Summary
Background
Global concern about vitamin D deficiency has fuelled debates on photoprotection and the importance of solar exposure to meet vitamin D requirements.
Objectives
To review the published evidence to reach a consensus on the influence of photoprotection by sunscreens on vitamin D status, considering other relevant factors.
Methods
An international panel of 13 experts in endocrinology, dermatology, photobiology, epidemiology and biological anthropology reviewed the literature prior to a 1‐day meeting in June 2017, during which the evidence was discussed. Methods of assessment and determining factors of vitamin D status, and public health perspectives were examined and consequences of sun exposure and the effects of photoprotection were assessed.
Results
A serum level of ≥ 50 nmol L−1 25(OH)D is a target for all individuals. Broad‐spectrum sunscreens that prevent erythema are unlikely to compromise vitamin D status in healthy populations. Vitamin D screening should be restricted to those at risk of hypovitaminosis, such as patients with photosensitivity disorders, who require rigorous photoprotection. Screening and supplementation are advised for this group.
Conclusions
Sunscreen use for daily and recreational photoprotection does not compromise vitamin D synthesis, even when applied under optimal conditions.
What's already known about this topic?
Knowledge of the relationship between solar exposure behaviour, sunscreen use and vitamin D is important for public health but there is confusion about optimal vitamin D status and the safest way to achieve this.
Practical recommendations on the potential impact of daily and/or recreational sunscreens on vitamin D status are lacking for healthy people.
What does this study add?
Judicious use of daily broad‐spectrum sunscreens with high ultraviolet (UV) A protection will not compromise vitamin D status in healthy people.
However, photoprotection strategies for patients with photosensitivity disorders that include high sun‐protection factor sunscreens with high UVA protection, along with protective clothing and shade‐seeking behaviour are likely to compromise vitamin D status.
Screening for vitamin D status and supplementation are recommended in patients with photosensitivity disorders.
Short abstract
Linked Comment: https://doi.org/10.1111/bjd.18126.
https://doi.org/10.1111/bjd.18494 available online
The prevention of rickets and osteoporosis by vitamin D has long been established. More recently, vitamin D has been implicated in many metabolic and immunological disorders as well as many cancers. Its pleiotropic activity may be mediated by modulation of ~1000 genes via the vitamin D receptor (VDR),1, 2 which is expressed by at least 60 human cell types.3 The VDR controls many cellular functions including growth, differentiation and apoptosis. However, the role of vitamin D in the prevention of nonskeletal diseases remains highly controversial.4, 5, 6, 7, 8
Terrestrial ultraviolet radiation (UVR) is the main determinant of vitamin D status. Stratospheric ozone absorbs all solar UVC (100–280 nm), attenuates UVB (280–315 nm) but not UVA (315–400 nm). The sun's height determines the UVR pathlength through the ozone layer. Thus, UVB intensity (irradiance) depends mainly on latitude, season and time of day. The ratio of UVA to UVB also varies with the sun's height because of the differential effect of the ozone layer. Thus, terrestrial UVR typically contains ≤ 5% UVB (~295–315 nm) and ≥ 95% UVA.
The minor UVB component is responsible for vitamin D synthesis,9 the initiating event of which is the isomerization of the epidermal chromophore (a UVR‐absorbing molecule) 7‐dehydrocholesterol (7‐DHC) into pre‐vitamin D3, which is thermally converted into cholecalciferol (vitamin D3).10 Pre‐vitamin D3 increases linearly as a function of time of exposure to UVR (i.e. dose) over a period of 30 min.11 Vitamin D3 enters the circulation via the vitamin D binding protein (DBP) and is hydroxylated into 25‐hydroxyvitamin D3 [25(OH)D3] in the liver [by vitamin D3‐25‐hydroxylase (CYP2R1)], and then in the kidney [by 25(OH)D3‐1α‐hydroxylase (CYP27B1)] to 1,25‐dihydroxyvitamin D3 [1,25(OH)2D3], the active form of vitamin D (calcitriol), which in fact is a hormone. However, many tissues including the skin12 also contain both hydroxylases for the synthesis of calcitriol.
Multiple intrinsic and extrinsic factors modulate vitamin D synthesis and overall status, including genetic polymorphisms, age, geographical location, sun exposure behaviour, UVB dose, clothing, body surface area (BSA) exposed.13 These are summarized in Figure 1, 14 and Appendix S1 (see Supporting Information). Vitamin D3 may also be obtained from supplementation and/or animal‐based foods (e.g. oily fish) and undergoes the same hydroxylations. Alternatively, vitamin D2 from nonanimal dietary uptake (e.g. mushrooms), is hydroxylated into 25(OH)D2 and then converted into 1,25(OH)2D2 (ergocalciferol). However, in general, intake from diet is low. For example, food intake in the U.S.A. between 2005 and 2006 in 19–30‐year‐old males and females was 204 IU ± 12 (5·1 μg) and 144 IU ± 12 (3·6 μg), respectively, which represents 34% and 24% of the recommended dietary allowance (RDA).15
Figure 1.
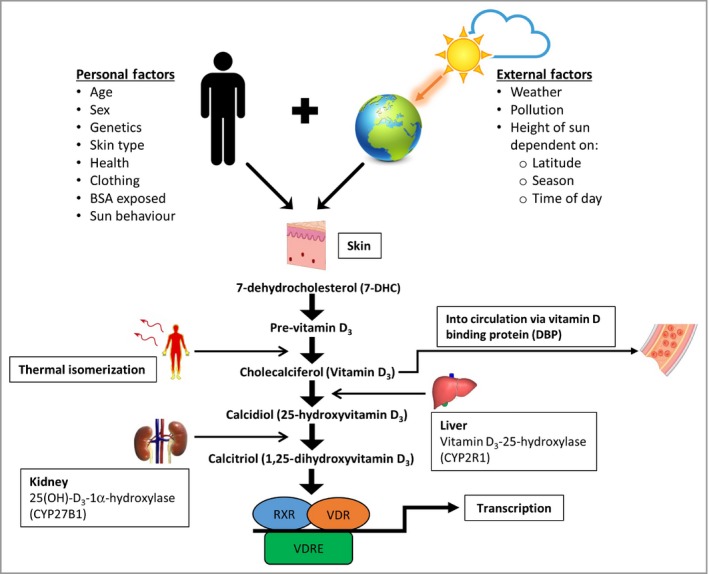
Factors that affect the synthesis of vitamin D3. Many factors determine vitamin D3 production. The most important external factor is UVB dose, which is the product of UVB intensity (irradiance) and exposure time. Cutaneous pre‐vitamin D3 is synthesized from 7‐dehydrocholesterol after UVB exposure. Thermally converted into vitamin D3, it then binds to vitamin D binding protein (DBP) in the blood to be activated sequentially by the liver and kidney. Cytochrome P450 (CYP) enzymes are crucial for the synthesis of biologically active vitamin D3 (calcitriol), which binds to intracellular vitamin D receptor (VDR) in most cells in the body. Adapted from Jolliffe et al.14 More details of these factors are given in the Supporting Information. BSA, body surface area; RXR, retinoid X receptor; VDRE, vitamin D response element.
Solar UVR has many adverse effects, the most obvious of which is sunburn (erythema). The World Health Organization has defined the global solar UV index (UVI) (http://www.who.int/uv/publications/en/UVIGuide.pdf) to allow comparisons of erythemal potential at various geographical locations (latitudes), seasons and times of day.16 This is a numerical index of the erythemally weighted irradiance of terrestrial UVR. It is divided into five bands: ‘low’ (1–2), ‘moderate’ (3–5), ‘high’ (6–7), ‘very high’ (8–10) and ‘extreme’ (≥ 11). The UVI is primarily an index of UVB irradiance because this spectral region is the main cause of erythema (see Conclusions and recommendations: Spectral considerations: Ultraviolet B, below) and sun protection is advised when the UVI is ≥ 3.17
Global concern about vitamin D deficiency has fuelled debates on the importance of solar exposure to meet vitamin D requirements.18, 19, 20, 21 The acute and chronic health benefits of using sunscreens are established22 but there has been concern about their possible impact on vitamin D status. An international panel was tasked to review the published evidence to reach a consensus on the influence of photoprotection by sunscreens on vitamin D status, considering other relevant factors.
Methods
The panel comprised experts from diverse disciplines including vitamin D, endocrinology, dermatology, photoprotection, experimental photobiology, epidemiology and anthropology. Panel members made a comprehensive search of literature published from January 1996 to May 2017, using the Scopus database, with the following search term categories individually and in combination: vitamin D, status, level, values, deficiency, measurement, assay, dosage, evaluation, polymorphisms, genetics, diet, phototype, pigmentation, lifestyle, location, latitude, sun, UV, UVR, ultraviolet, health, diseases, sunscreen, photoprotection or sun protection. Members of the panel used their specific areas of expertise to identify relevant papers and presented and discussed their results at a meeting in Paris in June 2017. The panel discussion was recorded by a scientific writer and used as the basis of the manuscript. Additional 2017–19 references were included during the writing process. This article summarizes the consensus and provides clinical recommendations in terms of photoprotection in order to ensure optimal vitamin D status.
Conclusions and recommendations from panel discussions
What is optimal vitamin D status and the best method to determine it?
Serum 25(OH)D is the best indicator of vitamin D status but there is no international consensus on its optimal value, with recommendations varying from 25 nmol L−1 to > 100 nmol L−1.23 Figure 2 summarizes the definitions of vitamin D status by various international bodies.23 The most widely held consensus for the boundary between insufficiency and sufficiency is 50 nmol L−1. According to the Institute of Medicine (IOM),15 a serum concentration of 50 nmol L−1 25(OH)D meets or exceeds the requirement of 97·5% of the U.S. population, but it is not possible to specify desired individual status.23 The determination of vitamin D status is discussed in Appendix S2 (see Supporting Information).
Figure 2.
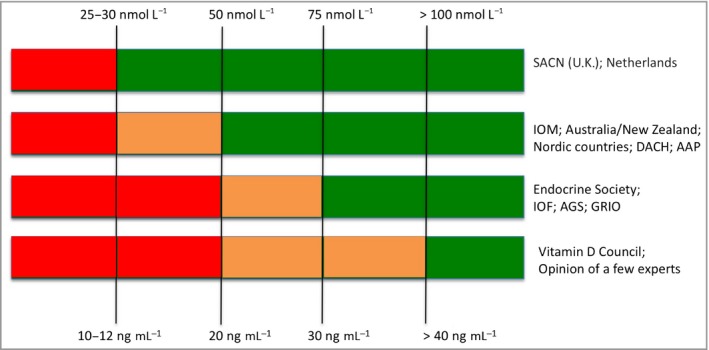
Thresholds of serum 25(OH)D concentration recommended by different bodies for definitions of vitamin D status (adapted from Bouillon23). Red, deficiency; orange, insufficiency; green, sufficiency. AAP, American Academy of Pediatrics; AGS, American Geriatrics Society; DACH, Deutschland, Austria and Confederation Helvetica; GRIO, French Research and Information Group on Osteoporosis; IOF, International Osteoporosis Foundation; IOP, Institute of Medicine; SACN, Scientific Advisory Committee on Nutrition (U.K.).
Public health perspectives
Hypovitaminosis D is prevalent globally.22, 24, 25 A systematic review covering 168 000 people from 44 countries reported serum 25(OH)D < 50 nmol L−1 in 37% of studies.26 This was mainly in the Middle East27 and Asia despite high insolation, emphasizing the importance of human behaviour.
Medical conditions and treatments with high risk of vitamin D deficiency are summarized in Table S1 (see Supporting Information). Concern about vitamin D status has resulted in increased screening with financial consequences.28 Clinical practice guidelines from the Endocrine Society advise screening only for those at risk of deficiency.29 In France, the Research and Information Group on Osteoporosis (GRIO) recommends systematic vitamin D supplementation without screening in everyone over 65 years.30
Disagreement on recommended doses for vitamin D supplementation arises, in part, from discrepancies of opinion on optimal serum 25(OH)D levels. The doses recommended for supplementation are discussed in Appendix S2 (see Supporting Information), but in case of deficiency, vitamin D supplementation should be 600–800 IU (15–20 μg) daily [but 400 IU (10 μg) in those less than 1‐year‐old] to achieve at least a target serum level of 50 nmol L−1.
Sunscreens and sun protection indices
Sunscreens are topical formulations that contain chemicals that attenuate solar UVR.31, 32 Global regulatory authorities have defined the sun protection factor (SPF) of a sunscreen as a universal quantitative index of protection against erythema, assessed after a single exposure of solar‐simulated radiation (SSR; Fig. 3a). In effect, the SPF is the ratio of SSR dose necessary for a minimal erythema dose (MED) with and without sunscreen application. SPF should be the primary driver of sunscreen choice. These authorities also require UVA protection (see Spectral considerations: Ultraviolet A). A given sunscreen, applied according to prescribed SPF test conditions at 2 mg cm−2, transmits 1⁄SPF of the erythemally effective UVR. One MED is equivalent to about three standard erythema doses (1 SED = 100 J m−2 of erythemally weighted UVR33) in a fair‐skinned person.34 Thus, assuming a possible ambient exposure of 30 SED during a sunbathing session, the correct use of SPF 20 sunscreen will allow a suberythemal 1·5 SED to reach the skin. However, people typically apply very much less with a commensurate reduction of actual labelled SPF. For example, a study of Danes on holiday in Egypt reported a mean application thickness of 0·79 mg cm−2.35 This paradoxically means that sunscreen use may be associated with sunburn as a result of more time in the sun.36, 37 Additional protection factors have been proposed, such as immune protection factor, DNA protection factor31 and a protection factor for visible light.38
Figure 3.
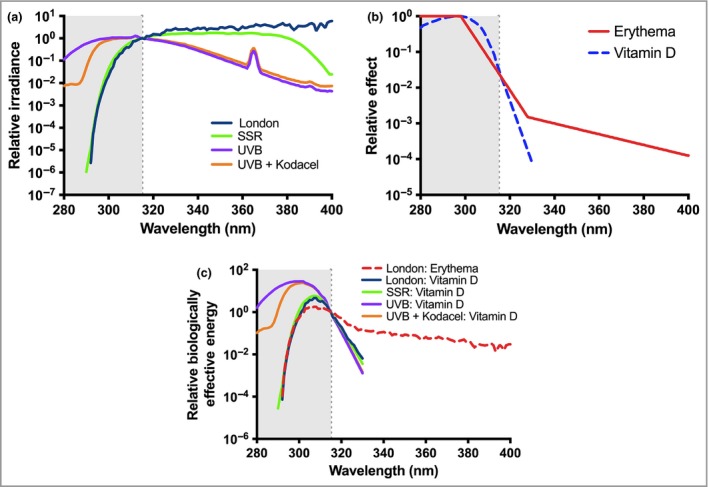
Ultraviolet radiation (UVR) spectra and their interactions with action spectra. (a) UVR emission spectra of natural temperate noon summer sunlight (London, U.K.; 51·5° N), solar simulated radiation (SSR) from a Solar® Light 16S‐001 v4·0 (Solar® Light, Glenside, PA, U.S.A.) with an emission spectrum compliant for sun‐protection factor (SPF) testing with the International Organization for Standardization (ISO) Standard 24444 and Cosmetics Europe 2006 and a UVB phototherapy source (Philips TL20W/12 fluorescent tubes in combination with and without a UVC blocking filter (Kodacel) that has been widely used in vitamin D studies. Spectra are normalized at 315 nm (CIE boundary between UVB and UVA). (b) CIE action spectra for erythema57 and formation of pre‐vitamin D3.58 (c) UVR emission spectra weighed for erythema and pre‐vitamin D3 using the emission spectra in Figure 3a and action spectra in Figure 3b. These products give biologically effective energy and are normalized at 315 nm (CIE boundary between UVB and UVA). Comparisons of the UVB source, with and without Kodacel, weighted with the pre‐vitamin D action spectrum show the large influence of nonsolar UVR in many laboratory studies. Comparisons of the London solar spectrum weighted with the erythema and pre‐vitamin D action spectra show that UVA filters have no influence on vitamin D production.
The benefits of sunscreens in photoprotection strategies
The acute and chronic adverse effects of solar UVR, especially to those with fair skins, are well established and can be inhibited by effective sun protection.22, 25, 39, 40 This includes (i) sun avoidance or seeking shade; (ii) clothing; and (iii) sunscreen use. When used optimally sunscreens can prevent erythema during a week‐long holiday, even when the UVI is very high.41 Laboratory studies have shown than sunscreens can prevent UVR‐induced immunosuppression42 and the formation of DNA damage43, 44 [specifically cyclobutane pyrimidine dimers (CPD), the action spectrum of which is very similar to erythema].45 CPD are thought to be important in many skin cancers. Those with cancer‐prone fair skin are especially sensitive to CPD formation, whereas the higher melanin content in dark skin affords much better protection against CPD, especially in the basal layer.46, 47, 48, 49, 50 A recent study with a high SPF sunscreen and high‐dose SSR for 5 consecutive days showed significant protection against CPD, even when the sunscreen was applied at 0·75 mg cm−2 to simulate typical use.44 A large Norwegian cohort showed that sunscreen use reduced the risk of melanoma.51 Extensive randomized controlled trials in Australia, with long‐term follow‐up, have demonstrated the protective properties of a sunscreen against photoageing, melanoma and squamous cell carcinoma, but not basal cell carcinoma.52, 53, 54, 55, 56
Spectral considerations
Ultraviolet B
Action spectroscopy shows that UVB is orders of magnitude more effective than UVA for erythema (see Fig. 3b57, 58).45 This means that the SPF is primarily, but not exclusively, a measure of UVB protection.31 Such protection is essential when UVB doses are high with recreational solar exposure, and in countries with high UVI.
Ultraviolet A
There has been an increasing trend over recent years for better UVA protection, with the aim of designing the ideal ‘neutral density’ sunscreen with ‘spectral homeostasis’ that mimics shade, i.e. it does not distort the natural solar UVR spectrum.59 There is no global standard for UVA protection and requirements vary with regulatory domain.31 The U.S. Food and Drug Administration has recently proposed greater UVA protection.60 A UVA protection factor (UVA‐PF) can be obtained using a sunscreen's ability to inhibit persistent pigment darkening in vivo.61 Spectral approaches, based on UVB/UVA absorption ratios and bandwidth cover, give qualitative but not quantitative information on UVA protection.
UVA irradiance is at least 20‐fold greater than UVB in sunlight.62 Furthermore, because UVA is not attenuated by the ozone layer, it is much less prone than UVB to daily, seasonal and geographical variation. Efficient UVA protection is highly recommended in recreational and daily photoprotection strategies, because good UVB protection, which inhibits sunburn, enables prolonged solar exposure and the accumulation of unnaturally high UVA doses. UVA1 (340–400 nm) preferentially induces CPD in the basal layer, which contains stem cells and melanocytes,63 as well as damaging DNA repair enzymes.64 Increasing UVA protection for a given SPF results in a de facto reduction of UVB protection, which might be expected to be beneficial for vitamin D synthesis.
Studies in vivo or in 3D skin models, have shown that for a given SPF a high UVA‐PF sunscreen offers better protection against pigmentation, photoageing and DNA damage compared with low UVA‐PF, and that low SPF sunscreens with high UVA‐PF offer such protection (Table 1).65, 66, 67, 68, 69, 70 One study, on a reconstructed skin model exposed to daily SSR, showed that a sunscreen with a lower SPF but strong UVA protection was more effective in preventing photodamage compared with a sunscreen with a higher SPF but low UVA protection.66 Thus, overall there seems to be biological and clinical advantages from increasing UVA protection for a given SPF.
Table 1.
Daily photoprotection studies with solar type UVR sources and emphasis on impact of ultraviolet (UV) A protection; summary of main conclusions from laboratory photoprotection studies
| First author, year | Study model | Exposure | Sunscreena , b | Conclusion |
|---|---|---|---|---|
| Young 200765 | Healthy volunteers FST I/II | Daily suberythemal SSR exposure (11 days) | Broad‐spectrum SS: SPF 7·5 UVA 4* | Prevention of DNA damage, p53 accumulation and Langerhans cell depletion |
| Lejeune 200866 | 3D human skin models | DUVRc dose–response (0–90 J cm−2) | SS with SPF 15 but high and low UVA‐PF with SPF/UVA‐PF ratio ≤ 3 or > 3 | High UVA‐PF (SPF/UVA‐PF ratio ≤ 3) showed better prevention of dermal alterations |
| Seité 201067 | Healthy volunteers FST II/III | Daily suberythemal DUVRc exposure (19 days over 4 weeks) | Broad‐spectrum SS: SPF 8 UVA‐PF 7 UVA 3* | Prevention of p53‐positive cells, melanin increase, loss of HLA‐DR‐positive cells and induction of dermal modifications (GAG) |
| Fourtanier 201268 | Asian (FST III) volunteersd | DUVRc | SS with SPF 19, 30 and 50, each with high and low UVA‐PF | Better inhibition of pigmentation (at 7 days) with high UVA‐PF (SPF/UVA‐PF ratio ≤ 3) |
| Marionnet 201270 | 3D human skin models | DUVRc exposure. 12 J cm−2 | SS with SPF 13 and high UVA‐PF (SPF/UVA‐PF ratio ≤ 3) | Inhibition of gene expression for adverse effects of DUVR |
DUVR, daylight UVR; FST, Fitzpatrick skin type; GAG, glycosaminoglycans; HLA‐DR, human leukocyte antigen – DR isotype; SPF, sun protection factor; SS, sunscreen; SSR, solar simulating radiation, UVA‐PF, UVA protection factor; UVR, ultraviolet radiation
SPF/UVA‐PF ratios from L'Oréal: ≤ 3, well‐balanced UVB–UVA protection (according to EC requirements); > 3, unbalanced SS with low UVA protection.
UVA star (*) rating refers to a sunscreen's UVA : UVB absorbance ratio (Boots star rating method). The higher the rating, the better the UVA protection with a maximal value of 5 (which represents a more or less neutral density sunscreen).
DUVR has a UVA/UVB ratio of ~ 27 (96·5% UVA, 3·5% UVB), which is more typical of temperate sunlight compared with SSR used for SPF testing.
Does photoprotection by sunscreens have an influence on vitamin D status?
Sunscreen use and vitamin D status
Given that solar UVB is the main source of vitamin D,71, 72 a possible adverse effect of sunscreen use on vitamin D synthesis has important public health implications. This has been studied using the different approaches described below. Reviews on sunscreen use and vitamin D synthesis have concluded that sunscreen use is likely to have minimal impact on vitamin D status,9, 73, 74 even though the action spectra (Fig. 3b) for erythema and pre‐vitamin D show considerable UVB overlap.57, 58 One reason suggested for this is suboptimal sunscreen application, which reduces its efficacy. However, little is known about the minimal UVB dose and exposed BSA requirements to maintain optimal vitamin D status.
Action spectroscopy shows that UVA protection will have no effect on vitamin D synthesis (Figs 3b, c), although one in vitro study has suggested that UVA2 (315–340 nm) may cause vitamin D degradation,75 in which case UVA protection may be beneficial for vitamin D production.
Laboratory and modelling studies have shown that serum 25(OH)D can be increased with repeated suberythemal UVR exposure;76, 77, 78 such doses can be as low as four exposures of 0·375 SED over 24% BSA.79 A study of Polish children, who did apply sunscreen, on holiday by the Baltic Sea showed that daily borderline erythemal exposure results in a highly significant increase of serum 25(OH)D3.80 These studies suggest that vitamin D synthesis occurs with low UVR doses and therefore sufficient UVR may be transmitted through a sunscreen for vitamin D synthesis.
Sunscreen use and vitamin D status in patients with photosensitivity with strict photoprotection
Patients with genetic and acquired photosensitivity disorders, and those at risk of and/or with a history of skin cancer are advised to practice strict photoprotection, including sunscreen use. This population is an ideal group to assess the effects of rigorous photoprotection. Table 2 shows some of these conditions,81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93 in which patients present with low levels of 25(OH)D3 except in the study of Ulrich et al.,86 in which 25(OH)D3 was > 132·5 nmol L−1 in 120 organ transplant recipients. However, it is impossible to attribute low serum 25(OH)D3 to a given photoprotection strategy because more than one was used. Furthermore, for the most part there were no controls, and supplementation was given or taken in many of the studies. Overall, it is not possible to use these studies for sunscreen guidance for the general population.
Table 2.
Vitamin D status in patients with photosensitivity under strict photoprotection
| First author, year | Pathology and patients (n) | Follow‐up | Location, latitude | Vit D intake | Photoprotection strategies | Vit D status/conclusions |
|---|---|---|---|---|---|---|
| Sollitto 199781 | XP, n = 8 | 6 years | U.S.A.: all parts | 7·7 μg daily |
SPF > 15 daily Clothing, shade‐seeking |
Mean 25(OH)D: 44·5 nmol L−1 |
| Querings 200482 |
XP, n = 3 BCNS, n = 1 |
End of winter | Germany: Homburg, 49° N | NA | Not specified | Mean 25(OH)D: 23·8 nmol L−1 |
| Querings 200683 |
Patients with kidney transplant, n = 31 Controls, n = 31 |
End of winter | Germany: Homburg, 49° N | NA | SS + clothing | Mean 25(OH)D: 27·3 nmol L−1 vs. 50·0 nmol L−1 in controls |
| Cusack 200884 |
Cutaneous lupus erythematous, n = 52 FST I–IV |
3 months in summer | Ireland: Dublin, 53° N | 40·4% took minimum 10 μg daily |
4 groups: SS user Shade seeker Non‐SS user Non‐shade seeker |
25(OH)D:57·9 nmol L−1 58·8 nmol L−1 73·5 nmol L−1 81·8 nmol L−1 |
| Holme 200885 | Erythropoietic protoporphyria, n = 201 | 7 months, January to July | U.K.: 51–57·5° N | 3 took fish liver oil daily | 80% shade‐seeking 68% used SS when sunny | 25(OH)D: 63% < 50 nmol L−1 17% < 25 nmol L−1 |
| Ulrich 200986 |
Organ transplant recipients Applied SS, n = 60 No SS, n = 60FST II‐III |
2 years | Germany: Berlin, 53° N | NA | SPF 50+, 2 mg cm−2 | 25(OH)D: lower in SS users (132·5 vs. 150·0 nmol L−1). Note: these values are very high |
| DeLong 201087 | Skin cancer patients n = 143 FST I‐II, 12 FST IV‐VI, n = 144FST I‐IV | 2 years (September to December period) | U.S.A.: Atlanta, GA, 34° N |
94% < 10 μg daily from diet 60% taking supplements |
Adherent or no sun protection |
Mean 25(OH)D: Adherent: 70 nmol L−1 (18% < 50 nmol L−1), Nonadherent: 73 nmol L−1 (16% < 50 nmol L−1) |
| Hoesl 201088 | XP, n = 15 | NA | Germany: Tubignen, 49° N | NA | Sun protection | Mean 25(OH)D: 27 nmol L−1 |
| Tang 201089 |
BCNS, n = 41 FST I–III NHANES controls, n = 360 |
2 years | U.S.A.: all parts | 34% daily multivitamin | 80% used daily SPF > 15 daily | 25(OH)D: 56% with < 50 nmol L−1 compared with 18% controls |
| Reid 201290 | Patients with photosensitivity, n = 165 (of which n = 35 with strict photoprotection) n = 143 &!#6;FST I‐III, 12 FST IV‐VI | 1 year | Scotland: Dundee, 56° N | Supplements used by 14 patients | None, sensible, strict |
Mean 25(OH)D: 41·9 nmol L−1 40% with < 50 nmol L−1 25% with < 25 nmol L−1 Supplementation associated with significantly higher 25(OH)D (57·5 vs. 39·5 nmol L−1) Strict vs. sensible photoprotection associated with lower 25(OH)D (33·4 vs. 42·1 nmol L−1) |
| Gentzsch 201491 | Gorlin (incl. multiple BCCs), n = 1 (case report) | NA | Germany: Freiburg, 48° N | Supplementation initiated | SS + clothing and shade‐seeking | 25(OH)D < 10 nmol L−1 |
| Kuwabara 201592 | XP‐A, n = 21Japanese | 2 days for vit D intake | Japan: Kobe, 35° N | Mean dietary intake of 4·1 μg day−1 | SPF > 30 + clothing | 25(OH)D: 76% < 25 nmol L−1 |
| Bogaczewicz 201693 | SLE: n = 104, Controls: n = 34FST II‐III | 16 weeks | Poland: Lodz, 52° N | After study | SS + clothing and hats | Summer 25(OH)D:SLE: median 56·8 nmol L−1, Controls: median 73·2 nmol L−1 |
25(OH)D, 25‐hydroxyvitamin D; BCC, basal cell carcinoma; BCNS, basal cell naevus syndrome; FST, Fitzpatrick skin type; NA, data not available or not applicable; NHANES, National Health and Nutrition Examination Survey; SLE, systemic lupus erythematosus; SPF, sun protection factor; SS, sunscreen; XP, xeroderma pigmentosum, vit, vitamin
Sunscreen use and vitamin D3 synthesis in studies using nonsolar ultraviolet radiation from artificial sources
Laboratory studies offer an obvious way to study the effects of sunscreens under controlled conditions. Five studies have shown that sunscreen application (0·5–2 mg cm−2) inhibited the synthesis of vitamin D (Table 3).94, 95, 96, 97 However, the sources used were mainly UVB‐rich (Fig. 3a), including nonsolar UVB (< 295 nm), which is very effective at pre‐vitamin D production (Fig. 3b). Figure 3c shows that such nonsolar wavelengths have a disproportionally large effect, and thus do not reflect environmental reality. Of note, one study showed that 25(OH)D synthesis is dependent on application thickness when 25% of BSA is exposed.96 It was recently shown that sunscreens block cutaneous vitamin D3 (cholecalciferol) production with only a minimal effect on circulating 25(OH)D after a single narrowband UVB (~313 nm) exposure.97 In general, the UVR dose of these studies is low, e.g. this was 0·8 MED (estimated to be ~3 SED in skin type III volunteers) with SPF 50 at 2 mg cm−2 in the study of Libon et al.97 Taking the SPF at face value means the dose through the sunscreen is 3/50 = 0·06 SED. However, it should be noted that the labelled SPF value is specific to SSR sources used for SPF testing that meet certain spectral specifications. The ‘actual SPF’ with nonsolar UVB‐rich sources may be considerably higher98 than labelled SPF in sunlight. This means that the labelled SPFs are in fact meaningless with nonsolar sources. Overall, when taking photobiological considerations into account, the use of sunscreens with non‐SSR sources cannot provide reliable data on their effect on vitamin D synthesis for public health purposes. The only way to do such studies reliably would be to use SSR as used in SPF testing, or a fluorescent SSR source.99 It should be noted that the higher UVB content of SSR than ‘typical’ terrestrial UVR may also influence results.100 Furthermore, the SSR doses given should be environmentally realistic and represent a serious challenge to the sunscreen under test.
Table 3.
Sunscreen use and vitamin D status in studies using normal human volunteers (FST I‐III) exposed to UVR from artificial sources
| First author, year | In vivo / ex vivo, n, age | UVR source | Dose | Exposed area | SPF | Amount of sunscreen | Time of assessment | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Matsuoka 198794 |
Ex vivo (skin from 1 donor) n = 3 (SS + UVR) n = 3 (vehicle + UVR) n= 3 (control) |
SSR | 1 MED | 6·2 cm−2 | NA | 5% (w/v) PABA | Pre and post UVR | SS blocked photoisomerization of stratum corneum 7‐DHC |
| Matsuoka 198794 |
In vivo n = 8 (SS, n = 4; placebo, n = 4) 21–45 years |
UVB phototherapy tubes (260–360 nm with peak at 313 nm) | 1 MED | Whole body | SPF 8 | Cannot say with confidence | 24 h, 2 h prior UVR, 1, 2, 3, 7, 14 days post exposure | Without SS there was a 17‐fold increase in serum vit D peaking at 1 day post UVR. SS totally blocked serum vit D increase |
| Matsuoka 199095 |
In vivo n = 27 23–32 years |
UVB phototherapy tubes (260–360 nm with peak at 313 nm) | Slightly < 1 MED |
Six groups with different SS application zones: G1, whole body; G2, except head & neck; G3, except arms; G4, except trunk; G5, except buttocks & legs; G6, whole body no SS |
SPF 15 | No data | 1 h before and 24 h after exposure | In the absence of whole‐body SS there was a ~5‐fold significant increase of serum vit D. Whole‐body SS totally blocked vit D formation. Lack of SS on the legs and trunk allowed significant synthesis. But synthesis not significant when arms and head & neck were spared |
| Faurschou 201296 |
In vivo n = 37 18–49 years |
UVB phototherapy tubes (290–360 nm with peak at 320 nm) |
4 × 3 SED 2–3 days interval |
25% BSA (upper front and back) | SPF 8 | 0, 0·5, 1·0, 1·5, 2·0 mg cm−2 | 3 days after final irradiation | Increase of 25(OH)D is dependent on SS application thickness. All increases significantly greater than baseline apart from SS at 2·0 mg cm−2 |
| Libon 201797 |
In vivo n = 72 19–25 years |
Narrowband UVB phototherapy tubes (311–313 nm) | 0·8 MED | Different body areas with and without SS (9–96%) | SPF 50+ | 2 mg cm−2 | Pre and post UVR up to 5 days | SS use decreased serum 25(OH)D by 8–13% and decreased cutaneous vit D by 76–93% |
25(OH)D, 25‐hydroxyvitamin D; 7‐DHC, 7‐dehydrocholesterol; BSA, body surface area; MED, minimal erythema dose; NA, not applicable; PABA, para‐aminobenzoic acid; SED, standard erythema dose; SPF, sun protection factor; SS, sunscreen; SSR, solar simulated radiation; vit D, vitamin D [not 25(OH)D], UVR, ultraviolet radiation
Sunscreen use for daily and recreational photoprotection and vitamin D status
Questionnaire‐based studies
Table S2 (see Supporting Information) shows that most questionnaire‐based studies report no correlation between sunscreen use and serum 25(OH)D3 levels. However, two studies showed a negative correlation and a positive correlation was observed in three studies. The negative correlation, in a Brazilian study, reported that 25(OH)D3 was sufficient (73 nmol L−1) in the sunscreen group.101 Godar et al. reported, from a modelling study, that young Americans (≤ 19 years) using sunscreen with SPF > 15 had insufficient vitamin D3 status, and concluded that most American children may not get sufficient solar exposure to meet their minimal vitamin D requirements.102 One explanation for the positive correlations, including one large Danish study of 2625 adults and 569 children,103 is increased solar exposure without erythema.
Questionnaire‐based studies have obvious limitations including compliance, unknown confounding factors, the use of nonsunscreen photoprotection and recall bias. UVR exposure was based on proxies such as time outdoors.
Controlled studies
Controlled field studies with real sun exposure are the best way to determine the effect of sunscreen use on vitamin D synthesis. Such studies present ethical considerations when considering control groups because lack of sunscreen use could result in sunburn and increased skin cancer risk. Results of such studies are shown in Table 4,41, 104, 105, 106, 107, 108, 109, 110, 111 which reports that most studies showed no change in serum 25(OH)D3 with sunscreen use,106, 107, 108 but two showed reduction.104, 105 These studies mostly ignore the most important factors that influence outcome, namely personal UVR exposure, sunscreen application thickness and BSA exposed. Marks et al.,105 who found no difference between sunscreen and control groups, measured UVR exposure in the last week of a 7‐week study in Australia using polysulphone badge personal dosimeters. The UVR exposures in the sunscreen and control groups were not different, but the last week's exposure is unlikely to have been critical for the outcome because serum 25(OH)D3 was best predicted in Australian adults by solar exposure 6 weeks prior to measurement.112
Table 4.
Sunscreen use and vitamin D status/outcomes in real sun exposure (controlled studies) in skin cancer patients and healthy volunteers
| First author, year | Participants: n (age) | Location, latitude | SPF | Assessment period | Baseline values | UVR monitored | Conclusions |
|---|---|---|---|---|---|---|---|
| Matsuoka 1988104 |
n = 20 (65 ±3 years) Controls: n = 20 (58 ± 3 years) |
U.S.A.: Springfield, IL, 40° N; Philadelphia, PA, 40° N | Not given |
Summer after SS use > 1 year |
No | No | 25(OH)D significantly lower (44%) in SS group than controls |
| Marks 1995105 | n = 113 (≥ 40 years) | Australia: Maryborough, 37° S |
SPF 17 Controls given base cream |
7 months (after summer) 1,25(OH)2D also assessed |
Yes |
Yes Dosimeter badges (last week) |
25(OH)D not significantly different between groups. SS group had significantly lower 1,25(OH)2D but still in the reference range. No difference in UVR exposure between groups |
| Farrerons 1998106 |
n = 24 (71 ± 8 years) Controls: n = 19 (59 ± 7 years) |
Spain: Barcelona, 41° N | SPF 15 |
2 years, 4 time points 1,25(OH)2D, PTH, bone markers also measured |
Yes |
No Outdoors ≥ once daily |
25(OH)D significantly lower in SS users at 3 time points. Overall, no other differencesb |
| Farrerons 2001107 |
n = 10 (74 ± 18 years)a Controls: n = 18 (59 ± 7 years) |
Spain: Barcelona, 41° N | SPF 15 |
2 years Bone mass |
Yes |
No Outdoors ≥ once daily |
No significant differences in bone mass between the two groups |
| Azizi 2012108 |
Outdoor male workers (~ 40 years) 3 sun protection intervention groups:c
|
Israel, 30–33° N 3 locations |
SPF 42 | Two successive winters (8 and 20 months) |
No (samples lost) |
Yes Ambient SED/day 40 ± 10 spring 15 ± 4 winter |
25(OH)D not significantly different at any time or between any intervention group |
| Jayaratne 2012110 |
n = 556 (daily sunscreen) vs. n = 557 (discretionary use) (19–70+ years) |
Australia: Nambour, 26° S | SPF 16 | End of a 4·5‐year RCT | No | No | 25(OH)D not significantly different in daily vs. discretionary SS use |
| Narbutt 201941 and Young 2019111 | n = 79 (34 ± 8 years) |
Participants: Spain: Tenerife, 28° N Controls: Poland: Łódź, 52° N |
SPF 15 (≥ 2 mg cm−2) Intervention: (i) High UVA‐PF (ii) Low UVA‐PF Discretionary SS use |
1‐week holiday in March | Yes |
Yes Personal electronic dosimeters measuring SED |
SS SPF 15 (no sunburn) reduced 25(OH)D compared with discretionary use (sunburn) but all increases highly significant. Significantly more 25(OH)D with high UVA‐PF SS. No difference in UVR exposure between holiday groups No change in control group in Poland |
1,25(OH)2D, 1,25‐dihydroxyvitamin D; 25(OH)D, 25‐hydroxyvitamin D; RCT, randomized control trial; SED, standard erythema dose; SPF, sun protection factor; SS, sunscreen; UVA‐PF, UVA protection factor; UVR, ultraviolet radiation
Paper gives 2 different values for age. This considered more likely.
Significant reduction of bone turnover marker (osteocalcin) in one autumn measurement.
The interventions are described in more detail in Azizi et al.,109 an earlier paper by the authors relating to the same study. SS included sunglasses and wide‐brimmed hats.
One factor that has been ignored in all types of study described above, except for the study of Faurschou et al.,96 is the effect of baseline 25(OH)D3 on the response to UVR. The lower the baseline, the greater the response to UVR113 and this must be considered in the statistical analyses. A similar observation has been made in vitamin D supplementation studies.114
A holiday study in Tenerife (Canary Islands) during a week of very high UVI was designed to take the above factors into account, including a discretionary sunscreen‐use control group. This showed that intervention with optimal SPF 15 sunscreen use (≥ 2 mg cm−2), which inhibited erythema,41 still enabled very considerable vitamin D production111 compared with the discretionary sunscreen‐use group that had sunburn. A comparison of high vs. low UVA‐PF showed greater vitamin D synthesis with the former. Thus, optimal UVA+B protection does not compromise vitamin D increase during recreational exposure. It was estimated that the daily UVR dose through the sunscreen was 0·4 SED, which is equivalent to 0·1 MED in a fair‐skinned person.41 Thus, the UVB doses needed for the biosynthesis of vitamin D3 are indeed very low. Overall, this study shows that it is possible to have the benefits or solar exposure while minimizing the risks.
In conclusion, effective sunscreens must attenuate UVB to prevent erythema. In theory, this should inhibit vitamin D3 biosynthesis. However, the doses of UVB necessary are low (i.e. substantially suberythemal) so that typical sunscreen use does not lead to vitamin D insufficiency in practice in healthy people. Indeed, even optimal sunscreen use allows good vitamin D synthesis under high UVI conditions. Better UVA protection for a given SPF results in a de facto reduction of UVB protection. UVA protection will have no impact on vitamin D synthesis (see Fig. 3b), and indeed may prevent photodegradation. Increased UVB for a given SPF should in theory and in practice result in better vitamin D synthesis. Studies done to date have been with lighter‐skinned individuals, and conclusions may not apply to those with darker skin types IV–VI who use sunscreens. In such cases, oral supplementation may be advisable.
Summary
Cutaneous vitamin D3 synthesis is initiated by terrestrial‐range UVB and can be achieved with suberythemal exposures to a relatively small BSA. Daily sunscreen use, for nonintentional solar exposure, is mainly based on products with low SPF and high UVA‐PF. This is unlikely to impact on vitamin D production. In fact, most studies published to date have shown no association between sunscreen use and vitamin D deficiency, even with regular use of SPF > 15. Some studies have even reported a positive association between sunscreen use and 25(OH)D3, suggesting that their use may have increased sun exposure. Indeed, time spent outdoors and BSA exposed to sun have been positively correlated with vitamin D status. Overall, other photoprotection behaviours (such as seeking shade, wearing protective clothing and long sleeves) may have more impact on vitamin D status than sunscreen use. The recommendations of the panel for daily and recreational photoprotection, as well as the need for vitamin D screening and supplementation, are summarized in Table 5.
Table 5.
General recommendations
Key messages
|
25(OH)D, 25‐hydroxyvitamin D; SPF, sun protection factor; UVA‐PF, ultraviolet A protection factor
Supporting information
Appendix S1 Intrinsic and extrinsic factors that determine serum 25(OH)D.
Appendix S2 What is optimal vitamin D status and the best method to determine it? Public health perspectives.
Table S1 Indications for 25(OH)D screening.
Table S2 Sunscreen use and vitamin D status in real sun exposure (questionnaire‐based and modelled studies).
Acknowledgments
We wish to thank Marielle Romet PhD and Françoise Nourrit‐Poirette PhD, from Santé Active Edition, France, who provided medical writing assistance on behalf of L'Oréal France. We also thank Karl Lawrence PhD for preparing the figures.
Funding sources This review was financed by L'Oréal Company.
Conflicts of interest B.A.B., F.B., L.M., M.N., M.V. are employees of L'Oréal Company. T.P. has received grants, funding and/or speaking fees from Bioderma, Beiersdorf, Galderma, ISDIN, ISIS pharma, L'Oréal, Pierre Fabre, SVR and Symrise. T.C. has received grants, funding or speaking fees from Abbvie, Janssen Cilag, L'Oréal, Sanofi Genzyme and Vichy Laboratories. R.B., V.C., T.D., J.v.d.P., F.L., N.G.J. and A.R.Y. received honoraria from L'Oréal for this project.
https://doi.org/10.1111/bjd.18494 available online
References
- 1. Maestro MA, Molnar F, Mourino A et al Vitamin D receptor 2016: novel ligands and structural insights. Expert Opin Ther Pat 2016; 26:1291–306. [DOI] [PubMed] [Google Scholar]
- 2. Bouillon R, Carmeliet G, Verlinden L et al Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008; 29:726–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–81. [DOI] [PubMed] [Google Scholar]
- 4. Bjelakovic G, Gluud LL, Nikolova D et al Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014; 1:CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cardoso AT, Nanji L, Costa J et al [Analysis of the Cochrane Review: Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014, 6:CD007469]. Acta Med Port 2014; 27:411–13. [PubMed] [Google Scholar]
- 6. Rosen CJ, Adams JS, Bikle DD et al The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 2012; 33:456–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Autier P, Mullie P, Macacu A et al Effect of vitamin D supplementation on non‐skeletal disorders: a systematic review of meta‐analyses and randomised trials. Lancet Diabetes Endocrinol 2017; 5:986–1004. [DOI] [PubMed] [Google Scholar]
- 8. Manson JE, Cook NR, Lee IM et al Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019; 380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Springbett P, Buglass S, Young AR. Photoprotection and vitamin D status. J Photochem Photobiol B 2010; 101:160–8. [DOI] [PubMed] [Google Scholar]
- 10. Haussler MR, McCain TA. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med 1977; 297:974–83. [DOI] [PubMed] [Google Scholar]
- 11. Holick MF, Chen TC, Lu Z et al Vitamin D and skin physiology: a D‐lightful story. J Bone Miner Res 2007; 22 (Suppl. 2):V28–33. [DOI] [PubMed] [Google Scholar]
- 12. Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 2011; 347:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen B, Wulf HC, Triguero‐Mas M et al Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage. J Invest Dermatol 2014; 134:2806–13. [DOI] [PubMed] [Google Scholar]
- 14. Jolliffe DA, Walton RT, Griffiths CJ et al Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non‐skeletal health outcomes: review of genetic association studies. J Steroid Biochem Mol Biol 2016; 164:18–29. [DOI] [PubMed] [Google Scholar]
- 15. IOM (Institute of Medicine) . Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- 16. WHO . Global Solar UV Index: A practical guide. A joint recommendation of the World Health Organization, World Meteorological Organization, United Nations Environment Programme, and the International Commission on Non‐Ionizing Radiation Protection. Geneva: WHO, 2002; 1–32. [Google Scholar]
- 17. McKenzie RL, Lucas RM. Reassessing impacts of extended daily exposure to low level solar UV radiation. Sci Rep 2018; 8:13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy KK, Gilchrest BA. What is all this commotion about vitamin D? J Invest Dermatol 2010; 130:321–6. [DOI] [PubMed] [Google Scholar]
- 19. Linos E, Keiser E, Kanzler M et al Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006. Cancer Causes Control 2012; 23:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janda M, Kimlin MG, Whiteman DC et al Sun protection messages, vitamin D and skin cancer: out of the frying pan and into the fire? Med J Aust 2007; 186:52–4. [DOI] [PubMed] [Google Scholar]
- 21. Liang G, Nan H, Qureshi AA et al Pre‐diagnostic plasma 25‐hydroxyvitamin D levels and risk of non‐melanoma skin cancer in women. PLoS One 2012; 7:e35211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lucas RM, Yazar S, Young AR et al Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem Photobiol Sci 2019; 18:641–80. [DOI] [PubMed] [Google Scholar]
- 23. Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol 2017; 13:466–79. [DOI] [PubMed] [Google Scholar]
- 24. Gel‐H Fuleihan, Bouillon R, Serum Clarke B et al 25‐hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res 2015; 30:1119–33. [DOI] [PubMed] [Google Scholar]
- 25. United Nations Environment Programme, Environmental Effects Assessment Panel . Environmental effects of ozone depletion and its interactions with climate change: progress report, 2016. Photochem Photobiol Sci 2017; 16:107–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilger J, Friedel A, Herr R et al A systematic review of vitamin D status in populations worldwide. Br J Nutr 2014; 111:23–45. [DOI] [PubMed] [Google Scholar]
- 27. Lips P, Cashman KD, Lamberg‐Allardt C et al Management of endocrine disease: current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency; a position statement of the European Calcified Tissue Society. Eur J Endocrinol 2019; 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 28. Zhao S, Gardner K, Taylor W et al Vitamin D assessment in primary care: changing patterns of testing. London J Prim Care (Abingdon) 2015; 7:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holick MF, Binkley NC, Bischoff‐Ferrari HA et al Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–30. [DOI] [PubMed] [Google Scholar]
- 30. Benhamou CL, Souberbielle JC, Cortet B et al for le Groupe de recherche et d'information sur les ostéoporoses (GRIO). La vitamine D chez l'adulte: recommandations du GRIO. Presse Med 2011; 40:673–82. [Google Scholar]
- 31. Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol 2017; 76:S100–9. [DOI] [PubMed] [Google Scholar]
- 32. Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed 2014; 30:62–80. [DOI] [PubMed] [Google Scholar]
- 33. Diffey BL, Jansen CT, Urbach F et al The standard erythema dose: a new photobiological concept. Photodermatol Photoimmunol Photomed 1997; 13:64–6. [DOI] [PubMed] [Google Scholar]
- 34. Harrison GI, Young AR. Ultraviolet radiation‐induced erythema in human skin. Methods 2002; 28:14–19. [DOI] [PubMed] [Google Scholar]
- 35. Petersen B, Datta P, Philipsen PA et al Sunscreen use and failures – on site observations on a sun‐holiday. Photochem Photobiol Sci 2013; 12:190–6. [DOI] [PubMed] [Google Scholar]
- 36. Petersen B, Thieden E, Philipsen PA et al Determinants of personal ultraviolet‐radiation exposure doses on a sun holiday. Br J Dermatol 2013; 168:1073–9. [DOI] [PubMed] [Google Scholar]
- 37. Petersen B, Thieden E, Philipsen PA et al A sun holiday is a sunburn holiday. Photodermatol Photoimmunol Photomed 2013; 29:221–4. [DOI] [PubMed] [Google Scholar]
- 38. Duteil L, Esdaile J, Maubert Y et al A method to assess the protective efficacy of sunscreens against visible light‐induced pigmentation. Photodermatol Photoimmunol Photomed 2017; 33:260–6. [DOI] [PubMed] [Google Scholar]
- 39. Lucas RM, Norval M, Neale RE et al The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem Photobiol Sci 2015; 14:53–87. [DOI] [PubMed] [Google Scholar]
- 40. Bais AF, Lucas RM, Bornman JF et al Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem Photobiol Sci 2018; 17:127–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Narbutt J, Philipsen PA, Harrison GI et al Sunscreen applied at ≥ 2 mg cm−2 during a sunny holiday prevents erythema, a biomarker of ultraviolet radiation‐induced DNA damage and suppression of acquired immunity. Br J Dermatol 2019; 180:604–14. [DOI] [PubMed] [Google Scholar]
- 42. Fourtanier A, Moyal D, Maccario J et al Measurement of sunscreen immune protection factors in humans: a consensus paper. J Invest Dermatol 2005; 125:403–9. [DOI] [PubMed] [Google Scholar]
- 43. Olsen CM, Wilson LF, Green AC et al Prevention of DNA damage in human skin by topical sunscreens. Photodermatol Photoimmunol Photomed 2017; 33:135–42. [DOI] [PubMed] [Google Scholar]
- 44. Young AR, Greenaway J, Harrison GI et al Sub‐optimal application of a high SPF sunscreen prevents epidermal DNA damage in vivo . Acta Dermato‐Venereol 2018; 98:880–7. [DOI] [PubMed] [Google Scholar]
- 45. Young AR, Chadwick CA, Harrison GI et al The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J Invest Dermatol 1998; 111:982–8. [DOI] [PubMed] [Google Scholar]
- 46. Del Bino S, Sok J, Bernerd F. Assessment of ultraviolet‐radiation‐induced DNA damage within melanocytes in skin of different constitutive pigmentation. Br J Dermatol 2013; 168:1120–3. [DOI] [PubMed] [Google Scholar]
- 47. Del Bino S, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol 2013; 169 (Suppl. 3):33–40. [DOI] [PubMed] [Google Scholar]
- 48. Fajuyigbe D, Lwin SM, Diffey BL et al Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J 2018; 32:3700–6. [DOI] [PubMed] [Google Scholar]
- 49. Tadokoro T, Kobayashi N, Zmudzka BZ et al UV‐induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J 2003; 17:1177–9. [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi Y, Takahashi K, Zmudzka BZ et al Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J 2006; 20:1486–8. [DOI] [PubMed] [Google Scholar]
- 51. Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer 1997; 71:600–4. [DOI] [PubMed] [Google Scholar]
- 52. Green A, Williams G, Neale R et al Daily sunscreen application and betacarotene supplementation in prevention of basal‐cell and squamous‐cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–9. [DOI] [PubMed] [Google Scholar]
- 53. van der Pols JC, Williams GM, Pandeya N et al Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–8. [DOI] [PubMed] [Google Scholar]
- 54. Green AC, Williams GM, Logan V et al Reduced melanoma after regular sunscreen use: randomized trial follow‐up. J Clin Oncol 2011; 29:257–63. [DOI] [PubMed] [Google Scholar]
- 55. Iannacone MR, Hughes MC, Green AC. Effects of sunscreen on skin cancer and photoaging. Photodermatol Photoimmunol Photomed 2014; 30:55–61. [DOI] [PubMed] [Google Scholar]
- 56. Sanchez G, Nova J, Rodriguez‐Hernandez AE et al Sun protection for preventing basal cell and squamous cell skin cancers. Cochrane Database Syst Rev 2016; 7:CD011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. CIE . Erythema Reference Action Spectrum and Standard Erythema Dose. Vienna, Austria: Commission Internationale de l'Eclairage (CIE) Central Bureau, 1998. [Google Scholar]
- 58. Bouillon R, Eisman J, Garabedian M et al Action spectrum for production of previtamin D3 in human skin. Vienna, Austria: Commission Internationale de l'Eclairage (CIE) Central Bureau, 2006. [Google Scholar]
- 59. Stengel F. Homeostasis in topical photoprotection: getting the spectral balance right. Am J Clin Dermatol 2018; 19:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. U.S. Food and Drug Administration . Sunscreen drug products for over the counter use. Proposed Rules (Document no. 2019‐03019). Federal Register 2019; 84:6204–75. [Google Scholar]
- 61. Cole C. Sunscreens – what is the ideal testing model? Photodermatol Photoimmunol Photomed 2014; 30:81–7. [DOI] [PubMed] [Google Scholar]
- 62. Dupont E, Gomez J, Bilodeau D. Beyond UV radiation: a skin under challenge. Int J Cosmet Sci 2013; 35:224–32. [DOI] [PubMed] [Google Scholar]
- 63. Tewari A, Sarkany RP, Young AR. UVA1 induces cyclobutane pyrimidine dimers but not 6‐4 photoproducts in human skin in vivo . J Invest Dermatol 2012; 132:394–400. [DOI] [PubMed] [Google Scholar]
- 64. McAdam E, Brem R, Karran P. Oxidative stress‐induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy. Mol Cancer Res 2016; 14:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Young AR, Orchard GE, Harrison GI et al The detrimental effects of daily sub‐erythemal exposure on human skin in vivo can be prevented by a daily‐care broad‐spectrum sunscreen. J Invest Dermatol 2007; 127:975–8. [DOI] [PubMed] [Google Scholar]
- 66. Lejeune F, Christiaens F, Bernerd F. Evaluation of sunscreen products using a reconstructed skin model exposed to simulated daily ultraviolet radiation: relevance of filtration profile and SPF value for daily photoprotection. Photodermatol Photoimmunol Photomed 2008; 24:249–55. [DOI] [PubMed] [Google Scholar]
- 67. Seité S, Christiaens F, Bredoux C et al A broad‐spectrum sunscreen prevents cumulative damage from repeated exposure to sub‐erythemal solar ultraviolet radiation representative of temperate latitudes. J Eur Acad Dermatol Venereol 2010; 24:219–22. [DOI] [PubMed] [Google Scholar]
- 68. Fourtanier A, Moyal D, Seite S. UVA filters in sun‐protection products: regulatory and biological aspects. Photochem Photobiol Sci 2012; 11:81–9. [DOI] [PubMed] [Google Scholar]
- 69. Moyal D . Prevention of pigmentation in Asian skin exposed to ultraviolet daylight. Proceedings of the International Federation of the Societies of Cosmetic Chemists (IFSCC) meeting. Osaka, Japan, October 2006 [poster].
- 70. Marionnet C, Pierrard C, Lejeune F et al Modulations of gene expression induced by daily ultraviolet light can be prevented by a broad spectrum sunscreen. J Photochem Photobiol B 2012; 116:37–47. [DOI] [PubMed] [Google Scholar]
- 71. Ashwell M, Stone EM, Stolte H et al UK Food Standards Agency Workshop Report: an investigation of the relative contributions of diet and sunlight to vitamin D status. Br J Nutr 2010; 104:603–11. [DOI] [PubMed] [Google Scholar]
- 72. Jamil NA, Yew MH, Noor Hafizah Y et al Estimated vitamin D synthesis and dietary vitamin D intake among Asians in two distinct geographical locations (Kuala Lumpur, 3 degrees N v. Aberdeen, 57 degrees N) and climates. Public Health Nutr 2018; 21:3118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol 2009; 161:732–6. [DOI] [PubMed] [Google Scholar]
- 74. Neale RE, Khan SR, Lucas RM et al The effect of sunscreen on vitamin D: a review. Br J Dermatol 2019; 10.1111/bjd.17980. [DOI] [PubMed] [Google Scholar]
- 75. Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab 1989; 68:882–7. [DOI] [PubMed] [Google Scholar]
- 76. Felton SJ, Cooke MS, Kift R et al Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low‐level summer sunlight exposures. Br J Dermatol 2016; 175:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Farrar MD, Webb AR, Kift R et al Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure. Am J Clin Nutr 2013; 97:1210–16. [DOI] [PubMed] [Google Scholar]
- 78. Webb AR, Kazantzidis A, Kift RC et al Meeting vitamin D requirements in white Caucasians at UK latitudes: providing a choice. Nutrients 2018; 10:pii:E497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bogh MK, Schmedes AV, Philipsen PA et al Vitamin D production depends on ultraviolet‐B dose but not on dose rate: a randomized controlled trial. Exp Dermatol 2011; 20:14–18. [DOI] [PubMed] [Google Scholar]
- 80. Narbutt J, Philipsen PA, Lesiak A et al Children sustain high levels of skin DNA photodamage, with a modest increase of serum 25‐hydroxyvitamin D3, after a summer holiday in Northern Europe. Br J Dermatol 2018; 179:940–50. [DOI] [PubMed] [Google Scholar]
- 81. Sollitto RB, Kraemer KH, DiGiovanna JJ. Normal vitamin D levels can be maintained despite rigorous photoprotection: six years’ experience with xeroderma pigmentosum. J Am Acad Dermatol 1997; 37:942–7. [DOI] [PubMed] [Google Scholar]
- 82. Querings K, Reichrath J. A plea for the analysis of vitamin‐D levels in patients under photoprotection, including patients with xeroderma pigmentosum (XP) and basal cell nevus syndrome (BCNS). Cancer Causes Control 2004; 15:219. [DOI] [PubMed] [Google Scholar]
- 83. Querings K, Girndt M, Geisel J et al 25‐hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab 2006; 91:526–9. [DOI] [PubMed] [Google Scholar]
- 84. Cusack C, Danby C, Fallon JC et al Photoprotective behaviour and sunscreen use: impact on vitamin D levels in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed 2008; 24:260–7. [DOI] [PubMed] [Google Scholar]
- 85. Holme SA, Anstey AV, Badminton MN et al Serum 25‐hydroxyvitamin D in erythropoietic protoporphyria. Br J Dermatol 2008; 159:211–13. [DOI] [PubMed] [Google Scholar]
- 86. Ulrich C, Jürgensen JS, Degen A et al Prevention of non‐melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case–control study. Br J Dermatol 2009; 161 (Suppl. 3):78–84. [DOI] [PubMed] [Google Scholar]
- 87. DeLong LK, Wetherington S, Hill N et al Vitamin D levels, dietary intake, and photoprotective behaviors among patients with skin cancer. Semin Cutan Med Surg 2010; 29:185–9. [DOI] [PubMed] [Google Scholar]
- 88. Hoesl M, Dietz K, Rocken M et al Vitamin D levels of XP‐patients under stringent sun‐protection. Eur J Dermatol 2010; 20:457–60. [DOI] [PubMed] [Google Scholar]
- 89. Tang JY, Wu A, Linos E et al High prevalence of vitamin D deficiency in patients with basal cell nevus syndrome. Arch Dermatol 2010; 146:1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reid SM, Robinson M, Kerr AC et al Prevalence and predictors of low vitamin D status in patients referred to a tertiary photodiagnostic service: a retrospective study. Photodermatol Photoimmunol Photomed 2012; 28:91–6. [DOI] [PubMed] [Google Scholar]
- 91. Gentzsch S, Kern JS, Loeckermann S et al Iatrogenic vitamin D deficiency in a patient with Gorlin syndrome: the conundrum of photoprotection. Acta Derm Venereol 2014; 94:459–60. [DOI] [PubMed] [Google Scholar]
- 92. Kuwabara A, Tsugawa N, Tanaka K et al High prevalence of vitamin D deficiency in patients with xeroderma pigmentosum‐A under strict sun protection. Eur J Clin Nutr 2015; 69:693–6. [DOI] [PubMed] [Google Scholar]
- 93. Bogaczewicz J, Karczmarewicz E, Pludowski P et al Requirement for vitamin D supplementation in patients using photoprotection: variations in vitamin D levels and bone formation markers. Int J Dermatol 2016; 55:e176–83. [DOI] [PubMed] [Google Scholar]
- 94. Matsuoka LY, Ide L, Wortsman J et al Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 1987; 64:1165–8. [DOI] [PubMed] [Google Scholar]
- 95. Matsuoka LY, Wortsman J, Hollis BW. Use of topical sunscreen for the evaluation of regional synthesis of vitamin D3. J Am Acad Dermatol 1990; 22:772–5. [DOI] [PubMed] [Google Scholar]
- 96. Faurschou A, Beyer DM, Schmedes A et al The relation between sunscreen layer thickness and vitamin D production after ultraviolet B exposure: a randomized clinical trial. Br J Dermatol 2012; 167:391–5. [DOI] [PubMed] [Google Scholar]
- 97. Libon F, Courtois J, Le Goff C et al Sunscreens block cutaneous vitamin D production with only a minimal effect on circulating 25‐hydroxyvitamin D. Arch Osteoporos 2017; 12:66. [DOI] [PubMed] [Google Scholar]
- 98. Farr PM, Diffey BL. How reliable are sunscreen protection factors? Br J Dermatol 1985; 112:113–18. [DOI] [PubMed] [Google Scholar]
- 99. Shih BB, Farrar MD, Cooke MS et al Fractional sunburn threshold UVR doses generate equivalent vitamin D and DNA damage in skin types I‐VI, but with epidermal DNA damage gradient correlated to skin darkness. J Invest Dermatol 2018; 138:2244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Young AR, Boles J, Herzog B et al A sunscreen's labeled sun protection factor may overestimate protection at temperate latitudes: a human in vivo study. J Invest Dermatol 2010; 130:2457–62. [DOI] [PubMed] [Google Scholar]
- 101. Maia M, Maeda SS, Marçon C. Correlation between photoprotection and 25 hydroxyvitamin D and parathyroid hormone levels. An Bras Dermatol 2007; 82:233–7. [Google Scholar]
- 102. Godar DE, Pope SJ, Grant WB et al Solar UV doses of young Americans and vitamin D3 production. Environ Health Perspect 2012; 120:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hansen L, Tjønneland A, Sun Køster B et al exposure guidelines and serum vitamin D status in Denmark: the StatusD study. Nutrients 2016; 8:pii:E266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matsuoka LY, Wortsman J, Hanifan N et al Chronic sunscreen use decreases circulating concentrations of 25‐hydroxyvitamin D. A preliminary study. Arch Dermatol 1988; 124:1802–4. [PubMed] [Google Scholar]
- 105. Marks R, Foley PA, Jolley D et al The effect of regular sunscreen use on vitamin D levels in an Australian population. Results of a randomized controlled trial. Arch Dermatol 1995; 131:415–21. [PubMed] [Google Scholar]
- 106. Farrerons J, Barnadas M, Rodriguez J et al Clinically prescribed sunscreen (sun protection factor 15) does not decrease serum vitamin D concentration sufficiently either to induce changes in parathyroid function or in metabolic markers. Br J Dermatol 1998; 139:422–7. [DOI] [PubMed] [Google Scholar]
- 107. Farrerons J, Barnadas M, Lopez‐Navidad A et al Sunscreen and risk of osteoporosis in the elderly: a two‐year follow‐up. Dermatology 2001; 202:27–30. [DOI] [PubMed] [Google Scholar]
- 108. Azizi E, Pavlotsky F, Kudish A et al Serum levels of 25‐hydroxy‐vitamin D3 among sun‐protected outdoor workers in Israel. Photochem Photobiol 2012; 88:1507–12. [DOI] [PubMed] [Google Scholar]
- 109. Azizi E, Flint P, Sadetzki S et al A graded work site intervention program to improve sun protection and skin cancer awareness in outdoor workers in Israel. Cancer Causes Control 2000; 11:513–21. [DOI] [PubMed] [Google Scholar]
- 110. Jayaratne N, Russell A, van der Pols JC. Sun protection and vitamin D status in an Australian subtropical community. Prevent Med 2012; 55:146–50. [DOI] [PubMed] [Google Scholar]
- 111. Young AR, Narbutt J, Harrison GI et al Optimal sunscreen use, during a sun holiday with a very high ultraviolet index, allows vitamin D synthesis without sunburn. Br J Dermatol 2019; 10.1111/bjd.17888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nair‐Shalliker V, Clements M, Fenech M et al Personal sun exposure and serum 25‐hydroxy vitamin D concentrations. Photochem Photobiol 2013; 89:208–14. [DOI] [PubMed] [Google Scholar]
- 113. Bogh MK, Schmedes AV, Philipsen PA et al Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol 2010; 130:546–53. [DOI] [PubMed] [Google Scholar]
- 114. Mazahery H, von Hurst PR. Factors affecting 25‐hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients 2015; 7:5111–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Intrinsic and extrinsic factors that determine serum 25(OH)D.
Appendix S2 What is optimal vitamin D status and the best method to determine it? Public health perspectives.
Table S1 Indications for 25(OH)D screening.
Table S2 Sunscreen use and vitamin D status in real sun exposure (questionnaire‐based and modelled studies).


