Abstract
Background
This study compared the efficacy and safety of lixisenatide with placebo as add‐on therapy to basal insulin (BI) in adults aged ≥70 years with type 2 diabetes (T2D), with or without moderate renal insufficiency.
Methods
This post hoc analysis evaluated data from non‐frail patients with T2D inadequately controlled on BI with or without oral antidiabetic drugs (n = 108), randomized to once‐daily lixisenatide 20 μg or placebo for 24 weeks (GetGoal‐O Study). The primary endpoint was the change in HbA1c from baseline to Week 24. Secondary endpoints included changes from baseline in fasting plasma glucose, 2‐hour postprandial plasma glucose (PPG), average seven‐point self‐monitored plasma glucose (SMPG), area under the curve for SMPG, daily BI dose, body weight, proportion of patients achieving HbA1c > 0.5%, and composite endpoints. Safety outcomes included the incidence of documented symptomatic hypoglycemia (plasma glucose <60 mg/dL) and gastrointestinal treatment‐emergent adverse events (TEAEs). Outcomes were also analyzed by the occurrence of moderate renal insufficiency.
Results
Compared with placebo, lixisenatide‐treated patients had significantly greater reductions in HbA1c, 2‐hour PPG, average seven‐point SMPG, and body weight. Documented symptomatic hypoglycemia was approximately two‐fold higher in patients treated with placebo than lixisenatide (12.7% vs 5.7%). GI TEAEs occurred more frequently in the lixisenatide‐ than placebo‐treated group (34% vs 9.1%). Moderate renal insufficiency (estimated glomerular filtration rate between ≥30 and <60 mL/min/1.73 m2) did not negatively affect lixisenatide efficacy or safety. A greater proportion of patients treated with lixisenatide than placebo achieved composite endpoints.
Conclusions
Add‐on therapy with lixisenatide in non‐frail patients aged ≥70 years with T2D uncontrolled with BI is effective, safe, and well tolerated and should be considered in this population.
Keywords: lixisenatide, older adults, type 2 diabetes
Highlights
This post hoc analysis of the GetGoal‐O trial suggests that add‐on therapy with lixisenatide in non‐frail patients aged ≥70 years with type 2 diabetes uncontrolled on basal insulin is safe and well tolerated and should be considered in this patient population as an alternative to rapid‐acting insulin. A fixed‐ratio combination of lixisenatide and insulin glargine U100 (iGlarLixi) is available.
The results suggest similar efficacy for lixisenatide in patients with moderate renal insufficiency without dose adjustment, with monitoring always advisable in this population
摘要
背景
这项研究在伴有或不伴中度肾功能不全的≥70岁2型糖尿病(type 2 diabetes, T2D)成人患者中, 比较了利西那肽与安慰剂作为基础胰岛素(basal insulin, BI)添加治疗的疗效及安全性。
方法
这项事后分析所评估的数据来自经BI联合或者不联合口服降糖药治疗后血糖仍控制不佳的非脆性T2D患者(n=108), 将其随机分配至每日一次20 μg利西那肽治疗组或安慰剂组, 治疗24周(GetGoal‐O研究)。主要终点为从基线至第24周的HbA1c变化。次要终点包括空腹血糖、餐后2小时血糖、平均7点自我监测血糖(self‐monitored plasma glucose, SMPG)、SMPG曲线下面积、每日BI剂量、体重、HbA1c降幅>0.5%的患者比例以及复合终点相对于基线的变化。安全性结局包括记录的症状性低血糖(血糖<60 mg/dl)及治疗期间胃肠道不良事件(treatment‐emergent adverse events, TEAEs)的发生率。还通过中度肾功能不全的发生对结果进行了分析。
结果
与安慰剂组相比, 利西那肽治疗组的HbA1c、餐后2小时血糖、平均7点SMPG以及体重下降均更显著。安慰剂组中记录的症状性低血糖发生率大约是利西那肽治疗组患者的2倍(分别为12.7%与5.7%)。利西那肽治疗组的胃肠道TEAEs发生率高于安慰剂组(分别为34%与9.1%)。中度肾功能不全(估算的肾小球滤过率≥30至<60 ml/min/1.73 m2)不会对利西那肽的疗效或安全性产生不良影响。利西那肽治疗组达到复合终点的患者比例高于安慰剂组。
结论
在≥70岁、既往使用BI治疗后血糖仍控制不佳的非脆性T2D患者中, 加用利西那肽治疗安全有效, 而且耐受性良好, 今后在这类人群中可考虑应用这种治疗方法。
Keywords: 利西那肽, 老年人, 2型糖尿病。
1. INTRODUCTION
Throughout the world, the prevalence of type 2 diabetes (T2D) is high among the older population.1 Despite this, older adults with T2D are often excluded from clinical trials. As a consequence, guidelines for pharmacological management of these patients are generally based on data extrapolated from studies in the general population.2, 3 However, diabetes in this population is often metabolically distinct from that of younger people.4 Furthermore, choice of antihyperglycemia therapy may be reduced in older people due to decline in renal function and changes to hepatic drug metabolism.5 The International Diabetes Federation1 and the American Diabetes Association (ADA)6 have both developed standards of care specifically for older people with T2D, recommending individualized treatment regimens with less stringent treatment goals compared with those for younger people with T2D. The recently published ADA/European Association for the Study of Diabetes (EASD) consensus statement does not discuss older adults specifically; however, it does recognize the importance of a patient‐centered approach of glycemic management in T2D, taking the individual's circumstances into account when formulating a treatment strategy.7
Basal insulin therapy can be effective in improving glycemic control in patients with T2D uncontrolled on oral antidiabetic drugs (OADs).8 However, a US clinical practice survey showed that on average only 38% of people with T2D achieve an HbA1c level < 7.0% with basal insulin alone during the first year of treatment.9 In adults of any age with T2D, ADA standard‐of‐care guidelines recommend combination therapy if basal insulin alone has been titrated to achieve acceptable fasting plasma glucose (FPG) levels or if the dose is >0.5 units/kg/d, yet is insufficient to control HbA1c.6 The recently published ADA/EASD consensus statement recommends glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs) as the first injectable before initiating basal insulin specifically, with the exception of patients with symptoms of hyperglycemia, evidence of autoimmune β‐cell destruction, or HbA1c ≥11%.7 In patients on dual or triple therapy unable to achieve goals, the position statement recommends initiation with a combination of an GLP‐1 RA and basal insulin if HbA1c is 2% over goals.7 In patients uncontrolled on basal insulin, therapeutic intensification of basal insulin with either a GLP‐1 RA, transition to an fixed‐ratio combination injectable (eg, basal insulin + GLP‐1 RA), or the addition of a rapid‐acting insulin is recommended.7
Despite these recommendations, clinical inertia, defined as the failure to intensify treatment when required, is common in clinical practice in patients with T2D.10, 11, 12, 13, 14, 15, 16, 17 In a UK study of patients treated with basal insulin who were clinically eligible for treatment intensification, only 31% had their treatment intensified, with a median time to intensification of 3.7 years.11 Delay in treatment intensification in patients with uncontrolled T2D has been shown to have a number of clinical consequences, including a significantly increased risk of myocardial infarction, heart failure, stroke, and a composite endpoint of cardiovascular events,18 as well as a higher incidence of (and significantly shorter median time to) progression of diabetic retinopathy.19 This progression of diabetes‐related complications with lack of treatment intensification in the overall T2D population is also seen in older adults.20, 21, 22
Many older adults require multiple medications; however, it has been reported that approximately 50% of older adults take at least one unneeded medication, with potentially harmful consequences.23 Therefore, it is important to ensure that older adults are only prescribed medications they need. Due to pathophysiological differences, blood glucose management for older people with T2D will most likely vary from that in younger patients and should be individualized to avoid prescribing unneeded medications. Notably, in older adults, the relative contribution of postprandial plasma glucose (PPG) to overall HbA1c appears to be higher than that of FPG.24
Lixisenatide (Adlyxin; Sanofi US, Bridgewater, New Jersey) is a once‐daily GLP‐1 RA that enhances glucose‐dependent insulin secretion and slows gastric emptying, resulting in a reduction in postprandial glucose exposure and a reduction in postprandial glucagon.25, 26, 27 The efficacy and safety of once‐daily lixisenatide (10 μg for 2 weeks, then 20 μg) in the treatment of T2D have been demonstrated in the global GetGoal Phase 3 clinical trials program, which included more than 5000 patients using a variety of background medications of OADs and/or basal insulin.28, 29 In the Phase 3 GetGoal‐O trial, which specifically recruited patients aged ≥70 years, among 350 randomized patients lixisenatide added to existing antihyperglycemia therapy was superior to placebo in reducing HbA1c and in targeting postprandial hyperglycemia, and had a favorable tolerability profile.3
In this article we discuss the efficacy and safety outcomes of lixisenatide as add‐on therapy to basal insulin in a subset of 108 patients aged ≥70 years who were uncontrolled on basal insulin with or without OADs in the GetGoal‐O trial.3
2. METHODS
2.1. Study design and participants
This was a post hoc analysis of the GetGoal‐O trial (http://Clinicaltrials.gov ID NCT01798706), details of which have been published previously.3 GetGoal‐O was a randomized, double blind, placebo‐controlled, two‐arm, parallel‐group, multinational, multicenter clinical trial in patients aged ≥70 years. Post hoc data analyzed for this subgroup analysis were from a subgroup of non‐frail participants with T2D (age ≥ 70 years), uncontrolled on their current therapy (basal insulin with or without OADs). Patients were randomized to receive additional once‐daily lixisenatide 20 μg (after 2 weeks at 10 μg) or matching placebo (sterile aqueous solution) for 24 weeks. The efficacy and safety of once‐daily lixisenatide (20 μg) were compared with placebo in these patients. The efficacy of lixisenatide was also compared with placebo in patients with and without moderate renal insufficiency (estimated glomerular filtration rate [eGFR] ≥30 to <60 and ≥60 mL/min/1.73 m2, respectively). Patients with severe renal insufficiency (eGFR <30 mL/min/1.73 m2) were excluded from the trial.
For details on ethics compliance and patients' informed consent, see the previously published study results.3
2.2. Statistical analysis
For continuous data, such as change from baseline in HbA1c, an analysis of covariance (ANCOVA) model was used with treatment groups (lixisenatide, placebo), randomization strata of HbA1c (<8.0%, ≥8.0%), and eGFR (≥30 to <60, ≥60 mL/min/1.73 m2) at screening, as well as country as fixed effects, with baseline value as a covariate. Patients with both baseline and at least one post‐baseline measurement were included (modified intention‐to‐treat population). Last‐observation‐carried‐forward was used to handle missing data. Categorical analyses, such as the proportion of patients achieving HbA1c reduction >0.5% without body weight gain, were performed using a Cochran‐Mantel‐Haenszel method stratified on the randomization strata of HbA1c (<8.0%, ≥8.0%) and eGFR (≥30 to <60, ≥60 mL/min/1.73 m2) at screening. Patients with missing data at Week 24 were treated as non‐responders.
2.3. Endpoints
The primary endpoint was the absolute change in HbA1c from baseline to Week 24 for lixisenatide vs placebo. Secondary endpoints included the change from baseline to Week 24 for lixisenatide vs placebo for FPG, 2‐hour PPG after a standardized breakfast meal, average seven‐point self‐monitored plasma glucose (SMPG), area under the curve (AUC) for SMPG, eGFR, daily dose of basal insulin/body weight, and body weight change. The proportion of patients achieving an HbA1c reduction >0.5% was also analyzed. Safety outcomes included the incidence of documented symptomatic hypoglycemia (plasma glucose <60 mg/dL) and gastrointestinal (GI) treatment‐emergent adverse events (TEAEs). Composite endpoints included the proportion of patients achieving HbA1c reduction >0.5% with no documented symptomatic hypoglycemia, the proportion of patients achieving HbA1c reduction >0.5% without body weight gain, and the proportion of patients achieving HbA1c reduction >0.5% with no documented symptomatic hypoglycemia or body weight gain.
3. RESULTS
3.1. Patient demographics and baseline characteristics
In all, 108 patients met the inclusion criteria for this subgroup analysis, of whom 53 were in the lixisenatide group and 55 were in the placebo group. Baseline demographics and characteristics were generally similar in both treatment groups (Table 1) and to those in the overall study population.3 One difference of note was that, compared with the lixisenatide group, the placebo group had a slightly higher body mass index (BMI) at baseline, and a greater proportion of patients had a BMI ≥30 kg/m2 (Table 1). In addition, the mean duration of diabetes was higher in both the placebo (16.7 years) and lixisenatide (16.8 years) subgroups than in the total GetGoal‐O study population (14.6 and 13.6 years, respectively).3 Patients included in the basal insulin subgroup also had a higher incidence of renal impairment compared with the total study population (45.3% and 40.0% in the lixisenatide and placebo groups, respectively, compared with 28.4% and 27.0% in the same groups for the overall study population).3
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Basal insulin subgroup | Overall study population | ||
|---|---|---|---|---|
| Placebo (n = 55) | Lixisenatide (n = 53) | Placebo (n = 174) | Lixisenatide (n = 176) | |
| Age (y) | 73 (70‐87) | 73 (70‐85) | 73 (70‐88) | 73 (70–87) |
| Age group | ||||
| < 75 y | 34 (61.8) | 36 (67.9) | 105 (60.3) | 114 (64.8) |
| ≥ 75 y | 21 (38.2) | 17 (32.1) | 69 (39.7) | 62 (35.2) |
| Sex | ||||
| Male | 24 (43.6) | 31 (58.5) | 90 (51.7) | 92 (52.3) |
| Female | 31 (56.4) | 22 (41.5) | 84 (48.3) | 84 (47.7) |
| Race | ||||
| Caucasian | 38 (69.1) | 37 (69.8) | 122 (70.1) | 128 (72.7) |
| African American | 0 (0.0) | 1 (1.9) | 0 (0.0) | 3 (1.7) |
| Asian | 6 (10.9) | 3 (5.7) | 11 (6.3) | 5 (2.8) |
| Other | 11 (20.0) | 12 (22.6) | 41 (23.6) | 40 (22.7) |
| Ethnicity | ||||
| Hispanic | 13 (23.6) | 17 (32.1) | 48 (27.6) | 51 (29.0) |
| Non‐Hispanic | 42 (76.4) | 36 (67.9) | 126 (72.4) | 125 (71.0) |
| Body weight (kg) | 82.1 ± 18.8 | 79.2 ± 12.8 | 80.1 ± 16.8 | 80.8 ± 14.5 |
| BMI (kg/m2) | 31.0 ± 4.6 | 29.4 ± 3.4 | 30.1 ± 4.5 | 29.9 ± 3.7 |
| BMI category | ||||
| < 30 kg/m2 | 24 (43.6) | 34 (64.2) | 96 (55.2) | 102 (58.0) |
| ≥ 30 kg/m2 | 31 (56.4) | 19 (35.8) | 78 (44.8) | 74 (42.0) |
| Duration of diabetes (y) | 16.7 ± 7.5 | 16.8 ± 7.3 | 14.6 ± 7.9 | 13.6 ± 7.3 |
| Background therapy | ||||
| Metformin | 43 (78.2) | 40 (75.5) | 150 (87.2) | 152 (86.4) |
| Sulfonylurea | 1 (1.8) | 0 (0.0) | 59 (34.3) | 70 (39.8) |
| Meglitinide | 0 (0.0) | 0 (0.0) | 2 (1.2) | 1 (0.6) |
| Pioglitazone | 0 (0.0) | 0 (0.0) | 4 (2.3) | 1 (0.6) |
| Basal insulina | 54 (98.2) | 53 (100.0) | 55 (32.0) | 54 (30.7) |
| Insulin glargine | 29 (52.7) | 27 (50.9) | 29 (16.9) | 28 (15.9) |
| Insulin human injection, isophane | 17 (30.9) | 22 (41.5) | 17 (9.9) | 22 (12.5) |
| Insulin detemir | 6 (10.9) | 3 (5.7) | 7 (4.1) | 3 (1.7) |
| Insulin lispro protamine suspension | 0 (0.0) | 2 (3.8) | 0 (0.0) | 2 (1.1) |
| Insulin degludec | 1 (1.8) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Isophane insulin | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (0.6) |
| Biphasic insulin | 1 (1.8) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Duration of BI treatment (y) | 3.8 ± 3.4 | 4.3 ± 5.0 | 3.8 ± 3.4 | 4.3 ± 4.9 |
| Daily dose of BI (units) | 38.7 ± 22.8 | 37.7 ± 21.9 | 38.5 ± 22.6 | 37.6 ± 21.7 |
| Daily dose of BI/body weight (units/kg) | 0.48 ± 0.31 | 0.47 ± 0.23 | 0.48 ± 0.30 | 0.47 ± 0.23 |
| HbA1c (%) | 8.2 ± 0.7 | 8.2 ± 0.8 | 8.1 ± 0.7 | 8.0 ± 0.7 |
| FPG | ||||
| mM | 8.4 ± 2.5 | 8.5 ± 2.8 | 8.9 ± 2.3 | 8.8 ± 2.4 |
| mg/dL | 150.9 ± 44.6 | 153.0 ± 50.3 | 160.2 ± 40.8 | 159.1 ± 42.9 |
| 2‐h PPG | ||||
| mM | 15.5 ± 4.3 | 15.4 ± 4.2 | 14.9 ± 3.7 | 15.2 ± 3.8 |
| mg/dL | 279.4 ± 76.8 | 276.8 ± 75.1 | 268.0 ± 66.5 | 273.5 ± 68.1 |
| Average seven‐point SMPG | ||||
| mM | 10.4 ± 2.5 | 10.4 ± 2.4 | 10.0 ± 2.0 | 9.8 ± 2.0 |
| mg/dL | 187.0 ± 44.4 | 186.7 ± 43.6 | 179.5 ± 35.6 | 176.3 ± 36.4 |
| AUC of SMPGb | 2605.8 ± 589.1 | 2653.8 ± 608.1 | 2475.7 ± 463.9 | 2471.1 ± 500.8 |
| eGFR categories at baseline | ||||
| ≥30 to <60 mL/min/1.73 m2 | 22 (40.0) | 24 (45.3) | 47 (27.0) | 50 (28.4) |
| ≥60 mL/min/1.73 m2 | 33 (60.0) | 29 (54.7) | 127 (73.0) | 126 (71.6) |
Note: Data are given as the median (range), mean ± SD, or as n (%).
Abbreviations: AUC, area under the curve; BI, basal insulin; BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; PPG, postprandial glucose; SMPG, self‐monitored plasma glucose.
One patient whose randomization strata for BI use was “No” was not included in the BI subgroup analysis.
Calculated based on US units (mg/dL) using a nominal time point.
3.2. Lixisenatide dose
The median (range) doses of lixisenatide at baseline and Week 24 were 10.0 μg (8.6‐10.8 μg) and 20.0 μg (10.0‐20.0 μg), respectively. This is in line with the recommended dosing for lixisenatide of 10 μg once daily for 2 weeks, then 20 μg once daily.30
3.3. Efficacy outcomes
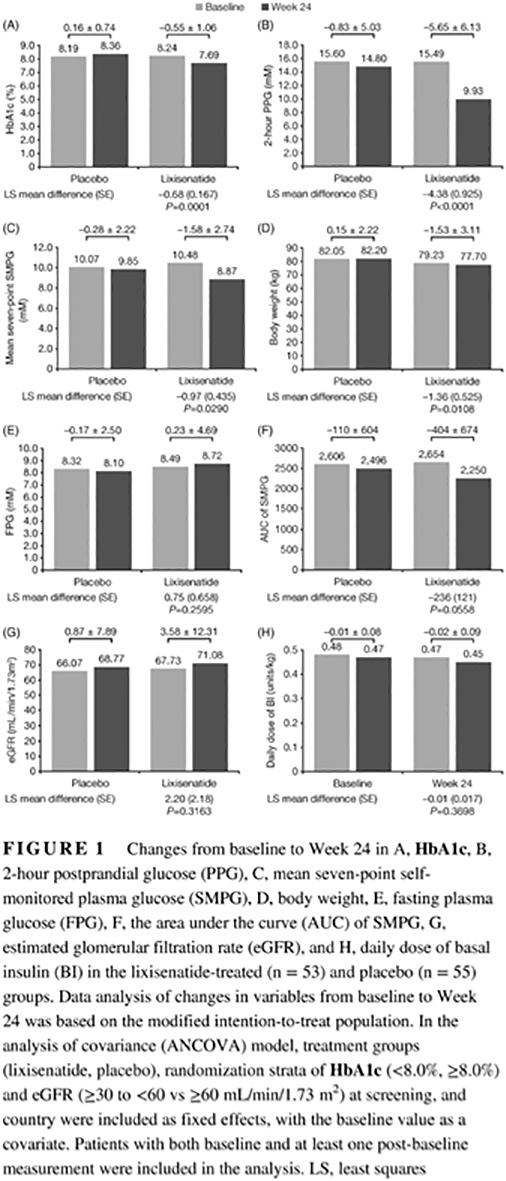
Lixisenatide was significantly better than placebo for achievement of the primary endpoint; at Week 24, lixisenatide therapy resulted in a significantly greater reduction in HbA1c compared with placebo: least squares (LS) mean difference (−0.68%, standard error [SE] 0.167%; P = 0.0001; Figure 1A). This was very similar in magnitude to the difference observed for the entire GetGoal‐O study population (LS mean difference − 0.64%; P < 0.0001).3 Patients randomized to lixisenatide also had significantly greater reductions in the secondary endpoints for 2‐hour PPG (P < 0.0001), average seven‐point SMPG (P = 0.0290), and body weight (P = 0.0108), with a trend toward significance for AUC SMPG at Week 24 compared with placebo (Figure 1). The efficacy findings from this subset analysis mirror the results from the GetGoal‐O study as a whole, where significant reductions were also observed with lixisenatide vs placebo in 2‐hour PPG (P < 0.0001), average seven‐point SMPG (P < 0.0001), and body weight (P < 0.0001).3
Figure 1.
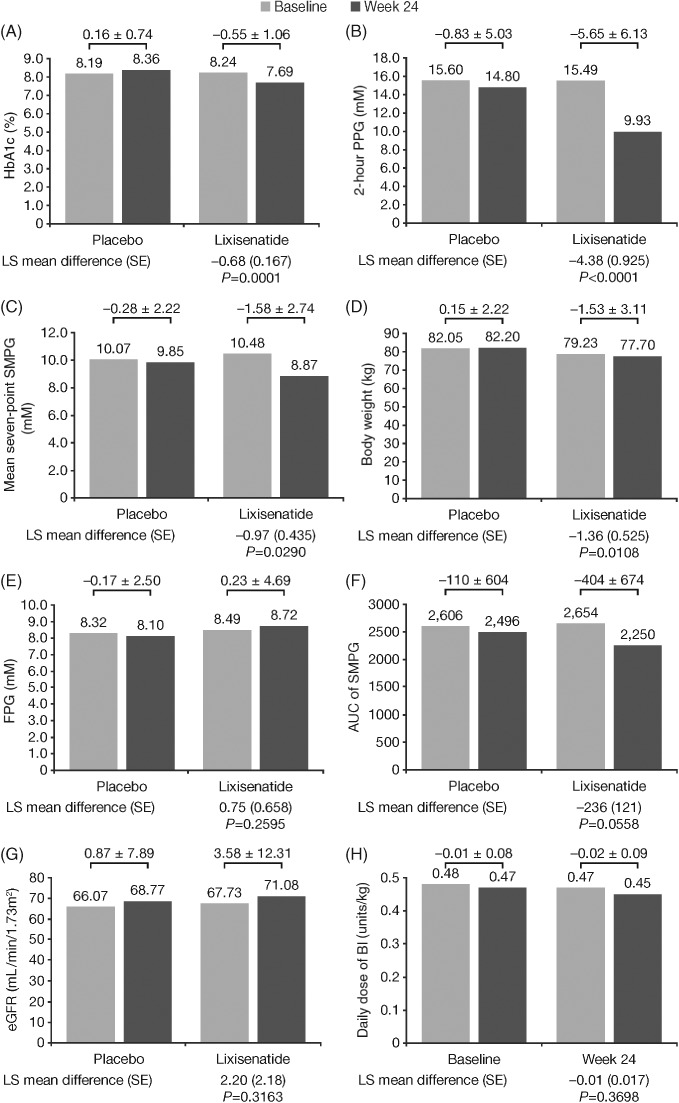
Changes from baseline to Week 24 in A, HbA1c, B, 2‐hour postprandial glucose (PPG), C, mean seven‐point self‐monitored plasma glucose (SMPG), D, body weight, E, fasting plasma glucose (FPG), F, the area under the curve (AUC) of SMPG, G, estimated glomerular filtration rate (eGFR), and H, daily dose of basal insulin (BI) in the lixisenatide‐treated (n = 53) and placebo (n = 55) groups. Data analysis of changes in variables from baseline to Week 24 was based on the modified intention‐to‐treat population. In the analysis of covariance (ANCOVA) model, treatment groups (lixisenatide, placebo), randomization strata of HbA1c (<8.0%, ≥8.0%) and eGFR (≥30 to <60 vs ≥60 mL/min/1.73 m2) at screening, and country were included as fixed effects, with the baseline value as a covariate. Patients with both baseline and at least one post‐baseline measurement were included in the analysis. LS, least squares
3.4. Safety outcomes
The incidence of documented symptomatic hypoglycemia (plasma glucose <60 mg/dL) was low in both treatment groups, but numerically higher in the placebo than lixisenatide group: seven patients (12.7%) vs three patients (5.7%), respectively. These corresponded to 0.38 and 0.29 events/patient‐year for the two groups, respectively (Table 2). This finding contrasted with the overall GetGoal‐O study population, where hypoglycemia was more frequently observed in the lixisenatide group.3 No instances of severe symptomatic hypoglycemia were recorded in either treatment group. As observed in the overall study population, within this subset, GI TEAEs occurred more frequently in the lixisenatide than placebo group (34.0% [18/53] vs 9.1% [5/55], respectively; Table 2), with the most commonly reported being nausea and diarrhea (Table 2). The rates of nausea and vomiting reported in the lixisenatide group were in line with those for the overall study population (26.1%), but fewer patients in the placebo group reported these TEAEs compared with the overall study population (1.8% vs 7.5%).3 The occurrence of GI TEAEs was reported in a median time to first GI event of 19 days (95% confidence interval [CI] 14.0‐34.0 days) in the lixisenatide group and 86 days (95% CI 6.0‐172.0 days) in the placebo group. The majority of first GI TEAEs occurred and were resolved within the first 8 weeks of treatment: 88.9% (16/18) in the lixisenatide group and 40.0% (2/5) in the placebo group. All GI TEAEs were either mild or moderate in intensity in both treatment groups. There were no instances of treatment discontinuation due to GI TEAEs in the placebo group and only two instances in the lixisenatide group, both of which were due to nausea (Table 2).
Table 2.
Documented symptomatic hypoglycemia (plasma glucose <60 mg/dL) and treatment‐emergent adverse events
| Characteristic | Placebo (n = 55) | Lixisenatide (n = 53) |
|---|---|---|
| Total patient years of exposurea | 23.9 | 24.0 |
| No. patients with events (%) | 7 (12.7) | 3 (5.7) |
| No. events per patient yearb | 0.38 | 0.29 |
| GI adverse events | ||
| No. patients with any GI TEAEc | 5 (9.1) | 18 (34.0) |
| Diarrhea | 4 (7.3) | 5 (9.4) |
| Nausea | 1 (1.8) | 14 (26.4) |
| Vomiting | 0 (0.0) | 2 (3.8) |
| Patients discontinuing treatment due to TEAE | 0 (0.0) | 2 (3.8) |
| Median (95% CI) time to first GI eventd (d) | 86.0 (6.0‐172.0) | 19.0 (14.0‐34.0) |
| Lixisenatide vs placebo | ||
| HR (95% CI)e | 4.77 (1.349‐16.835) | |
| P‐value | 0.0153 | |
| First TEAE GI events occurred within 8 weeks of treatment startc | 2 (40.0) | 16 (88.9) |
| First TEAE GI events resolved within 8 weeks of treatment startc | 2 (40.0) | 12 (66.7) |
Note: Unless indicated otherwise, data are given as n (%). Documented symptomatic hypoglycemia refers to symptomatic hypoglycemia recorded on the dedicated electronic case report form and meeting protocol definition for severe, or documented, or probable symptomatic hypoglycemia. Data analysis for this subpopulation was based on the safety population.
Abbreviations: CI, confidence interval; HR, hazard ratio; GI, gastrointestinal; TEAE, treatment‐emergent adverse event.
Patient years of exposure, calculated as the time from the first to the last injection of investigational medicinal product plus 3 days.
Calculated as the number of events divided by total patient years of exposure.
Number of patients with at least one reported TEAE.
Estimated by Kaplan‐Meier method. The P‐value for lixisenatide vs placebo was 0.0082.
The HR was estimated using a Cox regression model with treatment as the only factor.
3.5. Composite endpoints
A significantly greater proportion of patients treated with lixisenatide than placebo achieved the composite endpoints of HbA1c reduction >0.5% without documented symptomatic hypoglycemia, HbA1c reduction >0.5% without body weight gain, and HbA1c reduction >0.5% without body weight gain or symptomatic hypoglycemia (Figure 2A). Compared with the overall study population (Figure 2B), the differences between the lixisenatide and placebo groups were similar for the composite endpoint of >0.5% reduction in HbA1c without body weight gain (34.5% difference between the lixisenatide and placebo groups vs 30.6% in this subgroup). However, a smaller difference between the groups was seen for the proportion of patients achieving a >0.5% reduction in HbA1c without documented symptomatic hypoglycemia (27.0%) in the basal insulin subgroup compared with the total study population (35.8%), and hence in the combined endpoint of >0.5% reduction in HbA1c without either body weight gain or hypoglycemia (24.9% between‐group difference in this subgroup vs 31.0% in the total population).
Figure 2.
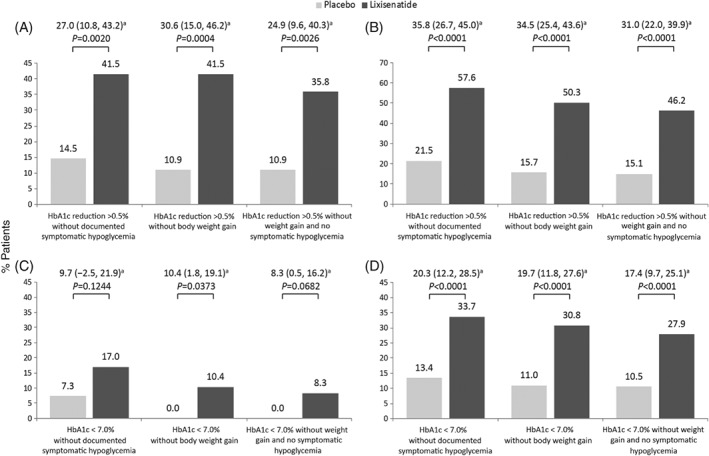
Composite endpoints of patients achieving HbA1c reductions >0.5% for the A, basal insulin subgroup or B, overall study population and patients achieving HbA1c <7.0% without documented symptomatic hypoglycemia, without body weight gain, and without documented symptomatic hypoglycemia or body weight gain for the C, basal insulin subgroup and D, overall study population. aProportion difference (95% confidence interval) between treatment groups calculated using Cochran‐Mantel‐Haenszel model
Similarly, a significantly greater proportion of patients in this subgroup treated with lixisenatide compared with placebo achieved the composite endpoints of HbA1c <7.0% without body weight gain (Figure 2C). Although more lixisenatide‐treated patients than placebo‐treated patients achieved the composite endpoint of HbA1c <7.0% without documented symptomatic hypoglycemia (17.0% vs 7.3%; P = 0.1244) and HbA1c <7.0% without body weight gain or symptomatic hypoglycemia (8.3% vs 0%; P = 0.0682), the differences did not reach statistical significance in these subgroups. The difference between the lixisenatide and placebo groups in the proportion of patients achieving HbA1c <7.0% for all three composite endpoints was lower than that seen for the overall study population, where statistical significance was reached for all these endpoints (P < 0.0001; Figure 2D).
3.6. Efficacy in patients with moderate renal insufficiency
Lixisenatide showed similar efficacy in patients with an eGFR of ≥60 mL/min/1.73 m2 (patients classified as having normal renal function to mild renal insufficiency) and patients with an eGFR between ≥30 and <60 mL/min/1.73 m2 (patients with moderate renal insufficiency) and tended to be more effective than placebo in both renal subgroups (Table 3). In both renal groups at Week 24, lixisenatide therapy resulted in a significantly greater reduction in HbA1c compared with placebo (P = 0.0219 and P = 0.0150, respectively; Table 3). Similarly, in both renal groups at Week 24, patients treated with lixisenatide showed a trend for greater reductions in 2‐hour PPG, average seven‐point SMPG, AUC of SMPG, daily dose of basal insulin, and body weight compared with placebo; however, the difference was only significant for 2‐hour PPG (P = 0.0004) and body weight (P = 0.0126) in patients without moderate renal insufficiency (Table 3). The nominal P‐values were reported without multiplicity adjustment. Due to the small sample size in these subgroups and multiple testing the results should be interpreted with caution.
Table 3.
Efficacy in patients with moderate renal insufficiency (estimated glomerular filtration rate [eGFR] ≥30 to <60 mL/min/1.73 m2) and with mild renal insufficiency to normal renal function (eGFR ≥60 mL/min/1.73 m2)
| eGFR ≥30 to <60 mL/min/1.73 m2 | eGFR ≥60 mL/min/1.73 m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Change from baseline to Week 24 | Placebo (n = 19) | Lixisenatide (n = 17) | LS mean difference ± SE | P‐valuea | Placebo (n = 36) | Lixisenatide (n = 36) | LS mean difference ± SE | P‐valuea |
| HbA1c (%) | 0.15 ± 0.64 | −0.65 ± 0.95 | −0.68 ± 0.278 | 0.0219 | 0.17 ± 0.80 | −0.50 ± 1.11 | −0.55 ± 0.219 | 0.0150 |
| 2‐h PPG (mM) | −1.31 ± 3.35 | −4.09 ± 6.01 | −3.23 ± 2.253 | 0.1702 | −0.63 ± 5.63 | −6.51 ± 6.12 | −4.28 ± 1.125 | 0.0004 |
| Average seven‐point SMPG (mM) | −0.35 ± 2.17 | −2.34 ± 3.51 | −0.16 ± 0.851 | 0.8553 | −0.25 ± 2.28 | −1.17 ± 2.20 | −0.87 ± 0.554 | 0.1253 |
| Body weight (kg) | 0.52 ± 2.72 | −2.00 ± 4.82 | 0.16 ± 1.549 | 0.9190 | −0.05 ± 1.93 | −1.31 ± 1.90 | −1.20 ± 0.465 | 0.0126 |
| FPG (mM) | 0.11 ± 1.22 | −0.26 ± 4.77 | 1.28 ± 1.013 | 0.2220 | −0.30 ± 2.92 | 0.46 ± 4.71 | 0.92 ± 0.890 | 0.3057 |
| AUC of SMPGa | −79.06 ± 584.65 | −519.45 ± 854.73 | 18.36 ± 306.844 | 0.9534 | −124.87 ± 625.27 | −350.58 ± 587.17 | −188.55 ± 144.767 | 0.2018 |
| eGFR (mL/min/1.73 m2) | 1.46 ± 7.10 | 0.46 ± 7.97 | −1.68 ± 3.311 | 0.6201 | 0.63 ± 8.28 | 4.89 ± 13.63 | 3.74 ± 2.920 | 0.2063 |
| Daily dose of BI (units) | 0.46 ± 8.75 | −3.92 ± 11.61 | −4.57 ± 4.018 | 0.2671 | −1.19 ± 5.48 | −1.86 ± 4.52 | −0.24 ± 1.140 | 0.8348 |
| Daily dose of BI/BW (units/kg) | 0.01 ± 0.10 | −0.04 ± 0.14 | −0.06 ± 0.049 | 0.2391 | −0.02 ± 0.06 | −0.02 ± 0.05 | 0.00 ± 0.013 | 0.7300 |
| HbA1c < 7.0% with | ||||||||
| No documented symptomatic hypoglycemia | 2 (10.5) | 4 (23.5) | ‐ | 2 (5.6) | 5 (13.9) | ‐ | ||
| No BW gain and no documented symptomatic hypoglycemia | 0 | 3 (17.6) | ‐ | 0 | 1 (3.2) | ‐ | ||
| No BW gain | 0 | 4 (23.5) | ‐ | 0 | 1 (3.2) | ‐ | ||
Note: Unless indicated otherwise, data are given as the mean ± SD or as n (%).
Abbreviations: AUC, area under the curve; BI, basal insulin; BW, body weight; FPG, fasting plasma glucose; LS, least squares; PPG, postprandial glucose; SMPG, self‐monitored plasma glucose.
All P‐values are reported as nominal P‐values without multiplicity adjustment.
4. DISCUSSION
The present study demonstrates the efficacy and safety of the GLP‐1 RA lixisenatide in participants with T2D aged ≥70 years who are uncontrolled on basal insulin with or without OADs. In line with the findings from analysis of the overall study population, which included patients on a variety of background treatment regimens, patients treated with lixisenatide achieved significantly greater HbA1c, PPG, body weight, and SMPG reductions than those in the placebo arm. Furthermore, a significantly greater proportion of patients treated with lixisenatide achieved a reduction in HbA1c >0.5% with no documented symptomatic hypoglycemia, no body weight gain, or both compared with placebo.
Decline in renal function is related to age. Similarly, changes to hepatic drug metabolism may reduce choice regarding antihyperglycemia therapies and increase the risk of hypoglycemia in older people with diabetes.5 Lixisenatide is eliminated through glomerular filtration, tubular reabsorption, and subsequent metabolic catabolism, and can lead to small increases in exposure with declining renal function.30 Although only limited data are available, previous clinical evidence suggests that lixisenatide is safe to use in patients with mild (eGFR ≥60 mL/min/1.73 m2) or moderate (eGFR ≥30 to <60 mL/min/1.73 m2) renal impairment; a meta‐analysis of nine lixisenatide trials also showed that clinical outcomes did not differ between the mild or moderate renal impairment categories, and efficacy outcomes were not significantly affected by mild or moderate renal impairment.31 Therefore, we also performed an analysis in which patients were stratified according to whether they had moderate renal insufficiency (eGFR 30‐59 mL/min/1.73 m2, chronic kidney disease [CKD] Stage 3A and B) or had an eGFR >60 mL/min/1.73 m2 (patients with mild renal insufficiency to normal renal function, CKD Stages 2 and 1, respectively). Lixisenatide provided similar efficacy in both stratification arms and remained more effective than placebo. In addition to efficacy outcomes, more patients treated with lixisenatide achieved composite endpoints of HbA1c <7.0% with no documented hypoglycemia, HbA1c <7.0% with no body weight gain, and HbA1c <7.0% with no body weight gain or documented hypoglycemia, regardless of renal insufficiency. Indeed, a higher proportion of lixisenatide‐treated patients achieved these endpoints in the moderate renal insufficiency group than in the composite mild renal insufficiency and normal renal kidney function group; however, sample sizes were low, making significant conclusions difficult. Therefore, the present analysis supports previous research and suggests that lixisenatide remains both efficacious and safe in people with moderate renal insufficiency. There are few data available regarding the use of lixisenatide in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2); it is recommended that these patients should be closely monitored for GI adverse reactions and changes in renal function. Lixisenatide is not recommended for patients with end‐stage renal disease (eGFR <15 mL/min/1.73 m2) due to a lack of therapeutic experience in this population.30
How well medication is tolerated is an important issue, especially in older adults. In the present analysis, nausea and diarrhea were identified as the most common GI TEAEs associated with lixisenatide treatment. Most reported GI TEAEs were transient, mild to moderate in intensity, and occurred and were resolved within 8 weeks of initiating treatment. Older adults, especially those with T2D, often must take multiple medications. In the present study, in addition to basal insulin (plus lixisenatide or placebo), most patients were taking OADs, and many were also taking various non–diabetes‐related medications. No differences in tolerability and safety were observed for patients on basal insulin compared with the study population as a whole.
In clinical trials, the complementary action of GLP‐1 RAs and basal insulin has demonstrated improvements in glycemic control while potentially reducing hypoglycemia risk and weight gain.32, 33, 34 Although many of the studies were performed in younger populations, the findings of the present study also identified a reduction in HbA1c, body weight, and hypoglycemia risk with add‐on lixisenatide. Similar to previous studies, the use of lixisenatide was associated with increased GI TEAEs. Despite these adverse events, there appears to be the possibility of a class effect of GLP‐1 RAs in reducing adverse cardiovascular events and mortality.35, 36 Consequently, this class of drugs is now generally recommended as the first‐line injectable medication for treatment of T2D, with some (eg, liraglutide and semaglutide) specifically recommended for patients with existing cardiovascular disease.7
In addition to individual GLP‐1 RAs, fixed‐ratio combination products can also be an efficient and safe option for some patients. Lixisenatide is a component of iGlarLixi, a once‐daily titratable fixed‐ratio combination of insulin glargine and lixisenatide.25, 26 The use of iGlarLixi could help reduce the burden of polypharmacy in these patients, and possibly improve compliance, due to a simpler therapeutic regimen. In addition, a recent study using indirect propensity score‐matched exploratory comparisons suggests that the use of iGlarLixi may be more effective (significantly greater reductions in HbA1c with associated body weight loss) with better GI tolerability (due to the more gradual titration of lixisenatide) than a sequential approach to treatment.37 Across the studies included in the propensity score‐matched manuscript, treatment with iGlarLixi, compared with insulin glargine and lixisenatide administered separately, resulted in lower incidences of nausea (9.2%‐10.0% vs 20.7%‐27.0%) and vomiting (1.1%‐3.3% vs 8.7%‐10.3%). Similarly, in a post hoc analysis of the LixiLan‐L and LixiLan‐O randomized clinical trials, iGlarLixi was associated with lower rates of GI TEAEs (nausea, vomiting, and/or diarrhea) in the first 8 weeks of treatment than lixisenatide alone. Overall, 9.6%‐11.7% of patients reported GI TEAEs with iGlarLixi across the two trials, compared with 27.5% of patients taking lixisenatide alone in the LixiLan‐O trial.38
In conclusion, the present post hoc analysis suggests that add‐on therapy with lixisenatide in non‐frail patients aged ≥70 years with T2D uncontrolled with basal insulin is safe and well tolerated and should be considered in this patient population. This combination was shown to be a safe and effective treatment option for patients with mild to moderate renal insufficiency, despite the expected slightly higher exposure rates without the required dose adjustments; regardless of this fact, monitoring is always advised in this patient population. In addition, the fixed‐ratio combination of insulin glargine U100 and lixisenatide (iGlarLixi) in a single pen is a simpler method of administration of these agents worthy of consideration in older adults.
CONFLICT OF INTERESTS
DG reports consulting fees from Novo Nordisk and Sanofi, is a member of the speakers bureaus for Astra Zeneca and Sanofi, and has received investigator fees from Eli Lilly and Company, Novo Nordisk, and Sanofi, but has no other financial interest, including individual stocks owned in any pharmaceutical or medical device company. TAD, MR, and ML are employees of Sanofi US, Inc. GM reports consulting fees from Abbott, Merck, Novo Nordisk, and Sanofi, as well as speaker fees from Boehringer Ingelheim, Eli Lilly and Company, and Merck.
ACKNOWLEDGEMENT
The authors received writing and editorial support from Martina Fuchsberger (Excerpta Medica, Amsterdam, The Netherlands), funded by Sanofi US, Inc.
Dailey GE, Dex TA, Roberts M, Liu M, Meneilly GS. Efficacy and safety of lixisenatide as add‐on therapy to basal insulin in older adults with type 2 diabetes in the GetGoal‐O Study. Journal of Diabetes. 2019;11:971–981. 10.1111/1753-0407.12952
Funding information This study was funded by Sanofi US, Inc.
REFERENCES
- 1. International Diabetes Federation . Global guideline for managing older people with type 2 diabetes. https://www.idf.org/e-library/guidelines/78-global-guideline-for-managing-older-people-with-type-2-diabetes.html. Published 2013. Accessed May 11, 2018.
- 2. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meneilly GS, Roy‐Duval C, Alawi H, et al. Lixisenatide therapy in older patients with type 2 diabetes inadequately controlled on their current antidiabetic treatment: the GetGoal‐O randomized trial. Diabetes Care. 2017;40:485‐493. [DOI] [PubMed] [Google Scholar]
- 4. Meneilly GS, Elahi D. Metabolic alterations in middle‐aged and elderly lean patients with type 2 diabetes. Diabetes Care. 2005;28:1498‐1499. [DOI] [PubMed] [Google Scholar]
- 5. Gates BJ, Walker KM. Physiological changes in older adults and their effect on diabetes treatment. Diabetes Spectr. 2014;27:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . Standards of medical care in diabetes ‐ 2018. Diabetes Care. 2018;41(suppl 1):S119‐S125. [DOI] [PubMed] [Google Scholar]
- 7. Davies MJ, D'Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khunti K, Caputo S, Damci T, et al. The safety and efficacy of adding once‐daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012;14:1129‐1136. [DOI] [PubMed] [Google Scholar]
- 9. Blonde L, Meneghini L, Peng XV, et al. Probability of achieving glycemic control with basal insulin in patients with type 2 diabetes in real‐world practice in the USA. Diabetes Ther. 2018;9:1347‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80 000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khunti K, Millar‐Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11:3‐12. [DOI] [PubMed] [Google Scholar]
- 13. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantalone KM, Misra‐Hebert AD, Hobbs TM, et al. Clinical inertia in type 2 diabetes management: evidence from a large, real‐world data set. Diabetes Care. 2018;41:e113‐e114. [DOI] [PubMed] [Google Scholar]
- 15. Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43:501‐511. [DOI] [PubMed] [Google Scholar]
- 16. Strain WD, Cos X, Hirst M, et al. Time to do more: addressing clinical inertia in the management of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;105:302‐312. [DOI] [PubMed] [Google Scholar]
- 17. Strain WD, Blüher M, Paldánius P. Clinical inertia in individualising care for diabetes: is there time to do more in type 2 diabetes? Diabetes Ther. 2014;5:347‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes. 2017;9:267‐274. [DOI] [PubMed] [Google Scholar]
- 20. Ajmera M, Raval A, Zhou S, et al. A real‐world observational study of time to treatment intensification among elderly patients with inadequately controlled type 2 diabetes mellitus. J Manag Care Spec Pharm. 2015;21:1184‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balkau B, Bouée S, Avignon A, et al. Type 2 diabetes treatment intensification in general practice in France in 2008‐2009: the DIAttitude Study. Diabetes Metab. 2012;38(Suppl 3):S29‐S35. [DOI] [PubMed] [Google Scholar]
- 22. Balkau B, Halimi S, Blickle JF, et al. Reasons for non‐intensification of treatment in people with type 2 diabetes receiving oral monotherapy: outcomes from the prospective DIAttitude study. Ann Endocrinol (Paris). 2016;77:649‐657. [DOI] [PubMed] [Google Scholar]
- 23. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munshi MN, Pandya N, Umpierrez GE, DiGenio A, Zhou R, Riddle MC. Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61:535‐541. [DOI] [PubMed] [Google Scholar]
- 25. Lorenz M, Pfeiffer C, Steinsträsser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes: relationship to postprandial glycemia. Regul Pept. 2013;185:1‐8. [DOI] [PubMed] [Google Scholar]
- 26. Meier JJ, Rosenstock J, Hincelin‐Méry A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38:1263‐1273. [DOI] [PubMed] [Google Scholar]
- 27. Werner U. Effects of the GLP‐1 receptor agonist lixisenatide on postprandial glucose and gastric emptying: preclinical evidence. J Diabetes Complicat. 2014;28:110‐114. [DOI] [PubMed] [Google Scholar]
- 28. Bain SC. The clinical development program of lixisenatide: a once‐daily glucagon‐like peptide‐1 receptor agonist. Diabetes Ther. 2014;5:367‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blonde L, Chava P, Dex T, Lin J, Nikonova EV, Goldenberg RM. Predictors of outcomes in patients with type 2 diabetes in the lixisenatide GetGoal clinical trials. Diabetes Obes Metab. 2017;19:275‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanofi‐aventis. Adlyxin prescribing information . https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471Orig1s000lbl.pdf. Published July 2016. Accessed March 20, 2019.
- 31. Hanefeld M, Arteaga JM, Leiter LA, et al. Efficacy and safety of lixisenatide in patients with type 2 diabetes and renal impairment. Diabetes Obes Metab. 2017;19:1594‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldenberg RM, Berard L. Adding prandial GLP‐1 receptor agonists to basal insulin: a promising option for type 2 diabetes therapy. Curr Med Res Opin. 2018;34:1‐10. [DOI] [PubMed] [Google Scholar]
- 33. Davies ML, Pham DQ, Drab SR. GLP1‐RA add‐on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36:893‐905. [DOI] [PubMed] [Google Scholar]
- 34. Wysham CH, Lin J, Kuritzky L. Safety and efficacy of a glucagon‐like peptide‐1 receptor agonist added to basal insulin therapy versus basal insulin with or without a rapid‐acting insulin in patients with type 2 diabetes: results of a meta‐analysis. Postgrad Med. 2017;129:436‐445. [DOI] [PubMed] [Google Scholar]
- 35. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:105‐113. [DOI] [PubMed] [Google Scholar]
- 36. Zweck E, Roden M. GLP‐1 receptor agonists and cardiovascular disease: drug‐specific or class effects? Lancet Diabetes Endocrinol. 2019;7:89‐90. [DOI] [PubMed] [Google Scholar]
- 37. Rosenstock J, Handelsman Y, Vidal J, et al. Propensity score‐matched comparative analyses of simultaneously administered fixed‐ratio iGlarLixi (LixiLan) vs sequential administration of insulin glargine and lixisenatide in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2018;20:2821‐2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trujillo JM, Roberts M, Dex T, Chao J, White J, LaSalle J. Low incidence of gastrointestinal adverse events over time with a fixed‐ratio combination of insulin glargine and lixisenatide vs lixisenatide alone. Diabetes Obes Metab. 2018;20:2690‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]


