Abstract
Objective
To assess long‐term efficacy and safety of canakinumab and the response to vaccination in children ages ≤5 years with cryopyrin‐associated periodic syndrome (CAPS).
Methods
CAPS patients (ages ≤5 years) received 2 mg/kg canakinumab subcutaneously every 8 weeks; patients with neonatal‐onset multisystem inflammatory disease (NOMID) received a starting dose of 4 mg/kg in this open‐label trial. Efficacy was evaluated using physician global assessment of disease activity and serum levels of C‐reactive protein (CRP) and amyloid A (SAA). Adverse events (AEs) were recorded. Vaccination response was evaluated using postvaccination antibody titers at 4 and 8 weeks after immunization.
Results
Of the 17 patients enrolled, 12 (71%) had Muckle‐Wells syndrome, 4 (24%) had NOMID, and 1 (6%) had familial cold autoinflammatory syndrome. All 17 patients had a complete response to canakinumab. Disease activity improved according to the physician global assessment, and for 65% of the patients autoinflammatory disease was characterized as “absent” at the end of the study. Median CRP levels decreased over time. No such change was evident in SAA levels. During the extension study, postvaccination antibody titers increased above protective levels in 16 (94%) of 17 assessable vaccinations. Ten of the patients (59%) had AEs suspected to be related to canakinumab; 8 (47%) experienced at least 1 serious AE (SAE). None of the AEs or SAEs required interruption of canakinumab therapy.
Conclusion
Our findings indicate that canakinumab effectively maintains efficacy through 152 weeks and appears to have no effect on the ability to produce antibodies against standard childhood non‐live vaccines. The safety profile of canakinumab was consistent with previous studies, supporting long‐term use of canakinumab for CAPS in children ≤5 years of age.
Introduction
Cryopyrin‐associated periodic syndrome (CAPS) is a rare autosomal‐dominant autoinflammatory disorder that includes a group of 3 overlapping inflammatory disorders: familial cold autoinflammatory syndrome (FCAS), Muckle‐Wells syndrome (MWS), and chronic infantile neurologic, cutaneous, articular (CINCA) syndrome/neonatal‐onset multisystem inflammatory disease (NOMID) (hereafter referred to as NOMID) 1. CAPS is caused by gain‐of‐function mutations in the NLRP3 gene, which lead to increased production of interleukin‐1β (IL‐1β) 2, 3, 4. The clinical manifestations of CAPS include characteristic urticaria‐like rash, recurrent fever episodes, ocular inflammation, musculoskeletal manifestations, and central nervous system (CNS) involvement 5.The milder symptoms include repeated episodes of fever and chills with malaise, intense fatigue, headaches, conjunctivitis, diffuse urticaria‐like rash, and generalized limb pain 6. These symptoms, when considered separately, often lead to a diagnostic delay of CAPS, which compromises quality of life and exposes patients to the risk of neurologic complications (including deafness) and, in the longer term, renal failure from secondary AA amyloidosis 7, 8, 9.
Early diagnosis of autoinflammatory diseases and effective treatment to control inflammation and prevent irreversible damage, such as deafness, severe joint deformity, growth retardation, and AA amyloidosis, are critical 10. With the well‐established efficacy of IL‐1 blockade and the widespread availability of genetic testing (helpful in atypical or complex cases), patients with CAPS are increasingly being diagnosed and treated in early life 5. Although the later‐stage manifestations related to NOMID, such as bony overgrowth and bone deformities, are not reversible, most CAPS‐specific symptoms may be reversible if patients are treated early. Growth retardation, CNS inflammation, and hearing loss have been reported to improve in some patients if treatment is provided in a timely manner 10, 11, 12. Early detection and consequent treatment for CAPS patients are critical to improve the patient's quality of life.
Current data on treatment in infants and children <2 years of age are limited and largely based on case reports 13, 14. Prior to the widespread use of biologic agents, basic treatments to relieve the symptoms of CAPS consisted of nonsteroidal antiinflammatory drugs, glucocorticoids, and immunosuppressants, which were partially successful at best 15. Agents targeting IL‐1β are now the standard of care 2, 16, 17. IL‐1 blockers, such as anakinra, rilonacept, and canakinumab, are currently licensed for therapy for CAPS in different parts of the world and have been shown to induce prompt and stable disease remission 18, 19, 20.
Canakinumab, a selective, human anti–IL‐1β monoclonal antibody, has been proven to be efficacious in patients with CAPS ≥2 years of age 21, 22, 23 but long‐term follow‐up data for this age group have not previously been published. Canakinumab has also been reported to not affect the induction or persistence of antibody responses after immunization with unadjuvanted influenza or alum‐adjuvanted meningococcal vaccines in healthy subjects ages 18–45 years 24. Recently and unexpectedly, pneumococcal vaccines were reported to trigger severe local and systemic inflammation in some patients with CAPS 25.
This report summarizes the long‐term efficacy and safety of canakinumab in pediatric patients with CAPS ≤5 years of age, including 6 patients ≤2 years of age, with follow‐up of 3 years, including the core study (http://www.ClinicalTrials.gov identifier: NCT01302860) and extension study (http://www.ClinicalTrials.gov identifier: NCT01576367), with an emphasis on response to vaccination. In particular, infection risk and the effectiveness of and risk of reactions to immunizations with inactivated vaccines were assessed.
Patients and Methods
Study design
The core study was a 56‐week, multicenter, open‐label, phase III trial, conducted in 7 countries (Germany, Belgium, Spain, France, Switzerland, the UK, and Canada). Patients who completed the core study entered the long‐term extension study, with a minimum treatment duration of 6 months and a maximum treatment duration of 24 months, until 152 weeks (see Supplementary Figure [Link], [Link], available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41004/abstract). Patients received 2 mg/kg canakinumab subcutaneously every 8 weeks for the entire study period (including the core and extension studies). Patients in whom a complete response was not achieved after the first dose of canakinumab, and those who experienced a relapse before the next scheduled dose, were eligible for a stepwise dose up‐titration (4 to 6 to 8 mg/kg). For patients previously treated with an anti–IL‐1 agent and for NOMID patients, a starting dose of 4 mg/kg was administered to mitigate against the risk of rebound flares anecdotally observed following withdrawal of IL‐1 blockade in CAPS patients, and since more severe CAPS phenotypes have previously been observed to require higher doses of IL‐1 blockade, respectively 22, 26. The study protocol was reviewed by the independent ethics committee or institutional review board of each center, and the study was conducted according to the ethics principles of the Declaration of Helsinki. Written informed consent was obtained from each patient's legal guardian before randomization.
Patients
Inclusion criteria
Patients with CAPS ages 28 days to 60 months, with confirmed NLRP3 mutations and a body weight of ≥2.5 kg, were included in the core study. If patients were receiving the anti–IL‐1 agents anakinra or rilonacept before enrollment, these were discontinued prior to the baseline visit and the patients had to demonstrate active disease prior to enrollment. Patients who completed the core study with no major protocol deviations and were ≥1 year of age were rolled over to the extension study.
Exclusion criteria
Preterm neonates, patients with a history of recurrent infections and/or evidence of active infections or latent tuberculosis infection, patients with neutropenia, and those who had been treated with immunosuppressive drugs or received live vaccinations up to 3 months before screening were excluded.
Assessment of treatment response
The main objective of the core study was to assess the efficacy of canakinumab with respect to treatment response (Table 1). The extension study assessed the long‐term efficacy of canakinumab with respect to relapse in patients who completed the core study. Complete response was defined as a physician global assessment of autoinflammatory disease activity as less than or equal to “minimal” (on a 5‐point scale of absent, minimal, mild, moderate, and severe) and assessment of skin disease as less than or equal to “minimal” (on a 5‐point scale of absent, minimal, mild, moderate, and severe) and serologic response indicated by serum C‐reactive protein (CRP) <15 mg/liter or serum amyloid A protein (SAA) <10 mg/liter 21. Relapse was defined for complete responders based on clinical features (physician global assessment greater than “minimal” or physician global assessment greater than or equal to “minimal” and assessment of skin disease greater than “minimal”) and serologic features (serum CRP >30 mg/liter or SAA >30 mg/liter).
Table 1.
Assessment of response to canakinumab in patients with cryopyrin‐associated periodic syndromea
| Definition of complete response |
| PGA of autoinflammatory disease activity less than or equal to “minimal” AND |
| Skin assessment score less than or equal to “minimal” AND |
| CRP <15 mg/liter OR SAA <10 mg/liter |
| Definition of relapseb |
| PGA of autoinflammatory disease activity greater than “minimal” OR PGA of autoinflammatory disease activity greater than or equal to “minimal” and skin assessment score greater than “minimal” AND |
| Serum CRP AND/OR SAA >30 mg/liter |
Physician global assessment (PGA) of autoinflammatory disease activity and skin assessment scores were summarized by severity code (absent, minimal, mild, moderate, severe). CRP = C‐reactive protein; SAA = serum amyloid A.
In patients in whom a complete response had been achieved.
Physician global assessment of autoinflammatory disease activity
For the physician global assessment, the following 8 features were each graded on a 5‐point scale (ranging from absent to severe): skin disease (urticaria‐like skin rash), arthralgia, myalgia, headache/migraine, conjunctivitis, fatigue/malaise, other symptoms related to autoinflammatory syndrome, and symptoms not related to autoinflammatory syndrome.
Evaluation of markers of inflammation
Serum levels of the inflammation markers CRP and SAA were determined in a central laboratory for all patients, regardless of age. The normal ranges for CRP and SAA levels were 0–6 mg/liter and 0–6.7 mg/liter, respectively.
Safety assessment
Safety of canakinumab was assessed in terms of adverse events (AEs) and serious AEs (SAEs) according to Medical Dictionary for Regulatory Activities (MedDRA; version 17.1) reporting criteria for clinical trials.
Immunogenicity assessment
Anticanakinumab antibody concentrations were assessed in serum, and their potential correlation with any AEs or SAEs and/or loss of efficacy was analyzed.
Assessment of antibody titers against vaccine antigen
The ability to attain or maintain protective antibody levels was assessed for inactivated vaccines, which the patients received as part of national vaccination programs, and thus potentially included the following antigens for the core study: Corynebacterium diphtheriae, Bordetella pertussis, Neisseria meningitidis, Clostridium tetani, influenza A (H1N1 and H3N2), influenza B, Haemophilus influenzae B, Streptococcus pneumoniae, and hepatitis B. The extension study included assessment of antibody titers against diphtheria, pertussis, Meningococcus, tetanus, Haemophilus influenzae B (polysaccharide or conjugate), influenza, Streptococcus pneumoniae, and hepatitis A and B. No live vaccinations were allowed throughout the course of the study or up to 3 months after the last canakinumab dose.
Assessment of vaccination response
Patients were assessed for vaccination response if antibody titer was measured 0–14 days after vaccination (referred to as the “predose assessment”), and on at least 1 subsequent occasion (at 4 weeks and/or 8 weeks after vaccination). Patients were not assessed for a vaccination response if the antibody titer was already sufficient before dosing and maintained during the study.
Assessment of neurologic, ophthalmologic, and auditory features
At screening and week 24, magnetic resonance imaging (MRI) of the brain and inner ear was performed, and neurologic, ophthalmologic, and auditory brainstem responses were assessed. A final assessment was performed for all evaluations at the end‐of‐study visits in the core and extension studies.
Evaluation of pharmacokinetics/pharmacodynamics
Canakinumab concentration data were collected and analyzed. A pharmacokinetics‐binding model was applied to estimate pharmacokinetics parameters such as clearance, apparent volume of distribution, and first‐order absorption rate constant for canakinumab in CAPS patients.
Statistical analysis
No inferential analyses were performed, and categorical variables are presented as absolute frequencies and percentages. Continuous variables are presented as the mean ± SD; median, minimum, maximum; and number of nonmissing data points. Tests were nonparametric. “End of study” refers to the end of the extension study.
AEs were coded using the MedDRA version 17.1. The number and percentage of patients with AEs were summarized, regardless of relationship to the study drug. Patients who experienced multiple events for a given term were counted once. Data are presented as rounded values.
Results
Patient disposition and baseline clinical characteristics
Seventeen patients (with a median age of 31 months [range 1−59 months]) with CAPS were enrolled in the core study. Six of the patients were younger than 2 years of age. All enrolled patients completed the core study and entered the extension study. Most patients (14 of 17 [82%]) completed the extension study; of the 3 patients who discontinued during the extension study, 2 discontinued owing to unsatisfactory therapeutic effect (and were thus managed outside the clinical protocol as per local standard of care) and 1 had no reason for discontinuation recorded (and data were also subsequently unavailable). Screening for NLRP3 mutations was performed by routine genetic sequencing as part of regular clinical care for all patients. The mean time from diagnosis to study entry was 2.6 years. All 17 patients had at least 1 confirmed pathologic NLRP3 mutation (Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41004/abstract). An additional TNFRS1 variant (p.R121Q, also referred to as the R92Q variant) was observed in 1 MWS patient. Of the 17 patients enrolled, 12 (71%) had MWS, 4 (24%) had NOMID (based on neurologic involvement in 4, on papilledema in 3, and overall severity of the phenotype in all 4 as judged by the local investigator), and 1 (6%) had FCAS (Table 2). Median baseline CRP and SAA levels were abnormal (7.0 mg/liter for CRP and 9.1 mg/liter for SAA).
Table 2.
Baseline characteristics of the 17 patients with cryopyrin‐associated periodic syndrome treated with canakinumaba
| Age, median (range) months | 31 (1–59) |
| Sex, no. (%) male | 12 (71) |
| Race, no. (%) | |
| Caucasian | 16 (94) |
| Asian | 1 (6) |
| Weight, no. (%) | |
| <15 kg | 14 (82) |
| ≥15 kg | 3 (18) |
| Molecular diagnosis of NLRP3 mutation, no. (%) | 17 (100) |
| Phenotype, no. (%) | |
| FCAS | 1 (6) |
| MWS | 12 (71) |
| NOMID | 4 (24) |
| Time from diagnosis to study entry, mean ± SD years | 2.6 ± 1.5 |
| , median (range)b | |
| CRP, mg/literc | 7 (0–165) |
| SAA, mg/literd | 9 (0–861) |
FCAS = familial cold autoinflammatory syndrome; MWS = Muckle‐Wells syndrome; NOMID = neonatal‐onset multisystem inflammatory disease; CRP = C‐reactive protein; SAA = serum amyloid A.
Data were available for 16 patients.
Normal range 0–6 mg/liter.
Normal range 0–6.7 mg/liter.
Dosage
Patients with the FCAS or MWS phenotype received a starting dose of 2 mg/kg of canakinumab every 8 weeks, with the exception of 3 MWS patients who received a starting dose of 4 mg/kg due to prior exposure to anti–IL‐1 agents. Patients with NOMID received a starting dose of 4 mg/kg due to clinical concern about severe phenotype, with the exception of 1 patient who received a starting dose of 6 mg/kg as an a priori agreed protocol deviation, again based on clinical concern regarding particularly severe phenotype (Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41004/abstract). A total of 9 (53%) of 17 patients did not require any dose up‐titration during the core study. Three of 4 NOMID patients and 5 of 12 MWS patients required dose up‐titration. During the extension study, 7 (41%) of the 17 patients did not require any dose up‐titration, whereas 10 patients (6 with MWS, 3 with NOMID, and 1 with FCAS) did require dose up‐titration (Figure 1).
Figure 1.
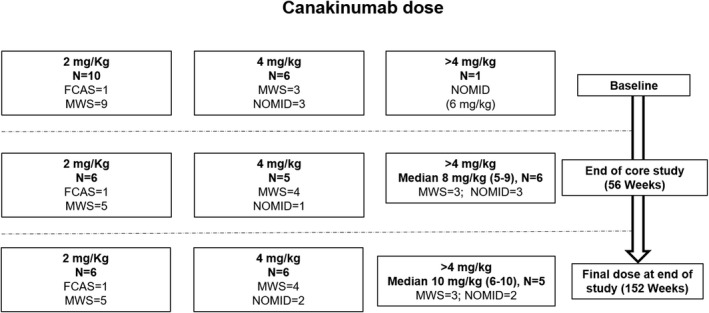
Canakinumab dosing at baseline, the end of the core study, and the end of the extension study (final dose) in patients with familial cold autoinflammatory syndrome (FCAS), Muckle‐Wells syndrome (MWS), and neonatal‐onset multisystem inflammatory disease (NOMID). Doses are rounded up to the nearest whole number. Values in the >4 mg/kg category at the end of the core study and for the final dose are the median (range). See Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41004/abstract for exact doses in individual patients.
Pharmacokinetics/pharmacodynamics
Data relating to pharmacokinetics and pharmacodynamics evaluation were analyzed and will be reported separately. Comparison of dose‐normalized drug concentrations among patients who received a consistent canakinumab dose demonstrated that exposure to canakinumab in patients ages 2 years or younger (n = 5) was similar to that observed in patients older than 2 years (n = 4).
Efficacy
Treatment responses
Overall, a complete response was achieved in 16 (94%) of the 17 patients by the end of the core study (week 56). In the extension study, a complete response was achieved in 100% of the patients within 72 weeks from the start of the study (Figure 2A), and no new relapses occurred after week 96 (Figure 2B).
Figure 2.
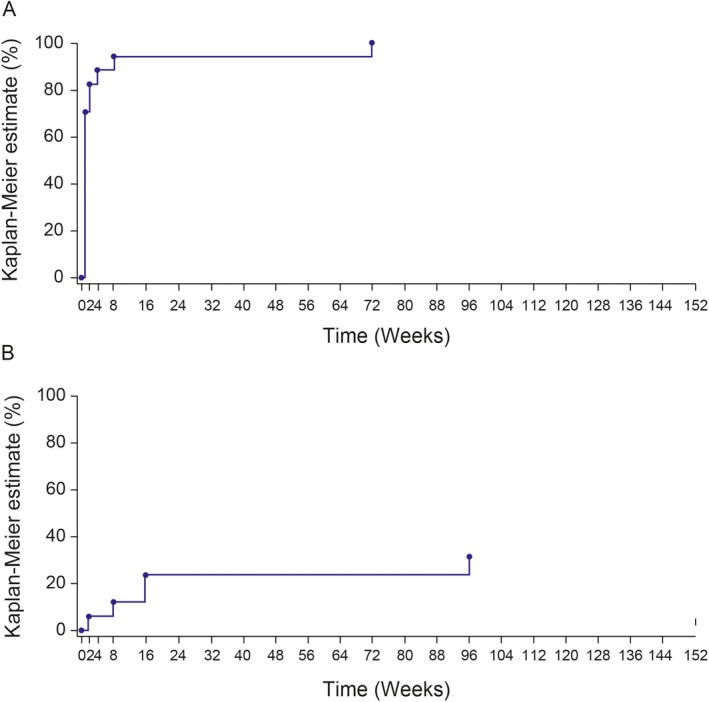
Percentage of patients with a complete response to treatment in the entire study period (152 weeks). A, Kaplan‐Meier estimate of the time to first response. Complete response was defined as physician global assessment of autoinflammatory disease activity as less than or equal to “minimal” (on a 5‐point scale of absent, minimal, mild, moderate, and severe) and assessment of skin disease as less than or equal to “minimal” (on a 5‐point scale of absent, minimal, mild, moderate, and severe) and serologic response indicated by serum C‐reactive protein (CRP) <15 mg/liter or serum amyloid A protein (SAA) <10 mg/liter. B, Kaplan‐Meier estimate of the time to first relapse. Relapse was defined for complete responders as a physician global assessment of autoinflammatory disease greater than “minimal,” or physician global assessment greater than or equal to “minimal” and assessment of skin disease greater than “minimal,”and serum CRP >30 mg/liter or SAA >30 mg/liter.
Physician global assessment of disease activity
The physician global assessment of autoinflammatory disease activity in the overall population decreased from baseline to the end of the study (Figure 3). The proportion of patients without autoinflammatory disease (absent) increased from 24% (4 of 17 patients) at baseline in the core study to 65% (11 of 17 patients) at the end of the study. The proportion of patients with mild and moderate autoinflammatory disease decreased from 47% (8 of 17 patients) at baseline to 6% (1 of 17 patients) at the end of the study and from 24% (4 of 17 patients) to 0% at the end of the study, respectively. There was 1 report of severe autoinflammatory disease flare at week 72, but none from week 80 to the end of the study.
Figure 3.
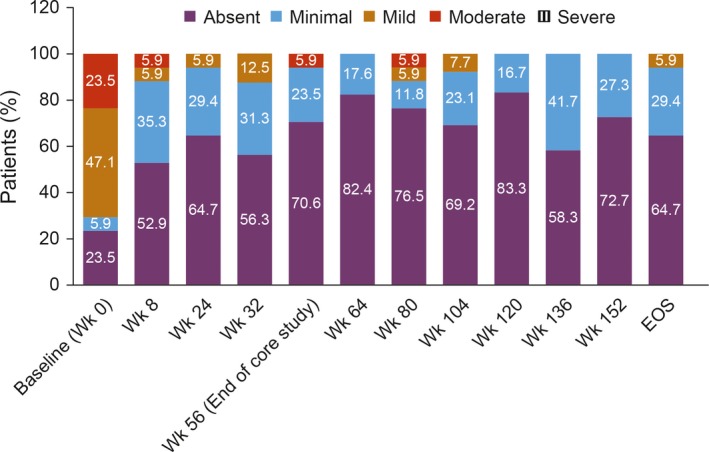
Physician global assessment of autoinflammatory disease in patients with cryopyrin‐associated periodic syndrome. Values are the percentage of patients in each category at the indicated week. EOS = end of study.
Assessment of skin disease
The proportion of patients without skin disease (absent) increased from 29% (5 of 17 patients) at baseline in the core study to 94% (16 of 17 patients) at the end of the study. The proportion of patients with moderate skin disease decreased from 29% (5 of 17 patients) at baseline to 0% at the end of the study. Severe skin disease was reported for 6% (1 of 17 patients) at baseline and was not reported for any patient at any visit thereafter.
Change in levels of serum markers of inflammation (CRP and SAA)
The median CRP level (normal range 0–6 mg/liter) decreased from 7.0 mg/liter at baseline (precanakinumab) (range 0.0–165.0) to 1.0 mg/liter at the end of the core study (range 0.0–22.0) and 3.0 mg/liter at the end of the study (range 0.0–47.0). The median SAA level (normal range 0–6.7 mg/liter) decreased from 9.1 mg/liter at baseline (range 0.0–861.0) to 2.2 mg/liter at the end of the core study (range 0.0–331.0). However, the median SAA level increased to 9.0 mg/liter at the end of the study (range 0.0–173.0).
Immunogenicity
Antibodies against canakinumab were not detected in any of the patients in the core or extension studies.
Findings of vaccination assessments
In the core study, 7 (41%) of the 17 patients (ages 5–59 months) received 10 types of inactivated vaccines. These 7 patients received a total of 31 vaccinations, 18 of which were assessable for a vaccination response. For the remaining 13 vaccinations, no predose antibody titer was available and, therefore, response was not assessed. All assessable patient vaccination cases showed a positive response (antibody titers increased above protective level) (Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41004/abstract). For all 31 patient vaccinations, including those without a predose antibody titer, a protective antibody titer level was observed during the core study, which was maintained throughout the trial. In the extension study, 4 (24%) of the 17 patients (ages 0–5 years) received 8 types of inactivated vaccines. These 4 patients received a total of 20 vaccinations, and 17 of these unique patient vaccinations were assessable for response; 16 (94%) showed a positive response, with antibody titers increasing above the protective level (Supplementary Table 2, available at http://onlinelibrary.wiley.com/doi/10.1002/art.41004/abstract). Furthermore, in 19 patient vaccination cases, including those without a predose antibody titer, a protective antibody titer level was observed during the study, which was maintained throughout the extension study, as seen in the core study. Predose antibody titers and the last measured antibody titer for each patient and corresponding vaccinations during the extension study are shown in Supplementary Table 2.
In one patient who received a Tetravac formulation (diphtheria, tetanus, acellular pertussis, and inactivated polio combination), a positive response to Bordetella pertussis and Corynebacterium diphtheriae was achieved, but not to Clostridium tetani; the values provided from the laboratory represented optical density rather than antibody concentrations and hence were considered nonevaluable. This patient had no AEs associated with this vaccination, nor lymphopenia or neutropenia at the time of last vaccination and antibody titer assessments. There was no disease flare induced by vaccination.
Neurologic, ophthalmologic, and auditory findings
Three patients had clinically significant audiometry abnormalities at baseline and were also classified as having abnormalities during the extension study. The hearing loss reported for 1 NOMID patient continued to worsen during the extension study.
On neurologic assessment, 4 patients were found to have the following abnormalities: delay in motor function and coordination and language delay in 1 NOMID patient, gap of acquisitions in 1 MWS patient, intention tremors in 1 MWS patient, and lack of responsiveness in 1 MWS patient. The ophthalmologic assessment showed abnormal findings in 2 NOMID patients who had clinically significant abnormalities during the extension study. One patient had uveitis in the right eye, and the second patient had a swollen and thick‐ended retinal fiber layer in the left eye, which was recorded as a clinically insignificant abnormality on days 946 and 1,125.
All but 1 patient underwent brain MRI. The findings were interpreted as normal for all of the patients, except for 1 patient with NOMID who had dilatation of ventricles, bilateral T2 hypersignal in parietal white matter, and delay of myelination on day 579 of the extension study.(At a later assessment, on day 629, this finding was recorded as a clinically insignificant abnormality.)
Safety
Overall, the mean exposure was 951 days per patient with a total of 44 patient‐years for the core and extension study periods, which comprised a mean exposure of 429 days per patient and 20 patient‐years for the core study and 546 days per patient and 25 patient‐years for the extension study. In the core study, all 17 patients exposed to canakinumab experienced at least 1 AE. The most common AEs were nasopharyngitis (in 7 [41%] of the17 patients) and upper respiratory tract infection (in 7 [41%]) (Table 3). Overall, in the core study 9 (53%) of the 17 patients had AEs that were suspected to be related to the study drug, with 2 cases each of bronchitis and molluscum contagiosum; all other drug‐related AEs were reported as single cases. In the extension study, 16 patients experienced at least 1 AE, and 10 (59%) of the 17 patients had AEs that were suspected to be related to the study drug, with the most common (observed in at least 3 of the patients) being diarrhea, pneumonia, rhinitis, pyrexia, and cough.
Table 3.
AEs and SAEs experienced by patients with CAPS treated with canakinumab in the core and extension studiesa
| Core study | Extension study | |
|---|---|---|
| Exposure, mean ± SD daysb | 428.6 ± 46 | 546.1 ± 210 |
| Total AEs, no. (%) | 17 (100) | 16 (94) |
| Total SAEs, no. (%) | 4 (23) | 8 (47) |
| Common AEs, no. (%)c | ||
| Nasopharyngitis | 7 (41) | 7 (41) |
| Upper respiratory tract infection | 7 (41) | |
| Diarrhea | 7 (41) | |
| Pyrexia | 6 (35) | 6 (35) |
| Rhinitis | 6 (35) | |
| Vomiting | 6 (35) | |
| Headache | 6 (35) | |
| SAEs | ||
| CAPS | 1 (5.9) | 0 (0) |
| Cryptorchidism | 1 (5.9) | 0 (0) |
| Diarrhea | 1 (5.9) | 0 (0) |
| Vomiting | 1 (5.9) | 1 (5.9) |
| Influenza | 1 (5.9) | 0 (0) |
| Lung infection | 1 (5.9) | 0 (0) |
| Wound infection (staphylococcal) | 1 (5.9) | 0 (0) |
| Femur fracture | 1 (5.9) | 0 (0) |
| Conductive deafness | 0 (0) | 1 (5.9) |
| Abdominal pain | 0 (0) | 1 (5.9) |
| Papillitis | 0 (0) | 1 (5.9) |
| Pneumonia | 0 (0) | 2 (11.8) |
| Bronchitis | 0 (0) | 1 (5.9) |
| Meningitis aseptic | 0 (0) | 1 (5.9) |
| Limb injury | 0 (0) | 1 (5.9) |
| Hematoma | 0 (0) | 1 (5.9) |
Except where indicated otherwise, values are the number (%) of patients. AEs = adverse events; SAEs = serious AEs; CAPS = cryopyrin‐associated periodic syndrome.
Since study enrollment.
Experienced by at least 6 patients (>35%).
In the core study, 4 (24%) of the 17 patients experienced at least 1 SAE; routine hospitalization for operative correction of congenital cryptorchidism (complicated by staphylococcal wound infection), diarrhea, vomiting, femur fracture, flare of CAPS disease activity, influenza, and lung infection were reported. None of the SAEs were reported for more than 1 patient. In the extension study, 8 (47%) of the patients experienced at least 1 SAE. Pneumonia was reported in 2 patients (12%), while all other SAEs were reported in 1 patient each. The proportion of patients with SAEs during the extension study is depicted in Table 3. There were no deaths or discontinuations due to AEs or SAEs during the entire study.
Discussion
CAPS is a rare autoinflammatory disease, caused by mutations in the NLRP3 gene, and is often challenging to diagnose due to unfamiliarity among many physicians, the rarity of the disease, overlapping symptoms with other diseases 27, and the presence of low‐penetrance genetic variants and poly‐morphisms 28. This study assessed the long‐term efficacy and safety of canakinumab in pediatric patients with CAPS ≤5 years of age across 7 countries and demonstrated clinical efficacy over a 3‐year period.
Overall, at least one complete response was achieved in the majority of the patients (9 of 10 patients <2 years of age and 7 of 7 patients >2 years of age) over the 56 weeks of the core study, and complete response was achieved in all patients by the end of the study. During the core study, no discontinuations were observed. In the extension study no patient discontinued due to AEs, but 2 patients discontinued due to an unsatisfactory therapeutic effect. These results are consistent with the findings of various studies demonstrating sustained efficacy of subcutaneous canakinumab administered every 8 weeks 21, 29. Another phase III study 22 demonstrated clinically and serologically inactive disease in 30% of patients after 2 months and 60% of patients after 6 months of canakinumab treatment. In our study, 75% of the NOMID patients (3 of 4) and 42% of the MWS patients (5 of 12) required dose up‐titration, emphasizing the need for higher doses in severely affected patients. In addition, the improvement in disease activity, as measured by physician global assessment of disease activity, that was observed during the core study was sustained until the end of the study. This improvement was also reflected in the assessment of skin disease (urticaria‐like skin rash).
Although CRP levels decreased over time until the end of the study, such a pattern of change was not evident in SAA levels. This was due to the fact that although all patients had clinically active disease, several patients had low prestudy SAA levels. Additionally, underpowering and missing data probably contributed to this seemingly discrepant SAA response to treatment. In periodic fever syndromes such as CAPS, pretreatment SAA levels do not always capture systemic inflammation, even when patients have clinically active disease, as previously observed in other studies of children with CAPS 22.
No new safety signals were observed, and the safety profile was consistent with that reported in previous canakinumab studies 21. No deaths were reported during the study. There were few clinically significant findings on audiograms or neurologic or ophthalmologic assessments. In this study, all but 1 of the patients underwent MRI of the brain and inner ear, and all MRI findings were reported as normal. This is consistent with the results of previous studies of the effect of canakinumab treatment on neurologic symptoms in CAPS patients 21, 30. Although it has been suggested that anakinra may have superior efficacy for the amelioration of inflammatory cerebrospinal fluid (CSF) biomarkers in patients with NOMID based on observations in a small number of patients who underwent serial CSF examination while sequentially receiving anakinra or canakinumab 31, there are no hard data to suggest that this translates into worse neurologic outcomes for NOMID patients treated with canakinumab compared to those treated with anakinra. This is clearly an area that will require ongoing surveillance in the longer term. As compared with the β‐Confident Registry 32 (which assessed 288 patients across 13 countries and different age groups, all treated with canakinumab), the frequency of AEs and SAEs was significantly lower in the present study of very young pediatric patients; however, it is likely explained by small patient numbers in our study (and hence wider confidence limits regarding this observation) compared with the β‐Confident Registry data.
In our study, canakinumab did not show any negative impact on postvaccination antibody production following the administration of non‐live vaccines in 9 pediatric patients. Recent studies have suggested that pneumococcal vaccines can trigger severe local and systemic inflammation 25 in CAPS patients, who are genetically prone to overactivation of inflammasomes 32. Hence, the potential benefits of pneumococcal immunization need to be assessed against safety signals. Of the 3 patients who received pneumococcal vaccine in this study, none developed an inflammatory flare. This study excluded inoculation with live attenuated vaccines because the current consensus is that these vaccines are contraindicated in patients treated with biologic agents. Interestingly, a 4‐month‐old patient with CAPS (NOMID) treated with canakinumab was recently reported to have been immunized with live attenuated vaccines (measles, rubella, varicella, and mumps) and achieved sufficient antibody titer without any complications 33.
Given the rarity of CAPS and the vulnerable nature of this young pediatric patient population, this was a nonrandomized and nonblinded study of a pragmatically small sample size. Although an extension study was included, the follow‐up period was still very limited for what is likely to be lifelong treatment. We acknowledge that the long‐term impact of canakinumab treatment on hearing loss and other potential late sequelae remains uncertain. Ultimately, much longer phase IV registry type studies may provide more data on the impact of canakinumab over decades of treatment.
Overall, our results demonstrate that canakinumab is an effective treatment for patients with CAPS ≤5 years of age. Canakinumab appears to have no effect on the ability to produce antibodies against standard childhood non‐live vaccines, and no vaccine‐associated disease flares were observed. The safety profile of canakinumab was thus acceptable and similar in this very young pediatric cohort to that observed in previous studies of older patients.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Brogan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Wei.
Acquisition of data
Wei.
Analysis and interpretation of data
Brogan, Hofer, Kuemmerle‐Deschner, Koné‐Paut, Roesler, Kallinich, Horneff, Calvo Penadés, Sevilla‐Perez, Goffin, Lauwerys, Lachmann, Uziel, Wei, Laxer.
Role of the Study Sponsor
Novartis Pharma AG facilitated the study design, provided writing assistance for the manuscript, and reviewed and approved the manuscript prior to submission. The authors independently collected the data, interpreted the results, and had the final decision to submit the manuscript for publication. Writing assistance was provided by Deepak Pakalapati and Anupama Tamta (Novartis Healthcare Pvt. Ltd, India). Publication of this article was not contingent upon approval by Novartis Pharma AG.
Additional Disclosures
Author Wei is an employee of China Novartis Institutes for Biomedical Research Company, Ltd.
Supporting information
Supplementary Figure 1
Supplementary Table 1
Supplementary Table 2
Supplementary Figure 1 legend
Acknowledgments
We thank Isabelle Marie, research assistant (Bicêtre Hospital, Le Kremlin‐Bicêtre, France), Beta López Montesinos, medical assistant, and M. Isabel Gonzalez Fernandez, research assistant (Hospital Universitario y Politécnico La Fe, Valencia, Spain), and Lawrence Ng, research assistant (The Hospital for Sick Children, Toronto, Canada).
http://www.ClinicalTrials.gov identifiers: NCT01302860 and NCT01576367.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Supported by Novartis Pharma AG, Switzerland. Research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health was supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre.
Dr. Brogan has received consulting fees from Sobi, Roche, and UCB (less than $10,000 each) and has received research support from Novartis, Sobi, NovImmune, and Roche. Dr. Hofer has received consulting fees from Novartis (less than $10,000). Dr. Kuemmerle‐Deschner has received consulting fees from Novartis (less than $10,000) and has received research support from Novartis. Dr. Koné‐Paut has received consulting fees from Novartis, Sobi, AbbVie, NovImmune, Pfizer, LFB, and Chugai (less than $10,000 each). Dr. Kallinich has received consulting fees, speaking fees, and/or honoraria from Novartis, Roche, Sobi, and CSL Behring. Dr. Horneff has received research support from AbbVie, Chugai, MSD/Janssen, Novartis, Pfizer, and Roche. Dr. Calvo Penadés has received research support from Novartis, AbbVie, Roche, Pfizer, MSD, GlaxoSmithKline, and Bristol‐Myers Squibb. Dr. Lachmann has received consulting fees from Novartis, Sobi, and Ionis (less than $10,000 each). Dr. Uziel has received consulting fees from Novartis (less than $10,000). Dr. Laxer has received consulting fees from Novartis, Sobi, Sanofi, Eli Lilly Canada, and Alexion (less than $10,000 each), has received consulting fees from Guidepoint Global, has received research support from Novartis, and is coauthor of Textbook of Pediatric Rheumatology, for which he receives royalties. No other disclosures relevant to this article were reported.
References
- 1. Kuemmerle‐Deschner JB, Ramos E, Blank N, Roesler J, Felix SD, Jung T, et al. Canakinumab (ACZ885, a fully human IgG1 anti‐IL‐1β mAb) induces sustained remission in pediatric patients with cryopyrin‐associated periodic syndrome (CAPS). Arthritis Res Ther 2011;13:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dodé C, Le Dû N, Cuisset L, Letourneur F, Berthelot JM, Vaudour G, et al. New mutations of CIAS1 that are responsible for Muckle‐Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet 2002;70:1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gattorno M, Tassi S, Carta S, Delfino L, Ferlito F, Pelagatti MA, et al. Pattern of interleukin‐1β secretion in response to lipopolysaccharide and ATP before and after interleukin‐1 blockade in patients with CIAS1 mutations. Arthritis Rheum 2007;56:3138–48. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin‐like protein causes familial cold autoinflammatory syndrome and Muckle‐Wells syndrome. Nat Genet 2001;29:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyamae T. Cryopyrin‐associated periodic syndromes: diagnosis and management. Paediatr Drugs 2012;14:109–17. [DOI] [PubMed] [Google Scholar]
- 6. Kone‐Paut I, Quartier P, Fain O, Grateau G, Pillet P, Le Blay P, et al. Real‐world experience and impact of canakinumab in cryopyrin‐associated periodic syndrome: results from a French observational study. Arthritis Care Res (Hoboken) 2017;69:903–11. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol 2001;108:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koné‐Paut I. Cryopyrine‐associated periodic syndrome: CAPS seen from adulthood. Rev Med Interne 2015;36:277–82. [DOI] [PubMed] [Google Scholar]
- 9. Muckle TJ, Well SM. Urticaria, deafness, and amyloidosis: a new heredo‐familial syndrome. Q J Med 1962;31:235–48. [PubMed] [Google Scholar]
- 10. Kuemmerle‐Deschner JB. CAPS: pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol 2015;37:377–85. [DOI] [PubMed] [Google Scholar]
- 11. Kuemmerle‐Deschner JB, Koitschev A, Ummenhofer K, Hansmann S, Plontke SK, Koitschev C, et al. Hearing loss in Muckle‐Wells syndrome. Arthritis Rheum 2013;65:824–31. [DOI] [PubMed] [Google Scholar]
- 12. Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal‐onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three‐ and five‐year outcomes. Arthritis Rheum 2012;64:2375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanariou M, Tantou S, Varela I, Raptaki M, Petropoulou C, Nikas I, et al. Successful management of cryopyrin‐associated periodic syndrome with canakinumab in infancy. Pediatrics 2014;134:e1468–73. [DOI] [PubMed] [Google Scholar]
- 14. Paccaud Y, Berthet G, Von Scheven‐Gête A, Vaudaux B, Mivelaz Y, Hofer M, et al. Neonatal treatment of CINCA syndrome. Pediatr Rheumatol Online J 2014;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landmann EC, Walker UA. Pharmacological treatment options for cryopyrin‐associated periodic syndromes. Expert Rev Clin Pharmacol 2017;10:855–64. [DOI] [PubMed] [Google Scholar]
- 16. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin‐1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012;11:633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koné‐Paut I, Galeotti C. Current treatment recommendations and considerations for cryopyrin‐associated periodic syndrome. Expert Rev Clin Immunol 2015;11:1083–92. [DOI] [PubMed] [Google Scholar]
- 18. Cantarini L, Lucherini OM, Cimaz R, Galeazzi M. Recurrent pericarditis caused by a rare mutation in the TNFRSF1A gene and with excellent response to anakinra treatment [letter]. Clin Exp Rheumatol 2010;28:802. [PubMed] [Google Scholar]
- 19. Gattorno M, Pelagatti MA, Meini A, Obici L, Barcellona R, Federici S, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor–associated periodic syndrome. Arthritis Rheum 2008;58:1516–20. [DOI] [PubMed] [Google Scholar]
- 20. Kuemmerle‐Deschner JB, Haug I. Canakinumab in patients with cryopyrin‐associated periodic syndrome: an update for clinicians. Ther Adv Musculoskelet Dis 2013;5:315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lachmann HJ, Kone‐Paut I, Kuemmerle‐Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin‐associated periodic syndrome. N Engl J Med 2009;360:2416–25. [DOI] [PubMed] [Google Scholar]
- 22. Russo RA, Melo‐Gomes S, Lachmann HJ, Wynne K, Rajput K, Eleftheriou D, et al. Efficacy and safety of canakinumab therapy in paediatric patients with cryopyrin‐associated periodic syndrome: a single‐centre, real‐world experience. Rheumatology (Oxford) 2014;53:665–70. [DOI] [PubMed] [Google Scholar]
- 23. Yokota S, Imagawa T, Nishikomori R, Takada H, Abrams K, Lheritier K, et al. Long‐term safety and efficacy of canakinumab in cryopyrin‐associated periodic syndrome: results from an open‐label, phase III pivotal study in Japanese patients. Clin Exp Rheumatol 2017;35 Suppl 108:19–26. [PubMed] [Google Scholar]
- 24. Chioato A, Noseda E, Felix SD, Stevens M, Del Giudice G, Fitoussi S, et al. Influenza and meningococcal vaccinations are effective in healthy subjects treated with the interleukin‐1β‐blocking antibody canakinumab: results of an open‐label, parallel group, randomized, single‐center study. Clin Vaccine Immunol 2010;17:1952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaeger VK, Hoffman HM, van der Poll T, Tilson H, Seibert J, Speziale A, et al. Safety of vaccinations in patients with cryopyrin‐associated periodic syndromes: a prospective registry based study. Rheumatology (Oxford) 2017;56:1484–91. [DOI] [PubMed] [Google Scholar]
- 26. Imagawa T, Nishikomori R, Takada H, Takeshita S, Patel N, Kim D, et al. Safety and efficacy of canakinumab in Japanese patients with phenotypes of cryopyrin‐associated periodic syndrome as established in the first open‐label, phase‐3 pivotal study (24‐week results). Clin Exp Rheumatol 2013;31:302–9. [PubMed] [Google Scholar]
- 27. Yu JR, Leslie KS. Cryopyrin‐associated periodic syndrome: an update on diagnosis and treatment response. Curr Allergy Asthma Rep 2011;11:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ter Haar NM, Oswald M, Jeyaratnam J, Anton J, Barron KS, Brogan PA, et al. Recommendations for the management of autoinflammatory diseases. Ann Rheum Dis 2015;74:1636–44. [DOI] [PubMed] [Google Scholar]
- 29. Kuemmerle‐Deschner J, Blank N, Roesler J, Ramos E, Lachmann HJ, Hoyer JD, et al. Canakinumab (ACZ885), a new IL‐1β blocking monoclonal antibody, provides sustained remission in patients with cryopyrin associated periodic fever syndrome (CAPS): results of an open label phase II study [abstract]. Ann Rheum Dis 2009;68 Suppl 3:366. [Google Scholar]
- 30. Mamoudjy N, Maurey H, Marie I, Koné‐Paut I, Deiva K. Neurological outcome of patients with cryopyrin‐associated periodic syndrome (CAPS). Orphanet J Rare Dis 2017;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez‐Smith J, Lin YC, Tsai WL, Kim H, Montealegre‐Sanchez G, Chapelle D, et al. Cerebrospinal fluid cytokines correlate with aseptic meningitis and blood–brain barrier function in neonatal‐onset multisystem inflammatory disease: central nervous system biomarkers in neonatal‐onset multisystem inflammatory disease correlate with central nervous system inflammation. Arthritis Rheumatol 2017;69:1325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffman HM, Kuemmerle‐Deschner JB, Hawkins PN, van der Poll T, Walker UA, Speziale A, et al. Safety and efficacy of long‐term canakinumab therapy in patients with CAPS: final results from β‐confident registry [abstract]. Arthritis Rheumatol 2016;68 Suppl 10 URL: https://acrabstracts.org/abstract/safety-and-efficacy-of-long-term-canakinumab-therapy-in-patients-with-caps-final-results-from-beta-confident-registry/. [Google Scholar]
- 33. Watanabe M, Nishikomori R, Fujimaki Y, Heike T, Ohara A, Saji T. Live‐attenuated vaccines in a cryopyrin‐associated periodic syndrome patient receiving canakinumab treatment during infancy. Clin Case Rep 2017;5:1750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Table 1
Supplementary Table 2
Supplementary Figure 1 legend


