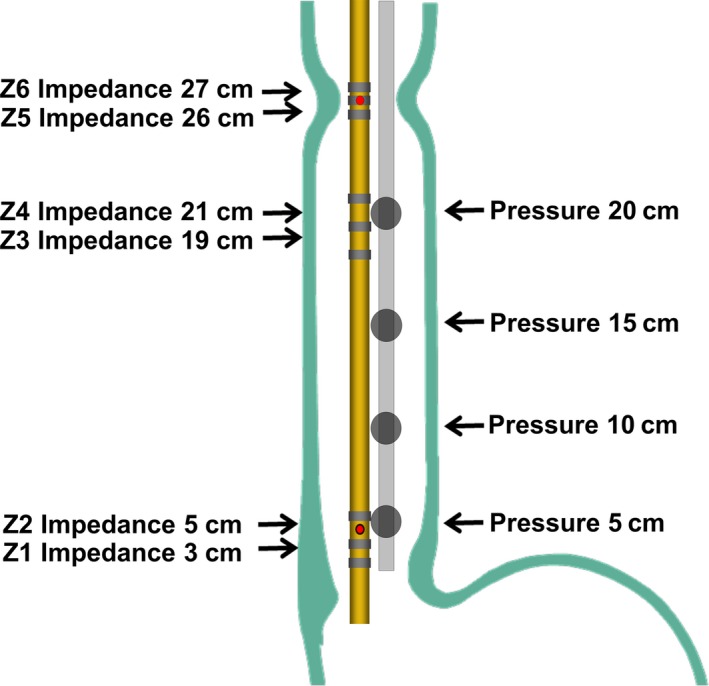
Positions of electrodes of the ambulatory pH‐impedance‐pressure monitoring system. Red spot: pH electrodes; grey loop: impedance electrodes; black spot: pressure sensors. The values in the figure refer to the distance from the upper border of the lower esophageal sphincter
Each participant was supplied with three standard nutritious test meals by the diet center of our hospital. During the 24‐hours of measurement, only the test meals and water were consumed. The participants were instructed to note down times of reflux symptoms, meals, and sleep and were encouraged to maintain their normal activities throughout recording. All participants tolerated and completed the measurements.
2.3. Data analysis
2.3.1. Assessment of reflux symptoms
Reflux symptoms during the previous month were scored as described by Vigneri et al23 as follows: reflux symptom score = severity score × frequency score; separate scores for heartburn and acid reflux are added together. The severity of each symptom is graded as follows: 0 = no symptoms; 1 = mild symptoms with spontaneous remission and no interference with normal activity or sleep; 2 = moderate symptoms with spontaneous but slow remission and mild interference with normal daily activities or sleep; and 3 = severe symptoms without spontaneous remission and marked interference with normal daily activities or sleep. The frequency of each symptom was scored as follows: 0 = none, 1 = <2 days per week, 2 = 2‐4 days per week, and 3 = >4 days per week.
2.3.2. Reflux variables
Gastroesophageal reflux (GER) variables were analyzed by an MMS Solar GI acquisition system from recorded pH‐impedance data. Esophageal acid reflux was defined as pH < 4 and expressed as acid exposure time (AET) in minutes and durations of acid reflux episodes. Acid exposure lasting ≥5 minute was defined as prolonged acid reflux. Severity of acid reflux was expressed as DeMeester scores.24 In accordance with the Lyon Consensus,12 AET >6% or more than 80 impedance‐detected reflux episodes was considered conclusive evidence for pathologic reflux.
Impedance‐detected reflux was classified on the basis of pH monitoring data as acidic reflux when pH < 4, weakly acid reflux when pH 4‐7, and non‐acidic reflux when pH > 7. Distal reflux was defined as reflux limited to within 19 cm of the LES, proximal reflux as reflux reaching further than 19 cm from the LES, and high reflux as reflux reaching further than 26 cm from the LES.
2.3.3. Esophageal motility and cough
Esophageal peristalsis was identified as primary or secondary peristalsis according to its association with swallowing during ambulatory pressure monitoring. Primary peristalsis is peristalsis induced by swallowing and transporting of boluses inside the esophagus, whereas secondary peristalsis is triggered by various intra‐esophageal stimuli (ie, refluxate in this study) in the absence of swallowing.25, 26 In this study, we identified those peristalsis as primary in which the bolus entry at each specific level obtained at the 50% point between 3 seconds pre‐swallow impedance baseline and impedance nadir during bolus presence and bolus exit determined as return to this 50% point on the impedance‐recovery curve.27 We detected swallows to distinguish primary from secondary peristalsis by deglutitive impedance gradient and impedance traces. There was no impedance change at the most proximal impedance electrode when secondary peristalsis happened. We analyzed primary and secondary peristalsis only during the longest period of acid reflux in each patient.
Further, ineffective peristalsis,28, 29 also called ineffective esophageal motility (IEM), was defined as contraction amplitude of P1 and P2 in the proximal esophagus <12 mm Hg or that of P3 and P4 in the distal esophagus <25 mm Hg, or antiperistalsis or synchronous contraction occurred in two or more channels during ambulatory pressure monitoring.
A cough was defined as a rapid, short duration, simultaneous pressure peak with time to peak <1 second19, 21 and with the same pressure configuration at all intra‐esophageal recording sites on ambulatory esophageal manometry. A coughing paroxysm was defined as two or more rapid simultaneous pressure peaks within 3 seconds. Only coughing paroxysms were analyzed in this study.
2.3.4. Association between reflux and coughing paroxysms
Coughing paroxysms were considered related to a reflux episode if they occurred within 2 minutes of a reflux episode.19, 20 Coughing paroxysms within 2 minutes after the onset of a reflux episode were considered reflux‐cough episodes (Figure 2A). Reflux episodes occurring within 2 minutes after a coughing paroxysm were defined as cough‐reflux episodes (Figure 2B). When there was more than 2 minutes between a reflux episode and coughing paroxysm, they were defined as being unrelated.
Figure 2.
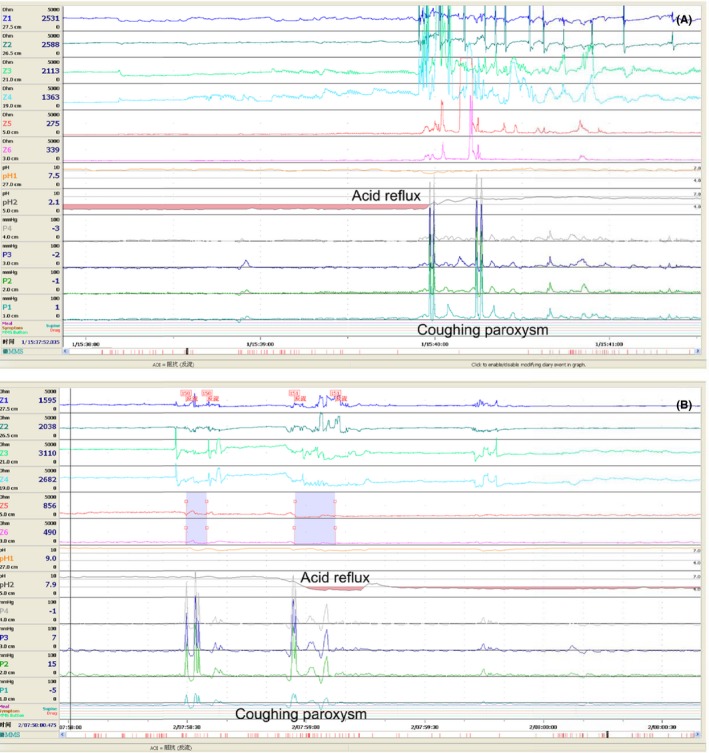
Tracings of ambulatory esophageal pH‐impedance‐pressure monitoring revealing a temporal correlation with reflux‐coughing paroxysms. A, Reflux‐cough episode; B, Cough‐reflux episode
2.4. Statistical analysis
SPSS 18.0 (IBM) was used for statistical analysis of data. Parametric and non‐parametric data are presented as the mean ± standard deviation (SD) and median and interquartile ranges (IQRs), respectively. Normally distributed continuous variables were compared using paired samples t tests and non‐normally distributed data using Mann–Whitney U tests. The chi‐square test was used for categorical variables. P < .05 was considered to denote statistical significance.
3. RESULTS
3.1. Patient cohort
The study cohort comprised 78 patients with GERD, 31 of whom had CC and were accordingly assigned to the GERD‐CC group, the remaining 47 (without CC) being assigned to the GERD group. Relevant patient characteristics were shown in Table 1. There were no significant differences in gender, age, bodyweight, height, and body mass index between the two groups.
Table 1.
Relevant patient characteristics
|
GERD‐CC group (n = 31) |
GERD group (n = 47) |
P value | |
|---|---|---|---|
| Female (n, [%]) | 17 (54.8%) | 24 (51.1%) | .74 |
| Age (y) | 52.1 ± 10.8 | 51.1 ± 12.0 | .70 |
| Body weight (Kg) | 66.7 ± 10.2 | 66.6 ± 14.3 | .88 |
| Height (cm) | 165.9 ± 8.6 | 165.5 ± 7.1 | .84 |
| BMI (Kg/m2) | 24.2 ± 3.0 | 24.0 ± 4.2 | .87 |
Data are presented as the mean ± SD or number (percentage).
Abbreviations: BMI, body mass index; GERD, gastroesophageal reflux disease; GERD‐CC, gastroesophageal reflux disease with chronic cough.
3.2. Gastroesophageal reflux characteristics according to CC status
The patients in the GERD‐CC group had significantly higher reflux symptom scores than those in the GERD group (P = .007). Gastroscopy showed no esophageal erosion (NERD) in 25 patients (80.6%) in the GERD‐CC group and in 34 patients (72.3%) in the GERD group (Table 2). Five patients in GERD‐CC group had LA‐A and one LA‐B esophagitis, whereas six, two, three, and two patients in the GERD group had LA‐A, LA‐B, LA‐C, and LA‐D esophagitis, respectively.
Table 2.
Reflux characteristics according to chronic cough status
|
GERD‐CC group (n = 31) |
GERD group (n = 47) |
P value | |
|---|---|---|---|
| Reflux symptom scores | 6.4 ± 2.4 | 4.6 ± 3.1 | .007 |
| NERD (%) | 80.6 | 72.3 | .400 |
| Esophageal acid reflux | |||
| AET (min) | 69.9 (30.6‐128.7) | 27.7 (4.3‐78.9) | .030 |
| >6% (%, n) | 25.8% (8) | 29.8% (14) | .702 |
| 4%‐6% (%, n) | 32.3% (10) | 17.0% (8) | .675 |
| <4% (%, n) | 41.9% (13) | 53.2% (25) | .330 |
| Episodes of acid reflux | 39.0 (21.0‐61.0) | 19.5 (7‐34.5) | .020 |
| Episodes of prolonged acid reflux | 2.0 (0‐5.0) | 1.0 (0‐4.0) | .301 |
| Longest reflux episode (min) | 10.0 (4.8‐17.9) | 6.8 (2.0‐13.3) | .102 |
| DeMeester score | 15.4 (6.1‐27.7) | 7.1 (2.1‐17.0) | .070 |
| Total episodes of refluxa | 143.0 (104.0‐211.0) | 103.0 (68.0‐141.0) | .008 |
| Distal extent | 111.0 (89.0‐162.8) | 92.0 (52.8‐123.3) | .084 |
| Proximal extent | 15.0 (6.0‐28.5) | 8.5 (5.0‐18.0) | .028 |
| High extent | 1.0 (0.75‐3.25) | 1.0 (0‐2.25) | .385 |
Data are presented as the median and interquartile range or percentage (number).
Abbreviations: AET, acid exposure time; GERD, gastroesophageal reflux disease; GERD‐CC, gastroesophageal reflux disease with chronic cough.
All reflux episodes detected by impedance per patient.
Patients in the GERD‐CC group had significantly longer AET (P = .03), more frequent acid reflux episodes (P = .02) and higher DeMeester scores (P = .07) than those in the GERD group (Table 2). However, there was no significant difference in AET >6% (25.8% vs 29.8%), AET <4%, or AET 4%‐6% between the two groups (Table 2). Even though the rates of AET >6% were relatively low in both groups, more than 80 reflux episodes were detected by impedance in those patients with AET <6%, indicating that all enrolled patients had objective evidences of gastroesophageal reflux.
According to pH‐impedance, patients in the GERD‐CC group had significantly more reflux episodes and proximal reflux episodes than those in the GERD group (both P < .05, Table 2). There were no significant differences in numbers of distal reflux (P = .084) or high‐reflux episodes between the two groups. The refluxates were acidic in 63.3% of high‐reflux episodes in the GERD‐CC group, which is significantly higher than 49.2% in the GERD group (P = .005, Figure 3). However, the percentage of acidity in distal and proximal reflux did not differ markedly between the two groups (P = .73 and P = .84, respectively).
Figure 3.
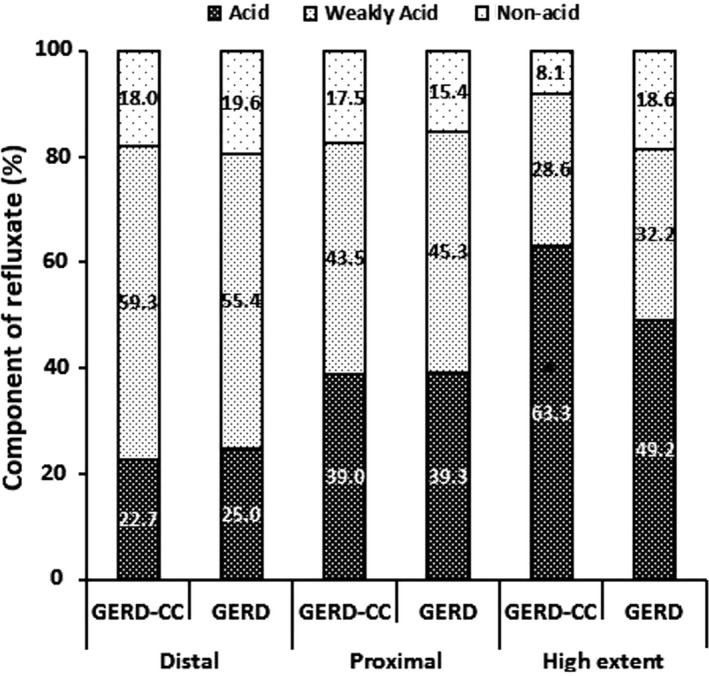
Component of refluxate according to reflux extent according to chronic cough status. GERD: gastroesophageal reflux disease, GERD‐CC: gastroesophageal reflux disease with chronic cough. * P < .05 compared with GERD group
3.3. Esophageal motility according to CC status
Synchronous ambulatory esophageal manometry detected 366 peristalsis waves during 78 prolonged acid reflux episodes. In the GERD‐CC group, 145 primary peristalsis and 67 secondary peristalsis waves occurred during the longest acid reflux episodes of each patient, whereas in the GERD group 110 primary peristalsis and 44 secondary peristalsis waves occurred during the longest acid episodes (Table 3). IEM, presenting as low pressure in both distal and proximal esophagus, occurred significantly more commonly during primary peristalsis (Figure 4A) in the GERD‐CC than in the GERD group (P < .001, Table 3), whereas low pressure in the distal esophagus was more common in the GERD group (P < .01). As for secondary peristalsis, IEM, mostly presenting as low pressure in the distal esophagus, was more common in the GERD than GERD‐CC group; however, in the GERD‐CC group, 63.9% of IEM presented as synchronous contraction (Figure 4B), significantly more frequently than in the GERD group (9.1%, P < .001).
Table 3.
Features of ambulatory esophageal motility during prolonged acid exposure in GERD‐CC and GERD groups
|
GERD‐CC group (n = 31) |
GERD group (n = 47) |
P value | |
|---|---|---|---|
| Primary peristalsis | 145 | 110 | |
| Ineffective peristalsis (%) | 104 (71.7%) | 68 (61.8%) | .095 |
| Distal low pressure (%) | 28 (26.9%) | 33 (48.5%) | .004 |
| Proximal low pressure (%) | 8 (7.7%) | 3 (4.4%) | .390 |
| Distal and proximal low pressure (%) | 40 (38.5%) | 8 (11.8%) | .000 |
| Synchronous contraction (%) | 28 (26.9%) | 24 (35.3%) | .243 |
| Secondary peristalsis | 67 | 44 | |
| Ineffective peristalsis (%) | 47 (70.1%) | 39 (88.6%) | .023 |
| Distal low pressure (%) | 17 (36.1%) | 35 (90.9%) | .000 |
| Proximal low pressure (%) | 0 | 0 | – |
| Synchronous contraction (%) | 30 (63.9%) | 4 (9.1%) | .000 |
Figure 4.
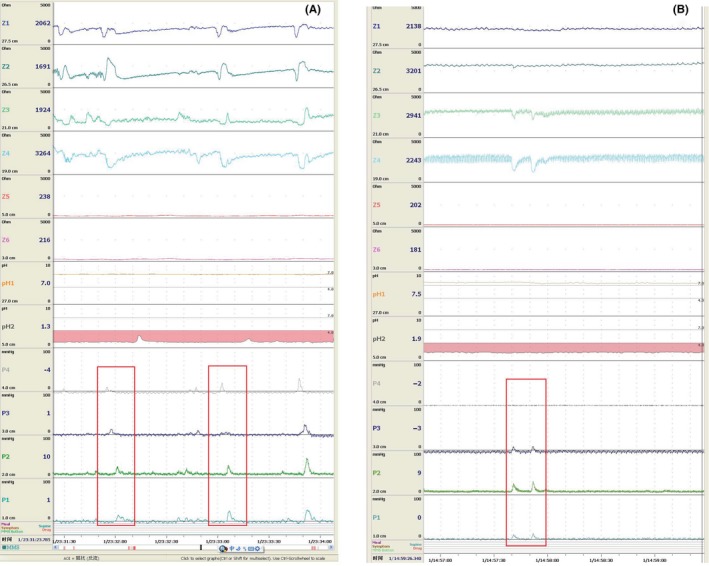
The ambulatory esophageal pH‐impedance‐pressure monitoring tracings show the low pan‐esophageal pressure of primary peristalsis (A) in a GERD‐CC patient and synchronous contraction of secondary peristalsis in a GERD patient (B). Abbreviations: GERD, gastroesophageal reflux disease; GERD‐CC, gastroesophageal reflux disease with chronic cough. Note: The typical low pan‐esophageal pressure waves and a synchronous contraction are marked with red frame
3.4. Characteristics of reflux‐induced cough episodes
Monitoring of pH‐impedance‐pressure detected 206 coughing paroxysms, 126 (61.2%) of which occurred within two minutes of a reflux episode. Sixty‐three of these episodes were reflux‐cough episodes and 63 cough‐reflux episodes. All 63 reflux‐cough episodes occurred in 23/31 patients (74.2%) in the GERD‐CC group. Fourteen of the 23 patients with reflux‐cough episodes also had cough‐reflux episodes and 10 of these unrelated reflux and cough. Additionally, nine other also had unrelated reflux and cough. Three patients only had cough‐reflux episodes and five had no coughing paroxysms during the 24 hours of monitoring.
Among 63 reflux‐cough episodes, 54.0% of the reflux episodes were acidic, 36.5% weakly acidic, and 9.5% non‐acidic. Furthermore, 74.6% reflux‐cough episodes resulted from distal extent reflux, 48.9% of these episodes being associated with acidic reflux, 40.4% with weakly acidic reflux, and 10.6% with non‐acidic reflux. Additionally, 23.8% reflux‐cough episodes resulted from proximal extent reflux, 66.7% of these episodes being associated with acidic reflux, 26.7% with weakly acidic reflux, and 6.6% with non‐acidic reflux. Only one reflux‐episode was associated with acidic high‐extent reflux (Figure 5).
Figure 5.
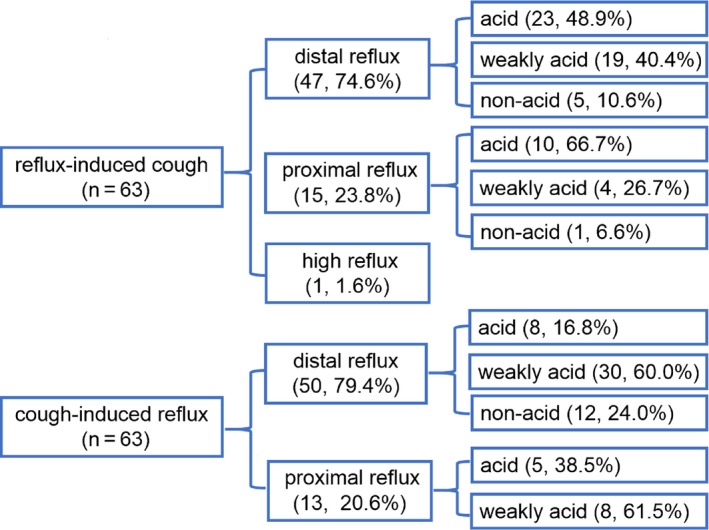
Characteristics of reflux in reflux‐induced cough and cough‐induced reflux episodes in patients with GERD‐CC. Note: n refers to the number of reflux‐induced coughing paroxysms or cough‐induced reflux episodes, not number of patients; values in the figures refer to the number of episodes and percentage (n, %)
Also, we monitored 16 reflux‐cough episodes in total of 823 proximal/high reflux while 47 reflux‐cough episodes in total of 4120 distal reflux (2.00% vs. 1.14%, P < .05).
3.5. Characteristics of cough‐induced reflux episodes
We found that 20.6% of the 63 cough‐reflux episodes that induced reflux were acidic reflux, 60.3% weakly acidic, and 19.1% non‐acidic. Furthermore, 79.4% reflux episodes were distal extent reflux, 60.0% of these being weakly acidic. Additionally, 20.6% episodes were proximal extent reflux, 61.5% of the refluxates being weakly acidic. No high‐extent reflux was recorded as associated with cough (Figure 5).
4. DISCUSSION
The relationship between gastroesophageal reflux and chronic cough is complex. Although there have been many studies and consensus has been reached on reflux‐cough syndrome, it is still difficult to diagnose reflux‐induced cough in an individual. Most previous studies19, 20, 21, 22 have focused on individuals with unexplained coughs and investigated the effects of reflux on cough. In contrast, we enrolled patients with GERD in our study and explored the differences between those with and without cough. We found that patients with GERD‐CC had more severe reflux episodes and proximal extent reflux than did GERD patients without CC, and that most high‐extent reflux is acidic. Proximal acid reflux and distal reflux jointly contribute to inducing reflux‐induced coughing in patients with GERD. Esophageal dysmotility, especially pan‐esophageal low pressure during primary peristalsis and synchronous contraction during secondary peristalsis, play important roles in GERD‐associated CC.
One important hypothesis concerning the mechanism of reflux‐induced cough is that proximal reflux and micro‐aspiration of gastric refluxate stimulate coughing by direct irritation of the respiratory tract. Previous studies have found that proximal acid reflux30 and aspiration of gastric contents (pepsin, bile acid, or lipid‐laden macrophages)31, 32, 33 can induce CC events. A recent study also found that volume clearance time and reflux burden play key roles in inducing coughing.20 However, other studies7, 34 found no difference between patients with CC and controls in proximal reflux events or bronchoalveolar lavage (BAL) pepsin or bile acids. Most studies have focused on the characteristics of reflux in patients with CC, whereas in our study we compared the characteristics of reflux in patients with GERD‐CC. A new finding of our study was that coughing paroxysms induced by proximal and high‐extent reflux occurred in 6/31 (19.4%) of patients with GERD‐CC and in 6/23 (26.1%) of reflux‐cough patients, most associated refluxate being acidic. We also found 16 reflux‐cough episodes in total of 823 proximal/high reflux, the percentage was higher than reflux‐cough episodes in distal reflux. These findings provide more detailed supportive evidence for acidic reflux of proximal and high‐extent inducing coughing in patients with GERD.
Another hypothesis for reflux‐cough episodes is stimulation of a vagal esophagobronchial reflex by reflux, triggering the cough reflex. Saline and acid infusion studies35, 36 have shown that cough frequency and amplitude are greater with acid than saline; infusion of acid into the esophagus increases cough sensitivity in patients with GERD and cough. Also, decreases in distal reflux and coughs after anti‐GERD therapy in patients with unexplained cough supports the distal‐reflux reflex mechanism.37 However, there is little evidence for transient distal reflux inducing cough or the associated reflux characteristics. We found distal reflux contributed to 74.6% of reflux‐cough episodes, the refluxate being acidic in almost half and weakly acidic in 40.4%. We also tried to compare additional characteristics of distal reflux that did or did not cause cough, but failed because of the huge difference in frequency of these episodes (47 distal refluxes causing cough vs. 3735 distal refluxes not causing cough).
Esophageal dysmotility is another important mechanism in GERD. Ineffective esophageal motility38, 39 and large breaks10, 40 are associated with reflux‐cough events. Meanwhile, long‐term exposure to acid is negatively correlated with esophageal body motility.41, 42 To the best of our knowledge, few studies have investigated esophageal motility during long‐term acid reflux in individuals with CC and GERD. We found that most primary and secondary peristalsis is ineffective (61.8%‐88.6%), low pressure amplitude in both the distal and proximal esophagus results in 38.5% IEM of primary peristalsis, and more synchronous contractions in secondary peristalsis result in IEM in patients with GERD‐CC. Those findings are consistent with impairment of primary and secondary peristalsis leading to ineffective esophageal clearance and prolonged exposure to acid.43, 44 Thus, we might extrapolate that low pan‐esophageal pressure amplitude in primary peristalsis and synchronous contraction in secondary peristalsis have important effects on reflux‐induced cough in patients with GERD‐CC.
Combining esophageal pH‐impedance and manometry monitoring is a proven diagnostic tool for identifying reflux and cough and guiding treatment of patients with reflux‐cough.7, 19, 20, 21, 22, 45, 46 We used a time window of two minute as indicating a temporal association between reflux episodes and coughing paroxysms.7, 19, 20, 21, 22, 45, 46 We found that 74.2% (23/31) of patients with GERD‐CC have reflux‐induced cough, 45.2% (14/31) having both reflux‐cough episodes and cough‐reflux episodes and 9.7% (3/31) having only cough‐reflux episodes. No coughing was recorded in 16.1% of patients with GERD‐CC. Distal reflux and weakly acidic reflux were more common in cough‐reflux events. The results of monitoring provided strong evidence that proton pump inhibitors (PPIs) are the optimal therapy for patients with GERD‐CC and cough caused by reflux, that is, adequate doses of more potent PPIs and prolonged treatment are indicated. As for reflux caused by cough, comprehensive antitussive measures might be more effective than overuse of PPIs whereas, if available, a prokinetic (ie, mosapride or prucalopride) may be indicated for patients with esophageal dysmotility (ie, IEM).26, 47
Our study has several limitations. Less than expected high‐extent reflux was detected, this discrepancy possibly being attributable to the high position of impedance loops (26 cm above the LES being defined as high extent in our study as compared with 15 cm above the LES being considered proximal, but not high‐extent reflux in a previous study).30 The highest pressure sensor was located 20 cm above LES, which could not detect swallows directly, so we used the impedance curve to identify the primary peristalsis. Another limitation was the relatively small sample size and small number of coughing paroxysms during which we recorded dynamic esophageal peristalsis. We only analyzed esophageal motility during the longest acid reflux episode of each patient, not during all prolonged episodes of long acid reflux. Moreover, we did not record and analyze self‐reports of cough symptom by patients; however, there have been some studies on the relationship between cough symptoms and reflux events.14, 15
5. CONCLUSIONS
In this study comparing patients with GERD with and without CC, we found that those with GERD‐CC had more severe reflux episodes and proximal extent reflux and that most high‐extent reflux was acidic. Proximal acid reflux and distal reflux‐reflex jointly contributed to occurrence of reflux‐induced cough in patients with GERD. Low pan‐esophageal pressure during primary peristalsis and synchronous contraction during secondary peristalsis during prolonged acid reflux play important roles in patients with GERD and CC. Thus, ambulatory pH‐impedance‐pressure monitoring may provide diagnostic and therapeutic evidence in patients who have failed PPI therapy, assisting optimization of PPI and/or indicating addition of prokinetic therapy in those with GERD‐CC and obvious dysmotilities, thus enhancing the integrated treatment in this subset of patients.
CONFLICT OF INTEREST
No competing interests declared.
AUTHOR CONTRIBUTIONS
XL and XF designed the study; SL and ZW performed pH‐impedance‐pressure monitoring; XL, HZ, XS, JL, DW, and XF enrolled patients and collected clinical data; XL and SL analyzed data and wrote the manuscripts; MK supervised and critically reviewed the manuscript; XF revised and finally approved the manuscript. All authors approved the final version of the manuscript. The abstract was presented at Digestive Disease Week, 2016 (Li X, Lin S, Wang Z, et al Gastroesophageal reflux disease and chronic cough: A possible mechanism elucidated by ambulatory pH‐impedance‐pressure monitoring. Gastroenterology 2016,150, 4 [Suppl 1]:S269).
ACKNOWLEDGMENTS
We thank Dr Trish Reynolds, MBBS, FRACP, from Edanz Group for language editing a draft of this manuscript.
Li X, Lin S, Wang Z, et al. Gastroesophageal reflux disease and chronic cough: A possible mechanism elucidated by ambulatory pH‐impedance‐pressure monitoring. Neurogastroenterol Motil. 2019;31:e13707 10.1111/nmo.13707
Xiaoqing Li and Sihui Lin equally contributed to this study.
Funding information
This study was funded by grants from the National High Technology Research and Development Program of China (No. 2010AA023007), Fund of Janssen Research Council (JRCC2015G103), and Youth Research Fund of Peking Union Medical College Hospital (pumch‐2013‐131).
REFERENCES
- 1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am J Gastroenterol. 2006;101:1900‐1920. [DOI] [PubMed] [Google Scholar]
- 2. Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364‐1374. [DOI] [PubMed] [Google Scholar]
- 3. Xu X, Yu LI, Chen Q, Lv H, Qiu Z. Diagnosis and treatment of patients with nonacid gastroesophageal reflux‐induced chronic cough. J Res Med Sci. 2015;20:885‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park HJ, Park YM, Kim J‐H, et al. Effectiveness of proton pump inhibitor in unexplained chronic cough. PLoS ONE. 2017;12:e0185397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu LI, Xu X, Hang J, et al. Efficacy of sequential three‐step empirical therapy for chronic cough. Ther Adv Respir Dis. 2017;11:225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mainie I, Tutuian R, Agrawal A, et al. Fundoplication eliminates chronic cough due to non‐acid reflux identified by impedance pH monitoring. Thorax. 2005;60:521‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sifrim D, Dupont L, Blondeau K, et al. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut. 2005;54:449‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fornari F, Blondeau K, Durand L, et al. Relevance of mild ineffective oesophageal motility (IOM) and potential pharmacological reversibility of severe IOM in patients with gastro‐oesophageal reflux disease. Aliment Pharmacol Ther. 2007;26:1345‐1354. [DOI] [PubMed] [Google Scholar]
- 9. Simren M, Silny J, Holloway R, et al. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almansa C, Smith JA, Morris J, et al. Weak peristalsis with large breaks in chronic cough: association with poor esophageal clearance. Neurogastroenterol Motil. 2015;27:431‐442. [DOI] [PubMed] [Google Scholar]
- 11. Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. 2018;67:1351‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahrilas PJ, Altman KW, Chang AB, et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest. 2016;150:1341‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough‐reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139:754‐762. [DOI] [PubMed] [Google Scholar]
- 15. Qiu Z, Yu LI, Xu S, et al. Cough reflex sensitivity and airway inflammation in patients with chronic cough due to non‐acid gastro‐oesophageal reflux. Respirology. 2011;16:645‐652. [DOI] [PubMed] [Google Scholar]
- 16. Sifrim D, Castell D, Dent J, et al. Gastro‐oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non‐acid, and gas reflux. Gut. 2004;53:1024‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weusten BL, Roelofs J, Akkermans L, Van Berge‐Henegouwen GP, Smout AndréJPM. The symptom‐association probability: an improved method for symptom analysis of 24‐hour esophageal pH data. Gastroenterology. 1994;107:1741‐1745. [DOI] [PubMed] [Google Scholar]
- 18. Taghavi SA, Ghasedi M, Saberi‐Firoozi M, et al. Symptom association probability and symptom sensitivity index: preferable but still suboptimal predictors of response to high dose omeprazole. Gut. 2005;54:1067‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herregods T, Pauwels A, Jafari J, et al. Ambulatory pH‐impedance‐pressure monitoring as a diagnostic tool for the reflux‐cough syndrome. Dis Esophagus. 2018;31:1‐7. [DOI] [PubMed] [Google Scholar]
- 20. Herregods T, Pauwels A, Jafari J, et al. Determinants of reflux‐induced chronic cough. Gut. 2017;66:2057‐2062. [DOI] [PubMed] [Google Scholar]
- 21. Herregods T, Pauwels A, Tack J, Smout A, Bredenoord AJ. Reflux‐cough syndrome: assessment of temporal association between reflux episodes and cough bursts. Neurogastroenterol Motil. 2017;29(12):e13129. [DOI] [PubMed] [Google Scholar]
- 22. Rosen R, Amirault J, Giligan E, Khatwa U, Nurko S. Intraesophageal pressure recording improves the detection of cough during multichannel intraluminal impedance testing in children. J Pediatr Gastroenterol Nutr. 2014;58:22‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106‐1110. [DOI] [PubMed] [Google Scholar]
- 24. Johnson LF, DeMeester TR. Development of the 24‐hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol. 1986;8(suppl 1):52‐58. [DOI] [PubMed] [Google Scholar]
- 25. Chen CL, Yi CH, Liu TT. Influence of intraluminal acidification on esophageal secondary peristalsis in humans. Dig Dis Sci. 2013;58:1948‐1954. [DOI] [PubMed] [Google Scholar]
- 26. Lei W‐Y, Hung J‐S, Liu T‐T, Yi C‐H, Chen C‐L. Influence of prucalopride on esophageal secondary peristalsis in reflux patients with ineffective motility. J Gastroenterol Hepatol. 2018;33:650‐655. [DOI] [PubMed] [Google Scholar]
- 27. Cho YK, Choi M‐G, Lim CH, et al. Impaired esophageal bolus transit in patients with gastroesophageal reflux disease and abnormal esophageal acid exposure. Gut Liv. 2012;6(4):440‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kahrilas PJ, Dodds WJ, Hogan WJ, et al. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94:73‐80. [DOI] [PubMed] [Google Scholar]
- 29. Schoeman MN, Holloway RH. Integrity and characteristics of secondary oesophageal peristalsis in patients with gastro‐oesophageal reflux disease. Gut. 1995;36:499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee J‐H, Park S‐Y, Cho S‐B, et al. Reflux episode reaching the proximal esophagus are associated with chronic cough. Gut Liv. 2012;6:197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grabowski M, Kasran A, Seys S, et al. Pepsin and bile acids in induced sputum of chronic cough patients. Respir Med. 2011;105:1257‐1261. [DOI] [PubMed] [Google Scholar]
- 32. Özdemir P, Erdinç M, Vardar R, et al. The role of microaspiration in the pathogenesis of gastroesophageal reflux‐related chronic cough. J Neurogastroenterol Motil. 2017;23:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farrell S, McMaster C, Gibson D, Shields MD, McCallion WA. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastro‐oesophageal reflux‐related pulmonary aspiration. J Pediatr Surg. 2006;41:289‐293. [DOI] [PubMed] [Google Scholar]
- 34. Decalmer S, Stovold R, Houghton LA, et al. Chronic cough: relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest. 2012;142:958‐964. [DOI] [PubMed] [Google Scholar]
- 35. Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149:160‐167. [DOI] [PubMed] [Google Scholar]
- 36. Javorkova N, Varechova S, Pecova R, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro‐oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20:119‐124. [DOI] [PubMed] [Google Scholar]
- 37. Irwin RS, Zawacki JK, Curley FJ, French CL, Hoffman PJ. Chronic cough as the sole presenting manifestation of gastroesophageal reflux. Am Rev Respir Dis. 1989;140:1294‐1300. [DOI] [PubMed] [Google Scholar]
- 38. Fouad YM, Katz PO, Hatlebakk JG, Castell DO. Ineffective esophageal motility: the most common motility abnormality in patients with GERD‐associated respiratory symptoms. Am J Gastroenterol. 1999;94:1464‐1467. [DOI] [PubMed] [Google Scholar]
- 39. Knight RE, Wells JR, Parrish RS. Esophageal dysmotility as an important co‐factor in extraesophageal manifestations of gastroesophageal reflux. Laryngoscope. 2000;110:1462‐1466. [DOI] [PubMed] [Google Scholar]
- 40. Bennett MC, Patel A, Sainani N, Wang D, Sayuk GS, Gyawali CP. Chronic cough is associated with long breaks in esophageal peristaltic integrity on high‐resolution manometry. J Neurogastroenterol Motil. 2018;24:387‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang L, Ye B, Wang M, et al. Acid exposure in patients with gastroesophageal reflux disease is associated with esophageal dysmotility. J Dig Dis. 2019;1: 10.1111/1751-2980. [DOI] [PubMed] [Google Scholar]
- 42. Jiang L, Ye B, Wang Y, Wang M, Lin L. Esophageal body motility for clinical assessment in patients with refractory gastroesophageal reflux symptoms. J Neurogastroenterol Motil. 2017;23:64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aben‐Athar CG, Dantas RO. Primary and secondary esophageal contractions in patients with gastroesophageal reflux disease. Braz J Med Biol Res. 2006;39:1027‐1031. [DOI] [PubMed] [Google Scholar]
- 44. Iwakiri K, Hayashi Y, Kotoyori M, et al. Defective triggering of secondary peristalsis in patients with non‐erosive reflux disease. J Gastroenterol Hepatol. 2007;22:2208‐2211. [DOI] [PubMed] [Google Scholar]
- 45. Blondeau K, Dupont LJ, Mertens V, Tack J, Sifrim D. Improved diagnosis of gastro‐oesophageal reflux in patients with unexplained chronic cough. Aliment Pharmacol Ther. 2007;25:723‐732. [DOI] [PubMed] [Google Scholar]
- 46. Blondeau K, Mertens V, Dupont L, et al. The relationship between gastroesophageal reflux and cough in children with chronic unexplained cough using combined impedance‐pH‐manometry recordings. Pediatr Pulmonol. 2011;46:286‐294. [DOI] [PubMed] [Google Scholar]
- 47. Chen C‐L, Yi C‐H, Liu T‐T, Orr WC. Effects of mosapride on secondary peristalsis in patients with ineffective esophageal motility. Scand J Gastroenterol. 2013;48:1363‐1370. [DOI] [PubMed] [Google Scholar]


