Summary
Background
Weekly adalimumab (Humira®) is approved for the treatment of hidradenitis suppurativa (HS) based on the 12‐week placebo‐controlled periods of the two phase III PIONEER trials.
Objectives
Using PIONEER integrated trial results, we aimed to evaluate the optimal medium‐term adalimumab maintenance dosing strategy for moderate‐to‐severe HS.
Methods
Each trial had two double‐blind periods; 12‐week Period A and 24‐week Period B. Patients randomized to adalimumab 40 mg every week (ADAew) (Period A), were rerandomized in Period B to ADAew (ADAew/ew), ADA every other week (ADAew/eow), or placebo (ADAew/pbo). Placebo‐randomized patients were reassigned in Period B to ADAew (PIONEER I) or placebo (PIONEER II). The primary outcome was HS Clinical Response (HiSCR). Patients who lost response during Period B were discontinued from the study and offered an option to enter the open‐label extension (OLE) to receive ADAew. Results are reported across the two study periods, and data were combined from the two study periods and the OLE.
Results
For week‐12 HiSCR achievers, the HiSCR week‐36 rate was 48·1% (ADAew/ew) vs. 46·2% (ADAew/eow) and 32·1% (ADAew/pbo). Combining (post hoc) these patients with week‐12 partial responders further differentiated outcomes in Period B (ADAew/ew 55·7% vs. ADAew/eow 40·0% and ADAew/pbo 30·1%). Period‐B adverse‐event rates were ADAew/ew 59·6% vs. ADAew/eow 57·4% and ADAew/pbo 65·0%. One patient (ADAew/ew) reported a serious infection.
Conclusions
Weekly adalimumab treatment, effective throughout 36 weeks, was the optimal maintenance medium‐term dosing regimen for this population. At least partial response after 12 weeks with continued weekly dosing had better outcomes than dose reduction or interruption. Patients who do not show at least a partial response to weekly adalimumab by week 12 are unlikely to benefit from continued therapy. No new safety risks were identified.
What's already known about this topic?
Hidradenitis suppurativa (HS) is a chronic inflammatory disease, commonly misinterpreted as an infection and treated with long‐term antibiotic regimens or surgical incisions.
Based on the chronicity of HS and the lack of evidence for efficacious and safe long‐term HS treatments, it is important to evaluate medium‐ to long‐term therapies for HS.
Weekly adalimumab (Humira®) is approved for the treatment of moderate‐to‐severe HS based on the two phase III PIONEER trials.
What does this study add?
This study pooled data from the two PIONEER trials, providing a more robust assessment of outcomes.
After at least partial treatment success with weekly adalimumab short‐term therapy (12 weeks), continuing weekly dosing during the subsequent 24 weeks had better outcomes than dose reduction or treatment interruption.
Patients who do not show at least a partial response to weekly adalimumab by week 12 are unlikely to benefit from continued therapy.
Short abstract
Linked Comment: https://doi.org/10.1111/bjd.18437.
Hidradenitis suppurativa (HS) or acne inversa is a serious, painful, systemic chronic skin disease, which may persist for decades.1, 2, 3 Inflammatory skin lesions including abscesses, fistulas and nodules, may exhibit purulent, malodorous drainage, and develop tunnels (sinus tracts)4 and scarring as disease severity increases.2, 5 Lesions may flare, resolve and recur in different body areas. As a result, patients with moderate‐to‐severe HS carry a substantial disease burden.6, 7
HS is not an infection. Evidence suggests that it is an inflammatory disease with a pathogenesis that is multifactorial.8 However, antibiotic treatment has historically played a central role in managing this disease.9 In view of the chronicity of the disease and the lack of evidence for efficacy and safety of any type of HS therapy beyond short‐term therapy,9 it is important to evaluate medium‐ to long‐term therapies.10, 11, 12, 13, 14, 15
The tumour necrosis factor (TNF)‐α antibody, adalimumab (Humira®, AbbVie Inc., North Chicago, IL, U.S.A.), is currently the only approved pharmacological therapy for the treatment of adults with moderate‐to‐severe HS. In initial trials, 40 mg adalimumab every‐week treatment was efficacious in controlling objective signs of moderate‐to‐severe disease and in reducing pain during the first 12–16 weeks.16, 17 The objective of this analysis was to evaluate the optimal medium‐term adalimumab maintenance dosing strategy from integrated results of the PIONEER I and II trials, and from the initial 36‐week portion of the open‐label extension (OLE) trial that paralleled the PIONEER trials. The safety and efficacy of adalimumab weekly dosing with dosage reduction and with maintenance of treatment response off therapy were also explored.
Patients and methods
Study design and participants
PIONEER I and II had similar study designs. Each was 36 weeks in duration with two double‐blind periods, i.e. 12‐week Period A and 24‐week Period B (Fig. 1). Patients who lost response or had worsening or absence of improvement in Period B (defined in Fig. 1) were allowed to enter the OLE.
Figure 1.
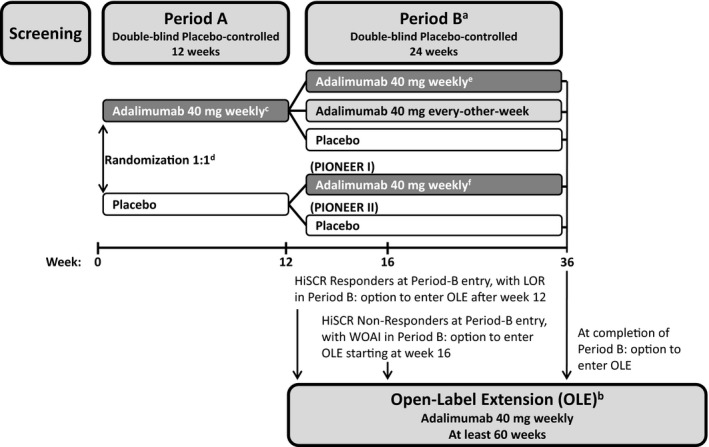
Study design.
aWeek‐12 Hidradenitis Suppurativa (HS) Clinical Response (HiSCR) responders through Period B to week 36 or until loss of response [loss of 50% of the abscess and inflammatory nodule (AN) count improvement gained between baseline and week 12], and week‐12 HiSCR nonresponders continued Period B to at least week 26 (and up to week 36). bPatients could enter the multicentre 60‐week phase III OLE trial (which evaluated long‐term safety, tolerability and efficacy of adalimumab for patients with moderate‐to‐severe HS), if (i) they completed Period B of their respective PIONEER trial; (ii) achieved HiSCR at entry to Period B of their respective PIONEER trial and then experienced a loss of response (LOR); or (iii) did not achieve HiSCR at the entry of Period B and then experienced worsening or absence of improvement (WOAI) (greater or equal to the baseline AN count on two consecutive visits after week 12, occurring at least 14 days apart). cStarting at week 4 after 160 mg (week 0), 80 mg (week 2). dStratified by baseline Hurley stage II vs. Hurley stage III (PIONEER I and II) and baseline concomitant antibiotic use (PIONEER II). eRerandomization for patients treated with adalimumab in Period A was stratified by week‐12 HiSCR status at entry into Period B and by baseline Hurley stage II vs. Hurley stage III. fAdalimumab 40 mg starting at week 16 after 160 mg (week 12), 80 mg (week 14).
Adults were enrolled if they were anti‐TNF‐α naive, had moderate‐to‐severe disease [total abscess and inflammatory nodule (AN) count of at least three at baseline, and HS lesions in two distinct body areas, one of which was classified as Hurley stage II or III],18 and had an inadequate response to oral antibiotics used to treat HS. Baseline antibiotics (tetracycline class) in stable doses were allowed in PIONEER II. Complete eligibility criteria and ethical standards have been published elsewhere.17
At enrolment, patients were randomized to receive adalimumab 40 mg weekly dosing (ADAew) or matching placebo. Patients treated with adalimumab continuing to Period B were rerandomized at week 12 to ADAew, adalimumab every‐other‐week dosing (ADAeow), or matching placebo (pbo) (1 : 1 : 1 ratio); patients who received placebo were reassigned to ADAew in PIONEER I or remained on placebo in PIONEER II. All patients were assigned in a blinded fashion. Randomization and blinding details have been previously published elsewhere.17
Assessments
This is an integrated (pooled) analysis from Period B of the two PIONEER trials and the initial portion of the OLE that paralleled the 36 weeks of the PIONEER trials. The statistical analysis plan prespecified the primary end point as achievement of HS Clinical Response (HiSCR) at week 36; assessed by nonresponder imputation (missing or early transfer to OLE were imputed as nonresponders). HiSCR was defined as at least a 50% reduction from baseline in total AN count, with no increase in abscess or draining‐fistula counts; this represents clinically meaningful lesion changes and has been previously described.17, 19, 20
Statistical analysis
The primary population for the integrated analysis of efficacy was the intention‐to‐treat population (ITT). In Period A, this included all patients randomized at week 0, and in Period B, all patients who received ADAew in Period A and were rerandomized to Period B (Fig. 2). Randomization in Period B was stratified based on patients’ HiSCR status at the end of Period A (week 12) and baseline Hurley stage. Treatment groups in Period B were categorized according to the dose received in Periods A and B (ADAew/ew, ADAew/eow, ADAew/pbo).
Figure 2.
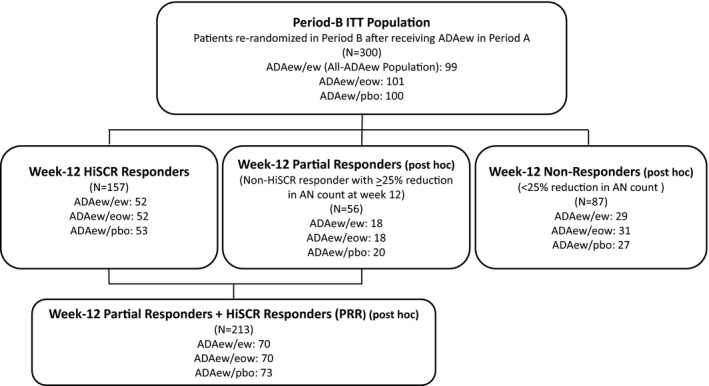
Efficacy analysis population and subpopulations. ITT, intention to treat; ADA, adalimumab; ew, weekly dosing; pbo, placebo; eow, every‐other‐week dosing; HiSCR, Hidradenitis Suppurativa Clinical Response; AN, total abscess and inflammatory nodule count.
Period‐B efficacy analysis subpopulations (Fig. 2) included (i) patients who achieved HiSCR at week 12 (week‐12 HiSCR responders); (ii) patients who did not achieve HiSCR at week 12 (week‐12 HiSCR nonresponders); and (iii) patients who received continuous ADAew dosing during both periods (all‐ADAew population). HiSCR up to week 36 was also evaluated for patients who transferred early to the OLE.
An additional population was defined post hoc to identify the most clinically appropriate patient group for continued treatment of ADAew (continued from Period A to Period B) over the medium term vs. adalimumab discontinuation (ADAew/pbo) (Fig. 2 and File S1; see Supporting Information).21, 22 The resulting population combined the week‐12 HiSCR responders with patients who did not achieve HiSCR at week 12, but did achieve a partial response at week 12, i.e. ≥ 25% reduction in AN count relative to baseline (week‐12 partial responders) to form the week‐12 partial responders plus HiSCR responders (PRR) population (Fig. 2).
The integrated efficacy for Period B adjusted for study, baseline Hurley stage, and week‐12 HiSCR status. Analysis of covariance was used for continuous variables, and the Cochran–Mantel–Haenszel test analysed discrete variables. The efficacy analysis in Period B conducted pairwise comparisons of each adalimumab arm vs. placebo in the Period‐B ITT population. According to the study design, patients who lost response during Period B were required to discontinue the study and enter the OLE to receive ADAew, and were counted as nonresponders in the subsequent Period‐B visit, even if the patients regained response while continuing ADAew treatment in the OLE. To adjust for this potential study‐design artifact, medium‐term efficacy of ADAew treatment was also summarized in the all‐ADAew population to include data from both the initial PIONEER studies and the OLE study.
Safety was analysed for all patients in the Period‐A ADAew group who received at least one dose of study drug in Period B.
Results
Of the 633 patients from PIONEER I and II who were randomized in Period A, 300 of the 316 patients assigned to ADAew entered Period B and were included in the Period B efficacy analyses; 300 patients were treated and included in the Period B safety analysis (Fig. S1; see Supporting Information). The primary reason for study discontinuation in Period B was meeting the protocol‐specified criteria for loss of response or worsening or absence of improvement (defined in Fig. 1). These patients were allowed to transfer early to the OLE. More in the ADAew/pbo group transferred early compared with the other groups (50 ADAew/pbo, 40 ADAew/eow, 35 ADAew/ew). Compliance (ratio of number of received vs. planned injections of study drug, verified by patient diaries) with study drug administration was high; the overall mean rate in Period B was 98·6% for ADAew/pbo, 98·3% for ADAew/eow and 97·5% for ADAew/ew.
Baseline demographics, disease characteristics, and comorbidity were generally balanced across the Period‐B treatment groups and were typical for a study population with moderate‐to‐severe HS (Table 1). The majority of these patients were female (62·3%), white (82·7%) and obese [mean body mass index = 32·2 kg m−2 (n = 299)]. Median duration of HS was 8·9 years (range 1–43·5), and the mean number of ANs at baseline markedly exceeded the minimum number required for study entry (at least three). Mean high‐sensitivity C‐reactive protein was elevated [17·0 mg L−1 (SD 23·9)].
Table 1.
Patient characteristics and comorbidities at baseline
| Period B (all patients receiving ADAew in Period A), N = 300 | ||||
|---|---|---|---|---|
| ADAew/pbo, N = 100 | ADAew/eow, N = 101 | ADAew/ew, N = 99 | Total, N = 300 | |
| Male, n (%) | 44 (44) | 36 (35·6) | 33 (33·3) | 113 (37·7) |
| Female, n (%) | 56 (56) | 65 (64·4) | 66 (66·7) | 187 (62·3) |
| Ethnicity, n (%) | ||||
| White | 81 (81) | 77 (76·2) | 90 (90·9) | 248 (82·7) |
| Black | 13 (13) | 19 (18·8) | 6 (6·1) | 38 (12·7) |
| Othera | 6 (6) | 5 (4·9) | 3 (5·8) | 12 (4·0) |
| Age, years, median (range) | 35 (20–67) | 36 (19–63) | 34 (18–64) | 35 (18–67) |
| BMI, kg m−2, median (range) | 32·6 (17·4–53·5); (N = 99) | 30·5 (18·3–53·4) | 31·5 (20·3–54·5) | 31·6 (17·4–54·5); (N = 299) |
| BMI,b kg m−2; n (%) | ||||
| Normal weight (< 25) | 21 (21·2) | 21 (20·8) | 14 (14·1) | 56 (18·7) |
| Overweight (25 to < 30) | 19 (19·2) | 26 (25·7) | 26 (26·3) | 71 (23·7) |
| Obese (30 to < 40) | 44 (44·4) | 39 (38·6) | 48 (48·5) | 131 (43·8) |
| Morbidly obese (≥ 40) | 15 (15·2) | 15 (14·9) | 11 (11·1) | 41 (13·7) |
| Current nicotine use, n (%) | 54 (54·0) | 65 (64·4) | 59 (59·6) | 178 (59·3) |
| Disease characteristics | ||||
| Hurley stage II, n (%) | 55 (55) | 52 (51·5) | 49 (49·5) | 156 (52·0) |
| Hurley stage III, n (%) | 45 (45) | 49 (48·5) | 50 (50·5) | 144 (48·0) |
| Modified Sartorius score, median (range) | 107 (18–397) | 100 (19–433) | 104 (20–1093) | 159·5 (18–1093) |
| Family history of HS, n (%) | 23 (23) | 21 (21·0) | 29 (29·3) | 73 (24·4) |
| Median disease duration, years (range) | 8·2 (1·1–43·5) | 8·5 (1·1–33·3) | 10·1 (1·0–40·4) | 8·9 (1·0–43·5) |
| HS lesions; mean (SD) | ||||
| AN | 13·1 (9·97) | 12·1 (10·52) | 12·1 (10·14) | 12·5 (10·19) |
| Abscess | 2·8 (3·59) | 2·6 (3·06) | 2·0 (2·61) | 2·4 (3·12) |
| Draining fistula | 4·1 (4·90) | 3·8 (5·11) | 3·6 (4·23) | 3·8 (4·75) |
| Inflammatory nodule | 10·3 (7·96) | 9·7 (9·74) | 10·1 (9·44) | 10·0 (9·05) |
| Daily pain at worst, median (range 0–10) | 4·7 (0–10); (N = 97) | 5·0 (0–10); (N = 100) | 4·4 (0–9·7); (N = 97) | 4·7 (0–10); (N = 294) |
| Prior surgery for HS, n (%) | 16 (16) | 17 (16·8) | 11 (11·1) | 44 (14·7) |
| hsCRP, mg L−1 mean (SD) | 16·4 (19·12); (N = 52) | 17·7 (22·80) | 16·9 (24·82) | 17·0 (22·31); (N = 299) |
pbo, placebo; ADAew, adalimumab every‐week dosing; BMI, body mass index; HS, hidradenitis suppurativa; AN, abscesses and inflammatory nodules; hsCRP, high‐sensitivity C‐reactive protein. a‘Other’ includes Asian [n = 4 (4%) ADAew/pbo; n = 3 (3·0%) ADAew/eow; and n = 7 (2·3%) all ADAew] and other ethnicities than those mentioned in this table and table footnote [n = 1, (1·0%) ADAew/pbo, n = 2, (2·0%) ADAew/eow, n = 2, (2·0%) ADAew/ew, and n = 5, (1·7%) all ADAew]. bMissing data for one patient (ADAew/pbo).
Efficacy
For all patients rerandomized after ADAew treatment in Period A (Period‐B ITT population), the proportion achieving HiSCR at week 36 in the ADAew/ew group was higher (43·4%) compared with the ADAew/eow group (30·7%) and significantly higher (P < 0·05) compared with the ADAew/pbo group (28·0%) (Fig. 3a). Patients remaining on continuous weekly treatment, did experience fluctuation in disease activity, as shown by a median AN‐count increase of one (interquartile range 0–4) from week 12, at the time point with highest activity during Period B. In comparison, those who withdrew from weekly dosing in Period B had a median AN‐count increase of three (interquartile range 1–6).
Figure 3.
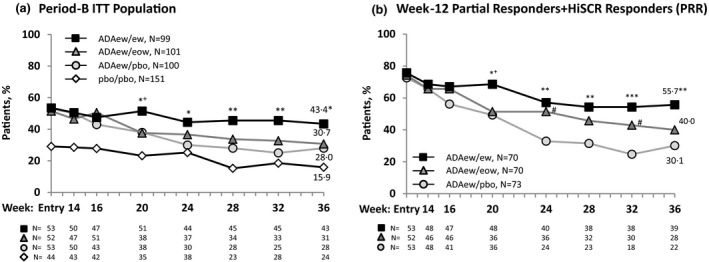
Achievement of Hidradenitis Suppurativa Clinical Response (HiSCR) at week 36 for patients who received adalimumab (ADA) every week (ew) in Period A. (a) Period‐B intention‐to‐treat population. Overall, patients rerandomized to ADAew did better in Period B than those rerandomized to ADA every other week (eow) or placebo (pbo). The pbo/pbo group was included only in the PIONEER II study; hence, a comparison between the other treatment groups and the pbo/pbo group was not performed (it is shown here for illustration purposes only). (b) Week‐12 partial responder plus HiSCR responders (PRR). This population was identified as the patients most likely to benefit from weekly adalimumab treatment continued past week 12. Nonresponder imputation. Statistical significance at ***P = 0·001, **P = 0·01, *P = 0·05 for ADAew/ew vs. ADA ew/pbo; + P = 0·05 for ADAew/ew vs. ADAew/eow; and # P = 0·05 for ADAew/eow vs. ADAew/pbo. P‐values were calculated using the Cochran–Mantel–Haenszel test adjusted for study, baseline Hurley stage and HiSCR responder at entry of Period B (Fig. 3a), and HiSCR status at rerandomization (Fig. 3b).
The HiSCR rate reduced over time during Period B for all treatment groups (Fig. 2a). For the week‐12 HiSCR responders, all treatment groups also had a reduction in the HiSCR rate over time during Period B. Respective HiSCR rates at week 14 and week 36 were 79·2% and 32·1% for ADAew/pbo; 78·8% and 46·2% for ADAew/eow; 73·1% and 48·1% for ADAew/ew. The HiSCR rate at week 36 in the ADAew/ew group was higher (48·1%, n/N = 25/52) compared with the ADAew/eow (46·2%, n/N = 24/52) and ADAew/pbo groups (32·1%, n/N = 17/53).
Our statistical modelling (File S1; see Supporting Information) identified the week‐12 PRR population as the group of patients who would benefit most from continuous weekly adalimumab treatment. For this population, the HiSCR rate for the ADAew/ew group at week 36 was higher (55·7%, n/N = 39/70) compared with the ADAew/eow group (40·0%, n/N = 28/70) and significantly higher (P < 0·01) compared with the ADAew/pbo group (30·1%, n/N = 22/73) (Fig. 3b). HiSCR rates declined slightly for all groups during Period B.
For the few week‐12 HiSCR nonresponders (< 25% reduction in AN count), the HiSCR rates at week 36 were 13·8% (n/N = 4/29) for ADAew/ew, 9·7% (n/N = 3/31) for ADAew/eow and 22·2% (n/N = 6/27) for ADAew/pbo.
Patients in the all‐ADAew population (N = 99) who received continuous ADAew dosing during Periods A and B, including records from the OLE period for those who discontinued Period B early and continued ADAew in the OLE, maintained treatment response through week 36 (53·5%, 53·5%, 58·6% and 55·6% achieved HiSCR at weeks 12, 20, 32 and 36, respectively; Fig. 4).
Figure 4.
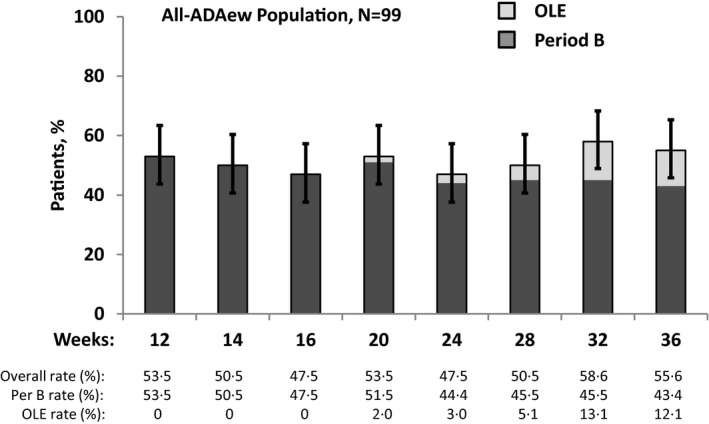
Maintenance of Hidradenitis Suppurativa Clinical Response (HiSCR) for patients receiving continuous adalimumab (ADA) every week (ADAew) across PIONEER I, II and open‐label extension (OLE). Patients in this analysis are those who received ADAew in both study periods (all‐ADAew population, N = 99). This analysis includes data from both study periods, in addition to the OLE data for the all‐ADAew population who discontinued Period B owing to loss of response, entered the OLE, and regained response in the OLE. This demonstrates maintenance of treatment response for patients who may regain response following temporary loss of response.
Safety
Treatment‐emergent adverse events included worsening of HS (Table 2). In Period B, the rate of any adverse event was lowest for the ADAew/ew group (59·6%) compared with the ADAew/eow (57·4%) and ADA/pbo groups (65·0%). Overall, the adverse event profile was similar between the ADAew/ew and ADAew/eow groups. The most frequently reported serious adverse event was worsening of HS (Period B: n = 2 ADAew/pbo, n = 3 ADAew/eow). The percentage reporting a serious infection was highest in the ADAew/ew group (1·0%) compared with the ADAew/eow (0%) and placebo groups (0%). One patient (ADAew/ew group) reported a serious infection and one patient (ADAew/eow group) reported nonmelanoma skin cancer (squamous cell carcinoma of the nasal slope). A fatal event of cardiorespiratory arrest occurred in a patient (ADAew/eow group) who had multiple cardiovascular risk factors. No patients reported opportunistic infection, tuberculosis (active or latent), lymphoma or demyelinating disorder.
Table 2.
Treatment emergent adverse events in Period B
| For patients who received ADAew in Period A, n (%) | ADAew/pbo, (N = 100) | ADAew/eow, (N = 101) | ADAew/ew (N = 99) |
|---|---|---|---|
| Any adverse event | 65 (65) | 58 (57·4) | 59 (59·6) |
| Serious adverse eventsa | 2 (2) | 5 (5·0) | 3 (3·0) |
| Adverse event leading to study drug discontinuation | 2 (2) | 2 (2·0) | 2 (2·0) |
| Infection | 29 (29) | 31 (30·7) | 32 (32·3) |
| Serious infectionb | 0 | 0 | 1 (1·0) |
| Malignancy | 0 | 1 (1·0) | 0 |
| Nonmelanoma skin cancer | 0 | 1 (1·0) | 0 |
| Psoriasis‐related adverse eventsc | 1 (1) | 1 (1·0) | 3 (3·0) |
| Adverse events leading to deathd | 0 | 1 (1·0) | 0 |
| Adverse events in ≥ 10% of patients in any group | |||
| Nasopharyngitis | 10 (10) | 4 (4·0) | 6 (6·1) |
| Worsening of hidradenitis suppurativa (HS) | 20 (20) | 18 (17·8) | 5 (5·1) |
ADA, adalimumab; ew, every‐week dosing; eow, every‐other‐week dosing; pbo, placebo. aSerious adverse events included the following: lymphadenitis, acute myocardial infarction, cardiorespiratory arrest, abortion induced (ADAew/eow, n = 1 for each); pneumonia, ectopic pregnancy (ADAew/ew, n = 1 for each); HS (ADAew/pbo, n = 2; ADAew/eow, n = 3) and rash (ADAew/ew, n = 1). bSerious infections included pneumonia (ADAew/ew, n = 1). cEvents of worsening or new onset included dermatitis psoriasiform (ADAew/pbo, n = 1; ADAew/ew, n = 2); psoriasis (ADAew/eow, n = 1; ADAew/ew, n = 1). dOne death owing to cardiorespiratory arrest occurred 42 days after the last dose of ADA in a 35‐year‐old man with a history of diabetes mellitus, smoking and a family history of coronary heart disease. Events include worsening of underlying HS disease.
Discussion
HS is a chronic disease that often begins in young adulthood and lasts for decades.23 As a result, developing optimally effective medium‐ and long‐term evidence‐based treatment strategies remains an active and acute need for these patients. Although antibiotics are often effective in the short term, there is a lack of evidence of medium‐ to long‐term antibiotic treatment for HS.24, 25
This pooled analysis from weeks 12 to 36 of PIONEER I and II is consistent with an optimal medium‐term adalimumab dosing strategy of continuing weekly adalimumab after week 12, as efficacy outcomes generally favoured the weekly dosing arm, and safety outcomes for those patients were not worse compared with the other dosing arms. Pooling the observations was appropriate based on the similarities of the studies, and provided a more robust assessment by combining the somewhat limited sample sizes in Period B of each study.
Overall, for patients who received ADAew in Period A, those who continued on ADAew in Period B had better HiSCR outcomes compared with those rerandomized to every‐other‐week dosing or to treatment discontinuation (placebo). Weekly treatment also resulted in significantly better HiSCR outcomes compared with placebo in all subgroups, except where subgroup sizes were small. In addition, week‐12 HiSCR responders who continued ADAew in Period B, showed better maintenance of response than those rerandomized to a reduced dosing regimen or to treatment discontinuation. From a clinical perspective, individual patients should expect fluctuations in their response to treatment, which clinicians should take into consideration when administering long‐term treatment with weekly adalimumab dosing.
A systematic reduction in HiSCR was observed in all treatment groups during Period B of the PIONEER trials. The apparent rate reduction over time using only the assessments from Period B for week‐12 HiSCR responders during Period B, could be due in part to the study design, which forced patients who lost response during Period B to discontinue the study and enter the OLE, even if the loss of response may have resulted from temporary disease exacerbation, which is common owing to the waxing and waning nature of HS.26, 27 This was addressed by integrating data from the PIONEER studies and from the OLE, which allowed these patients to demonstrate whether they could achieve HiSCR during continued treatment with ADAew throughout the OLE. The result showed maintenance of treatment response from week 12 (53·5%) to week 36 (55·6%). This may represent a more clinically relevant picture of treatment as it accounts for disease‐related fluctuations in treatment response, and illustrates that a loss of treatment response is not necessarily permanent.
Despite small patient numbers in the week‐12 partial responders (≥ 25% reduction in AN count) population who continued on ADAew, when this group was combined with the week‐12 HiSCR responders (i.e. PRR), the response at week 36 with continued weekly dosing was greater than for the week‐12 HiSCR responders alone, and was also greater than every‐other‐week dosing or placebo. This suggests that continuous weekly dosing is the most effective strategy over the subsequent 24 weeks for patients with at least a partial response to an initial 12 weeks of weekly treatment.
For those not achieving HiSCR by week 12, a partial response (≥ 25% reduction in AN count) at week 12 appeared to be predictive of later response, and HiSCR response was more likely with continued ADAew treatment vs. dose reduction or dose withdrawal, so allowing these patients enough time to respond to treatment is an important consideration. Continuing weekly dosing for patients with partial response is supported by the outcomes of other end points for this population, including reduced rates of flare, pain and lower lesion counts among patients rerandomized to weekly vs. every‐other‐week dosing or placebo.28 One caveat is that the small number of patients who achieved a partial HiSCR response precludes the possibility of robust, reliable inferences about characteristics that could predict an eventual HiSCR response.
Adalimumab weekly treatment provides an effective treatment option for patients with HS, but there is room for improvement. Although a clinical benefit was shown in the long term in 43% of patients overall at week 36, it is clear that further research for treatment options and targets should be pursued.
There appeared to be little safety risk with continuing weekly adalimumab therapy for patients with HS who had at least a partial response (≥ 25% reduction in AN count) by 12 weeks of weekly treatment, as the number and nature of adverse events for patients who received medium‐term (up to 36 weeks) weekly treatment (all‐ADAew population) did not show an increasing incidence. Additional benefits from weekly dosing during the first 12 weeks of the trials compared with placebo, included significant pain reduction and significantly better treatment response across subgroups. Regardless of whether patients were at Hurley stage II or III and whether antibiotic therapy was used,17 the baseline burden of disease was very similar, as were efficacy results.
For patients with a < 25% reduction in AN count at week 12, continuing weekly adalimumab treatment beyond week 12 yielded outcomes similar to placebo, suggesting that continuation of adalimumab treatment for patients without at least a partial response at week 12 cannot be recommended.
This analysis was limited by the small number of patients in the week‐12 HiSCR partial responder population who were rerandomized to weekly adalimumab dosing, and in the week‐12 HiSCR nonresponder population, and by the post hoc nature of the analyses for the PRR group.
After an induction dose, continuous adalimumab 40 mg weekly dosing was an effective medium‐term treatment for patients with moderate‐to‐severe HS throughout the two PIONEER trials. When achieving at least partial treatment success with adalimumab short‐term therapy, patients who continued on weekly dosing had better outcomes than those who were switched to every‐other‐week dosing or those whose treatment was interrupted. Patients with a < 25% reduction in AN count after 12 weeks of initial adalimumab weekly treatment did not demonstrate benefit from further adalimumab treatment. Conversely, it is important to note that the response trajectory for patients achieving > 25% but < 50% reduction in AN count in the first 12 weeks, may be delayed and, therefore, longer treatment periods may be needed to optimize disease improvement in this group. No new safety risks were identified with adalimumab weekly dosing through 36 weeks of treatment.
Supporting information
File S1. Methodology to identify population for continued adalimumab treatment.
Fig. S1. Patient disposition.
Acknowledgments
The authors would like to acknowledge Jody Bennett, employed by AbbVie, for medical writing support in the production of this publication.
Conflicts of interest
G.B.E.J. received honoraria from AbbVie, MSD and Pfizer for participation on advisory boards, grants from AbbVie, Actelion, Janssen‐Cilag, InflaRx, Leo Pharma, Novartis, Pierre Fabre, UCB and Regeneron for participation as an investigator, speaker honoraria from AbbVie, Galderma, Leo Pharma and MSD; and unrestricted research grants from AbbVie, Leo Pharma and Novartis. M.M.O. received honoraria from AbbVie for advisory board participation and for speaker services, and from AbbVie, Crescendo Biosciences, Gilead, UCB, GSK and Innovaderm for consultant services. M.M.O. was an AbbVie employee during this study. S.B.F. has received compensation from AbbVie, AstraZeneca, Janssen, Novartis, Promius, Celgene and Regeneron for research; from AbbVie for speaker services and from Galderma for consultation services. W.P.F.G. received honoraria from AbbVie, Actelion, Amgen, Arylide, Boehringer, Celgene, Cipher, Eli Lilly, Galderma, Janssen, Novartis and Tribute for participation on advisory boards and for consultant and speaker services, and received research grants from AbbVie, Amgen, Eli Lilly and Novartis. E.P.P. received honoraria from AbbVie, Amgen, Celgene, Janssen, Galderma, Novartis and Pfizer for participation as a speaker and serving on advisory boards and also received investigator‐initiated grants (paid to the Erasmus MC) from AbbVie, AstraZeneca, Janssen and Pfizer. U.M. received payment for work from the following commercial organizations either directly or indirectly through an intermediary: AbbVie, Almirall‐Hermal, Amgen, BASF, Biogen, Boehringer‐Ingelheim, Celgene, Centocor, Eli Lilly, Foamix, Forward Pharma, Galderma, Janssen, Leo Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL and Xenoport. A.W.A. served as an investigator and/or consultant for AbbVie, Amgen, Janssen, Merck, Lilly, Celgene, Novartis, Pfizer and Modernizing Medicine. Y.G., Z.G., D.A.W. and H.D.T. were full‐time employees of AbbVie Inc, and owned AbbVie stock and/or stock options. A.B.K. received honoraria as a consultant for AbbVie, Janssen, Lilly and Novartis, and grants as an investigator from AbbVie, Janssen, Novartis and UCB, and received fellowship funding from AbbVie and Janssen.
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial‐level data (analysis datasets), in addition to other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan and execution of a Data Sharing Agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing.html.
Funding sources AbbVie Inc. funded this study and participated in the study design, study research, collection, analysis and interpretation of data, and writing of this publication. All authors had access to the study data and collaborated in the manuscript preparation with support from a professional medical writer funded by the sponsor. All authors and AbbVie reviewed and approved the manuscript before submission, and the authors maintained control over the final content. The corresponding author had the final responsibility for the decision to submit for publication.
Conflicts of interest See Appendix.
References
- 1. Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2009; 23:985–98. [DOI] [PubMed] [Google Scholar]
- 2. Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012; 366:1580–64. [DOI] [PubMed] [Google Scholar]
- 3. Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart‐verified case‐control analysis. J Am Acad Dermatol 2014; 71:1144–50. [DOI] [PubMed] [Google Scholar]
- 4. Lipsker D, Severac F, Freysz M et al The ABC of hidradenitis suppurativa: a validated glossary on how to name lesions. Dermatology 2016; 232:137–42. [DOI] [PubMed] [Google Scholar]
- 5. Kurzen H, Kurokawa I, Jemec GB et al What causes hidradenitis suppurativa? Exp Dermatol 2008; 17:455–72. [DOI] [PubMed] [Google Scholar]
- 6. Jemec GB, Heidenheim M, Nielsen NH. Hidradenitis suppurativa–characteristics and consequences. Clin Exp Dermatol 1996; 21:419–23. [DOI] [PubMed] [Google Scholar]
- 7. von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol 2001; 144:809–13. [DOI] [PubMed] [Google Scholar]
- 8. Ring HC, Thorsen J, Saunte DM et al The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol 2017; 153:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zouboulis CC, Desai N, Emtestam L et al European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29:619–44. [DOI] [PubMed] [Google Scholar]
- 10. Delage M, Guet‐Revillet H, Duchatelet S et al Deciphering the microbiology of hidradenitis suppurativa: a step forward towards understanding an enigmatic inflammatory skin disease. Exp Dermatol 2015; 24:736–7. [DOI] [PubMed] [Google Scholar]
- 11. Ring HC, Riis Mikkelsen P, Miller IM et al The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol 2015; 24:727–31. [DOI] [PubMed] [Google Scholar]
- 12. Hessam S, Sand M, Georgas D et al Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol 2016; 29:161–7. [DOI] [PubMed] [Google Scholar]
- 13. Ring HC, Emtestam L. The microbiology of hidradenitis suppurativa. Dermatol Clin 2016; 34:29–35. [DOI] [PubMed] [Google Scholar]
- 14. Fischer AH, Haskin A, Okoye GA. Patterns of antimicrobial resistance in lesions of hidradenitis suppurativa. J Am Acad Dermatol 2017; 76:309–13.e2. [DOI] [PubMed] [Google Scholar]
- 15. Ring HC, Bay L, Kallenbach K et al Normal skin microbiota is altered in pre‐clinical hidradenitis suppurativa. Acta Derm Venereol 2017; 97:208–13. [DOI] [PubMed] [Google Scholar]
- 16. Kimball AB, Kerdel F, Adams D et al Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157:846–55. [DOI] [PubMed] [Google Scholar]
- 17. Kimball AB, Okun MM, Williams DA et al Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016; 375:422–34. [DOI] [PubMed] [Google Scholar]
- 18. Hurley HJ. Hidradenitis suppurativa In: Dermatologic Surgery: Principles and Practice, (Roenigk RK, Roenigk HH, Jr, eds), 2nd edn New York, NY: Marcel Dekker, 1996; 623–45. [Google Scholar]
- 19. Kimball AB, Jemec GB, Yang M et al Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014; 171:1434–42. [DOI] [PubMed] [Google Scholar]
- 20. Zouboulis CC, Del Marmol V, Mrowietz U et al Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015; 231:184–90. [DOI] [PubMed] [Google Scholar]
- 21. Chen G, Zhong H, Belousov A, Devanarayan V. A PRIM approach to predictive‐signature development for patient stratification. Stat Med 2015; 34:317–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKeegan EM, Ansell PJ, Davis G et al Plasma biomarker signature associated with improved survival in advanced non‐small cell lung cancer patients on linifanib. Lung Cancer 2015; 90:296–301. [DOI] [PubMed] [Google Scholar]
- 23. Kromann CB, Deckers IE, Esmann S et al Risk factors, clinical course and long‐term prognosis in hidradenitis suppurativa: a cross‐sectional study. Br J Dermatol 2014; 171:819–24. [DOI] [PubMed] [Google Scholar]
- 24. European Centre for Disease Prevention and Control . Antimicrobial resistance surveillance in Europe 2015. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS‐Net). Available at: https://www.ecdc.europa.eu (last accessed 16 June 2017).
- 25. World Health Organization . Global Action Plan on Antimicrobial Resistance, 2015. Available at https://www.who.int/antimicrobial-resistance/publications (last accessed 16 June 2017).
- 26. Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther 2004; 17:50–4. [DOI] [PubMed] [Google Scholar]
- 27. Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin 2010; 28:779–93. [DOI] [PubMed] [Google Scholar]
- 28. Gulliver W, Okun MM, Martorell A et al Therapeutic response guided dosing strategy to optimize long‐term adalimumab treatment in patients with hidradenitis suppurativa: integrated results from the PIONEER phase 3 trials. J Invest Dermatol 2016; 136 (Suppl. 2):S161. [Google Scholar]
- 29. World Health Organization . BMI Classification. Available at http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (last accessed 3 October 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Methodology to identify population for continued adalimumab treatment.
Fig. S1. Patient disposition.


