Abstract
Background
Few clinical trials have evaluated long‐term treatment of nail psoriasis with biologics.
Objective
Safety and efficacy of adalimumab [ADA; Humira AbbVie Inc, North Chicago, IL, USA)] long‐term treatment (52 weeks) was evaluated in a phase‐3, randomized trial in patients with moderate‐to‐severe plaque psoriasis and concomitant moderate‐to‐severe fingernail psoriasis. Results from the first 26 weeks (Period A) have been reported.
Methods
Patients receiving 40 mg ADA every other week or placebo in Period A, continued with or switched to 40 mg ADA every‐other‐week treatment in the subsequent 26‐week open‐label extension (OLE) period. Main efficacy evaluations were ≥75% improvement in total‐fingernail modified Nail Psoriasis Severity Index (mNAPSI 75) and achievement of Physician's Global Assessment for Fingernail Psoriasis of clear or minimal disease (PGA‐F 0/1) with a ≥2‐grade improvement from baseline, across the trial for patients who continued ADA from Period A through the OLE (Continuous‐ADA Population). Safety was evaluated during the OLE and for patients receiving ADA at any time during the study (All‐ADA Population).
Results
Of the 217 patients initially randomized in Period A, 188 (86.6%; 94 in each treatment group) entered the OLE after completion of or early escape from Period A. For the Continuous‐ADA Population (N = 109), endpoint achievement rates improved from OLE entry (Week 26) to Week 52, including total‐fingernail mNAPSI 75 (47.4–54.5%); PGA‐F 0/1 (51.1–55.6%) and total‐fingernail mNAPSI = 0 (6.6–17.9%). Serious adverse event and serious infection rates for the All‐ADA Population (N = 203) were 6.9% and 3.4%, respectively.
Conclusions
In this population of psoriasis patients with concomitant, moderate‐to‐severe nail psoriasis, long‐term efficacy and improvement in signs and symptoms of nail disease were demonstrated after every‐other‐week ADA treatment, including incremental improvements in rate of total clearance of nail disease. No new safety risks were identified for patients receiving at least one ADA dose across 52 weeks.
Short abstract
Linked Commentary: D. Rigopoulos. J Eur Acad Dermatol Venereol 2019; 33: 2014–2015. https://doi.org/10.1111/jdv.15988.
Introduction
Nail disease in patients with psoriasis is a chronic, painful condition that can result in impaired function, restrictions in daily life activities, and can aggravate the reduced quality of life, including psychological stress and negative effects on social activities inherent to psoriasis.1, 2, 3, 4 Concomitant skin or scalp psoriasis, or psoriatic arthritis (PsA) can occur with nail psoriasis.2, 5, 6 Approximately half of patients with skin psoriasis can have nail involvement,7 and approximately 80% of patients with both psoriasis and PsA also have nail involvement.8, 9, 10 Longer‐term nail disease may precede more severe psoriasis or PsA.11 Higher rates of nail disease are associated with more severe psoriasis and longer psoriasis duration.1, 6, 11 Quality of life for psoriasis patients can be much worse for those with concomitant nail psoriasis.12
Treatment of nail psoriasis differs depending on disease severity. Systemic therapy has been recommended for patients with psoriasis that is limited to the nails or who have not responded to topical therapy, as well as for more severe disease and when multiple body areas are involved (skin, nails and joints).13 Time to treatment response is slow because of the length of time involved in nail growth (5–7 months for complete nail growth14). Several clinical trials have evaluated long‐term treatment and effectiveness of biologic agents for nail psoriasis. Most have evaluated subpopulations of psoriasis patients who had concomitant moderate‐to‐severe nail psoriasis [tumour necrosis factor alpha (TNF‐α) inhibitors etanercept15 and infliximab16, 17; IL 12/23 inhibitor ustekinumab,18 IL‐17A inhibitor ixekizumab19, 20 and JAK inhibitor tofacitinib21]; however, none evaluated long‐term safety in their respective nail psoriasis populations. Two trials prospectively evaluated patients with moderate‐to‐severe nail disease: one in patients treated with secukinumab22, 23 and the other in the current phase‐3 trial described below.
The anti‐TNF‐α agent adalimumab [Humira (AbbVie Inc, North Chicago, IL, USA)] has demonstrated efficacy and safety up to 28 weeks for the treatment of nail psoriasis in studies of patients with moderate‐to‐severe psoriasis and PsA.24, 25, 26, 27 A phase‐3, randomized, controlled trial of adalimumab treatment of patients with moderate‐to‐severe plaque psoriasis and concomitant fingernail psoriasis reported efficacy and safety results from the first 26 weeks.28 Here, we report long‐term efficacy and safety results from the second 26‐week, open‐label extension (OLE) portion of that trial.
Materials and methods
Study design
This was a phase‐3, multicenter, double‐blind, randomized, parallel‐arm, placebo‐controlled trial.28 In the initial 26‐week Period A, patients were randomized in a 1 : 1 ratio to 40‐mg adalimumab every other week (starting at Week 1, after an 80 mg dose at Week 0) or to placebo. Patients were required to escape early from Period A into the OLE if starting from Week 16, they experienced significant worsening of their skin psoriasis [increase in body surface area (BSA) involvement by at least 25% over the baseline measurement]. This allowed the study to remain blinded and enabled patients receiving placebo to avoid undue disease burden. Upon completion of Period A, patients entered the 26‐week OLE and either continued with or switched to 40‐mg adalimumab every other week. Efficacy and safety outcomes throughout the 52 weeks of the study are reported here.
The study was conducted in accordance with International Council for Harmonisation guidelines, applicable regulations and the principles of the Declaration of Helsinki. Patients reviewed and signed an informed consent statement. The study protocol was approved by an independent ethics committee or institutional review board at each site.
Enrolment
As previously described,28 enrolment criteria included BSA ≥10% or ≥5% with a total modified Nail Psoriasis Severity Index (mNAPSI) score ≥20; target fingernail mNAPSI score of ≥8; a score of at least moderate severity for the Physician's Global Assessment for Fingernail Psoriasis (PGA‐F29) and the Physician's Global Assessment for Skin Psoriasis (PGA‐S) scores; and a score of >3 for the Nail Psoriasis Physical Functioning Severity (NPPFS) score or Nail Psoriasis Pain Numeric Rating Scale (NRS) score. Patients were not allowed previous adalimumab treatment or concomitant treatment for nail psoriasis during the study.
Efficacy assessments
The main efficacy analyses across 52 weeks were achievement of 75% or more improvement relative to baseline in total‐fingernail mNAPSI30 (mNAPSI 75), and achievement of PGA‐F 0/1 (clear or minimal) with a ≥2‐grade improvement from baseline. The mNAPSI endpoint was chosen because nail disease severity and nail involvement are reflected in the scoring. Other efficacy analyses across 52 weeks included per cent improvement from baseline in total‐fingernail NAPSI,31 achievement of total‐fingernail mNAPSI score of 0 and achievement of at least 50% improvement in the scalp component of the Brigham Scalp Nail Inverse Palmo‐Plantar Psoriasis Index32 [B‐SNIPI50 (scalp)] relative to baseline for patients with baseline scalp psoriasis score of ≥6 (assessed only for patients in US and Puerto Rico). A description of the methods that were used in this study to score nail disease has been published.28 Patient‐reported outcomes across 52 weeks included the following scores: improvement from baseline in Nail Psoriasis Pain numerical rating scale (NRS) using a scale of 0 (no pain) to 10 (severe pain); mean change from baseline in Nail Psoriasis Physical Functioning Severity (NPPFS) score, which measured the impact of fingernail psoriasis on the ability to perform physical tasks, using a scale of 0 (none) to 10 (severe); mean change from baseline in Nail Assessment of Psoriasis and Psoriatic Arthritis Quality of Life (NAPPA QoL) score,33 which measured the impact of nail psoriasis on hands and/or feet in terms of patient suffering and ability to function, using a scale of 0 (no effect) to 4 (very much of an effect) for each question; and mean change from baseline in Nail Psoriasis Quality of Life (Nail Ps QoL), which measured the impact of nail psoriasis on patient quality of life, using a scale of 0 (none) to 10 (severe).
Post hoc analyses of efficacy during the OLE were performed for a subgroup with a history of PsA. These included achievement at Week 52 of mNAPSI 75, mean change from baseline in Nail Psoriasis Pain NRS and mean change from baseline in Nail Ps QoL.
Statistical analyses
Efficacy of long‐term treatment with adalimumab across the trial was determined by evaluating continuous adalimumab treatment from Week 0 to Week 52 for all patients who were randomized to adalimumab at Week 0 (Continuous‐ADA Population, N = 109; Fig. 1). Missing data for the long‐term treatment analysis were handled by multiple imputation for all endpoints. As‐observed results are also reported.
Figure 1.
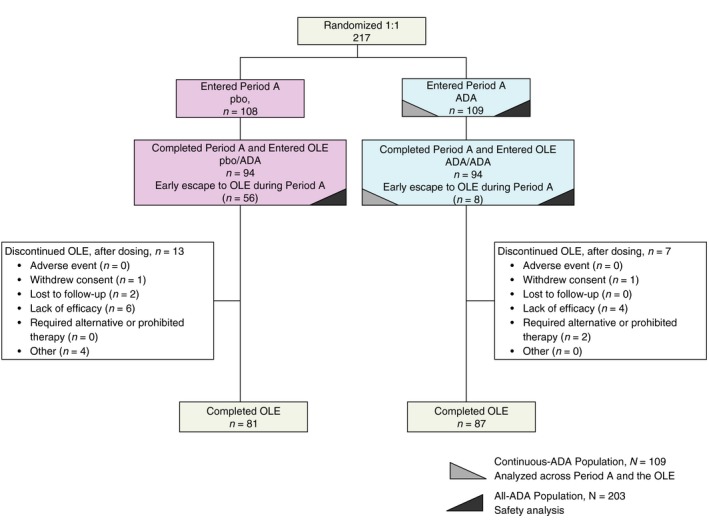
Patient Disposition in the OLE. For those patients who discontinued from study participation for more than one reason, only the primary reason is listed. Abbreviations: ADA, adalimumab; OLE, open‐label extension; pbo, placebo.
Efficacy of adalimumab was also analysed in the OLE. The two dose groups included patients who received placebo in Period A (pbo/ADA, N = 94) and patients who received adalimumab in Period A (ADA/ADA, N = 94; Fig. 1). Efficacy for the subgroup with a history of PsA was evaluated post hoc for the two dose groups in the OLE (pbo/ADA and ADA/ADA). Missing data for all OLE efficacy analyses were handled by non‐responder imputation (NRI) for categorical variables and by last observation carried forward (LOCF) for continuous variables.
Safety was evaluated by treatment‐emergent adverse events for the two OLE treatments and for all patients who received at least one dose of adalimumab during the entire trial (All‐ADA Population, N = 203; Fig. 1).
Results
Baseline
Of the 217 randomized patients, 188 (86.6%) entered the OLE after reaching Week 26 or escaping early to the OLE (94 in each OLE treatment group) and 168 completed the study (81 pbo/ADA, 87 ADA/ADA; Fig. 1). Demographics and baseline characteristics for the 188 OLE patients showed that the majority were male (84.6%) and White (95.2%) with a mean age of 47.2 years. Mean BMI of 29.4 kg/m2 indicated that patients were on average, preobese.34 To evaluate the long‐term efficacy of adalimumab treatment, the 109 patients initially randomized to adalimumab were evaluated continuously from baseline to Week 52 (Continuous‐ADA Population).
Patients had substantial nail disease and pain at baseline as measured by total‐fingernail mNAPSI and NAPSI scores and by Nail Psoriasis Pain NRS score. The effect of nail psoriasis at baseline on patient's quality of life was measured by Nail Ps QoL, NAPPA QoL and NPPFS scores (Table 1).
Table 1.
Key demographics and baseline characteristics for patients in the OLE
| Demographics | By dose received in period A and the OLE | ||
|---|---|---|---|
|
pbo/ADA N = 94 |
ADA/ADA N = 94 |
||
| n (%) | n (%) | ||
| Sex | Male | 76 (80.9) | 83 (88.3) |
| Female | 18 (19.1) | 11 (11.7) | |
| Race | White | 90 (95.7) | 89 (94.7) |
| Asian | 3 (3.2) | 4 (4.3) | |
| Other† | 1 (1.1) | 1 (1.1) | |
| BSA of Ps | 5% to < 10% | 34 (36.2) | 37 (39.4) |
| ≥10% | 60 (63.8) | 57 (60.6) | |
| PGA‐F | Moderate | 53 (56.4) | 44 (46.8) |
| >Moderate | 41 (43.6) | 50 (53.2) | |
| PGA‐S | Moderate | 55 (58.5) | 56 (59.6) |
| >Moderate | 39 (41.5) | 37 (39.4) | |
| Scalp psoriasis | 80 (85.1) | 80 (85.1) | |
| Psoriatic arthritis | 26 (27.7) | 28 (29.8) | |
| Characteristics | Mean [SD]‡ | Mean [SD]‡ | |
|---|---|---|---|
| Age, years | 47.1 [11.44] | 47.3 [11.82] | |
| Body mass index, kg/m2 |
(N = 93) 29.2 [6.88] |
(N = 93) 29.6 [5.09] |
|
| Weight, kg | 88.6 [20.07] | 91.7 [17.27] | |
| Duration of psoriasis, years | 18.7 [13.73] | 20.7 [12.34] | |
| Duration of nail psoriasis, years | 12.0 [11.12] | 12.4 [9.65] | |
| PASI score | 13.3 [9.87] | 12.8 [9.00] | |
| Total‐fingernail mNAPSI score (range 0–130)‡ | 58.6 [20.78] | 57.0 [18.34] | |
| Total‐fingernail mNAPSI score (range 0–130)‡, median [Q1, Q3] | 57.0 [46, 73] | 57.0 [44, 68] | |
| Onycholysis or oil‐drop dyschromia | 16.5 [12, 23] | 17.5 [12, 22] | |
| Pitting | 13.0 [10, 18] | 14.0 [9, 18] | |
| Nail plate crumbling | 8.0 [4, 16] | 9.0 [5, 14] | |
| Leukonychia | 6.0 [2, 9] | 5.0 [2, 8] | |
| Splinter haemorrhage | 4.0 [2, 7] | 5.0 [3, 7] | |
| Nail bed hyperkeratosis | 6.5 [2, 10] | 6.0 [2, 9] | |
| Red spots in lunula | 0 [0, 2] | 0 [0, 2] | |
| Total‐fingernail NAPSI score (range 0–80)§ | 46.6 [15.40] | 48.0 [15.67] | |
| Nail psoriasis pain (NRS) score (range 0–10)§ | 5.7 [2.25] | 5.0 [2.48] | |
| B‐SNIPI, scalp component (range 0–20)§ |
(N = 16) 7.9 [5.82] |
(N = 23) 9.7 [5.32] |
|
| NPPFS score (range 0–10)§ | 5.2 [2.15] | 5.23 [2.63] | |
| Nail Ps QoL (range 0–10)¶ | 5.2 [2.28] | 4.9 [2.79] | |
| NAPPA QoL (range 0–4)§ | 3.1 [0.83] | 3.1 [0.87] |
†Other includes multirace (n = 1 for pbo/ADA) and black (n = 1 for ADA/ADA). ‡All are mean [SD] unless otherwise indicated. §Higher score indicates higher severity or impairment. ¶0 indicates no impact on quality of life; 10 indicates severe impact.
ADA, adalimumab; B‐SNIPI, Brigham Scalp Nail Inverse Palmo‐Plantar Psoriasis Index; DLQI, Dermatology Life Quality Index; NAPPA, Nail Assessment of Psoriasis and Psoriatic Arthritis; NAPSI, Nail Psoriasis Severity Index (m, modified); NPPFS, Nail Psoriasis Physical Functioning Severity; NRS, Numeric Rating Scale; OLE, open‐label extension; PASI, Psoriasis Area Severity Index; pbo, placebo; PGA, Physician's Global Assessment of psoriasis (‐F, fingernail; ‐S, skin); Ps, psoriasis; Q, quartile; QoL, quality of life, SD, standard deviation.
Efficacy
For the Continuous‐ADA Population (N = 109), the rate of achievement of total‐fingernail mNAPSI 75 was 25.9% at Week 16 and increased to 47.4% at Week 26 and to 54.5% at Week 52. Similar results were seen with the rate of achievement of PGA‐F 0/1 with ≥2 grades of improvement from baseline (Fig. 2). Improvements in response to adalimumab long‐term treatment were also seen from Weeks 16–52 for the other efficacy endpoints in this analysis, including total‐fingernail mNAPSI = 0 (Table 2). The observed mean improvement in patient‐reported outcomes increased across the trial to Week 52 (Fig. 3). The per cent improvement relative to baseline at Weeks 4 and 52 were 37% and 76% for Nail Psoriasis Pain NRS, 28% and 74% for NPPFS, 28% and 74% for Nail Ps QoL, and 16% and 48% for NAPPA QoL.
Figure 2.
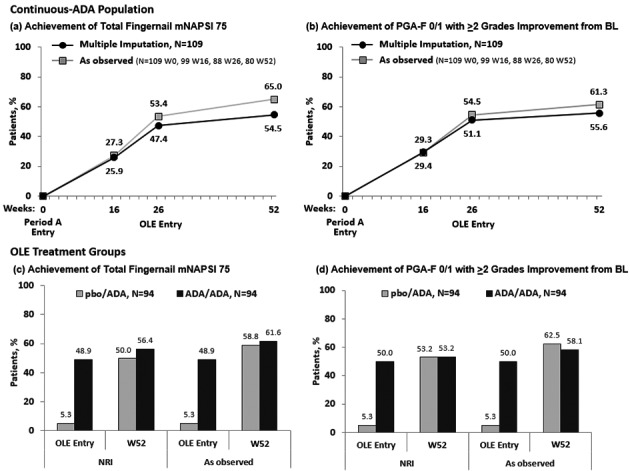
Main Efficacy outcomes over 52 weeks. (a) Achievement of Total‐Fingernail mNAPSI 75 (Continuous‐ADA Population). (b) Achievement of PGA‐F 0/1 with ≥2 Grades Improvement from BL (Continuous‐ADA Population). (c) Achievement of Total‐Fingernail mNAPSI 75 (OLE). (d) Achievement of PGA‐F 0/1 with ≥2 Grades Improvement from BL (OLE). Missing data were handled by multiple imputation. Abbreviations: ADA, adalimumab; BL, baseline; mNAPSI, modified Nail Psoriasis Severity Index; NRI, non‐responder imputation; OLE, open‐label extension; pbo, placebo; PGA‐F, Physician's Global Assessment of Fingernail Psoriasis; W, week.
Table 2.
Other efficacy results
| Continuous‐ADA population, N = 109 | OLE treatment groups | ||||||
|---|---|---|---|---|---|---|---|
| pbo/ADA, N = 94 | ADA/ADA, N = 94 | ||||||
| W16 | W26 | W52 | W26 (entry) | W52 | W26 (entry) | W52 | |
| Total‐fingernail NAPSI; mean % improvement [SE] | 44.8 [2.84] | 57.0 [3.86] | 65.5 [3.52] |
(N = 94) 8.8 [3.28] |
(N = 88) 67.4 [3.28] |
(N = 94) 57.3 [3.25] |
(N = 90) 68.3 [3.22] |
| Total‐fingernail mNAPSI = 0; achievement; % for Continuous‐ADA; n (%) for OLE | 2.8 | 6.6 | 17.9 | 0 | 11 (11.7) | 7 (7.4) | 19 (20.2) |
| B‐SNIPI 50 (scalp) achievement; % for Continuous‐ADA; n (%) for OLE |
(N = 18) 66.4 |
(N = 18) 57.8 |
(N = 18) 65.8 |
(N = 9) 0 |
(N = 9) 4 (44.4) |
(N = 16) 10 (62.5) |
(N = 16) 11 (68.8) |
Mean improvement is based on mean change in relation to baseline. Missing data were handled by multiple imputation for long‐term analysis and for the OLE analysis by non‐responder imputation for categorical variables and last observation carried forward for continuous variables.
ADA, adalimumab; B‐SNIPI, Brigham Scalp Nail Inverse Palmo‐Plantar Psoriasis Index; NAPSI, Nail Psoriasis Severity Index (m, modified); OLE, open‐label extension; pbo, placebo; W, week.
Figure 3.
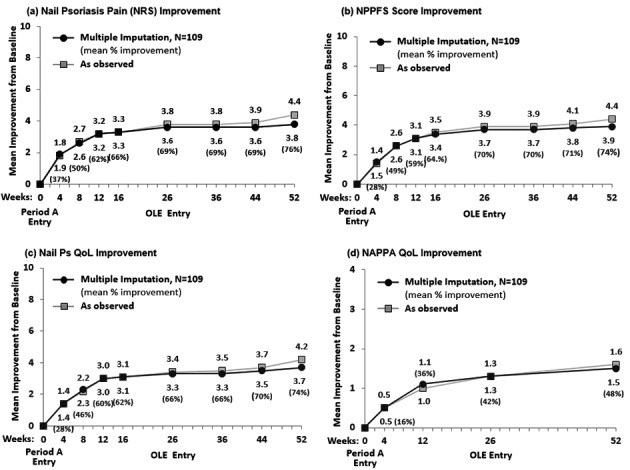
Patient‐reported outcomes over 52 weeks. (a) Nail Psoriasis Pain (NRS) Improvement. (b) NPPFS Score Improvement. (c) Nail Ps QoL Improvement. (d) NAPPA QoL Improvement. Results are reported as mean improvement from baseline. Missing data were handled by multiple imputation. NAPPA, Nail Assessment of Psoriasis and Psoriatic Arthritis; NPPFS, Nail Psoriasis Physical Functioning Severity; NRS, Numeric Rating Scale; OLE, open‐label extension; Ps, psoriasis; QoL, quality of life.
Patients who were randomized to placebo in Period A and entered the OLE (pbo/ADA) showed an increase in total‐fingernail mNAPSI 75 response starting at the time they switched to ADA at OLE entry (Week 26) (5.3%) and continuing to Week 52 (50.0%) (Fig. 2). Similar rates were seen for the pbo/ADA group in achievement of PGA‐F 0/1 with ≥2 grades of improvement from baseline, at OLE entry (5.3%) to Week 52 (53.2%) (Fig. 2). Increased response was also demonstrated across the other efficacy outcomes from OLE entry to Week 52 for the pbo/ADA group (Table 2).
The rate of achievement of total‐fingernail mNAPSI 75 and of PGA‐F 0/1 increased substantially from OLE entry to Week 52 for the pbo/ADA group, regardless of PsA history, and for the ADA/ADA group with a history of PsA (Fig. 4). The rate of achievement of total‐fingernail mNAPSI 75 and PGA‐F 0/1 for the ADA/ADA group with no history of PsA was stable from OLE entry up to Week 52. Mean improvement in patient‐reported outcomes of nail pain NRS, NPPFS, Nail Ps QoL score and NAPPA QoL score also increased from OLE entry to Week 52 for both treatment groups, regardless of PsA history (Fig. 4).
Figure 4.
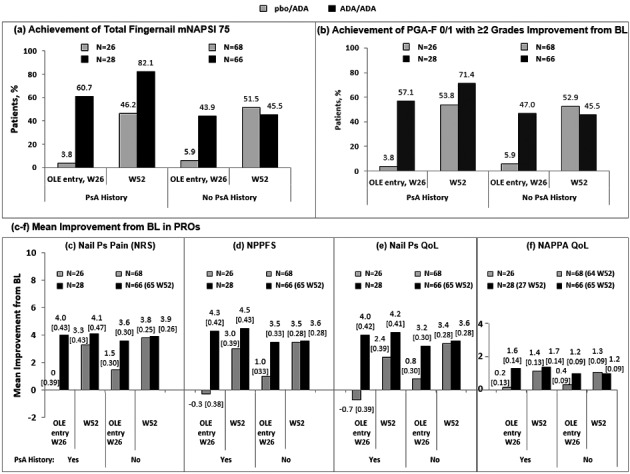
Efficacy and patient‐reported outcomes by history of psoriatic arthritis, OLE. (a) Achievement of Total‐Fingernail mNAPSI 75. (b) Achievement of PGA‐F 0/1 with ≥2 Grades Improvement from BL. (c) Mean Improvement from BL in Nail Ps Pain (NRS). (d) Mean Improvement from BL in NPPFS score. (e) Mean Improvement from BL in Nail Ps QoL. (f) Mean Improvement from BL in NAPPA QoL. Missing data were handled by NRI for mNAPSI 75 and PGA‐F 0/1 and by LOCF for Nail Psoriasis Pain NRS, NPPFS, Nail Ps QoL and NAPPA QoL; LOCF data include standard error. Range of scores for Nail Ps Pain NRS, NPPFS and Nail Ps QoL scores are 0–10, and for NAPPA QoL, 0–4. ADA, adalimumab; DLQI, Dermatology Life Quality Index; LOCF, last observation carried forward; mNAPSI, modified Nail Psoriasis Severity Index; NAPPA, Nail Assessment of Psoriasis and Psoriatic Arthritis; NPPFS, Nail Psoriasis Physical Functioning Severity; NRI, non‐responder imputation; NRS, Numeric Rating Scale; OLE, open‐label extension; pbo, placebo; PGA‐F, Physician's Global Assessment of Psoriasis of the Fingernail; Ps, psoriasis; PsA, psoriatic arthritis; QoL, quality of life; W, week.
Safety
For the 203 patients in the All‐ADA population, the majority of adverse events were mild (46, 22.7%) or moderate (64, 31.5%) in severity. The most common events were nasopharyngitis (12.3%) and upper respiratory tract infection (8.4%). The rates of serious adverse events and of serious infections were 6.9% and 3.4%, respectively (Table 3).
Table 3.
Treatment‐emergent adverse events
| Adverse events, n (%) | During OLE by dose group, n (%) | During the trial | |
|---|---|---|---|
| pbo/ADA, N = 94 | ADA/ADA, N = 94 | All‐ADA,† N = 203 | |
| Any adverse event | 44 (46.8) | 47 (50.0) | 121 (59.6) |
| Events leading to study drug discontinuation | 0 | 0 | 6 (3.0) |
| Serious | 3 (3.2) | 3 (3.2) | 14 (6.9) |
| Infections | 25 (26.6) | 30 (31.9) | 77 (37.9) |
| Serious infection | 2 (2.1) | 1 (1.1) | 7 (3.4) |
| Diverticulitis | 1 (1.1) | 0 | 2 (1.0) |
| Bronchitis | 0 | 0 | 1 (0.5) |
| Endocarditis | 0 | 0 | 1 (0.5) |
| Erysipelas | 0 | 0 | 1 (0.5) |
| Influenza | 0 | 1 (1.1) | 1 (0.5) |
| Lung infection | 1 (1.1) | 0 | 1 (0.5) |
| Pneumonia | 0 | 1 (1.1) | 1 (0.5) |
| Special interest ‡ | |||
| Worsening/new onset of psoriasis | 4 (4.3) | 1 (1.1) | 7 (3.4) |
| Injection site reaction | 2 (2.1) | 2 (2.1) | 8 (3.9) |
| Allergic reaction including angio‐oedema/anaphylaxis | 1 (1.1) | 0 | 2 (1.0) |
| Any haematologic disorders including pancytopenia | 1 (1.1) | 0 | 2 (1.0) |
†Patients who received at least one dose of ADA during Period A plus any patient who received ADA in the OLE. ‡For ≥2 patients in any treatment group.
ADA, adalimumab; OLE, open‐label extension; pbo, placebo.
Rates of adverse events for the 188 patients in the OLE (Table 3) were similar to those for the All‐ADA population. For these patients, most of the events were mild (40, 21.3%) or moderate (45, 23.9%) in severity. The most common were nasopharyngitis (9.6% pbo/ADA vs. 12.8% ADA/ADA) and upper respiratory tract infection (6.4% pbo/ADA; 4.3% ADA/ADA). The rates of serious adverse events and of serious infections were similar between the two OLE treatment groups (3.2% and 2.1% pbo/ADA vs. 3.2% and 1.1% ADA/ADA).
Serious non‐infectious adverse events for the All‐ADA Population were cardiac failure, myocardial infarction, diverticular perforation, anaphylactic reaction, arthropod sting, tenosynovitis, seizure, major depression, suicidal ideation, bladder sphincter atony, stress urinary incontinence, prostatitis and hypertensive crisis (n = 1 for each event). Serious non‐infectious adverse events in the OLE were myocardial infarction (pbo/ADA, n = 1) and tenosynovitis, seizure and stress incontinence (ADA/ADA, n = 1 for each).
There were no events of tuberculosis, opportunistic infections, demyelinating disorders or deaths. There was 1 event of benign breast neoplasm in the ADA/ADA group.
Discussion
In this trial, adalimumab at the dose approved for treatment of moderate‐to‐severe plaque psoriasis also treated patients with moderate‐to‐severe nail psoriasis across 52 weeks of treatment. The treatment response appeared sustainable, and incremental improvements were observed in the rate of achievement of total clearance of nail disease across 52 weeks. The OLE results corroborate and extend the results reported from the placebo‐controlled portion of the study and suggest that adalimumab efficacy after 52 weeks of continuous therapy may be higher than after 26 weeks.
As postulated in Elewski et al.,28 patients who had a partial treatment response at 26 weeks may have experienced additional response to longer‐term treatment, given the slower growth dynamics of the nail apparatus than of skin plaques, and therefore, the time to achieve ultimate improvement in nail disease could be beyond 26 weeks We observed that the response rate to adalimumab treatment increased from OLE entry to Week 52.
Patients who had been randomized to placebo in Period A were able to achieve similar results by the end of the OLE after being switched to adalimumab in the OLE. Week‐52 efficacy for patients randomized to placebo in Period A and receiving adalimumab starting at Week 26 (i.e. efficacy for these patients after 26 weeks of continuous adalimumab) was numerically similar to the efficacy observed at Week 26 for patients who had been randomized to adalimumab in Period A, indicating that the results across the study are internally consistent.
Efficacy outcomes in this trial as measured by mean per cent improvement from baseline in NAPSI (Table 2) were reported in other randomized, controlled trials of long‐term biologic treatment of nail psoriasis. These include a trial (REACH) of adalimumab 40 mg every‐other‐week treatment for psoriasis of the hands and feet (Week 28, 54.6%),24 and a trial (TRANSFIGURE) of the IL‐17A inhibitor, secukinumab 150 and 300 mg once weekly for 5 weeks then every 4 weeks (Week 32, 52.6% for 150 mg and 63.2% for 300 mg).35 Published results from other long‐term trials may help to further contextualize the results from our trial (Table 4), although psoriasis was the primary analysis in those trials, and nail psoriasis was a secondary or post hoc analysis in subsets of the psoriasis patients.
Table 4.
Summary of clinical trials with primary analysis in psoriasis and additional analyses in subsets with nail psoriasis
| Study drug | Study analysis | Dose | Mean % improvement in NAPSI |
|---|---|---|---|
| Infliximab, TNF‐α inhibitor16 |
Phase 3, RCT Retrospective |
5 mg Q8W | W50, 67.8% |
| Etanercept, TNF‐α inhibitor15 |
Randomized, open‐label Post hoc |
25 mg biw | W54, 51.0% |
| Ustekinumab, IL‐2/23 inhibitor18 |
Phase 2/3, RCT Secondary endpoint |
45 mg and 90 mg Q12W |
W64, 56.6% (45 mg) W64, 67.8% (90 mg) |
| Ixekizumab, IL‐17A inhibitor19 |
Phase 2, RCT Post hoc |
120 mg Q4W | W48, 75.4% |
| Ixekizumab, IL‐17A inhibitor20 |
Phase 3, RCT Subgroup |
80 mg Q4W | W60, 81.8% |
| Tofacitinib, JAK inhibitor21 |
Phase 3, RCT Post hoc |
5 mg and 10 mg bid |
W52, 65.6% (5 mg) W52, 75.5% (10 mg) |
bid, twice daily; biw, twice weekly; eow, every other week; NAPSI, Nail Psoriasis Severity Index; Ps, psoriasis; RCT, randomized controlled trial; QnW, every n week(s); W, week.
Improvements in treatment response were generally also observed regardless of whether patients had a prior history of PsA or not. Improved treatment response to adalimumab regardless of baseline PsA status was also seen in a post hoc analysis of psoriasis patients from another phase‐3 trial of adalimumab; mean NAPSI scores improved after 16 weeks of adalimumab treatment.27
Like nail psoriasis, scalp psoriasis is associated with the development of PsA,36is frequently a manifestation of skin psoriasis, is difficult to treat and can have a negative impact on patient quality of life.37, 38In the current trial, the incidence of concomitant scalp psoriasis was similar to the published rate in patients with chronic plaque psoriasis.39, 40Improvement in scalp psoriasis was achieved by over 60% of patients during the OLE, including patients who were switched from placebo to adalimumab upon entry to the OLE and patients receiving long‐term adalimumab treatment.
The safety results from this study are consistent with the known adalimumab safety profile, although the serious infection rate for adalimumab was higher than in other adalimumab trials evaluating patients with moderate‐to‐severe psoriasis41, 42, 43and with moderate‐to‐severe psoriasis of the hands and feet.24 Safety results over 52 weeks were comparable to those after 26 weeks of adalimumab treatment.28 No unexpected safety risk was identified.24, 44 Adverse event rates are not comparable to the other trials of long‐term, biologics treatment for nail psoriasis mentioned earlier, as they did not report safety results for the nail psoriasis populations in their trials.
This study was limited by the requirement of at least 5% affected BSA involvement and the small sample size of patients with scalp psoriasis.
In conclusion, 52 weeks of every‐other‐week treatment with 40‐mg adalimumab improved patient response to the study endpoints, signs and symptoms of moderate‐to‐severe nail psoriasis and patient‐reported quality of life. The achievement rates of mNAPSI and total clearance of nail disease (mNAPSI = 0) improved across 52 weeks. No new safety risks were identified with adalimumab treatment in this population.
Acknowledgements
The authors would like to acknowledge Yihua Gu, MS, for statistical support and Jody Bennett for medical writing support in the production of this publication. Both are AbbVie employees.
Conflicts of interest
B Elewski received grants from AbbVie, Anaptys‐Bio, Boehringer Ingelheim, Celgene, Incyte, Leo, Lilly, Merck, Novartis, Pfizer, Regeneron, Sun and Valeant for investigator services, and honoraria from Boehringer Ingelheim, Celgene, Leo, Lilly, Novartis, Pfizer, Sun and Valeant Verrica for consultant services. C Baker received honoraria from AbbVie, Pfizer, Novartis, Lilly and Janssen for participation on ad boards and for investigator and speaker services. J Crowley received honoraria for consultant and speaker services and grants for investigator services from AbbVie, Janssen, Lilly, Novartis, Regeneron, Sanofi‐Aventis and UCB; received honoraria and grants for consultant and investigator services, respectively, from Amgen, Celgene and Sun Pharma; and received grants for investigator services from MC2 Therapeutics, Merck, Pfizer, Sandoz and Verrica Pharmaceuticals. Y Poulin received grants for investigator services from AbbVie, Amgen, Baxalta, Celgene, Janssen, Eli Lilly, Glaxo Smith Kline, Incyte, Leo Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi and UCB Pharma, and received honoraria for speaker and/or advisory board services from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis and Leo Pharma. MM Okun received honoraria from AbbVie for advisory board participation and for speaker services, and from AbbVie, Gilead, UCB, GSK, InflaRx, Genentech, Innovaderm and Seattle Genetics for consultant services. PA Rich received grants for investigator services from AbbVie, Allergan, Amgen, Anacor, Cassiopea, Dusa, Eli Lilly, Galderma, Janssen, Leo, Meiji, Merck, Novartis, Pfizer, Psolar, Ranbaxy, Sandoz and Viamet, honoraria for advisory board participation from AbbVie, Eli Lilly, Novartis, Sandoz and Valeant, and honoraria for consultant services from AbbVie, Novartis and Polichem. B Calimlim, Z Geng and O Servin received a salary as AbbVie employees and may have also received stocks and/or stock options.
Funding source and Data sharing
AbbVie Inc. funded this study and participated in the study design; study research; collection, analysis and interpretation of data; and writing, reviewing and approving of this publication. All authors had access to the data and participated in the development, review, and approval and in the decision to submit this publication. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial‐level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1. de Jong EM, Seegers BA, Gulinck MK et al Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patients. Dermatology 1996; 193: 300–303. [DOI] [PubMed] [Google Scholar]
- 2. Augustin M, Reich K, Blome C et al Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol 2010; 163: 580–585. [DOI] [PubMed] [Google Scholar]
- 3. Radtke MA, Langenbruch AK, Schafer I et al Nail psoriasis as a severity indicator: results from the PsoReal study. Patient Relat Outcome Meas 2011; 2: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawry M. Biological therapy and nail psoriasis. Dermatol Ther 2007; 20: 60–67. [DOI] [PubMed] [Google Scholar]
- 5. Richert B, Caucanas M. Epidemiology of nail psoriasis In Rigopoulos D, Tosti A, eds. Nail Psoriasis: A to Z, Springer, Cham, Heidelberg, New York, Dordrecht, London, 2014: 1–8. [Google Scholar]
- 6. Hallaji Z, Babaeijandaghi F, Akbarzadeh M et al A significant association exists between the severity of nail and skin involvement in psoriasis. J Am Acad Dermatol 2012; 66: e12–e13. [DOI] [PubMed] [Google Scholar]
- 7. Jiaravuthisan MM, Sasseville D, Vender RB et al Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol 2007; 57: 1–27. [DOI] [PubMed] [Google Scholar]
- 8. Radtke MA, Beikert FC, Augustin M. Nail psoriasis – a treatment challenge. J Dtsch Dermatol Ges 2013; 11: 203–219. [DOI] [PubMed] [Google Scholar]
- 9. Williamson L, Dalbeth N, Dockerty JL et al Extended report: nail disease in psoriatic arthritis–clinically important, potentially treatable and often overlooked. Rheumatology (Oxford) 2004; 43: 790–794. [DOI] [PubMed] [Google Scholar]
- 10. McGonagle D, Tan AL, Benjamin M. The nail as a musculoskeletal appendage–implications for an improved understanding of the link between psoriasis and arthritis. Dermatology 2009; 218: 97–102. [DOI] [PubMed] [Google Scholar]
- 11. Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol 2009; 23(Suppl 1): 15–21. [DOI] [PubMed] [Google Scholar]
- 12. Callis‐Duffin K, Karki C, Mason M et al The burden of nail psoriasis: a real‐world analysis from the Corrona Psoriasis Registry. J Am Acad Dermatol 2018; 79(Supplement 1): 283. [Google Scholar]
- 13. Crowley JJ, Weinberg JM, Wu JJ et al Treatment of nail psoriasis: best practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol 2015; 151: 87–94. [DOI] [PubMed] [Google Scholar]
- 14. Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol 2010; 24: 420–423. [DOI] [PubMed] [Google Scholar]
- 15. Luger TA, Barker J, Lambert J et al Sustained improvement in joint pain and nail symptoms with etanercept therapy in patients with moderate‐to‐severe psoriasis. J Eur Acad Dermatol Venereol 2009; 23: 896–904. [DOI] [PubMed] [Google Scholar]
- 16. Reich K, Ortonne JP, Kerkmann U et al Skin and nail responses after 1 year of infliximab therapy in patients with moderate‐to‐severe psoriasis: a retrospective analysis of the EXPRESS Trial. Dermatology 2010; 221: 172–178. [DOI] [PubMed] [Google Scholar]
- 17. Rich P, Griffiths CE, Reich K et al Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol 2008; 58: 224–231. [DOI] [PubMed] [Google Scholar]
- 18. Igarashi A, Kato T, Kato M et al Efficacy and safety of ustekinumab in Japanese patients with moderate‐to‐severe plaque‐type psoriasis: long‐term results from a phase 2/3 clinical trial. J Dermatol 2012; 39: 242–252. [DOI] [PubMed] [Google Scholar]
- 19. Langley RG, Rich P, Menter A et al Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 20. van de Kerkhof P, Guenther L, Gottlieb AB et al Ixekizumab treatment improves fingernail psoriasis in patients with moderate‐to‐severe psoriasis: results from the randomized, controlled and open‐label phases of UNCOVER‐3. J Eur Acad Dermatol Venereol 2017; 31: 477–482. [DOI] [PubMed] [Google Scholar]
- 21. Merola JF, Elewski B, Tatulych S et al Efficacy of tofacitinib for the treatment of nail psoriasis: Two 52‐week, randomized, controlled phase 3 studies in patients with moderate‐to‐severe plaque psoriasis. J Am Acad Dermatol 2017; 77: 79–87 e71. [DOI] [PubMed] [Google Scholar]
- 22. Reich K, Arenberger P, Mrowietz U et al Secukinumab shows high and sustained efficacy in nail psoriasis: week 80 results from the TRANSFIGURE study. J Am Acad Dermatol 2017; 1: AB232. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong AW, Vender R, Kircik L. Secukinumab in the treatment of palmoplantar, nail, scalp, and pustular psoriasis. J Clin Aesthet Dermatol 2016; 9: S12–S16. [PMC free article] [PubMed] [Google Scholar]
- 24. Leonardi C, Langley RG, Papp K et al Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo‐controlled, double‐blind trial. Arch Dermatol 2011; 147: 429–436. [DOI] [PubMed] [Google Scholar]
- 25. Thaci D, Unnebrink K, Sundaram M et al Adalimumab for the treatment of moderate to severe psoriasis: subanalysis of effects on scalp and nails in the BELIEVE study. J Eur Acad Dermatol Venereol 2015; 29: 353–360. [DOI] [PubMed] [Google Scholar]
- 26. Saraceno R, Pietroleonardo L, Mazzotta A et al TNF‐alpha antagonists and nail psoriasis: an open, 24‐week, prospective cohort study in adult patients with psoriasis. Expert Opin Biol Ther 2013; 13: 469–473. [DOI] [PubMed] [Google Scholar]
- 27. Paul C, van de Kerkhof P, Puig L et al Influence of psoriatic arthritis on the efficacy of adalimumab and on the treatment response of other markers of psoriasis burden: subanalysis of the BELIEVE study. Eur J Dermatol 2012; 22: 762–769. [DOI] [PubMed] [Google Scholar]
- 28. Elewski BE, Okun MM, Papp K et al Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo‐controlled trial. J Am Acad Dermatol 2018; 78: 90–99 e91. [DOI] [PubMed] [Google Scholar]
- 29. Hudgens S, Sundaram M, Williams DA. Evaluation of a novel clinician reported outcome in nail psoriasis. Value Health 2016; 19: A127. Presented at ISPOR 121st Annual International Meeting, May 121‐125, 2016, Washington DC. [Google Scholar]
- 30. Cassell SE, Bieber JD, Rich P et al The modified Nail Psoriasis Severity Index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol 2007; 34: 123–129. [PubMed] [Google Scholar]
- 31. Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003; 49: 206–212. [DOI] [PubMed] [Google Scholar]
- 32. Patel M, Liu SW, Qureshi A, Merola JF. The Brigham Scalp Nail Inverse Palmoplantar Psoriasis Composite Index (B‐SNIPI): a novel index to measure all non‐plaque psoriasis subsets. J Rheumatol 2014; 41: 1230–1232. [DOI] [PubMed] [Google Scholar]
- 33. Augustin M, Blome C, Costanzo A et al Nail Assessment in Psoriasis and Psoriatic Arthritis (NAPPA): development and validation of a tool for assessment of nail psoriasis outcomes. Br J Dermatol 2014; 170: 591–598. [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization . Body Mass Index (BMI) classifications. Accessed at http://www.who.int (last accessed: 20 August 2019).
- 35. Reich K, Sullivan J, Arenberger P et al Effect of secukinumab on clinical activity and disease burden of nail psoriasis: 32‐week results from the randomized placebo‐controlled TRANSFIGURE trial. Br J Dermatol 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36. Guenther L, Gulliver W. Psoriasis comorbidities. J Cutan Med Surg 2009; 13(Suppl 2): S77–87. [DOI] [PubMed] [Google Scholar]
- 37. Ortonne JP, Ganslandt C, Tan J, et al. Quality of life in patients with scalp psoriasis treated with calcipotriol/betamethasone dipropionate scalp formulation: a randomized controlled trial. J Eur Acad Dermatol Venereol 2009; 23(8): 919–26. [DOI] [PubMed] [Google Scholar]
- 38. Van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. Am J Clin Dermatol 2001; 2(3): 159–65. [DOI] [PubMed] [Google Scholar]
- 39. Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol 2008; 26(5): 448–59. [DOI] [PubMed] [Google Scholar]
- 40. Wozel G, Klein E, Mrowietz U, et al. Scalp psoriasis. J Dtsch Dermatol Ges 2011; 9(1): 70–4. [DOI] [PubMed] [Google Scholar]
- 41. Thaçi D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol 2010; 163(2): 402–11. [DOI] [PubMed] [Google Scholar]
- 42. Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158(3): 558–66. [DOI] [PubMed] [Google Scholar]
- 43. Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58(1): 106–15. [DOI] [PubMed] [Google Scholar]
- 44. Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013; 72(4): 517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


