Abstract
Aims
To identify clinically useful associations between HbA1c levels and various continuous glucose monitoring‐derived metrics.
Methods
We retrospectively analysed end‐of‐study HbA1c levels and >2 weeks of continuous glucose monitoring data collected from 530 adults with Type 1 diabetes or insulin‐requiring Type 2 diabetes during four randomized trials. Each trial lasted ≥24 weeks and provided central laboratory end‐of‐study HbA1c levels and continuous glucose monitoring data from the preceding 3 months. Participants were assigned to groups based on either HbA1c levels or continuous glucose monitoring‐derived glucose values.
Results
HbA1c was strongly correlated with mean glucose value (r=0.80), time spent with glucose values in the 3.9–10.0 mmol/l range (time in range; r=–0.75) and percentage of glucose values >13.9 mmol/l (r=0.72), but was weakly correlated with the percentage of glucose values <3.9 mmol/l (r=–0.39) or <3.0 mmol/l (r=–0.21). The median percentage of glucose values <3.0 mmol/l was <1.2% (<20 min/day) for all HbA1c‐based groups, but the median percentage of values >13.9 mmol/l varied from 2.5% (0.6 h/day) to 27.8% (6.7 h/day) in the lowest and highest HbA1c groups, respectively. More than 90% of participants with either <2% of glucose values >13.9 mmol/l, mean glucose <7.8 mmol/l, or time in range >80% had HbA1c levels ≤53 mmol/mol (≤7.0%). For participants with HbA1c ≥64 mmol/mol (≥8.0%), the median time in range was 44%, with 90% of participants having a time in range of <59%.
Conclusions
The associations shown in the present study suggest that continuous glucose monitoring‐derived metrics may help guide diabetes therapy intensification efforts in an HbA1c‐independent manner.
What's new?
Glycaemic control can be assessed with HbA1c or with descriptive statistics from continuous glucose monitoring (CGM) data. HbA1c is highly correlated with the average CGM‐derived glucose value.
Using HbA1c and CGM data from recently completed clinical trials, we found HbA1c to be highly correlated with the percentage of CGM values indicating hyperglycaemia, but poorly correlated with the percentage of CGM values indicating hypoglycaemia.
Because CGM data revealed hypoglycaemia among participants with HbA1c values ≥69 mmol/mol (≥8.5%), relaxation of HbA1c goals is not an effective strategy for hypoglycaemia prevention.
CGM‐based heuristics to guide therapy intensification efforts independently of HbA1c are also described.
What's new?
Glycaemic control can be assessed with HbA1c or with descriptive statistics from continuous glucose monitoring (CGM) data. HbA1c is highly correlated with the average CGM‐derived glucose value.
Using HbA1c and CGM data from recently completed clinical trials, we found HbA1c to be highly correlated with the percentage of CGM values indicating hyperglycaemia, but poorly correlated with the percentage of CGM values indicating hypoglycaemia.
Because CGM data revealed hypoglycaemia among participants with HbA1c values ≥69 mmol/mol (≥8.5%), relaxation of HbA1c goals is not an effective strategy for hypoglycaemia prevention.
CGM‐based heuristics to guide therapy intensification efforts independently of HbA1c are also described.
Introduction
HbA1c is a valuable indirect biomarker of average glycaemia, and informs the relationship between glycaemic control and the chronic vascular complications of diabetes 1; however, inter‐individual variations in the ratio between mean glucose and HbA1c 2, combined with the insensitivity of HbA1c to the timing, amplitude, and frequency of glucose concentration swings 3, limit the precision with which it can be used to guide therapy intensification efforts. By contrast, continuous glucose monitoring (CGM) allows the direct and nearly instantaneous assessment of mean glucose concentrations and the glycaemic responses to interventions; as such, it can empower and motivate people with diabetes 4 and help to improve glycaemic control 5. The recently described glucose management indicator is a linear function of CGM‐derived mean glucose values and is intended to reduce potential confusion by supplanting the earlier ‘estimated HbA1c’ metric 6. Other metrics for characterization of short‐term glycaemic control with CGM data were proposed by an international consensus conference 7 and include time in range (TIR; usually expressed as the percentage of glucose values from 3.9 to 10.0 mmol/l), as well as the time spent above or below various thresholds indicating clinically significant or immediately actionable hypoglycaemia or hyperglycaemia. In the present study, we report on the relationship between CGM‐derived glycaemic variables and the corresponding HbA1c levels by analysing individual‐level data from recently completed randomized clinical trials.
Participants and methods
Four recently completed randomized controlled studies of adults with diabetes provided data for the present study. Phase I of the DIAMOND study enrolled participants with Type 1 diabetes 8 or participants with Type 2 diabetes 9 using multiple daily injections of insulin, and compared the effects of CGM to those of usual care based on self‐monitoring of blood glucose. Phase II of the DIAMOND study enrolled participants with Type 1 diabetes who had completed the CGM arm in phase I. All continued with CGM; they were randomized to either continue on multiple daily injections or to switch to insulin pump therapy 10. The REPLACE‐BG study 11 enrolled participants with well‐controlled Type 1 diabetes and compared the safety and efficacy of CGM used as an adjunct to self‐monitoring of blood glucose to that of CGM used non‐adjunctively for therapeutic decisions. The HypoDE study 12 enrolled participants with Type 1 diabetes and a history of impaired hypoglycaemia awareness or severe hypoglycaemia, and studied the effectiveness of real‐time CGM for reducing the number of hypoglycaemic events. All of these studies lasted at least 24 weeks and used current or recent‐generation CGM systems (G4 Platinum or G5 Mobile; Dexcom, Inc., San Diego, CA, USA). The DIAMOND and REPLACE‐BG studies excluded participants with evidence of decreased renal function (estimated glomerular filtration rate (GFR) of <45 or <30 ml/min in the respective studies); the DIAMOND study also excluded participants with conditions affecting the reliability of HbA1c measurements. Data from participants for whom <2 weeks of CGM data were available were excluded. Baseline characteristics of participants in the four studies and the number of participants from each study whose data were used in the present analysis are given in Table 1.
Table 1.
Baseline characteristics of participants
| Study | DIAMOND phase I | DIAMOND phase II | REPLACE‐BG | HypoDE | |||
|---|---|---|---|---|---|---|---|
| Subgroup | Continuous subcutaneous insulin infusion | Multiple daily injections | CGM only | CGM + blood glucose monitoring | Control | Real‐time CGM | |
| Assigned to CGM, n | 105 | 37 | 38 | 149 | 77 | 74 | 75 |
| Age, years | 46±14 | 46±15 | 45±12 | 44±14 | 45±13 | 47±12 | 46±12 |
| Diabetes duration, years | 19 (9–29) | 22 (12–29) | 15 (6–29) | 23±12 | 25±12 | 22±14 | 21±14 |
| HbA1c, mmol/mol | 70±7.7 | 60±7.7 | 60±9.8 | 54±7.7 | 53±7.7 | 57±10.6 | 59±10.9 |
| HbA1c, % | 8.6±0.7 | 7.6±0.7 | 7.6±0.9 | 7.1±0.7 | 7.0±0.7 | 7.3±1.0 | 7.6±1.0 |
| Analysed, n | 104 | 69 | 216 | 141 | |||
Values are reported as mean ± sd or median (interquartile range).
End‐of‐study HbA1c values and corresponding CGM metrics from the last 3 months of study participation were available from 104 completers of the DIAMOND phase I study (29 with Type 1 diabetes who did not continue to phase II and 75 with Type 2 diabetes who were not eligible for phase II), from 69 completers of the DIAMOND phase II study, from 216 completers of the REPLACE‐BG study, and from 141 completers of the HypoDE study. For the DIAMOND and REPLACE‐BG studies, HbA1c values were determined at Northwest Lipid Research Laboratories, Seattle, with the DCCT standardized analyser (Tosoh Bioscience, South San Francisco, CA, USA). For the HypoDE study, HbA1c values were determined with a certified high‐performance liquid chromatography method at MLM Medical Laboratories (Mönchengladbach, Germany).
The variable TIR is expressed as a percentage of glucose values in the range 3.9–10.0 mmol/l. Hyperglycaemic exposure is expressed as the percentage of glucose values > 13.9 mmol/l, while hypoglycaemic exposure is expressed as the percentage of glucose values below either 3.9 mmol/l or 3.0 mmol/l. Participants were categorized based on end‐of‐study HbA1c levels ranging from <48 mmol/mol (<6.5%) to ≥69 mmol/mol (≥8.5%), and separately into categories based on CGM‐derived metrics.
Results
Among the 530 participants, the median number of reported glucose values was 8567, equivalent to 29.7 complete days of data per participant, assuming that points were collected at 5‐min intervals. The mean (±sd; range) HbA1c value was 56 (±9; 33–98) mmol/mol [7.3 (±0.8; 5.2–11.1)%]. The mean (±sd; range) glucose value was 9.3 (±1.5; 5.2–16.2) mmol/l. The average glucose value was highly correlated with both TIR (r=–0.93) and percentage of glucose values >13.9 mmol/l (r=0.92). Higher levels of glycaemic variability (measured as the standard deviation of glucose values) were associated with higher mean glucose values (r=0.66) and lower TIR values (r=–0.76).
Relationships between HbA1c and four CGM‐based glycaemic variables are shown in Fig. 1. There was a strong inverse correlation between HbA1c and TIR, such that every 10% change in TIR was associated with a 7mmol/mol (0.7%) change in HbA1c. There were strong positive correlations between HbA1c and both mean glucose and the percentage of glucose values >13.9 mmol/l, and a weak inverse correlation between HbA1c and the percentage of glucose values <3.9 mmol/l. Of the 139 individuals with a TIR of ≥70%, 130 had a glucose management indicator of ≤7% and 111 had an HbA1c of ≤53 mmol/mol (7%).
Figure 1.
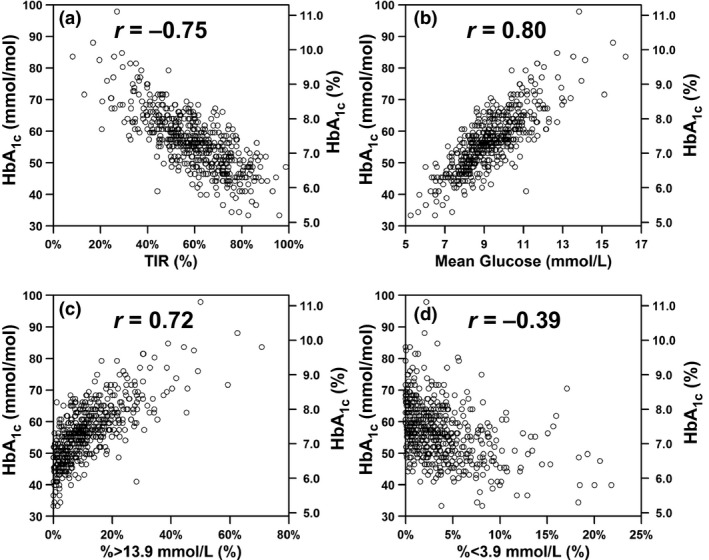
Relationships between HbA1c and (a) time in range (TIR), (b) mean glucose, (c) percentage of glucose values >13.9 mmol/l and (d) percentage of glucose values <3.9 mmol/l.
The median and interdecile (10th to 90th percentile) ranges of CGM‐derived variables for participants in six different HbA1c‐based groups are shown in Fig. 2. For the 76 participants with HbA1c values <48 mmol/mol (<6.5%), the median TIR was 74.9%. In this group, the median frequency of glucose values <3.9 mmol/l was 5.6% and the median frequency of glucose values >13.9 mmol/l was 2.5%. For the 188 participants with HbA1c values ≤53 mmol/mol (≤7.0%), the median TIR was 72.1%, with 90% of participants having a TIR of >57%. This group of participants with relatively good glycaemic control had a mean glucose level of 8.0 mmol/l, median percentage of glucose values <3.9 mmol/l of 4.4%, and a median percentage of glucose values >13.9 mmol/l of 3.7%. For the 42 participants with HbA1c values ≥69 mmol/mol (≥8.5%), the median TIR was 35.5%. In this group, the median frequency of glucose values <3.9 mmol/l was 1.1%, and the median frequency of glucose values >13.9 mmol/l was 27.8%. Overall, the median percentage of glucose values <3.0 mmol/l was <1.2% (<20 min/day), but the median percentage of glucose values >13.9 mmol/l ranged from 2.5% (0.6 h/day) to 27.8% (6.7 h/day) in groups with the lowest to the highest HbA1c values, respectively. For participants with HbA1c values ≥64 mmol/mol (≥8.0%), the median TIR was 43.8%, with 90% of participants having a TIR of <59%.
Figure 2.
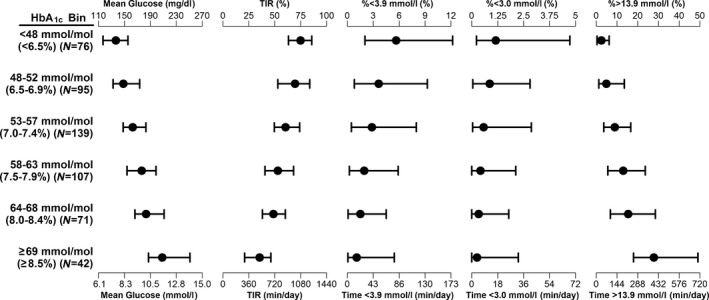
Median and interdecile ranges of various continuous glucose monitoring (CGM)‐derived variables for participants with HbA1c values in different bins.
Median and interdecile ranges of HbA1c values for participants grouped according to various CGM‐derived metrics are shown in Fig. 3. At least 90% of the individuals in several CGM‐based groups had HbA1c values ≤53 mmol/mol (≤7.0%), including groups with mean glucose values <7.8 mmol/l, with TIR values of > 80%, and <2% of glucose values >13.9 mmol/l. By contrast, >90% of the individuals in other CGM‐based groups had HbA1c values >53 mmol/mol (>7.0%), including groups with mean glucose values >10.0 mmol/l, with TIR values of <50%, and with ≥16% of glucose values >13.9 mmol/l. Of the 113 individuals with HbA1c values ≥64 mmol/mol (≥8.0%), only nine (8%) had TIR values of ≥60%, and 57 (50%) had 20% or more of their glucose values >13.9 mmol/l. By contrast, of the 188 individuals with HbA1c values ≤53 mmol/mol (≤7.0%), 159 (85%) had TIR values of ≥60%, and only two (1%) had ≥20% of their glucose values >13.9 mmol/l.
Figure 3.
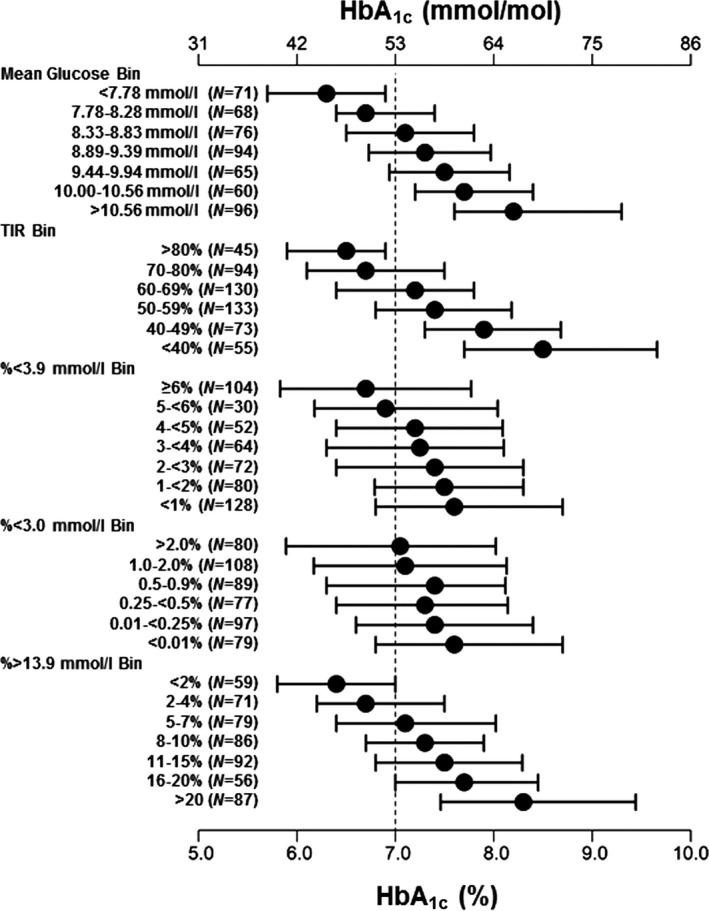
Median and interdecile ranges of HbA1c values for participants with various continuous glucose monitoring (CGM)‐derived variables in different bins.
These relationships were considered separately for individuals with Type 1 diabetes and those with Type 2 diabetes (n=455 and n=75, respectively). Although all participants with Type 2 diabetes were on intensive insulin therapy, they experienced less hypoglycaemia than participants with Type 1 diabetes at both the 3.9 mmol/l threshold (1.2% vs 4.1%) and the 3.0 mmol/l threshold (0.3% vs 1.3%).
Discussion
The present analysis adds detail to the close relationship that has been observed between several CGM‐derived glycaemic variables and HbA1c, with an especially strong correlation between HbA1c and mean glucose (and the glucose management indicator, which is derived from the mean glucose). HbA1c was strongly related to hyperglycaemic exposure and inversely related to TIR. The correlations between HbA1c and hypoglycaemic exposure were inverse and relatively weak, consistent with the insensitivity of HbA1c to hypoglycaemic events. The present analysis also provides clinicians with heuristics for guiding therapy intensification efforts, such as the value of minimizing the percentage of glucose values >13.9 mmol/l and helping define optimal TIR goals. Consistent with earlier observations from the T1D Exchange registry regarding severe hypoglycaemia in people with poor glycaemic control 13, 14, glucose values <3.0 mmol/l were recorded in individuals with HbA1c levels ≥69 mmol/mol (≥8.5%), showing that relaxing HbA1c goals is an ineffective strategy for hypoglycaemia prevention. The range of mean glucose values for specific HbA1c values, and the range of HbA1c values for specific mean glucose values, confirm and extend earlier observations of individual variation in glycation ratios 2, 3 and justify incorporating CGM‐derived metrics into routine care discussions.
Strengths of the present study include the fact that the four clinical trials providing data for this analysis addressed different questions, enrolled adults with either Type 1 or Type 2 diabetes, and observed a wide range of end‐of‐study HbA1c values. The comparability of CGM systems and the central laboratory measurements of HbA1c levels were additional strengths of the present study. The choice to analyse participants with at least 14 days of CGM data was justified by separate studies 15, 16 showing that this amount of data provides a good estimate of glucose metrics for a 3‐month period; however, most participants evaluated in the present study provided more than twice this amount.
Our results are consistent with those reported in the study by Vigersky and McMahon 17 regarding the relationship between HbA1c and TIR, in which the two were highly correlated (r=0.84) and each 10% change in TIR was associated with a 9mmol/mol (0.8%) change in HbA1c. A recent report on children and adolescents in Sweden 18 noted similar relationships between HbA1c and CGM‐derived metrics such as TIR and time spent in the narrower target range of 3.9–7.8 mmol/l, and suggested that participants aged 6–18 years should aim to keep their time in this range at or above 50%.
These results confirm prior studies showing that people with similar HbA1c levels may have widely disparate exposure to hypoglycaemia and hyperglycaemia, and emphasize the value of CGM studies when evaluating people with diabetes. CGM‐derived outcomes such as TIR are increasingly recognized as drivers of improved diabetes management and mindset 19, and CGM‐based estimates of hypoglycaemia and hyperglycaemia can be very meaningful and feasible outcome measures for clinical trials 20. CGM data can also help manage people with impaired renal function, haemoglobinopathies, or other conditions in which HbA1c levels might be misleading. When using CGM reports to inform therapy intensification strategies in people with suboptimally controlled diabetes, clinicians may wish to focus on strategies to increase TIR and limit the duration of hyperglycaemic excursions.
Funding sources
None.
Competing interests
I.B.H. has served as a consultant to Abbott Diabetes Care, Adocia, Bigfoot Biomedical and Roche. His institution has received research grant support from Medtronic. J.B.W., P.C., S.P., T.C.W. and D.A.P. are current or former employees of Dexcom, Inc.
Ethical approval
Ethics approvals were obtained separately for the DIAMOND, HypoDE, and REPLACE‐BG studies, and informed consents were obtained from all participants. Because all participants consented to re‐analysis of their de‐identified data such as those reported here, no additional ethics approval was required. [Correction added on 8 September 2019, after first online publication: Ethical approval text has been corrected].
Diabet. Med. 36, 1637–1642 (2019)
Prior publication: Data in this article were published in abstract form and presented at the 78th Scientific Sessions of the American Diabetes Association (Diabetes 2018;67(S1):A234) and the 54th Annual Meeting of the European Association for the Study of Diabetes (Diabetologia 2018;61(S1):S393).
References
- 1. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2. Argento NB, Nakamura K, Sala RD, Simpson P. Hemoglobin A1C, mean glucose, and persistence of glycation ratios in insulin‐treated diabetes. Endocr Pract 2014; 20: 252–260. [DOI] [PubMed] [Google Scholar]
- 3. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care 2017; 40: 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawton J, Blackburn M, Allen J, Campbell F, Elleri D, Leelarathna L et al Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self‐management: qualitative study. BMC Endocr Disord 2018; 18(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fonseca VA, Grunberger G, Anhalt H, Bailey TS, Blevins T, Garg SK et al Continuous Glucose Monitoring: A Consensus Conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract 2016; 22: 1008–1021. [DOI] [PubMed] [Google Scholar]
- 6. Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A et al Glucose Management Indicator (GMI): A New Term for Estimating A1C From Continuous Glucose Monitoring. Diabetes Care 2018; 41: 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH et al International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S et al Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA 2017; 317: 371–378. [DOI] [PubMed] [Google Scholar]
- 9. Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D et al Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann Intern Med 2017; 167: 365–374. [DOI] [PubMed] [Google Scholar]
- 10. Beck RW, Riddlesworth TD, Ruedy KJ, Kollman C, Ahmann AJ, Bergenstal RM et al Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 700–708. [DOI] [PubMed] [Google Scholar]
- 11. Aleppo G, Ruedy K, Riddlesworth T, Kruger D, Peters A, Hirsch I et al REPLACE‐BG: A Randomized Trial Comparing Continuous Glucose Monitoring With and Without Routine Blood Glucose Monitoring in Well‐Controlled Adults with Type 1 Diabetes. Diabetes Care 2017; 40: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinemann L, Freckmann G, Ehrmann D, Faber‐Heinemann G, Guerra S, Waldenmaier D et al Real‐time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018; 391: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 13. Weinstock RS, Xing D, Maahs DM, Michels A, Rickels MR, Peters AL et al Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013; 98: 3411–3419. [DOI] [PubMed] [Google Scholar]
- 14. Cengiz E, Xing D, Wong JC, Wolfsdorf JI, Haymond MW, Rewers A et al Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes 2013; 14: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riddlesworth TD, Beck RW, Gal RL, Connor CG, Bergenstal RM, Lee S et al Optimal Sampling Duration for Continuous Glucose Monitoring to Determine Long‐Term Glycemic Control. Diabetes Technol Ther 2018; 20: 314–316. [DOI] [PubMed] [Google Scholar]
- 16. Xing D, Kollman C, Beck RW, Tamborlane WV, Laffel L, Buckingham BA et al Optimal sampling intervals to assess long‐term glycemic control using continuous glucose monitoring. Diabetes Technol Ther 2011; 13: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vigersky RA, McMahon C. The Relationship of Hemoglobin A1C to Time‐in‐Range in Patients with Diabetes. Diabetes Technol Ther 2019; 21: 81–85. [DOI] [PubMed] [Google Scholar]
- 18. Petersson J, Åkesson K, Sundberg F, Särnblad S. Translating Glycated Hemoglobin A1c into Time Spent in Glucose Target Range: a Multicenter Study. Pediatr Diabetes 2019; 20: 339–344. [DOI] [PubMed] [Google Scholar]
- 19. Runge AS, Kennedy L, Brown AS, Dove AE, Levine BJ, Koontz SP et al Does Time‐in‐Range Matter? Perspectives From People With Diabetes on the Success of Current Therapies and the Drivers of Improved Outcomes. Clin Diabetes 2018; 36: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beck RW, Calhoun P, Kollman C. Use of continuous glucose monitoring as an outcome measure in clinical trials. Diabetes Technol Ther 2012; 14: 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]


