Abstract
Aims
To investigate how the cardiovascular (CV) risk benefits of dapagliflozin translate into healthcare costs compared with other non‐sodium–glucose cotransporter‐2 inhibitor glucose‐lowering drugs (oGLDs) in a real‐world population with type 2 diabetes (T2D) that is similar to the population of the DECLARE‐TIMI 58 trial.
Methods
Patients initiating dapagliflozin or oGLDs between 2013 and 2016 in Swedish nationwide healthcare registries were included if they fulfilled inclusion and exclusion criteria of the DECLARE‐TIMI 58 trial (DECLARE‐like population). Propensity scores for the likelihood of dapagliflozin initiation were calculated, followed by 1:3 matching with initiators of oGLDs. Per‐patient cumulative costs for hospital healthcare (in‐ and outpatient) and for drugs were calculated from new initiation until end of follow‐up.
Results
A total of 24 828 patients initiated a new GLD; 6207 initiated dapagliflozin and 18 621 initiated an oGLD. After matching based on 96 clinical and healthcare cost variables, groups were balanced at baseline. Mean cumulative 30‐month healthcare cost per patient was similar in the dapagliflozin and oGLD groups ($11 807 and $11 906, respectively; difference, −$99; 95% CI, −$629, $483; P = 0.644). Initiation of dapagliflozin rather than an oGLD was associated with significantly lower hospital costs (−$658; 95% CI, −$1169, −$108; P = 0.024) and significantly higher drug costs ($559; 95% CI, $471, $648; P < 0.001). Hospital cost difference was related mainly to fewer CV‐ and T2D‐associated complications with use of dapagliflozin compared with use of an oGLD (−$363; 95% CI, −$665, −$61; P = 0.008).
Conclusion
In a nationwide, real‐world, DECLARE‐like population, dapagliflozin was associated with lower hospital costs compared with an oGLD, mainly as a result of reduced rates of CV‐ and T2D‐associated complications.
1. INTRODUCTION
There is currently an epidemic of type 2 diabetes (T2D), and its increasing prevalence has resulted in a rapid increase in related healthcare costs over the last decade. In Sweden, healthcare costs for diabetes doubled, from €835 million to €1684 billion between 2006 and 2014,1, 2, 3 mainly driven by costs associated with hospital care for cardiovascular (CV) complications, in particular heart failure.2 Recent clinical trials have demonstrated that some newer glucose‐lowering drugs (GLDs) have a beneficial effect on CV outcomes in patients with T2D,4, 5, 6, 7, 8, 9 and these paradigm‐changing effects could have the potential to reduce healthcare costs.
Dapagliflozin is a sodium–glucose cotransporter‐2 inhibitor (SGLT‐2i) that has been shown to be safe and to effectively reduce CV disease in both high‐ and low‐CV‐risk patients in clinical trial and real‐world settings.7, 10, 11, 12, 13, 14, 15, 16 In addition, dapagliflozin is increasingly prescribed worldwide10, 17 and has been observed to be effective in reducing blood glucose levels in various clinical settings.18, 19, 20, 21, 22, 23, 24, 25, 26, 27
The DECLARE‐TIMI 58 trial (http://clinicaltrials.gov NCT01730534)28 was the largest CV outcomes trial (CVOT) concerning an SGLT‐2i to date (dapagliflozin, n = 17 160) and applied broad eligibility criteria, resulting in a study population with established CV disease or with multiple CV risk factors.7, 14, 15, 16 In this trial, use of dapagliflozin reduced the risk of CV death or heart failure compared with placebo (4.9% and 5.8%, respectively; hazard ratio [HR], 0.83; 95% CI, 0.73, 0.95), as well as reducing kidney disease progression (1.5% and 2.8%, respectively; HR, 0.53; 95% CI, 0.43, 0.66) in patients with or without established CV disease.7, 14, 15 Subsequent to the DECLARE‐TIMI 58 trial, several observational studies have been conducted to investigate the use of dapagliflozin in a real‐world clinical setting and its potential impact on CV outcomes. Firstly, a multinational study of more than 800 000 patients reported that the broad eligibility criteria of the DECLARE‐TIMI 58 trial were applicable to 59% of the Swedish population with T2D.29 This representativeness was two‐ to four‐fold greater than that of other SGLT‐2i CVOTs.29 Secondly, assessment of the external validity of the results of the DECLARE‐TIMI 58 trial demonstrated that the beneficial CV effects of dapagliflozin could be translated into a real‐world population with T2D.11 Differences in CV mortality benefits have been shown between CVOTs6, 8, 9 and observational studies,10, 12 and this is may be explained by differences in the frailty of the patients included. This is supported by post hoc analyses of the DECLARE‐TIMI 58 trial (Figure S1).14, 16 While the safety, clinical benefits, representativeness and external validity of the DECLARE‐TIMI 58 trial have been evaluated,7, 11, 12, 14, 15, 29 the way in which the observed beneficial effects of dapagliflozin might impact healthcare costs is not known.
The aim of the present analysis was to compare the hospital healthcare costs of using dapagliflozin and those of using other glucose‐lowering drugs (oGLD) in a nationwide, real‐world DECLARE‐like population based on the main eligibility criteria of the DECLARE‐TIMI 58 trial.11
2. MATERIALS AND METHODS
This nationwide, observational study is part of the D360 Nordic program, a large‐scale epidemiological investigation that aims to obtain full‐coverage understanding of T2D and its treatment.3, 30 This program utilizes the unique features of the mandatory healthcare registries and corresponding healthcare systems in Sweden to identify all patients with T2D with filled prescriptions for a glucose‐lowering drug (GLD) (Appendix, Section S1).31
2.1. Data sources
Sweden has a comprehensive, nationwide public healthcare system. All citizens have a unique personal identification number (person‐ID), which is mandatory for all administrative purposes (including any contact with the healthcare system and drug dispensaries), thus providing a complete medical history from a population perspective. This study included data from the Swedish Prescribed Drug Register, the Cause of Death Register and the National Patient Register covering all hospitalizations with discharge diagnoses and all out‐patient hospital visits (Appendix, Section S1).11 Individual patient‐level data from the national registers were linked using the person‐ID. The linked anonymized database was managed separately by Statisticon AB, Uppsala, Sweden. The study was approved by the Stockholm regional ethics committee (registration number 2013/2206–31).
2.2. Study population
All incident new‐user episodes of filled prescriptions for either dapagliflozin or a non‐SGLT‐2i GLD (oGLD) in Sweden between 2013 and 2016, in patients with T2D who were at least 18 years of age were eligible for inclusion in the analysis.10, 17, 32 Patients with type 1 diabetes, gestational diabetes, polycystic ovarian syndrome or cancer (current or prior history) were excluded (Appendix, Section 2).11 The DECLARE‐like study population for evaluation was defined by the main inclusion and exclusion criteria of the DECLARE‐TIMI 58 trial (≥40 years of age with established CV disease, or with multiple risk factors: men ≥55 and women ≥60 years with hypertension or dyslipidaemia); adoption from these trial criteria to registry data is shown in the online Appendix, Section S3.
The new‐user date (index date) was defined as the date of the initial filled prescription for dapagliflozin or oGLD, and, for participants to be considered a new user, this date had to be preceded by a 12‐month period without any filled prescription for the same drug class. This definition allowed for several possible new‐user dates for a patient within the observation period, both within drug class and between classes, which eliminates the risk of immortal time bias while maximizing the number of observations.17
2.3. Baseline data
Patient characteristics included age at the date of index drug initiation, sex, index year and year of first registered GLD dispense (detailed definitions are given in Table S5a).3, 33, 34 Year of first registered GLD dispense was used in the propensity score as a proxy for duration of T2D and index year was used to ensure that the treatments of interest were initiated at the same point in time. Comorbidities were searched for in all available data prior to and including the index date, with the exception of severe hypoglycaemia, which was included only if it occurred within the 12 months prior to index date, and cancer, which was included only if it occurred within five years prior to index date (detailed definitions are given in Table S5b). Prior medications were defined as any drugs dispensed within the 12 months prior to, and including, the index date (detailed definitions are given in Table S5c).
2.4. Healthcare cost outcomes
Healthcare costs for inpatient and outpatient hospital care were estimated using Diagnosis Related Groups (DRGs). The DRG‐price level for 2016 (1 US$ [US dollar] = 8.56 SEK [Swedish Krona]) was applied throughout the study. Drug costs were based on the actual costs of dispensing at the pharmacy and were adjusted for inflation to the general price level for 2016 using the Consumer Price Index (CPI). Baseline costs were defined as mean per‐patient costs for both the three and 12 months preceding initiation of dapagliflozin or an oGLD.
Cumulative healthcare cost per patient was calculated for incremental three‐month intervals up to 30 months (ie, cost for 0–3 months, 0–6 months, etc., up to 0–30 months). For patients with a shorter follow‐up period than the end of the time interval of interest, the observed cost was divided by the fraction of time the patient contributed, to get the expected cost for the full time period of interest, and then weighted according to the relative time in study up to the time point of interest, in order to avoid influence of extreme values. As an example, if a patient was followed for exactly 13‐months, the patient would contribute with the actual cost for all time intervals up to 12 months. For the 0 to 15‐month interval, the cost was then estimated as . For the remaining time intervals, the cost was estimated using the same approach, but modifying the denominator to include the time interval of interest.
In the calculation of average cost, individual cost estimates were weighted according to relative time‐in‐study up to the interval of interest. Thus, for all time points up to 12 months in the current example the weight was 1. For the 0 to 15‐month interval, the patient contributed with exposure time during 13/15 (~87%) months and, therefore, had the weight of 0.87 for the estimation of mean cost at 15 months. For the 0 to 18‐month interval, the weight would be 0.72, and so on, up to 30 months when the weight would be 0.43. As the aim of the study was to evaluate actual cost, and patients who die have no healthcare‐related cost after the date of death, only the cost until death was calculated for patients who died during the study, and the weight was 1 for all subsequent time points. The justification for this simplistic imputation of costs is that all censoring occurs at the end of follow‐up and is therefore completely uninformative. As a result, the cost after censoring is expected to be the same as before censoring.
2.5. Statistical analysis
Baseline characteristics are presented as mean and standard deviation for continuous variables, and as absolute and relative frequencies for categorical variables. In order to compare baseline characteristics between groups, standardized differences were calculated for all baseline variables. A difference of more than 10% was considered a non‐negligible group imbalance, based on current standards.35
\A propensity score for each new user of dapagliflozin was calculated using a logistic regression model with patient characteristics, age, time since dispense of first GLD, three‐ and 12‐month healthcare costs prior to index date, comorbidities, coronary revascularization, frailty, all separate classes of GLDs, dispense of CV disease preventive drugs and drugs associated with treatment of heart failure, and date of both index drug and first‐line initiation as independent variables (Appendix, Section S4). Use of healthcare cost at both three‐ and 12‐months in the propensity score ensured a balance of both the short‐term (three‐month) and long‐term (12‐month) costs between patients. For detailed information concerning variables included in the propensity score see Tables S5a‐c. To maximize the number of eligible patients receiving dapagliflozin, while at the same time avoiding immortal time bias, all new user episodes (new drugs) are included prior to matching. One patient might, therefore, contribute with more than one index for different drugs and different time points. Propensity scores were then used to match each incident user of dapagliflozin with incident users of an oGLD (1:3 match; caliper of 0.2) using the Match function in the R package Matching.36 Confidence intervals (CIs) and P values for cost estimations and differences between groups were constructed using 500 bootstrap iterations. To explore the impact of patients with early censoring, a sensitivity analysis was performed which included only episodes with an index date during 2013 and 2014 (ie, with a minimum of 24 months of follow‐up). All analyses were conducted using R statistical software (R version 3.5.0).37
3. RESULTS
Initially, 287 180 new‐user episodes of any GLD were identified. After matching, 24 828 episodes (6207 dapagliflozin and 18 621 oGLD) remained for analysis (Figure 1). Both groups were well balanced at baseline (Table 1 and Table S7), with a mean age of 66 years, 33% having CV disease, and with similar mean three‐ and 12‐month healthcare costs prior to index ($987 and $3863, respectively).
Figure 1.
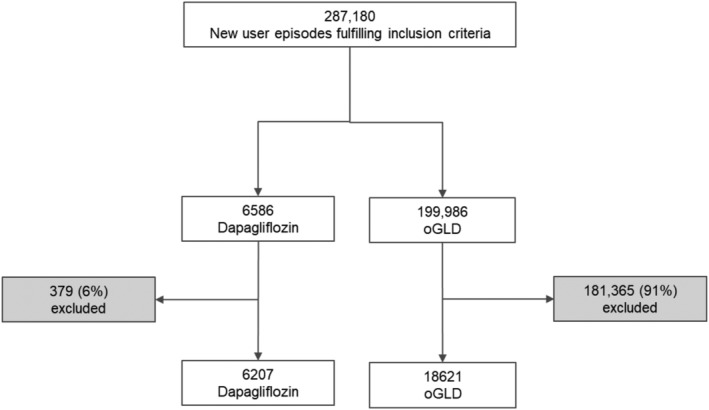
Patient flow‐chart. Grey boxes show how many dapagliflozin patients were excluded because no propensity score other glucose lowering drug (oGLD) match was found
Table 1.
Baseline characteristics of new users of dapagliflozin vs other glucose‐lowering drugs (oGLD), propensity score matched 1:3
| Dapagliflozin N = 6207 | oGLD N = 18 621 | Standardized difference (%)a | |
|---|---|---|---|
| Age, years (SD) | 66.1 (7.5) | 66.2 (8.0) | 0.4 |
| Sex, female, n (%) | 2077 (33.5%) | 6287 (33.8%) | 0.5 |
| Years since first glucose‐lowering drug (SD) | 7.3 (3.1) | 7.4 (3.0) | 3.2 |
| Healthcare cost | |||
| Total healthcare cost last 12 months | 3922.5 (7541.5) | 3843.2 (7719.7) | 1.0 |
| Hospital care cost | 2805.9 (7336.2) | 2763.8 (7552.4) | 0.6 |
| Glucose‐lowering drug cost | 732.6 (784.6) | 698.7 (812.9) | 4.2 |
| Other drugs cost | 383.9 (425.9) | 380.7 (488.8) | 0.7 |
| Total healthcare cost last 3 months | 1013.5 (3483.6) | 978.8 (2405.1) | 1.2 |
| Hospital care cost | 728.7 (3450.2) | 705.1 (2367.0) | 0.8 |
| Glucose‐lowering drug cost | 185.8 (228.4) | 176.0 (236.0) | 4.2 |
| Other drugs cost | 99.0 (126.9) | 97.7 (160.8) | 0.9 |
| Cardiovascular disease | 2035 (32.8%) | 6289 (33.8%) | 1.7 |
| Myocardial infarction | 793 (12.8%) | 2366 (12.7%) | 0.2 |
| Unstable angina | 397 (6.4%) | 1172 (6.3%) | 0.3 |
| Angina pectoris | 962 (15.5%) | 2840 (15.3%) | 0.6 |
| Heart failure | 504 (8.1%) | 1496 (8.0%) | 0.3 |
| Atrial fibrillation | 607 (9.8%) | 1802 (9.7%) | 0.3 |
| Stroke | 615 (9.9%) | 1918 (10.3%) | 1.1 |
| Peripheral artery disease | 355 (5.7%) | 1099 (5.9%) | 0.6 |
| Chronic kidney disease | 71 (1.1%) | 224 (1.2%) | 0.4 |
| Microvascular complications | 2276 (36.7%) | 6894 (37.0%) | 0.6 |
| Severe hypoglycemia | 31 (0.5%) | 105 (0.6%) | 0.7 |
| Lower limb amputations | 25 (0.4%) | 81 (0.4%) | 0.4 |
| Glucose‐lowering drugs | |||
| Metformin | 4898 (78.9%) | 15 009 (80.6%) | 3.5 |
| Sulphonylurea | 1494 (24.1%) | 4653 (25.0%) | 1.7 |
| DPP‐4i | 1628 (26.2%) | 4812 (25.8%) | 0.7 |
| GLP‐1RA | 1003 (16.2%) | 2784 (15.0%) | 2.7 |
| Meglitinides | 314 (5.1%) | 965 (5.2%) | 0.5 |
| Thiazolidinediones | 149 (2.4%) | 436 (2.3%) | 0.3 |
| Acarbose | 45 (0.7%) | 144 (0.8%) | 0.5 |
| Insulin | 2611 (42.1%) | 7795 (41.9%) | 0.3 |
| Short‐acting | 1011 (16.3%) | 2970 (15.9%) | 0.8 |
| Intermediate‐acting | 1182 (19.0%) | 3548 (19.1%) | 0.0 |
| Premixed insulin | 709 (11.4%) | 2127 (11.4%) | 0.0 |
| Long‐acting | 1011 (16.3%) | 2980 (16.0%) | 0.6 |
| CV risk treatment | 6207 (100.0%) | 18 621 (100.0%) | N/A |
| Low‐dose aspirin | 2683 (43.2%) | 8000 (43.0%) | 0.4 |
| Statins | 4770 (76.8%) | 14 397 (77.3%) | 0.9 |
| Antihypertensives | 5627 (90.7%) | 16 901 (90.8%) | 0.3 |
| ACE inhibitors | 2564 (41.3%) | 7718 (41.4%) | 0.2 |
| ARB | 2712 (43.7%) | 8135 (43.7%) | 0.0 |
| Dihydropyridines | 2444 (39.4%) | 7308 (39.2%) | 0.2 |
| Thiazides | 552 (8.9%) | 1637 (8.8%) | 0.3 |
| Beta blockers | 3198 (51.5%) | 9533 (51.2%) | 0.5 |
| Loop diuretics | 1070 (17.2%) | 3228 (17.3%) | 0.2 |
| Aldosterone antagonists | 427 (6.9%) | 1260 (6.8%) | 0.4 |
| Warfarin | 436 (7.0%) | 1336 (7.2%) | 0.5 |
| Receptor P2Y12 antagonists | 424 (6.8%) | 1279 (6.9%) | 0.1 |
All numbers in parenthesis are percentage if not stated otherwise.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CV, cardiovascular; DPP‐4i, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; oGLD, other glucose lowering drugs; SD, standard deviation; SGLT‐2i, Sodium‐glucose‐cotransporter‐2‐inhibitors.
Standardized difference of >10% is considered to represent a non‐negligible group imbalance.
3.1. Hospital healthcare costs
At baseline, the three‐month hospital healthcare costs per patient were well balanced between the dapagliflozin and oGLD groups ($729 and $705, respectively) (Table 2). Hospital healthcare cost was already significantly lower for patients treated with dapagliflozin compared with that for those treated with an oGLD at 12 months, and this difference further increased towards 30 months (−$658 (95% CI, −$1169, −$108; P = 0.024) (Figure 1 and Table 2). Diabetes‐ and CV‐related hospital healthcare costs accounted for the highest proportion of the difference (−$363; 95% CI, −$665, −$61; P = 0.008); heart failure (−$102; 95% CI, −$184, −$16; P = 0.024), myocardial infarction (−$85; 95% CI, −$173, $1; P = 0.052) and kidney disease (−$78; 95% CI, −$170, $50; P = 0.156) were the most prominent contributors to the difference (Table 2). There was no difference in healthcare costs related to stroke between patients receiving dapagliflozin and those receiving an oGLD. Other diseases accounted for –$295 (95% CI, −$660, $81; P = 0.120) of the difference in costs between the groups.
Table 2.
Healthcare costs for new initiation of dapagliflozin vs other glucose‐lowering drugs
| Baseline costs (US$) | 12‐month costs (US$) | 30‐month costs (US$) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dapa | oGLD | Dapa | oGLD | Diff. | 95%CI | p‐value | Dapa | oGLD | Diff. | 95%CI | p‐value | |
| Total healthcare cost | 1014 | 979 | 4968 | 5054 | −86 | −351 to 224 | .500 | 11 807 | 11 906 | −99 | −629 to 483 | .644 |
| Hospital costs | 729 | 705 | 3136 | 3456 | −321 | −587 to −19 | .028 | 7481 | 8140 | −658 | −1169 to −108 | .024 |
| CV and diabetes | 374 | 378 | 1410 | 1569 | −160 | −303 to −2 | .048 | 3334 | 3698 | −363 | −665 to −61 | .008 |
| Heart failure | 29 | 27 | 101 | 142 | −40 | −82 to 1 | .064 | 241 | 343 | −102 | −184 to −16 | .024 |
| Myocardial infarction | 32 | 29 | 100 | 153 | −53 | −107 to −4 | .036 | 240 | 324 | −85 | −173 to 1 | .052 |
| Stroke | 49 | 38 | 121 | 113 | 8 | −34 to 55 | .772 | 250 | 257 | −7 | −83 to 63 | .724 |
| Kidney | 6 | 20 | 55 | 86 | −31 | −76 to 32 | .260 | 124 | 203 | −78 | −170 to 50 | .156 |
| Other | 355 | 327 | 1726 | 1887 | −161 | −333 to 61 | .148 | 4147 | 4442 | −295 | −660 to 81 | .120 |
| Drugs costs | 285 | 274 | 1832 | 1597 | 235 | 202 to 272 | <.001 | 4326 | 3766 | 559 | 471 to 648 | <.001 |
| Glucose‐lowering drugs | 186 | 176 | 1422 | 1161 | 262 | 233 to 289 | <.001 | 3323 | 2725 | 597 | 527 to 673 | <.001 |
| Dapagliflozin | 0 | 0 | 648 | 19 | 629 | 621 to 639 | <.001 | 1325 | 70 | 1255 | 1231 to 1281 | <.001 |
| DPP‐4i | 29 | 29 | 103 | 194 | −91 | −98 to −84 | <.001 | 244 | 411 | −67 | −183 to −149 | <.001 |
| GLP‐1RA | 48 | 45 | 201 | 422 | −221 | −241 to −203 | <.001 | 567 | 954 | −387 | −435 to −339 | <.001 |
| Insulin | 87 | 85 | 372 | 434 | −62 | −79 to −44 | <.001 | 952 | 1082 | −130 | −176 to −82 | <.001 |
| Other GLD | 22 | 17 | 99 | 91 | 7 | 3 to 12 | <.001 | 234 | 208 | 26 | 16 to 36 | <.001 |
| Other drugs | 99 | 98 | 410 | 437 | −27 | −40 to −12 | <.001 | 1003 | 1041 | −38 | −71 to −3 | .032 |
Abbreviations: CV, cardiovascular; dapa, dapagliflozin; DPP‐4i, dipeptidyl‐peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; oGLD, other glucose‐lowering drugs; SGLT‐2i, sodium glucose‐cotransporter‐2‐inhibitor.
3.2. Drug costs
At baseline, GLD costs per‐patient were well‐balanced between the dapagliflozin and oGLD groups (Table 1). Drug cost was higher for the dapagliflozin group during the entire 30‐month follow‐up period (Table 2 and Figure 2). The difference in drug cost was largely driven by GLDs ($597; 95% CI, $527, $673; P < 0.001), with little contribution from other drugs, including those used for CV prevention. The majority of the difference could be explained by dapagliflozin cost, but balanced >50% by less use of other costly glucose lowering drugs compared to the oGLD group; lower average per‐patient costs for dipeptidyl peptidase‐4 inhibitors (DPP‐4i) (−$167; 95% CI, −$183, −$149]; P < 0.001), glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) (−$387; 95% CI, −$435, −$339; P < 0.001) and insulin (−$130; 95% CI, −$176, −$82; P < 0.001).
Figure 2.
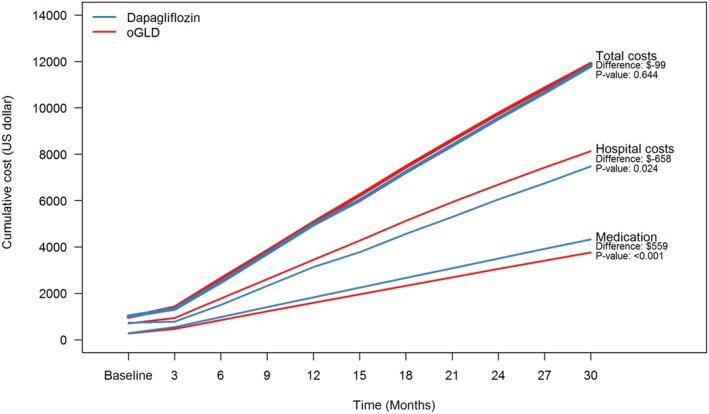
Cumulative health care costs in new users of dapagliflozin vs other glucose lowering drugs (oGLD)
3.3. Total healthcare costs
Total mean cumulative healthcare cost per patient, based on costs of hospital healthcare and drugs, was similar in the dapagliflozin group and the oGLD group during the full observation period and at 30 months (−$99; 95% CI, −$629, $483; P = 0.644) (Figure 2 and Table 2). In the sensitivity analysis, which included only patients with at least 24 months of follow‐up, the number of new‐user episodes was reduced from 24 828 to 8880 (2220 dapagliflozin; 6660 oGLD). In this analysis, nearly identical results for total mean cumulative healthcare costs were observed (Figure S2).
4. DISCUSSION
In this nationwide, observational, real‐world study, a novel approach was used to evaluate the estimated total healthcare costs for patients with T2D who initiated treatment with either dapagliflozin or a non‐SGLT‐2i oGLD in a population with a patient profile to similar to that of the DECLARE‐TIMI 58 trial.7 This DECLARE‐like population, defined by applying the main eligibility criteria from the DECLARE‐TIMI 58 trial to a real‐world population, has been described previously.11
In the DECLARE‐like population, initiation of dapagliflozin was associated with significantly lower hospital healthcare costs compared with initiation of oGLDs. This lower cost was driven mainly by lower costs for CV and diabetes care, and these are related to the clinical benefits with dapagliflozin reported in previous studies, including beneficial effects on CV outcomes in clinical trials and observational studies.7, 10, 11, 12, 14, 15, 16 These lower hospital healthcare costs for patients initiating dapagliflozin are of importance because approximately 30% of hospital healthcare costs are related to CV‐related diseases.2 Although the lower hospital healthcare costs were balanced by the cost of dapagliflozin treatment, there was less need for other costly GLDs (eg, GLP‐1RAs, DPP‐4is or insulin) in the dapagliflozin group compared with the oGLD group, resulting in a more than 50% compensation for the higher cost of dapagliflozin.
A smaller study using US data (n = 5444) that compared healthcare costs related to initiation of either dapagliflozin or sitagliptin reported similar findings, despite using a different methodology and using data from a country with a different healthcare infrastructure (eg, more than three‐fold healthcare costs and insurance‐based healthcare than in the present study).38 They also concluded that the lower hospital healthcare cost associated with dapagliflozin treatment was offset by the higher drug costs.38 The present study evaluated a larger, well‐matched population (based on 96 clinical and cost variables at baseline, using propensity score matching) with a longer duration of follow‐up, thus providing important additional detailsed insights beyond those from the US study.38
The present study is based on the cumulative healthcare costs reported to authorities by healthcare providers for reimbursement purposes, that is, DRG costs for hospital visits and the historic costs of drugs dispensed by the pharmacy.39 This analysis was based on historic data and on the cost of drugs and procedures, and no updated costs to reflect modern pricing have been used. Consequently, total healthcare cost savings could be different if updated with a lower drug cost. In addition, the costs to hospitals for specific procedures might vary over time, but this is unlikely to vary as much as drug costs. Moreover, the variation in costs for specific procedures would impact both groups and have little impact on between‐group differences in hospital healthcare costs. However, as all costs in this study are historic, the difference in hospital and drug costs could be considered conservative estimates.
Unlike cost‐effectiveness analyses, the analysis reported here does not include a measure of the outcomes, for example, Quality Adjusted Life Years (QALYs). However, as previously shown in a DECLARE‐like population,11 treatment with dapagliflozin has beneficial CV effects compared with treatment with oGLDs, and this would lead to a QALY benefit. When considered along with this QALY benefit, the cost neutrality between dapagliflozin and oGLDs observed in this study indicates that dapagliflozin is cost‐effective compared with oGLDs.
4.1. Strengths of the study
This was a population‐based, nationwide, real‐world observational study that provides a high external validity and a large enough population to enable propensity‐score matched analyses; few patients initiating dapagliflozin were lost (n = 379; 6%) during the matching process (Figure 1). The national register used, from an established and complete public healthcare system, included records of full coverage for hospitalizations, for filled drug prescriptions and for cause of death. In addition, cardiovascular diagnoses have been validated in the Swedish hospital care registry, showing high validity.40
4.2. Limitations of the study
There are a number of important limitations to the current analysis. Because of the observational nature of the present study, causal relationships cannot be fully investigated as the presence of confounding factors such as selection bias or lack of available variables, impacting the CV risk at baseline, cannot be fully excluded. The close matching on a large number of essential variables ensures that some confounding factors were controlled for, but even propensity score matching does not eliminate all potential confounding, for example, residual confounding by indication. Furthermore, the present work provides no information concerning laboratory measurements, lifestyle parameters, primary healthcare data, socioeconomic data or duration of diabetes (where a proxy for time since diagnosis was used, matching for age at index date, time since first registered GLD treatment and classes of GLD at baseline).
The results of the current analysis are representative only of patients in a DECLARE‐like population in Sweden and, therefore, cannot be extended to all patients with T2D in other countries. In addition, we had no information concerning emigration, which would mean that we will underestimate the cost for those who emigrated during follow‐up. It has been suggested that primary analyses should be performed in single databases and discussed in the context of cross‐national comparisons.41 We would, therefore, encourage multinational analyses similar to the DECLARE‐like study presented here to be performed using healthcare registry data from across the world.17, 32
As this was a cost study, the effects of mortality have not been fully accounted for. As a result of this, the lower mortality rate associated with initiation of dapagliflozin rather than an oGLD11 will have increased the healthcare costs for dapagliflozin as more patients remained alive, while the individual clinical benefit for each patient remaining alive is not accounted for.
Finally, information on primary healthcare costs, indirect costs, including sick leave, and other costs associated with CV disease and T2D were not captured. It is estimated that primary healthcare costs and indirect costs for T2D patients account for approximately 25%2 and 35%42 of total healthcare‐related cost, respectively. Assuming that hospital and primary healthcare resources are positively correlated, the present study may have underestimated the cost differences.
In summary, several important limitations might have contributed to an underestimation of the favourable healthcare cost associated with dapagliflozin compared with an oGLD.
In conclusion, in this nationwide, observational study, initiation of dapagliflozin was associated with significantly lower hospital healthcare costs compared with initiation of oGLDs, mainly driven by lower costs for CV‐ and T2D‐related care. These lower hospital healthcare costs were balanced by higher drug costs for dapagliflozin. However, a lower use of other costly GLDs was observed in patients initiating dapagliflozin. These results indicate that dapagliflozin, having well documented clinical benefits, can be prescribed to patients with T2D without increasing the total cost of healthcare. The observational nature of the study did not allow full exploration of causal relationships, because of the risk of confounding, and further studies are encouraged.
CONFLICT OF INTEREST
T. N. has received unrestricted grants from AstraZeneca and NovoNordisk, and serves on the national board of NovoNordisk, Sanofi‐Aventis, Eli Lilly, Boehringer Ingelheim, Amgen and MSD. J. W. E. has received honoraria or research grants from AstraZeneca, NovoNordisk, Bayer, Sanofi and MSD. D. N. has received consultancy fees from Novo Nordisk, Astra Zeneca and Eli Lilly. M. T. is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic‐Baltic is a client. A. N. has received honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. J. B. holds a full‐time position at AstraZeneca as an epidemiologist.
AUTHOR CONTRIBUTIONS
All authors participated in the research design. M. T. performed data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility in the decision to submit for publication. All authors are guarantors of the manuscript.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
We are grateful to Susanna Jerström and Helena Goike at AstraZeneca for logistic support and valuable comments on the manuscript. Urban Olsson, Statisticon AB, is acknowledged for database management. Copy editing was performed by Alexander Jones, inScience Communication, and was funded by AstraZeneca.
Norhammar A, Bodegard J, Nyström T, et al. Dapagliflozin vs non‐SGLT‐2i treatment is associated with lower healthcare costs in type 2 diabetes patients similar to participants in the DECLARE‐TIMI 58 trial: A nationwide observational study. Diabetes Obes Metab. 2019;21:2651–2659. 10.1111/dom.13852
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13852.
Funding information This work was sponsored by AstraZeneca.
REFERENCES
- 1. IDF Diabetes Atlas Eighth Edition 2017. International Diabetes Federation. Available at http://www.idf.org/diabetesatlas. Published 2017. Accessed April 1, 2019.
- 2. Nathanson D, Sabale U, Eriksson JW, et al. Healthcare cost development in a type 2 diabetes patient population on glucose‐lowering drug treatment: a Nationwide observational study 2006–2014. Pharmacoecon Open. 2018;2:393‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norhammar A, Bodegard J, Nystrom T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose‐lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006‐2013. Diabetologia. 2016;59:1692‐1701. [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 5. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 InvestigatorsDapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‐357. [DOI] [PubMed] [Google Scholar]
- 8. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31‐39. [DOI] [PubMed] [Google Scholar]
- 9. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 10. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709‐717. [DOI] [PubMed] [Google Scholar]
- 11. Norhammar A, Bodegard J, Nystrom T, Thuresson M, Nathanson D, Eriksson JW. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE‐TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21:1136‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20:344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eriksson JW, Norhammar A, Bodegard J, et al. Dapagliflozin is associated with lower risk of hospitalization for kidney disease, heart failure and all‐cause death compared to DPP‐4i: CVD‐REAL Nordic. 53rd EASD Annual Meeting, 11–15 September 2017, Lisbon, Portugal; 2017. https://www.easd.org/virtualmeeting/home.html#!resources/dapagliflozin‐compared‐to‐dpp4i‐treatment‐is‐associated‐with‐lower‐risk‐of‐kidney‐disease‐heart‐failure‐and‐all‐cause‐death‐cvd‐real‐nordic. Accessed April 1, 2019.
- 14. Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub‐analysis from DECLARE TIMI‐58 trial. Circulation. 2019;139:2516‐2527. [DOI] [PubMed] [Google Scholar]
- 15. Kato ET, Silverman MG, Mosenzon O, et al. Effect of Dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528‐2536. [DOI] [PubMed] [Google Scholar]
- 16. Verma S, McMurray JJV. The serendipitous story of SGLT2 inhibitors in heart failure: new insights from DECLARE‐TIMI 58. Circulation. 2019;139:2537‐2541. [DOI] [PubMed] [Google Scholar]
- 17. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71:2628‐2639. [DOI] [PubMed] [Google Scholar]
- 18. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 19. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care. 2010;33:2217‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446‐456. [DOI] [PubMed] [Google Scholar]
- 21. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with Dapagliflozin add‐on to Saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 23. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care. 2011;34:2015‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2011;13:928‐938. [DOI] [PubMed] [Google Scholar]
- 26. Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care. 2009;32:1656‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilding JP, Woo V, Soler NG, et al. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405‐415. [DOI] [PubMed] [Google Scholar]
- 28. Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the Dapagliflozin effect on cardiovascular events (DECLARE)‐TIMI 58 trial. Am Heart J. 2018;200:83‐89. [DOI] [PubMed] [Google Scholar]
- 29. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2018;1:e00036 10.1111/dom.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Persson F, Bodegard J, Lahtela JT, et al. Different patterns of second‐line treatment in type 2 diabetes after metformin monotherapy in Denmark, Finland, Norway and Sweden (D360 Nordic): a multinational observational study. Endocrinol Diabetes Metab. 2018;1:e00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindh A, Persson F, Sobocki P, Bodegard J, Lindarck N. Nordic longitudinal data from electronic medical records and full population national registers: unique opportunities for new insights in benefit of diabetes patients. Value Health. 2015;18:A726. [Google Scholar]
- 32. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose Cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose Cotransporter‐2 inhibitors). Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eriksson JW, Bodegard J, Nathanson D, Thuresson M, Nystrom T, Norhammar A. Sulphonylurea compared to DPP‐4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all‐cause mortality. Diabetes Res Clin Pract. 2016;117:39‐47. [DOI] [PubMed] [Google Scholar]
- 34. Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Second line initiation of insulin compared with DPP‐4 inhibitors after metformin monotherapy is associated with increased risk of all‐cause mortality, cardiovascular events, and severe hypoglycemia. Diabetes Res Clin Pract. 2017;123:199‐208. [DOI] [PubMed] [Google Scholar]
- 35. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387‐398. [DOI] [PubMed] [Google Scholar]
- 36. Sekhon J. Multivariate and propensity score matching software with automated balance optimization. J Stat Softw. 2011;42:1‐52. [Google Scholar]
- 37. R: A Language and Environment for Statistical Computing [Computer Program]. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/. Accessed April 1, 2019. [Google Scholar]
- 38. Parker ED, Wittbrodt ET, McPheeters JT, Frias JP. Comparison of healthcare resource utilization and costs in patients with type 2 diabetes initiating dapagliflozin versus sitagliptin. Diabetes Obes Metab. 2019;21:227‐233. [DOI] [PubMed] [Google Scholar]
- 39. Socialstyrelsen . Weight Lists for NordDRG. The National Board of Health and Welfare. Available at https://www.socialstyrelsen.se/klassificeringochkoder/norddrg/vikter. Published 2019. Accessed April 23, 2019.
- 40. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raschi E, Poluzzi E, Fadini GP, Marchesini G, De Ponti F. Observational research on sodium glucose co‐transporter‐2 inhibitors: a real breakthrough? Diabetes Obes Metab. 2018;20:2711‐2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bommer C, Heesemann E, Sagalova V, et al. The global economic burden of diabetes in adults aged 20‐79 years: a cost‐of‐illness study. Lancet Diabetes Endocrinol. 2017;5:423‐430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


