Abstract
Objective
Patients diagnosed with advanced larynx cancer face a decisional process in which they can choose between radiotherapy, chemoradiotherapy, or a total laryngectomy with adjuvant radiotherapy. Clinicians do not always agree on the best clinical treatment, making the decisional process for patients a complex problem.
Methods
Guided by the International Patient Decision Aid (PDA) Standards, we followed three developmental phases for which we held semi‐structured in‐depth interviews with patients and physicians, thinking‐out‐loud sessions, and a study‐specific questionnaire. Audio‐recorded interviews were verbatim transcribed, thematically coded, and analyzed. Phase 1 consisted of an evaluation of the decisional needs and the regular counseling process; phase 2 tested the comprehensibility and usability of the PDA; and phase 3 beta tested the feasibility of the PDA.
Results
Patients and doctors agreed on the need for development of a PDA. Major revisions were conducted after phase 1 to improve the readability and replace the majority of text with video animations. Patients and physicians considered the PDA to be a major improvement to the current counseling process.
Conclusion
This study describes the development of a comprehensible and easy‐to‐use online patient decision aid for advanced larynx cancer, which was found satisfactory by patients and physicians (available on http://www.treatmentchoice.info). The outcome of the interviews underscores the need for better patient counseling. The feasibility and satisfaction among newly diagnosed patients as well as doctors will need to be proven. To this end, we started a multicenter trial evaluating the PDA in clinical practice (http://ClinicalTrials.gov Identifier: NCT03292341).
Level of Evidence
NA Laryngoscope, 129:2733–2739, 2019
Keywords: Patient decision aid, counseling, health communication, larynx cancer, chemoradiotherapy, radiotherapy, laryngectomy
INTRODUCTION
A major shift from current population‐/guidelines‐based medicine to personalized and participative medicine is underway. This transition is being supported by the development of clinical decision support systems based on prediction models of treatment outcome.1, 2 In parallel, shared decision making (SDM) is gradually taking over the traditional paternalistic patient–doctor relationship. SDM represents the process in which patients and healthcare professionals make healthcare choices in which the best available evidence regarding risks and benefits of the possible options are both taken into account, as well as the patients’ personal values and situation.3, 4 There is level 1 evidence that SDM improves patient satisfaction and patient–doctor communication and leads to better patient outcomes.5, 6, 7, 8, 9 However, SDM is challenging. Doctors have limited consultation time, and physicians find it difficult to assess patients’ treatment preferences.10, 11, 12 Making a shared decision can be difficult, especially for patients diagnosed with advanced cancer for whom there is no best choice.
An example of a condition for which there is not always a best choice is the treatment decision for advanced larynx cancer. Historically, patients were treated by a total laryngectomy (TL). This leads to loss of normal voice, social and adaptation problems, and associated distress. In the last decades, concurrent chemoradiotherapy (CRT) or radiotherapy (RT) alone have been shown to be successful in sparing the larynx in the majority of patients while reaching almost similar overall survival (OS) rates.13 Recent publications, however, demonstrated that in more advanced tumors TL still seems to give the best OS rates.14, 15 These publications have led to an update in the American Society of Clinical Oncology guidelines in 2018, which now states that extensive (T)3 or large T4a lesions might achieve better survival rates following total laryngectomy.16 Despite these results, organ preservation is still widely applied,14, 15, 17 and patients sometimes are willing to trade off survival in order to preserve their larynx.18 However, (C)RT sometimes fails, which necessitates salvage surgery; in these cases, rehabilitation is even more complicated and less successful.19 It therefore seems difficult—if not impossible—for a doctor to transfer all this information and the associated uncertainty to patients while at the same time helping them capture all the information and make a well‐balanced treatment choice.
A patient decision aid (PDA) can support this decisional process by transferring medical information in an easy‐to‐understand way. PDAs aim to inform patients about the different treatment options and help them to clarify their personal preferences. A recent Cochrane review reported that patients using a PDA had more knowledge about the treatment options and expected benefits and harms, experienced less decisional conflict, and became less passive decision makers.7
To empower patients and improve shared decision making, we developed a comprehensive, interactive Web‐based PDA for patients with primary T3 to T4 larynx cancer receiving curative treatment. In this article, we describe the development process and evaluation of the PDA among patients and doctors in two dedicated head and neck cancer centers.
MATERIALS AND METHODS
The development of the PDA was based on the quality criteria as set out by the International Patient Decision Aid Standards (IDPAS) collaboration.20, 21 We followed three phases in the development process (see Fig. 1). In phase 1, we reviewed relevant literature on advanced larynx cancer and compared this to currently used counseling papers. Furthermore, we held semi‐structured in‐depth interviews with patients and doctors to evaluate patients’ decisional needs and the regular counseling process. We stopped inclusion of participants after reaching data saturation, meaning that additional participants did not contribute anything new to our knowledge as obtained by previous interviews. Based on these results, a hospital‐based Web designer constructed the first version of the PDA.
Figure 1.
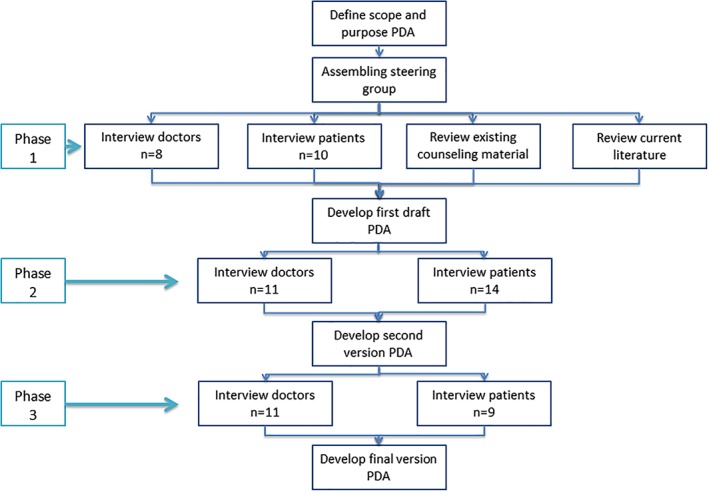
Developmental process. Flowchart of the developmental process of the PDA (analogy of IPDAS checklist).21 IPDAS = International Patient Decision Aid Standards; PDA = Patient Decision Aid.
In phase 2, we alpha tested the comprehensibility and usability of the first version using a mixed method approach. Similar to phase 1, we interviewed patients and doctors. Next, we demonstrated the PDA using a thinking‐out‐loud session during which the research assistant guided the participant through the PDA while asking for feedback. Participants then filled in a study‐specific questionnaire containing 38 statements regarding satisfaction with the PDA and effectiveness, comprehensibility, usability, and value of the information (see online Supporting Appendix 2). Each statement was phrased in a positive way ranging from 1 (totally disagree) to 5 (totally agree); therefore, agreement conferred a positive evaluation of the PDA. Furthermore, participants were asked to rank the tool on overall satisfaction ranging from 0 (very unsatisfied) to 10 (very satisfied).
In phase 3, we beta tested the feasibility of the second and last version of the tool by using the same mixed method approach as described for phase 2.
All patients participating in this study were recruited by their treating physician or by the Dutch Patient Society for Head and Neck Cancer; had been treated with TL, CRT or RT for larynx cancer; and gave written informed consent. Interviews were audio recorded, verbatim transcribed without personal data, and thematically coded using MAXQDA software for qualitative data analysis (VERBI Software GmbH, Berlin, Germany). Thematically coding the interviews enabled us to identify patterns with respect to decisional needs, the counseling process, and the PDA. These developmental steps allowed us to identify critical flaws in the PDA and supplement missing information after discussions within the developmental team.
Ethics
This study does not fall under the scope of the Medical Research Involving Human Subjects Act, which was confirmed by the institutional review board. The institutional review board of both hospitals approved this study.
RESULTS
Phase 1: Needs Assessment and Barriers to the Counseling Process
Doctors
Characteristics of participants can be found in Table 1. All doctors agreed that the need for a PDA is increasing. In terms of development of the PDA, doctors indicated that it should be as complete and objective as possible, clear, and contain easy‐to‐understand numbers or figures regarding survival and possible side effects for different treatments. It should not push the patient in a particular direction by asking them questions such as “Is OS most important to you?” or “Do you want to preserve your larynx at any costs?” Regarding the layout, the optimal PDA should be visually supported by images and be easy to navigate through: “Yes, I believe there is a need for something like that, if everything is nicely illustrated for patients and can be explained in a simple way” Head and Neck Surgeon (HNS1)
Table 1.
Participant Characteristics.
| Phase | No. Participants | Mean Age | Male/Female | Treatment/Type Physician |
|---|---|---|---|---|
| Phase 1 | 9 patients | 74 | 2 female/7 male | 2 CRT, 1 TL, 6 RT |
| Phase 1 | 8 physicians | – | 1 female/7 male | 4 HNS, 4 RTO |
| Phase 2 | 14 patients | 70 | 2 female/12 male | 2 CRT, 2 CRT and salvage TL, 8 RT, 2 RT and salvage TL |
| Phase 2 | 11 physicians | – | 2 female/9 male | 4 HNS, 4 RTO, 3 MO |
| Phase 3 | 9 patients | 66 | 1 female/8 male | 3 TL, 1 RT, 4 RT and salvage TL, 1 CRT and salvage TL |
| Phase 3 | 11 physicians | – | 2 female/9 male | 4 HNS, 4 RTO, 3 MO |
CRT = chemoradiotherapy; HNS = head and neck surgeon; MO = medical oncologist; RT = radiotherapy; RTO = radiation oncologists; TL = total laryngectomy.
Perceived barriers for good patient counseling for advanced larynx cancer were the relatively low average educational level of the typical patient. Most doctors doubted that patients would remember the information provided during the counseling process. Another experienced barrier was difficulty gaining insight into personal values and coping strategies of the patient: “In a conversation it is often difficult to understand what is most important for the patient. That is where I see the biggest challenge” (HNS3)
Patients
Most patients were positive about the intended development of a PDA and would have wanted to use it if it would have been available to them. One patient, however, did not want to know any details regarding treatment, although the patient agreed the details could be useful for other patients. Most patients had searched for more information on the Internet during their counseling process. The majority of patients indicated repetition of information as useful to reconfirm the received information and said they often did not remember information received during counseling. Reasons for not remembering consisted of the amount of information given at once and the impact of the diagnosis, which made them forget about the rest: “You are occupied with the disease. Not with the information; that you do not remember. When you are told it is that serious, it is almost like you are numb. The whole thinking process does not work anymore” Total laryngectomy patient no. 4 (PtTL04).
Development PDA
After combining the information found in the literature, existing patient counseling flyers, and the interviews, the first version of the online PDA was constructed (see Fig. 2).
Figure 2.
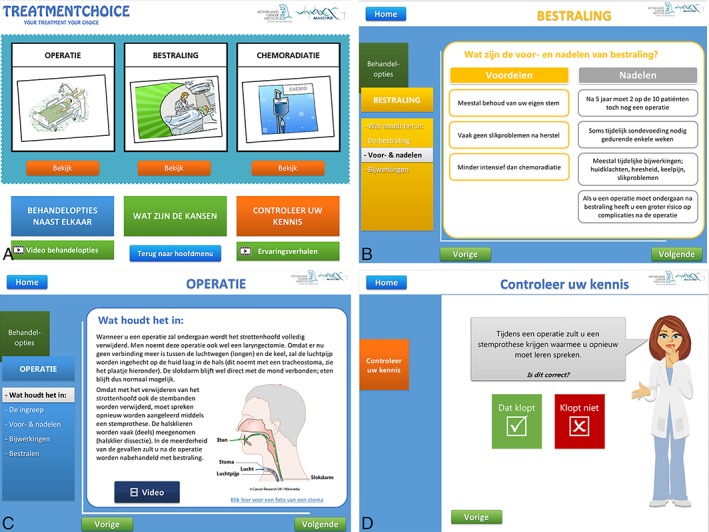
Layout of the first version of the PDA. (A) Home page of the PDA. For each treatment option, we included videos of doctors explaining the treatment and videos of patients who are interviewed on their decisional process, the treatment, and their quality of life. (B) The PDA contains a short summary with the risks and benefits of each option laid out next to each other and estimated overall survival rates per treatment and tumor characteristics (based on the tumor‐node‐metastasis classification). (C) All the treatment options are explained using text, pictures, and videos (D). At the end, patients can fill in a knowledge and preference test. They are encouraged to take the results of these tests to their physician to identify potential gaps in their knowledge and discuss personal preferences. PDA = Patient Decision Aid.
Phase 2: Alpha Testing of Comprehensibility and Usability
Doctors
Due to time restraints, most doctors only thoroughly evaluated the medical information of their own specialty and recommended on the usability of the PDA in general. They estimated that it would take patients a median of 60 minutes to complete the PDA. In general, the feedback was positive, with a median mark of 7 of 10. However, several adjustments—mostly small—were suggested by all participants. The participants were generally satisfied with the medical information given, although several participants made some corrections to the text. Furthermore, almost half of them were afraid there was too much text. Also, two participants felt that the treatment from their specialty was described too negatively, although the other nine other participants did not consider this to be the case. With regard to navigation, improvements were suggested to add a homepage with an index of all the chapters and to alter the use of colors.
Patients
Fourteen patients evaluated the first draft and filled in a study‐specific questionnaire. All statements were ranked with a median score of 4 (out of 5), and the PDA got a median 8 of 10 score for overall satisfaction. The patients identified several strong points of the tool. They expected it would provide future patients with a clear picture of the different treatment options and the diagnostic procedures, which would improve communication with the doctor. They considered the information as very reliable compared to information on the Internet for which they would otherwise have searched. Furthermore, patients were happy that they could consult all this information at home again, also during the process, instead of waiting for a doctor's appointment to answer a simple question: “Yes, but indeed it is sometimes easier to not … err … if you think you have to consult the doctor to ask a simple question, this is a more accessible tool” Radiotherapy patient no. 01 (PtRT01).
Regarding improvements to be made to the PDA, the most important issue was that some patients were concerned that low‐educated patients might have difficulty interpreting the abundance of text in the PDA. They suggested summarizing the text or looking for other ways to present the information.
Improvements to the PDA
Based on the findings from the alpha testing, the PDA underwent major revisions (see Fig. 3). We replaced almost all text slides with animation videos, drastically changed the layout, and made some usability adjustments.
Figure 3.
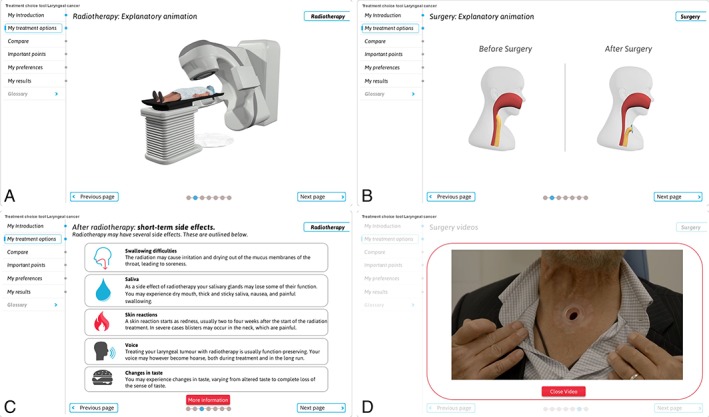
Layout of the final version of the PDA. With the results of the interviews, major changes were made. The majority of text was replaced by animation videos (A, B) explaining the details of all the different treatment options, and textual corrections suggested by the physicians were adjusted. We added a voic‐over so patients would not have to read the text, and the structure of the PDA is now explained at the homepage with an introduction animation video. (C) Large texts were summarized, but the more comprehensive text was still available on request via an extra information button. (D) Furthermore, bright colors were replaced with blue and white tones. An extra patient video was added. PDA = Patient Decision Aid.
Phase 3: Beta Testing of the Feasibility
Doctors
All doctors were satisfied with the new PDA and thought of it as an effective tool for new patients. All items in the questionnaire were scored with a median score of either 4 of 5 or 5 of 5. The median time that doctors indicated would be necessary to use the tool was 60 minutes, and their median mark for overall satisfaction was 8 of 10. In the interviews, they indicated that the PDA gave a good and detailed overview of the different treatment options; the interface was clear; and the simple structure used in the PDA made navigating through the different treatment options an intuitive process.
Contrary to how the majority of doctors evaluated the PDA, two of them commented that it took them too long to go through all the options. Interestingly, one doctor also said it should be made clearer that sometimes patients do not have a choice in treatment. Another suggestion was to quantify the frequency and incidence of certain side effects. Overall, the doctors agreed it was a good tool that would aid the regular counseling process and thereby improve the quality of patient care.
Patients
The new version of the PDA was tested again among patients from both clinics. All patients were very satisfied. “Fantastic, yes I really mean it, I really think it is fantastic, I believe it's fantastic counseling. And I tell you, they have failed the counseling in my case” Total laryngectomy patient no. 2 (PtTL02). The median score of all items in the study‐specific questionnaire was 4 of 5; the usability and comprehensibility questions scored a median of 5 of 5.
Patients indicated that they could complete the whole tool in 60 minutes and gave the PDA a median score 8 of 10. The animations were considered a good improvement because they made it easier to understand and visualize, for example, the changed anatomy after TL. Other improvements mentioned were the easy navigation and the leaner layout with less bright and flashy colors.
In responding to the question of what could be improved in the tool, one patient described having missed information about expressing emotions, such as the inability to make sound laughing or crying after a TL. Also, a comparison of speech rehabilitation methods was suggested, as well as the desire for information on other related care, such as physical therapy or dentistry. TL patients expressed concern that the patient in the TL video seemed to have above average quality of life, which might give unrealistic expectations regarding rehabilitation after TL. Other than that, all patients would advise new patients to use the tool. They indicated that the information provided is easy to understand and gives enough details to make a well‐reasoned treatment choice.
Final Corrections to the Tool
Final corrections to the tool were made, with the most important change being the addition of a new video of a TL patient to manage expectations of recovery after a TL. Furthermore, minor editorial changes were made, for example, in the representation of the OS rates. The final version of the tool will be accessible on http://www.treatmentchoice.info/.
DISCUSSION
In this article, we have described the developmental process and qualitative evaluation of a Web‐based PDA for advanced larynx cancer using a mixed methods approach. We followed the process as outlined by the IPDAS guidelines and performed several semi‐structured interviews with patients and doctors.21 All participants who evaluated the last version agreed on the usefulness and quality of the tool and thought it would make a great contribution to the process of medical decision making. Patients agreed it would clarify the possible outcomes of treatment, improve communication with the doctor, and help them make a choice. These results are in line with studies evaluating PDAs developed for other medical decisions.7
The necessity for improvement of the regular counseling process seems evident. Stafford et al. performed a national survey among surgeons in the United Kingdom and revealed that 84% gave the diagnosis and discussed TL at the same consultation, which lasted approximately 15 minutes.22 Perhaps not surprisingly, a recent review on preoperative counseling for TL patients demonstrated that the majority of patients and their spouses considered the current preoperative counseling inadequate. Up to 20% of patients were unaware that loss of normal voice would occur, and up to 41% noted that they had not received any counseling at all.11 Although this might have been forgotten by the patients because patients from our study also indicated that they often did not remember information received during counseling, the implications for improvements are clear.
Evaluation of patients’ preferences is a difficult task and is quite often overlooked or forborne in the era of national guidelines and results from multidisciplinary meetings in which strong emphasis is placed on survival outcomes. Patients, however, may have other considerations and might not always prefer the treatment option with the highest expected OS.18, 23, 24 Furthermore, treatment choices can be highly dependent on the type of information provided during counseling. In 2014, Laccourreye et al. evaluated how giving more specific information regarding the risk on a feeding tube or tracheotomy after primary radiotherapy altered the treatment decision made by patients, and they demonstrated significant changes in their preferred treatment after obtaining more specific information.25
In order to make a medical decision on treatment that is in line with personal values and preferences of the patient, there are certain conditions that need to be met. First, a sufficient number of decisional needs must be fulfilled. These are, for example, adequate knowledge, realistic expectations, and clear information regarding the risks and benefits of each treatment. If patients lack one or more of these basic decisional needs, this leads to decisional conflict. When less decisional needs are met, patients are more likely to postpone decisions, feel regret, and/or blame others for their potential poor outcome.26, 27, 28 Indeed, patients from our study who had not been informed about the different treatment options at the time of their treatment felt they had been mistreated by their physician, and some even felt resentful of them.
Focusing on the head and neck cancer patient group, lack of health literacy might be a problem, a concern that was also expressed during the interviews. Health literacy is defined as the “degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions.”29 (p. 3) Low health literacy is associated with increased hospital rates and even mortality30 and is related to the educational level of patients, which is relatively low among head and neck cancer patients.31, 32 Yet, Narwani et al. evaluated online available patient information for larynx cancer and demonstrated that it was written at an advanced level, similar to that of Time magazine.30 Indeed, also after the first evaluation of our PDA, participants recommended simplifying the PDA to make it more readable and understandable. These findings underscore the value of a simple and understandable PDA for this population.
Limitations
There are certain limitations to our study. Patients who participated in our study were recruited by their treating physician and the National patient society. Although we tried to get a mix of patients, some bias is almost unavoidable because patients who are not interested in improving counseling were not participating in this study. Furthermore, because the developmental team conducted the majority of the interviews, patients and doctors might have hesitated to give too much negative feedback on the tool. However, by following the steps as set out by the IPDAS and interviewing several different patients and doctors, we have reached a saturation level in the feedback that gives us confidence in the usability of the tool.
CONCLUSION
The results of our study suggest that a Web‐based PDA for advanced larynx cancer can be a valuable addition to the regular counseling process. The feasibility and actual satisfaction among newly diagnosed patients as well as doctors or trained paramedics have yet to be proven. To this end, a multicenter trial has now started in the Netherlands comparing regular care to patients receiving the PDA (http://ClinicalTrials.gov Identifier: NCT03292341). Results are expected in 2020.
Supporting information
Appendix S1 The following terms were used in PubMed.
Appendix S2. During the developmental phase several questionnaires were used for the patient interviews. Section A. was used during the needs assessment of phase 1. Sections B‐E. Were used during phases 2 and 3 for alpha and beta testing of the patient decision aid.
Acknowledgment
The authors would like to thank all patients and doctors who participated in the interviews to optimize the PDA. A special thanks to Gili Yaron, MA, PhD, for useful comments on the structure of the manuscript and the language editing.
Preliminary results of this study presented at the 7th European Conference on Head and Neck Oncology (ECHNO), Budapest, Hungary, September 9th 2016; at the European Society for Radiotherapy and Oncology (ESTRO) conference 2017; and at the Dutch Working Group Head and Neck Cancer yearly research meetings (NWHHT) in October 13th 2016, May 10th 2017, and October 9th 2018.
The authors acknowledge financial support from the European Program H2020‐2015‐17 (BD2Decide ‐ PHC30‐689715) and Alpe d'HuZes‐KWF (DESIGN). The Netherlands Cancer Institute receives a research grant from ATOS Medical Sweden, which contributes to the existing infrastructure for health‐related quality‐of‐life research of the Department of Head and Neck Oncology and Surgery. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Lambin P, van Stiphout RG, Starmans MH, et al. Predicting outcomes in radiation oncology—multifactorial decision support systems. Nat Rev Clin Oncol 2013;10:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambin P, Zindler J, Vanneste BG, et al. Decision support systems for personalized and participative radiation oncology. Adv Drug Deliv Rev 2017;109:131–153. [DOI] [PubMed] [Google Scholar]
- 3. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681–692. [DOI] [PubMed] [Google Scholar]
- 4. Legare F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2014:CD006732. [DOI] [PubMed] [Google Scholar]
- 5. Basch E. Patient‐reported outcomes—harnessing patients' voices to improve clinical care. N Engl J Med 2017;376:105–108. [DOI] [PubMed] [Google Scholar]
- 6. Stewart MA. Effective physician‐patient communication and health outcomes: a review. CMAJ 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 7. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 8. Llewellyn CD, McGurk M, Weinman J. How satisfied are head and neck cancer (HNC) patients with the information they receive pre‐treatment? Results from the satisfaction with cancer information profile (SCIP). Oral Oncol 2006;42:726–734. [DOI] [PubMed] [Google Scholar]
- 9. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fowler FJ Jr, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood) 2011;30:699–706. [DOI] [PubMed] [Google Scholar]
- 11. Fitzgerald E, Perry A. Pre‐operative counselling for laryngectomy patients: a systematic review. J Laryngol Otol 2016;130:15–20. [DOI] [PubMed] [Google Scholar]
- 12. Hahlweg P, Harter M, Nestoriuc Y, Scholl I. How are decisions made in cancer care? A qualitative study using participant observation of current practice. BMJ Open 2017;7:e016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer . The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 1991;324:1685–1690. [DOI] [PubMed] [Google Scholar]
- 14. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 2006;116:1–13. [DOI] [PubMed] [Google Scholar]
- 15. Timmermans AJ, van Dijk BA, Overbeek LI, et al. Trends in treatment and survival for advanced laryngeal cancer: A 20‐year population‐based study in The Netherlands. Head Neck 2016;38(suppl 1):E1247–E1255. [DOI] [PubMed] [Google Scholar]
- 16. Forastiere AA, Ismaila N, Wolf GT. Use of larynx‐preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract 2018;14:123–128. [DOI] [PubMed] [Google Scholar]
- 17. Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope 2014;124:2064–2069. [DOI] [PubMed] [Google Scholar]
- 18. McNeil BJ, Weichselbaum R, Pauker SG. Speech and survival: tradeoffs between quality and quantity of life in laryngeal cancer. N Engl J Med 1981;305:982–987. [DOI] [PubMed] [Google Scholar]
- 19. Theunissen EA, Timmermans AJ, Zuur CL, et al. Total laryngectomy for a dysfunctional larynx after (chemo)radiotherapy. Arch Otolaryngol Head Neck Surg 2012;138:548–555. [DOI] [PubMed] [Google Scholar]
- 20. Elwyn G, O'Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006;333:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elwyn G, Kreuwel I, Durand MA, et al. How to develop web‐based decision support interventions for patients: a process map. Patient Educ Couns 2011;82:260–265. [DOI] [PubMed] [Google Scholar]
- 22. Stafford ND, Lewin RJ, Nash P, Hardman GF. Surgeon information giving practices prior to laryngectomy: a national survey. Ann R Coll Surg Engl 2001;83:371–375. [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton DW, Pedersen A, Blanchford H, et al. A comparison of attitudes to laryngeal cancer treatment outcomes: a time trade‐off study. Clin Otolaryngol 2018;43:117–123. [DOI] [PubMed] [Google Scholar]
- 24. Laccourreye O, Malinvaud D, Holsinger FC, Consoli S, Menard M, Bonfils P. Trade‐off between survival and laryngeal preservation in advanced laryngeal cancer: the otorhinolaryngology patient's perspective. Ann Otol Rhinol Laryngol 2012;121:570–575. [DOI] [PubMed] [Google Scholar]
- 25. Laccourreye O, Malinvaud D, Menard M, Consoli S, Giraud P, Bonfils P. Total laryngectomy or laryngeal preservation for advanced laryngeal cancer. Impact of the functional risk upon the patient's preferences. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131:93–97. [DOI] [PubMed] [Google Scholar]
- 26. Brehaut JC, O'Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making 2003;23:281–292. [DOI] [PubMed] [Google Scholar]
- 27. Ferron Parayre A, Labrecque M, Rousseau M, Turcotte S, Legare F. Validation of SURE, a four‐item clinical checklist for detecting decisional conflict in patients. Med Decis Making 2014;34:54–62. [DOI] [PubMed] [Google Scholar]
- 28. Gattellari M, Ward JE. Will men attribute fault to their GP for adverse effects arising from controversial screening tests? An Australian study using scenarios about PSA screening. J Med Screen 2004;11:165–169. [DOI] [PubMed] [Google Scholar]
- 29. Kutner M, Greenberg E, Jin Y, Paulsen C. The health literacy of America's adults: results from the 2003 National Assessment of Adult Literacy. In: Education USDo , ed. Washington, DC: National Center for Education Statistics; 2006. [Google Scholar]
- 30. Narwani V, Nalamada K, Lee M, Kothari P, Lakhani R. Readability and quality assessment of internet‐based patient education materials related to laryngeal cancer. Head Neck 2016;38:601–605. [DOI] [PubMed] [Google Scholar]
- 31. Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the larynx in England and Wales up to 2001. Br J Cancer 2008;99(suppl 1):S35–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hwang E, Johnson‐Obaseki S, McDonald JT, Connell C, Corsten M. Incidence of head and neck cancer and socioeconomic status in Canada from 1992 to 2007. Oral Oncol 2013;49:1072–1076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 The following terms were used in PubMed.
Appendix S2. During the developmental phase several questionnaires were used for the patient interviews. Section A. was used during the needs assessment of phase 1. Sections B‐E. Were used during phases 2 and 3 for alpha and beta testing of the patient decision aid.


