Abstract
Genetically determined myoclonus disorders are a result of a large number of genes. They have wide clinical variation and no systematic nomenclature. With next‐generation sequencing, genetic diagnostics require stringent criteria to associate genes and phenotype. To improve (future) classification and recognition of genetically determined movement disorders, the Movement Disorder Society Task Force for Nomenclature of Genetic Movement Disorders (2012) advocates and renews the naming system of locus symbols. Here, we propose a nomenclature for myoclonus syndromes and related disorders with myoclonic jerks (hyperekplexia and myoclonic epileptic encephalopathies) to guide clinicians in their diagnostic approach to patients with these disorders. Sixty‐seven genes were included in the nomenclature. They were divided into 3 subgroups: prominent myoclonus syndromes, 35 genes; prominent myoclonus syndromes combined with another prominent movement disorder, 9 genes; disorders that present usually with other phenotypes but can manifest as a prominent myoclonus syndrome, 23 genes. An additional movement disorder is seen in nearly all myoclonus syndromes: ataxia (n = 41), ataxia and dystonia (n = 6), and dystonia (n = 5). However, no additional movement disorders were seen in related disorders. Cognitive decline and epilepsy are present in the vast majority. The anatomical origin of myoclonus is known in 64% of genetic disorders: cortical (n = 34), noncortical areas (n = 8), and both (n = 1). Cortical myoclonus is commonly seen in association with ataxia, and noncortical myoclonus is often seen with myoclonus‐dystonia. This new nomenclature of myoclonus will guide diagnostic testing and phenotype classification. © 2019 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Keywords: genetics, hyperekplexia, myoclonic epilepsy, myoclonus, nomenclature
Myoclonus is a hyperkinetic movement disorder characterized by sudden, brief, involuntary jerks of a single or multiple muscles.1, 2, 3 It can be caused by muscle contraction (positive myoclonus) or sudden interruption of muscle activity during intended isometric contraction (negative myoclonus).4 The myoclonic jerks can be difficult to distinguish from other hyperkinetic movement disorders.5 Electrophysiological testing has proven helpful for discriminating myoclonus from other hyperkinetic movement disorders and for classifying the myoclonus subtype.6 Myoclonus can be classified based on anatomical origin: cortical, subcortical (or noncortical7), spinal, and peripheral myoclonus.6 So far, in genetic myoclonus syndromes only cortical (CM) and subcortical subtypes have been described.8
Determination of the etiology of myoclonus is challenging, and recently, a novel diagnostic 8‐step algorithm was proposed to help clinicians accurately, efficiently, and cost‐effectively diagnose myoclonus.8 Once the acquired forms and late‐onset neurodegenerative disorders (such as Alzheimer's disease and parkinsonian disorders) of myoclonus are excluded in this diagnostic workup, a large number of genetically determined disorders with wide clinical variation remain. In almost all genetic syndromes, myoclonus is not the sole feature, but it is accompanied or even overshadowed by another movement disorder.5 This is likely the reason systematic nomenclature similar to PARK (for parkinsonism) or DYT (for dystonia) has not been established for myoclonus. In many of the suspected genetic myoclonus syndromes, the genetic cause is (still) unknown, but next‐generation sequencing (NGS) has revolutionized molecular genetic diagnosis and has produced an exponential increase in known genetic causes and expansion of movement disorder phenotypes, including myoclonus. However, NGS frequently produces genetic variants for which pathogenicity is unclear. This emphasizes the importance of good clinical phenotyping and weighting of NGS results in the context of the presenting clinical syndrome.
In 2012, the International Parkinson and Movement Disorder Society Task Force for Nomenclature of Genetic Movement Disorders was established to revise the system of locus symbols, as the current movement disorders system had become outdated with the advances in NGS, the lack of established criteria for conferring locus symbols, or ongoing revision of the list.9
Here we present a new myoclonus nomenclature. We also include groups of related disorders that can present in the outpatient clinic of a movement disorder specialist with jerks as a prominent symptom. First, there are the hyperekplexias, as the excessive startle reflex closely resembles reticular reflex myoclonus, both clinically and neurophysiologically.10 Second are the genetic epilepsy syndromes with myoclonic jerks, specifically the epileptic encephalopathies. Patients with myoclonic epilepsy encephalopathies exhibit, next to their clear epileptic attacks, often spontaneous, reflex or action myoclonus, with evidence of a cortical origin. These cortically driven epileptic jerks resemble isolated cortical myoclonus, as both are characterized by short‐lasting (<100‐millisecond) jerks with a cortical discharge on the electroencephalogram (EEG). Historically, it is not clear if there is a neurobiological distinction between the 2 phenomena, and therefore we decided to include them both in the current myoclonus nomenclature.
The first 2 papers of the task force included the proposed nomenclature for genetic parkinsonism, dystonia, autosomal‐dominant and ‐recessive cerebellar ataxia, hereditary spastic paraplegia, paroxysmal movement disorders, neurodegeneration with brain iron accumulation, and primary familial brain calcification.1, 2 Here, we present the genetically determined myoclonus syndromes nomenclature based on the same principles, criteria, and recommendations.
Methods
Inclusion
Our recommendations are based on a systematic literature search. All articles regarding genetic causes of myoclonus syndromes were identified by a PubMed, Online Mendelian Inheritance in Man, and Textbook search, including all the additional relevant references cited in the articles found. The key search terms “myoclonus,” “myoclonic epilepsy,” and “startle” were used in combination with the term “genetic causes.” For the period to June 2015, we used our previously published systematic review with the same search terms.8 In addition, an identical search was performed for the period between June 2015 and October 2018 to identify newly discovered genes. All reviewed articles and abstracts were restricted to those published in English.
Following the recommendations of the task force, the criteria for gene inclusion are that mutations in the gene must be causative (ie, risk factor genes were excluded), and myoclonus must be a prominent feature. In determining the pathogenicity, no specific threshold for the level of penetrance of a mutation was designated by the Movement Disorder Society (MDS) Task Force and was determined for each gene based on standards prevailing in the field. In the field of myoclonus, we decided that genes related to myoclonus or myoclonic epilepsy with medium or low penetrance were excluded. In Table 1 we included genetic disorders DYT‐ANO3 and CHOR‐NKX2‐1, although the penetrance of these genes is reduced. The reason to include them is that the previous nomenclature of the MDS Task Force decided to include lower penetrance, as it is more common in dystonic syndromes and these 2 genes present with the clinical syndrome of myoclonus‐dystonia.
Table 1.
The proposed new list of genetically determined myoclonus syndromes
| New designation | Name | Myoclonus | Ataxia | Dystonia | Epilepsy | Cognitive problems | Clinical clues | Myoclonic subtype | OMIM | Inheritance pattern | Locus symbol | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prominent myoclonus syndromes | ||||||||||||
| MYC‐CLN3 11 | CLN3 disease | + | −/+ | − | ++ | ++ | Juvenile onset, parkinsonian signs, retinal degeneration, neuropsychiatric symptoms | CMa | 607042 | AR | CLN3 | |
| MYC‐CLN5 12 | CLN5 disease | ++ | −/+ | − | ++ | ++ | Late‐infantile onset, blindness | CMa | 608102 | AR | CLN5 | |
| MYC‐CLN6 13 | CLN6 disease | ++ | +/++ | − | ++ | ++ | Early juvenile or adult14 onset, visual failure | CMa | 606725 | AR | CLN6 | |
| MYC‐CLN8 15 | CLN8 disease | ++ | +/++ | − | ++ | ++ | Late infantile onset, retinopathy | CMa | 607837 | AR | CLN8 | |
| MYC‐DNAJC5 16 | CLN4 disease | ++ | +/++ | − | ++ | ++ | Adult‐onset | CMa | 611203 | AD | CLN4 | |
|
MYC‐GLRA1 17; MYC‐SLC6A5 18; MYC‐GLRB 19 |
Hyperekplexia | + | − | − | − | − | Generalized stiffness at birth and following startle, neonatal tonic cyanotic attacks, periodic limb movement during sleep, and hypnagogic myoclonus | BSM |
138491 604159 138492 |
AD, AR AD, AR AR |
HKPX1 HKPX3 HKPX2 |
|
| MYC‐KCNC1 20 | MEAK | ++ | ++ | − | + | −/+ | − | CM | 176258 | AD | None | |
| MYC‐PRICKLE1 21 | EPM 1B | ++ | ++ | − | + | −/+ | Upward gaze palsy | UN | 608500 | AR | None | |
|
MYC‐SAMD12,c MYC‐RAPGEF2 22 |
FCMTE | + | − | − | +/++ | −/+ | Adult‐onset, anxiety, and depression23 | CM |
618073 609530 |
AD | None | |
| MYC‐SCARB2 24 | AMRF syndrome | ++ | +/++ | − | +/++ | −/+ | Tremor, renal failure, peripheral neuropathy | CM | 602257 | AR | None | |
| ATX/HSP‐FOLR1 25 | Cerebral folate transport deficiency | −/+ | ++ | − | ++ | ++ | Chorea, drop attacks26 | UN | 136430 | AR | None | |
| CARS2 27 | CARS2 | −/+ | − | − | ++ | ++ | Tetraparesis, visual and hearing impairment, areflexia, hypotonia28 | UN | 612800 | AR | None | |
| CHD2 29 | CHD2 encephalopathy | − | − | − | +/++ | +/++ | Photosensitivity, multiple seizure types of which atonic‐myoclonic‐absence is most common30 | CM | 602119 | AD | None | |
| CUX231 | Myoclonic DEE | − | − | − | ++ | ++ | Infantile‐onset myoclonic and absence seizures, stereotypies and dyskinesias | CM | 610648 | AD | None | |
|
GLDC 32; AMT 33 |
Classic non‐ketotic hyperglycinemia | − | − | − | ++ | ++ | Neonatal onset: progressive lethargy, hypotonia | CM |
238300 238310 |
AR | None | |
| mt‐MTTK 34 d | MERRF | − | + | − | ++ | −/+ | Muscle weakness, hearing loss, peripheral neuropathy, optic atrophy, axial lipomas, and variable other neurological manifestations (heterogeneous disease, multiple genes associated with phenotype)35 | CM | 590060 | Mt | None | |
| PIGA 36 | MCAHS2 | − | − | − | ++ | ++ | Dysmorphic features, neonatal hypotonia | CM | 311770 | XLR | None | |
| POLG 37 | POLG‐related disorders | −/+ | −/+ | −/+ | ++ | ++ | Parkinsonism, chorea, migraine, stroke‐like episodes, hearing and visual impairment, myopathy, neuropathy, endocrine and gastrointestinal disorders | UN | 174763 | AD or AR | None | |
| SCN1A 38 e | Dravet syndrome | −/+ | −/+ | − | ++ | +/++ | Febrile and prolonged seizures with alternating pattern | CM | 607208 | AD | None | |
| SERPINI1 39, 40 | FENIB | − | −/+ | − | ++ | ++ | − | CM | 602445 | AD | None | |
| SLC6A1 41 | Doose syndrome | − | − | − | ++ | + | Atonic drop attacks | CM | 137165 | AD | None | |
| TBC1D24 42 | TBC1D24‐related disorders | −/+ | −/+ | −/+ | +/++ | +/++ | Variable types of seizures, muscle hypotonia, extrapyramidal signs, hearing and visual loss43 | UN | 613577 | AR | None | |
| Combined myoclonus syndromesf | ||||||||||||
| MYC/ATX‐CSTB 44 | Unverricht‐Lundborg | ++ | ++ | − | + | −/+ | Periodicity of symptoms45 | CM | 601145 | AR | None | |
| MYC/ATX‐EPM2A 46 | Lafora disease | ++ | ++ | − | ++ | ++ | Focal visual seizures, drop attacks, psychosis47 | CM | 607566 | AR | None | |
| MYC/ATX‐GOSR2 48 | North Sea PME | ++ | ++ | − | +/++ | −/+ | Scoliosis, areflexia, pes cavus, syndactyly, drop attacks | CM | 614018 | AR | None | |
| MYC/ATX‐KCTD7 49 | EPM 3 | ++ | ++ | − | ++ | ++ | Pyramidal signs, micorcephaly50 | UN | 611726 | AR | None | |
| MYC/ATX‐NEU1 51 | Sialidosis | ++ | ++ | − | −/+ | +/++ | Cherry‐red spots52 | CM | 608272 | AR | None | |
| MYC/ATX‐NHLRC1 53 | Lafora disease | ++ | ++ | − | ++ | ++ | See MYC‐EPM2A | CM | 608072 | AR | None | |
| MYC/ATX‐TPP1 54 | CLN2 disease | ++ | ++ | − | ++ | ++ | Late infantile onset, retinopathy, spasticity, hypotonia, extended vegetative state | CMa | 204500 | AR | CLN2 | |
| MYC/DYT‐SGCE 55 | Myoclonus‐dystonia (M‐D) | + | − | + | − | − | M‐D predominantly in upper body, psychiatric disorders | SCM | 604149 | AD | DYT11 | |
| MYC/DYT‐KCTD17 56 | Myoclonus‐dystonia | + | − | + | − | − | M‐D predominantly in upper body, laryngeal involvement can occur, psychiatric symptoms |
SCM |
616386 | AD | None | |
| Disorders that usually present with other phenotypes but can manifest as a prominent myoclonus syndrome | ||||||||||||
| ATX‐ATM 57 | Variant Ataxia‐telangiectasia | + | + | ++ | − | −/+ |
M‐D phenotype, chorea58 Systemic abnormalities: immunodeficiency, malignancies, and oculocutaneous telangiectasias |
SCM | 607585 | AR | None | |
| ATX‐ATN1 59 g | DRPLA, PME phenotype | +/++ | ++ | − | +/++ | ++ | PME phenotype especially in patients with age of onset < 20 years. Other phenotypes are an ataxochoreoathetoid form and a pseudo‐Huntington form | CM | 607462 | AD | None | |
| ATX‐NPC1 60 | Niemann‐Pick type C | ++ | ++ | −/+ | −/+ | +/++ | PMA‐phenotype, chorea, and tremor,61 hepatosplenomegaly, vertical supranuclear gaze palsy | CM | 607623 | AR | None | |
| ATX‐PRKCG 62 g | SCA 14 | + | + | −/+ | − | −/+ | M‐D phenotype, sensory loss, hyperactive tendon reflexes, depression63 | SCM | 176980 | AD | SCA14 | |
| DYT‐ANO3 64 | Tremorous cervical dystonia | + | − | ++ | − | − | M‐D predominantly in upper body, tremor | SCM | 610110 | AD | DYT24 | |
| CHOR/DYT‐ADCY5 65 | FDFM | + | − | + | − | −/+ | M‐D phenotype with episodic mixed hyperkinetic disorder of the face characterized by myoclonus‐chorea,66 axial hypotonia | UN | 600293 | AD | None | |
| CHOR‐HTT 67 | Juvenile Huntington's disease | ++ | ++ | − | −/+ | +/++ | Behavioral symptoms and parkinsonian signs68 | CM | 613004 | AD | None | |
| CHOR‐NKX2‐1 69 | Benign hereditary chorea | ++ | + | +/++ | − | + | M‐D phenotype, chorea more prominent at young age, in adult life myoclonus most disabling if present. Tics, brain‐lung‐thyroid syndrome. | UN | 600635 | AD | None | |
| HSP‐KIF5A 70 | Neonatal myoclonus | ++ | − | − | −/+ | ++ | Neonatal onset. Eye movement abnormalities, apnea, ptosis, optic nerve pallor, hypotonia. Leukoencephalopathy may be seen.71 | UN | 602821 | AD | SPG10 | |
| HSP‐SACS 20 | ARSACS | ++ | ++ | − | ++ | ++ | Pyramidal signs72 | CMa | 604490 | AR | None | |
| PARK‐GBA 73 | Neuronopathic Gaucher disease | +/++ | +/++ | − | ++ | ++ | Spasticity, horizontal gaze abnormalities, visceral involvement74 | CMa | 606463 | AR | None | |
| APP 75 | Familial Alzheimer's disease | + | −/+ | − | + | ++ | − | CM | 104760 | AD | None | |
| ASAH1 76 | Spinal muscular atrophy | ++ | − | − | ++ | −/+ | Progressive lower motor neuron disease manifestations | CMa , h | 613468 | AR | None | |
| CSNK2B 77 | CSNK2B‐related disorders | − | − | − | ++ | + | Infantile onset of myoclonic seizures. Speech and language disorder. | CM | 115441 | AD | None | |
| CTSA 78 | Galactosialidosis | ++ | ++ | − | +/++ | ++ | Coarse facies, vertebral changes, cherry‐red spots, corneal clouding, absence of visceromegaly, angiokeratoma79 | CM | 613111 | AR | None | |
| FARS2 80 | FARS2‐related disorders | − | − | − | ++ | ++ | Early infantile onset of myoclonic seizures, GTCS, and infantile spasms. | CM | 611592 | AR | None | |
| PRNP 81 i | Familial Creutzfeldt‐Jakob disease | ++ | ++ | − | −/+ | ++ | Chorea, visual impairment, akinetic mutism, sleep disturbances, psychiatric disorders, peripheral neuropathy82 | CM & SCM | 176640 | AD | None | |
| PSEN1 83 | Familial Alzheimer's disease | + | −/+ | − | + | ++ | Spastic paraparesis, rigidity, behavioral symptoms, language and dysexecutive deficits84 | CM | 104311 | AD | None | |
| RPS6KA3 85 | Coffin‐Lowry syndrome | + | − | − | − | + | Stimulus‐induced drop episodes,86 dysmorphism, progressive skeletal changes, hearing loss, mitral valve deformity | UN | 300075 | XLD | None | |
| SLC2A1 87 | Glucose transport type 1 deficiency | − | −/+ | − | ++ | +/++ | Myoclonic, myoclonic‐astatic, GTC, and absence seizures starting in early up to middle childhood. Other phenotypes include paroxysmal exertion‐induced dyskinesia, absence epilepsy or episodic choreoathetosis, and spasticity.88 | CM | 138140 | AD | None | |
| SYNGAP1 89 | SYNGAP1‐associated intellectual disability and epilepsy | − | −/+ | − | +/++ | +/++ | Early infantile onset of drop attacks, massive myoclonic jerks, and (myoclonic)‐absence seizures. Hypotonia, behavioral disorder, ASD, orthopedic problems. | CM | 603384 | AD | None | |
| UBE3A 90 | Angelman syndrome | + | −/+ | − | ++ | ++ | Myoclonic, myoclonic absence, and myoclonic‐tonic seizures in early childhood; nonepileptic myoclonus first presenting in adolescence. Sleep dysfunction, absent or limited expressive language.91 | CMa | 601623 | b | None | |
| mUDPC792 | Silver‐Russell syndrome | + | − | + | − | − | Growth retardation, dysmorphism, M‐D predominantly located in upper body | UN | 180860 | IC | None | |
++, Severe/progressive presentation of symptom; +, mild presentation of symptom; −/+, symptom can be present or absent; ‐ symptom is absent.
AMRF, action myoclonus renal failure; ARSACS, autosomal‐recessive spastic ataxia of Charlevoix‐Saguenay; BSM, brain stem myoclonus; CM, cortical origin of myoclonus; DEE, developmental and epileptic encephalopathy; DRPLA, dentate‐rubro‐pallido‐luysianatrophy; EPM, progressive myoclonus epilepsy; FCMTE, familial cortical myoclonic tremor with epilepsy; FDFM, familial dyskinesia with facial myokymia; FENIB, familial encephalopathy with neuroserpin inclusion bodies; ICs, isolated cases; MCAHS2, multiple congenital anomalies‐hypotonia‐seizures syndrome‐2; M‐D, myoclonus‐dystonia; MEAK, myoclonus epilepsy and ataxia from potassium (K+) channel mutation; MERRF, myoclonic epilepsy with ragged red fibers; SCM, subcortical origin of myoclonus; UN, myoclonic subtype is unknown; XLD, X‐linked dominant; XLR, X‐linked recessive.
Myoclonic subtype could not be assigned according to the official criteria stated by Zutt et al (2018)83; therefore, the subtype stated in the literature was adopted but accentuated as presumed using an asterisk.
Loss of the maternally inherited UBE3A gene.
Recently, authors have proven the pentanucleotide repeat TTTCA (and TTTTA) to be causative of FCMTE in the intron of MYC‐SAMD12 and MYC‐RAPGEF2.12 Although the authors believe the intronic pentanucleotide repeat to be pathogenic irrespective of the gene, we have stated the 2 genes that have been confirmed in the literature.
The following additional genetic mutations are able to cause MERRF: mt‐MTTL11 (OMIM 590050), mt‐MTTH1 (OMIM 590040), mt‐MTTS11 (OMIM 590080), mt‐MTTS21 (OMIM 590085), mt‐MTTF1 (OMIM 590070), mt‐MTTW (OMIM 590095)1.
The following genes have been reported to cause a DS‐like phenotype by at 2 independent research groups: SCN1B (OMIM 600235), PCDH19 (OMIM 300460), GABRA1 (OMIM 615744).
The phenotype of a combined myoclonus syndrome is characterized by multiple predominant movement disorders including myoclonus.
Because of recent suggestions of the Task Force Nomenclature, the previously proposed prefix SCA for autosomal‐dominant ataxias was replaced by ATX, resulting in the replacement of prefixes of 2 genes, ATN1 and PRCKG. SCA‐ATN1 has been changed to ATX‐ATN1 and SCA‐PRKCG to ATX‐PRKCG.
Patients diagnosed with a genetic defect of ASAH1 were described by Topaloglu et al (2016) as having subcortical myoclonic epileptiform abnormalities. However, based on the clinical characteristics we suspect a cortical origin of the myoclonic jerks and have classified this gene accordingly.
Opposed to the previously assigned prefix CHOR in CHOR‐PRNP, the prefix CHOR was removed, and the name was altered to PRNP, as this gene causes multiple phenotypes including myoclonus and in which chorea only dominates in a minority of cases.
Cognitive problems include both cognitive decline and psychomotor retardation. The myoclonic subtype was determined unknown if neither an official myoclonic subtype could be assigned or a myoclonic subtype was stated in the literature.
Prominent myoclonus was present if either (1) the literature stated that myoclonic jerks were a prominent feature of the phenotype, (2) the myoclonic jerks were the main reason for disability, and/or (3) the myoclonic jerks were the main focus of treatment. In addition to this, the predominance of myoclonus in the disorder had to be confirmed in the literature by a second independent group of researchers.1
This adjudication process included 2 persons (S.V. and R.Z.). All genes included in the new nomenclature were reviewed by 6 experts within the field of myoclonus to reach a broadly supported consensus (H.S., J.C., S.B., P.T., T.K., M.T.).
Classification
Following the recommendation of the task force and to guide clinicians in daily practice, the genetic disorders were allocated based on clinical presentation into 1 of the following 3 groups: (1) prominent myoclonus syndromes, genetic disorders that present with prominent myoclonus in the majority of cases; (2) combined myoclonus syndromes, genetic disorders that present with prominent myoclonus and another prominent movement disorder (eg, dystonia/ataxia) in the majority of cases; and (3) disorders that usually present with other phenotypes but can manifest as a prominent myoclonus syndrome, genetic disorders that present with prominent myoclonus only in a minority of cases as part of the phenotypic spectrum of this disorder.
Prefixes
In accordance with the recommendations of the task force, the prefix MYC was given to genes in which myoclonus is a prominent feature in the majority of the patients. In addition, we added a second prefix to genes and consequently allocated it to the subgroup combined myoclonus syndromes, in which another movement disorder is an additional prominent feature, resulting in a double prefix if both movement disorders are prominent (eg, MYC/ATX‐GOSR2). Overlapping genes with double prefixes were discussed among the appropriate experts from the MDS Task Force to reach consensus. The symbol prefix is followed by the gene name. For clarity and to allow comparison with former classifications, we provided the old locus symbol (eg, DYT11) in the last column of Table 1, when appropriate. Genes that present with myoclonic epilepsy were not given any prefix, because the dominant feature of the phenotype is epilepsy rather than a movement disorder.
Additional Clinical and Electrophysiological Items
A brief description of the clinical presentation of disorders linked to each gene is listed in Table 1 with special emphasis on the most common accompanying signs and symptoms including ataxia, dystonia, cognitive problems, or epilepsy. Furthermore, we added the myoclonic anatomical subtype, cortical or subcortical (ie, noncortical), if known, for each genetic disorder based on reported clinical and electrophysiological features to further improve the classification of myoclonus. Experts have argued against the term “subcortical” myoclonus, as its anatomical origin is still undetermined; however, the term “subcortical” myoclonus will still be used in the new nomenclature because of the absence of a widely supported alternative.7 See Supplementary Table 1 for the anatomical classification criteria for myoclonus.93
Results
Gene Selection
One hundred sixty‐six genes linked to a myoclonus syndrome were found in the systematic literature review. An extensive overview of all genes associated with myoclonus with reason for inclusion or exclusion can be found in Supplementary Table 3, and see Figure 1 for an overview. Nighty‐nine genes were excluded because of the absence of prominent myoclonus (n = 45), lack of confirmation of the phenotype with prominent myoclonus by a second independent research group (n = 31), and questionable pathogenicity (n = 23).
Figure 1.
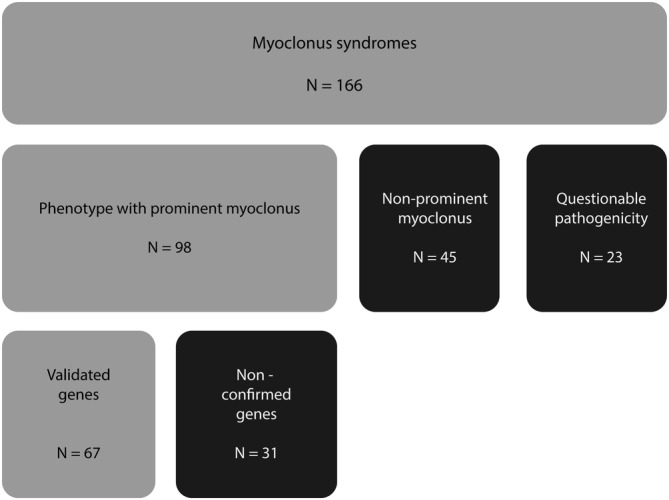
In and exclusion of genes associated with myoclonus syndromes.
Sixty‐seven genes were included in the new nomenclature for myoclonus syndromes (see Table 1). (1) In the subgroup prominent myoclonus syndromes, 35 genes were included; (2) in the subgroup combined myoclonus syndromes, 9 genes were included; and (3) in the subgroup disorders that usually present with other phenotypes but can manifest as a prominent myoclonus syndrome, 23 genes were included.
Prefix Allocation
The locus symbol prefix MYC was assigned to 22 genes. Genes in which the predominant phenotype showed wide heterogeneity or was dominated by epileptic or nonmotor symptoms were not assigned any prefix. For myoclonus epilepsy with ragged red fibers syndrome, only the most frequent causative gene (mt‐MTTK) is listed. The remaining causative genes are stated in the caption of Table 1, as they are associated with a similar phenotype as mt‐MTTK.
Additional Clinical and Electrophysiological Clues
The following most common accompanying signs and symptoms observed overall were cognitive decline in 90% (n = 60), epilepsy in 82% (n = 55), ataxia in 61% (n = 41), ataxia and dystonia in 9% (n = 6), and dystonia in 7% (n = 5). The anatomical location of myoclonic origin could be allocated in 64% of genes (n = 43) because of support of strong electrophysiological data, and in the cortex in 51% (n = 34), noncortical areas in 12% (n = 8), and both cortical and noncortical areas in 1% (n = 1) of all genes. Three of the 8 genes with jerks originating from noncortical areas were classified as originating from the brain stem (hyperekplexia).
Discussion
In this article we propose a nomenclature of genetically determined myoclonus according to the new naming system presented by the MDS Task Force.1 This myoclonus list currently includes 67 genes. Thirty‐five genes presented with prominent myoclonus syndromes, 9 with combined myoclonus syndromes, and 23 with disorders that usually present with other phenotypes but can manifest as a prominent myoclonus syndrome. Co‐occurrence of movement disorders, especially ataxia and dystonia, was seen in almost all except for familial cortical myoclonus tremor with epilepsy (FCMTE, or BAFME, benign adult familial myoclonus epilepsy), hyperekplexia, and (myoclonic) epileptic encephalopathies. Epilepsy and cognitive decline were the most frequently observed accompanying clinical features for the disorders listed in this new nomenclature.
The literature search detected 166 genes linked to a myoclonus syndrome, but only 67 were used for the nomenclature list. Filtering using strict criteria (independent confirmation and predominant myoclonus) to arrive at a list of confirmed entities that can present with predominant myoclonus is meant to help the clinician with the selection of test procedures and assist in the interpretation of results of genetic testing.2 In our opinion, the requirement for independent confirmation by a second research group is an important criterion, as it diminishes erroneous genotype‐phenotype linkages. At present, with the widespread use of NGS in research and clinical diagnostics, many potentially new myoclonus genes are reported. Still, we had to exclude 31 genes (19%) that require validation. A significant proportion of patients with myoclonus syndromes still remain unsolved (progressive myoclonus ataxias in 36%94 and progressive myoclonus epilepsies in 28%95), in which excluded genes could be considered.
A new clinical diagnostic approach in patients with myoclonus has recently been described.8 After establishing that the myoclonus in a patient has a genetic cause, Table 1 can be used as a diagnostic framework for physicians in clinical practice to select candidate genes for individual patients based on the absence or presence of accompanying signs and symptoms.
FCMTE/BAFME is the only genetically determined myoclonus syndrome with relatively pure myoclonus, although it is accompanied by infrequent epilepsy in a majority of but not all patients. This genetic disorder is caused by 2 recently confirmed genes (MYC‐SAMD12 and MYC‐RAPGEF2) with intronic expansions of noncoding TTTCA and TTTTA pentanucleotide repeats. It presents with a phenotype of benign CM with infrequent tonic‐clonic and sometimes focal seizures. RNA‐mediated toxicity resulting in diffuse loss of Purkinje cells in the cerebellum is suggested to be the underlying pathogenesis of this disorder.96, 97 The potential role of the cerebellum in CM has been pointed out multiple times in the literature, supported by the frequent phenotypical co‐occurrence of CM and cerebellar ataxia.98
Ataxia is the most common accompanying movement disorder in myoclonus syndromes (24 genes). Almost all patients in whom the genetic disorder consists of a combination of ataxia and myoclonus present with the clinical syndrome of progressive myoclonus ataxia (PMA) or progressive myoclonus epilepsy (PME). The most common and best characterized are Unverricht‐Lundborg disease (MYC/ATX‐CSTB), Lafora disease (MYC/ATX‐EMP2A), neuronal ceroid lipofuscinosis (multiple genes), sialidosis (MYC/ATX‐NEU 1), and dentatorubral pallidoluysian atrophy (ATX‐ATN1).99
The anatomical origin of myoclonus in most patients with ataxia is thought to be cortical. Clinically, cortical myoclonic jerks present typically in the distal limbs and face, jerks are provoked by action and are stimulus sensitive.93 Of the genetic disorders in which ataxia and myoclonus co‐occur, we found that cortical origin was supported by strong electrophysiological evidence in 54% (n = 14), and it was suspected in 33% (n = 8). Mechanistic hypotheses for cortical myoclonus include: (1) loss of Purkinje cells with astrocytosis, resulting in disinhibition via the cerebello‐thalamico‐cortical pathway, (2) neuronal cell loss in the dentate nuclei leading to impaired cerebellar projections to the cortex, or (3) a reduction in the concentration of γ‐aminobutyric acid (GABA)‐ergic synapses in the sensory‐motor cortex.100 On a molecular level, most genetic disorders presenting with both ataxia and myoclonus have impaired posttranslational modification of proteins to which certain neuronal groups might be particularly vulnerable compared with others.100 This could play a role in the characteristic phenotype of PMA, including a fixed order of signs, starting with ataxia, subsequently CM, and eventually by infrequent epilepsy.94
Dystonia is the second type of prominent movement disorder accompanying myoclonus. The combination of myoclonus and dystonia is known as myoclonus‐dystonia syndrome (M‐D). The classical myoclonus‐dystonia phenotype is based on genetic defects in the MYC/DYT‐SGCE gene in about 50% of cases.101 Other disorders that can give rise to a myoclonus‐dystonia phenotype include MYC/DYT‐KCTD17, DYT‐ANO3, ATX‐PRKCG, ATX‐ATM, CHOR/DYT‐ADCY5, CHOR‐NKX2‐1, and maternal uniparental disomy with regions of heterodisomy and isodysomy on chromosome 7 (mUPD7), which is based on the loss of function of the SGCE gene.
The anatomical locus of myoclonus in M‐D is subcortical. Clinically, the myoclonus and dystonia in M‐D are located mainly in the trunk and proximal upper limbs, and the myoclonus is not stimulus sensitive. The noncortical origin of the myoclonus is supported electrophysiologically in 5 genetic disorders presenting with M‐D (MYC/DYT‐SGCE, MYC/DYT‐KCTD17, DYT‐ANO3, ATX‐ATM, ATX‐PRKCG) and unknown in 2 others (CHOR/DYT‐ADCY5 and CHOR‐NKX2‐1). The pathophysiology of subcortical myoclonus includes circuit abnormalities in the basal ganglia and involvement of the cerebellum. Disruptions in neurotransmission pathways have been hypothesized to play a role, particularly the unbalanced homeostasis of GABA, serotonin, and dopamine‐related pathways.102 In contrast to myoclonus of cortical origin, cortical excitability and intracortical inhibition were found to be normal or less profoundly disturbed.103
The overlap between types of accompanying movement disorders and the anatomical origins of the myoclonic jerks is remarkable. Currently, the anatomical origin can be assigned in only 64% of genetic disorders. We encourage movement disorder specialists to classify the subtype of myoclonus by a thorough clinical description (eg, distribution, stimulus sensitivity) of the myoclonic jerks and if possible electrophysiological testing (eg, corticomuscular coherence or jerk‐locked back‐averaging). We realize that availability of the tests varies considerably between centers and countries.6 However, the myoclonic subtype guides the clinician toward a more precise differential diagnosis (see Table 1) and effective treatment strategy,104 and it helps to unravel the pathogenesis of myoclonus by creating homogenous groups.
Epilepsy is an additional feature in 82% of myoclonus syndromes, presenting either as CM in combination with epilepsy or myoclonic jerks as part of a myoclonic seizure. It is only described in genes with jerks originating from the cortex, as mutations in genes linked to noncortical myoclonus (hyperekplexia, all M‐D syndromes, and Coffin‐Lowry syndrome) rarely present with epileptic manifestations. The distinction between myoclonus and (myoclonic) epilepsy can be difficult to make, and seemingly minor differences in terminology can create confusion. Myoclonus epilepsy is a condition in which CM, often continuously present, and epilepsy occur independently, whereas myoclonic epilepsy is an attack of generalized convulsions starting with myoclonic jerks or predominantly characterized by myoclonic jerks. Jerks in both CM and myoclonic epilepsy are associated with EEG polyspikes or spike/polyspike‐wave complexes before the onset of an EMG burst.105 Confusion is not only the case in clinical practice but also in the literature, making it difficult to interpret many of the clinical presentations described. For instance, the phenotype associated with MYC/ATX‐GOSR2 has been called an epileptic syndrome with myoclonic seizures (progressive myoclonus epilepsy type 6) in articles from the field of epilepsy,106 as opposed to a syndrome with prominent cortical myoclonus in combination with epilepsy (progressive myoclonus ataxia) in articles from the field of movement disorders.107 Particularly in the fields of movement disorders and epilepsy, the phenotype is a decisive factor for further diagnostics, and inaccuracy of descriptions can lead to erroneous genotype‐phenotype relationships. Ongoing discussion and consensus meetings between experts in both fields are necessary to accomplish a consistent terminology with clear definitions that could easily be implemented in clinical practice.
Cognitive problems including cognitive decline and psychomotor retardation have been reported in all but 5 genetic disorders, MYC/DYT‐SGCE, MYC/DYT‐KCTD17, mUDP7 (based on loss of SGCE‐gene), DYT‐ANO3, and the hyperekplexias. Other nonmotor features, particularly psychiatric disorders and behavioral problems, are also being recognized as part of the phenotype of certain movement disorders (eg, dystonia). In disorders with cortical myoclonus, almost half the patients experience symptoms of depression or anxiety.108 Underestimation of these nonmotor features is likely, as we have only recently started considering this to be part of the phenotype. Future case descriptions of myoclonus syndromes should include details on cognition, psychiatric symptoms, and behavioral changes. The clinician should be aware of the high occurrence of nonmotor features in patients with myoclonus syndromes. These are features that impact the patient's life and his or her family, and they require proper guidance and counseling.109
Just as the presence of accompanying signs and symptoms can guide clinicians to a refined differential diagnosis, absence of an accompanying movement disorder proves a useful observation, as it points toward the related disorders, hyperekplexia and myoclonic epileptic encephalopathies. Hyperekplexia is characterized by 3 clinical symptoms: generalized stiffness at birth, excessive startle reflexes, and generalized stiffness following a startle. Genetic studies have shown mutations in different parts of the inhibitory glycine receptor complex, located in the postsynaptic membrane of glycinergic and mixed GABAergic neurons. Synaptic inhibition in the brain stem and spinal cord is impaired as a result of a defect in 1 of these 3 genes.10 With regard to the genes identified in epileptic encephalopathies with prominent myoclonic jerks, a majority of these disorders share a phenotype that includes early disease onset (in the first 18 months of life) and a progressive course resulting in refractory epilepsy and severe cognitive decline. However, some genetic disorders are extremely rare (eg, CARS2), and those phenotypes are likely to be expanded in the coming years.
Conclusion
In collaboration with the MDS Task Force, we present a new nomenclature that includes 67 genetically determined myoclonus syndromes. As is apparent from this current list, numerous genes are linked to myoclonus syndromes, and prioritizing putative causative genes based on corresponding accompanying signs or symptoms and clinical clues could accelerate the identification of a molecular diagnosis in individual cases. Furthermore, it shows the additional value of electrophysiological testing in patients with myoclonus syndromes, as it may lead to a more refined differential diagnosis and therapeutic strategy. The current nomenclature can be used as a framework to add newly discovered genes in a systematic way and can be used for movement disorder (myoclonus) next‐generation sequencing diagnostics. In the near future, genetically determined myoclonus syndromes can be uploaded in the searchable online database, the Movement Disorder Society Genetic Mutation Database, MDSGene (http://www.mdsgene.org), to provide an online, browsable database of hereditary myoclonus syndromes.110
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
S.V.: 1B, 1C, 3A, 3B.
R.Z.: 1A, 1B, 1C, 3B.
C.K.: 1A, 3B.C.M.: 1A, 3B.
S.F.B.: 3B.
J.N.C.: 3B.
H.S.: 3B.
T.J.K.: 1A, 1B, 1C, 3B.
M.A.J.T.: 1A, 1B, 1C, 3B.
Full financial disclosure for the previous 12 months
C.K. is a medical adviser to Centogene, received honoraria from the Wellcome Trust Review Board and the Scientific Advisory Board of the Else Kroener Fresenius Foundation, and received grants from the Hermann and Lilly Schilling Foundation, the German Research Foundation, the BMBF, the German Research Foundation, the European Community, and intramural funds from the University of Luebeck. She receives royalties from the Oxford University Press and is employed at the University of Luebeck. C.M. is consultant for Acorda, received honoraria for teaching from EMD Serono and has received grants from the Michael J. Fox Foundation, Canadian Institutes of Health Research, and National Parkinson Foundation. She was site principal investigator for a clinical trial sponsored by the National Institutes of Health, International Parkinson and Movement Disorder Society and Parkinson Disease Foundation. She has received contracts from Horizon Pharma and is employed with the University Health Network. For this article, she received support from the International Parkinson and Movement Disorder Society. T.J.K. received grants from Metabolic Power Foundation, Metakids Foundation, and Ride4Kids Foundation (all nonprofit) for studying movement disorders in metabolic diseases. He received research grants from Actelion Pharmaceuticals (profit) for studying movement disorders in Niemann‐Pick‐C disease and received an honorarium for presenting at a sponsored meeting on Niemann‐Pick‐C. M.A.J.T. has received research grants from the Dystonia Medical Research Foundation, Stichting Wetenschapsfonds Dystonie Vereniging, Prinses Beatrix Foundation, Fonds NutsOhra, Jacques and Gloria Gossweiler Foundation, Fonds Psychische Gezondheid, and Phelps Stichting and unrestricted grants for education and for the national DystonieNet from Ipsen & Allergan Farmaceutics, Merz, Medtronic, and Actelion. S.V., R.Z., S.F.B., J.N.C., and H.S. have nothing to disclose.
Supporting information
Supplementary table 1 The electrophysiological and clinical features of myoclonus, its subtypes and mimic.
Supplementary table 2. The clinical and electrophysiological features of myoclonic jerks stated for each gene.
Supplementary table 3 Overview of genes presenting with myoclonus
Acknowledgments
The authors are grateful to P.D. Thompson of the University of Adelaide and Royal Adelaide Hospital in Adelaide, Australia, for his critical review of the manuscript and helpful comments. The authors also thank the International Parkinson and Movement Disorder Society for supporting the Task Force on Nomenclature and Classification of Inherited Movement Disorders.
Relevant conflicts of interest/financial disclosures: None of the authors have potential conflicts of interest to be disclosed.
Funding agencies: This research received support from International Parkinson and Movement Disorders Society.
References
- 1. Marras C, Lang A, van de Warrenburg BP, et al. Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force. Mov Disord 2016;31:436–457. [DOI] [PubMed] [Google Scholar]
- 2. Rossi M, Anheim M, Durr A, et al. The genetic nomenclature of recessive cerebellar ataxias. Mov Disord 2018;33:1056–1076. [DOI] [PubMed] [Google Scholar]
- 3. Caviness JN. Myoclonus. Mayo Clin Proc 1996;71:679–688. [DOI] [PubMed] [Google Scholar]
- 4. Shibasaki H, Hallet M. The Neurological Examination: Scientific Basis for Clinical Diagnosis. New York: Oxford University Press; 2016. [Google Scholar]
- 5. Van Egmond ME, Elting JWJ, Kuiper A, et al. Myoclonus in childhood‐onset neurogenetic disorders: The importance of early identification and treatment. Eur J Paediatr Neurol 2015;19:726–729. [DOI] [PubMed] [Google Scholar]
- 6. Zutt R, Elting JW, van Zijl JC, et al. Electrophysiologic testing aids diagnosis and subtyping of myoclonus. Neurology 2018;90:e647–e657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shibasaki H, Thompson PD. Milestones in myoclonus. Mov Disord 2011;26:1142–1148. [DOI] [PubMed] [Google Scholar]
- 8. Zutt R, van Egmond ME, Elting JW, et al. A novel diagnostic approach to patients with myoclonus. Nat Rev Neurol 2015;11:687–697. [DOI] [PubMed] [Google Scholar]
- 9. Marras C, Lohmann K, Lang A, Klein C. Fixing the broken system of genetic locus symbols: Parkinson disease and dystonia as examples. Neurology 2012;78:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dreissen YEM, Tijssen MAJ. The startle syndromes: Physiology and treatment. Epilepsia 2012;53:3–11. [DOI] [PubMed] [Google Scholar]
- 11. Munroe PB, Mitchison HM, O'Rawe AM, et al. Spectrum of mutations in the Batten disease gene, CLN3. Am J Hum Genet 1997;61:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peltonen L, Savukoski M, Klockars T, Holmberg V, Santavuori P, Lander ES. CLN5, a novel gene encoding a putative transmembrane protein mutatedin Finnish variant late infantile neuronal ceroid lipofuscinosis. Nat Genet 1998;19:286–288. [DOI] [PubMed] [Google Scholar]
- 13. Gao H, Boustany R‐MN, Espinola JA, et al. Mutations in a novel CLN6‐encoded transmembrane protein cause variant neuronal ceroid lipofuscinosis in man and mouse. Am J Hum Genet 2002;70:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arsov T, Smith KR, Damiano J, et al. Kufs Disease, the Major Adult Form of Neuronal Ceroid Lipofuscinosis, Caused by Mutations in CLN6. Am J Hum Genet 2011;88:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranta S, Zhang Y, Ross B, et al. The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat Genet 1999;23:233–236. [DOI] [PubMed] [Google Scholar]
- 16. Cadieux‐Dion M, Andermann E, Lachance‐Touchette P, et al. Recurrent mutations in DNAJC5 cause autosomal dominant Kufs disease. Clin Genet 2013;83:571–575. [DOI] [PubMed] [Google Scholar]
- 17. Shiang R, Ryan SG, Zhu Y‐Z, Hahn AF, O'Connell P, Wasmuth JJ. Mutations in the alpha‐1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat Genet 1993;5:351–357. [DOI] [PubMed] [Google Scholar]
- 18. Rees MI, Harvey K, Pearce BR, et al. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat Genet 2006;38:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rees MI, Lewis TM, Kwok JBJ, et al. Hyperekplexia associated with compound heterozygote mutations in the beta‐subunit of the human inhibitory glycine receptor (GLRB). Hum Mol Genet 2002;11:853–860. [DOI] [PubMed] [Google Scholar]
- 20. Muona M, Berkovic SF, Dibbens LM, et al. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat Genet 2015;47:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bassuk AG, Wallace RH, Buhr A, et al. A Homozygous Mutation in Human PRICKLE1 Causes an Autosomal‐Recessive Progressive Myoclonus Epilepsy‐Ataxia Syndrome. Am J Hum Genet 2008;83:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishiura H, Doi K, Mitsui J, et al. Expansions of intronic TTTCA and TTTTA repeats in benign adult familial myoclonic epilepsy. Nat Genet 2018;50:581–590. [DOI] [PubMed] [Google Scholar]
- 23. Lei XX, Liu Q, Lu Q, et al. TTTCA repeat expansion causes familial cortical myoclonic tremor with epilepsy. Eur J Neurol 2018:26(3):513–518. [DOI] [PubMed] [Google Scholar]
- 24. Berkovic SF, Dibbens LM, Oshlack A, et al. Array‐Based Gene Discovery with Three Unrelated Subjects Shows SCARB2/LIMP‐2 Deficiency Causes Myoclonus Epilepsy and Glomerulosclerosis. Am J Hum Genet 2008;82(3):673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinfeld R, Grapp M, Kraetzner R, et al. Folate Receptor Alpha Defect Causes Cerebral Folate Transport Deficiency: A Treatable Neurodegenerative Disorder Associated with Disturbed Myelin Metabolism. Am J Hum Genet 2009;85:354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortigoza Escobar JD, Pérez Dueñas B. Treatable Inborn Errors of Metabolism Due to Membrane Vitamin Transporters Deficiency. Semin Pediatr Neurol 2016;23:341–350. [DOI] [PubMed] [Google Scholar]
- 27. Hallmann K, Zsurka G, Moskau‐Hartmann S, et al. A homozygous splice‐site mutation in CARS2 is associated with progressive myoclonic epilepsy. Neurology 2014;83:2183–2187. [DOI] [PubMed] [Google Scholar]
- 28. Coughlin CR, Scharer GH, Friederich MW, et al. Mutations in the mitochondrial cysteinyl‐tRNA synthase gene, CARS2, lead to a severe epileptic encephalopathy and complex movement disorder. J Med Genet 2015;52:532–540. [DOI] [PubMed] [Google Scholar]
- 29. Carvill GL, Heavin SB, Yendle SC, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 2013;45:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas RH, Zhang LM, Carvill GL, et al. CHD2 myoclonic encephalopathy is frequently associated with self‐induced seizures. Neurology 2015;84:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chatron N, Møller RS, Champaigne NL, et al. The epilepsy phenotypic spectrum associated with a recurrent CUX2 variant. Ann Neurol 2018;83:926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tada K, Kure S, Kume A, Hiraga K. Genomic Analysis of Non‐ketotic Hyperglycinaemia: A Partial Deletion of P‐protein Gene. J lnher Metab Dis 1990;13(5):766–770. [DOI] [PubMed] [Google Scholar]
- 33. Nanao K, Takada G, Takahashi E‐I, et al. Structure and Chromosomal Localization of the Aminomethyltransferase Gene (AMT). Genomics 1994;19:27–30. [DOI] [PubMed] [Google Scholar]
- 34. Shoffner JM, Lott MT, Lezza a M, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged‐red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 1990;61:931–937. [DOI] [PubMed] [Google Scholar]
- 35. Tranchant C, Anheim M. Movement disorders in mitochondrial diseases. Rev Neurol 2016;172:524–529. [DOI] [PubMed] [Google Scholar]
- 36. Johnston JJ, Gropman AL, Sapp JC, et al. The Phenotype of a Germline Mutation in PIGA: The Gene Somatically Mutated in Paroxysmal Nocturnal Hemoglobinuria. Am J Hum Genet 2012;90:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Goethem G, Dermaut B, Löfgren A, Martin J‐J, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 2001;28:211–212. [DOI] [PubMed] [Google Scholar]
- 38. Claes L, Del‐Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De Novo Mutations in the Sodium‐Channel Gene SCN1A Cause Severe Myoclonic Epilepsy of Infancy. Am J Hum Genet 2001;68:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davis RL, Shrimpton a E, Holohan PD, et al. Familial dementia caused by polymerization of mutant neuroserpin. Nature 1999;401:376–379. [DOI] [PubMed] [Google Scholar]
- 40. Davis RL, Shrimpton AE, Carrell RW, et al. Association between conformational mutations in neuroserpin and onset and severity of dementia. Lancet 2002;359:2242–2247. [DOI] [PubMed] [Google Scholar]
- 41. Carvill GL, McMahon JM, Schneider A, et al. Mutations in the GABA Transporter SLC6A1 Cause Epilepsy with Myoclonic‐Atonic Seizures. Am J Hum Genet 2015;96:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Falace A, Filipello F, La Padula V, et al. TBC1D24, an ARF6‐Interacting Protein, Is Mutated in Familial Infantile Myoclonic Epilepsy. Am J Hum Genet 2010;87:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Balestrini S, Milh M, Castiglioni C, et al. TBC1D24 genotype – phenotype correlation Epilepsies and other neurologic features. Neurology 2016:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pennacchio LA, Lehesjoki AE, Stone NE, et al. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science 1996;271:1731–1734. [DOI] [PubMed] [Google Scholar]
- 45. Crespel A, Ferlazzo E, Franceschetti S, et al. Unverricht‐Lundborg disease. Epileptic Disord 2016;18:28–37. [DOI] [PubMed] [Google Scholar]
- 46. Minassian BA, Lee JR, Herbrick JA, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet 1998;20:171–174. [DOI] [PubMed] [Google Scholar]
- 47. Turnbull J, Tiberia E, Striano P, et al. Lafora disease. Epileptic Disord 2016;18:S38–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Corbett MA, Schwake M, Bahlo M, et al. A mutation in the Golgi Qb‐SNARE gene GOSR2 causes progressive myoclonus epilepsy with early ataxia. Am J Hum Genet 2011;88:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Bogaert P, Azizieh R, Désir J, et al. Mutation of a potassium channel‐related gene in progressive myoclonic epilepsy. Ann Neurol 2007;61:579–586. [DOI] [PubMed] [Google Scholar]
- 50. Van Bogaert P. KCTD7‐related progressive myoclonus epilepsy. Epileptic Disord 2016;18:115–119. [DOI] [PubMed] [Google Scholar]
- 51. Bonten E, Van Der Spoel A, Fornerod M, Grosveld G, D'Azzo A. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev 1996;10:3156–3169. [DOI] [PubMed] [Google Scholar]
- 52. Franceschetti S, Canafoglia L. Sialidoses. Epileptic Disord 2016;18:89–93. [DOI] [PubMed] [Google Scholar]
- 53. Chan EM, Young EJ, Ianzano L, et al. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet 2003;35:125–127. [DOI] [PubMed] [Google Scholar]
- 54. Rawlings ND, Barrett AJ. Tripeptidyl‐peptidase I is apparently the CLN2 protein absent in classical late‐infantile neuronal ceroid lipofuscinosis. Biochim Biophys Acta 1999;1429:496–500. [DOI] [PubMed] [Google Scholar]
- 55. Zimprich A, Grabowski M, Asmus F, et al. Mutations in the gene encoding epsilon‐sarcoglycan cause myoclonus‐dystonia syndrome. Nat Genet 2001;29:66–69. [DOI] [PubMed] [Google Scholar]
- 56. Mencacci NE, Rubio‐Agusti I, Zdebik A, et al. A missense mutation in KCTD17 causes autosomal dominant myoclonus‐dystonia. Am J Hum Genet 2015;96:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Savitsky K, Bar‐shira A, Gilad S, et al. A Single Ataxia Telangiectasia Gene with a Product Similar to PI‐3 Kinase. Science 1995;268:1749–1753. [DOI] [PubMed] [Google Scholar]
- 58. Pearson TS. More Than Ataxia: Hyperkinetic Movement Disorders in Childhood Autosomal Recessive Ataxia Syndromes. Tremor Other Hyperkinet Mov (N Y) 2016;6:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koide R, Ikeuchi T, Onodera O, et al. Unstable expansion of CAG repeat in hereditary dentatorubral‐pallidoluysian atrophy (DRPLA). Nat Genet 1994;6:9–13. [DOI] [PubMed] [Google Scholar]
- 60. Carstea ED, Morris JA, Coleman KG, et al. Niemann‐Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 1997;277:228–231. [DOI] [PubMed] [Google Scholar]
- 61. Anheim M, Lagha‐Boukbiza O, Fleury‐Lesaunier M‐C, et al. Heterogeneity and frequency of movement disorders in juvenile and adult‐onset Niemann‐Pick C disease. J Neurol 2014;261:174–179. [DOI] [PubMed] [Google Scholar]
- 62. Chen DH, Brkanac Z, Verlinde CLMJ, et al. Missense mutations in the regulatory domain of PKC gamma: a new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet 2003;72:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miura S, Nakagawara H, Kaida H, et al. Expansion of the phenotypic spectrum of SCA14 caused by the Gly128Asp mutation in PRKCG. Clin Neurol Neurosurg 2009;111:211–215. [DOI] [PubMed] [Google Scholar]
- 64. Stamelou M, Charlesworth G, Cordivari C, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord 2014;29:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen YZ, Matsushita MM, Robertson P, et al. Autosomal dominant familial dyskinesia and facial myokymia: single exome sequencing identifies a mutation in adenylyl cyclase 5. Arch Neurol 2012;69:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tunc S, Brüggemann N, Baaske MK, et al. Facial twitches in ADCY5‐associated disease — Myokymia or myoclonus? An electromyography study. Parkinsonism Relat Disord 2017;40:73–75. [DOI] [PubMed] [Google Scholar]
- 67. Anon. The Huntington's Disease Collaborative Researches Group . A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosom. Cell 1993;72:971–983. [DOI] [PubMed] [Google Scholar]
- 68. Rossi Sebastiano D, Soliveri P, Panzica F, et al. Cortical myoclonus in childhood and juvenile onset Huntington's disease. Parkinsonism Relat Disord 2012;18:794–797. [DOI] [PubMed] [Google Scholar]
- 69. Breedveld GJ, van Dongen JWF, Danesino C, et al. Mutations in TITF‐1 are associated with benign hereditary chorea. Hum Mol Genet 2002;11:971–979. [DOI] [PubMed] [Google Scholar]
- 70. Duis J, Dean S, Applegate C, et al. KIF5A mutations cause an infantile onset phenotype including severe myoclonus with evidence of mitochondrial dysfunction. Ann Neurol 2016;80:633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rydzanicz M, Jagła M, Kosinska J, et al. KIF5A de novo mutation associated with myoclonic seizures and neonatal onset progressive leukoencephalopathy. Clin Genet 2017;91:769–773. [DOI] [PubMed] [Google Scholar]
- 72. Nascimento FA, Canafoglia L, Aljaafari D, et al. Progressive myoclonus epilepsy associated with SACS gene mutations. Neurol Genet 2016;2:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tsuji S, Choudary P V., Martin BM, et al. A Mutation in the Human Glucocerebrosidase Gene in Neuronopathic Gaucher's Disease. N Engl J Med 1987;316:570–575. [DOI] [PubMed] [Google Scholar]
- 74. Park JK, Orvisky E, Tayebi N, et al. Myoclonic epilepsy in Gaucher disease: Genotype‐phenotype insights from a rare patient subgroup. Pediatr Res 2003;53:387–395. [DOI] [PubMed] [Google Scholar]
- 75. Goate A, Chartier‐Harlin M‐C, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 1991;349:704–706. [DOI] [PubMed] [Google Scholar]
- 76. Zhou J, Tawk M, Tiziano FD, et al. Spinal Muscular Atrophy Associated with Progressive Myoclonic Epilepsy Is Caused by Mutations in ASAH1. Am J Hum Genet 2012. 91:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Poirier K, Hubert L, Viot G, et al. CSNK2B splice site mutations in patients cause intellectual disability with or without myoclonic epilepsy. Hum Mutat 2017:932–941. [DOI] [PubMed] [Google Scholar]
- 78. Takano T, Shimmoto M, Fukuhara Y, et al. Galactosialidosis: clinical and molecular analysis of 19 Japanese patients. Brain Dysfunct 1991;4:271–280. [Google Scholar]
- 79. Annunziata I and d'Azzo A. Galactosialidosis: historic aspects and overview of investigated and emerging treatment options. Expert Opin Orphan Drugs 2017;5:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elo JM, Yadavalli SS, Euro L, et al. Mitochondrial phenylalanyl‐tRNA synthetase mutations underlie fatal infantile Alpers encephalopathy. Hum Mol Genet 2012;21:4521–4529. [DOI] [PubMed] [Google Scholar]
- 81. Goldgaber D, Goldfarb LG, Brown P, et al. Mutations in familial Creutzfeldt‐Jakob disease and Gerstmann‐Sträussler‐Scheinker's syndrome. Exp Neurol 1989;106:204–206. [DOI] [PubMed] [Google Scholar]
- 82. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt‐Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus 2015;39:E2. [DOI] [PubMed] [Google Scholar]
- 83. Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early‐onset familial Alzheimer's disease. Nature 1995;375:754–760. [DOI] [PubMed] [Google Scholar]
- 84. Ryan NS, Nicholas JM, Weston PSJ, et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer's disease: a case series. Lancet Neurol 2016;15:1326–1335. [DOI] [PubMed] [Google Scholar]
- 85. Trivier E, De Cesare D, Jacquot S, et al. Mutations in the kinase Rsk‐2 associated with Coffin‐Lowry syndrome. Nature 1996;384:567–570. [DOI] [PubMed] [Google Scholar]
- 86. Van Egmond ME, Elting JWJ, Kuiper A, et al. Myoclonus in childhood‐onset neurogenetic disorders: The importance of early identification and treatment. Eur J Paediatr Neurol 2015;19:726–729. [DOI] [PubMed] [Google Scholar]
- 87. Mullen SA, Marini C, Suls A, et al. Glucose Transporter 1 Deficiency as a Treatable Cause of Myoclonic Astatic Epilepsy. Arch Neurol 2011;68:1152. [DOI] [PubMed] [Google Scholar]
- 88. Koch H and Weber YG. The glucose transporter type 1 (Glut1) syndromes. Epilepsy Behav 2018;1:4–7. [DOI] [PubMed] [Google Scholar]
- 89. Vissers LELM, de Ligt J, Gilissen C, et al. A de novo paradigm for mental retardation. Nat Genet 2010;42:1109–1112. [DOI] [PubMed] [Google Scholar]
- 90. Kishino T, Lalande M, Wagstaff J. UBE3A/E6‐AP mutations cause Angelman syndrome. Nat Genet 1997;15:70–73. [DOI] [PubMed] [Google Scholar]
- 91. Pollack SF, Grocott OR, Parkin KA, Larson AM, Thibert RL. Myoclonus in Angelman syndrome. Epilepsy Behav 2018;82:170–174. [DOI] [PubMed] [Google Scholar]
- 92. Kotzot D, Schmitt S, Bernasconi F, et al. Uniparental disomy 7 in Silver‐Russell syndrome and primordial growth retardation. Hum Mol Genet 1995;4:583–587. [DOI] [PubMed] [Google Scholar]
- 93. Zutt R, Elting JW, van der Hoeven JH, Lange F, Tijssen MAJ. Myoclonus subtypes in tertiary referral center. Cortical myoclonus and functional jerks are common. Clin Neurophysiol 2017;128:253–259. [DOI] [PubMed] [Google Scholar]
- 94. Van der Veen S, Zutt R, Becker CE, Elting JWJ, De Koning TJ, Tijssen MAJ. Progressive Myoclonus Ataxia Time for a New Definition? MovDisord 2018;33:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Franceschetti S, Michelucci R, Canafoglia L, et al. Progressive myoclonic epilepsies Definitive and still undetermined causes. Neurology 2014;82:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ishiura H, Doi K, Mitsui J, et al. Expansions of intronic TTTCA and TTTTA repeats in benign adult familial myoclonic epilepsy. Nat Genet 2018;50:581–590. [DOI] [PubMed] [Google Scholar]
- 97. Van Rootselaar AF, Van Der Salm SMA, Bour LJ, et al. Decreased cortical inhibition and yet cerebellar pathology in “familial cortical myoclonic tremor with epilepsy.” Mov Disord 2007;22:2378–2385. [DOI] [PubMed] [Google Scholar]
- 98. Ganos C, Kassavetis P, Erro R, Edwards MJ, Rothwell J and Bhatia KP. The role of the cerebellum in the pathogenesis of cortical myoclonus. Mov Disord 2014;29:437–443. [DOI] [PubMed] [Google Scholar]
- 99. Bhat S and Ganesh S. New discoveries in progressive myoclonus epilepsies: a clinical outlook. Expert Rev Neurother 2018;18:649–667. [DOI] [PubMed] [Google Scholar]
- 100. Corbett MA, Schwake M, Bahlo M, et al. A mutation in the Golgi Qb‐SNARE gene GOSR2 causes progressive myoclonus epilepsy with early ataxia. Am J Hum Genet 2011;88:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nardocci N, Zorzi G, Barzaghi C, et al. Myoclonus‐dystonia syndrome: clinical presentation, disease course, and genetic features in 11 families. Mov Disord 2008;23:28–34. [DOI] [PubMed] [Google Scholar]
- 102. Roze E, Lang AE, Vidailhet M. Myoclonus‐dystonia: classification, phenomenology, pathogenesis, and treatment. Curr Opin Neurol 2018;31:484–490. [DOI] [PubMed] [Google Scholar]
- 103. Eberhardt O, Topka H. Myoclonic disorders. Brain Sci 2017;7(8): pii: E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dijk JM, Tijssen MAJ. Management of patients with myoclonus: Available therapies and the need for an evidence‐based approach. Lancet Neurol 2010;9:1028–1036. [DOI] [PubMed] [Google Scholar]
- 105. Apartis E and Vercueil L. To jerk or not to jerk: A clinical pathophysiology of myoclonus. Rev Neurol (Paris) 2016;172:465–476. [DOI] [PubMed] [Google Scholar]
- 106. Boissé Lomax L, Bayly MA, Hjalgrim H, et al. “North Sea” progressive myoclonus epilepsy: phenotype of subjects with GOSR2 mutation. Brain 2013;136:1146–1154. [DOI] [PubMed] [Google Scholar]
- 107. van Egmond ME, Verschuuren‐Bemelmans CC, Nibbeling EA, et al. Ramsay hunt syndrome: Clinical characterization of progressive myoclonus ataxia caused by GOSR2 mutation. Mov Disord 2014;29:139–143. [DOI] [PubMed] [Google Scholar]
- 108. Zutt R, Gelauff JM, Smit M, Van Zijl JC, Stone J and Tijssen MAJ. The presence of depression and anxiety do not distinguish between functional jerks and cortical myoclonus. Parkinsonism Relat Disord 2017;45:90–93. [DOI] [PubMed] [Google Scholar]
- 109. Skorvanek M, Rosenberger J, Minar M, et al. Relationship between the non‐motor items of the MDS‐UPDRS and Quality of Life in patients with Parkinson's disease. J Neurol Sci 2015;353:87–91. [DOI] [PubMed] [Google Scholar]
- 110. Lill CM, Mashychev A, Hartmann C, et al. Launching the movement disorders society genetic mutation database (MDSGene). Mov. Disord 2016;31:607–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1 The electrophysiological and clinical features of myoclonus, its subtypes and mimic.
Supplementary table 2. The clinical and electrophysiological features of myoclonic jerks stated for each gene.
Supplementary table 3 Overview of genes presenting with myoclonus


