ABSTRACT
The fungal kingdom comprises a hyperdiverse clade of heterotrophic eukaryotes characterized by the presence of a chitinous cell wall, the loss of phagotrophic capabilities and cell organizations that range from completely unicellular monopolar organisms to highly complex syncitial filaments that may form macroscopic structures. Fungi emerged as a ‘Third Kingdom’, embracing organisms that were outside the classical dichotomy of animals versus vegetals. The taxonomy of this group has a turbulent history that is only now starting to be settled with the advent of genomics and phylogenomics. We here review the current status of the phylogeny and taxonomy of fungi, providing an overview of the main defined groups. Based on current knowledge, nine phylum‐level clades can be defined: Opisthosporidia, Chytridiomycota, Neocallimastigomycota, Blastocladiomycota, Zoopagomycota, Mucoromycota, Glomeromycota, Basidiomycota and Ascomycota. For each group, we discuss their main traits and their diversity, focusing on the evolutionary relationships among the main fungal clades. We also explore the diversity and phylogeny of several groups of uncertain affinities and the main phylogenetic and taxonomical controversies and hypotheses in the field.
Keywords: Fungi, taxonomy, phylogeny, phylogenomics, diversity
I. Introduction
If we were to review the most recent advances in animal or plant phylogeny and taxonomy, a paragraph detailing the relevance of any of those groups would be superfluous. Animals and plants are studied not only in specialized biology degrees, but even in primary education. Fungi, though, only merit a brief mention in high school text books, and very rarely occupy a central position in university‐level biology degrees, generally falling between the fields of botany and microbiology (Editorial, 2017; Freimoser, 2017). Yet Fungi are literally everywhere, shaping the world as we know it. They can be found in the stratosphere (Wainwright, Wickramasinghe, & Rajaratnam, 2003) and the bottom of the Dead Sea (Oren & Gunde‐Cimerman, 2012), from antarctic glaciers (Freeman et al., 2009) to torrid deserts (Gonçalves et al., 2016), from the gut of flies (Blackwell, 2017) to deep oceanic sediments (Nagahama et al., 2011), and anywhere in between. Fungi are powerful players in global bio‐geochemistry, recycling carbon and mobilizing nitrogen, phosphorus and other bio‐elements. They provide essential support to plant life in the form of endophytes and mycorrhizae, while fungal pathogens can decimate plant and animal populations, threatening food supplies and even pushing some species to the brink of extinction. The metabolic singularities of many fungi have provided humanity with fermented foods and beverages to feed us and delight our senses, medicines to cure our bodies, and many compounds with important industrial usages. Fungi themselves are an important and valued source of food, and in the near future fungal biomass might even help to clothe and shelter us (Wojciechowska, 2017; Jones et al., 2018).
Fungal taxonomy has undergone major changes since the recognition of this group in Linnean taxonomy, where it was considered part of the ‘Regnum Vegetabile’ (Linnaeus, 1767). Early classifications included several groups of heterotrophic eukaryotes characterized by their osmotrophic nutrition with diverse phylogenetic affinity, as well as a core of clades collectively deemed the ‘true fungi’, or Eumycota (Whittaker, 1969). True fungi generally share the following traits: (i) the presence of a β‐glucan and (generally) chitin cell wall, at least in their spores; (ii) they are usually unicellular, or grow as a mycelium – a multinucleated, walled, cylindrical cell of variable size; (iii) the presence of the amino adipidic pathway for the biosynthesis of lysine; and (iv) the presence of flattened mitochondrial crystae (Adl et al., 2012, 2018). Nevertheless, numerous exceptions exist for virtually all these traits, both in the form of secondary losses within fungi, as well as by their presence in other eukaryotic groups (Richards, Leonard, & Wideman, 2017). Early on, four major phyla were defined within the true fungi, based on their morphological and reproductive traits: Chytridiomycota, Zygomycota, Ascomycota and Basidiomycota (Whittaker, 1969). Later, molecular phylogenies proved the paraphyly of Zygomycota and Chytridiomycota (Tanabe, Watanabe, & Sugiyama, 2005; James et al., 2006a , b , White et al., 2006), as well as the affinity of Microsporidia and the chytrid‐like Rozella to the fungal kingdom (Keeling, Luker, & Palmer, 2000; Fischer & Palmer, 2005; James et al., 2006b). More recently, the advent of environmental‐sequencing‐based technologies have brought about the recognition of a novel highly diverse and cosmopolitan clade of fungal‐like organisms that include Rozella and some related genera, for which the terms Rozellidea, Rozellomycota and Cryptomycota have been used (Lara, Moreira, & López‐García, 2010; Jones et al., 2011; Adl et al., 2012; James & Berbee, 2012; Corsaro et al., 2014b). Finally, the Aphelidea, a poorly studied clade of amoeboid parasitoids of unicelular algae was found to be sister group to Microsporidia and Rozella, completing the fungal family portrait (Karpov et al., 2014a).
The most up‐to‐date taxonomy comprises the described diversity of known true fungi, dividing it into nine major lineages: Opisthosporidia, Chytridiomycota, Neocallimastigomycota, Blastocladiomycota, Zoopagomycota, Mucoromycota, Glomeromycota, Ascomycota and Basidiomycota. Together, these lineages form a monophyletic clade, the true fungi (Fig. 1), which is sister to a group of amoeboid protozoans consisting of the Nucleariida (Nuclearia, Micronuclearia, Parvularia) and Funticulida (Fonticula) (Karpov et al., 2014a; Spatafora et al., 2017a; Tedersoo et al., 2018). Below, we describe the main features of these nine fungal lineages, plus several other groups that might represent additional independent lineages or whose affinity to any of the well‐defined groups is still not fully resolved.
Figure 1.
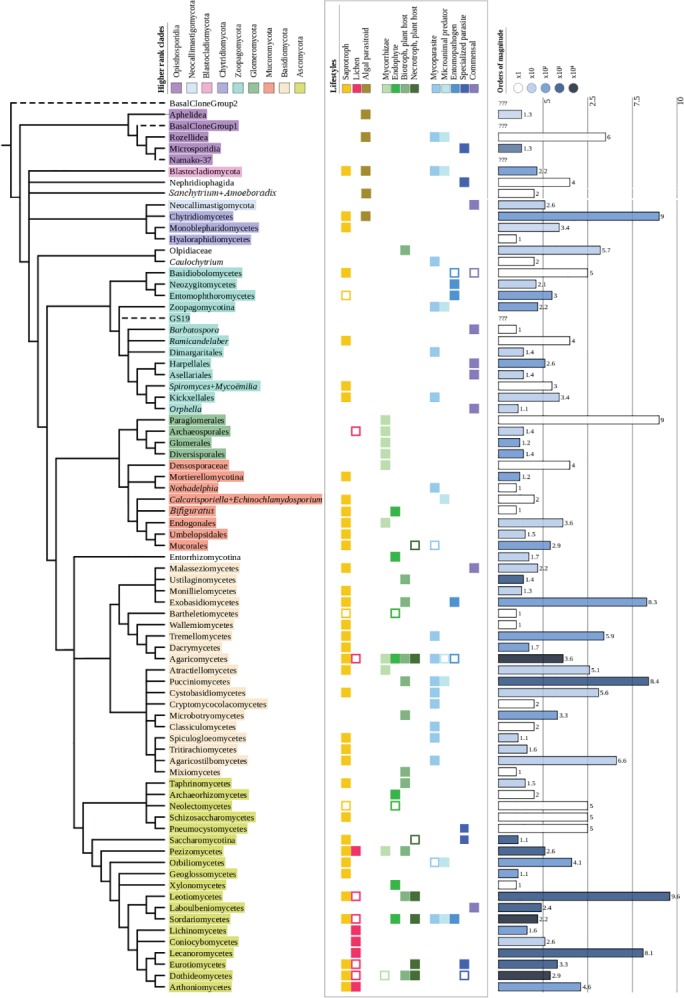
The fungal tree of life. Tree showcasing currently described groups within the Kingdom Fungi up to the class level, as well as incertae sedis lineages that cannot be assigned to any other class. In the case of zygomycetous fungi, due to historical reasons, we have included lineages up to order level. The first column uses colours to cluster clades in corresponding phyla. The second column compiles the lifestyles present in each group. Empty squares indicate that the given lifestyle is anecdotic or hypothetical. The third column shows the number of described species in each group according to the Catalogue of Life (Bisby & Roskov, 2010) or, for certain groups that are not represented in this database, Wikispecies (Wikimedia, 2011). Since the number of species might vary by several orders of magnitude, species number bars are coded using different colours. Tree generated using the interactive Tree of Life (iTOL) server (Letunic & Bork, 2016).
II. Zoosporic fungi
All Fungi must descend from an organism that was single celled and, at least at some point in its life cycle, able to swim with the use of posterior flagella. Several fungal lineages still present this lifestyle, although in some cases secondary loses have occurred. The evolutionary relationships among these lineages remain unresolved, mostly due to the deep divergence of these lineages, the current incomplete sampling, and the parasitic nature of many. Figure 2 illustrates some of these organisms, and the main lineages are listed in Table 1.
Figure 2.
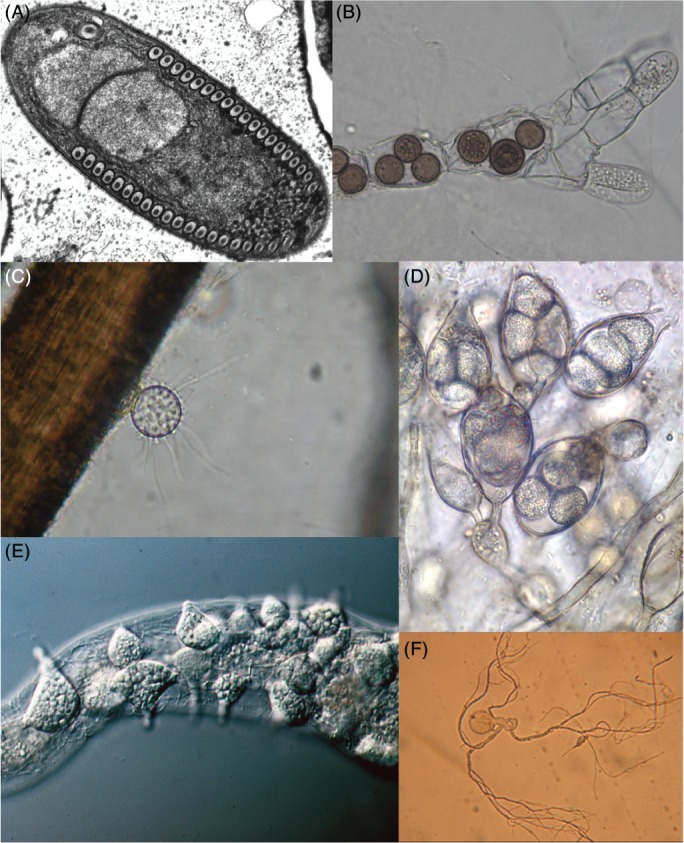
Diversity of zoosporic Fungi. (A) Transmission electron micrograph of a sporoblast of Fibrillanosema crangonycis (Microsporidia). The nucleus is clearly visible in the image and a series of concentrical structures with a highly electrodense core that appear tightly packed around the perimeter of the cell. This peculiar structure corresponds to a coiled polar tube, an infective harpoon‐like structure characteristic of Microsporidia. Original photograph taken by Leon White, CC BY‐SA 3.0 license. (B) Mature zoosporangia of Rozella allomycis (Rozellidea) during the last stages of infection of a mycelium of Allomyces sp. (Blastocladiomycota). Like many zoosporic fungi, Rozella is a parasitoid that invades and consumes the host cytoplasm, after which it produces sporangia. Original photograph by Timothy Y. James, CC BY‐SA 3.0 license. (C) Zoosporangium of Rhizophidium keratinophylum (Chytridiomycetes), appearing as a globular structure, growing on a human hair (fibrous brown structure). Beyond their parasitic roles, many chytrids have important roles in aquatic environments as saprotrophs specialized for degrading highly recalcitrant organic matter, such as pollen grains, arthropod exuviae or keratin. Original photograph by Wikipedia user TelosCricket, CC BY‐SA 4.0 license. (D) Micrograph of a group of oogonia from Gonapodya polymorpha (Monoblepharidomycetes, Chytridiomycota). The Monoblepharidomycetes are the only group of Fungi that present morphologically distinct gametes (i.e. anisogamy). They are also the only group within Chytridiomycota that have developed true hyphae, which evolved independently from those of terrestrial Fungi. Original photograph by Marilyn R. N. Mollicone. All rights reserved. (E) Mature sporangia of Catenaria anguillulae (Blastocladiomycota) growing inside a nematode alongside a true mycelium. Despite its relatively low number of species, Blastocladiomycota is a highly diverse group in terms of ecology, including saprotrophs, plant pathogens, algal parasitoids and even animal parasites. Catenaria, in particular, has been studied for its potential use as a pest‐control agent in agriculture. Original photograph by George Barron. Licensed for non‐commercial academic and research use only. (F) Microscopic preparation of a monocentric thallus from Neocallimastix frontalis (Neocallimastigomycota) isolated from deer faeces. The thallus possess a bulbous structure that corresponds with the zoosporangia and a series of root‐like protrusions, the rhizoids. The Neocallimastigomycota are a group of Fungi almost exclusively associated with the gut of herbivorous mammals. They have lost their mitochondria and present a highly expanded repertoire of cellulolytic enzymes. Original photograph from Atanasova‐Pancevska & Kungulovski (2017), CC BY‐NC 4.0 license.
Table 1.
Main lineages within Opisthosporidia and the zoosporic fungi. Due to changes in the scope of certain historical clades, references might not refer to the initial published description but rather to more recent bibliography
| Name and references | Main described lifestyles | Main traits | Representative genera |
|---|---|---|---|
|
Opisthosporidia (Karpov et al., 2014a) |
Intracellular parasites and parasitoids | Many are zoosporic or amoeboid; lacking chitin‐based cell wall, although chitin might be present in resting stages | |
|
Aphelida |
Parasitoids of photosynthetic unicellular eukaryotes | Intracellular unwalled stage as a phagotrophic amoeba; characteristic food vacuole with excretory body; ciliated or amoeboid dispersal cells; tubular or lamellar cristae | Aphelidium, Amoebaphelidium |
|
Rozellida (Lara et al., 2010; Jones et al., 2011) syn. Cryptomycota, Rozellomycota |
Parasitoids of diverse organisms | Zoosporic; intracellular unwalled stage with digitiform protrusions, may be phagotrophic | Rozella, Nucleophaga |
|
Microsporidia (Adl et al., 2012) |
Intracellular parasites of metazoans | Mitochondria reduced to mitosome; polar tube | Nosema, Spraguea, Encephalitozoon, Amphiamblys, Metchnikovella |
|
Chytridiomycota (Hibbett et al., 2007) |
Free‐living saprobes and parasitoids | Zoosporic, uniflagellated, sometimes as crawling cells; rhizoid formation; stacked Golgi apparatus | |
|
Chytridiomycetes (Hibbett et al., 2007) |
Free‐living saprobes and parasitoids | Thallus monocentric or rhizomycelial polycentric | Spizellomyces, Chytridium, Batrachochytrium, Homoloaphlyctis, Rhizophlyctis, Arkaya, Kappamyces |
|
Hyaloraphidiomycetes (Ustinova et al., 2000) |
Unknown | Lack of flagellum or rhizoid; crescent‐shaped cells; reproduction by 4–8 spores within cells that present the same structure as the mother cell; stacked Golgi dictyosomes | Hyaloraphidium |
|
Monoblepharidomycetes (Adl et al., 2012; Tedersoo et al., 2018) syn. Neocallimastigomycotina |
Free‐living saprobes and parasitoids | Thallus filamentous, sometimes forming true hyphae or unbranched; two centrosomes in parallel; flagellum unique steming from one centrosome; second centrosome is striated with radiating microtubules; oogamy | Gonapodya, Monoblepharis, Oedogoniomyces |
|
Neocallimastigomycota (Hibbett et al., 2007) syn. Neocallimastigomycotina, Neocallimastigomycetes within Chytridiomycota |
Associated with the gut of mammals and some reptiles; anaerobic cellulose decomposer | Zoosporic, sometimes with many flagella; mitochondria reduced to hydrogenosomes; extremely low %GC; highly developed carbohydrate metabolism | Neocallimastix, Piromyces, Orpinomyces |
|
Blastocladiomycota (James et al., 2006b) |
Saprobes; animal parasites, algal parasitoids, plant pathogens | Zoosporic, uniflagellated; cone‐shaped nucleus with particular kinetocore ultrastructure; some species produce true hyphae | Allomyces, Catenaria, Blastocladia, Physoderma |
(1). Opisthosporidia
This group is formed by three main lineages: Aphelidea, Rozellidea and Microsporidia (also known as the ARM clade). All known species in this clade are intracellular parasites or parasitoids of a wide range of eukaryotes. The term Cryptomycota was proposed to describe a series of cosmopolitan aquatic organisms related to Rozella and to highlight an apparent diversity comparable to that of known fungi (Jones et al., 2011). We herein use the term Rozellidea to describe Rozella and related environmental sequences and discard the use of Cryptomycota henceforth. The inclusion of Aphelidea created the Opisthosporidia (Karpov et al., 2014a). However, some phylogenetic studies suggest that Opisthosporidia are paraphyletic, with Rozellidea + Microsporidia as the earliest splitting branch, followed by Aphelidea (Tedersoo et al., 2018); or the opposite, with Aphelidea as sister to the true Fungi and the two together sister to Rozellida + Microsporidia (Torruella et al., 2018).
(a). Microsporidia
Microsporidia is a diverse group of intracellular obligate parasites of metazoans and occasionally gregarines (Metchnikovellidae). Microsporidian parasites of insects and vertebrates have been widely studied, with dozen of fully sequenced genomes available. However, microsporidians outside this range of hosts are poorly described, and some environmental studies suggest that they must present a large, undescribed diversity based on their host range and endemicity (Ardila‐Garcia et al., 2013). As a result of their lifestyle, they present very reduced genomes, to the point that they have some characteristics typical of prokaryotic genomes, such as overlapping genes (Peyretaillade et al., 2011). They also lack motile structures and, with the exception (so far) of Mitosporidium daphniae, true mitochondria (Haag et al., 2014), possessing instead mitochondria‐derived organelles called mitosomes. Mitosomes lack a genome, and their main function seems to be the assembly of iron–sulfur clusters (Stairs, Leger, & Roger, 2015). Many members of this clade have lost the ability to perform glycolysis and the tricarboxylic acid cycle, relying on scavenging ATP directly from the host cell via an array of horizontally acquired genes (Cuomo et al., 2012; Alexander et al., 2016). They present a highly specialized harpoon‐like penetration structure termed the polar tube that is a highly modified Golgi apparatus (Xu & Weiss, 2005; Beznoussenko et al., 2007). Simplified cellular morphology, lack of mitochondria and long‐branch‐attraction phylogenetic artefacts caused by their parasitic nature, led to the hypothesis that Microsporidia were early‐branching eukaryotes, whose divergence preceded the acquisition of mitochondria (Corradi & Keeling, 2009). Later on, the description of mitosomes and several mitochondria‐related genes in their genomes refuted such hypothesis, and further phylogenetic studies pointed at a close relatedness to fungi, either as a highly derived fungal clade or as a sister group to the rest of the kingdom. Phylogenomic analyses favoured the latter hypothesis, joining Rozella allomycis as sister to all other fungi (Capella‐Gutiérrez, Marcet‐Houben, & Gabaldón, 2012; James et al., 2013). This prompted the official adoption of Microsporidia by mycologists (McNeill et al., 2012). Yet the nomenclatural rules for this group still follow protist conventions instead of classical botanical rules applied to other fungi. May this legacy remind us of the taxonomic rollercoaster they have gone through.
(b). Rozellidea
Rozella is a genus of flagellated parasitoids of zoosporic fungi (Chytridiomycota and Blastocladiomycota), Oomycetes, and some green algae (Gleason et al., 2012). Rozella presents a zoosporic infectious stage that attaches to the host cell. After this, the protoplasm of Rozella invades the host until it has occupied all available space. At this point, the parasite sporulates, completing the life cycle (Foust, 1937; Letcher et al., 2017, 2018; Powell, Letcher, & James, 2017). Some species can form resting spores, sometimes presenting spines. The genome of Rozella allomycis, a parasitoid of the blastoclad Allomyces was published in 2013 (James et al., 2013). Unlike most Microsporidia, Rozella presents a non‐reduced genome and true mitochondria (James et al., 2013), although with reduced mitochondrial metabolism. Rozellidea also includes the recently described Paramicrosporidium and Nucleophaga, which are microsporidian‐like parasites of amoebozoa (Corsaro et al., 2014a, b , 2016). It is noteworthy that the trophobiont stages of Nucleophaga and Rozella are covered by digitiform protrusions (Powell, 1984; Corsaro et al., 2014a, 2016), suggesting some form of phagocytic capabilities. Environmental sequences phylogenetically related to Rozella have been found in virtually all aquatic environments, comprising a very high sequence divergence. Such distribution and divergence was interpreted as the existence of a highly species‐rich and ecologically meaningful hidden clade, which could be comparable in diversity to the rest of true fungi (Corsaro et al., 2016). However, given the known characteristics of Nucleophaga and Paramicrosporidium, such conclusions may be premature. If we extrapolate our knowledge of Microsporidia to these microsporidian‐like organisms, it is plausible to assume that high evolutionary rates may inflate estimations of real taxonomic diversity, leading to incorrect interpretations of large sequence divergence as evidence of diversity at high taxonomical rank.
(c). Evolutionary relationships between Microsporidia and Rozellidea
The relationship between Microsporidia and Rozellidea and the existence of both groups as independent phylogenetic lineages is currently the subject of debate (Corsaro et al., 2016; Torruella et al., 2018). While Rozella has several morphological traits that set it apart from Microsporidia, the same cannot be said about Paramicrosporidium and Nucleophaga. Similarities between the two groups run deeper than morphology. Several important metabolic characteristics such as the horizontally acquired strategies for nucleoside scavenging from the host, or loss of amino acid biosynthetic pathways and mitochondrial electron transport chain are found in both Rozellidea and Microsporidia (Quandt et al., 2017). However, the distribution of several of these traits is rather patchy, implying parallel reductive evolution. Mitosporidium, a parasite of the water flea Daphnia pulex, has been placed closer to Rozella than to the core Microsporidia (Corsaro et al., 2016). The partial sequence of the metchnikovellidan Amphiamblys suggests a phylogenetic position even further away from the core of Microsporidia than Mitosporidium, which would probably imply affinity with Rozellidea (Corsaro et al., 2016). Phylogenomic analyses of Paramicrosporidium recover it as a sister to core Microsporidia + Mitosporidium (Mikhailov, Simdyanov, & Aleoshin, 2017). Genomic analyses of Metchnikovella incurvata confirm Metchnikovellidae as a distinct and early branching clade within Microsporidia (Galindo et al., 2018). No phylogeny including both Rozellidea and any member of the Metchnikovellidae has been published to date. Evaluation and description of additional microsporidian‐like parasites of hosts outside insects and vertebrates are likely to blur the line between Rozellidea and Microsporidia even further. This situaton has led to some authors expanding the definition of Microsporidia to include Paramicrosporidium and Nucleophaga, leaving Microsporidia and Rozella as two separate monophyletic clades (Quandt et al., 2017; Bass et al., 2018).
(d). Aphelidea
The last major lineage to join the fungal family are the Aphelidea. Only four genera have been described in this group to date: Aphelidium, Amoebaphelidium, Paraphelidium and Pseudaphelidium (Karpov et al., 2017b). Their life cycle consists of a motile cell that is either flagellated (Aphelidium, Pseudaphelidium), amoeboid (Amoebaphelidium) or both (Paraphelidium). Occasionally, the zoospore may form a cyst, which can act either as a resistance form or as part of the penetration mechanism (Paraphelidium). Once inside the host, the parasitoid develops as a multinucleated plasmodium. The plasmodium divides into uninucleated zoospores after it has consumed the host cytoplasm by phagocytosis and releases the zoospores through the penetration site (Karpov et al., 2014b, 2017b). Despite having just a few formally described species, environmental sampling suggests that Aphellidea is indeed a highly diverse and cosmopolitan clade (Karpov et al., 2014b). It remains unknown whether deviations to the described life cycle exist in nature.
(2). Chytridiomycota
Chytridiomycota are divided into three main classes: Chytridiomycetes, Monoblepharidomycetes and Hyaloraphidiomycetes (James et al., 2006a; Sekimoto et al., 2011). Chytrids present a zoosporic disemination stage and usually a growing non‐flagellated stage. Chytrid cells can present different degrees of apical growth, such as filopodia and rhizoids, but in such cases the cell has a single nucleus and the protrusions are not cylindrical. Despite the presence of filopodia in several chytrid groups (e.g. Batrachochytrium) (Fritz‐Laylin, Lord, & Mullins, 2017), true phagocytosis has never been described. Multinucleated non‐cylindrical growth forms, known as rhizomycelia, have been observed in several clades. True mycelial growth is restricted to certain genera within the Monoblepharidomycetes (Dee et al., 2015). Chytrids are important pathogens of plants (e.g. Synchitrium), animals (e.g. Batrachochytrium), parasites of several groups of algae (e.g. Chytridium, Dinomyces), as well as decomposers of highly recalcitrant organic matter, such as pollen (e.g. Spizellomyces, Rhyzophidium), cellulose (e.g. Rhizophlyctis), arthropod exoskeletons, and fungal spores. Parasitic chytrids seem to play a key role in aquatic environments, controlling algal biomass and blooms, recycling nutrients, and acting as food for small animals in a nutrient loop that has been termed the ‘mycoloop’ (Gleason, Schmidt, & Marano, 2010; Rasconi, Niquil, & Sime‐Ngando, 2012; Kagami, Miki, & Takimoto, 2014; Frenken et al., 2016, 2017). The mycoloop is not restricted to members of the Chytridiomycota sensu stricto, as involvement of other zoosporic fungi such as members of the Aphelidea and Rozellidea is known (Gleason et al., 2012, 2014; Ishida et al., 2015). In land environments their presence is usually minor, although they form the main component of the fungal fraction in certain soil environments, such as periglaciar soils (Freeman et al., 2009). Environmental studies have found a myriad of putative novel clades within the phylum or as phylum‐level lineages closely related to chytrids, particularly in marine and soil environments (Nagahama et al., 2011; Manohar et al., 2013; Richards et al., 2015; Tedersoo et al., 2017). Sequence information on zoosporic fungi is currently very limited, which poses challenges to obtaining a robust chytrid tree of life. This situation fortunately is changing, and thanks to the application of single‐cell‐based techniques genomic and environmental sampling is steadily increasing (Grossart et al., 2016; Ahrendt et al., 2018).
(a). Chytridiomycetes
Chytridiomycetes is by far the largest class of zoosporic fungi with around 1000 described species. Based on phylogenetic analyses and the ultrastructure of the zoosporic stage, several lineages have been raised to the level of orders. The phylogenetic affinity of several genera, as well as the relationships of the different orders within the class is still not fully resolved (Misra, Tewari, & Deshmukh, 2012; Powell & Letcher, 2014). The picture will become further complicated as new environmental chytrids are described. Chytridiomycetes have received considerable attention in recent years owing to Batrachochytrium dendrobatidis, a parasite that is devastating populations of amphibians worldwide (Longcore, Pessier, & Nichols, 1999; Berger et al., 2005). The genome of Batrachochytrium dendrobatidis, published in 2009, represented the first sequenced chytrid (Cuomo & Birren, 2010).
(b). Monoblepharidomycetes
Monoblepharidomycetes comprise a group of freshwater, zoosporic fungi that can present either unicellular or mycelial growth. The mycelial Monoblepharidomycetes (Gonapodya, Monoblepharys and Monoblepharella) form a monophyletic clade within the class (James et al., 2006a; Sekimoto et al., 2011). They are the only described chytrids that form true hyphae, which in turn present some unique cytological characteristics, such as the presence of centrioles and the absence of Spitzenkörper, which point to an independent origin of these traits from the other fungi (Sekimoto et al., 2011; Dee et al., 2015). An oogonic sexual cycle (i.e. the presence of morphologically different gametes) is common in Monoblepharidomycetes, a unique feature among fungi. Hyaloraphidium is a poorly studied organism with an unclear lifestyle that was classified originally as a colourless green algae (Ustinova, Krienitz, & Huss, 2000), but molecular phylogenies show affinity with Monoblepharidomycetes (Forget et al., 2002; Sekimoto et al., 2011). As such, it has been classified as either a member of the Monoblepharidomycetes or as its own class, Hyaloraphidiomycetes (Schoch et al., 2014; Tedersoo et al., 2018). It is important to note that this lineage has lost its flagellum independently from the main terrestrial fungi (Ustinova et al., 2000; James et al., 2006b).
(3). Neocallimastigomycota
Neocallimastigomycota comprises a small group of flagellated, obligate anaerobic, non‐parasitic fungi. The group is formed by a single family currently comprising 18 recognized genera (Powell & Letcher, 2014; Hanafy et al., 2017; Wang, Liu, & Groenewald, 2017; Hanafy, Elshahed, & Youssef, 2018; Joshi et al., 2018; Hanafy et al., 2019), of which some may be paraphyletic (Wang et al., 2017). They lack true mitochondria, harbouring mitochondria‐derived hydrogenosomes instead. Some genera are multiflagellated. The nuclear envelope remains intact during mitosis. As a result of adaptation to anaerobic environments, members of this group do not synthesize ergosterol, whose biosynthetic pathway requires oxygen, but use tetrahymenol instead (Weete, Abril, & Blackwell, 2010). Similarly to Chytridiomycota, pluricellular forms seem to lack true hyphal organization. Neocallimastigomycota present large genomes (101 Mb in Orpinomyces) with high content of repetitive elements and a very low GC content (as low as 17% in Orpinomyces) (Billon‐Grand et al., 1991; Youssef et al., 2013). These genomes harbour a very wide and rich repertoire of carbohydrate‐degrading enzymes (Youssef et al., 2013; Gruninger et al., 2014), shaped by gene expansions and horizontal gene‐transfer events (Garcia‐Vallvé, Romeu, & Palau, 2000; Murphy et al., 2019; Wang et al., 2019). Members of this phylum have been isolated or detected almost exclusively in the gut of herbivorous mammals and iguanas, where they decompose plant organic matter. Outside this environment, there is a single report of the presence of these fungi in the gut of a sea urchin, based on morphological identification (Thorsen, 1999), indicating the potential of yet to be sampled diversity within the group. Unlike most fungal lineages, members of this phylum have rather high optimal growth temperatures as a consequence of their animal‐associated lifestyle.
The phylogenetic position of Neocallimastigomycota remains elusive, and thus its status as a phylum is debated. Some studies place them as the sister branch to Chytridiomycota sensu stricto (James et al., 2006a , b ; Ebersberger et al., 2012; Bauer et al., 2015), while other phylogenetic analyses place Neocallimastigomycota within Chytridiomycota sensu stricto, with Monoblepharidomycetes as sister to the rest of Chytridiomycota + Neocallimastigomycota (Sekimoto et al., 2011) (Fig. 3). Molecular dating suggests that the group is rather modern, having diversified in association with the emergence of grasses (Poaceae) and herbivorous mammals (Wang et al., 2019). This recent origin might suggest that the Neocallimastigomycota are in fact a highly specialized lineage that has emerged from within another zoosporic lineage, but the specifics of this presumed affinity are still unresolved.
Figure 3.

Phylogenetic position of Neocallimastigomycota in different studies. Simplified topology from several phylogenetic studies covering the phylogenetic position of Neocallimastigomycota. Numbers inside triangles represent the number of sampled species within the clade. (A) Topology obtained from James et al. (2006b). Phylogeny constructed from a concatenation of 18S rRNA, 28S rRNA and 5.8S rRNA, using Bayesian inference. (B) Topology obtained from James et al. (2006a). Phylogeny constructed from a concatenation of 18S rRNA, 28S rRNA, 5.8S rRNA, EF1‐α, RPB1 and RPB2, using Bayesian inference. (C) Topology obtained from Sekimoto et al. (2011). Phylogeny constructed from RPB1, RPB2, EF1‐α, rRNA and actin genes, using a maximum‐likelihood approach. (D) Topology obtained from Ebersberger et al. (2012). Phylogeny reconstructed from a supermatrix of 46 single‐copy genes, using a maximum‐likelihood approach.
(4). Blastocladiomycota
The phylum Blastocadiomycota comprises a relatively small group of zoosporic fungi with diverse morphological and ecological traits. Before its promotion to a phylum level based on molecular phylogenetic studies (James et al., 2006b), the group was already recognized as monophyletic based on ultrastructural similarities (Cavalier‐Smith, 1981), uniting them with terrestrial fungi. Unlike other zoosporic fungi, Blastocladiomycota present alternation of gametophytic and sporophytic generations. They present up to three types of uniflagellated zoospores, asexual meiospores and sexual gametes. Sexual reproduction is not known in all groups. True mycelial growth (sometimes presenting pseudosepta) is recognized, while amoeboid crawling stages have not been described. The zoospores present a characteristic nuclear cap that, in hyphal species, produces bipolar growth. The resting sporangium is typically darkly pigmented and usually presents spines and other ornamentations (James, Porter, & Martin, 2014). In other respects, Blastocladiomycota are very similar in their ecological and general morphological characteristics to members of the Chytridiomycota sensu stricto, with species that are saprobes in soils and freshwater environments, invertebrate parasites, and plant and algal pathogens (Porter et al., 2011; James et al., 2014). The group contains two formerly popular model organisms, Allomyces macrogynus and Blastocladiella emersonii, which are two saprotophs with well‐defined and well‐studied alternation of generations. The genus Coelomomyces grows as unwalled tubular thalli in an insect or crustacean host, a cellular organization that has been compared to that observed in members of the Entomophthoromycotina (Gleason et al., 2010).
Historically associated with the other zoosporic fungi, molecular studies initially showed great divergence from them (Bowman et al., 1992; Bruns et al., 1992; James et al., 2000; Lutzoni et al., 2004). Blastocladiomycota were dragged into controversies regarding the paraphyly of Zygomycota, appearing as the sister branch to Entomophthoromycotina in some studies (Tanabe et al., 2005; Tretter et al., 2013). This placement was shown to be caused by high evolutionary rates in certain genera in the Zoopagomycota. Correction of these artefacts and the availability of additional sequences for molecular analyses led to the recovery in most phylogenetic studies, albeit with low support, of Blastocladiomycota as the sister branch to all terrestrial fungi + Olpidium (Sekimoto et al., 2011; Ebersberger et al., 2012; Torruella et al., 2012; Spatafora et al., 2016; Tedersoo et al., 2018). The phylum contains a single order, Blastocladiales, and five morphologically defined families that were validated with minor changes by molecular studies (Porter et al., 2011; James et al., 2014). Environmental sampling has detected Blastocladiomycota clades at high abundances in aquatic environments, both marine and fresh water (Tedersoo et al., 2017). Some studies suggest that they might be prevalent in oxygen‐poor environments (James et al., 2014).
III. Zygomycetous fungi
Loss of their flagellar apparatus and the development of hyphal growth allowed a particular group of Fungi to conquer emerged lands. These terrestrial Fungi include most described diversity, included in the subkingdom Dikarya (Basidiomycota + Ascomycota + Entorrhizomycota) plus several lineages collectively called zygomycetous Fungi. ‘Zygomycetous’ refers to a paraphyletic phylum (Zygomycota), and this in turn to a sexual structure, the zygospore, that is common to most lineages ascribed to it. We include here the Glomeromycota, despite their historical separation from the ‘Zygomycota’ due to the absence of observed zygospores (or any sexual structure), for the sake of simplicity and in light of recent taxonomic revisions that advocate the incorporation of Glomeromycota into the Mucoromycota. With this in mind, zygomycetous Fungi form two main lineages, one that is composed mostly of parasites of opisthokonts (Zoopagomycota; see Table 2) and a second that is composed mostly of plant symbionts and saprotrophs (Glomeromycota + Mucoromycota; see Table 3). Figure 4 illustrates some of these organisms.
Table 2.
Main lineages within Zoopagomycota. Due to changes in the scope of certain historical clades, references might not refer to the initial published description but to more recent bibliography. For historical reasons we have decided to keep the ordinal assignation within zygomycetous fungi, although we consider that these clades are likely to be elevated to a higher taxonomic rank as proposed by several authors
| Name | Main described lifestyles | Main traits | Representative genera |
|---|---|---|---|
|
Zoopagomycota |
Saprobes, invertebrate parasites, mycoparasites, amoebophagous | Thallus mycelial, mostly separated into cells with complete or uniperforate septa; sexual reproduction, if present, via zygospores by gametangial conjugation | |
|
Zoopagomycotina (Hibbett et al., 2007) |
Parasites of small invertebrates, amoebae and fungi | Very small thallus, generally coenocytic; uniperforated septa appear in certain nematode‐trapping genera; sexual reproduction with globose zygospores | Zoopage, Piptocephalis, Rhopalomyces, Amoebophylus |
|
Entomophthoromycotina (Hibbett et al., 2007) |
Insect parasites; occasionally saprobes or plant parasites | Coenocytic hyphae, yeast or unwalled syncitia growing within the host | |
|
Basidiobolomycetes (Humber, 2012) |
Saprotrophs and facultative insect parasites; sometimes associated with reptiles and amphibians | Hyphal or yeast‐like; large nucleus with large central nucleolus; zygospores with thick bi‐layered cell walls; globose conidia, released by a rocket‐like mechanism | Basidiobolus, Schizangiella |
|
Entomophthoromycetes (Hibbett et al., 2007) |
Obligate insect parasites; Conidiobolus lives as a saprotroph and facultative insect parasite | Filamentous without septa; grow as an unwalled syncitia within host; ballistic conidia; 24‐methyl cholesterol as main membrane sterol | Entomophthora, Pandora, Massospora, Conidiobolus |
|
Neozygitomycetes (Humber, 2012) |
Parasites of hemipterans and orthopterans | Melanized spores; vermiform, moderately sized chromosomes that condense during mitosis on a central metaphase plate but uncoil during interphase; nuclear numbers in vegetative cells and conidia are low and apparently controlled at 3–5 | Neozygites, Thaxterosporium |
|
Kickxellomycotina (Hibbett et al., 2007) |
Parasites or symbionts of arthropods, mycoparasites, saprobes | Presence of septa with plugs, morphology diagnostic of the different clades; mycelium regularly septated | |
|
Asellariales (Hibbett et al., 2007) |
Associated with the gut of isopods and springtails | Filamentous, branched thalli; asexual reproduction by arthrospore‐like cells that disarticulate; lenticular septa | Asellaria, Baltomyces, Orchesellaria |
|
Dimargaritales (Hibbett et al., 2007) |
Haustorial mycoparasites, mostly on Mucoromycotina | Thallus branched, with septate hyphae, producing septate sporangiophores; septa with median disciform cavities containing biconvex plugs with polar protuberances; asexual reproduction by bisporous merosporangia | Dimargaris, Dispira, Tieghemiomyces |
|
Harpellales (Hibbett et al., 2007) |
Associated with the gut of aquatic insects | Basal cell attached to the host from which a filamentous septate thallus emerges; septa with lenticular plugs; asexual reproduction by lateral elongate monosporous trichospores; sexual reproduction by conical or biconical zygospores | Harpella, Smittium |
|
Kickxellales (Hibbett et al., 2007) |
Saprobes or mycoparasites | Thallus branched, with septate hyphae; septa with median disciform cavities containing biconvex plugs; asexual reproduction by monospored sporangiola on sporocladia; sexual reproduction by nearly globose zygospores | Coemansia, Kickxella, Martensiomyces, Ramicandelaber |
|
Ramicandelaber (Tedersoo et al., 2018) |
Saprobe | Sporangiophores septate, verticillately branched, forming supporting septated hyphae with rhizoids; branches cylindrical or ellipsoid, with further irregular branching | Ramicandelaber |
|
Barbatospora (Tretter et al., 2014) |
Associated with aquatic larvae of Simuliidae | Branched septate thallus with basal cell; cap‐like structure at the end of the trichospores, which falls away at maturity to reveal a set of appendage‐like structures; unknown zygospores and septal morphology | Barbatospora |
|
Orphella (Tretter et al., 2014) |
Associated with aquatic larvae and nymphs of Plecoptera | Coiled asexual spores and zygospores; basal cell attached to the host from which a filamentous branched septate thallus emerges; unknown septal morphology | Orphella |
|
Spiromyces + Mycöemilia clade |
Saprobes, isolated from dung and soil |
Septate hyphae; lenticular septal plug; sporophores erect, septate, branched or unbranched, producing one to several fertile parts |
Spiromyces, Mycöemilia |
Table 3.
Main lineages within Mucoromycota and Glomeromycota. Due to changes in the scope of certain historical clades, references might not refer to the initial published description but to more recent bibliography. For historical reasons we have decided to keep the ordinal assignation within zygomycetous fungi, although we consider that these cladess are likely to be elevated to a higher taxonomic rank as proposed by several authors
| Name | Main described lifestyles | Main traits | Representative genera |
|---|---|---|---|
|
Mucoromycota |
Mostly filamentous saprobes; occasionally mycoparasites, plant pathogens or mycorrhizal | Coenocytic hyphae, able to perform anastomosis; mature hyphae sometimes irregularly septated; rhizoids common | |
|
Mortierellomycotina (Hoffmann et al., 2011) |
Filamentous saprobes | Absence of columella; dichotomous branching | Mortierella, Aquamortierella, Dissophora |
|
Mucoromycotina (Hibbett et al., 2007) |
Filamentous saprobes; occasional mycoparasites, plant pathogens or ectomycorrhizal | Chitosan as main structural polysaccharide; sporangia with well‐developed columella | |
|
Endogonales |
Ectomycorrhizal and saprobe | Zygospores with apposed suspensors in a subterranean sporocarp | Endogone |
|
Umbelopsidales (Spatafora et al., 2016) |
Saprobes | Thallus branched; hyphae initially without septa but developing near the branch point; asexual reproduction via sporangia; sporangiophores densely branched, with septa distant from the sporangium; sporangia reddish or ochraceous, globose or elongate, multispored or single‐spored; columella usually conspicuous; chlamydospores abundant, filled with lipids in culture; unknown sexual stages | Umbelopsis |
|
Mucorales (Adl et al., 2012) |
Saprobes, ocasionally mycoparasites or plant pathogens | Filamentous, septa absent except in older hyphae; plasmodesmata at septal pores | Mucor, Phycomyces, Saksaena, Lichtheimia |
|
Glomeromycota (Schüβler et al., 2001) syn. Glomeromycotina within Mucoromycta |
Endomycorrhizal, except Geosiphon, which forms a symbiosis with cyanobacteria | Coenocytic hyphae, able to perform anastomosis; multinucleated spores; asexually formed chlamydospore‐like spores are borne terminally, laterally, or intercalary on specialized hyphae; form specialized haustoria‐like branched structures termed arbuscular mycorrhizae to interact with the host | |
|
Diversisporales (Schüβler et al., 2001) |
Endomycorrhizal |
Fungi hypogeous, forming endomycorrhizae with arbuscules, often lacking vesicles; with or without hypogeous auxiliary cells; forming either complex spores produced within a sporiferous saccule, complex spores developing from a bulbous base on the sporiferous hypha, or glomoid spores |
Acaulaspora, Gigaspora, Diversispora |
|
Glomerales (Schüβler et al., 2001) |
Endomycorrhizal |
Fungi mostly hypogeous, sometimes epigeous, forming endomycorrhizae or mycorrhiza‐like symbioses with spores, vesicles or arbuscules in plants; hyphae of vegetative mycelium mostly non‐septate; asexual reproduction by glomoid spores, mainly terminal, but sometimes intercalary; spores solitary or formed in clusters, or in sporocarps |
Glomus, Rhizophagus, Funneliformis |
|
Paraglomerales (Schüβler et al., 2001) |
Endomycorrhizial |
Fungi hypogeous, forming endomycorrhizae with arbuscules and intraradical mycelium, rarely with vesicles; non‐pigmented glomoid spores |
Paraglomus |
|
Archaeosporales (Schüβler et al., 2001) |
Endomycorrhizal, except Geosiphon, which forms a symbiosis with cyanobacteria |
Fungi hypogeous, forming endocytosymbioses with photoautotrophic prokaryotes, or endomycorrhizal; with or without vesicles; spores lacking pigmentation or reaction to Melzer's reagent; glomoid spores formed singly or in loose clusters on the soil, acaulosporoid complex spores formed singly in the soil; dense spore clusters unknown |
Archaeospora, Ambispora, Geosiphon |
Figure 4.
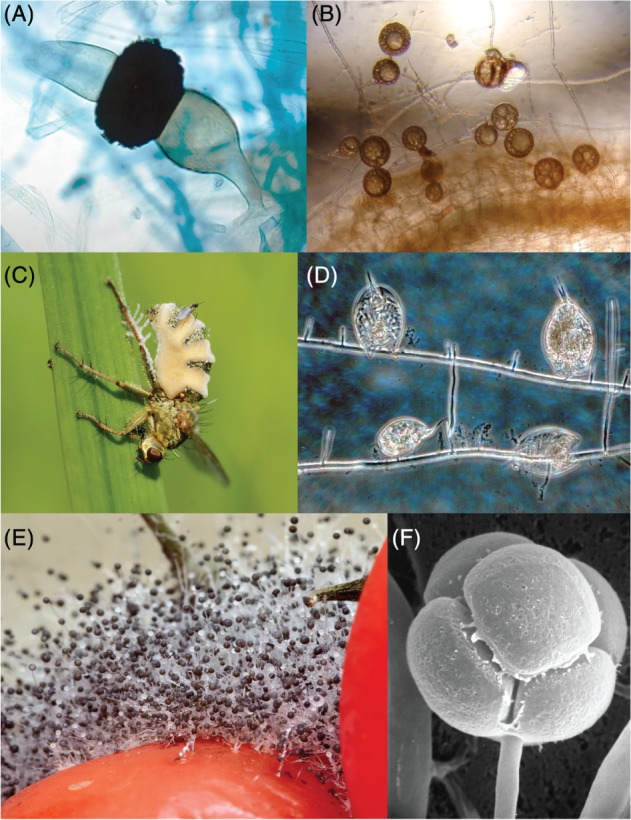
Diversity of zygomycetous Fungi. (A) Zygospore from Rhizopus stolonifer (Mucorales, Mucoromycotina). Zygospores are naked sexual spores formed in the intersection of two mating hyphae in both Zoopagomycota and Mucoromycota. Original photograph by George Barron. Licensed for non‐commercial academic and research use only. (B) Mycelium and multinucleated spores from Rhizophagus intrarradices (Glomerales, Glomeromycotina) growing in association with a plant root, appearing as a foamy structure in the lower part of the picture. The spores, appearing as dark brown globular structures, contain multiple nuclei that are thought to form a chimeric population (heterokaryon). Original photograph by Banco de Glomeromycota in vitro, CC BY‐NC‐ND 2.5 AR license. (C) Entomophthora muscae (Entomophthorales, Entomophthoromycotina) growing in a fly. The Entomophthorales include mostly entomopathogenic species that form an unwalled coenocytic mycelium that invades the host body before killing it. Original photograph by Hans Hillewaert, CC BY‐SA 4.0 license. (D) Hyphae from Zoophagus insidians (Zoopagales, Zoopagomycotina) attacking a group of rotifers. Zoopagales is a group of parasitic fungi that mostly infect other fungi, protozoans and microinvertebrates. Original photograph by George Barron. Licensed for non‐commercial academic and research use only. (E) Pin mould [probably Rhizopus stolonifer (Mucorales, Mucoromycotina)] growing on a tomato. Most members of the mucorales are fast‐growing saprotrophs that present very large sporangia, appearing here as dark globose structures at the end of long aerial hyphae. Original photograph by Wikipedia user Calimo, CC BY‐SA 3.0 license. (F) Scanning electron micrograph of a Mortierella hyalina (Mortierellales, Mortierellomycotina) sporangium. Members of the Mortierellomycotina have similar ecologies to Mucorales, but they can be easily differentiated by the absence of an inflated base to their sporangia (columella). Original photograph by flickr user ZygoLife Research Consortium, CC BY‐SA 2.0 license.
(1). Zoopagomycota
This phylum is the earliest diverging group of non‐flagellated fungi, and includes three main lineages: Zoopagomycotina, Entomophthoromycotina and Kickxellomycotina (Hibbett et al., 2007; Spatafora et al., 2016). These three lineages have the ability to form true mycelia. Most members are either saprotophs or parasites of metazoans, amoebae or other fungi, including highly specialized forms. Virtually no morphological characteristics unite the three subphyla, which are classified together based on phylogenetic affinity and their general metazoan‐associated lifestyle.
(a). Entomophthoromycotina
Entomophthoromycotina comprises three main classes: Basidiobolomycetes, Neozygitomycetes and Entomophthoromycetes (Humber, 2012). All groups in the Entomophthoromycotina present 24‐methyl cholesterol as their main membrane sterol (Weete et al., 2010). Basidiobolomycetes is the earliest‐splitting lineage and comprises the genus Basidiobolus, a saprotrophic gut commensal of amphibians and reptiles and opportunistic human pathogen (Manning, Waters, & Callaghan, 2007; Manning & Callaghan, 2008), and the yeast‐like snake pathogen Schizangiella (Gryganskyi et al., 2013; Benny, Humber, & Voigt, 2014). Basidiobolus is so far the only genus in the Entomophthoromycotina to present septate hyphae. Basidiobolus and Conidiobolus are unique among the zygomycetous fungi for possessing a true Spitzenkörper (Roberson et al., 2011; Fisher et al., 2018). The second class, Neozygitomycetes, comprises several genera of parasites of mites and aphids. Unfortunately, very little sequencing data are available for this group, raising doubts about its taxonomic uniqueness, its phylogenetic placement and even its membership in this subphylum (White et al., 2006; Gryganskyi et al., 2013).
The Entomophthoromycetes is the most species‐rich and best‐characterized class in the subphylum. It comprises mainly the genus Conidiobolus (family Ancylistaceae) (a saprobe, facultative insect parasite and occasional human pathogen), several families of mostly specialized insect parasites that form a well‐defined monophyletic group, and some small genera with diverse ecological strategies: Ancylistes (Ancylistaceae) is a parasite of desmid algae, Macrobiotophthora (Ancylistaceae) parasitises nematodes and tardigrades, Completoria (Completoriaceae) is a fern biotrophic parasite, and Meristacrum (Meristacraceae) is a nematode parasite (Gryganskyi et al., 2012, 2013; Humber, 2012). Nuclear characters (size, presence, stainability, nucleolar characteristics and pattern of mitosis) and modes of germination of resting spores are important for their systematics at a family level (Humber, 1989; Benny et al., 2014). The available genetic information on these lineages is very limited, and thus their taxonomy might be revised in the near future (Gryganskyi et al., 2013; Benny et al., 2014). The family Ancylistaceae was the earliest to split within the Entomophthoromycetes, although sequencing data are mostly limited to Conidiobolus species and phylogenetic analyses have typically failed to recover monophyly of this genus (Gryganskyi et al., 2013; Spatafora et al., 2016). Another common ecological strategy is found in a monophyletic group of highly specialized entomopathogenic fungi comprising around 200 species (Entomophthora, Massospora, Pandora, Entomophaga). These parasites usually grow as unwalled mycelia within the host's coelomatic cavity, where they commonly reach considerable biomass before killing the host. This intimate association with the host for most members of the Entomophthoromycotina has made the collection of samples for DNA‐based analyses challenging, but morphological studies have proved to be generally consistent with molecular phylogenies (Humber, 2012; Gryganskyi et al., 2013).
(b). Zoopagomycotina
Zoopagomycotina comprises a single order, Zoopagales, that includes five families and around 20 genera (Hibbett et al., 2007; Benny et al., 2014, 2016; Degawa, 2014). The genus Basidiolum is suspected to belong to this subphylum (Benny et al., 2016). They live as parasites of free‐living amoebae (Zoopagaceae, Cochlonemataceae), soil microinvertebrates (Helicocephalidaceae, Zoopagaceae and Cochlonemataceae) or other fungi (Piptocephalidaceae and Sigmoideomycetaceae) (Benny et al., 2016). Several members of the Cochlonemataceae are endoparasites of amoebae or small animals, requiring engulfment by phagocytosis or ingestion by their host (Benny et al., 2014). Inside the host, they grow as a poorly developed thallus. The remainder of the subphylum grow as a mycelium, often with a foamy appearance, use sticky substances to attach the host and produce haustoria to penetrate them (Duddington, 1956; Saikawa, 2011; Benny et al., 2014). Most species show a narrow host range. Septa can be found in some genera (e.g. Euryancale, Cystopage). Both sexual and asexual spores have been described. The evolutionary relationships among the different families are not well resolved, mostly due to the difficulty of working with such small and usually unculturable fungi (Davis et al., 2019b). Additionally, molecular analyses suggest that members of this clade show high evolutionary rates (Ahrendt et al., 2018; Davis et al., 2019a), and some of the most commonly used primers for barcode sequences of fungi or eukaryotes work poorly for this group (Tedersoo & Lindahl, 2016). This amplification problem is caused by their unusually long internal transcribed spacer (ITS) sequences, so the recent development of more‐specific primers (Lazarus et al., 2017) will help the study of these fungi to catch up with the rest of the kingdom. Finally, phylogenetic evaluation of genera in the Piptocephalidaceae (Piptocephalis, Syncephalis, Kuzuhaea) (Lazarus et al., 2017), Zoopagaceae (Zoopage, Zoophagus, Acaulopage) and Cochlonemataceae (Cochlonema) (Lazarus et al., 2017; Reynolds et al., 2019) suggests that morphological characters might be insufficient for species or even genus delimitation in this subphylum and thus a large cryptic diversity is to be expected.
(c). Kickxellomycotina
Kickxellomycotina was created as a subphylum unifying several poorly studied fungal groups united by the presence of septated mycelia that present unique septal pores with a lenticular plug (Tanabe et al., 2004; Hibbett et al., 2007; Benny et al., 2014). The morphology of these pore plugs, as well as the characteristics of the sporangia are diagnostic traits for the main groups within the subphylum (Tretter et al., 2014). The group comprises four recognized orders (Kickxellales, Harpellales, Asellariales and Dimargaritales) plus several genera of still unresolved phylogenetic placement (Ramicandelaber, Barbatospora, Orphella, Spiromyces and Mycoëmilia) (Tretter et al., 2013, 2014). New orders or higher taxonomical assignation for the Ramicandelaber and Spiromyces + Mycoëmilia clades have been proposed but have yet to find support (Benny et al., 2016; Tedersoo et al., 2018). Harpellales, Asellariales, Orphella, and Barbatospora are fungi with poorly developed thalli that are found in association with the gut of several groups of arthropods. Dimargaritales and Martensella (Kickxellales) are mycoparasites. The rest of Kickxellales, Spiromyces, Mycöemilia and Ramicandelaber grow as saprobes. The genus Coemansia (Kickxellaceae, Kickxellales) (Chuang et al., 2017), a dung‐associated saprotroph with intrincate asexual structures, was the first sequenced Kickxellomycotina (Chang et al., 2015). The placement of some orders within the group has been difficult due to the existence of long‐branch‐attraction artefacts in molecular phylogenies (e.g. in Dimargaritales) (Tanabe et al., 2000; Tretter et al., 2013) and the difficulty of obtaining DNA for several lineages. The saprotrophic members of the group are common in soil and dung (Benny et al., 2014).
(d). Future prospects in the Zoopagomycota
The phylogenetic placement and definition of Zoopagomycota has been controversial. They were originally placed within the Zygomycota, a group that was later shown to be paraphyletic, comprising up to 10 independent orders whose relationships were uncertain (Benny et al., 2014). In this context, the relationship between Zoopagomycotina and Kickxellomycotina was established (White et al., 2006; Hibbett et al., 2007; Spatafora et al., 2016). Finally, the monophyly of Entomophthoromycotina, as well as its relationships with the rest of the phylum were resolved, and the phylum Zoopagomycota was proposed (Spatafora et al., 2016). In addition to the described groups, environmental sequencing studies have described Clade GS19, which may represent a novel lineage distinct from any of the subphyla (Spatafora et al., 2016) or a distinct lineage within Kickxellomycotina (Tedersoo et al., 2017). Given the general association of these fungi with metazoans and other organisms in soil, we expect their presence in soil to be low in terms of biomass, and associated with their hosts. This seems to be the case for rainforest (Tedersoo et al., 2018) and prairie soils (Dunthorn et al., 2017). Contrary to this assumption, however, there are reports of high abundance of sequences from amoebophagous fungi (genus Kuzuhaea, Zoopagales) in permafrost soils, suggesting that these microfungi are indeed highly abundant in at least certain environments (Penton et al., 2013). The known diversity of their usual hosts (i.e. amoebae, insects, fungi and nematodes) suggests that the undescribed diversity of these fungi might be very large, particularly considering that many of these interactions seem to be quite specific. Outside parasitic relationships, members of this group [Basidiobolus (Basidiobolomycetes), Coemansia, (Kickxellales)], together with Mortierella (Mortierellomycotina), were reported to be enriched on the surfaces of two species soil acari compared to the surrounding soil, forming distinct communities for each species (Werner, Peršoh, & Rambold, 2018). While their small size imposes challenges to traditional sequencing approaches, they are prime candidates for single‐cell sequencing‐based techniques. Using the latter methods, several genomes in these lineages have been sequenced recently (Ahrendt et al., 2018).
(2). Glomeromycota
Virtually all known members of this phylum live as obligate symbionts of land plants, forming a particular type of symbiosis termed arbuscular mycorrhizae. Glomeromycota was separated from the rest of Zygomycota based on early ribosomal protein phylogenies (Schüβler, Schwarzott, & Walker, 2001). The fungus mycelia grow inside the root of the plant, penetrating the cells of the host. The mycelium is always non‐septate and presents anastomoses (Redecker & Schüßler, 2014). Members of this group have 24‐ethyl‐cholesterol as the main membrane sterol, apparently lacking ergosterol (Weete et al., 2010). The fungus helps the plant with the acquisition of phosphorus, nitrogen and water in exchange for photosynthesis‐derived metabolites. Functionally similar associations exist for members of Mucoromycota, Ascomycota and Basidiomycota, but Glomeromycota is by far the most common symbiont group associating with nearly three‐quarters of land plants (Bidartondo et al., 2011). Four orders are recognized within the Glomeromycota (Paraglomerales, Archaeosporales, Diversisporales and Glomerales) that include 11 families and around 230 morphospecies (Stürmer, 2012). Their spores are multinucleated (with up to hundreds of nuclei that are often genetically distinct populations), very large, and filled with lipid and protein globules. Spore morphology defines the different groups.
Geosiphon pyriforme (Geosiphonaceae, Archaeosporales) is the only member of the phylum that does not form arbuscular mycorhizae (Gehrig, Schüßler, & Kluge, 1996). It grows as a symbiont of colonies of cyanobacteria in the genus Nostoc in a fashion that some authors have considered functionally similar to a lichen (; Kluge et al., 2002). The symbiosis is photosynthetically active (Kluge, Mollenhauer, & Mollenhauer, 1991) and is able to fix atmospheric nitrogen (Kluge et al., 1992). However, the ultrastructure of the symbiosis is more akin to arbuscular mycorrhizae than to a prototypical lichen (Schüßler et al., 2007).
Earlier studies placed Glomeromycota as the sister clade to Dikarya (Ascomycota + Basidiomycota + Entorrhizomycota) (Schüβler et al., 2001; Lutzoni et al., 2004; White et al., 2006), while most recent phylogenies place them as sister to or within Mucoromycota (Liu, Hodson, & Hall, 2006; Nadimi et al., 2012; Lin et al., 2014; Spatafora et al., 2016). Spatafora et al. (2016) classified this group as subphylum Glomeromycotina within Mucoromycota. However, we consider that elevation to phylum status is justified by their phenotypical peculiarities and historical use, and that such nomenclature is compatible with either phylogenetic scenario. The discovery of several novel lineages (see Section V) will eventually force taxonomists to reevaluate the Glomeromycota and Mucoromycota, but we consider it premature to merge these two groups before resolving the phylogenetic placement of these new lineages on the fungal tree of life. As biotrophic organisms, they cannot be grown axenically, and must be cultured using experimentally tractable plants. In general terms, most described species show low host specificity and low endemism (Davison et al., 2015), although is highly likely that sampling and identification methodologies are biased towards generalist and highly resilient species (Ohsowski et al., 2014). Environmental studies show that this group is ubiquitous and probably much more diverse than previously thought (Ohsowski et al., 2014; Tedersoo et al., 2017). Solid indirect evidence of sexual recombination has been described, although no sexual structures have ever be observed (Sanders, 2011; Tisserant et al., 2013).
(3). Mucoromycota
Mucoromycota comprises the largest and best‐studied group of zygomycetous fungi. Most species grow as saprobes, with some species being non‐haustorial parasites of plants and other fungi, or ectomycorrhizal. It includes two subphyla: Mortierellomycotina and Mucoromycotina (Hoffmann, Voigt, & Kirk, 2011; Benny et al., 2014; Spatafora et al., 2016). They grow as coenocytic and anostomosing hyphae. Certain members of this phylum are important in the food industry as causes of food spoilage (Filtenborg, Frisvad, & Thrane, 1996; Moss, 2008; Garnier, Valence, & Mounier, 2017) or in the preparation of certain fermented foods (Londoño‐Hernández et al., 2017). Some can also cause rare but highly invasive infections in humans and animals (Kwon‐Chung, 2012; Fisher, Gow, & Gurr, 2016; Serris et al., 2019). Several members of this lineage, specially the orders Mucorales (Mucoromycotina) and Mortierellales (Morteirellomycotina) are used in industrial fermentations for the production of chitosan, lipids or carotenoids (Conti et al., 2001; Kuzina & Cerdá‐Olmedo, 2007; Papanikolaou et al., 2007; Karimi & Zamani, 2013).
(a). Mortierellomycotina
Mortierellomycotina are morphologically and ecologically similar to the rest of the group, but their phylogenetic positioning has been historically convoluted (Rosewich & Kistler, 2000; Voigt & Wöstemeyer, 2001; Tanabe et al., 2005; Kwaśna, Ward, & Bateman, 2006; Nadimi et al., 2012; Wagner et al., 2013). The subphylum includes one family, 13 genera and more than 100 currently recognized species (Wagner et al., 2013). Mortierellomycotina are diferentiated from Mucoromycotina by the morphology of the zygospore, and the absence of a columella, which is a basally inflated sporangiophore. Many produce a characteristic colony morphology and garlic‐like odour when grown in culture. The elevation to subphylum for this group is based exclusively on phylogenetic analyses (White et al., 2006; Hoffmann et al., 2011; Spatafora et al., 2016) that suggest that it constitutes a phylogenetically distinct lineage, sister to Mucoromycota. Most species of this clade are ascribed to the paraphyletic genus Mortierella (Petkovits et al., 2011). The group also includes some specialized forms, such as Aquamortierella, which is found only in aquatic habitats (Embree & Indoh, 1967).
(b). Mucoromycotina
Mucoromycotina includes two orders: Endogonales and Mucorales. It is noteworthy that many older references use Mucorales in a sense that roughly equates to the modern circumscription of Mucoromycota or Mucoromycotina. Some authors recognize an additional order, Umbelopsidales, that includes the single family Umbelopsidaceae (Spatafora et al., 2016). Endogonales comprises a single family, four genera and about 30 species, while Mucorales spans 14 families comprising 56 genera and around 300 species (Benny et al., 2014). Some members of Endogonales form ectomycorrhizal associations with some plants, and in particular with certain lineages of liverworts (Bidartondo et al., 2011; Field et al., 2015; Orchard et al., 2017). Compared to most fungi, cell walls of Mucoromycotina are known to contain chitosan, a deacetylated form of chitin, as the main structural component (Ruiz‐Herrera & Ortiz‐Castellanos, 2010; Mélida et al., 2015). They also present an extrusion of the sporangiophore termed a columella that is synapomorphic for the subphylum. Porous, plasmodesmata‐containing septa may appear in reproductive structures and senescent hyphae. Most species are saprotrophs, and occasionally can be facultative parasites of animals, plants and other fungi. At least three genera (Dicranophora, Spinellus and Syzygites) are obligate parasites of mushrooms (Benny et al., 2014).
(c). Future prospects in the Mucoromycota
Environmental studies reveal that Mucoromycotina and Mortierellomycotina are ubiquous in most environments and suggest that a large fraction of the diversity in both subphyla remains unexplored (Tedersoo et al., 2014, 2017; Ziaee et al., 2016). As mentioned above, the relationship with other phyla is debated (Liu et al., 2006; Nadimi et al., 2012; Lin et al., 2014; Spatafora et al., 2016). Several genera have recently been assigned to the Mucoromycota based on molecular studies, which probably implies that a taxonomic overhaul of the Mucoromycota and the establishment of novel orders or classes is necessary. These genera and their phylogenetic affiliations are discussed further in Section V.
IV. Dikarya
Dikarya is by far the most species‐rich and best‐studied group of Fungi. It includes two main phyla, Basidiomycota and Ascomycota. Additionally, a small group of root endophytes has recently been proposed to represent a third phylum, Entorrhizomycota (see Section V). Dikarya are characterized for a sexual cycle that includes hyphal fusion uncoupled with meiosis, which in turn produces hyphae that contain two independent nuclear populations (dikaryotic hyphae). Most also present septated hyphae, ergosterol as the membrane sterol, and several lineages are even able to form multicellular reproductive or vegetative structures. Figure 5 provides examples of Basidiomycota, while Fig. 6 does the same for Ascomycota, while the main lineages of these groups are listed in Tables 4 and 5, respectively.
Figure 5.

Diversity of Basidiomycota. (A) Basidia from Coprinus (Agaricomycetes, Agaricomycotina). Basidia are reproductive structures formed by a cell attached to the (typically four) derived spores produced by meiosis, appearing here as dark structures. Original photograph by Wikipedia user Jon Houseman, CC BY‐SA 3.0 license. (B) Puccinia recondita (Pucciniomycetes, Pucciniomycotina) growing on the back of a leaf. Pucciniomycetes are a diverse class of biotrophic plant pathogens within the Pucciniomycotina. Original photograph by flickr user Line Sabroe, CC BY 2.0 license. (C) Micrograph of a skin cell infected by Malassezia furfur (Malasseziomycetes, Ustilaginomycotina). Although most Ustilaginomycotina are plant pathogens, the genus Malassezia is commonly found in the skin of mammals. Original photograph in the public domain. (D) Fruiting bodies of Amanita muscaria (Agaricomycetes, Agaricomycotina), a poisonous mushroom famous for its bright white and red colour and its hallucinogenic properties. Original photograph in the public domain. (E) Micrograph of Wallemia ichthyophaga (Wallemiomycetes, Wallemiomycotina), appearing as a rounded mass. Wallemiomycetes contains a few species of highly extremotolerant fungi. W. ichthyophaga in particular requires high salinity to grow, as can be seen from the presence of cubic salt crystals in the picture. Photograph by Wikipedia user Anticicklon, CC BY‐SA 3.0 license. (F) A Ginkgo biloba leaf covered by clustersof black Bartheletia paradoxa telia. B. paradoxa represents a divergent lineage that has probably co‐evolved with Ginkgopsida, an ancient plant lineage of which there is only one extant species. Original photograph by flicker user AJC1, CC BY‐SA 2.0 license.
Figure 6.
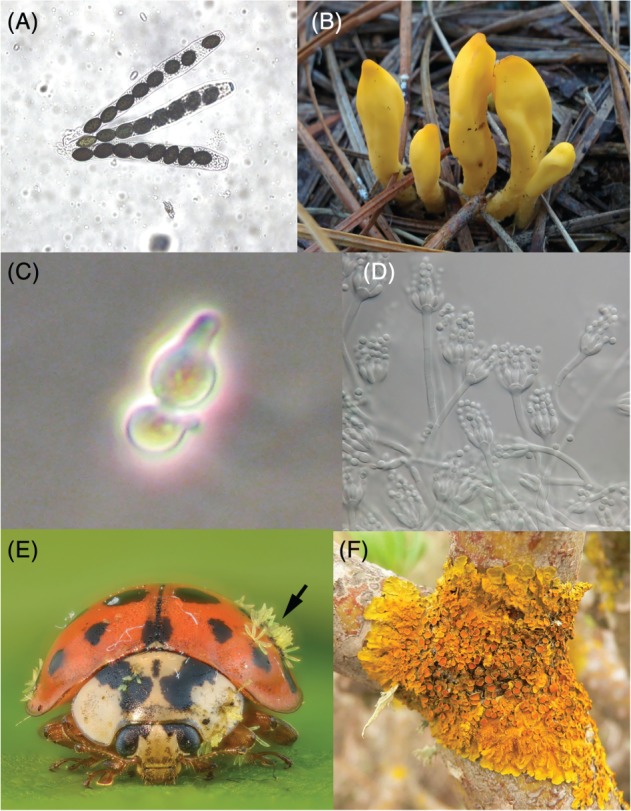
Diversity of Ascomycota. (A) Asci from Sordaria fimicola (Sordariomycetes, Pezizomycotina). Asci are reproductive structures that enclose (typically four or eight) spores produced by meiosis, appearing here as dark structures. Original photograph by Wikipedia user CarmelitaLevin CC BY‐SA 4.0 license. (B) Fruiting bodies of Neolecta vitellina (Neolectomycetes, Taphrinomycotina). Taphrinomycotina includes several lineages with a wide range of body plans, ranging from intracellular parasites to complex multicellular fungi. Original photograph by Mushroom Observer user gillow2e, CC BY‐SA 3.0 license. (C) Mating cells (Shmoo) of Saccharomyces cerevisiae. Under the right conditions haploid cells enter the shmoo mating state and fuse with a mating cell of the opposite mating type, producing a diploid cell. The diploid cell can enter meiosis, producing an ascus with four spores, from which haploid cells germinate. Original photograph by Wikipedia user Pilarbini, CC BY‐SA 4.0 license. (D) Micrograph of a group of conidia from Penicillium spinulosum (Eurotiomycetes, Pezizomycotina). Penicillium is a genus of cosmopolitan moulds that mostly propagate by producing high numbers of asexual conidiospores. Original photograph by Wikipedia user Medmyco, CC BY‐SA 4.0 license. (E) Photograph of a ladybird infected with Hesperomyces virescens (Laboulbeniomycetes, Pezizomycotina), appearing here as light‐coloured digitiform structures (see arrow). Laboulbeniales are a diverse order of fungi associated with arthropod surfaces that present determinate growth and separate sexes. Original photograph by flickr user Gilles San Martin, CC BY‐SA 2.0 license. (F) Xanthoria parietina, a lichen, growing on a branch. In the picture, disk‐like structures can be observed sprouting prefentially in the centre of the formation. These correspond with the apothecia, support tissues containing the asci. Original photograph by Wikipedia user Marianne Perdomo, CC BY‐SA 2.0 license.
Table 4.
Main lineages within Basidiomycota. Due to changes in the scope of certain historical clades, references might not refer to the initial published description of a clade but to more recent bibliography
| Name | Main described lifestyles | Main traits | Representative genera |
|---|---|---|---|
|
Pucciniomycotina (Adl et al., 2012) |
Unicellular and filamentous; biotrophic plant pathogens, insect parasites, saprobes, endophytes and mycorrhizal | Karyogamy typically in probasidium or teliospore, followed by meiosis commonly in a separate compartment; simple septal pores occluded by a pore; presence of mannose and absence of xylose as cell wall component; centrosome multilayered | |
|
Tritirachiomycetes (Schell et al., 2011) |
Saprobes | Mycelial; uniperforate simple septa; conidiophores subhyaline to dematiaceous; teleomorph not known | Tritirachium |
|
Mixiomycetes (Adl et al., 2012) |
Biotrophic parasites of ferns | Multinucleated hyphae and multiple spores produced simultaneously in sporogeneous cells | Mixia |
|
Agaricostilbomycetes (Wang et al., 2015c) |
Saprotrophic yeast‐like or dimorphic, mycoparasites | Dimorphic; fucose as cell wall carbohydrate component, septal pores without associated microbodies, aseptate basidiospores during germination and no colacosomes, teliospores, curved holobasidia, and radiate conidia; nucleoplasmic spindle‐pole‐body separation, metaphasic spindle‐pole body intranuclear | Agaricostylbum, Chionosphaera, Ruinenia, Jianyunia, Kondoa |
|
Cystobasidiomycetes |
Mycoparasites, saprobes | Absence of fucose in cell wall; cytoplasmic spindle‐pole‐body separation; metaphasic spindle‐pole body intranuclear; presence of mycosporines | Cystobasidium, Bannoa, Erythrobasidium, Naohidea, Sakaguchia, Cyrenella |
|
Microbotryomycetes |
Biotrophic plant pathogens, yeast‐like | Presence of colacosomes and septal pores without microbodies; etaphasic spindle pole bodies intranuclear | Microbotryum, Heterogastridium, Mastigobasidium, Sporidiobolus |
|
Classiculomycetes (Adl et al., 2012) |
Aquatic, probably mycoparasite | Septal bodies associated with microbodies and tremelloid haustorial cells | Classicula, Jaculispora |
|
Cryptomycocolacomycetes (Adl et al., 2012) |
Mycoparasites | Colacosomes and septal pores with microbodies | Cryptomycocolax |
|
Atractiellomycetes |
Saprobes, mycorrhizae with orchids | Presence of symphlechosomes; filamentous; some genera form fruiting bodies | Atractiella, Saccoblastia, Helicogloea, Hobsonia |
|
Pucciniomycetes |
Biotrophic plant pathogens, insect parasites, mycoparasites | Metaphasic intermeiotic spindle‐pole‐body duplication | Puccinia, Septobasidium, Melampsora, Cronartium, Pachnocybe, Platygloea |
|
Spiculogloeomycetes (Wang et al., 2015c) |
Mycoparasitic, saprobe | Defined phylogenetically; teleomorphic members that may form tremelloid haustorial cells | Spiculogloea, Mycogloea, Sporobolomyces |
|
Ustilagomycotina (Adl et al., 2012) |
Unicellular and filamentous; saprobes or biotrophic plant pathogens | Mostly yeasts or dimorphic yeasts; glucose‐rich cell walls; simple septal pores | |
|
Malasseziomycetes (Wang et al., 2014) |
Lipophylic fungi; associated with vertebrate skin, commonly found in the environment | Cells are globose, ovoid or cylindrical; budding is typically monopolar, enteroblastic and percurrent; cell wall multilamellate, inner layer of the cell wall corrugated with a groove spiralling from the bud site; lipid dependent or lipophilic | Malassezia |
|
Exobasidiomycetes (Adl et al., 2012) |
Plant biotrophic pathogens, saprobes, animal parasites | Presence of interaction zones and no intracellular hyphal coils; probably paraphyletic | Ceraceosorus, Exobasidium, Doassansia, Entyloma |
|
Ustilaginomycetes (Adl et al., 2012) |
Biotrophic plant pathogens | Glucose as main cell wall carbohydrate, xylose absent; parenthesomes absent from septal pores; centrosomes globosed, unlayered | Ustilago, Urocystis, Floromyces, Tilletia |
|
Moniliellomycetes (Wang et al., 2014) |
Saprobes, some species are xerophilic | Sexual morph unknown; smooth or velvety colonies, greyish to olivaceous black; budding cells ellipsoidal; true hyphae disarticulate with arthroconidia;. pseudohyphae and chlamydospores may be present; multi‐lamellar cell wall; hyphal septa typically possess dolipores with an arch of endoplasmic reticulum, micropore‐like structures may also be present | Moniliella |
|
Agaricomycotina (Adl et al., 2012) |
Filamentous, yeasts or dimorphic yeasts; saprobes, plant parasites and ectomycorrhizal; also endophytes, mycoparasites, amoebophagous, symbionts and lichens | Many produce macroscopic fruiting bodies; pore septa with endoplasmic reticulum‐derived structures; xylose in cell wall; B type 5S rRNA | |
|
Agaricomycetes (Adl et al., 2012) |
Filamentous fungi; saprobes, plant parasites, mycorrhizal; also endophytes, mycoparasites, amoebophagous, symbionts and lichens | Often presenting macroscopic and complex fruiting bodies; commonly found as a dikaryon; many present lignin‐degrading capabilities | Amanita, Agaricus, Auricularia, Geastrum, Rhizoctonia, Trametes, Lentinula |
|
Dacrymycetes (Adl et al., 2012) |
Wood‐decaying saprobes | Gelatinous fruiting bodies; basidia furcate, rarely unisporous; parenthesomes unperforated | Dacryopinax, Cerinomyces |
|
Tremellomycetes (Adl et al., 2012) |
Saprobes, mycoparasites | Dimorphic, fruiting body gelatinous or absent; basidia septate or non‐septate; parenthesomes sacculate or absent | Tremella, Cryptococcus, Filobasidium, Mrakia |
|
Wallemiomycotina (Nguyen et al., 2013) syn. Wallemiomycetes, within Agaricomycotina |
Extremotolerant, filamentous or yeast‐like |
Basidiomata absent; basidiospores produced by some genera; arthroconidial or basauxic anamorphs are produced in some species |
Wallemia, Basidioascus, Geminibasidium |
|
Bartheletiomycetes (within Agaricomycotina) (Scheuer et al., 2008; Mishra et al., 2018) syn. Bartheletiomycotina |
Associated with fallen leaves of Ginkgo biloba | Septa with multiple plasmodesma‐like perforations; sexual reproduction by thick walled teliospores with longitudinally septated basidia | Bartheletia |
Table 5.
Main lineages within Ascomycota. Due to changes in the scope of certain historical clades, references might not refer to the initial published description of a clade but to more recent bibliography
| Name | Main described lifestyles | Main traits | Representative genera |
|---|---|---|---|
|
Taphrinomycotina (Adl et al., 2012) syn. Archiascomycetes (obsolete) |
Filamentous or yeast; plant pathogens, saprotrophs, endophytes, animal pathogens | Asci produced from binucleate cells; do not form croziers or interascal tissue | |
|
Taphrinomycetes (Adl et al., 2012) |
Biotrophic plant pathogens or saprotrophic yeasts | Poorly developed mycelium or yeast‐like; dikaryotic mycelium infective, develops directly into asci globose with eight spores; yeast‐like monokaryotic anamorph | Taphrina, Protomyces, Saitoella |
|
Archaeorhizomycetes (also known as Soil Clone Group I) (Rosling et al., 2011) |
Root endophytes; seems to have a large and unexplored diversity in soils | Filamentous, simple septa | Archaeorhyzomyces |
|
Schizosaccharomycetes (Adl et al., 2012) |
Saprotrophic yeasts on sugary substrates; sometimes dimorphic | Unicellular fungi dividing by fission; mycelium absent or poorly developed; sexual reproduction by fusion of two vegetative cells to form an ascus; karyogamy and meiosis inside the ascus to produce four nuclei, which might divide to form eight | Schizosaccharomyces |
|
Pneumocystomycetes (Adl et al., 2012) |
Obligate pulmonary extracellular parasite of mammals | Thin cell wall, irregular shape; sexual reproduction initiated by fusion of two cells followed by karyogamy and cyst wall formation; cholesterol as main membrane sterol | Pneumocystis |
|
Neolectomycetes |
Unclear; probably saprobe or root associated | Filamentous, forming stalked fruiting bodies; cylindrical asci formed from binucleate cells that undergo karyogamy, meiosis, and one mitotic division to produce eight cylindrical ascospores; septa with Woronin body | Neolecta |
|
Saccharomycotina (Adl et al., 2012) syn. Hemiascomycota (obsolete) |
Saprobes, commensals, extremotolerants and parasites; most are yeasts | Yeast‐like or poorly developed mycelium; sexual reproduction by fusion of vegetative cell; ascomata absent; asci separated by endomembranes; genome reduction; several groups have modifications in their genetic code | Saccharomyces, Candida, Yarrowia, Zygosaccharomyces, Pichia, Lipomyces |
|
Pezizomycotina (Adl et al., 2012) syn. Euascomycota (obsolete) |
Mostly filamentous; saprobes and lichens; also plant necrotrophic or biotrophic parasites, animal parasites, mycorrhizal, endophytes, amoebophagous and extremophiles | Filaments present septa with Woronin bodies; asci protected by multicelullular structures | |
|
Arthoniomycetes (Adl et al., 2012) |
Lichen forming or saprobes | Ascomata usually apothecial; interascal tissue of branched paraphysoids in a gel matrix; asci thick‐walled, fissitunicate | Arthonia, Melaspilea, Opegrapha, Roccella |
|
Coniocybomycetes (Prieto et al., 2013) |
Lichen forming |
Apothecia stalked, excipulum poorly to well developed, formed as a continuation of the stalk tissue; capitulum spherical to obconical; mazaedium present, brown to pale; asci cylindrical, ellipsoid, or irregular, dissolving at an early stage, forming from ascogenous hyphae with or without croziers, either singly or in chains; spores simple, spherical, or ellipsoidal, or rarely cylindrical with 1–5 septa, pale to brown, smooth or with a verrucose or cracked ornamentation |
Coniocybe, Sclerophora |
|
Dothideomycetes (Adl et al., 2012) |
Saprobes, extremotolerant black fungi, plant pathogens, occasionally lichen forming | Ascomata variable, formed lysigenously from stromatic tissue; asci cylindrical to saccate, thick‐walled, fissitunicate, rarely with apical structures; ascospores septate or muriform | Mycosphaerella, Cladosporium, Venturia, Holmiella, Botryosphaeria, Pseudogymnoascus |
|
Eurotiomycetes |
Saprobes, extremotolerant black fungi, animal parasites, plant pathogens, occasionally lichen forming | Morphologically diverse, delimited by phylogenetic criteria | Aspergillus, Penicillium, Capronia, Endocarpon, Onygena, Mycocalicium |
|
Geoglossomycetes (Adl et al., 2012) |
Saprobes | Fruiting bodies cylindrical, dark coloured, 2–8 cm long; septate ascospores, commonly pigmented | Geoglossum, Trichoglossum |
|
Laboulbeniomycetes (Adl et al., 2012) |
Ectoparasites of insects and other terrestrial or aquatic arthropods, mycoparasites | Mycelium absent or poorly developed; basal haustorium; ascomata perithecial, usually surrounded by complex appendages; ascospores two‐celled, elongated, one end adapted to attach to the host | Laboulbenia, Herpomyces, Pyxidophora, Ceratomyces, Cochliomyces |
|
Lecanoromycetes (Adl et al., 2012) |
Lichen forming, occasionally saprobes | Asci fissitunicate, thick‐walled, with thickened cap‐like appendage; septate ascospores | Acarospora, Cladonia, Candelaria, Graphis, Pertusaria |
|
Lichinomycetes (Adl et al., 2012) |
Lichen forming with cyanobacteria | Ascomata apothecial, setose and fleshy; asci simple, thin‐walled, usually surrounded by a gelatinous layer; thallus usually gelatinous | Lichina, Peltula, Eremithallus |
|
Leotiomycetes (Adl et al., 2012) |
Saprobes, plant pathogens, occasionally lichen forming | Ascomata apothecial, discoid, cleistothecial, elongated or absent, usually fleshy, commonly hairy or with appendages; thin‐walled peridium; asci typically inorpeculate, cylindrical, thin‐walled | Erysiphe, Botryotinia, Sclerotinia, Thelebolus, Leotia, Macroderma |
|
Orbiliomycetes (Adl et al., 2012) |
Filamentous saprobes; amoeba and nematode trapping | Ascomata apothecial, small, waxy, translucent or lightly pigmented; interascal tissue of simple paraphyses, usually with knob‐like apices, united by a matrix; many species form specialized trapping structures | Orbilia, Arthrobotrys, Dactylellina |
|
Pezizomycetes (Adl et al., 2012) |
Saprobes, ectomycorrhizal or biotrophic plant pathogens |
Ascomata apothecial or cleistothecial, usually visible with unaided eye, leathery or fleshy and often brightly pigmented; interascal tissue present; asci not fissitunicate, usually elongated, cylindrical, thin‐walled, without wall thickening or apical apparatus, with operculum or vertical slit, forcibly discharging ascospores; cleisthotecial species present globose asci, lack operculum or vertical slit and do not discharge ascospores; scospores usually ellipsoidal or globose, aseptate |
Peziza, Tuber, Pyronema, Ascobolus |
|
Sordariomycetes (Adl et al., 2012) |
Saprobes, plant pathogens, animal parasites, mycoparasites, endophytes, occasionally lichen forming | Morphologically diverse, delimited by phylogenetic criteria | Neurospora, Colletotrichum, Nectria, Cordyceps, Hypocrea, Bertia, Ceratocystis, Ophiostoma |
|
Xylonomycetes (Gazis et al., 2012) |
Endophytes, beetle‐associated symbionts |
Defined by phylogenetic criteria |
Xylona, Symbiotaphrina |
(1). Basidiomycota
Basidiomycota is the second most species‐rich phylum of Fungi with nearly 32,000 described species (Hibbett et al., 2007; Adl et al., 2012, 2018; Zhao et al., 2017), which present a wide array of lifestyles and cell‐organization strategies. Basidiomycota comprise the most complex fungi in terms of cell cycle (e.g. Puccinia) and multicellularity (e.g. the mushroom‐forming fungi, particularly the genus Armilliaria). The main characteristic of the group is the production of specialized club‐like cells called basidia, that usually produce four sexual spores (Hibbett et al., 2007; Adl et al., 2012; McLaughlin & Spatafora, 2014). Mating usually implies anastomosis between the two mating hyphae and formation of a dikaryon – hyphae with two stable populations of nuclei. The nuclei in the dikaryon remain in association before undergoing karyogamy and meoisis to produce the basidiospores. Hyphae, when present, are septate. In some of the groups, septa present dolipores – dome‐shaped modified endoplasmic reticulum (parenthesome). The formation of basidia is shared with the Entorhizomycota (see Section V.5). The phylum Basidiomycota contains at at least three well‐defined lineages: Pucciniomycotina, Ustilagomycotina, and Agaricomycotina. Most phylogenies recover Ustilaginomycotina as sister to Agaricomycotina, with Pucciniomycotina as the earliest diverging branch. This relationship remains debated as it seems to be greatly influenced by methodological biases (Prasanna et al., 2019). Wallemiomycotina is a recently recognized subphylum that includes a few genera (Wallemia, Basidioascus, Geminibasidium) of thermoresistant and xerotolerant moulds or yeast‐like fungi (Zalar et al., 2005; Padamsee et al., 2012; Zajc et al., 2013; Nasr et al., 2014). The ultrastructure of the septal pore in Wallemia is similar to members of Tremellomycetes (Zalar et al., 2005; Matheny et al., 2006; Zhao et al., 2017). The phylogenetic positioning of this clade is currently uncertain (Fig. 7). Some phylogenies have placed them as the earliest branching class (Wallemiomycetes) within Agaricomycotina (or, equally, as sister to Agaricomycotina) (Matheny et al., 2006; Padamsee et al., 2012; Nguyen, Nickerson, & Seifert, 2013; Zajc et al., 2013; Nguyen et al., 2015; Zhao et al., 2017; Mishra, Choi, & Thines, 2018). Other phylogenies place them as sister to the other three subphyla (Matheny et al., 2006; Wang et al., 2014; Zhao et al., 2017), with some studies reporting both topologies depending on the phylogenetic markers used, or as sister to Ustilaginomycotina (Bauer et al., 2015; Tedersoo et al., 2018). The largest differences stem from the use of ribosomal markers versus protein‐coding genes, with genome‐wide data sets preferentially supporting a sister relationship with Agaricomycotina. We here use the subphylum category, as it is compatible with either phylogenetic hypothesis. The three genera form two deeply divergent clades classified as distinct orders (McLaughlin & Spatafora, 2014) or classes (Nguyen et al., 2013), with Wallemia as sister to Basidioascus + Geminibasidium. Finally, Bartheletia paradoxa is an enigmatic filamentous fungus with a unique septal structure, consisting of multiple plasmodema‐like perforations, that lives in association with fallen leaves of Ginkgo biloba (Scheuer et al., 2008). Recent phylogenomic analyses place Bartheletia as sister to Agaricomycotina + Wallemiomycotina (Mishra et al., 2018). The latter authors suggest retaining Wallemiomycetes within the subphylum Agaricomycotina, and raising a new class Bartheletiomycetes to include Bartheletia (Mishra et al., 2018) in that subphylum.
Figure 7.
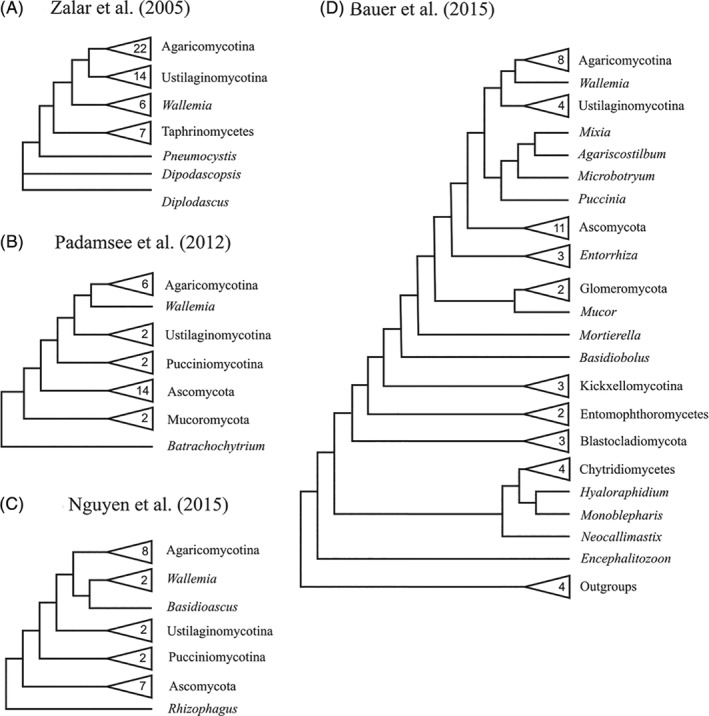
Phylogenetic position of Wallemia in different studies. Simplified topology from several phylogenetic studies covering the phylogenetic position of Wallemiomycetes. Numbers inside triangles represent the number of sampled species within the clade. (A) Topology extracted from Zalar et al. (2005). Phylogeny constructed using a maximum‐parsimony approach. (B) Topology extracted from Padamsee et al. (2012). Phylogeny constructed from a data set of 71 protein‐coding genes, using a Bayesian inference approach. (C) Topology extracted from Nguyen et al. (2015). Pylogeny constructed from a data set of 35 single‐copy protein‐coding genes, using a Bayesian inference approach. (D) Topology extracted from Bauer et al. (2015). Phylogeny constructed from a concatenation of 18S rRNA, 28S rRNA, 5.8S rRNA, RPB1 and RPB2, using a combination of Bayesian inference, maximum‐likelihood and maximum‐parsimony approaches.
(a). Pucciniomycotina
There are more than 8,400 described species of Pucciniomycotina, classified in 10 classes (Agaricostilbomycetes, Atractiellomycetes, Classiculomycetes, Cryptomycocolacomycetes, Cystobasidiomycetes, Microbotryomycetes, Mixiomycetes, Pucciniomycetes, Spiculogloeomycetes and Tritirachiomycetes), 20 orders and 35 families (Aime et al., 2006; Schell, Lee, & Aime, 2011; Wang et al., 2015b, c ). Some members of the group, particularly in the Pucciniomycetes, have very large genomes (Tavares et al., 2014) and several have highly complex life cycles (e.g. Puccinia) involving several hosts and free‐living stages. Septal pores are simple, without dolipores. The cell wall contains mannose, but lacks xylose (Aime, Toome, & McLaughlin, 2014). Pucciniomycotina is generally considered the earliest‐splitting lineage within the Basidiomycota, and consistent with this view they share some characteristics in their cell division and spindle‐pole‐body formation with the Ascomycota. Woronin body‐like structures have been described in Agaricostilbomycetes and Cryptomycocolacomycetes (Aime et al., 2014). Pucciniomycotina contains a widely diverse clade of mostly biotrophic plant pathogens called rusts (class Pucciniomycetes), as well as some free‐living species that usually grow as a saprotrophic yeasts. Mycoparasites, insect pathogens and mycorrhizae are also described (Aime et al., 2014). The relationship between the different classes of Pucciniomycotina is poorly resolved, with different phylogenetic studies producing highly contradictory results (Aime et al., 2006; Schell et al., 2011; Wang et al., 2015c; Zhao et al., 2017) (Fig. 8). Environmental studies suggest the existence of several undescribed major clades within the subphylum (Tedersoo et al., 2017), and taxonomists are fully aware that the lack of phenotypic characteristics of many of these fungi hides a wide diversity of cryptic species.
Figure 8.
Phylogenetic relationships among the different clades within Pucciniomycotina in different studies. Numbers inside triangles represent the number of sampled species within the clade. (A) Topology extracted from Aime et al. (2006). Phylogeny reconstructed from LSU rRNA and SSU rRNA genes, using a maximum‐parsimony approach. (B) Topology extracted from Wang et al. (2015c). Phylogeny reconstructed from a concatenation of SSU rRNA and LSU rRNA D1/D2, using a maximum‐likelihood approach. (C) Topology extracted from Schell et al. (2011). Phylogeny constructed from a concatenation of EF1‐α, LSU rRNA and SSU rRNA genes, using a maximum‐parsimony approach. (D) Topology extracted from Zhao et al. (2017). Phylogeny reconstructed from a concatenation of LSU rRNA, SSU rRNA, 5.8S rRNA, TEF1, RPB1 and RPB2, using a maximum‐likelihood approach.
(b). Ustilaginomycotina
Ustilaginomycotina comprises around 1,700 species of mostly anamorphic or dimorphic yeasts. The majority of described species are plant pathogens, typically biotrophic (smuts), while others live as saprotrophic free‐living yeasts or animal pathogens (e.g. Malassezia). Plant pathogens usually have asexual yeast states, often with saprobic capabilities, and an infecting dikaryotic mycelial stage (Begerow et al., 2014). For most of the clades with only yeast forms, a sexual cycle has never been described (Begerow et al., 2014; Wang et al., 2015a). Septa are poreless and present a membrane cap or a true dolipore in some species of Moniliella (Moniliellomycetes) (Wang et al., 2014). The group includes four classes: Ustilaginomycetes, Exobasidiomycetes, Malasseziomycetes and Moniliellomycetes, that span 10 orders, plus several incertae sedis genera (Begerow et al., 2014). Some studies have raised doubts regarding the monophyly of Exobasidiomycetes (Hibbett et al., 2007; Begerow et al., 2014; Wang et al., 2014), but so far they have been considered premature as grounds to reshape the taxonomy of the class.
(2). Agaricomycotina
Agaricomycotina is the largest group of Basidiomycota, containing around two‐thirds of all described Basidiomycota. The subphylum includes three classes: Tremellomycetes, Dacrymycetes and Agaricomycetes (Hibbett et al., 2007; Adl et al., 2012, 2018), with Tremellomycetes the earliest‐diverging clade. The Tremellomycetes include yeast species that can be dimorphic (e.g. Cryptococcus, Tremella, Cystofilobasidium), often forming macroscopic gelatinous fruiting bodies, although some species without a yeast stage are also known (Hibbett, 2006; Hibbett et al., 2007; Adl et al., 2012). Many species are mycoparasitic, living inside the fruiting bodies of other fungi and infecting them through a particular type of haustoria (tremelloid haustoria) (McLaughlin & Spatafora, 2014; Liu et al., 2015). Others are free‐living yeasts or animal pathogens. They present dolipores with endoplasmic reticulum projections (McLaughlin & Spatafora, 2014; Liu et al., 2015). Five orders are recognized within the Tremellomycetes (McLaughlin & Spatafora, 2014). Many important genera in this group (e.g. Cryptococcus, Tremella) are paraphyletic (Liu et al., 2015). The taxonomy of the group is currently highly volatile and many changes are expected in the near future.
The Dacrymycetes include a small group of wood‐decaying fungi that produce gelatinous and usually highly pigmented fruting bodies. They are characterized by a unique basidial morphology (Hibbett, 2006; Shirouzu et al., 2013, 2016). Shirouzu et al. (2013) described a second order, Unilacrymales, although its monophyly is questioned (Zhao et al., 2017).
Agaricomycetes is by far the largest and most diverse class within the Agaricomycotina. The class comprises 22 orders and over 21,000 species (Shirouzu et al., 2013; McLaughlin & Spatafora, 2014). Agaricomycetes possess dolipores, a trait shared with the Dacrymycetes (van Driel et al., 2008, 2009; McLaughlin & Spatafora, 2014). The presence and structure of parenthesomes help define taxonomic groups. Mating‐type loci systems can reach extreme levels of sophistication, with many species of mushrooms harbouring thousands of intercompatible mating types (Kothe, 1996; Brown & Casselton, 2001; Raudaskoski & Kothe, 2010; Ni et al., 2011; Coelho et al., 2017). Many produce a high diversity of fruiting‐body types whose morphologies have had traditional taxonomic value. Secondary metabolites are highly diverse in this group (Wisecaver, Slot, & Rokas, 2014; Wisecaver & Rokas, 2015).
Most agaricomycetes live as saprotrophs, plant pathogens or ectomycorrizae (McLaughlin & Spatafora, 2014; de Mattos‐Shipley et al., 2016). As for saprotrophs, many species are wood decaying, and are broadly classified as brown or white rots. White‐rot fungi have developed a wide array of enzymes that allow them to degrade lignin, a metabolic feat unique to this group in the whole biosphere (Martínez et al., 2005; Dashtban et al., 2010; Floudas et al., 2012; Sigoillot et al., 2012; Riley et al., 2014). Beyond these lifestyles, there are also nematode‐trapping species (e.g. Coprinus, Pleurotus), insect symbionts (e.g. Termitomyces), lichens (e.g. Dyctionema), lichen‐associated forms (e.g. Burgella), endophytes (e.g. Piriformospora), mycoparasites (e.g. Pseudoboletus) (McLaughlin & Spatafora, 2014) and amoebophagous species (e.g. Pagidospora, Tulasnella) (Duddington, 1956; Corsaro et al., 2017). The phylogenetic backbone of Agaricomycetes is well resolved, at least to the order level (Hibbett, 2006; Zhao et al., 2008, 2017). However, morphology‐based identification of fruiting bodies hides a poorly explored pool of cryptospecies. Many species are discovered every year in undersampled areas and environmental studies (Zhao et al., 2017), revealing an even greater hidden diversity of Agaricomycetes, particularly within early‐diverging orders.
(3). Ascomycota
Ascomycota is the largest fungal phylum comprising roughly two‐thirds of all described species (Lutzoni et al., 2004; Schoch et al., 2009; McLaughlin & Spatafora, 2015). The diversity of this group, combined with relative ease of experimental manipulation, has made Ascomycota the ‘default’ fungi. Certain species (e.g. Saccharomyces cerevisiae, Neurospora crassa, Emericella nidulans, Schizosaccharomyces pombe) have been used extensively as model organisms, for which extensive genetic studies have been performed and which have served to drive breakthrough discoveries in the field of biology. Mating induces the formation of dikaryon hyphae that, unlike in Basidiomycota, are normally very short lived. The dikaryon hypha leads to the formation of the ascus, a sac‐like structure that contains the (usually eight) meiosis‐derived spores. Asexual spores or other means of asexual propagation are very common, and sexual stages are unknown for many members of the phylum. Ascomycota range from simple yeasts to fungi with highly complex macroscopic fruiting bodies. The phylum contains three main classes: Taphrinomycotina, Saccharomycotina and Pezizomycotina (Stajich et al., 2009; Spatafora et al., 2017b), with Saccharomycotina and Pezizomycotina being sister lineages. While environmental studies suggest the existence of a large amount of unknown diversity, all clades up to the class level have cultured or morphologically characterized reresentatives (Hibbett et al., 2007; Adl et al., 2012, 2018).
(a). Taphrinomycotina
The Taphrinomycotina (previously known as Archiascomycota) is a species‐poor but physiologically diverse group of Ascomycota. It currently includes several genera spread over five classes: Taphrinomycetes includes eight genera of biotrophic plant pathogens (Taphrina, Protomyces) and saprotrophic yeasts (Saitoella) (Sugiyama, Hosaka, & Suh, 2006; Liu et al., 2008b; Adl et al., 2012; Spatafora et al., 2017b); Neolectomycetes includes fruiting‐body‐forming species (Neolecta) of uncertain lifestyle and multicellular structures that have arisen independently to those found in Pezizomycotina or Agaricomycotina (Landvik et al., 2003; Healy et al., 2013; Nguyen et al., 2017); Schizosaccharomycetes contain a single genus of saprotrophic yeasts (Schizosaccharomyces) that include the fission yeast S. pombe, an important model organism (Rhind et al., 2011; Kurtzman & Sugiyama, 2015); Pneumocystidomycetes contain a genus of biotrophic lung parasites of mammals (Pneumocystis) (Sugiyama et al., 2006; Hauser et al., 2010; Porollo et al., 2014); and Archaeorhizomycetes, with only two cultivated species of filamentous root endophytes in the genus Archaeorhizomyces (Rosling et al., 2011; Menkis et al., 2014). Archaeorhizomycetes was first described based on environmental sequences as a cosmopolitan and diverse clade of soil fungi termed the Soil Clone Group I (Porter et al., 2008). Analyses in soil suggest they are at relatively low abundance and are probably associated with the rhizosphere (Porter et al., 2008; Rosling et al., 2011; Menkis et al., 2014). At least one member of each described class of Taphrinomycotina has been sequenced, and so far all possess reduced and compact genomes. The monophyly of the group has been a matter of intense debate (Hibbett et al., 2007; Liu et al., 2008b; Ebersberger et al., 2012; Menkis et al., 2014; Ren et al., 2016) mostly due to incongruences between mitochondrial and nuclear phylogenies and usually an unstable phylogenetic position of Schizosaccharomyces caused by its long branches. While the monophyly of Taphrinomycotina is well supported, the relationships among the different classes within the subphylum are still subject to debate (Liu et al., 2008b; Ebersberger et al., 2012; Kurtzman & Sugiyama, 2015). The only class within the group that is not monotypic, the Taphrinomycetes, might not be monophyletic, as the exact affiliation of Saitoella remains elusive.
(b). Saccharomycotina
Nearly all members of Saccharomycotina (previously known as Hemiascomycota) grow as yeasts, although many can switch to a filamentous form with varying degrees of complexity. Asci are typically formed inside the mother cell, surrounded by simple membranes (Hibbett et al., 2007; Adl et al., 2012, 2018). Many members of the Ascoideaceae, Cephaloascaceae, Endomycetaceae and Saccharomycetaceae are always filamentous (Deák & Péter, 2013). Filamentous forms contain septa with multiple micropores. Their genomes are highly streamlined and gene rich showing extensive gene loss and a great reduction of transposable elements and introns (Dujon et al., 2004; Dujon, 2010; Dujon & Louis, 2017). Most lineages present only one ribosomal DNA (rDNA) locus that contain tens or hundreds of copies in tandem and which usually contains the 5S rRNA loci (Proux‐Wéra, Byrne, & Wolfe, 2013; Dujon & Louis, 2017). Several lineages have deviations from the canonical genetic code that affect sense codons (Sugita & Nakase, 1999; Mühlhausen & Kollmar, 2014; Mühlhausen et al., 2016). Sex is well studied within this group, and many examples of hybrids exist in nature and under laboratory conditions (Morales & Dujon, 2012; Ga et al., 2013; Hittinger et al., 2015a; Mixão & Gabaldón, 2018).
Saccharomycotina is, by far, the best represented eukaryotic lineage in terms of genomic information. Saccharomyces cerevisiae was the first sequenced eukaryote (Goffeau et al., 1996) and about 10% of the described Saccharomycotina have now been sequenced (Dujon & Louis, 2017). In stark contrast with our understanding of their physiology, biochemistry, genetics and evolution, our knowledge about the ecology of this group is fairly limited (Kurtzman & Sugiyama, 2015; Treseder & Lennon, 2015; Hittinger et al., 2015a; Shen et al., 2016; Dujon & Louis, 2017). Many species seem to associate with certain microniches, such as animal mucosae, animal gut, flowers, fruits or trees, while others are bona fide plant pathogens (e.g. Eremothecium). Several species are adapted to extreme environments, including growth at high osmotic pressure, high temperature, high carbon dioxide concentration, in the presence of toxic compounds or on unusual carbon sources. Most species are unable to exploit complex polysaccarides, and metabolic clusters for the production of complex secondary metabolites (e.g. alkaloids, polyketides or non‐ribosomal peptides) are practically non‐existent. Genomic data in the last two decades has allowed us to tackle the problematic phylogeny and taxonomy of yeasts, which are devoid of phenotypic traits (Dujon et al., 2004; Wolfe, 2006; Dujon, 2010; Mühlhausen & Kollmar, 2014; Hittinger et al., 2015b; Shen et al., 2016; Dujon & Louis, 2017). This has proved the paraphyly of certain important genera (e.g. Candida, Pichia), currently undergoing nomenclatural redefinition from established names with decades of usage to newer ones based on phylogenetic evidence. Currently, Saccharomycotina is circumscribed into a single class and order (Saccharomycetes, Saccharomycetales), spanning 14 families and around 1500 species (Kurtzman, Fell, & Boekhout, 2011).
(c). Pezizomycotina
Pezizomycotina (previously known as Euascomycota) is the most diverse subphylum of Ascomycota. The basic body plan of this subphylum is filamentous and anastomosed, with septa that present a peroxisome‐derived electrodense organelle called the Woronin body (Liu et al., 2008a; Adl et al., 2012; Healy et al., 2013). Asci are typically protected and supported by multicellular structures named ascocarps or ascomata. Some members are unicellular. Comparative genomics has revealed a unique mode of genome evolution termed mesosynteny, in which genomic regions mantain a conserved gene content without conservation of the gene order. Mesosynteny has only been described in pezizomycotina, and seems to be particularly strong in members of the Dothideomycetes (Hane et al., 2011). Compared to other fungi, they tend to contain a high abundance of enzymes for secondary metabolism (Wisecaver et al., 2014; Wisecaver & Rokas, 2015). Pezizomycotina are currently circumscribed into 67 orders in 13 classes (Hibbett et al., 2007; Adl et al., 2012, 2018; Gazis et al., 2012; Spatafora et al., 2017b): Arthoniomycetes, Coniocybomycetes, Dothideomycetes, Eurotiomycetes, Geoglossomycetes, Laboulbeniomycetes, Lecanoromycetes, Leothiomycetes, Lichinomycetes, Orbiliomycetes, Pezizomycetes, Sordariomycetes and Xylonomycetes. An important fraction of Pezizomycotina remains unclassified. For instance, the Catalogue of Life (Bisby & Rosko, 2010, accession date June 2019) contains more than 5000 species whose affiliation to any of these classes is unknown, many not even assigned to a family level.
Their living strategies vary wildly, but special mention must be made of lichenized Pezizomycotina. Approximately 40% of the group are lichens and around 98% of lichens are Pezizomycotina. Since lichens form macroscopic thalli with well‐defined morphological traits, the amount of lichen hidden diversity is probably lower than for other types of Ascomycota. A lichenic lifestyle appears in six classes (Grube & Wedin, 2016), and for the classes Arthoniomycetes, Coniocybomycetes, Lichinomycetes and Lecanoromycetes only lichenic species are known. Most non‐lichenic members of the Pezizomycotina have saprobic capabilities to a certain degree, although more‐specialized facultative lifestyles are common. Many are mycorrhizal, plant pathogens, endophytes, animal parasites and symbionts, mycoparasites, amoebophagous, endolichenic or endolythic (Stajich et al., 2009; Corsaro et al., 2017; Spatafora et al., 2017b).
V. Fungi incertae sedis
We here discuss some groups whose classification, even to the level of broad taxonomic affiliation, remains elusive. See Table 6 for a list of these groups.
Table 6.
Fungi incertae sedis and environmental taxa. Due to changes in the scope of certain historical clades, references might not refer to the initial published description of a clade but to more recent bibliography
| Name | Main described lifestyles | Main traits | Representative genera | Main phylogenetic hypotheses |
|---|---|---|---|---|
|
NCLC1 (also known as Basal Clone Group 1) |
Unknown, detected in marine environments | Unknown | None described | Probable sister lineage to Rozellidea + Microsporidia |
|
Basal Clone Group 2 (also known as GS01) (Monchy et al., 2011; Tedersoo et al., 2017, 2018; Bass et al., 2018) |
Unknown, detected in soil and freshwater environments | Unknown | None described | Probable sister lineage to all Fungi |
|
Namako‐37 |
Unknown, first detected in anoxic sediments from a lake | Unknown | None described | Distinct lineage within Rozellidea or Microsporidia; branches closer to traditional Microsporidia than to Rozella; might rise as a novel high‐level taxon after revision of Opisthosporidian taxonomy |
|
Nephridiophagida (Lange, 1993) |
Extracellular parasites in the nephridia of certain arthropods | Multicellular plasmodia; endocytic germination with nuclear functional differentiation | Nephridiophaga | Fungi incertae sedis; all evidence suggests they are members of Eumycota |
|
Amoeboradix + Sanchytrium (Karpov et al., 2018) |
Algal parasitoids | Amoeboid zoospores; large kinetosome | Amoeboradix, Sanchytrium | Fungi incertae sedis |
|
Olpidiaceae (Tedersoo et al., 2018) |
Biotrophic plant pathogens | Zoosporic, single flagellum; thallus monocentric, holocarpic or eucarpic; two parallel centrioles linked to nucleus by shared, tapering, striated rhizoplast; porangium single, endobiotic | Olpidium, Cibdelia | Independent lineage of zoosporic fungi; early phylogenies clustered them with Zoopagomycota; most modern phylogenies recover them as sister to terrestrial fungi |
|
GS19 (Tedersoo et al., 2017) |
Unknown, detected from soil | Unknown | None described | Falls within Zoopagomycota, either as a novel lineage or as an unexplored lineage within Kickxellomycotina |
|
Caulochytrium |
Mycoparasite | Presence of aerial sporangia; monocentric thallus and eucarpic, presence of rhizoids | Caulochytrium | Probably related to Olpidium |
|
Nothadelphia (Degawa & Gams, 2004) |
Mycoparasite | Haustorial parasite; mycelium irregularly septated and scarcely branched; absence of collumella; sporangia leave a minute collarette after dequiescence | Nothadelphia | Proposed as a member of the Mortierellomycotina; no phylogenetic studies are available |
|
Calcarisporiella + Echinochlamydosporium (Tedersoo et al., 2018) |
Saprobes, nematophagous | Thallus branched and septate; thin‐walled hyphae; simple sporangiophores; globose, spiny chlamydospores borne laterally on short hyphae | Calcarisporiella, Echinochlamydosporium | Distinct lineage branching sister to Mucoromycotina; formerly classified as members of Mortierellomycotina; Tedersoo et al. (2018) elevated them to the subphylum Calcarisporiellomycotina |
|
Densosporaceae |
Ectomycorrhizal |
Sporocarps hypogeal, with numerous small blastospores; blastospores globose, terminal or intercalary, developed on thin hyphae, with the lumen of the hyphal appendages completely occluded; in some mature blastospores, wall irregularly thickened and lumen deformed; blastospores with thickened walls often deformed and contents viscid |
Densospora, Sphaerocreas | Morphological characteristics suggest ambiguous affiliation to either Mucoromycotina or Glomeromycotina; Desirò et al. (2017) recovered it as an independent lineage related to Endogonales |
|
Bifiguratus (Torres‐Cruz et al., 2017) |
Soilborne or endophyte in mosses; living in association with a wide array of bacteria; dimorphic | Coenocytic hyphae with ornamentations; intercalary and terminal chlamydospores; no sporangia observed to date | Bifiguratus | Torres‐Cruz et al. (2017) recovered it as an early‐splitting lineage within Mucoromycotina |
|
Entorrhizomycota (Bauer et al., 2015) syn. Entorrhizomycotina within Basidiomycota |
Root‐associated endophytes | Presence of basidia; forms intracellular septate hyphal coils | Entorrhiza, Talbotiomyces | Bauer et al. (2015) recovered it as sister to the rest of Basidiomycota or the rest of Dikarya and proposed elevation to phylum Entorhizomycota; Zhao et al. (2017) and Tedersoo et al. (2018) recovered it as sister to Basidiomycota and retained the phylum level |
(1). Early‐splitting environmental lineages
The use of metabarcoding approaches, mostly using ribosomal RNA (rRNA) genes, has revealed the existence of several deep branches in the fungal tree of life for which we still do not have any cultured representative. Enviromental studies have identified two early‐diverging and relatively diverse clades of fungi termed the Basal Clone Group 1 (BCG1) (Nagahama et al., 2011; Tedersoo et al., 2018) and BCG2 (Monchy et al., 2011; Tedersoo et al., 2017, 2018; Bass et al., 2018). BCG1 is a marine clade that appears related to Rozellidea, and thus it could be considered a novel lineage within the Opisthosporidia. BCG2 on the other hand, has been detected from soils and fresh water, and might be the sister group to all non‐Opisthosporidia Fungi. Finally, the environmental clade Namako‐37 is an unexplored lineage related to Rozellidea and Microsporidia (Takishita et al., 2007; Bass et al., 2018). The identification and study of these groups would provide invaluable information about the origin and early evolution of Fungi.
(2). Nephridiophagida
Nephridiophagida is an enigmatic and understudied group of intracellular parasites historically classified as related to Haplosporidia or Microsporidia, to the point that some species were originally described within microsporidian genera (Radek et al., 2017). To date, members of this group have been only described as extracellular parasites associated with the Malpighian tubules of some insects and the millipede Xenobolus. Four genera are currently described: Nephridiophaga, Coelosporidium, Oryctospora and Peltomyces (Lange, 1993; Radek et al., 2017). Nephridiophaga periplanetae has been described as a multinucleated mitochondriated amoeboid stage with the ability to attach to microvilli of the host cell and with endocytic germination with somatic and germinative differentiation of nuclei (Lange, 1993; Radek et al., 2017). The first phylogenetic analysis of this group based on the 18S rRNA gene placed them within zygomycetous fungi, and they have been proposed to be related to Dimargaritales or Harpellales (Wylezich, Radek, & Schlegel, 2004). A second analysis using small subunit (SSU) rRNA genes returned an affiliation with Chytridiomycota, albeit with low support (Radek et al., 2017).
(3). Zoosporic fungi incertae sedis
The phylogenetic positioning of several chytrid‐like lineages is highly debated. Olpidium is a morphologically reduced obligate biotrophic plant pathogen (Powell & Letcher, 2014), which makes it relatively difficult to study. Its lifestyle has likely shaped its genome, making it susceptible to phylogenetic artefacts (James et al., 2006b; Sekimoto et al., 2011). Caulochytrium is an enigmatic parasite of other fungi that presents aerial sporangia, a unique trait within the Chytridiomycota (Olive, 1980). Its phylogenetic position has not been studied, although a relationship with Olpidium has been proposed based on the structural characteristics of their zoospores. The algal parasites Sanchytrium and Amoeboradix were recovered as a divergent lineage based on ribosomal rRNA phylogenies (Karpov et al., 2017a, 2018). These two organisms are united by the presence of amoeboid zoospores. Several groups of uncultured chytrids have been described from environmental studies that seem to stem from different points of the chytrid tree of life (Richards et al., 2015; Tedersoo et al., 2017). Of particular interest are soil clades GS17 and GS18, which are related to Olpidium. The phylogenetic peculiarities of Olpidium and several related plant‐pathogenic genera have led to the recent creation of a new phylum Olpidiomycota to accommodate them (Tedersoo et al., 2018). Future phylogenetic work should aim to address the relationship of Olpidium with other fungi in order to establish the position and boundaries of this nascent phylum.
(4). Zygomycetous fungi incertae sedis
A few zygomycetous fungi currently hold an uncertain phylogenetic affinity, particularly in the Mucoromycotina, Mortierellomycotina and Glomeromycota. Recent phylogenetic studies based on 18S rRNA place the genus Sphaerocreas as a sister branch to a group of uncultured symbionts of liverworts, forming a distinct lineage within Mucoromycotina (Hirose et al., 2014; Benny et al., 2016). Densospora (McGee, 1996) is an ectomycorrhizal fungus isolated from Australia that contains several morphological traits that suggest ambiguous affiliation to either Endogonales or Glomeromycota (Gleason & McGee, 2004; Desirò et al., 2017). A multigene phylogeny recovered an affiliation between Sphaerocreas and Densospora – a relationship that should further be explored in the near future (Desirò et al., 2017). Bifiguratus is a recently described fungus with an endophytic and saprotrophic lifestyle that appears as a distinct and early‐splitting lineage within the Mucoromycotina (Torres‐Cruz et al., 2017). Calcarisporiella was traditionally classified as an Ascomycota, and was recently shown to form a distinct lineage in an uncertain position within Mucoromycota (Hirose et al., 2012), and related to Echinochlamydosporium (Hirose et al., 2014). Due to the position of these lineages, it is very likely that mycologists will erect higher‐level taxonomic categories to accommodate them once their phylogenetic position has been determined with precision. Finally, Nothadelphia is an unplaced biotrophic parasite of Mortierella (Degawa & Gams, 2004). The description of Nothadelphia suggest similarities with mycotrophic chytrids, but the authors classified it as Zygomycota incertae sedis due to its apparent inability to form zoospores, suggesting a possible affiliation with Mortierellomycotina.
(5). Entorhizomycota
The genus Entorrhiza contains about a dozen species that are associated with the roots of members of Cyperaceae and Juncaceae (Bauer et al., 2015; Riess et al., 2015). Talbotiomyces is another root endophyte isolated as root galls in several families of Caryophyllales (Vánky, Bauer, & Begerow, 2007). The phylogenetic placement of Entorrhiza has been historically controversial, being variously placed as a distinct lineage of Ustilagomycotina (Bauer, Oberwinkler, & Vánky, 1997; Begerow, Stoll, & Bauer, 2006) or as a member of Tremellomycetes or Pucciniomycotina (Vánky et al., 2007). Recent phylogenetic analyses of Entorrhiza placed it as either sister to the rest of Basidiomycota or as sister to the rest of Dikarya, for which the authors proposed elevation to phylum level (Bauer et al., 2015); while a six‐gene phylogeny recovered them with high support as the sister branch to all Basidiomycota (Zhao et al., 2017). The classical relationship between Talbotiomyces and Entorrhiza based on morphological traits (Vánky et al., 2007) was recently confirmed by molecular phylogenies, and the order Talbotiomycetales was proposed (Riess et al., 2015). Environmental studies do not suggest the existence of a wide undescribed diversity of Entorrhizomycota, but novel genera associated with the roots of unsampled plants are to be expected (Tedersoo et al., 2017).
VI. Concluding remarks
The Fungi is a fascinating group of organisms entailing a vast diversity that have important roles in virtually all ecosystems. The answer to the question ‘what is a fungus’ does not have a simple answer.
Fungi started as unicellular, flagellated, eukaryvorous organisms. This lifestyle is still prevalent in the zoosporic lineages and implies a series of traits that are ancestral to the Kingdom. Elucidating the phylogenetic relationships among the zoosporic lineages and describing the ‘fungal dark matter’ is essential for our understanding of this group in a broad sense. The morphological and genomic reduction associated with a parasitic lifestyle makes it even more difficult to reconstruct the ancestral state of the different fungal lineages, or to find common features among the modern lineages. Most well‐studied fungi are terrestrial. This group represents a highly derived lifestyle that has been incredibly successful. The large number of described species in this lineage, together with their prevalence in terrestrial environments and the possession of well‐defined, widespread and ancestral morphological and biochemical traits has spawned a thriving academic community with more than a century of history. This in turn has led to the biased view that considers terrestrial fungi as representing the canonical fungal identity, while zoosporic lineages are regarded as evolutionary stepping stones towards that presumed ideal. While no serious evolutionary biologist will recognize this view as scientifically valid, it still permeates and conditions our collective view of the Fungi. The search for synapomorphies to unify the Fungi has failed (Richards et al., 2017) and we must get used to this lack of hard taxonomic boundaries. More important, in our view, is to understand what evolutionary transitions define the main fungal lineages and how they relate to their particular adaptations.
The phylogenetic backbone of fungi has steadily grown more and more solid as genomic information has accumulated during the last decade. Such information has confirmed the monophyly of the Ascomycota and Basidiomycota and the delimitations of their main subphyla, recognized the phylogenetic identity of the Blastocladiomycota, and turned the disorganized assemblage of orders that were the ‘Zygomycota’ into a defined set of phylogenetic lineages. From single‐gene‐based phylogenetic approaches we have moved to full phylogenomic studies that have helped to resolve some of the most intractable evolutionary puzzles in the group, such as the placement of Microsporidia and the relationships among the different clades within the zygomycetous fungi. Environmental sequencing has provided us with new and exciting prospects in fungal taxonomy, with the description of the Cryptomycota and the Archaeorrhizomycetes as two highly diverse and virtually uncultured taxa with an important presence in the environment. Such studies suggest that most major lineages of Fungi contain species described through traditional means (Tedersoo et al., 2017). However, this does not imply that we possess an adequate understanding of fungal diversity, which remains very unevenly explored and is reflected in the taxonomy of the different clades that form the kingdom. For instance, information on sequence divergence of different species of Schizosaccharomyces (Taphrinomycotina) (Rhind et al., 2011; Naumov, Kondratieva, & Naumova, 2015) or Rozella (Rozellomycota) (Gleason et al., 2012) should allow us to split those genera, likely raising new higher taxonomic ranks. However, in the absence of information regarding existing genetic and physiological diversity within these groups, a proper taxonomic revision is still not possible. This is particularly urgent for Schizosaccharomyces, given its wide use as an experimental model.
We live in a time of transition for fungal taxonomy, as we continue to characterize new fungal diversity but struggle to provide a coherent and unified framework. The next few decades will bring an avalanche of information from several early‐diverging lineages, data that might imply a re‐evaluation of at least some of the main groups discussed herein. Such is the case for the Microsporidia and Rozellidea, which very likely will be merged, for uncultured deep‐branching lineages of Chytridiomycota and other zoosporic fungi, such as Olpidium and associated sequences or novel environmental lineages, for which we still lack even a basic phylogeny, for the GS19 clade within the Zoopagomycota, for the Endogonales and the genera Densospora, Nothadelphia, Calcarisporiella and Echinochlamydosporium within the boundaries of Mucoromycotina, Mortierellomycotina and Glomeromycota, and for the genera Entorrhiza and Talbotiomyces at the base of Basidiomycota. The placement of some other groups is yet to be investigated, including BCG1 and BCG2, the Nephridiophagida, and the Meristacraceae. Solving these relationships satisfactorily will surely produce great taxonomic revisions. In this regard, a recent work (Tedersoo et al., 2018) proposed the use of a diverged‐time‐based approach to fungal taxonomy and, together with rRNA phylogenetics, supports a radical nomenclatural shift that we must reject at present, at least in broad terms. While we understand and value their proposed strategy, we advise caution regarding the robustness of their phylogenetic results and consider that several of their new taxonomic proposals introduce unnecessary changes in otherwise well‐established taxa. Because of this, we herein adopted a more orthodox and conservative taxonomic approach, prioritizing the use of older names in cases where the phylogenetic delineation of the taxa is volatile and attempting to use naming conventions that are consistent with the main phylogenetically conflicting topologies when possible. Environmental sampling is also increasing our understanding of the sister groups of Fungi, holding the promise of expanding our knowledge of the evolution of not just Fungi, but of Opisthokonta in particular and of Eukaryotes in general.
VII. Conclusions
Solving the phylogenetic relationships among the zoosporic fungi is an on‐going challenge. Genomic sampling in these lineages is still poor, many are plagued with long branches due to their parasitic lifestyle, and environmental sequences suggest the existence of a large unsampled diversity within these groups.
By contrast, recent studies have produce great advances in our knowledge regarding the relationships among the main lineages of zygomycetous fungi. However, several lineages are still to be placed. In particular, the boundaries between Mucoromycotina, Mortierellomyctina and the Glomeromycotina seem to contain several poorly studied groups that will force re‐evaluation of the phylogenetic relationships among these groups.
Both Basidiomycota and Ascomycota have suffered recent drastic taxonomic revisions that have affected primarily the Pucciniomycotina, Agaricomycotina and the Pezizomycotina. The phylogeny of both groups is fairly well resolved and environmental data suggest that there are no major (at least to a class level) unsampled lineages. The phylogenetic placement of certain lineages of Basidiomycota is still controversial (Bartheletia, Wallemiomycetes). Ascomycota, on the other hand, contains many poorly studied low‐rank taxa whose position is unknown and for which sequence data are still unavailable.
Several lineages have not yet been assigned robustly to any of the known phyla, some of which might be erected as novel phylum‐level clades in the near future. Herein we highlight the Nephridiophagida, a poorly studied group of arthropod parasites that appear in two phylogenetic works as bona fide fungi but have been largely ignored by mycologists.
VIII. ACKNOWLEDGEMENTS
T.G. acknowledges support from: the Spanish Ministry of Economy, Industry, and Competitiveness (MEIC) for the EMBL partnership, and grants ‘Centro de Excelencia Severo Ochoa 2013‐2017’ SEV‐2012‐0208, and BFU2015‐67107 cofunded by the European Regional Development Fund (ERDF); the CERCA Programme/Generalitat de Catalunya; the Catalan Research Agency (AGAUR) SGR857; the European Union's Horizon 2020 research and innovation programme under grant agreement ERC‐2016‐724173; and the Marie Sklodowska‐Curie grant agreement No H2020‐MSCA‐ITN‐2014‐642095. The authors wish to thank David Hibbett for his suggestions. Finally, special thanks for the work of Alexandra Elbakyan and all her collaborators. Without their labour this review would have been impossible.
IX. REFERENCES
- Adl, S. M. , Simpson, A. G. , Lane, C. E. , Lukeš, J. , Bass, D. , Bowser, S. S. , Brown, M. , Burki, F. , Dunthorn, M. , Hampl, V. , Heiss, A. , Hoppenrath, M. , Lara, E. , Le Gall, L. , Lynn, D. H. , et al. (2012). The revised classification of eukaryotes. Journal of Eukaryotic Microbiology 59, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adl, S. M. , Bass, D. , Lane, C. E. , Lukeš, J. , Schoch, C. L. , Smirnov, A. , Agatha, S. , Berney, C. , Brown, M. W. , Burki, F. , Cárdenas, P. , Čepička, I. , Chistyakova, L. , del Campo, J. , Dunthorn, M. , et al. (2018). Revisions to the classification, nomenclature, and diversity of eukaryotes. Journal of Eukaryotic Microbiology 66, 4–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrendt, S. R. , Quandt, C. A. , Ciobanu, D. , Clum, A. , Salamov, A. , Andreopoulos, B. , Cheng, J.‐F. , Woyke, T. , Pelin, A. , Henrissat, B. , Reynolds, N. K. , Benny, G. L. , Smith, M. E. , James, T. Y. , Grigoriev, I. V. , et al. (2018). Leveraging single‐cell genomics to expand the fungal tree of life. Nature Microbiology 3, 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime, M. C. , Matheny, P. B. , Henk, D. A. , Frieders, E. M. , Nilsson, R. H. , Piepenbring, M. , Mclaughlin, D. J. , Szabo, L. J. , Begerow, D. , Sampaio, J. P. , Bauer, R. , Weiß, M. , Oberwinkler, F. & Hibbett, D. (2006). An overview of the higher level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences. Mycologia 98, 896–905. [DOI] [PubMed] [Google Scholar]
- Aime, M. C. , Toome, M. & McLaughlin, D. J. (2014). 10 Pucciniomycotina In Systematics and Evolution (eds Karl Esser), pp. 271–294. Springer, Berlin Heidelberg. [Google Scholar]
- Alexander, W. G. , Wisecaver, J. H. , Rokas, A. & Hittinger, C. T. (2016). Horizontally acquired genes in early‐diverging pathogenic fungi enable the use of host nucleosides and nucleotides. Proceedings of the National Academy of Sciences 113, 4116–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila‐Garcia, A. M. , Raghuram, N. , Sihota, P. & Fast, N. M. (2013). Microsporidian diversity in soil, sand, and compost of the Pacific Northwest. Journal of Eukaryotic Microbiology 60, 601–608. [DOI] [PubMed] [Google Scholar]
- Atanasova‐Pancevska, N. & Kungulovski, D. (2017). Biodiversity of anaerobic fungi from ruminants in Republic of Macedonia. Biodiversity International Journal 1, 100–107. [Google Scholar]
- Bass, D. , Czech, L. , Williams, B. A. P. , Edric Berney, C. , Dunthorn, M. , Mah E G, F. , Torruella, G. E. , Stentiford, G. D. & Williams, T. A. (2018). Clarifying the relationships between Microsporidia and Cryptomycota. The Journal of Eukaryotic Microbiology 0, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, R. , Oberwinkler, F. & Vánky, K. (1997). Ultrastructural markers and systematics in smut fungi and allied taxa. Canadian Journal of Botany 75, 1273–1314. [Google Scholar]
- Bauer, R. , Garnica, S. , Oberwinkler, F. , Riess, K. , Weiß, M. & Begerow, D. (2015). Entorrhizomycota: a new fungal phylum reveals new perspectives on the evolution of Fungi. PLoS One 10, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begerow, D. , Stoll, M. & Bauer, R. (2006). A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia 98, 906–916. [DOI] [PubMed] [Google Scholar]
- Begerow, D. , Schäfer, A. M. , Kellner, R. , Yurkov, A. , Kemler, M. , Oberwinkler, F. & Bauer, R. (2014). 11 Ustilaginomycotina In Systematics and Evolution (eds Karl Esser), pp. 295–329. Springer, Berlin Heidelberg. [Google Scholar]
- Benny, G. L. , Humber, R. A. & Voigt, K. (2014). Zygomycetous Fungi: phylum Entomophthoromycota and subphyla Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina In Systematics and Evolution: Part A, Second Edition (eds Karl Esser), pp. 209–250. Springer, Berlin Heidelberg. [Google Scholar]
- Benny, G. L. , Smith, M. E. , Kirk, P. M. , Tretter, E. D. & White, M. M. (2016). Challenges and future perspectives in the systematics of Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina In Biology of Microfungi, pp. 65–126. Springer, Cham. [Google Scholar]
- Berger, L. , Hyatt, A. D. , Speare, R. & Longcore, J. E. (2005). Life cycle stages of Batrachochytrium dendrobatidis Longcore et al. 1999, the amphibian chytrid. Diseases of Aquatic Organisms 68, 51–63. [DOI] [PubMed] [Google Scholar]
- Beznoussenko, G. V. , Dolgikh, V. V. , Seliverstova, E. V. , Semenov, P. B. , Tokarev, Y. S. , Trucco, A. , Micaroni, M. , Di Giandomenico, D. , Auinger, P. , Senderskiy, I. V. , Skarlato, S. O. , Snigirevskaya, E. S. , Komissarchik, Y. Y. , Pavelka, M. , De Matteis, M. A. , et al. (2007). Analogs of the Golgi complex in microsporidia: structure and avesicular mechanisms of function. Journal of Cell Science 120, 1288–1298. [DOI] [PubMed] [Google Scholar]
- Bidartondo, M. I. , Read, D. J. , Trappe, J. M. , Merckx, V. , Ligrone, R. & Duckett, J. G. (2011). The dawn of symbiosis between plants and fungi. Biology Letters 7, 574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon‐Grand, G. , Fiol, J. B. , Breton, A. , Bruyère, A. & Oulhaj, Z. (1991). DNA of some anaerobic rumen fungi: G + C content determination. FEMS Microbiology Letters 82, 267–270. [DOI] [PubMed] [Google Scholar]
- Bisby, F. A. & Roskov, Y. R. (2010). The Catalogue of Life: towards an integrative taxonomic backbone for biodiversity In Biodiversify. Biodiversity: Progress and Problems (eds Nimis P. L. and Vignes Lebbe R.), pp. 37–42. [Google Scholar]
- Blackwell, M. (2017). Made for each other: ascomycete yeasts and insects In The Fungal Kingdom (eds Joseph Heitman, Barbara J. Howlett, Pedro W. Crous, Eva H. Stukenbrock, Timothy Y. James and Neil A. R. Gow), pp. 945–962. American Society for Microbiology, Washington, DC. [Google Scholar]
- Bowman, B. H. , Taylor, J. W. , Brownlee, A. G. , Lee, J. , Lu, S. D. & White, T. J. (1992). Molecular evolution of the fungi: relationship of the Basidiomycetes, Ascomycetes, and Chytridiomycetes. Molecular Biology and Evolution 9, 285–296. [DOI] [PubMed] [Google Scholar]
- Brown, A. J. & Casselton, L. A. (2001). Mating in mushrooms: increasing the chances but prolonging the affair. Trends in Genetics 17, 393–400. [DOI] [PubMed] [Google Scholar]
- Bruns, T. D. , Vilgalys, R. , Barns, S. M. , Gonzalez, D. , Hibbett, D. S. , Lane, D. J. , Simon, L. , Stickel, S. , Szaro, T. M. & Weisburg, W. G. (1992). Evolutionary relationships within the fungi: analyses of nuclear small subunit rRNA sequences. Molecular Phylogenetics and Evolution 1, 231–241. [DOI] [PubMed] [Google Scholar]
- Capella‐Gutiérrez, S. , Marcet‐Houben, M. & Gabaldón, T. (2012). Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi. BMC Biology 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier‐Smith, T. (1981). Eukaryote kingdoms: Seven or nine? Biosystems 14, 461–481. [DOI] [PubMed] [Google Scholar]
- Chang, Y. , Wang, S. , Sekimoto, S. , Aerts, A. L. , Choi, C. , Clum, A. , LaButti, K. M. , Lindquist, E. A. , Yee Ngan, C. , Ohm, R. A. , Salamov, A. A. , Grigoriev, I. V. , Spatafora, J. W. & Berbee, M. L. (2015). Phylogenomic analyses indicate that early Fungi evolved digesting cell walls of algal ancestors of land plants. Genome Biology and Evolution 7, 1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, S.‐C. , Ho, H.‐M. , Reynolds, N. , Smith, M. E. , Benny, G. L. , Chien, C.‐Y. & Tsai, J.‐L. (2017). Preliminary phylogeny of Coemansia (Kickxellales), with descriptions of four new species from Taiwan. Mycologia 109, 815–831. [DOI] [PubMed] [Google Scholar]
- Coelho, M. A. , Bakkeren, G. , Sun, S. , Hood, M. E. & Giraud, T. (2017). Fungal sex: the Basidiomycota In The Fungal Kingdom (eds J. Heitman, B. J. Howlett, P. W. Crous, E. H. Stukenbrock, T. Y. James and N. A. R. Gow), pp. 147–175. American Society for Microbiology, Washington, DC. [Google Scholar]
- Conti, E. , Stredansky, M. , Stredanska, S. & Zanetti, F. (2001). γ‐Linolenic acid production by solid‐state fermentation of Mucorales strains on cereals. Bioresource Technology 76, 283–286. [DOI] [PubMed] [Google Scholar]
- Corradi, N. & Keeling, P. J. (2009). Microsporidia: a journey through radical taxonomical revisions. Fungal Biology Reviews 23, 1–8. [Google Scholar]
- Corsaro, D. , Walochnik, J. , Venditti, D. , Müller, K.‐D. , Hauröder, B. & Michel, R. (2014a). Rediscovery of Nucleophaga amoebae, a novel member of the Rozellomycota. Parasitology Research 113, 4491–4498. [DOI] [PubMed] [Google Scholar]
- Corsaro, D. , Walochnik, J. , Venditti, D. , Steinmann, J. , Müller, K.‐D. & Michel, R. (2014b). Microsporidia‐like parasites of amoebae belong to the early fungal lineage Rozellomycota. Parasitology Research 113, 1909–1918. [DOI] [PubMed] [Google Scholar]
- Corsaro, D. , Michel, R. , Walochnik, J. , Venditti, D. , Müller, K.‐D. , Hauröder, B. & Wylezich, C. (2016). Molecular identification of Nucleophaga terricolae sp. nov. (Rozellomycota), and new insights on the origin of the Microsporidia. Parasitology Research 115, 3003–3011. [DOI] [PubMed] [Google Scholar]
- Corsaro, D. , Köhsler, M. , Wylezich, C. , Venditti, D. , Walochnik, J. & Michel, R. (2017). New insights from molecular phylogenetics of amoebophagous fungi (Zoopagomycota, Zoopagales). Parasitology Research 117, 157–167. [DOI] [PubMed] [Google Scholar]
- Cuomo, C. A. & Birren, B. W. (2010). The fungal genome initiative and lessons learned from genome sequencing. Methods in Enzymology 470, 833–855. [DOI] [PubMed] [Google Scholar]
- Cuomo, C. A. , Desjardins, C. A. , Bakowski, M. A. , Goldberg, J. , Ma, A. T. , Becnel, J. J. , Didier, E. S. , Fan, L. , Heiman, D. I. , Levin, J. Z. , Young, S. , Zeng, Q. & Troemel, E. R. (2012). Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Research 22, 2478–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtban, M. , Schraft, H. , Syed, T. A. & Qin, W. (2010). Fungal biodegradation and enzymatic modification of lignin. International Journal of Biochemistry and Molecular Biology 1, 36–50. [PMC free article] [PubMed] [Google Scholar]
- Davis, W. J. , Amses, K. R. , Benny, G. L. , Carter‐House, D. , Chang, Y. , Grigoriev, I. , Smith, M. E. , Spatafora, J. W. , Stajich, J. E. & James, T. Y. (2019a). Genome‐scale phylogenetics reveals a monophyletic Zoopagales (Zoopagomycota, Fungi). Molecular Phylogenetics and Evolution 133, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, W. J. , Amses, K. R. , James, E. S. & James, T. Y. (2019b). A new 18S rRNA phylogeny of uncultured predacious fungi (Zoopagales). Mycologia 111, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, J. , Moora, M. , Öpik, M. , Adholeya, A. , Ainsaar, L. , Bâ, A. , Burla, S. , Diedhiou, A. G. , Hiiesalu, I. , Jairus, T. , Johnson, N. C. , Kane, A. , Koorem, K. , Kochar, M. , Ndiaye, C. , et al. (2015). Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349, 970–973. [DOI] [PubMed] [Google Scholar]
- Deák, T. & Péter, G. (2013). Developments in yeast taxonomy. Acta Alimentaria 42, 55–68. [Google Scholar]
- Dee, J. M. , Mollicone, M. , Longcore, J. E. , Roberson, R. W. & Berbee, M. L. (2015). Cytology and molecular phylogenetics of Monoblepharidomycetes provide evidence for multiple independent origins of the hyphal habit in the Fungi. Mycologia 107, 710–728. [DOI] [PubMed] [Google Scholar]
- Degawa, Y. (2014). Verrucocephalum, a new nematophagous genus in the Helicocephalidaceae (Zoopagales). Mycoscience 55, 144–148. [Google Scholar]
- Degawa, Y. & Gams, W. (2004). A new species of Mortierella, and an associated sporangiiferous mycoparasite in a new genus, Nothadelphia . Studies in Mycology 50, 567–572. [Google Scholar]
- Desirò, A. , Rimington, W. R. , Jacob, A. , Pol, N. V. , Smith, M. E. , Trappe, J. M. , Bidartondo, M. I. & Bonito, G. (2017). Multigene phylogeny of Endogonales, an early diverging lineage of fungi associated with plants. IMA Fungus 8, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel, K. G. A. , van Peer, A. F. , Grijpstra, J. , Wösten, H. A. B. , Verkleij, A. J. , Müller, W. H. & Boekhout, T. (2008). Septal pore cap protein SPC18, isolated from the basidiomycetous fungus Rhizoctonia solani, also resides in pore plugs. Eukaryotic Cell 7, 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel, K. G. A. , Humbel, B. M. , Verkleij, A. J. , Stalpers, J. , Müller, W. H. & Boekhout, T. (2009). Septal pore complex morphology in the Agaricomycotina (Basidiomycota) with emphasis on the Cantharellales and Hymenochaetales. Mycological Research 113, 559–576. [DOI] [PubMed] [Google Scholar]
- Duddington, C. L. (1956). The predacious fungi: Zoopagales and Moniliales. Biological Reviews 31, 152–193. [Google Scholar]
- Dujon, B. (2010). Yeast evolutionary genomics. Nature 11, 512–524. [DOI] [PubMed] [Google Scholar]
- Dujon, B. A. & Louis, E. J. (2017). Genome diversity and evolution in the budding yeasts (Saccharomycotina). Genetics 206, 717–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon, B. , Sherman, D. , Fischer, G. , Durrens, P. , Casaregela, S. , Lafentaine, I. , De Montigny, J. , Marck, C. , Neuvéglise, C. , Talla, E. , Goffard, N. , Frangeul, L. , Algie, M. , Anthouard, V. , Babour, A. , et al. (2004). Genome evolution in yeasts. Nature 430, 35–44. [DOI] [PubMed] [Google Scholar]
- Dunthorn, M. , Kauserud, H. , Bass, D. , Mayor, J. & Mahé, F. (2017). Yeasts dominate soil fungal communities in three lowland Neotropical rainforests. Environmental Microbiology Reports 9, 668–675. [DOI] [PubMed] [Google Scholar]
- Ebersberger, I. , de Matos Simoes, R. , Kupczok, A. , Gube, M. , Kothe, E. , Voigt, K. & von Haeseler, A. (2012). A consistent phylogenetic backbone for the Fungi. Molecular Biology and Evolution 29, 1319–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial (2017). Stop neglecting fungi. Nature Microbiology 2, 17120. [DOI] [PubMed] [Google Scholar]
- Embree, R. W. & Indoh, H. (1967). Aquamortierella, a New Genus in the Mucorales. Bulletin of the Torrey Botanical Club 94, 464–467. [Google Scholar]
- Field, K. J. , Pressel, S. , Duckett, J. G. , Rimington, W. R. & Bidartondo, M. I. (2015). Symbiotic options for the conquest of land. Trends in Ecology & Evolution 30, 477–486. [DOI] [PubMed] [Google Scholar]
- Filtenborg, O. , Frisvad, J. C. & Thrane, U. (1996). Moulds in food spoilage. International Journal of Food Microbiology 33, 85–102. [DOI] [PubMed] [Google Scholar]
- Fischer, W. M. & Palmer, J. D. (2005). Evidence from small‐subunit ribosomal RNA sequences for a fungal origin of Microsporidia. Molecular Phylogenetics and Evolution 36, 606–622. [DOI] [PubMed] [Google Scholar]
- Fisher, M. C. , Gow, N. A. R. & Gurr, S. J. (2016). Tackling emerging fungal threats to animal health, food security and ecosystem resilience. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 371, 20160332 10.1098/rstb.2016.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, K. E. , Romberger, I. , Lowry, D. , Shange, P. & Roberson, R. W. (2018). Hyphal tip growth and cytoplasmic characters of Conidiobolus coronatus (Zoopagomycota, Entomophthoromycotina). Mycologia 110, 31–38. [DOI] [PubMed] [Google Scholar]
- Floudas, D. , Binder, M. , Riley, R. , Barry, K. , Blanchette, R. A. , Henrissat, B. , Martínez, A. T. , Otillar, R. , Spatafora, J. W. , Yadav, J. S. , Aerts, A. , Benoit, I. , Boyd, A. , Carlson, A. , Copeland, A. , Coutinho, P. M. , de Vries, R. P. , Ferreira, P. , Findley, K. , Foster, B. , Gaskell, J. , Glotzer, D. , Górecki, P. , Heitman, J. , Hesse, C. , Hori, C. , Igarashi, K. , Jurgens, J. A. , Kallen, N. , Kersten, P. , Kohler, A. , Kües, U. , Kumar, T. K. , Kuo, A. , LaButti, K. , Larrondo, L. F. , Lindquist, E. , Ling, A. , Lombard, V. , Lucas, S. , Lundell, T. , Martin, R. , McLaughlin, D. , Morgenstern, I. , Morin, E. , Murat, C. , Nagy, L. G. , Nolan, M. , Ohm, R. A. , Patyshakuliyeva, A. , Rokas, A. , Ruiz‐Dueñas, F. J. , Sabat, G. , Salamov, A. , Samejima, M. , Schmutz, J. , Slot, J. C. , St John, F. , Stenlid, J. , Sun, H. , Sun, S. , Syed, K. , Tsang, A. , Wiebenga, A. , Young, D. , Pisabarro, A. , Eastwood, D. C. , Martin, F. , Cullen, D. , Grigoriev, I. V. & Hibbett, D. S. (2012). The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336, 1715–1719. [DOI] [PubMed] [Google Scholar]
- Forget, L. , Ustinova, J. , Wang, Z. , Huss, V. A. R. & Franz Lang, B. (2002). Hyaloraphidium curvatum: a linear mitochondrial genome, tRNA editing, and an evolutionary link to lower fungi. Molecular Biology and Evolution 19, 310–319. [DOI] [PubMed] [Google Scholar]
- Foust, F. K. (1937). A new species of Rozella parasitic on Allomyces . Journal of the Elisha Mitchell Scientific Society 53, 197–204. [Google Scholar]
- Freeman, K. R. , Martin, A. P. , Karki, D. , Lynch, R. C. , Mitter, M. S. , Meyer, A. F. , Longcore, J. E. , Simmons, D. R. & Schmidt, S. K. (2009). Evidence that chytrids dominate fungal communities in high‐elevation soils. Proceedings of the National Academy of Sciences of the United States of America 106, 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimoser, F . (2017). Start teaching mycology! Electronic file available at https://naturemicrobiologycommunity.nature.com/users/63137-florian-freimoser/posts/20287-start-teaching-mycology Accessed 21.06.2018.
- Frenken, T. , Velthuis, M. , de Senerpont Domis, L. N. , Stephan, S. , Aben, R. , Kosten, S. , van Donk, E. & Van de Waal, D. B. (2016). Warming accelerates termination of a phytoplankton spring bloom by fungal parasites. Global Change Biology 22, 299–309. [DOI] [PubMed] [Google Scholar]
- Frenken, T. , Alacid, E. , Berger, S. A. , Bourne, E. C. , Gerphagnon, M. M. , Grossart, H.‐P. , Gsell, A. S. , Ibelings, B. W. , Kagami, M. , Küpper, F. C. , Letcher, P. M. , Loyau, A. , Miki, T. , Nejstgaard, J. C. , Rasconi, S. , et al. (2017). Integrating chytrid fungal parasites into plankton ecology. Research gaps and needs. Environmental Microbiology 19, 3802–3822. [DOI] [PubMed] [Google Scholar]
- Fritz‐Laylin, L. K. , Lord, S. J. & Mullins, R. D. (2017). WASP and SCAR are evolutionarily conserved in actin‐filled pseudopod‐based motility. The Journal of Cell Biology 216, 1673–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ga, A. , Gabaldo, T. , Pryszcz, L. P. , Ne, T. , James, S. a. , Bond, C. J. , Stratford, M. , Roberts, I. N. , Louis, V. L. , Despons, L. , Friedrich, A. , Martin, T. , Durrens, P. , Casaregola, S. , Neuveglise, C. , et al. (2013). Evolutionary role of interspecies hybridization and genetic exchanges in yeasts. Current Opinion in Microbiology 10, 12875–12880. [Google Scholar]
- Galindo, L. J. , Torruella, G. , Moreira, D. , Timpano, H. , Paskerova, G. , Smirnov, A. , Nassonova, E. & López‐García, P. (2018). Evolutionary genomics of Metchnikovella incurvata (Metchnikovellidae), an early branching microsporidium. Genome Biology and Evolution 10, 2736–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Vallvé, S. , Romeu, A. & Palau, J. (2000). Horizontal gene transfer of glycosyl hydrolases of the rumen fungi. Molecular Biology and Evolution 17, 352–361. [DOI] [PubMed] [Google Scholar]
- Garnier, L. , Valence, F. & Mounier, J. (2017). Diversity and control of spoilage fungi in dairy products: an update. Microorganisms 5, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazis, R. , Miadlikowska, J. , Lutzoni, F. , Arnold, A. E. E. & Chaverri, P. (2012). Culture‐based study of endophytes associated with rubber trees in Peru reveals a new class of Pezizomycotina: Xylonomycetes. Molecular Phylogenetics and Evolution 65, 294–304. [DOI] [PubMed] [Google Scholar]
- Gehrig, H. , Schüßler, A. & Kluge, M. (1996). Geosiphon pyriforme, a fungus forming endocytobiosis with Nostoc (Cyanobacteria), is an ancestral member of the glomales: evidence by SSU rRNA Analysis. Journal of Molecular Evolution 43, 71–81. [DOI] [PubMed] [Google Scholar]
- Gleason, F. H. & McGee, P. A. (2004). The ultrastructure of cell walls in some sporocarpic species of Densospora, Glomus and Endogone . Australasian Mycologist 22, 73–75. [Google Scholar]
- Gleason, F. H. , Schmidt, S. K. & Marano, A. V. (2010). Can zoosporic true fungi grow or survive in extreme or stressful environments? Extremophiles 14, 417–425. [DOI] [PubMed] [Google Scholar]
- Gleason, F. H. , Carney, L. T. , Lilje, O. & Glockling, S. L. (2012). Ecological potentials of species of Rozella (Cryptomycota). Fungal Ecology 5, 651–656. [Google Scholar]
- Gleason, F.H. , Lilje, O. , Marano, A. V, Sime‐Ngando, T. , Sullivan, B.K. , Kirchmair, M. & Neuhauser, S. (2014). Ecological functions of zoosporic hyperparasites. Frontiers in Microbiology 5, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau, A. , Barrell, B. G. , Bussey, H. , Davis, R. W. , Dujon, B. , Feldmann, H. , Galibert, F. , Hoheisel, J. D. , Jacq, C. , Johnston, M. , Louis, E. J. , Mewes, H. W. , Murakami, Y. , Philippsen, P. , Tettelin, H. , et al. (1996). Life with 6000 genes. Science 274, 563–567. [DOI] [PubMed] [Google Scholar]
- Gonçalves, V. N. , Cantrell, C. L. , Wedge, D. E. , Ferreira, M. C. , Soares, M. A. , Jacob, M. R. , Oliveira, F. S. , Galante, D. , Rodrigues, F. , Alves, T. M. A. , Zani, C. L. , Junior, P. A. S. , Murta, S. , Romanha, A. J. , Barbosa, E. C. , Kroon, E. G. , Oliveira, J. G. , Gomez‐Silva, B. , Galetovic, A. , Rosa, C. A. & Rosa, L. H. (2016). Fungi associated with rocks of the Atacama Desert: taxonomy, distribution, diversity, ecology and bioprospection for bioactive compounds. Environmental Microbiology 18, 232–245. [DOI] [PubMed] [Google Scholar]
- Gromov, B. V. (2000). Algal parasites of the genera Aphelidium, Amoeboaphelidium, and Pseudaphelidium from the Cienkovski's ‘“monadinea”’ group as representatives of a new class. Zoologichesky Zhurnal 79, 517–525. [Google Scholar]
- Grossart, H.‐P. , Wurzbacher, C. , James, T. Y. & Kagami, M. (2016). Discovery of dark matter fungi in aquatic ecosystems demands a reappraisal of the phylogeny and ecology of zoosporic fungi. Fungal Ecology 19, 28–38. [Google Scholar]
- Grube, M. & Wedin, M. (2016). Lichenized fungi and the evolution of symbiotic organization. Microbiology Spectrum 4, 749–765. [DOI] [PubMed] [Google Scholar]
- Gruninger, R. J. , Puniya, A. K. , Callaghan, T. M. , Edwards, J. E. , Youssef, N. , Dagar, S. S. , Fliegerova, K. , Griffith, G. W. , Forster, R. , Tsang, A. , McAllister, T. & Elshahed, M. S. (2014). Anaerobic fungi (phylum Neocallimastigomycota ): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiology Ecology 90, 1–17. [DOI] [PubMed] [Google Scholar]
- Gryganskyi, A. P. , Humber, R. A. , Smith, M. E. , Miadlikovska, J. , Wu, S. , Voigt, K. , Walther, G. , Anishchenko, I. M. & Vilgalys, R. (2012). Molecular phylogeny of the Entomophthoromycota. Molecular Phylogenetics and Evolution 65, 682–694. [DOI] [PubMed] [Google Scholar]
- Gryganskyi, A. P. , Humber, R. A. , Smith, M. E. , Hodge, K. , Huang, B. , Voigt, K. & Vilgalys, R. (2013). Phylogenetic lineages in Entomophthoromycota. Persoonia 30, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag, K. L. , James, T. Y. , Pombert, J.‐F. , Larsson, R. , Schaer, T. M. M. , Refardt, D. & Ebert, D. (2014). Evolution of a morphological novelty occurred before genome compaction in a lineage of extreme parasites. Proceedings of the National Academy of Sciences of the United States of America 111, 15480–15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafy, R. A. , Elshahed, M. S. , Liggenstoffer, A. S. , Griffith, G. W. & Youssef, N. H. (2017). Pecoramyces ruminantium, gen. nov., sp. nov., an anaerobic gut fungus from the feces of cattle and sheep. Mycologia 109, 231–243. [DOI] [PubMed] [Google Scholar]
- Hanafy, R. A. , Elshahed, M. S. & Youssef, N. H. (2018). Feramyces austinii, gen. nov, sp. nov., an anaerobic gut fungus from rumen and fecal samples of wild Barbary sheep and fallow deer. Mycologia 110, 513–525. [DOI] [PubMed] [Google Scholar]
- Hanafy, R.A. , Lanjekar, V.B. , Dhakephalkar, P.K. , Callaghan, T.M. , Dagar, S.S. , Griffith, G.W. , Elshahed, M.S. & Youssef, N.H . (2019). Seven new Neocallimastigomycota genera from fecal samples of wild, zoo‐housed, and domesticated herbivores: Description of Ghazallomyces constrictus gen. nov., sp. nov., Aklioshbomyces papillarum gen. nov., sp. nov., Agriosomyces longus gen. nov., sp. Nov. Capellomyces foraminis gen. nov., sp. nov and Capellomyces elongatus sp. nov., Joblinomyces apicalis gen. nov., sp. nov., Khoyollomyces ramosus gen. nov., sp. nov. and Tahromyces munnarensis gen. nov., sp. nov. bioRxiv, doi: doi.org 10.1101/642694. [DOI]
- Hane, J. K. , Rouxel, T. , Howlett, B. J. , Kema, G. H. , Goodwin, S. B. & Oliver, R. P. (2011). A novel mode of chromosomal evolution peculiar to filamentous Ascomycete fungi. Genome Biology 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, P. M. , Burdet, F. X. , Cissé, O. H. , Keller, L. , Taffé, P. , Sanglard, D. & Pagni, M. (2010). Comparative genomics suggests that the fungal pathogen Pneumocystis is an obligate parasite scavenging amino acids from its host's lungs. PLoS One 5, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, R. A. , Kumar, T. K. A. , Hewitt, D. A. & McLaughlin, D. J. (2013). Functional and phylogenetic implications of septal pore ultrastructure in the ascoma of Neolecta vitellina . Mycologia 105, 802–813. [DOI] [PubMed] [Google Scholar]
- Hibbett, D. S. (2006). A phylogenetic overview of the Agaricomycotina. Mycologia 98, 917–925. [DOI] [PubMed] [Google Scholar]
- Hibbett, D. S. , Binder, M. , Bischoff, J. F. , Blackwell, M. , Cannon, P. F. , Eriksson, O. E. , Huhndorf, S. , James, T. , Kirk, P. M. , Lücking, R. , Thorsten Lumbsch, H. , Lutzoni, F. , Matheny, P. B. , Mclaughlin, D. J. , Powell, M. J. , et al. (2007). A higher‐level phylogenetic classification of the Fungi. Mycological Research 111, 509–547. [DOI] [PubMed] [Google Scholar]
- Hirose, D. , Degawa, Y. , Inaba, S. & Tokumasu, S. (2012). The anamorphic genus Calcarisporiella is a new member of the Mucoromycotina. Mycoscience 53, 256–260. [Google Scholar]
- Hirose, D. , Degawa, Y. , Yamamoto, K. & Yamada, A. (2014). Sphaerocreas pubescens is a member of the Mucoromycotina closely related to fungi associated with liverworts and hornworts. Mycoscience 55, 221–226. [Google Scholar]
- Hittinger, C. T. , Rokas, A. , Bai, F.‐Y. , Boekhout, T. , Gonçalves, P. , Jeffries, T. W. , Kominek, J. , Lachance, M.‐A. , Libkind, D. , Rosa, C. A. , Sampaio, J. P. & Kurtzman, C. P. (2015a). For the ‘genomes and evolution’ special issue of current opinion in genetics and development. Current Opinion in Genetics & Development 35, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittinger, C. T. , Rokas, A. , Bai, F. , Boekhout, T. , Gonc, P. , Jeffries, T. W. , Libkind, D. , Kominek, J. , Kurtzman, C. P. & Rosa, C. A. (2015b). Genomics and the making of yeast biodiversity. Current Opinion in Genetics & Development 35, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, K. , Voigt, K. & Kirk, P. M. (2011). Mortierellomycotina subphyl. Nov., based on multi‐gene genealogies. Mycotaxon 115, 353–363. [Google Scholar]
- Humber, R. A. (1989). Synopsis of a revised classification for the Entomophthorales (Zygomycotina). Mycotaxon 34, 441–460. [Google Scholar]
- Humber, R. a. (2012). Entomophthoromycota: a new phylum and reclassification for entomophthoroid fungi. Mycotaxon 120, 477–492. [Google Scholar]
- Ishida, S. , Nozaki, D. , Grossart, H.‐P. & Kagami, M. (2015). Novel basal, fungal lineages from freshwater phytoplankton and lake samples. Environmental Microbiology Reports 7, 435–441. [DOI] [PubMed] [Google Scholar]
- James, T. Y. & Berbee, M. L. (2012). No jacket required–new fungal lineage defies dress code: recently described zoosporic fungi lack a cell wall during trophic phase. BioEssays 34, 94–102. [DOI] [PubMed] [Google Scholar]
- James, T. Y. , Porter, D. , Leander, C. A. , Vilgalys, R. & Longcore, J. E. (2000). Molecular phylogenetics of the Chytridiomycota supports the utility of ultrastructural data in chytrid systematics. Canadian Journal of Botany 78, 336–350. [Google Scholar]
- James, T. Y. , Kauff, F. , Schoch, C. L. , Matheny, P. B. , Hofstetter, V. , Cox, C. J. , Celio, G. , Gueidan, C. , Fraker, E. , Miadlikowska, J. , Lumbsch, H. T. , Rauhut, A. , Reeb, V. , Arnold, A. E. , Amtoft, A. , Stajich, J. E. , Hosaka, K. , Sung, G. H. , Johnson, D. , O'Rourke, B. , Crockett, M. , Binder, M. , Curtis, J. M. , Slot, J. C. , Wang, Z. , Wilson, A. W. , Schüßler, A. , Longcore, J. E. , O'Donnell, K. , Mozley‐Standridge, S. , Porter, D. , Letcher, P. M. , Powell, M. J. , Taylor, J. W. , White, M. M. , Griffith, G. W. , Davies, D. R. , Humber, R. A. , Morton, J. B. , Sugiyama, J. , Rossman, A. Y. , Rogers, J. D. , Pfister, D. H. , Hewitt, D. , Hansen, K. , Hambleton, S. , Shoemaker, R. A. , Kohlmeyer, J. , Volkmann‐Kohlmeyer, B. , Spotts, R. A. , Serdani, M. , Crous, P. W. , Hughes, K. W. , Matsuura, K. , Langer, E. , Langer, G. , Untereiner, W. A. , Lücking, R. , Büdel, B. , Geiser, D. M. , Aptroot, A. , Diederich, P. , Schmitt, I. , Schultz, M. , Yahr, R. , Hibbett, D. S. , Lutzoni, F. , McLaughlin, D. J. , Spatafora, J. W. & Vilgalys, R. (2006a). Reconstructing the early evolution of Fungi using a six‐gene phylogeny. Nature 443, 818–822. [DOI] [PubMed] [Google Scholar]
- James, T. Y. , Letcher, P. M. , Longcore, J. E. , Mozley‐Standridge, S. E. , Porter, D. , Powell, M. J. , Griffith, G. W. & Vilgalys, R. (2006b). A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 98, 860–871. [DOI] [PubMed] [Google Scholar]
- James, T. Y. , Pelin, A. , Bonen, L. , Ahrendt, S. , Sain, D. , Corradi, N. & Stajich, J. E. (2013). Shared signatures of parasitism and phylogenomics unite Cryptomycota and Microsporidia. Current Biology 23, 1548–1553. [DOI] [PubMed] [Google Scholar]
- James, T. Y. , Porter, T. M. & Martin, W. W. (2014). 7 Blastocladiomycota In Systematics and Evolution (eds Karl Esser), pp. 177–207. Springer, Berlin Heidelberg. [Google Scholar]
- Jones, M. D. M. , Forn, I. , Gadelha, C. , Egan, M. J. , Bass, D. , Massana, R. & Richards, T. A. (2011). Discovery of novel intermediate forms redefines the fungal tree of life. Nature 474, 200–203. [DOI] [PubMed] [Google Scholar]
- Jones, M. , Bhat, T. , Huynh, T. , Kandare, E. , Yuen, R. , Wang, C. H. & John, S. (2018). Waste‐derived low‐cost mycelium composite construction materials with improved fire safety. Fire and Materials 42, 816–825. [Google Scholar]
- Joshi, A. , Lanjekar, V. B. , Dhakephalkar, P. K. , Callaghan, T. M. , Griffith, G. W. & Dagar, S. S. (2018). Liebetanzomyces polymorphus gen. et sp. nov., a new anaerobic fungus (Neocallimastigomycota) isolated from the rumen of a goat. MycoKeys 40, 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami, M. , Miki, T. & Takimoto, G. (2014). Mycoloop: chytrids in aquatic food webs. Frontiers in Microbiology 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, K. & Zamani, A. (2013). Mucor indicus: biology and industrial application perspectives: a review. Biotechnology Advances 31, 466–481. [DOI] [PubMed] [Google Scholar]
- Karpov, S. A. , Mamkaeva, M. A. , Aleoshin, V. V. , Nassonova, E. , Lilje, O. & Gleason, F. H. (2014a). Morphology, phylogeny, and ecology of the aphelids (Aphelidea, Opisthokonta) and proposal for the new superphylum Opisthosporidia. Frontiers in Microbiology 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov, S. A. , Mamkaeva, M. A. , Benzerara, K. , Moreira, D. & López‐García, P. (2014b). Molecular phylogeny and ultrastructure of Aphelidium aff. melosirae (Aphelida, Opisthosporidia). Protist 165, 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov, S. A. , Mamanazarova, K. S. , Popova, O. V. , Aleoshin, V. V. , James, T. Y. , Mamkaeva, M. A. , Tcvetkova, V. S. , Vishnyakov, A. E. & Longcore, J. E. (2017a). Monoblepharidomycetes diversity includes new parasitic and saprotrophic species with highly intronized rDNA. Fungal Biology 121, 729–741. [DOI] [PubMed] [Google Scholar]
- Karpov, S. A. , Tcvetkova, V. S. , Mamkaeva, M. A. , Torruella, G. , Timpano, H. , Moreira, D. , Mamanazarova, K. S. & López‐García, P. (2017b). Morphological and genetic diversity of Opisthosporidia: new Aphelid Paraphelidium tribonemae gen. et sp. nov. Journal of Eukaryotic Microbiology 64, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov, S. A. , López‐García, P. , Mamkaeva, M. A. , Klimov, V. I. , Vishnyakov, A. E. , Tcvetkova, V. S. & Moreira, D. (2018). The chytrid‐like parasites of algae Amoeboradix gromovi gen. et sp. nov. and Sanchytrium tribonematis belong to a new fungal lineage. Protist 169, 122–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling, P. J. , Luker, M. A. & Palmer, J. D. (2000). Evidence from beta‐tubulin phylogeny that Microsporidia evolved from within the Fungi. Molecular Biology and Evolution 17, 23–31. [DOI] [PubMed] [Google Scholar]
- Kluge, M. (2002). A fungus eats a cyanobacterium: the story of the Geosiphon pyriformis Endocyanosis. Royal Irish Academy. Biology and Environment: Proceedings of the Royal Irish Academy 102, 11–14. [Google Scholar]
- Kluge, M. , Mollenhauer, D. & Mollenhauer, R. (1991). Photosynthetic carbon assimilation in Geosiphon pyriforme (Kützing) F. v. Wettstein, an endosymbiotic association of fungus and cyanobacterium. Planta 185, 311–315. [DOI] [PubMed] [Google Scholar]
- Kluge, M. , Mollenhauer, D. , Mollenhauer, R. & Kape, R. (1992). Geosiphon pyriforme, an endosymbiotic consortium of a fungus and a cyanobacterium (Nostoc), fixes nitrogen. Botanica Acta 105, 343–344. [Google Scholar]
- Kluge, M. , Mollenhauer, D. , Wolf, E. & Schüβler, A. (2002). The Nostoc‐Geosiphon endocytobiosis In Cyanobacteria in Symbiosis, pp. 19–30. Springer, Dordrecht. [Google Scholar]
- Kothe, E. (1996). Tetrapolar fungal mating types: sexes by the thousands. FEMS Microbiology Reviews 18, 65–87. [DOI] [PubMed] [Google Scholar]
- Kottke, I. , Suá Rez, J. P. , Herrera, P. , Cruz, D. , Bauer, R. , Haug, I. & Garnica, S. (2010). Atractiellomycetes belonging to the ‘rust’ lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. Proceedings of the Royal Society Biological Sciences 277, 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara, Y. , Degawa, Y. & Tokumasu, S. (2004). Two novel kickxellalean fungi, Mycoëmilia Scoparia gen. sp. nov. and Ramicandelaber brevisporus sp. nov. Mycological Research 108, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Kurtzman, C. P. & Sugiyama, J. (2015). 1 Saccharomycotina and Taphrinomycotina: the yeasts and yeastlike fungi of the Ascomycota In The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research. VII Systematics and Evolution Part B (eds D. McLaughlin and J. Spatafora), pp. 3–33. Springer, Berlin, Heidelberg. [Google Scholar]
- Kurtzman, C. P. , Fell, J. W. & Boekhout, T. (2011). The Yeasts: A Taxonomic Study, 5th Edition, pp. 2354 Elsevier Science. [Google Scholar]
- Kuzina, V. & Cerdá‐Olmedo, E. (2007). Ubiquinone and carotene production in the Mucorales Blakeslea and Phycomyces. Applied Microbiology and Biotechnology 76, 991–999. [DOI] [PubMed] [Google Scholar]
- Kwaśna, H. , Ward, E. & Bateman, G. L. (2006). Phylogenetic relationships among Zygomycetes from soil based on ITS1/2 rDNA sequences. Mycological Research 110, 501–510. [DOI] [PubMed] [Google Scholar]
- Kwon‐Chung, K. J. (2012). Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clinical Infectious Diseases 54, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landvik, S. , Schumacher, T. K. , Eriksson, O. E. & Moss, S. T. (2003). Morphology and ultrastructure of Neolecta species. Mycological Research 107, 1021–1031. [DOI] [PubMed] [Google Scholar]
- Lange, C. E. (1993). Unclassified protists of arthropods: the ultrastructure of Nephridiophaga periplanetae (Lutz & Splendore, 1903) N. Comb., and the affinities of the Nephridiophagidae to other protists. Journal of Eukaryotic Microbiology 40, 689–700. [Google Scholar]
- Lara, E. , Moreira, D. & López‐García, P. (2010). The Environmental Clade LKM11 and Rozella form the deepest branching clade of Fungi. Protist 161, 116–121. [DOI] [PubMed] [Google Scholar]
- Lazarus, K. L. , Benny, G. L. , Ho, H.‐M. & Smith, M. E. (2017). Phylogenetic systematics of Syncephalis (Zoopagales, Zoopagomycotina), a genus of ubiquitous mycoparasites. Mycologia 109, 333–349. [DOI] [PubMed] [Google Scholar]
- Letcher, P. M. , Longcore, J. E. , Quandt, C. A. , da Silva Leite, D. , James, T. Y. & Powell, M. J. (2017). Morphological, molecular, and ultrastructural characterization of Rozella rhizoclosmatii, a new species in Cryptomycota. Fungal Biology 121, 1–10. [DOI] [PubMed] [Google Scholar]
- Letcher, P.M. , Longcore, J.E. , James, T.Y. , Leite, D.S. , Simmons, D.R. & Powell, M.J. (2018). Morphology, ultrastructure, and molecular phylogeny of Rozella multimorpha, a new species in Cryptomycota. Journal of Eukaryotic Microbiology 65, 180–190. [DOI] [PubMed] [Google Scholar]
- Letunic, I. & Bork, P. (2016). Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research 44, 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, K. , Limpens, E. , Zhang, Z. , Ivanov, S. , Saunders, D. G. O. O. , Mu, D. , Pang, E. , Cao, H. , Cha, H. , Lin, T. , Zhou, Q. , Shang, Y. , Li, Y. , Sharma, T. , van Velzen, R. , et al. (2014). Single nucleus genome sequencing reveals high similarity among nuclei of an endomycorrhizal fungus. PLoS Genetics 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnaeus, C. (1767). Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio duodecima. 1. Regnum Animale. 1 & 2 Holmiae, Laurentii Salvii In Holmiae [Stockholm], Laurentii Salvii p. 248.
- Liu, Y. J. , Hodson, M. C. & Hall, B. D. (2006). Loss of the flagellum happened only once in the fungal lineage: phylogenetic structure of kingdom Fungi inferred from RNA polymerase II subunit genes. BMC Evolutionary Biology 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Ng, S. K. , Lu, Y. , Low, W. , Lai, J. & Jedd, G. (2008a). Making two organelles from one: Woronin body biogenesis by peroxisomal protein sorting. The Journal of Cell Biology 180, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Leigh, J. W. , Brinkmann, H. , Cushion, M. T. , Rodriguez‐Ezpeleta, N. , Philippe, H. & Lang, B. F. (2008b). Phylogenomic analyses support the monophyly of Taphrinomycotina, including Schizosaccharomyces fission yeasts. Molecular Biology and Evolution 26, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.‐Z. , Wang, Q.‐M. , Göker, M. , Groenewald, M. , Kachalkin, A. V. , Lumbsch, H. T. , Millanes, A. M. , Wedin, M. , Yurkov, A. M. , Boekhout, T. & Bai, F.‐Y. (2015). Towards an integrated phylogenetic classification of the Tremellomycetes. Studies in Mycology 81, 85–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño‐Hernández, L. , Ramírez‐Toro, C. , Ruiz, H. A. , Ascacio‐Valdés, J. A. , Aguilar‐Gonzalez, M. A. , Rodríguez‐Herrera, R. & Aguilar, C. N. (2017). Rhizopus oryzae – ancient microbial resource with importance in modern food industry. International Journal of Food Microbiology 257, 110–127. [DOI] [PubMed] [Google Scholar]
- Longcore, J. E. , Pessier, A. P. & Nichols, D. K. (1999). Batrachochytrium dendrobatidis gen. et sp. nov., a Chytrid pathogenic to amphibians. Mycologia 91, 219–227. [Google Scholar]
- Lutzoni, F. , Kauff, F. , Cox, C. J. , McLaughlin, D. , Celio, G. , Dentinger, B. , Padamsee, M. , Hibbett, D. , James, T. Y. , Baloch, E. , Grube, M. , Reeb, V. , Hofstetter, V. , Schoch, C. , Arnold, A. E. , Miadlikowska, J. , Spatafora, J. , Johnson, D. , Hambleton, S. , Crockett, M. , Shoemaker, R. , Sung, G. H. , Lücking, R. , Lumbsch, T. , O'Donnell, K. , Binder, M. , Diederich, P. , Ertz, D. , Gueidan, C. , Hansen, K. , Harris, R. C. , Hosaka, K. , Lim, Y. W. , Matheny, B. , Nishida, H. , Pfister, D. , Rogers, J. , Rossman, A. , Schmitt, I. , Sipman, H. , Stone, J. , Sugiyama, J. , Yahr, R. & Vilgalys, R. (2004). Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. American Journal of Botany 91, 1446–1480. [DOI] [PubMed] [Google Scholar]
- Manning, R. J. & Callaghan, A. A. (2008). Pathogenicity of Conidiobolus spp. and Basidiobolus ranarum to arthropods co‐occurring in leaf litter. Fungal Ecology 1, 33–39. [Google Scholar]
- Manning, R. J. , Waters, S. D. & Callaghan, A. A. (2007). Saprotrophy of Conidiobolus and Basidiobolus in leaf litter. Mycological Research 111, 1437–1449. [DOI] [PubMed] [Google Scholar]
- Manohar, C. S. , Raghukumar, C. , Sumathi Manohar, C. & Raghukumar, C. (2013). Fungal diversity from various marine habitats deduced through culture‐independent studies. FEMS Microbiology Letters 341, 69–78. [DOI] [PubMed] [Google Scholar]
- Martínez, Á. T. , Speranza, M. , Ruiz‐Dueñas, F. J. , Ferreira, P. , Camarero, S. , Guillén, F. , Martínez, M. J. , Gutiérrez, A. & del Río, J. C. (2005). Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. International Microbiology 8, 195–204. [PubMed] [Google Scholar]
- Matheny, P. B. , Gossmann, J. A. , Zalar, P. , Kumar, T. K. A. & Hibbett, D. S. (2006). Resolving the phylogenetic position of the Wallemiomycetes: an enigmatic major lineage of Basidiomycota. Canadian Journal of Botany 84, 1794–1805. [Google Scholar]
- de Mattos‐Shipley, K. M. J. , Ford, K. L. , Alberti, F. , Banks, A. M. , Bailey, A. M. & Foster, G. D. (2016). The good, the bad and the tasty: the many roles of mushrooms. Studies in Mycology 85, 125–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee, P. P. A. (1996). The Australian zygomycetous mycorrhizal fungi: the genus Densospora gen. Nov. Australian Systematic Botany 9, 329–336. [Google Scholar]
- McLaughlin, D. J. & Spatafora, J. W. (2014). The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research. VII Systematics and Evolution Part A, 2nd Edition. [Google Scholar]
- McLaughlin, D. J. & Spatafora, J. W. (2015). The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research. VII Systematics and Evolution Part B, 2nd Edition. [Google Scholar]
- McNeill, J. , Barrie, F. R. , Buck, W. R. , Demoulin, V. , Greuter, W. , Hawksworths, D. L. , Herendeen, P. S. , Knapp, S. , Marhold, K. , Prado, J. , Van Reine, W. F. P. , Smith, G. F. , Wiersma, J. H. & Turland, N. J. (2012). International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 In Regnum Vegetabile no. 154 p. Koeltz Scientific Books, Königstein. [Google Scholar]
- Mélida, H. , Sain, D. , Stajich, J. E. & Bulone, V. (2015). Deciphering the uniqueness of Mucoromycotina cell walls by combining biochemical and phylogenomic approaches. Environmental Microbiology 17, 1649–1662. [DOI] [PubMed] [Google Scholar]
- Menkis, A. , Urbina, H. , James, T. Y. & Rosling, A. (2014). Archaeorhizomyces borealis sp. nov. and a sequence‐based classification of related soil fungal species. Fungal Biology 118, 943–955. [DOI] [PubMed] [Google Scholar]
- Mikhailov, K. V. , Simdyanov, T. G. & Aleoshin, V. V. (2017). Genomic survey of a hyperparasitic microsporidian Amphiamblys sp. (Metchnikovellidae). Genome Biology and Evolution 9, 454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, B. , Choi, Y.‐J. & Thines, M. (2018). Phylogenomics of Bartheletia paradoxa reveals its basal position in Agaricomycotina and that the early evolutionary history of basidiomycetes was rapid and probably not strictly bifurcating. Mycological Progress 17, 333–341. [Google Scholar]
- Misra, J. K. , Tewari, J. P. & Deshmukh, S. K. (2012). Systematics and Evolution of Fungi. CRC Press, Enfield, New Hampshire. [Google Scholar]
- Mixão, V. & Gabaldón, T. (2018). Yeast interspecies hybrids hybridization and emergence of virulence in opportunistic human yeast pathogens. Yeast 35, 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchy, S. , Sanciu, G. , Jobard, M. , Rasconi, S. , Gerphagnon, M. , Chabé, M. , Cian, A. , Meloni, D. , Niquil, N. , Christaki, U. , Viscogliosi, E. & Sime‐Ngando, T. (2011). Exploring and quantifying fungal diversity in freshwater lake ecosystems using rDNA cloning/sequencing and SSU tag pyrosequencing. Environmental Microbiology 13, 1433–1453. [DOI] [PubMed] [Google Scholar]
- Morales, L. & Dujon, B. (2012). Evolutionary role of interspecies hybridization and genetic exchanges in yeasts. Microbiology and Molecular Biology Reviews 76, 721–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, M. O. (2008). Fungi, quality and safety issues in fresh fruits and vegetables. Journal of Applied Microbiology 104, 1239–1243. [DOI] [PubMed] [Google Scholar]
- Mühlhausen, S. & Kollmar, M. (2014). Molecular phylogeny of sequenced Saccharomycetes reveals polyphyly of the alternative yeast codon usage. Genome Biology and Evolution 6, 3222–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlhausen, S. , Findeisen, P. , Plessmann, U. , Urlaub, H. & Kollmar, M. (2016). A novel nuclear genetic code alteration in yeasts and the evolution of codon reassignment in eukaryotes. Genome Research 26, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C. , Youssef, N. , Hanafy, R.A. , Couger, M. , Stajich, J.E. , Wang, Y. , Baker, K. , Dagar, S. , Griffith, G. , Farag, I. , Callaghan, T. & Elshahed, M.S. (2019). Horizontal gene transfer as an indispensible driver for Neocallimastigomycota evolution into a distinct gut‐dwelling fungal lineage. bioRxiv, 1–40. [DOI] [PMC free article] [PubMed]
- Nadimi, M. , Beaudet, D. , Forget, L. , Hijri, M. & Lang, B. F. (2012). Group I intron–mediated trans‐splicing in mitochondria of Gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with Mortierellales. Molecular Biology and Evolution 29, 2199–2210. [DOI] [PubMed] [Google Scholar]
- Nagahama, T. , Takahashi, E. , Nagano, Y. , Abdel‐Wahab, M. A. & Miyazaki, M. (2011). Molecular evidence that deep‐branching fungi are major fungal components in deep‐sea methane cold‐seep sediments. Environmental Microbiology 13, 2359–2370. [DOI] [PubMed] [Google Scholar]
- Nasr, S. , Soudi, M. R. , Nasrabadi, S. M. Z. , Nikou, M. M. , Salmanian, A. H. & Nguyen, H. D. T. (2014). Basidioascus persicus sp. nov., a yeast‐like species of the order Geminibasidiales isolated from soil. International Journal of Systematic and Evolutionary Microbiology 64, 3046–3052. [DOI] [PubMed] [Google Scholar]
- Naumov, G. I. , Kondratieva, V. I. & Naumova, E. S. (2015). Hybrid sterility of the yeast Schizosaccharomyces pombe: genetic genus and many species in statu nascendi? Microbiology 84, 159–169. [PubMed] [Google Scholar]
- Nguyen, H. D. T. , Nickerson, N. L. & Seifert, K. A. (2013). Basidioascus and Geminibasidium : a new lineage of heat‐resistant and xerotolerant basidiomycetes. Mycologia 105, 1231–1250. [DOI] [PubMed] [Google Scholar]
- Nguyen, H. D. T. , Chabot, D. , Hirooka, Y. , Roberson, R. W. & Seifert, K. A. (2015). Basidioascus undulatus: genome, origins and sexuality. International Microbiological Association Fungus 6, 215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. A. , Cissé, O. H. , Yun Wong, J. , Zheng, P. , Hewitt, D. , Nowrousian, M. , Stajich, J. E. & Jedd, G. (2017). Innovation and constraint leading to complex multicellularity in the Ascomycota. Nature Communications 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M. , Feretzaki, M. , Sun, S. , Wang, X. & Heitman, J. (2011). Sex in Fungi. Annual Review of Genetics 45, 405–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler, F. (2017). Yeasts in Pucciniomycotina. Mycological Progress 16, 831–856. [Google Scholar]
- Ohsowski, B. M. , Zaitsoff, P. D. , Öpik, M. & Hart, M. M. (2014). Where the wild things are: looking for uncultured Glomeromycota. New Phytologist 204, 171–179. [DOI] [PubMed] [Google Scholar]
- Olive, L. S. (1980). Caulochytrium protostelioides sp. nov., a new chytrid with aerial sporangia. American Journal of Botany 67, 568–574. [Google Scholar]
- Orchard, S. , Hilton, S. , Bending, G. D. , Dickie, I. A. , Standish, R. J. , Gleeson, D. B. , Jeffery, R. P. , Powell, J. R. , Walker, C. , Bass, D. , Monk, J. , Simonin, A. & Ryan, M. H. (2017). Fine endophytes (Glomus tenue) are related to Mucoromycotina, not Glomeromycota. New Phytologist 213, 481–486. [DOI] [PubMed] [Google Scholar]
- Oren, A. & Gunde‐Cimerman, N. (2012). Fungal life in the Dead Sea. Progress in Molecular and Subcellular Biology 53, 115–132. [DOI] [PubMed] [Google Scholar]
- Padamsee, M. , Arun Kumar, T. K. , Riley, R. , Binder, M. , Boyd, A. , Calvo, A. M. , Furukawa, K. , Hesse, C. , Hohmann, S. , James, T. Y. , Labutti, K. , Lapidus, A. , Lindquist, E. , Lucas, S. , Miller, K. , et al. (2012). The genome of the xerotolerant mold Wallemia sebi reveals adaptations to osmotic stress and suggests cryptic sexual reproduction. Fungal Genetics and Biology 49, 217–226. [DOI] [PubMed] [Google Scholar]
- Papanikolaou, S. , Galiotou‐Panayotou, M. , Fakas, S. , Komaitis, M. & Aggelis, G. (2007). Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. European Journal of Lipid Science and Technology 109, 1060–1070. [Google Scholar]
- Penton, C. R. , StLouis, D. , Cole, J. R. , Luo, Y. , Wu, L. , Schuur, E. A. G. , Zhou, J. & Tiedje, J. M. (2013). Fungal diversity in permafrost and tallgrass prairie soils under experimental warming conditions. Applied and Environmental Microbiology 79, 7063–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovits, T. , Nagy, L. G. , Hoffmann, K. , Wagner, L. , Nyilasi, I. , Griebel, T. , Schnabelrauch, D. , Vogel, H. , Voigt, K. , Vágvölgyi, C. & Papp, T. (2011). Data partitions, Bayesian analysis and phylogeny of the zygomycetous fungal family Mortierellaceae, inferred from nuclear ribosomal DNA sequences. PLoS One 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyretaillade, E. , El Alaoui, H. , Diogon, M. , Polonais, V. , Parisot, N. , Biron, D. G. , Peyret, P. & Delbac, F. (2011). Extreme reduction and compaction of microsporidian genomes. Research in Microbiology 162, 598–606. [DOI] [PubMed] [Google Scholar]
- Porollo, A. , Sesterhenn, T. M. , Collins, M. S. , Welge, J. A. & Cushion, M. T. (2014). Comparative genomics of Pneumocystis species suggests the absence of genes for myo‐inositol synthesis and reliance on inositol transport and metabolism. MBio 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, T. M. , Schadt, C. W. , Rizvi, L. , Martin, A. P. , Schmidt, S. K. , Scott‐Denton, L. , Vilgalys, R. & Moncalvo, J. M. (2008). Widespread occurrence and phylogenetic placement of a soil clone group adds a prominent new branch to the fungal tree of life. Molecular Phylogenetics and Evolution 46, 635–644. [DOI] [PubMed] [Google Scholar]
- Porter, T. M. , Martin, W. , James, T. Y. , Longcore, J. E. , Gleason, F. H. , Adler, P. H. , Letcher, P. M. & Vilgalys, R. (2011). Molecular phylogeny of the Blastocladiomycota (Fungi) based on nuclear ribosomal DNA. Fungal Biology 115, 381–392. [DOI] [PubMed] [Google Scholar]
- Powell, M. J. (1984). Fine structure of the unwalled thallus of Rozella polyphagi in its host Polyphagus euglenae . Mycologia 76, 1039–1048. [Google Scholar]
- Powell, M. J. & Letcher, P. M. (2014). Chytridiomycota, Monoblepharidomycota, and Neocallimastigomycota In Systematics and Evolution: Part A, Second Edition (eds Karl Esser), pp. 141–175. Springer, Berlin Heidelberg. [Google Scholar]
- Powell, M. J. , Letcher, P. M. & James, T. Y. (2017). Ultrastructural characterization of the host–parasite interface between Allomyces anomalus (Blastocladiomycota) and Rozella allomycis (Cryptomycota). Fungal Biology 121, 561–572. [DOI] [PubMed] [Google Scholar]
- Prasanna, A. N. , Gerber, D. , Kijpornyongpan, T. , Aime, M. C. , Doyle, V. P. & Nagy, L. G. (2019). Model choice, missing data, and taxon sampling impact phylogenomic inference of deep Basidiomycota relationships. Systematic Biology 0, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto, M. , Baloch, E. , Tehler, A. & Wedin, M. (2013). Mazaedium evolution in the Ascomycota (Fungi) and the classification of mazaediate groups of formerly unclear relationship. Cladistics 29, 296–308. [DOI] [PubMed] [Google Scholar]
- Proux‐Wéra, E. , Byrne, K. P. & Wolfe, K. H. (2013). Evolutionary mobility of the ribosomal DNA array in yeasts. Genome Biology and Evolution 5, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt, C. A. , Beaudet, D. , Corsaro, D. , Walochnik, J. , Michel, R. , Corradi, N. & James, T. Y. (2017). The genome of an intranuclear parasite, Paramicrosporidium saccamoebae, reveals alternative adaptations to obligate intracellular parasitism. eLife 6, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek, R. , Wurzbacher, C. , Gisder, S. , Nilsson, R. H. , Owerfeldt, A. , Genersch, E. , Kirk, P. M. & Voigt, K. (2017). Morphologic and molecular data help adopting the insect‐pathogenic nephridiophagids (Nephridiophagidae) among the early diverging fungal lineages, close to the Chytridiomycota. MycoKeys 25, 31–50. [Google Scholar]
- Rasconi, S. , Niquil, N. & Sime‐Ngando, T. (2012). Phytoplankton chytridiomycosis: community structure and infectivity of fungal parasites in aquatic ecosystems. Environmental Microbiology 14, 2151–2170. [DOI] [PubMed] [Google Scholar]
- Raudaskoski, M. & Kothe, E. (2010). Basidiomycete mating type genes and pheromone signaling. Eukaryotic Cell 9, 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redecker, D. & Schüßler, A. (2014). Glomeromycota In Systematics and Evolution, pp. 251–269. Springer, Berlin Heidelberg. [Google Scholar]
- Ren, R. , Sun, Y. , Zhao, Y. , Geiser, D. , Ma, H. & Zhou, X. (2016). Phylogenetic resolution of deep eukaryotic and fungal relationships using highly conserved low‐copy nuclear genes. Genome Biology and Evolution 8, 2683–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, N. K. , Benny, G. L. , Ho, H.‐M. , Hou, Y.‐H. , Crous, P. W. & Smith, M. E. (2019). Phylogenetic and morphological analyses of the mycoparasitic genus Piptocephalis . Mycologia 111, 54–68. [DOI] [PubMed] [Google Scholar]
- Rhind, N. , Chen, Z. , Yassour, M. , Thompson, D. A. , Haas, B. J. , Habib, N. , Wapinski, I. , Roy, S. , Lin, M. F. , Heiman, D. I. , Young, S. K. , Furuya, K. , Guo, Y. , Pidoux, A. , Chen, H. M. , Robbertse, B. , Goldberg, J. M. , Aoki, K. , Bayne, E. H. , Berlin, A. M. , Desjardins, C. A. , Dobbs, E. , Dukaj, L. , Fan, L. , FitzGerald, M. G. , French, C. , Gujja, S. , Hansen, K. , Keifenheim, D. , Levin, J. Z. , Mosher, R. A. , Muller, C. A. , Pfiffner, J. , Priest, M. , Russ, C. , Smialowska, A. , Swoboda, P. , Sykes, S. M. , Vaughn, M. , Vengrova, S. , Yoder, R. , Zeng, Q. , Allshire, R. , Baulcombe, D. , Birren, B. W. , Brown, W. , Ekwall, K. , Kellis, M. , Leatherwood, J. , Levin, H. , Margalit, H. , Martienssen, R. , Nieduszynski, C. A. , Spatafora, J. W. , Friedman, N. , Dalgaard, J. Z. , Baumann, P. , Niki, H. , Regev, A. & Nusbaum, C. (2011). Comparative functional genomics of the fission yeasts. Science 332, 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, T. A. , Leonard, G. , Mahé, F. , Del Campo, J. , Romac, S. , Jones, M. D. M. , Maguire, F. , Dunthorn, M. , De Vargas, C. , Massana, R. & Chambouvet, A. (2015). Molecular diversity and distribution of marine fungi across 130 European environmental samples. Proceedings of the Royal Society Biological Sciences 282, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, T. A. , Leonard, G. & Wideman, J. G. (2017). What defines the “Kingdom” Fungi? Microbiology Spectrum 5, 1–21. [DOI] [PubMed] [Google Scholar]
- Riess, K. , Bauer, R. , Kellner, R. , Kemler, M. , Piątek, M. , Vánky, K. & Begerow, D. (2015). Identification of a new order of root‐colonising fungi in the Entorrhizomycota: Talbotiomycetales ord. nov. on eudicotyledons. IMA Fungus 6, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, R. , Salamov, A. A. , Brown, D. W. , Nagy, L. G. , Floudas, D. , Held, B. W. , Levasseur, A. , Lombard, V. , Morin, E. , Otillar, R. , Lindquist, E. A. , Sun, H. , LaButti, K. M. , Schmutz, J. , Jabbour, D. , et al. (2014). Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white‐rot/brown‐rot paradigm for wood decay fungi. PNAS 111, 9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson, R. W. , Saucedo, E. , Maclean, D. , Propster, J. , Unger, B. , Oneil, T. A. , Parvanehgohar, K. , Cavanaugh, C. & Lowry, D. (2011). The hyphal tip structure of Basidiobolus sp.: a zygomycete fungus of uncertain phylogeny. Fungal Biology 115, 485–492. [DOI] [PubMed] [Google Scholar]
- Rosewich, U. L. & Kistler, H. C. (2000). Role of horizontal gene transfer in the evolution of Fungi. Annual Review of Phytopathology 38, 63. [DOI] [PubMed] [Google Scholar]
- Rosling, A. , Cox, F. , Cruz‐Martinez, K. , Ihrmark, K. , Grelet, G.‐A. , Lindahl, B. D. , Menkis, A. & James, T. Y. (2011). Archaeorhizomycetes: unearthing an ancient class of ubiquitous soil fungi. Science 333, 876–879. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Herrera, J. & Ortiz‐Castellanos, L. (2010). Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi. FEMS Yeast Research 10, 225–243. [DOI] [PubMed] [Google Scholar]
- Saikawa, M. (2011). Ultrastructural studies on zygomycotan fungi in the Zoopagaceae and Cochlonemataceae. Mycoscience 52, 83–90. [Google Scholar]
- Sanders, I. R. (2011). Fungal sex: meiosis machinery in ancient symbiotic Fungi. Current Biology 21, 896–897. [DOI] [PubMed] [Google Scholar]
- Schell, W. A. , Lee, A. G. & Aime, M. C. (2011). A new lineage in Pucciniomycotina: class Tritirachiomycetes, order Tritirachiales, family Tritirachiaceae. Mycologia 103, 1331–1340. [DOI] [PubMed] [Google Scholar]
- Scheuer, C. , Bauer, R. , Lutz, M. , Stabentheiner, E. , Mel'nik, V. A. & Grube, M. (2008). Bartheletia paradoxa is a living fossil on Ginkgo leaf litter with a unique septal structure in the Basidiomycota. Mycological Research 112, 1265–1279. [DOI] [PubMed] [Google Scholar]
- Schoch, C. L. , Sung, G.‐H. , López‐Giráldez, F. , Townsend, J. P. , Miadlikowska, J. , Hofstetter, V. , Robbertse, B. , Matheny, P. B. , Kauff, F. , Wang, Z. , Gueidan, C. , Andrie, R. M. , Trippe, K. , Ciufetti, L. M. , Wynns, A. , Fraker, E. , Hodkinson, B. P. , Bonito, G. , Groenewald, J. Z. , Arzanlou, M. , de Hoog, G. S. , Crous, P. W. , Hewitt, D. , Pfister, D. H. , Peterson, K. , Gryzenhout, M. , Wingfield, M. J. , Aptroot, A. , Suh, S. O. , Blackwell, M. , Hillis, D. M. , Griffith, G. W. , Castlebury, L. A. , Rossman, A. Y. , Lumbsch, H. T. , Lücking, R. , Büdel, B. , Rauhut, A. , Diederich, P. , Ertz, D. , Geiser, D. M. , Hosaka, K. , Inderbitzin, P. , Kohlmeyer, J. , Volkmann‐Kohlmeyer, B. , Mostert, L. , O'Donnell, K. , Sipman, H. , Rogers, J. D. , Shoemaker, R. A. , Sugiyama, J. , Summerbell, R. C. , Untereiner, W. , Johnston, P. R. , Stenroos, S. , Zuccaro, A. , Dyer, P. S. , Crittenden, P. D. , Cole, M. S. , Hansen, K. , Trappe, J. M. , Yahr, R. , Lutzoni, F. & Spatafora, J. W. (2009). The Ascomycota tree of life: a phylum‐wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58, 224–239. [DOI] [PubMed] [Google Scholar]
- Schoch, C. L. , Robbertse, B. , Robert, V. , Vu, D. , Cardinali, G. , Irinyi, L. , Meyer, W. , Nilsson, R. H. , Hughes, K. , Miller, A. N. , Kirk, P. M. , Abarenkov, K. , Aime, M. C. , Ariyawansa, H. A. , Bidartondo, M. , et al. (2014). Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüßler, A. , Martin, H. , Cohen, D. , Fitz, M. & Wipf, D. (2007). Arbuscular mycorrhiza: studies on the Geosiphon symbiosis lead to the characterization of the first glomeromycotan sugar transporter. Plant Signaling & Behavior 2, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüβler, A. , Schwarzott, D. & Walker, C. (2001). A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research 105, 1413–1421. [Google Scholar]
- Sekimoto, S. , Rochon, D. , Long, J. E. , Dee, J. M. & Berbee, M. L. (2011). A multigene phylogeny of Olpidium and its implications for early fungal evolution. BMC Evolutionary Biology 11, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serris, A. , Danion, F. , Lanternier, F. , Serris, A. , Danion, F. & Lanternier, F. (2019). Disease entities in mucormycosis. Journal of Fungi 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X.‐X. , Zhou, X. , Kominek, J. , Kurtzman, C. P. , Hittinger, C. T. & Rokas, A. (2016). Reconstructing the backbone of the Saccharomycotina yeast phylogeny using genome‐scale data. G3: Genes . Genomes, Genetics 6, 3927–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirouzu, T. , Hirose, D. , Oberwinkler, F. , Shimomura, N. , Maekawa, N. & Tokumasu, S. (2013). Combined molecular and morphological data for improving phylogenetic hypothesis in Dacrymycetes. Mycologia 105, 1110–1125. [DOI] [PubMed] [Google Scholar]
- Shirouzu, T. , Uno, K. , Hosaka, K. & Hosoya, T. (2016). Early‐diverging wood‐decaying fungi detected using three complementary sampling methods. Molecular Phylogenetics and Evolution 98, 11–20. [DOI] [PubMed] [Google Scholar]
- Sigoillot, J. C. , Berrin, J. G. , Bey, M. , Lesage‐Meessen, L. , Levasseur, A. , Lomascolo, A. , Record, E. & Uzan‐Boukhris, E. (2012). Fungal strategies for lignin degradation. Advances in Botanical Research 61, 263–308. [Google Scholar]
- Spatafora, J. W. , Chang, Y. , Benny, G. L. , Lazarus, K. , Smith, M. E. , Berbee, M. L. , Bonito, G. , Corradi, N. , Grigoriev, I. , Gryganskyi, A. , James, T. Y. , O'Donnell, K. , Roberson, R. W. , Taylor, T. N. , Uehling, J. , Vilgalys, R. , White, M. M. & Stajich, J. E. (2016). A phylum‐level phylogenetic classification of zygomycete fungi based on genome‐scale data. Mycologia 108, 1028–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora, J. W. , Aime, M. C. , Grigoriev, I. V. , Martin, F. M. , Stajich, J. E. & Blackwell, M. (2017a). The fungal tree of life: from molecular systematics to genome‐scale phylogenies In The Fungal Kingdom (eds J. Heitman, B. J. Howlett, P. W. Crous, E. H. Stukenbrock, T. Y. James and N. A. R. Gow), pp. 3–34. Springer, Cham. [DOI] [PubMed] [Google Scholar]
- Spatafora, J. W. , Aime, M. C. , Grigoriev, I. V. , Martin, F. , Stajich, J. E. & Blackwell, M. (2017b). The Fungal Tree of Life: from molecular systematics to senome‐scale phylogenies. Microbiology Spectrum 5, 3–34. [DOI] [PubMed] [Google Scholar]
- Stairs, C. W. , Leger, M. M. & Roger, A. J. (2015). Diversity and origins of anaerobic metabolism in mitochondria and related organelles. Philosophical Transactions of the Royal Society of London. Series B . Biological Sciences 370, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stajich, J. E. , Berbee, M. L. , Blackwell, M. , Hibbett, D. S. , James, T. Y. , Spatafora, J. W. & Taylor, J. W. (2009). The Fungi. Current Biology 19, 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer, S. L. (2012). A history of the taxonomy and systematics of arbuscular mycorrhizal fungi belonging to the phylum Glomeromycota. Mycorrhiza 22, 247–258. [DOI] [PubMed] [Google Scholar]
- Sugita, T. & Nakase, T. (1999). Non‐universal usage of the leucine CUG codon and the molecular phylogeny of the genus Candida . Systematic and Applied Microbiology 22, 79–86. [DOI] [PubMed] [Google Scholar]
- Sugiyama, J. , Hosaka, K. & Suh, S.‐O. (2006). Early diverging Ascomycota: phylogenetic divergence and related evolutionary enigmas. Mycologia 98, 996–1005. [DOI] [PubMed] [Google Scholar]
- Takishita, K. , Tsuchiya, M. , Kawato, M. , Oguri, K. , Kitazato, H. & Maruyama, T. (2007). Genetic diversity of microbial eukaryotes in anoxic sediment of the saline meromictic lake Namako‐ike (Japan): on the detection of anaerobic or anoxic‐tolerant lineages of eukaryotes. Protist 158, 51–64. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y. , O'Donnell, K. , Saikawa, M. & Sugiyama, J. (2000). Molecular phylogeny of parasitic zygomycota (Dimargaritales, zoopagales) based on nuclear small subunit ribosomal DNA sequences. Molecular Phylogenetics and Evolution 16, 253–262. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y. , Saikawa, M. , Watanabe, M. M. & Sugiyama, J. (2004). Molecular phylogeny of Zygomycota based on EF‐1α and RPB1 sequences: limitations and utility of alternative markers to rDNA. Molecular Phylogenetics and Evolution 30, 438–449. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y. , Watanabe, M. M. & Sugiyama, J. (2005). Evolutionary relationships among basal fungi (Chytridiomycota and Zygomycota): insights from molecular phylogenetics. Journal of General and Applied Microbiology 51, 267–276. [DOI] [PubMed] [Google Scholar]
- Tavares, S. , Ramos, A.P. , Pires, A.S. , Azinheira, H.G. , Caldeirinha, P. , Link, T. , Abranches, R. , Silva, M. do C., Voegele, R.T. , Loureiro, J. & Talhinhas, P. (2014). Genome size analyses of Pucciniales reveal the largest fungal genomes. Frontiers in Plant Science 5, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo, L. & Lindahl, B. (2016). Fungal identification biases in microbiome projects. Environmental Microbiology Reports 8, 774–779. [DOI] [PubMed] [Google Scholar]
- Tedersoo, L. , Bahram, M. , Põlme, S. , Kõljalg, U. , Yorou, N. S. , Wijesundera, R. , Ruiz, L. V. , Vasco‐Palacios, A. M. , Thu, P. Q. , Suija, A. , Smith, M. E. , Sharp, C. , Saluveer, E. , Saitta, A. , Rosas, M. , et al. (2014). Global diversity and geography of soil fungi. Science 346, 1256688. [DOI] [PubMed] [Google Scholar]
- Tedersoo, L. , Bahram, M. , Puusepp, R. , Nilsson, R. H. & James, T. Y. (2017). Novel soil‐inhabiting clades fill gaps in the fungal tree of life. Microbiome 45, 5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo, L. , Sánchez‐Ramírez, S. , Kõljalg, U. , Bahram, M. , Döring, M. , Schigel, D. , May, T. , Ryberg, M. & Abarenkov, K. (2018). High‐level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Diversity 1, 135–159. [Google Scholar]
- Thorsen, M. S. (1999). Abundance and biomass of the gut‐living microorganisms (bacteria, protozoa and fungi) in the irregular sea urchin Echinocardium cordatum (Spatangoida: Echinodermata). Marine Biology 133, 353–360. [Google Scholar]
- Tisserant, E. , Malbreil, M. , Kuo, A. , Kohler, A. , Symeonidi, A. , Balestrini, R. , Charron, P. , Duensing, N. , Frei dit Frey, N. , Gianinazzi‐Pearson, V. , Gilbert, L. B. , Handa, Y. , Herr, J. R. , Hijri, M. , Koul, R. , et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America 110, 20117–20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Cruz, T. J. , Billingsley Tobias, T. L. , Almatruk, M. , Hesse, C. N. , Kuske, C. R. , Desirò, A. , Benucci, G. M. N. , Bonito, G. , Stajich, J. E. , Dunlap, C. , Arnold, A. E. & Porras‐Alfaro, A. (2017). Bifiguratus adelaidae, gen. et sp. nov., a new member of Mucoromycotina in endophytic and soil‐dwelling habitats. Mycologia 109, 363–378. [DOI] [PubMed] [Google Scholar]
- Torruella, G. , Derelle, R. , Paps, J. , Lang, B. F. , Roger, A. J. , Shalchian‐Tabrizi, K. & Ruiz‐Trillo, I. (2012). Phylogenetic relationships within the Opisthokonta based on phylogenomic analyses of conserved single‐copy protein domains. Molecular Biology and Evolution 29, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruella, G. , Grau‐Bové, X. , Moreira, D. , Karpov, S. A. , Burns, J. A. , Sebé‐Pedrós, A. , Völcker, E. & López‐García, P. (2018). Global transcriptome analysis of the aphelid Paraphelidium tribonemae supports the phagotrophic origin of fungi. Communications Biology 1, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treseder, K. K. & Lennon, J. T. (2015). Fungal traits that drive ecosystem dynamics on land. Microbiology and Molecular Biology Reviews 79, 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter, E. D. , Johnson, E. M. , Wang, Y. , Kandel, P. & White, M. M. (2013). Examining new phylogenetic markers to uncover the evolutionary history of early‐diverging fungi: comparing MCM7, TSR1 and rRNA genes for single‐ and multi‐gene analyses of the Kickxellomycotina. Persoonia 30, 106–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter, E. D. , Johnson, E. M. , Benny, G. L. , Lichtwardt, R. W. , Wang, Y. , Kandel, P. , Novak, S. J. , Smith, J. F. & White, M. M. (2014). An eight‐gene molecular phylogeny of the Kickxellomycotina, including the first phylogenetic placement of Asellariales. Mycologia 106, 912–935. [DOI] [PubMed] [Google Scholar]
- Ustinova, I. , Krienitz, L. & Huss, V. A. R. (2000). Hyaloraphidium curvatum is not a green alga, but a lower fungus; Amoebidium parasiticum is not a fungus, but a member of the DRIPs. Protist 151, 253–262. [DOI] [PubMed] [Google Scholar]
- Vánky, K. , Bauer, R. & Begerow, D. (2007). Talbotiomyces, a new genus for Entorrhiza calospora (Basidiomycota). Mycologica Balcanica 4, 11–14. [Google Scholar]
- Voigt, K. & Wöstemeyer, J. (2001). Phylogeny and origin of 82 zygomycetes from all 54 genera of the Mucorales and Mortierellales based on combined analysis of actin and translation elongation factor EF‐1α genes. Gene 270, 113–120. [DOI] [PubMed] [Google Scholar]
- Voos, J. R. (1969). Morphology and life cycle of a new chytrid with aerial sporangia. American Journal of Botany 56, 898–909. [Google Scholar]
- Wagner, L. , Stielow, B. , Hoffmann, K. , Petkovits, T. , Papp, T. , Vágvölgyi, C. , de Hoog, G. S. , Verkley, G. & Voigt, K. (2013). A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia 30, 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright, M. , Wickramasinghe, N. C. , Narlikar, J. V. & Rajaratnam, P. (2003). Microorganisms cultured from stratospheric air samples obtained at 41 km. FEMS Microbiology Letters 218, 161–165. [DOI] [PubMed] [Google Scholar]
- Wang, Q.‐M. , Theelen, B. , Groenewald, M. , Bai, F.‐Y. & Boekhout, T. (2014). Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Persoonia 33, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q.‐M. , Begerow, D. , Groenewald, M. , Liu, X.‐Z. , Theelen, B. , Bai, F.‐Y. & Boekhout, T. (2015a). Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Studies in Mycology 81, 55–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q.‐M. , Groenewald, M. , Takashima, M. , Theelen, B. , Han, P.‐J. , Liu, X.‐Z. , Boekhout, T. & Bai, F.‐Y. (2015b). Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequence analyses. Studies in Mycology 81, 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q.‐M. , Yurkov, A. M. , Göker, M. , Lumbsch, H. T. , Leavitt, S. D. , Groenewald, M. , Theelen, B. , Liu, X.‐Z. , Boekhout, T. & Bai, F.‐Y. (2015c). Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Studies in Mycology 81, 149–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Liu, X. & Groenewald, J. Z. (2017). Phylogeny of anaerobic fungi (phylum Neocallimastigomycota), with contributions from yak in China. Antonie Van Leeuwenhoek 110, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Youssef, N. , Couger, M. , Hanafy, R. , Elshahed, M. & Stajich, J.E. (2019). Molecular dating of the emergence of anaerobic rumen fungi and the impact of laterally acquired genes. bioRxiv, 10.1101/401869. [DOI] [PMC free article] [PubMed]
- Weete, J. D. , Abril, M. & Blackwell, M. (2010). Phylogenetic distribution of fungal sterols. PLoS One 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, S. , Peršoh, D. & Rambold, G. (2018). Insights into fungal communities colonizing the acarosphere in a forest soil habitat. Mycological Progress 17, 1067–1085. [Google Scholar]
- White, M. M. , James, T. Y. , O'Donnell, K. , Cafaro, M. J. , Tanabe, Y. & Sugiyama, J. (2006). Phylogeny of the Zygomycota based on nuclear ribosomal sequence data. Mycologia 98, 872–884. [DOI] [PubMed] [Google Scholar]
- Whittaker, R. H. (1969). New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms. Science 163, 150–160. [DOI] [PubMed] [Google Scholar]
- wikimedia (2011). Wikimedia Strategic Plan ‐ A collaborative vision for the movement through 2015. Wikimedia Foundation Electronic file available at https://upload.wikimedia.org/wikipedia/commons/c/c0/WMF_StrategicPlan2011_spreads.pdf/nhttps://www.ndi.org/files/Handout 5 ‐ Wikimedia Strategic Plan.pdf. Accessed 01.11.2018.
- Wisecaver, J. H. & Rokas, A. (2015). Fungal metabolic gene clusters: caravans traveling across genomes and environments. Frontiers in Microbiology 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisecaver, J. H. , Slot, J. C. & Rokas, A. (2014). The evolution of fungal metabolic pathways. PLoS Genetics 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowska, I. (2017). The leather underground: biofabrication offers new sources for fabrics. AATCC Review 17, 18–23. [Google Scholar]
- Wolfe, K. H. (2006). Comparative genomics and genome evolution in yeasts. Philosophical Transactions of the Royal Society: Biological Sciences 361, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylezich, C. , Radek, R. & Schlegel, M. (2004). Phylogenetische Analyse der 18S rRNA identifiziert den parasitischen Protisten Nephridiophaga blattellae (Nephridiophagidae) als Vertreter der Zygomycota (Fungi). Denisia 13, 435–442. [Google Scholar]
- Xu, Y. & Weiss, L. M. (2005). The microsporidian polar tube: a highly specialised invasion organelle. International Journal for Parasitology 35, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, N. H. , Couger, M. B. , Struchtemeyer, C. G. , Liggenstoffer, A. S. , Prade, R. A. , Najar, F. Z. , Atiyeh, H. K. , Wilkins, M. R. & Elshahed, M. S. (2013). The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Applied and Environmental Microbiology 79, 4620–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajc, J. , Liu, Y. , Dai, W. , Yang, Z. , Hu, J. , Gostinčar, C. & Gunde‐Cimerman, N. (2013). Genome and transcriptome sequencing of the halophilic fungus Wallemia ichthyophaga: haloadaptations present and absent. BMC Genomics 14, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalar, P. , Sybren de Hoog, G. , Schroers, H. J. , Frank, J. M. & Gunde‐Cimerman, N. (2005). Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie Van Leeuwenhoek 87, 311–328. [DOI] [PubMed] [Google Scholar]
- Zhao, R.‐L. , Desjardin, D. E. , Soytong, K. & Hyde, K. D. (2008). Advances in the phylogenesis of Agaricales and its higher ranks and strategies for establishing phylogenetic hypotheses. Journal of Zhejiang University. Science. B 9, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R.‐L. , Li, G.‐J. , Sánchez‐Ramírez, S. , Stata, M. , Yang, Z.‐L. , Wu, G. , Dai, Y.‐C. , He, S.‐H. , Cui, B.‐K. , Zhou, J.‐L. , Wu, F. , He, M.‐Q. , Moncalvo, J.‐M. & Hyde, K. D. (2017). A six‐gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Diversity 84, 43–74. [Google Scholar]
- Ziaee, A. , Zia, M. , Bayat, M. & Hashemi, J. (2016). Identification of Mucorales isolates from soil using morphological and molecular methods. Current Medical Mycology 2, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


