Abstract
Background
Major hepatectomy is a complex surgical procedure with high morbidity. Intra‐abdominal infection (IAI) is common following hepatectomy and affects treatment outcomes. This study was performed to investigate perioperative factors and determine whether the preoperative serum albumin level is associated with IAI following major hepatectomy.
Methods
From January 2008 to December 2018, 268 patients underwent major hepatectomy. We retrospectively analyzed demographic data and preoperative and perioperative variables. IAI was defined as organ/space surgical site infection. Risk factors for IAI were analyzed by logistic regression analysis.
Results
In total, 268 patients were evaluated. IAI was observed in 38 patients (14.6%). The mortality rate in the IAI group was 15.7%. Multivariate logistic analysis confirmed that the serum albumin level (odds ratio 0.91; 95% confidence interval 0.84–0.97; P = 0.03) and operative duration (odds ratio 1.50; 95% confidence interval 1.18–1.91; P < 0.01) were independent factors associated with IAI. A logistic model using the serum albumin level and operative duration to estimate the probability of IAI was analyzed. The area under the receiver operating characteristic curve for predicting IAI was 0.78.
Conclusion
The serum albumin level and operative duration were independent factors predicting IAI following major hepatectomy.
Keywords: Hepatectomy, Intra‐abdominal infection, Risk factors, Serum albumin, Treatment outcome
Highlight
Rungsakulkij and colleagues set out to investigate the as yet controversial association between preoperative serum albumin levels and intra‐abdominal infection following major hepatectomy. Preoperative serum albumin levels and operative duration were predictors of postoperative intra‐abdominal infection, indicating the need for preoperative nutritional intervention in patients with low serum albumin levels.

Introduction
Hepatectomy is the standard treatment for both benign and malignant diseases of the liver with adequate functional reserve 1, 2. The mortality rate of hepatectomy is very low in high‐volume centers 2, 3, 4. Despite technical advances, improvements in perioperative management, a greater understanding of the physiology of the liver, and accumulation of experience with liver resection at specialized centers, the postoperative morbidity rate is still relatively high at up to 40% according to previous reports 3, 5. Major hepatectomy is a complex surgical procedure and has a higher complication rate than minor surgery 5, 6. Although no consensus has been reached regarding the definition of major hepatectomy, the removal of more than three liver segments is the definition generally accepted worldwide 6, 7.
Surgical site infection (SSI) is a common complication following hepatectomy and is classified as superficial SSI, deep or incisional SSI, and organ/space SSI 8. Organ/space SSI and intra‐abdominal infection (IAI) are lethal complications following hepatectomy, and previous studies have shown that the incidence of IAI following hepatectomy may reach 17% 9, 10, 11, 12. IAI is associated with poor long‐term outcomes in patients with both primary and secondary malignancies following hepatectomy 13, 14, 15. Various preoperative and perioperative factors are associated with IAI following hepatectomy. Preoperative risk factors for IAI include the body mass index, serum albumin level, smoking, dialysis, serum bilirubin level, anemia, and repeat hepatectomy. The operative duration, extent of surgery, bile leakage, concomitant bowel surgery, and blood loss are perioperative risk factors for IAI 12. The extent of hepatic resection is strongly associated with the development of IAI. Kenjo et al. 2 and Dokmak et al. 3 reported higher morbidity rates in patients undergoing major hepatectomy than minor hepatectomy.
The preoperative serum albumin level is strongly associated with outcomes following various types of gastrointestinal surgery, including gastric, pancreatic, and colorectal surgery 16, 17, as well as non‐gastrointestinal surgery 18. The association between serum albumin and IAI is controversial 2, 11, 12. To the best of our knowledge, no study has yet been performed to evaluate the association of the preoperative serum albumin level with IAI in patients undergoing major hepatectomy. The present study was performed to investigate perioperative factors and determine whether the preoperative serum albumin level is associated with IAI following major hepatectomy.
Methods
A total of 268 consecutive patients underwent major hepatectomy at the Department of Surgery, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand from January 2008 to December 2018, and their data were retrospectively analyzed. The study protocol was approved by the Institutional Ethical Committee at Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Thailand (protocol number, MURA2019/80). Major hepatectomy was defined as removal of four or more segments. The liver segments were defined according to the Brisbane classification 19. All patients underwent preoperative cross‐sectional dynamic imaging using either triple‐phase computed tomography or magnetic resonance imaging. Routine blood examinations included a complete blood count, coagulogram, liver and kidney function tests, and the preoperative serum alpha‐fetoprotein level. The low serum albumin was defined as serum albumin level <35 g/l. Preoperative characteristics including the American Society of Anesthesiologists (ASA) class, evidence of hepatitis virus B or C, and smoking were recorded. The preoperative nutritional status was assessed from the Nutritional Alert Form (NAF). The patients were classified to A (normal‐mild malnutrition), B (moderate malnutrition), C (severe malnutrition) 20. Preoperative indocyanine green retention test at 15 min (ICG‐R15) was also performed. Preoperative biliary intervention was defined as the performance of preoperative percutaneous biliary drainage, endoscopic biliary drainage, and previous operative procedures involving the biliary tract. The indication for preoperative biliary intervention is cholangitis and for preoperative biliary drainage in the patients who had perihilar obstruction. Makuuchi’s criteria are used to select patients for curative resection in our center 21. The extent of liver resection was based on the patient’s liver functional reserve as assessed mainly by Makuuchi’s criteria, including the preoperative ascites volume, Child–Pugh score, ICG‐R15 value, and occasionally, volumetric computed tomography analysis. A preoperative evaluation was conducted by a multidisciplinary team including a surgeon, gastroenterologist, medical oncologist, and interventionist every week at our hospital. The platelet‐to‐lymphocyte ratio (PLR), prognostic nutritional index (PNI) and neutrophil‐to‐lymphocyte ratio (NLR) are among the preoperative serum inflammatory indices included in the study. PLR was calculated as the platelet count divided by the lymphocyte count. PNI was calculated as albumin (g/l) + 0.005 × total lymphocyte count (/µl). NLR was calculated as neutrophil count divided by the lymphocyte count.
Perioperative method
Prophylactic antibiotics were routinely given with intravenous cefazolin or cefoxitin 1.0 g within 30 min before skin incision. The incision type depended on the surgeon’s preference. The Pringle maneuver was performed using intermittent clamping. Intraoperative ultrasound was routinely used to stage the disease and to guide parenchymal transection, which was performed using a Cavitron ultrasonic aspirator, the clamp–crushing technique, or a combined technique depending on the surgeon’s preference. A prophylactic drainage tube was routinely placed. The suture material was also dependent upon the surgeon’s preference. Blood loss was estimated by an anesthesiologist, who also assessed the need for blood transfusion. The operative time was defined as the period from the start of the incision to closure of the abdominal wound.
Postoperative complications
Intra‐abdominal infection was defined as an infection that occurred within 30 days postoperatively, appeared to be related to the operation and involved any part of the anatomy other than the incision that was opened or manipulated during the operation, and showed at least one of the following characteristics: purulent drainage from the tube placed through a stab wound into the organ space; isolation of organisms from an aseptically obtained culture of fluid or tissue from the organ space; development of an abscess or other evidence of infection involving the organ space as found on direct examination, during reoperation, or by histopathologic or radiologic examination; or diagnosis of an organ/space SSI made by the surgeon or attending doctor 8. Bile leakage was classified as grade A, B, and C according to the International Study Group of Liver Surgery 22. The drainage tube was removed after postoperative day 3. The patients who had bile leakage, the drainage tube was placed until there was no evidence of bile leakage. The IAI patients who had sepsis, the reoperation was performed. Postoperative mortality was recorded as 90‐day mortality and in‐hospital mortality.
Statistical analyses
Among the patient characteristics, continuous variables were compared by Student’s t‐test and categorical variables were compared by the χ2 test or Fisher’s exact test. A P‐value of <0.05 was considered statistically significant. The potential risk factors were analyzed by univariate and multivariate methods using a logistic regression model. Independent risk factors were expressed as odds ratio (OR) with 95% confidence interval (CI). The performance of the predictor for the variable factor was analyzed by the receiver operating characteristic (ROC) curves.
Internal validity of the final model’s performance in terms of area under the curve (AUC) of the ROC curve was assessed using the bootstrap cross‐validation method. Consequently, the data were resampled with replacement, and for each resample a logistic regression model with the same risk factor as the final model (assuming the final model to be true) was developed. This method was applied to the full, non‐resampled data set to obtain a validating AUC value. This method was repeated 10,000 times (10,000 replicates). The width of the 95% range of the validating AUCs of the resamples (2.5% and 97.5% quantiles for AUCs) was used as the internal validity measure.
Results
Patient characteristics and perioperative data
A total of 268 patients underwent major hepatectomy from January 2008 to December 2018, of whom 38 (14.6%) had IAI. The overall mortality rate was 4.85% (13/268). For the analyses, the patients were divided into two groups: IAI (n = 38) and non‐IAI (n = 230). The clinicopathological characteristics of the two groups are shown in Table 1. The IAI group contained more patients with a higher ASA class (class IV) (16.22% vs. 4.82%, P = 0.034), more patients who had undergone previous biliary intervention (34.11% vs. 12.17%, P < 0.001), a greater aspartate aminotransferase level (57 vs. 36.5 U/l, P = 0.003), a longer operative duration (512 vs. 395 min, P < 0.001), greater blood loss (1,800 vs. 1,000 ml, P < 0.001), and a greater length of hospital stay (LOH) (24 vs. 11 days, P < 0.001) than the non‐IAI group. The IAI group also had a lower hemoglobin level (12.1 vs. 12.9 g/dl, P = 0.015) and serum albumin level (33.1 vs. 37.7 g/l, P < 0.001) than the non‐IAI group. The IAI group had higher rates of reoperation (13.16% vs. 0.44%, P < 0.001), readmission (15.79% vs. 0.87%, P < 0.001), and mortality (15.79% vs. 3.04%, P = 0.001).
Table 1.
Patients’ perioperative characteristics
| Data | Total (n = 268) | Non‐IAI (n = 230) | IAI (n = 38) | P‐value |
|---|---|---|---|---|
| Sex | ||||
| Male | 156 (58.21) | 135 (58.70) | 21 (55.26) | 0.691 |
| Female | 112 (41.79) | 95 (41.30) | 17 (44.74) | |
| Age, years | 54.82 ± 12.61 | 54.42 ± 12.96 | 57.28 ± 10.08 | 0.194 |
| BMI (kg/m2), mean ± SD | 23.82 ± 3.69 | 23.84 ± 3.64 | 23.67 ± 4.08 | 0.798 |
| Comorbidities | ||||
| DM | 59 (22.01) | 49 (21.30) | 10 (26.32) | 0.490 |
| Hypertension | 97 (36.19) | 82 (35.65) | 15 (39.47) | 0.650 |
| Dyslipidemia | 33 (12.31) | 28 (12.17) | 5 (13.16) | 0.864 |
| CKD | 2 (0.75) | 2 (0.87) | 0 | 0.999 |
| Viral hepatitis | 63 (23.51) | 55 (23.91) | 8 (21.05) | 0.700 |
| COPD | 6 (2.24) | 6 (2.61) | 0 | 0.314 |
| Cirrhosis | 10 (3.73) | 8 (3.48) | 2 (5.26) | 0.591 |
| ASA class, n = 265 | ||||
| I | 25 (9.43) | 24 (10.53) | 1 (2.70) | 0.034 |
| II | 98 (36.98) | 84 (36.84) | 14 (37.84) | |
| III | 125 (47.17) | 109 (47.81) | 16 (43.24) | |
| IV | 17 (6.42) | 11 (4.82) | 6 (16.22) | |
| Smoking | 99 (36.94) | 85 (36.96) | 14 (36.84) | 0.989 |
| Cholangitis | 6 (2.24) | 6 (2.61) | 0 | 0.599 |
| Previous biliary intervention | 41 (15.30) | 28 (12.17) | 13 (34.11) | 0.000 |
| Platelet count ×103, n = 265 | 248 (87, 703) | 244 (87, 669) | 284 (108, 703) | 0.168 |
| Hb, g/dl, n = 261 | 12.8 (5.7, 40.9) | 12.9 (5.7, 40.9) | 12.1 (7.9, 15.1) | 0.015 |
| BUN, mg/dl, n = 256 | 12 (4, 43) | 12 (4, 43) | 13 (8, 40) | 0.245 |
| AST, U/l, n = 254 | 37 (8, 378) | 36.5 (8, 378) | 57 (10, 220) | 0.003 |
| ALT, U/l, n = 253 | 43 (10, 229) | 41 (10, 229) | 58.5 (13, 221) | 0.000 |
| Cholesterol, mg/dl, n = 210 | 199.30 ± 48.85 | 203.02 ± 49.67 | 174.46 ± 44.34 | 0.004 |
| eGFR, n = 258 | 91.53 (4.55, 133.50) | 92.05 (4.55, 133.5) | 80.00 (30.34, 129.96) | 0.077 |
| PT, n = 239 | 12.10 (9.70, 27.00) | 12.00 (9.70, 27.00) | 12.90 (10.00, 18.10) | 0.015 |
| TB, mg/dl, n = 242 | 0.6 (0.2, 24.7) | 0.6 (0.2, 24.7) | 0.7 (0.2, 5.1) | 0.293 |
| PNI, n = 214 | 93.78 ± 34.36 | 94.34 ± 34.85 | 90.50 ± 31.65 | 0.566 |
| PLR, n = 217 | 140..35 (49.49, 625.07) | 137.75 (49.49, 587.32) | 155.26 (63.98, 625.07) | 0.222 |
| NLR, n = 217 | 2.14 (0.57, 30.66) | 2.14 (0.57, 30.67) | 2.46 (0.80, 21.5) | 0.376 |
| Albumin, g/l, n = 261 | 37.2 (21.7, 49) | 37.7 (22.2, 49) | 33.1 (21.7, 45.7) | 0.000 |
| ≥35 | 173 (66.28) | 156 (69.64) | 17 (45.95) | 0.005 |
| <35 | 88 (33.72) | 68 (30.36) | 20 (54.05) | |
| ICG‐R15, n = 110 | 11.40 (0.1, 53.8) | 11.3 (0.1, 53.8) | 11.9 (1.9, 36.6) | 0.416 |
| NAF score, n = 267 | ||||
| A (normal‐mild malnutrition) | 192 (71.91) | 167 (72.93) | 25 (65.79) | 0.365 |
| B (moderate malnutrition) | 75 (28.09) | 62 (27.07) | 13 (34.21) | |
| Diagnosis | ||||
| Hepatocellular carcinoma | 84 (31.34) | 73 (31.74) | 11 (28.95) | 0.144 |
| Cholangiocarcinoma | 89 (33.21) | 69 (30.00) | 20 (52.63) | |
| Liver metastasis | 50 (18.66) | 45 (19.57) | 5 (13.16) | |
| Hepatolithiasis | 3 (1.12) | 2 (0.87) | 1 (2.63) | |
| Liver abscess | 1 (0.37) | 1 (0.43) | 0 | |
| Liver donor | 22 (8.21) | 22 (9.57) | 0 | |
| Benign hepatic tumor | 10 (3.73) | 9 (3.91) | 1 (2.63) | |
| Gallbladder cancer | 1 (0.37) | 1 (0.43) | 0 | |
| Hepatic cyst | 8 (2.99) | 8 (3.48) | 0 | |
| Biliary reconstruction, n = 265 | 4 (1.51) | 3 (1.32) | 1 (2.63) | 0.464 |
| Operative time, min | 411.30 ± 150.69 | 395.16 ± 145.78 | 512.63 ± 143.09 | 0.000 |
| Blood loss, ml | 1,200 (50, 32,000) | 1,000 (50, 3,200) | 1,800 (300, 16,000) | 0.000 |
| Antibiotic coated suture material, n = 220 | ||||
| Antibiotic coated | 206 (93.64) | 178 (93.19) | 28 (96.55) | 0.700 |
| Non‐antibiotic coated | 14 (6.36) | 13 (6.81) | 1 (3.45) | |
| Closure suture material, n = 219 | ||||
| Monofilament | 96 (43.84) | 79 (41.36) | 17 (60.71) | 0.054 |
| Multifilament | 123 (56.16) | 112 (58.64) | 11 (39.29) | |
| Bile leakage | ||||
| No | 235 (87.69) | 205 (89.13) | 30 (78.95) | 0.077 |
| Yes | 33 (12.31) | 25 (10.87) | 8 (21.05) | |
| Treatment, n = 267 | ||||
| Stitch off | 7 (2.62) | 5 (2.18) | 2 (5.26) | 0.271 |
| PCD | 18 (6.74) | 5 (2.18) | 13 (34.21) | 0.000 |
| Reoperation | 6 (2.25) | 1 (0.44) | 5 (13.16) | 0.000 |
| LOH, days | 11 (3, 106) | 11 (3, 106) | 24.5 (7, 90) | 0.000 |
| Readmission | 8 (2.99) | 2 (0.87) | 6 (15.79) | 0.000 |
| Death | 13 (4.85) | 7 (3.04) | 6 (15.79) | 0.001 |
Data are presented as n (%), mean ± standard deviation, or median (range)
ALT alanine aminotransferase, ASA American Society of Anesthesiologists, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, eGFR estimated glomerular filtration rate, Hb hemoglobin, IAI intra‐abdominal infection, ICG‐R15 indocyanine green retention test at 15 min, LOH length of hospital stay, NAF nutritional alert form, NLR neutrophil‐to‐lymphocyte ratio, PCD percutaneous drainage, PLR platelet‐to‐lymphocyte ratio, PNI prognostic nutritional index, PT prothrombin time, TB total bilirubin
Regarding to the preoperative serum albumin, the preoperative characteristics of patients associated with normal and low serum albumin level are displayed in Table 2. The low serum albumin group contained more patients with higher ASA class (class IV) (12.6 vs. 3.47%, P < 0.001), higher smoking populations (47.7 vs. 32.9%, P = 0.020), more patients who had undergone previous biliary intervention (29.5 vs. 8.09%, P < 0.001), higher platelet level (294 vs. 234 × 103, P < 0.001), higher aspartate aminotransferase level (42.5 vs. 34.5, P = 0.001), longer prothrombin time (12.8 vs. 11.8, P < 0.001), higher serum total bilirubin level (1.07 vs. 1.02 mg/dl, P = 0.008), greater malignant patients (86.3 vs. 72.2%, P = 0.010) than normal serum albumin group. The low serum albumin group also had lower hemoglobin level (11.8 vs. 13.1 g/dl, P < 0.001).
Table 2.
Patients’ perioperative characteristics compared between normal and low serum albumin groups
| Data | Total (n = 261) | Albumin ≥35 (n = 173) | Albumin <35 (n = 88) | P‐value |
|---|---|---|---|---|
| Sex | ||||
| Male | 153 (58.62) | 95 (54.91) | 58 (65.91) | 0.088 |
| Female | 108 (41.38) | 78 (45.09) | 30 (34.09) | |
| Age, years | 55.01 ± 12.42 | 54.26 ± 13.18 | 56.46 ± 10.70 | 0.148 |
| BMI | 23.8 ± 3.68 | 23.92 ± 3.47 | 23.76 ± 4.11 | 0.746 |
| Comorbidities | ||||
| DM | 59 (22.61) | 36 (20.81) | 23 (26.14) | 0.331 |
| Hypertension | 96 (36.78) | 62 (35.84) | 34 (38.64) | 0.658 |
| Dyslipidemia | 32 (12.26) | 21 (12.14) | 11 (12.50) | 0.933 |
| Chronic kidney disease | 2 (0.77) | 2 (1.16) | 0 | 0.551 |
| Viral hepatitis | 61 (23.37) | 41 (23.70) | 20 (22.73) | 0.861 |
| COPD | 5 (1.92) | 3 (1.73) | 2 (2.27) | 0.764 |
| Cirrhosis | 10 (3.83) | 7 (4.05) | 3 (3.41) | 0.800 |
| ASA class, n = 260 | ||||
| I | 24 (9.23) | 23 (13.29) | 1 (1.15) | 0.000 |
| II | 95 (36.54) | 72 (41.62) | 23 (26.44) | |
| III | 124 (48.69) | 72 (41.62) | 52 (59.77) | |
| IV | 17 (6.54) | 6 (3.47) | 11 (12.64) | |
| Smoking | 99 (37.93) | 57 (32.95) | 42 (47.73) | 0.020 |
| Cholangitis | 6 (2.30) | 3 (1.73) | 3 (3.41) | 0.408 |
| Previous biliary intervention | 40 (15.33) | 14 (8.09) | 26 (29.55) | 0.000 |
| Platelet count ×103, n = 260 | 247 (87, 703) | 234 (102, 630) | 294 (87, 703) | 0.000 |
| Hb, g/dl, n = 257 | 12.8 (5.7, 40.9) | 13.1 (5.7, 40.9) | 11.8 (7.9, 40.9) | 0.000 |
| BUN, mg/dl, n = 252 | 12 (4, 43) | 13 (4, 43) | 12 (5, 34.8) | 0.147 |
| AST, U/l, n = 260 | 37 (8, 378) | 34.5 (8, 378) | 42.5 (10, 235) | 0.001 |
| ALT, U/l, n = 259 | 43 (10, 229) | 41 (12, 229) | 50 (10, 224) | 0.105 |
| Cholesterol, mg/dl, n = 215 | 199.19 ± 49.97 | 202.90 ± 50.52 | 191.91 ± 48.39 | 0.132 |
| eGFR, n = 258 | 91.04 (4.55, 133.5) | 91.61 (4.55, 133.50) | 89.49 (33.60, 129.96) | 0.992 |
| PT, n = 239 | 12.10 (9.70, 27.00) | 11.8 (9.70, 27.00) | 12.80 (10.80, 26.1) | 0.000 |
| TB, mg/dl, n = 241 | 0.6 (0.2, 21.4) | 0.6 (0.2, 12) | 0.7 (0.2, 21.4) | 0.008 |
| PNI, n = 214 | 93.78 ± 34.36 | 96.27 ± 33.84 | 88.56 ± 35.11 | 0.125 |
| PLR, n = 214 | 140.23 (49.49, 625.07) | 128.06 (54.55, 587.32) | 176.69 (49.49, 625.07) | 0.000 |
| NLR, n = 214 | 2.15 (0.57, 30.67) | 1.96 (0.57, 30.67) | 2.62 (0.84, 21.50) | 0.001 |
| IAI | ||||
| No | 224 (85.82) | 156 (90.17) | 68 (77.27) | 0.005 |
| Yes | 37 (14.18) | 17 (9.83) | 20 (22.73) | |
| ICG‐R15, n = 110 | 11.4 (0.1, 53.8) | 11.3 (0.1, 29.5) | 12.2 (0.1, 53.8) | 0.811 |
| NAF score, n = 261 | ||||
| A (normal‐mild malnutrition) | 186 (71.26) | 129 (74.57) | 57 (64.77) | 0.098 |
| B (moderate malnutrition) | 75 (28.74) | 44 (25.43) | 31 (35.23) | |
| Diagnosis | ||||
| Hepatocellular carcinoma | 82 (31.42) | 51 (29.48) | 31 (35.23) | 0.015 |
| Cholangiocarcinoma | 87 (33.33) | 50 (28.90) | 37 (42.05) | |
| Liver metastasis | 50 (19.16) | 35 (20.23) | 15 (17.05) | |
| Hepatolithiasis | 3 (1.15) | 2 (1.16) | 1 (1.14) | |
| Liver abscess | 1 (0.38) | 0 | 1 (1.14) | |
| Liver donor | 20 (7.66) | 19 (10.98) | 1 (1.14) | |
| Benign hepatic tumor | 10 (3.83) | 9 (5.20) | 1 (1.14) | |
| Gallbladder cancer | – | – | – | – |
| Hepatic cyst | 8 (3.07) | 7 (4.05) | 1 (1.14) | |
| Biliary reconstruction, n = 260 | 4 (1.54) | 4 (2.33) | 0 | 0.303 |
| Operative time, min | 411.78 ± 152.03 | 382.94 ± 136.75 | 469.13 ± 1164.94 | 0.000 |
| Blood loss, ml | 1,200 (50, 32,000) | 1,000 (50, 16,000) | 1,600 (100, 32,000) | 0.000 |
| Antibiotic coated suture material, n = 217 | ||||
| Antibiotic coated | 203 (93.55) | 137 (94.48) | 66 (91.67) | 0.427 |
| Non‐antibiotic coated | 14 (6.45) | 8 (5.52) | 6 (8.33) | |
| Closure suture material, n = 216 | ||||
| Monofilament | 94 (43.52) | 60 (41.38) | 34 (47.89) | 0.365 |
| Multifilament | 122 (56.48) | 85 (58.62) | 37 (52.11) | |
| Bile leakage | ||||
| No | 228 (87.36) | 150 (86.71) | 78 (88.64) | 0.657 |
| Yes | 33 (12.64) | 23 (13.29) | 10 (11.36) | |
| Treatment | ||||
| Stitch off, n = 261 | 7 (2.68) | 6 (3.47) | 1 (1.14) | 0.270 |
| PCD, n = 261 | 17 (6.51) | 11 (6.36) | 6 (6.82) | 0.887 |
| Reoperation, n = 267 | 6 (2.30) | 2 (1.16) | 4 (4.55) | 0.194 |
| LOH, days | 11 (1, 106) | 10 (1, 106) | 15 (1, 90) | 0.000 |
| Readmission | 8 (3.07) | 6 (3.47) | 2 (2.27) | 0.721 |
| Death | 12 (4.60) | 2 (1.16) | 10 (11.36) | 0.000 |
Data are presented as n (%), mean ± standard deviation, or median (range)
ALT alanine aminotransferase, ASA American Society of Anesthesiologists, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, eGFR estimated glomerular filtration rate, Hb hemoglobin, IAI intra‐abdominal infection, ICG‐R15 indocyanine green retention test at 15 min, LOH length of hospital stay, NAF nutritional alert form, NLR neutrophil to lymphocyte ratio, PCD percutaneous drainage, PLR platelet‐to‐lymphocyte ratio, PNI prognostic nutritional index, PT prothrombin time, TB total bilirubin
Analysis of risk factors associated with IAI
The results of the univariate and multivariate analyses of potential predictors of IAI are shown in Table 3. The univariate analysis identified the following variables as significantly associated with IAI: ASA class IV (OR 13.0; 95% CI 1.40–122.2; P < 0.05), previous biliary intervention (OR 3.75; 95% CI 1.72–8.16; P < 0.01), hemoglobin concentration (OR 0.81; 95% CI 0.67–0.98; P < 0.05), serum albumin concentration (OR 0.887; 95% CI 0.83–0.94; P < 0.01), operative time (OR 1.612; 95% CI 1.28–2.02; P < 0.01), and blood loss (OR 1.153; 95% CI 1.03–1.28; P < 0.01). In the multivariate analysis, only the operative time (OR 1.506; 95% CI 1.18–1.91; P < 0.01) and serum albumin concentration (OR 0.911; 95% CI 0.84–0.97; P < 0.05) were significantly associated with IAI.
Table 3.
Univariate and multivariate analysis of predictors of intra‐abdominal infection
| Data | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Sex | ||||
| Male | 1 | |||
| Female | 1.150 (0.57–2.29) | 0.691 | ||
| Age, years | 1.018 (0.99–1.05) | 0.195 | ||
| BMI | 0.987 (0.89–1.08) | 0.798 | ||
| Comorbidities | ||||
| DM | 1.319 (0.59–2.90) | 0.491 | ||
| Hypertension | 1.177 (0.58–2.38) | 0.650 | ||
| Dyslipidemia | 1.093 (0.39–3.03) | 0.864 | ||
| Chronic kidney disease | – | – | ||
| Viral hepatitis | 0.848 (0.36–1.95) | 0.700 | ||
| COPD | – | – | ||
| Cirrhosis | 1.541 (0.31–7.55) | 0.593 | ||
| ASA class | ||||
| I | 1 | |||
| II | 4.000 (0.50–31.98) | 0.191 | ||
| III | 3.522 (0.44–27.86) | 0.233 | ||
| IV | 13.090 (1.40–122.2) | 0.024 | ||
| Smoking | 0.995 (0.48–2.02) | 0.989 | ||
| Cholangitis | – | – | ||
| Previous biliary intervention | 3.751 (1.72–8.16) | 0.001 | ||
| Platelet count x103 | 1.259 (0.92–1.70) | 0.136 | ||
| Hb, g/dl | 0.814 (0.67–0.98) | 0.035 | ||
| BUN, mg/dl | 1.038 (0.97–1.10) | 0.201 | ||
| AST, U/l | 1.006 (0.99–1.02) | 0.050 | ||
| ALT, U/l | 1.009 (1.00–1.02) | 0.023 | ||
| Cholesterol, mg/dl | 0.987 (0.97–0.99) | 0.006 | ||
| eGFR | 0.985 (0.97–1.00) | 0.068 | ||
| PT | 1.130 (0.96–1.32) | 0.124 | ||
| TB, mg/dl | 0.980 (0.84–1.14) | 0.794 | ||
| PNI | 0.996 (0.98–1.01) | 0.564 | ||
| PLR | 1.291 (0.88–1.88) | 0.185 | ||
| NLR | 1.066 (0.96–1.18) | 0.223 | ||
| Albumin, g/l | 0.887 (0.83–0.94) | 0.000 | 0.910 (0.83–0.99) | 0.031 |
| ICG‐R15 | 1.338 (0.91–1.95) | 0.134 | ||
| NAF score | ||||
| A | 1 | |||
| B | 1.400 (0.67–2.91) | 0.366 | ||
| Preoperative diagnosis | ||||
| Benign | 1 | |||
| Malignant | 1.828 (0.61–5.46) | 0.280 | ||
| Biliary reconstruction | 2.018 (0.20–19.92) | 0.548 | ||
| Operative time, min | 1.612 (1.28–2.02) | 0.000 | 1.580 (1.19–2.09) | 0.001 |
| Blood loss, ml | 1.153 (1.03–1.28) | 0.008 | ||
| Antibiotic coated suture material | ||||
| Antibiotic coated | 1 | |||
| Non‐antibiotic coated | 0.489 (0.06–3.88) | 0.499 | ||
| Closure suture material | ||||
| Monofilament | 1 | 0.058 | ||
| Multifilament | 0.456 (0.20–1.03) | |||
| Bile leakage | ||||
| No | 1 | |||
| Yes | 2.186 (0.90–5.29) | 0.083 | ||
ALT alanine aminotransferase, ASA American Society of Anesthesiologists, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, CI confidence interval, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, eGFR estimated glomerular filtration rate, Hb hemoglobin, IAI intra‐abdominal infection, ICG‐R15 indocyanine green retention test at 15 min, LOH length of hospital stay, NAF nutritional alert form, NLR neutrophil to lymphocyte ratio, OR odds ratio, PCD percutaneous drainage, PLR platelet‐to‐lymphocyte ratio, PNI prognostic nutritional index, PT prothrombin time, TB total bilirubin
Predictors of IAI based on preoperative serum albumin level and operative duration
The performance of the final prediction model of important risk factors for IAI was examined in 268 patients. When the final model was fitted using total logistic regression, the operative time and albumin concentration remained in the final model. According to our final prediction model, the predictive performance of the serum albumin level and operative duration for the risk of IAI based on 268 patients was shown by an AUC of 0.7846 (Fig. 1a). Moreover, a logistic regression model to estimate the probability of IAI based on preoperative serum albumin and operative duration in the 298 patients was constructed from those risk factors as described in the following formula:
Figure 1.
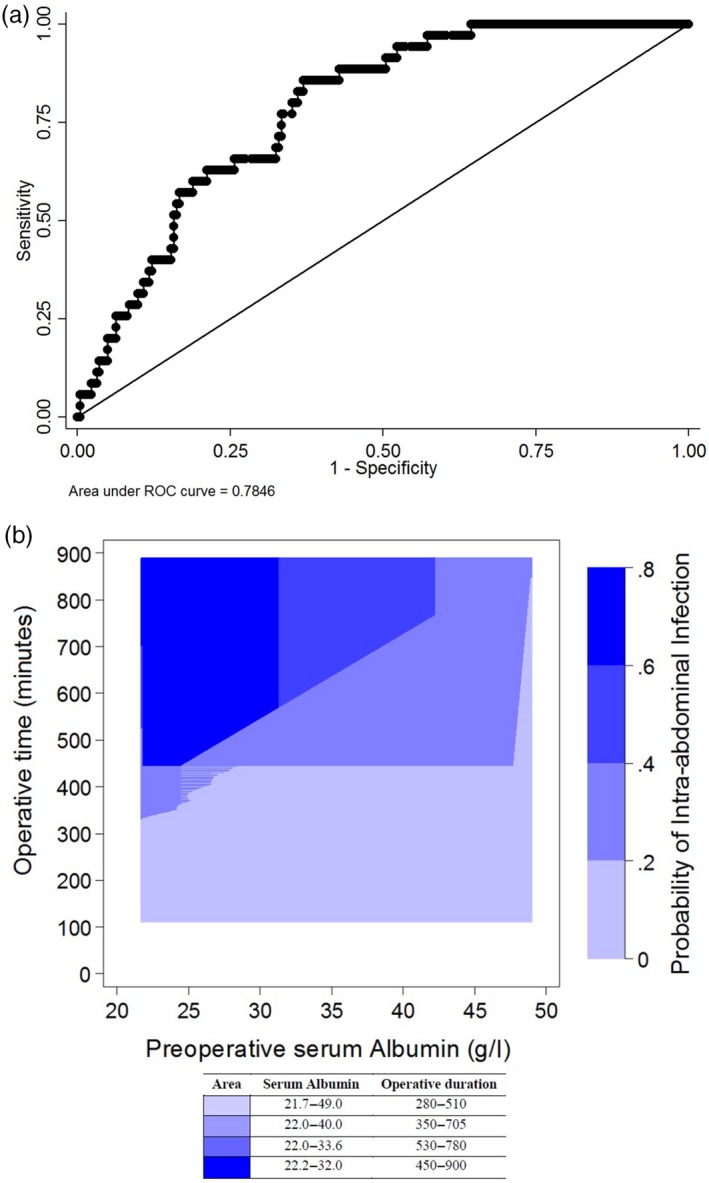
(a) Receiver operating characteristic curve for predicting intra‐abdominal infection based on the serum albumin level and operative duration. The area under the receiver operating characteristic curve is 0.7846. (b) This figure shows a contour plot the estimated probability of intra‐abdominal infection following major hepatectomy stratified according to the serum albumin concentration and operative duration, based on the logistic regression model. As an example, the darkest blue shaded are in the upper left had corner of the figure represents probabilities of intra‐abdominal infection between 0.6–0.8, in which the operative duration ranges between 450–900 min, and the preoperative albumin level ranges between 22–32 g/l
The performance of the final model for the probability of IAI in 268 patients in terms of the AUC showed the AUC to be 0.785, standard error, 0.035 (Fig. 1). Calculations for the Bootstrap cross‐validation showed the 2.5–97.5% quantiles (95%) interval of the 10,000 resamples to be between 0.741 and 0.787, which included the AUC of the full data (0.785). The width of this interval is only 0.046 and can be considered sufficiently narrow (of the same order as the standard error of the AUC estimate), confirming the validity of the final model.
Discussion
Major hepatectomy, which is defined as the removal of four or more liver segments, is a complex surgery associated with a high morbidity rate when compared with minor hepatectomy 5, 6, 7, 14. Previous studies have demonstrated that postoperative morbidity is associated with an increased LOH, a higher reoperation rate, a subsequently higher cost of the inpatient stay, and poor long‐term outcomes 23, 24. Our study results are consistent with these previous reports in that the LOH, reoperation rate, and mortality rate were significantly higher in the IAI group. Infectious complications following hepatectomy are common; according to one report, the incidence rate may reach 20% 13. A common infectious complication is IAI or organ/space SSI, and the long‐term outcomes are affected by this complication 13, 14. The mechanisms underlying the poor long‐term outcomes of patients with postoperative IAI are postulated to involve the inflammatory process. Postoperative infections are associated with excessive and sustained levels of proinflammatory cytokine release, and cell‐mediated immunity is suppressed by the effects of anesthesia and the postoperative stress response, which in turn increases the growth of occult micrometastasis 25. In the present study, the incidence of IAI following major hepatectomy was 14%, which is comparable with previous studies 10, 15. However, the incidence of IAI in our study was relatively higher than previous studies from Moreno Elola‐Olaso et al. 11 and Kenjo et al. 2. This may be explained by differences in the patient characteristics and operative time. The preoperative serum albumin in our study was lower (3.7 vs. 4.0 g/dl) and longer operative duration (411 vs. 253 min) than previous two studies. Moreover, patients in our study had a higher proportion of low serum albumin group (33.7 vs. 16%) than previous studies.
From our study, the multivariate analysis showed that the independent factors associated with IAI following major hepatectomy were the preoperative serum albumin level and the operative time. Serum albumin is a negatively charged protein that is synthesized by hepatocytes and released into the circulation, and the rate of albumin synthesis by hepatocytes is regulated by changes in the plasma colloid pressure, the metabolic and inflammatory state of the host, and various anabolic and catabolic hormones 26, 27. Serum albumin has been used as a surrogate marker of patients’ nutritional state worldwide. Fuhrman et al. 27 reported that inflammation exerts the most significant effects on the serum protein level by altering normal hepatic protein metabolism and inducing capillary leakage. They concluded that hepatic proteins are indicators of morbidity and mortality and recovery from acute and chronic disease. The preoperative serum albumin is strongly associated with postoperative complications in patients undergoing hepatectomy 4. However, the association of preoperative hypoalbuminemia as a predictor of postoperative IAI following hepatectomy is controversial. Moreover, most studies have included both minor and major hepatectomy 13, 23. In the present study, all patients underwent major hepatectomy, which is a complex surgery with a high morbidity rate. Our results showed that the serum albumin level was a significant independent factor associated with IAI. Regarding to the preoperative hypoalbuminemia (<35 g/l) patients had significantly greater incidence of IAI following major hepatic resection than normal groups. This result is consistent with previous reports from two large cohort studies from the United States. Moreno Elola‐Olaso et al. 11 performed a large‐population retrospective study of the predictors of SSI after liver resection using the American College of Surgeons National Surgical Quality Improvement Program (ACS‐NSQIP) database, and the results showed that serum albumin was a predictor of SSI (both superficial and deep or organ/space SSI). Neumayer et al. 28 performed a large‐population study and found that among 163,624 patients undergoing vascular and general surgical procedures, low serum albumin levels of <3.5 mg/dl was independent risk factors associated with increased risk of SSI. According to these reports, the preoperative serum albumin level is strongly predictive of postoperative IAI following hepatectomy, especially major hepatectomy. Hypoalbuminemia, which is commonly observed in malnutrition patients is associated with a series of physiological derangement that may lead to complications and death 29. Consequently, the malnourished patients who are undergoing major operations are at significant risk from perioperative complications such as infectious complications 30. The hypoalbuminemia associated with IAI following hepatectomy is explained by alterations in both innate and adaptive immune function contribute significantly to increased susceptibility to infections 31. Moreover, protein wasting during the postoperative period is believed to represent the metabolic cost of rapid mobilizing amino acids for wound healing and the synthesis of immune cells and proteins 32. The recent guideline from European Society for Clinical Nutrition and Metabolism (ESPEN) state that nutrition is critically important to recovery from major surgery and patients at a high risk due to impaired nutritional status should ideally receive oral supplementation prior to major surgery, even if this results in the delay of resection of a malignancy 33. Nevertheless, the serum albumin following preoperative nutritional intervention is not considered as a marker for well‐nourished patients, because of the nutritional support often fails to improve serum albumin levels 29. To the best of our knowledge, no randomized, controlled trial study has yet been performed to evaluate the increase of the preoperative serum albumin level with IAI in patients undergoing major hepatectomy. Thus, a further well‐designed prospective randomized controlled trial study comparing low and high serum albumin patients undergoing major hepatectomy should be conducted.
Our study also showed that the operative time was an independent factor associated with IAI following major hepatectomy. A prolonged operative time is associated with an increased risk of postoperative SSI 34, 35. Procter et al. 34 performed a retrospective study of 299,359 operations performed at 173 hospitals from the ACS‐NSQIP and found that the infectious complication rate increased linearly with the operative duration. They concluded that the operative duration is independently associated with increased infectious complications and the LOH after adjustment for procedure‐ and patient‐related risk factors 34. A prolonged operative time is associated with increased exposure of microbes to the environment, heightened surgical stress to the immune system, and diminished efficacy of antimicrobial prophylaxis over time 13, 35. Several studies have shown that the operative time is a significant independent factor associated with SSI, including organ/space SSI, in patients undergoing hepatectomy 11, 12. Determinants of the operative duration are multifactorial and include the operative type, proficiency of the surgeon, presence of surgical trainees, communication among operative professionals, and whether the operation is performed on an emergency basis 36. Major hepatectomy usually has a long operative time because of the complexity of the operation. With respect to improvements in the efficacy of the procedure to reduce the operative duration, Procter et al. 34 and Haynes et al. 37 recommended improvements in team communication and the consistency of operating room staffing using a surgical safety checklist program, technology, and process efficiency to reduce the operative duration.
This study had some limitations. First, because of its retrospective nature, some selection bias may have been present. Second, the population of our study was relatively small when compared with previous studies.
Conclusion
Major hepatectomy is associated with a high morbidity rate. The preoperative serum albumin level is an important marker of patients’ nutritional and inflammatory status. The present study indicates that the serum albumin level and operative duration are significant predictors of postoperative IAI. In patients with a low serum albumin level who are undergoing major hepatectomy, the surgeon should consider preoperative nutritional intervention with the use of a short‐term high‐protein supplement diet and closely monitor the patients for the development of postoperative IAI. A further prospective study of perioperative nutritional support to improve treatment outcomes in such patients should be conducted.
Conflict of interest
None declared.
Acknowledgment
We thank Angela Morben, DVM, ELS, from Edanz Group (http://www.edanzediting.com/ac), for editing a draft of this article.
References
- 1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. [DOI] [PubMed] [Google Scholar]
- 2. Kenjo A, Miyata H, Gotoh M, Kitagawa Y, Shimada M, Baba H, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg. 2014;218:412–22. [DOI] [PubMed] [Google Scholar]
- 3. Dokmak S, Fteriche FS, Borscheid R, Cauchy F, Farges O, Belghiti J. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford). 2013;15:908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben‐Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shubert CR, Habermann EB, Truty MJ, Thomsen KM, Kendrick ML, Nagorney DM. Defining perioperative risk after hepatectomy based on diagnosis and extent of resection. J Gastrointest Surg. 2014;18:1917–28. [DOI] [PubMed] [Google Scholar]
- 6. Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford). 2011;13:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bismuth H. Revisiting liver anatomy and terminology of hepatectomies. Ann Surg. 2013;257:383–6. [DOI] [PubMed] [Google Scholar]
- 8. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. [PubMed] [Google Scholar]
- 9. Shirata C, Hasegawa K, Kokudo T, Arita J, Akamatsu N, Kaneko J, et al. Surgical site infection after hepatectomy for hepatocellular carcinoma. Dig Surg. 2018;35:204–11. [DOI] [PubMed] [Google Scholar]
- 10. Kurmann A, Wanner B, Martens F, Klasen J, Stickel F, Montani M, et al. Hepatic steatosis is associated with surgical‐site infection after hepatic and colorectal surgery. Surgery. 2014;156:109–16. [DOI] [PubMed] [Google Scholar]
- 11. Moreno Elola‐Olaso A, Davenport DL, Hundley JC, Daily MF, Gedaly R. Predictors of surgical site infection after liver resection: a multicentre analysis using National Surgical Quality Improvement Program data. HPB (Oxford). 2012;14:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kokudo T, Uldry E, Demartines N, Halkic N. Risk factors for incisional and organ space surgical site infections after liver resection are different. World J Surg. 2015;39:1185–92. [DOI] [PubMed] [Google Scholar]
- 13. Tang H, Lu W, Yang Z, Jiang K, Chen Y, Lu S, et al. Risk factors and long‐term outcome for postoperative intra‐abdominal infection after hepatectomy for hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang XF, Bagante F, Chakedis J, Moris D, Beal EW, Weiss M, et al. Perioperative and long‐term outcome for intrahepatic cholangiocarcinoma: impact of major versus minor hepatectomy. J Gastrointest Surg. 2017;21:1841–50. [DOI] [PubMed] [Google Scholar]
- 15. Farid SG, Aldouri A, Morris‐Stiff G, Khan AZ, Toogood GJ, Lodge JP, et al. Correlation between postoperative infective complications and long‐term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91–100. [DOI] [PubMed] [Google Scholar]
- 16. Rungsakulkij N, Tangtawee P, Suragul W, Muangkaew P, Mingphruedhi S, Aeesoa S. Correlation of serum albumin and prognostic nutritional index with outcomes following pancreaticoduodenectomy. World J Clin Cases. 2019;7:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bendersky V, Sun Z, Adam MA, Rushing C, Kim J, Youngwirth L, et al. Determining the optimal quantitative threshold for preoperative albumin level before elective colorectal surgery. J Gastrointest Surg. 2017;21:692–9. [DOI] [PubMed] [Google Scholar]
- 18. Peacock MR, Farber A, Eslami MH, Kalish JA, Rybin D, Doros G, et al. Hypoalbuminemia predicts perioperative morbidity and mortality after infrainguinal lower extremity bypass for critical limb ischemia. Ann Vasc Surg. 2017;41:169–175.e4. [DOI] [PubMed] [Google Scholar]
- 19. Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2000;2:333–9. [Google Scholar]
- 20. Komindrg S, Tangsermwong T, Janepanish P. Simplified malnutrition tool for Thai patients. Asia Pac J Clin Nutr. 2013;22:516–21. [DOI] [PubMed] [Google Scholar]
- 21. Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–94. [DOI] [PubMed] [Google Scholar]
- 22. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–8. [DOI] [PubMed] [Google Scholar]
- 23. Okamura Y, Takeda S, Fujii T, Sugimoto H, Nomoto S, Nakao A. Prognostic significance of postoperative complications after hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2011;104:814–21. [DOI] [PubMed] [Google Scholar]
- 24. Ruan DY, Lin ZX, Li Y, Jiang N, Li X, Wu DH, et al. Poor oncologic outcomes of hepatocellular carcinoma patients with intra‐abdominal infection after hepatectomy. World J Gastroenterol. 2015;21:5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 26. Neel DR, McClave S, Martindale R. Hypoalbuminaemia in the perioperative period: clinical significance and management options. Best Pract Res Clin Anaesthesiol. 2011;25:395–400. [DOI] [PubMed] [Google Scholar]
- 27. Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–64. [DOI] [PubMed] [Google Scholar]
- 28. Neumayer L, Hosokawa P, Itani K, El‐Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–87. [DOI] [PubMed] [Google Scholar]
- 29. Franch‐Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20:265–9. [DOI] [PubMed] [Google Scholar]
- 30. Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–9. [DOI] [PubMed] [Google Scholar]
- 31. Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–44. [DOI] [PubMed] [Google Scholar]
- 32. de Luis DA, Culebras JM, Aller R, Eiros‐Bouza JM. Surgical infection and malnutrition. Nutr Hosp. 2014;30:509–13. [DOI] [PubMed] [Google Scholar]
- 33. Walcott‐Sapp S, Billingsley KG. Preoperative optimization for major hepatic resection. Langenbeck Arch Surg. 2018;403:23–35. [DOI] [PubMed] [Google Scholar]
- 34. Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk‐adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210:60–5.e2. [DOI] [PubMed] [Google Scholar]
- 35. Cheng H, Clymer JW, Po‐Han Chen B, Sadeghirad B, Ferko NC, Cameron CG, et al. Prolonged operative duration is associated with complications: a systematic review and meta‐analysis. J Surg Res. 2018;229:134–44. [DOI] [PubMed] [Google Scholar]
- 36. Campbell DA Jr, Henderson WG, Englesbe MJ, Hall BL, O'Reilly M, Bratzler D, et al. Surgical site infection prevention: the importance of operative duration and blood transfusion–results of the first American College of Surgeons‐National Surgical Quality Improvement Program Best Practices Initiative. J Am Coll Surg. 2008;207:810–20. [DOI] [PubMed] [Google Scholar]
- 37. Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–9. [DOI] [PubMed] [Google Scholar]


