Abstract
Oxidative stress is a key regulator of idiopathic pulmonary fibrosis. Paraquat (PQ)‐induced pulmonary fibrosis seriously endangers people's health. Rapamycin has been reported to alleviate PQ‐induced pulmonary fibrosis, but its underlying mechanism is unclear. The nuclear factor E2‐related factor 2 (Nrf2) plays an important regulatory role in the antioxidant therapy of PQ‐induced pulmonary fibrosis. In this study, we tried to confirm that rapamycin attenuates PQ‐induced pulmonary fibrosis by regulating Nrf2 pathway. In vivo, we proved that rapamycin could inhibit the degree of PQ‐induced oxidant stress as well as enhanced the expression of Nrf2. In vitro, rapamycin decreased the upregulated effects of cell death and apoptosis, fibrosis‐related factors expression and fibroblast‐to‐myofibroblast transformation by PQ treatment. In vivo, rapamycin treatment reduced fibrosis degree and the expression of fibrosis‐related factors in lung tissues of rat treated PQ. Furthermore, we also found that Nrf2 knockdown reduced the inhibitory effect of rapamycin on PQ‐induced pulmonary fibrosis, as well as decreased Nrf2 transfer from the cytoplasm into the nucleus. Our findings demonstrated that the protective effect of rapamycin is associated with the activation of the Nrf2 pathway in pulmonary fibrosis induced by PQ poisoning.
Keywords: Nrf2, oxidant stress, paraquat, pulmonary fibrosis, rapamycin
The protective effect of rapamycin is associated with the activation of the Nrf2 pathway in pulmonary fibrosis induced by PQ poisoning.
1. INTRODUCTION
Paraquat (1,1‐dimethyl‐4,4‐bipyridinium dichloride, PQ), which is proven safety record, is an effective and widely used herbicide properly applied to remove weeds. However, over the past decades, PQ has caused thousands of accidental deaths. From 2011 to 2014 at the national level in South Korea, epidemiologic data in South Korea regarding acute herbicide or insecticide poisoning in adults demonstrate that PQ is the most common pesticide poisoning and produced the highest rate of fatality followed by endosulfan (Moon, Chun, & Cho, 2016).
PQ accumulates particularly in human lungs, and the PQ concentration in human lungs can be 6–10 times higher than that in the plasma (Sun & Chen, 2016). Some studies strongly suggest that human lungs are a specific target for the pathological effects of PQ (Dinis‐Oliveira et al., 2008). The pathogenesis of PQ toxicity is involved in acute lung damages in the initial stage and pulmonary fibrosis in the final stage. The mechanism of PQ causing pulmonary fibrosis is complex and still unclear, but some experts think that oxidative stress and inflammation might be involved in the process (Toygar et al., 2015). Nuclear factor E2‐related factor 2 (Nrf2) is an important transcription factor, and kelch‐like ECH‐associated Protein1 (Keap1) is its specific repressor. Keap1/Nrf2 signaling mediates oxidative stress in cells, while also regulates genes associated with the scavenging of oxygen free radicals. Some studies have verified that Naringenin (Podder, Song, & Kim, 2014), alpha‐lipoic acid (Kim, Podder, & Song, 2013), cycloartenyl ferulate (Hong et al., 2013), Silymarin (Zhao, Shi, Li, Li, & Zhao, 2015), and resveratrol (He, Wang, Szklarz, Bi, & Ma, 2012) are able to inhibit PQ‐induced lung damages or pulmonary fibrosis via Nrf2‐mediated pathway.
Rapamycin (sirolimus) has profound immunosuppressive effects and is currently used to help organ transplant recipients prevent graft rejection. The potential value of rapamycin in PQ‐induced acute lung injuries and pulmonary fibrosis has been proved, but the mechanism is still elusive (Chen, Jiao et al., 2015; Chen, Ma et al., 2015; Shao et al., 2015). The rapamycin‐mediated protection against oxidative stress is due in part to an increase in the transcription of antioxidant genes mediated by Nrf2 pathway (Calap‐Quintana et al., 2015). However, the association between rapamycin and Nrf2, as well as their effects on PQ‐induced oxidative stress remain unstated.
In this study, we aimed to explore the possible mechanism of rapamycin's effect through the Nrf2 pathway on PQ‐induced pulmonary fibrosis. For this purpose, we established PQ‐induced lung fibrosis rat model, and gave the Nrf2 inhibitor treatment and observed the effects of rapamycin. To further explore the interplay between rapamycin and Nrf2 pathway, lung fibroblasts (LFs) and Nrf2 that are silenced by transfection LFs were exposed to PQ or/and rapamycin.
2. MATERIALS AND METHODS
2.1. Ethical statement
The experiment and procedures of the animal were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Ethics Committee of Kunming Medical University.
2.2. Animal model
Healthy male rats (200–220 g; Vital River, Beijing, China) were acclimated the environment with free eating and drinking for 1 week. Then the rats were randomly divided into six groups: (a) control group (Untreated animals), (b) PQ group was intragastric administration with paraquat once, (c) Rapamycin group was injected with rapamycin 0.2 mg per kilogram every day, (d) Rapamycin+PQ group which was intragastric administration with paraquat once and was injected with rapamycin 0.2 mg per kilogram every day, (e) PQ+si‐Nrf2 group was injected both PQ and brusatol 0.2 mg per kilogram every day, and (f) PQ+si‐Nrf2+rapamycin group was injected among PQ, rapamycin, and brusatol 0.2 mg per kilogram every day. A total of 48 rats were used in our study, each group has 8 rats.
2.3. Cell culture and transfection
LFs was purchased from Shanghai Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). LFs cells were cultured in 10% fetal bovine serum (Hyclone, Logan) and 1% PS (100 units/ml penicillin and 100 mg/ml streptomycin) medium with GlutaMAX (Dulbecco's modified Eagle medium [DMEM], Gibco BRL). Cells were habitually passaged every 3–4 days, incubated in an incubator with 5% CO2 at 37°C.
Twenty‐four hours before transfection, LFs cells were seeded in six‐well plates with optimum density and then incubated overnight. sh‐Nrf2 and negative control were transfected into LFs cells with Lipofectamine 2000 reagent and Opti‐MEM medium (Invitrogen Life Technologies) in light of the specification. The sh‐Nrf2 and inhibitor control were bought from Tolo Biotech (Shanghai, China).
2.4. Hematoxylin‐eosin (H&E) staining
The lung tissues from rats were fixed with 4% paraformaldehyde (Sinopharm, Beijing, China) overnight, washed for 6 hr with flowing water, and dehydrated in ethanol of 70% (2 hr), 80% (overnight), 90% (2 hr), and 100% (twice in an hour). Then the tissues were immersed in xylene (Sinopharm) until transparent, and saturated by paraffin at 60 ℃ for 2 hr. The tissue block was cut into pieces of 5 μm, spread in water, and dried on a glass slide at 60 ℃ for 24 hr. The slices were dewaxed in xylene, hydrated in ethyl alcohol of 100% for 5 min twice, 95% for 2 min, 85% for 2 min, 75% for 2 min, and water for 2 min. Then the slices were stained by hematoxylin (Solarbio, Beijing, China) for 5 min and eosin (Sinopharm) for 3 min, dehydrated in ethanol of 75% for 2 min, 85% for 2 min, 95% for 2 min, 100% for 5 min twice, xylene for 10 min twice, and mounted with neutral balsam. The sections were observed and photographed with a microscope (Olympus, Tokyo, Japan) at 200× magnification.
2.5. Masson staining
Lung tissues sections were subjected to Masson staining. Weigert's iron hematoxylin solution was added, followed by 1% hydrochloric acid (Sinopharm) in alcohol (Sinopharm) for differentiation. Then the sections were counterstained in xylidine‐ponceau 2R and sealed with neutral balsam (Sinopharm). Under the low‐power microscope, the collagen fibers, and nuclei were stained blue, and the cytoplasm, muscle fibers, and red blood cells were stained red.
2.6. Detection of superoxide dismutase, glutathione, and catalase
The tissue was added into phosphate‐buffered saline (PBS) of 9‐fold volume, scattered into cell suspension, freeze‐thawed for three times, and centrifuged in 12,000 rpm for 10 min. The supernatant was collected as the protein sample, and the concentration was detected by BCA protein quantitative kit (Beyotime Biotechnology, China) according to the protocol. Superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) content was detected by enzyme linked immunosorbent assay (ELISA) (Jiancheng, Nanjing, China) according to the manufacturer's protocol.
2.7. RT‐qPCR
RNA of cells was extracted using total RNA rapid extraction kit (BioTeke, Beijing, China) according to the manufacturer's protocol. After detecting the concentration, 1 μg of RNA sample was reversely transcribed into complementary DNA (cDNA) with M‐MLV reverse transcriptase (BioTeke) in the presence of oligo(dT) and random 50 primers (Invitrogen, Guangzhou, China). The instruments in this section were pro‐treated by surface RNase Erase (TIANDZ, Beijing, China) and the reagents were RNase‐free. The cDNA (1 μl for each reaction) was used for real‐time PCR to detect Nrf2 using 2× Power Taq PCR MasterMix (BioTeke) and SYBR Green (Solarbio), with β‐actin as the internal control. The PCR procedure was set as follows: 95°C for 10 min, 38 cycles of 95°C for 12 s, 60°C for 18 s and 72°C for 30 s, and finally 4°C for 5 min. Calculations were performed using the method. Information on real‐time primers as follows: β‐actin Forward primer: 5′‐GGAGATTACTGCCCTGGCTCCTAGC‐3′, β‐actin Reversed primer: 5′‐GGCCGGACTCATCGTACTCCTGCTT‐3′; Nrf2 Forward primer: 5′‐ CCCAGCACATCCAGACAGAC‐3′, Nrf2 Reversed primer: 5′‐TATCCAGGGCAAGCGACTC‐3′.
2.8. Western blot
Protein was extracted using a whole‐cell lysis kit (CWBio, Beijing, China) from cells and tissues, and the concentration of protein was measured using a BCA protein quantitative kit (Beyotime Biotechnology). After being denatured by boiling, the protein sample (40 μg for each lane) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Boston, MA). After blocking with 5% skim milk (YILI, Hohhot, Inner Mongolia, China) at room temperature for 1 hr, the membrane was incubated with the following antibodies at 4°C overnight: rabbit anti‐HO‐1 (1:500; Santa Cruz, CA), rabbit anti‐Keap1 (1:200; Santa Cruz), mouse anti‐α‐SMA (1:400; Boster, Wuhan, Hubei, China), rabbit anti‐Col I (1:400; Boster), mouse anti‐Col III (1:400; Boster), rabbit anti‐MMP‐2 (1:400; Santa Cruz), rabbit anti‐MMP‐9 (1:400; Santa Cruz), rabbit anti‐TIMP (1:400; Santa Cruz), and rabbit anti‐Nrf2 (1:200; (Santa Cruz). After rinsing with TBST, the membrane was incubated with goat antirabbit IgG labeled with horseradish peroxidase (HRP) (1:5,000; Santa Cruz) or goat antimouse IgG‐HRP (1:5,000; Santa Cruz) at 37°C for 45 min, and explored with ECL reagent (Thermo Fisher Scientific). After removing antibodies by stripping buffer (Beyotime Biotechnology), the membrane was incubated with mouse anti‐β‐actin (1:1,000; Santa Cruz), and goat antimouse IgG‐HRP (1:5,000; Santa Cruz) to detect the internal control, β‐actin. Optical density values of bands were analyzed by a gel image processing system (BioRad).
2.9. MTT assay
Cells were seeded into 96‐well plates with 5 × 103 per pore to culture. After adhering to the plate, the cells were treated with drug or laser (different in groups), then 3‐(4,5‐di‐methyl‐2‐thiazolyl)‐2,5‐diphenyl‐2‐H‐tetrazolium bromide (MTT; Macklin) was added into the medium with 0.2 mg/ml, and 24 hr later, the plate was centrifuged at 1,000 rpm for 10 min and the supernatant was discarded. The crystal was dissolved with dimethyl sulfoxide (DMSO; Sigma‐ Aldrich) (200 μl per pore), and the optical density of the solution was measured at 490 nm.
2.10. Immunofluorescence
Cells were seeded onto glass slide beforehand. When the confluence reached 70%, the cells were fixed with 4% paraformaldehyde (Sinopharm) for 15 min and permeated with 0.1% Triton X‐100 (Amresco, Solon, OH) at room temperature for 30 min. After blocking with goat serum (Solarbio) for 15 min, the cells were incubated with mouse anti‐α‐SMA (1:400; Boster) and rabbit anti‐Nrf2 (1:200; Santa Cruz) at 4°C overnight. After rinsing with PBS, the cells were incubated with goat antirabbit immunoglobulin G (IgG) labeled with Cy3 (1:200, diluted with PBS; Invitrogen) at room temperature in the dark for 60 min. After being counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Invitrogen, USA), the cells were mounted in the presence of anti‐fluorescence quenching agent (Abcam), observed and photographed with a fluorescence microscope (Olympus) at 400× magnification.
2.11. ROS generation
Single cell suspensions were obtained from lung tissues of rats by enzymatic dissociation and the number of cells counted no less than 1 × 105. Reactive oxygen species (ROS) was measured using the 2′,7′‐ dichlorofluorescin diacetate (DCFH‐DA) assay. The cells were incubated with 10 μM DCFH‐DA for 30–60 min at 37°C and then were washed with PBS. Fluorescence density was measured by FACScan instrument (Becton Dickinson) at the excitation wavelength of 485 nm and the emission wavelength of 530 nm.
2.12. Statistical analysis
The data in this study are presented as mean ± standard deviation (SD) of 3 or 5 individual experiments, and analyzed by one‐way ANOVA test, which was considered statistically significantly p < .05 or p < .01.
3. RESULTS
3.1. Rapamycin suppresses PQ‐induced oxidant stress in pulmonary fibrosis
Based on our previous study demonstrated rapamycin alleviates PQ‐induced pulmonary fibrosis through upregulating Nrf2 (Xu et al., 2017), we attempted to explore the effects of rapamycin on the oxidant stress by regulating Nrf2 pathway in the lung tissues of rat with PQ‐induced pulmonary fibrosis. As shown in Figure 1a, ROS generation in lung tissues of rat treated with PQ was significantly upregulated and more obvious on the 7th day than the 28th day compared with control group and rapamycin only group, but rapamycin was decreased PQ‐induced ROS generation. Besides, there is no significant difference between control group and rapamycin only group. Moreover, reverse transcriptase quantitative polymerase chain reaction (RT‐qPCR) analysis results show that the level of NQO1 was significantly increased on the 7th day after PQ administration (p < .01, Figure 1b), but decreased on the 28th day after PQ administration (p < .01, Figure 1b). ELISA analysis showed that PQ treatment for the 7th day was markedly reduced the levels of GSH and CAT, and SOD activity (Figure 1c–e), while increased on the 28th day after PQ treatment. Besides, rapamycin treatment was rescued the oxidative stress in PQ‐induced pulmonary fibrosis (Figure 1b–e). Furthermore, western blot results revealed that PQ treatment was significantly decreased the expression of Nrf2 and HO‐1 proteins, and enhanced the level of Keap1 protein (Figure 1f), whereas rapamycin reversed the expression of oxidant/antioxidant factors. Taken together, rapamycin relieves oxidant stress in PQ‐induced pulmonary fibrosis by regulating Nrf2 pathway.
Figure 1.
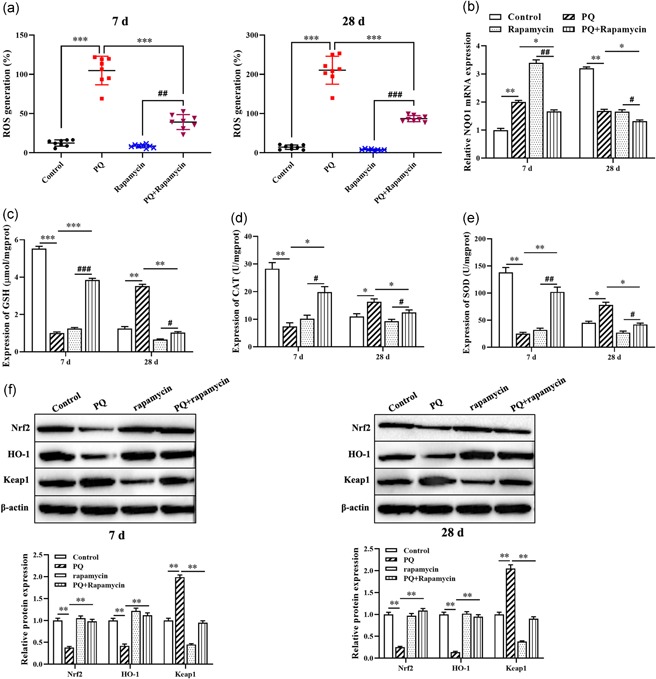
Rapamycin suppresses PQ‐induced oxidant stress in pulmonary fibrosis. (a) The expression of ROS level was detected by DCFH‐DA assay. (b) RT‐qPCR was used to measure the expression of NQO1. (c–e) The levels of GSH and CAT, and SOD activity were evaluated by ELISA kit. (f) Western blot was applied to detect the expression of Nrf2, HO‐1, and Keap1 proteins. *p < .05, **p < .01, ***p < .001, compared with control group; # p < .05, ## p < .01, ### p < .001, compared with rapamycin group. CAT, catalase; DCFH‐DA, 2’,7’‐dichlorofluorescin diacetate; ELISA, enzyme‐linked immunosorbent assay; GSH, glutathione; Nrf2, nuclear factor E2‐related factor 2; PQ, paraquat; ROS, reactive oxygen species; RT‐qPCR, reverse transcriptase quantitative polymerase chain reaction; SOD, superoxide dismutase [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Rapamycin alleviates cell death and apoptosis of LFs induced by PQ
Previous studies suggested that ROS generation induced cell death and apoptosis (Liao, Li, Zeng, & Lan, 2017), we next detected the effect of rapamycin on the proliferation ability and apoptosis of LFs treated with PQ by MTT assay and flow cytometry. Primarily, Western blot showed that knockdown of Nrf2 was significantly decreased the expression of Nrf2 protein compared with NC group (p < .01, Figure 2a). Moreover, MTT assay showed that PQ treated LFs was significantly inhibited the cell viability compared with control group (p < .01, Figure 2b), while rapamycin together treatment reversed the effect of PQ on the proliferation of LFs. Of note, knockdown of Nrf2 promoted PQ‐inhibited cell viability as well as decreased the protective effect of rapamycin on PQ‐treated LFs (p < .01, Figure 2b). In addition, flow cytometry analysis results revealed that PQ enhanced cell death and apoptosis compared with control group (p < .01, Figure 2c,d) but rapamycin decreased the upregulated effect of PQ or Nrf2 knockdown on apoptosis of LFs. Furthermore, to further explore the role of Nrf2 in PQ‐reduced cell death and apoptosis, we observed the location of Nrf2 by immunofluorescence. The results showed that PQ decreased the expression of Nrf2 in the nucleus as well as Nrf2 knockdown was enhanced the PQ blocking Nrf2 from cytoplasm to nucleus, while rapamycin reversed the situation and significantly promoted the expression of Nrf2 in the nucleus (Figure 2e). Taken together, rapamycin suppresses PQ‐induced cell death and apoptosis through activating Nrf2 pathway.
Figure 2.
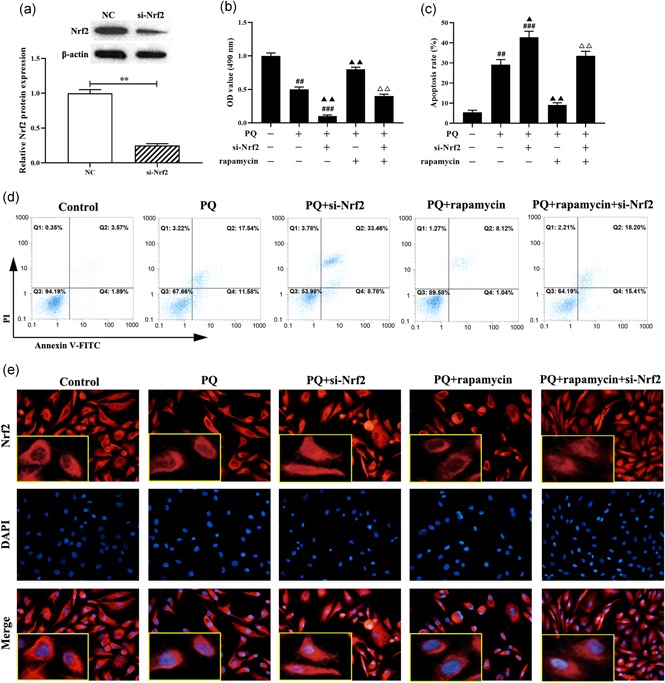
Rapamycin alleviates cell death and apoptosis of LFs induced by PQ treatment. (a) Western blot was used to detect the expression of Nrf2 protein. (b) MTT was applied to evaluate the cell viability. (c and d) The apoptosis of LFs was measured by flow cytometry. (e) Immunofluorescence was used to assess the localization of Nrf2 (Red). **p < .05, compared with NC group; # p < .05, ## p < 0.01, ### p < .001, compared with control group; ▲ p < .05, ▲▲ p < .01, ▲▲▲ p < .001, compared with PQ group; △△ p < .01, compared with PQ+rapamycin group. LFs, lung fibroblast; Nrf2, nuclear factor E2‐related factor 2; PQ, paraquat [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Rapamycin reversed PQ‐induced fibroblast‐to‐myofibroblast transformation
Transformation of fibroblast to myofibroblast is a critical cellular event in fibrosis. Western blot showed that PQ was significantly increased the expression of myofibroblast marker protein α‐SMA, and rapamycin exerted an inhibitory effect on PQ‐induced fibroblast‐to‐myofibroblast transformation (FMT) (p < .01, Figure 3a). Meanwhile, knockdown of Nrf2 expression on LFs, PQ significantly increased the expression of α‐SMA and attenuated the effectiveness of rapamycin inhibited FMT (Figure 3a). Besides, the expression of α‐SMA in LFs treated with PQ was evaluated by immunofluorescence, which was consistent with Western blot analysis (Figure 3b). Taken together, rapamycin decreased PQ‐induced FMT through upregulating Nrf2.
Figure 3.
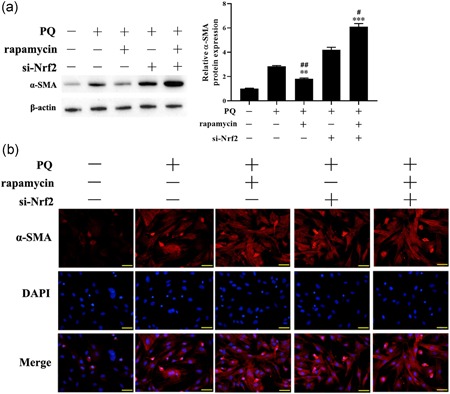
Rapamycin reversed PQ‐induced fibroblast‐to‐myofibroblast transformation. (a) Western blot was applied to detect the expression of α‐SMA protein. (b) The expression of α‐SMA was evaluated by immunofluorescence. **p < .01, ***p < .001, compared with PQ group; # p < .05, ## p < .01, compared with PQ+si‐Nrf2 group. Nrf2, nuclear factor E2‐related factor 2; PQ, paraquat [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Inhibitory effect of rapamycin on fibrosis‐related factors in LFs treated with PQ and lung tissues of rat with PQ‐stimulated pulmonary fibrosis
To further confirm the underlying mechanism of rapamycin relieves pulmonary fibrosis stimulated by PQ, we detected the expression of fibrosis related factors, including Col‐I, Col‐III, MMP2, MMP9, and TIMP by Western blot and immunofluorescence. PQ treatment results showed that the expression of Col‐I, Col‐III, MMP2, and MMP9 in lung tissues were higher than the control group and inhibited the expression of TIMP (Figure 4a). Meanwhile, rapamycin attenuated PQ‐induced the expression of profibrogenic factors. However, knockdown of Nrf2 has promoted the inhibitory effect of PQ on the expression of profibrogenic factors, and the function of rapamycin was further weakened. Furthermore, immunofluorescence analysis showed that the expression of Col‐I, Col‐III, MMP2, and MMP9 in LFs treated with PQ were significantly decreased after rapamycin treatment, while knockdown of Nrf2 was reversed the suppressor effect of rapamycin on fibrosis (Figure 4b). Taken together, rapamycin inhibited the PQ‐induced pulmonary fibrosis through activating Nrf2 pathway and thus exerting an anti‐fibrosis effect.
Figure 4.
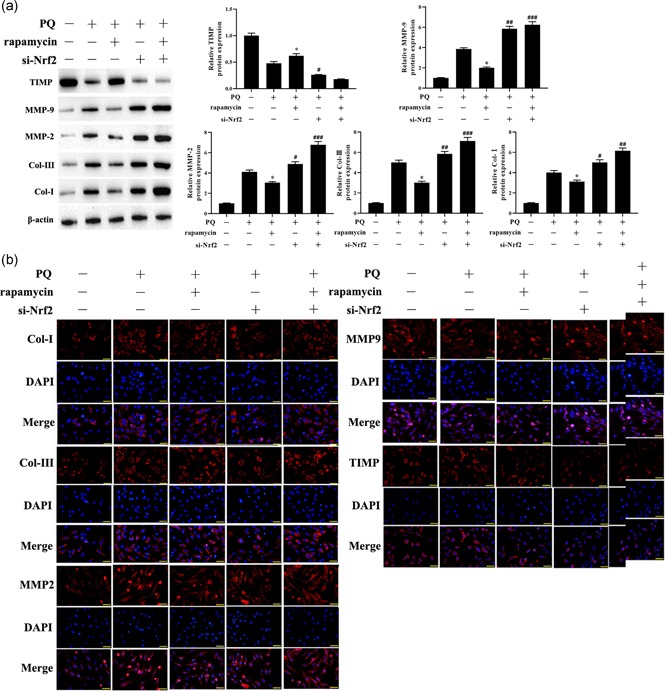
Inhibitory effect of rapamycin on fibrosis‐related factors in LFs treated with PQ and lung tissues of rat with PQ‐stimulated pulmonary fibrosis. (a) The expression of Col‐I, Col‐III, MMP2, MMP9, and TIMP in lung tissues were detected by Western blot. (b) Immunofluorescence micrographs of Col‐I, Col‐III, MMP2, MMP9, and TIMP in LFs, Red indicates the protein of interest, and blue indicates the cell nucleus. *p < .05, compared with PQ group; # p < .05, ## p < .01, ### p < .001, compared with PQ+rapamycin group. LFs, lung fibroblast; PQ, paraquat [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Rapamycin decreased pulmonary fibrosis stimulated by PQ in vivo
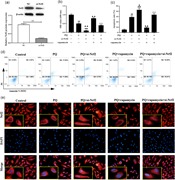
To assess rapamycin alleviated pulmonary fibrosis of rat treated with PQ, we observed the histopathological lung by H&E and Masson staining. H&E staining of lung sections from the rat treated PQ revealed that the diffuse alveolar has collapsed and thickened compared with control group and rapamycin only group (Figure 5a). Masson staining showed that diffuse perialveolar, peribronchial, and interstitial fibrosis in lung tissues sections of the PQ group (Figure 5b). Of note, this phenomenon was assuaged by rapamycin treatment. Besides, knockdown of Nrf2 aggravated PQ‐induced pulmonary fibrosis in rats as well as reduced the anti‐fibrosis function of rapamycin (Figure 5).
Figure 5.
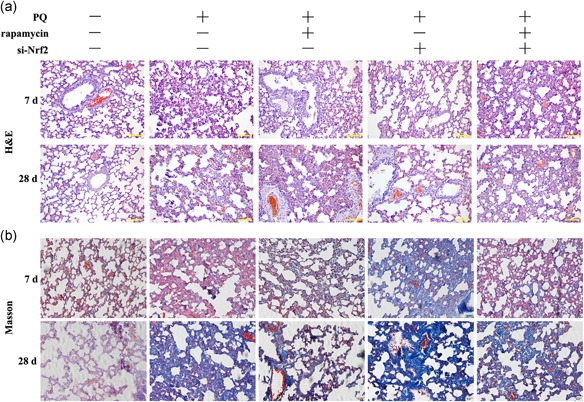
Rapamycin decreased pulmonary fibrosis stimulated by PQ in vivo. (a and b) H&E and Masson staining were used to evaluate the histological of rat lung ( ×200). PQ, paraquat [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this study, we demonstrated that rapamycin alleviates PQ‐induced pulmonary fibrosis as well as decreased the expression levels of Col I, Col III, MMP2, and MMP9. This result is similar to the previous study (28624451). These effects may be partly ascribed to the inhibition of TGF‐β1 (Shao et al., 2015). In the initial stage, rapamycin could also reverse PQ‐induced acute lung injuries by inhibiting the activation of NF‐κB (Chen, Ma et al., 2015).
A keap1/nrf2 signaling pathway is also involved in PQ‐induced lung fibrosis (Dou et al., 2016). He and many others have all found that Nrf2 is critical in terms of defending against PQ toxin in normal cells and the protective effects of resveratrol (He et al., 2012). Ding's study demonstrated that the protective effects of SIRT1 are associated with the activation of NRF2/ARE antioxidant pathway in lung injuries induced by PQ toxin (Ding et al., 2016). To explain the role of Nrf2 pathway in rapamycin inhibiting lung fibrosis, we injected the Nrf2 inhibitor into rats with PQ‐induced lung fibrosis, and subsequently, the effects of rapamycin were weakened. We selected the LFs as the targets for PQ, and results demonstrated that rapamycin is able to block PQ‐induced FMT and down‐regulate the expression of collagen III, collagen I, MMP2 and MMP9, and up‐regulate TIMP in the lung fibroblast. Meantime, the effect of rapamycin on lung fibroblast was inhibited by silencing the expression of Nrf2 in LFs cells. Furthermore, in these processes, rapamycin promoted Nrf2′ transfer from the cytoplasm to the nucleus.
Rapamycin, an inhibitor of mammalian target of rapamycin (mTOR), was initially introduced into clinical practice as an efficacious maintenance immunosuppressant in the treatment of transplant rejection (Webster, Lee, Chapman, & Craig, 2006). The antifibrotic effects of mTOR inhibition have been reported in several rat models of chronic kidney disease, including diabetic nephropathy, chronic glomerulosclerosis and tubulointerstitial fibrosis (Krämer et al., 2008; Lioberas et al., 2006; Wu et al., 2006). In the bleomycin‐induced pulmonary fibrosis rat model, rapamycin alleviates alveolitis and pulmonary fibrosis through decreasing the expression of MMP‐9 and tissue inhibitors of metalloproteinase‐1 in lung tissue (Jin et al., 2014). In our study, we also found that the expression of MMP‐2 and MMP‐9 was higher and that TIMP was lower when LFs were cocultured with PQ, but rapamycin reversed the results. Other studies demonstrated that mTOR is a major effector of epidermal growth factor receptor (EGFR)‐induced pulmonary fibrosis (Korfhagen et al., 2009). In the recent study, mTOR overactivation in alveolar epithelial cells (AECs) and compromised autophagy in the lungs are involved in the pathogenesis of pulmonary fibrosis (Gui et al., 2015). There is growing evidence to support the idea that the mTOR signaling pathway plays a key role in pulmonary fibrosis (Simler et al., 2002; Tulek et al., 2011; Yoshizaki et al., 2010).
In summary, our results demonstrated that rapamycin is able to alleviate PQ‐induced pulmonary fibrosis and that Nrf2 pathway is involved in this process. The effect of rapamycin on PQ‐induced lung fibrosis would be weakened if the expression of Nrf2 is inhibited. But this experiment could not further explain the mechanism of rapamycin regulating the expression of Nrf2.
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (grant number 81360015, 81560015 and 81860018); Yunnan applied basic research projects (grant number 2013FB049); Yunnan applied basic research projects‐joint special project (grant number 2014FB046, 2017FE468(‐210) and 2017FE468(‐005)), Young and middle‐aged academic and technical reserve talents in Yunnan province (grant number 2018HB18) and Yunnan medical science leader (grant number D‐201628).
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
Z. D. and T. Z. designed the study design and the experiments. W. T., W. W., and S. D. carried out PCR and Western blot. Z. L., W. Y., and T. Z. carried out the immunofluorescence staining and fluorescence microscopy. W. T., W. L., and C. V. participated in the design of the study and performed the statistical analysis. Z. D., T. Z., and C. V. draft the manuscript. All authors read and approved the final manuscript.
Tai W, Deng S, Wu W, et al. Rapamycin attenuates the paraquat‐induced pulmonary fibrosis through activating Nrf2 pathway. J Cell Physiol. 2019;235:1759–1768. 10.1002/jcp.29094
Contributor Information
Tao Zhang, Email: ZT6958@sina.com.
Zhaoxing Dong, Email: zhaoxingdong2222@126.com.
References
REFERENCES
- Calap‐Quintana, P. , Soriano, S. , Llorens, J. V. , Al‐Ramahi, I. , Botas, J. , Moltó, M. D. , & Martínez‐Sebastián, M. J. (2015). TORC1 inhibition by rapamycin promotes antioxidant defences in a drosophila model of friedreich's ataxia. PLoS One, 10(7), e0132376 10.1371/journal.pone.0132376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D. , Jiao, G. , Ma, T. , Liu, X. , Yang, C. , & Liu, Z. (2015). The mechanism of rapamycin in the intervention of paraquat‐induced acute lung injury in rats. Xenobiotica, 45(6), 538–546. 10.3109/00498254.2014.995149 [DOI] [PubMed] [Google Scholar]
- Chen, D. , Ma, T. , Liu, X. W. , Yang, C. , & Liu, Z. (2015). Rapamycin reverses paraquat‐induced acute lung injury in a rat model through inhibition of NF‐κB activation. International Journal of Clinical and Experimental Pathology, 8(5), 4627–4638. [PMC free article] [PubMed] [Google Scholar]
- Ding, Y. W. , Zhao, G. J. , Li, X. L. , Hong, G. L. , Li, M. F. , Qiu, Q. M. , … Lu, Z. Q. (2016). SIRT1 exerts protective effects against paraquat‐induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. International Journal of Molecular Medicine, 37(4), 1049–1058. 10.3892/ijmm.2016.2503 [DOI] [PubMed] [Google Scholar]
- Dinis‐Oliveira, R. J. , Duarte, J. A. , Sánchez‐Navarro, A. , Remião, F. , Bastos, M. L. , & Carvalho, F. (2008). Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Critical Reviews in Toxicology, 38(1), 13–71. 10.1080/10408440701669959 [DOI] [PubMed] [Google Scholar]
- Dou, T. , Yan, M. , Wang, X. , Lu, W. , Zhao, L. , Lou, D. … Zhou, Z. (2016). Nrf2/ARE Pathway Involved in Oxidative Stress Induced by Paraquat in Human Neural Progenitor Cells. Oxidative Medicine and Cellular Longevity, 2016, 8923860 10.1155/2016/8923860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, Y. S. , Wang, L. , Tian, X. , Li, X. , Ma, A. , Zhou, W. , … Xu, K. F. (2015). mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. PLoS One, 10(9), e0138625 10.1371/journal.pone.0138625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Wang, L. , Szklarz, G. , Bi, Y. , & Ma, Q. (2012). Resveratrol inhibits paraquat‐induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2‐related factor 2 pathway. Journal of Pharmacology and Experimental Therapeutics, 342(1), 81–90. 10.1124/jpet.112.194142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, G. L. , Liu, J. M. , Zhao, G. J. , Wang, L. , Liang, G. , Wu, B. , … Lu, Z. Q. (2013). The reversal of paraquat‐induced mitochondria‐mediated apoptosis by cycloartenyl ferulate, the important role of Nrf2 pathway. Experimental Cell Research, 319(8), 2845–2855. 10.1016/j.yexcr.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Jin, X. , Dai, H. , Ding, K. , Xu, X. , Pang, B. , & Wang, C. (2014). Rapamycin attenuates bleomycin‐induced pulmonary fibrosis in rats and the expression of metalloproteinase‐9 and tissue inhibitors of metalloproteinase‐1 in lung tissue. Chin Medical Journal, 127(7), 1304–1309. [PubMed] [Google Scholar]
- Kim, Y. S. , Podder, B. , & Song, H. Y. (2013). Cytoprotective effect of alpha‐lipoic acid on paraquat‐exposed human bronchial epithelial cells via activation of nuclear factor erythroid related factor‐2 pathway. Biological and Pharmaceutical Bulletin, 36(5), 802–811. [DOI] [PubMed] [Google Scholar]
- Korfhagen, T. R. , Le Cras, T. D. , Davidson, C. R. , Schmidt, S. M. , Ikegami, M. , Whitsett, J. A. , & Hardie, W. D. (2009). Rapamycin prevents transforming growth factor‐alpha‐induced pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 41(5), 562–572. 10.1165/rcmb.2008-0377OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer, S. , Wang‐Rosenke, Y. , Scholl, V. , Binder, E. , Loof, T. , Khadzhynov, D. , … Peters, H. (2008). Low‐dose mTOR inhibition by rapamycin attenuates progression in anti‐thy1‐induced chronic glomerulosclerosis of the rat. American Journal of Physiology. Renal Physiology, 294(2), F440–F449. 10.1152/ajprenal.00379.2007 [DOI] [PubMed] [Google Scholar]
- Liao, Y. R. , Li, Z. J. , Zeng, P. , & Lan, Y. Q. (2017). TLR7 deficiency contributes to attenuated diabetic retinopathy via inhibition of inflammatory response. Biochemical and Biophysical Research Communications, 493(2), 1136–1142. 10.1016/j.bbrc.2017.08.085 [DOI] [PubMed] [Google Scholar]
- Lioberas, N. , Cruzado, J. M. , Franquesa, M. , Herrero‐Fresneda, I. , Torras, J. , Alperovich, G. , … Grinyó, J. M. (2006). Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. Journal of the American Society of Nephrology, 17(5), 1395–1404. 10.1681/ASN.2005050549 [DOI] [PubMed] [Google Scholar]
- Moon, J. M. , Chun, B. J. , & Cho, Y. S. (2016). The characteristics of emergency department presentations related to acute herbicide or insecticide poisoning in South Korea between 2011 and 2014. Journal of Toxicology and Environmental Health, Part A, 79(11), 466–476. 10.1080/15287394.2016.1172529 [DOI] [PubMed] [Google Scholar]
- Podder, B. , Song, H. Y. , & Kim, Y. S. (2014). Naringenin exerts cytoprotective effect against paraquat‐induced toxicity in human bronchial epithelial BEAS‐2B cells through NRF2 activation. Journal of Microbiology and Biotechnology, 24(5), 605–613. [DOI] [PubMed] [Google Scholar]
- Shao, X. , Li, M. , Luo, C. , Wang, Y. Y. , Lu, Y. Y. , Feng, S. , … Chen, J. H. (2015). Effects of rapamycin against paraquat‐induced pulmonary fibrosis in mice. Journal of Zhejiang University. Science. B, 16(1), 52–61. 10.1631/jzus.B1400229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simler, N. R. , Howell, D. C. , Marshall, R. P. , Goldsack, N. R. , Hasleton, P. S. , Laurent, G. J. , … Egan, J. J. (2002). The rapamycin analogue SDZ RAD attenuates bleomycin‐induced pulmonary fibrosis in rats. European Respiratory Journal, 19(6), 1124–1127. [DOI] [PubMed] [Google Scholar]
- Sun, B. , & Chen, Y. G. (2016). Advances in the mechanism of paraquat‐induced pulmonary injury. European Review for Medical and Pharmacological Sciences, 20(8), 1597–1602. [PubMed] [Google Scholar]
- Toygar, M. , Aydin, I. , Agilli, M. , Aydin, F. N. , Oztosun, M. , Gul, H. , … Honca, M. (2015). The relation between oxidative stress, inflammation, and neopterin in the paraquat‐induced lung toxicity. Human and Experimental Toxicology, 34(2), 198–204. 10.1177/0960327114533808 [DOI] [PubMed] [Google Scholar]
- Tulek, B. , Kiyan, E. , Toy, H. , Kiyici, A. , Narin, C. , & Suerdem, M. (2011). Anti‐inflammatory and anti‐fibrotic effects of sirolimus on bleomycin‐induced pulmonary fibrosis in rats. Clinical and Investigative Medicine, 34(6), E341. [DOI] [PubMed] [Google Scholar]
- Webster, A. C. , Lee, V. W. , Chapman, J. R. , & Craig, J. C. (2006). Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: A systematic review and meta‐analysis of randomized trials. Transplantation, 81(9), 1234–1248. [DOI] [PubMed] [Google Scholar]
- Wu, M. J. , Wen, M. C. , Chiu, Y. T. , Chiou, Y. Y. , Shu, K. H. , & Tang, M. J. (2006). Rapamycin attenuates unilateral ureteral obstruction‐induced renal fibrosis. Kidney International, 69(11), 2029–2036. 10.1038/sj.ki.5000161 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Tai, W. , Qu, X. , Wu, W. , Li, Z. , Deng, S. , … Dong, Z. (2017). Rapamycin protects against paraquat‐induced pulmonary fibrosis: Activation of Nrf2 signaling pathway. Biochemical and Biophysical Research Communications, 490(2), 535–540. 10.1016/j.bbrc.2017.06.074 [DOI] [PubMed] [Google Scholar]
- Yoshizaki, A. , Yanaba, K. , Yoshizaki, A. , Iwata, Y. , Komura, K. , Ogawa, F. , … Sato, S. (2010). Treatment with rapamycin prevents fibrosis in tight‐skin and bleomycin‐induced mouse models of systemic sclerosis. Arthtitis and Rheumatism, 62(8), 2476–2487. 10.1002/art.27498 [DOI] [PubMed] [Google Scholar]
- Zhao, F. , Shi, D. , Li, T. , Li, L. , & Zhao, M. (2015). Silymarin attenuates paraquat‐induced lung injury via Nrf2‐mediated pathway in vivo and in vitro. Clinical and Experimental Pharmacology and Physiology, 42(9), 988–998. 10.1111/1440-1681.12448 [DOI] [PubMed] [Google Scholar]


