Abstract
Background
Cancer cachexia is characterized by weight loss and is associated with increased morbidity and mortality in patients with cancer. Anamorelin (ONO‐7643; ANAM) is a novel and selective ghrelin receptor agonist that improves appetite, lean body mass (LBM), body weight, and anorexia.
Methods
This multicenter, open‐label, single‐arm study investigated the efficacy and safety of 100 mg anamorelin in 50 Japanese patients with advanced and unresectable gastrointestinal (colorectal, gastric, or pancreatic) cancer. ANAM was administered once daily over 12 weeks. The primary endpoint was the proportion of patients that maintained or gained LBM over the course of the study. Secondary endpoints included changes in LBM, body weight, quality of life (QoL), and nutritional status biomarkers.
Results
The proportion of patients who responded to treatment was 63.3% (95% CI, 48.3%‐76.6%), with a least square mean ± SE change in LBM and body weight from baseline of 1.89 ± 0.36 kg and 1.41 ± 0.61 kg, respectively. Appetite‐related questions on the QoL questionnaire showed that ANAM improved appetite. Adverse events occurred in 79.6% of patients, and the most common treatment‐related adverse events were increased γ‐glutamyl transpeptidase (8.2%), diabetes mellitus (6.1%), hyperglycemia (6.1%), and prolonged QRS complex (6.1%).
Conclusions
ANAM improved anorexia and patients' nutritional status, resulting in rapid increases in LBM and body weight in patients with advanced gastrointestinal cancer who had cancer cachexia. ANAM treatment was well tolerated over 12 weeks. ANAM is a potential clinically beneficial pharmacotherapeutic option for patients with advanced gastrointestinal cancer who have cancer cachexia.
Keywords: anamorelin, anorexia, body weight, cachexia, gastrointestinal neoplasms
Short abstract
The receipt of 100 mg anamorelin increases lean body mass and improves symptoms of anorexia and nutritional status in patients with advanced gastrointestinal cancer who have cancer cachexia. Anamorelin is well tolerated in these patients.
Introduction
Up to 80% of patients with cancer develop cachexia, which is characterized by weight loss (mainly lean body mass [LBM]) and anorexia.1 Cachexia reflects a negative protein and energy balance driven by reduced food intake and hypermetabolism.2 This results in diminished physical function and a reduced ability to tolerate chemotherapy, and is associated with a poor prognosis and reduced quality of life (QoL).3, 4
Cachexia is prevalent in patients with gastrointestinal cancers and, despite its negative impact on patients, it is frequently under‐recognized and insufficiently treated, partly because its underlying mechanisms are not fully understood.5, 6, 7 Cachexia cannot be fully reversed by conventional nutritional support, and effective treatments are lacking, meaning that there is an unmet need for novel strategies to address the condition. Although corticosteroids and progestins can increase appetite and weight, they have been shown to be ineffective against cachexia, and their long‐term use is associated with adverse drug reactions.8, 9
Cachexia is a growing problem in Asia, primarily because of the rapidly increasing incidence of cancer, and there is a paucity of data in terms of its prevalence and management.10 In Western countries, megestrol acetate is administered to patients with acquired immunodeficiency syndrome‐related and cancer‐related cachexia but is associated with an increase in body weight mainly through increasing fat rather than LBM.11 Megestrol acetate is also associated with a high incidence of adverse events (AEs), such as thromboembolic events and edema.12 In addition, MABp1 (an interleukin 1α antibody) has shown some activity in phase 3 trials, although this agent is currently under development and has not yet been approved for use.13
More recently, ghrelin, which stimulates growth hormone (GH) release and increases appetite, has been suggested as a viable treatment target.14, 15, 16 Ghrelin receptor agonists have garnered interest for their potential effects on clinical conditions, such as anorexia and cancer‐related cachexia. It has been suggested that ghrelin receptor agonists positively affect LBM through the increased secretion of GH, insulin‐like growth factor 1 (IGF‐1), and insulin‐like growth factor‐binding protein 3 (IGFBP‐3) through activation of the ghrelin receptor.17, 18, 19
Anamorelin (ANAM) is a novel, orally active, selective ghrelin receptor agonist with appetite‐enhancing and anabolic activity. During clinical development in phase 1, 2, and 3 trials, it was confirmed that ANAM enhances appetite, increases LBM, and is well tolerated.20, 21, 22, 23 Two multinational phase 3 studies using 100 mg ANAM daily showed increases in LBM and body weight and improved anorexia/cachexia‐specific QoL of non–small‐cell lung cancer (NSCLC)‐associated cachexia.23, 24 Furthermore, in two Japanese phase 2 studies, the activity of 100 mg ANAM daily was demonstrated by an increase in LBM and body weight while improving appetite loss in patients who had NSCLC with cachexia.25, 26 However, ANAM has not been evaluated in Japanese patients with gastrointestinal cancer who have cachexia.
The purpose of this clinical trial was to determine the efficacy and safety of ANAM for the treatment of cachexia by evaluating the change in LBM from baseline and tolerability in Japanese patients with gastrointestinal cancers who received treatment with ANAM for 12 weeks.
Materials and Methods
Study Design
This was an open‐label, single‐arm study performed from February 2017 to April 2018 at 19 centers across Japan. Patients were administered 100 mg ANAM once daily for 12 weeks during the treatment period, which was followed by a 4‐week follow‐up period for assessment of AEs. Ethical approval was provided by the institutional review board at each study site, and the study adhered to the principles outlined in the Declaration of Helsinki and good clinical practice guidelines. This trial is registered in the Japan Primary Registries Network database (JapicCTI‐163426).
Patients
Patients who were eligible for inclusion in this study were Japanese patients (aged ≥20 years) with advanced, unresectable colorectal, gastric, or pancreatic cancer who were in the stage of cachexia by definition. Cachexia was defined as follows: involuntary weight loss ≥5% for 6 months before enrolling in the study, anorexia, and satisfaction of at least 2 of the following criteria: fatigue, generalized muscle weakness, arm muscle circumference (in centimeters) <10th percentile, and 1 or more of the following conditions: albumin <3.2 g/dL, C‐reactive protein >5.0 mg/L, or hemoglobin <12 g/dL. Anorexia, fatigue, and generalized muscle weakness were defined as grade 1 or higher based on the NCI Common Terminology Criteria for Adverse Events, version 4.0 (Japan Clinical Oncology Group; Japanese version). An additional criterion was an Eastern Cooperative Oncology Group performance status from 0 to 2 (or from 0 to 1 for patients with pancreatic cancer).27
Patients were excluded if they had an inability to orally ingest, digest, or absorb food and oral drugs; uncontrolled mental conditions; ascites, pleural effusions, or pericardial effusions requiring drainage or thoracentesis; uncontrolled edema; diminished cardiac function; or uncontrolled diabetes mellitus. All patients provided written informed consent before participation.
Prohibited Prior and Concomitant Therapies
Patients were requested not to take systemic corticosteroids (except when used to prevent anticancer drug‐induced nausea, vomiting, or hypersensitivity or to prevent hypersensitivity reactions to contrast agents), GH preparations, medroxyprogesterone acetate, megestrol acetate, Chinese traditional medicines indicated for anorexia, antiarrhythmic drugs, anthracycline antineoplastic drugs, or cytochrome p450 3A4 inhibitors and inducers (including food and beverages containing St John's wort) during the study period. Anticancer agents other than those specifically excluded above were permitted. Radiotherapy, except to treat brain metastases as long as concomitant systemic corticosteroids were administered at ≤5 mg daily (prednisolone equivalent dose), and palliative radiotherapy for bone metastases also were prohibited.
Outcomes
The primary endpoint was the proportion of patients classified as LBM responders. Responders were defined as patients who maintained or gained LBM (≥0 kg) from baseline to all evaluation time points, as measured by dual energy x‐ray absorptiometry (DEXA). Details of the models used for measurements and parameters measured have been described previously.26 DEXA was performed at baseline and at weeks 3, 6, 9, and 12 of the treatment period.
Secondary endpoints included changes in LBM, body weight, a QoL questionnaire for patients with cancer who were treated with anticancer drugs (QoL‐ACD) (appetite‐related questions 8, 9, and 11 and total score), and biomarkers (IGF‐1, IGFBP‐3, and prealbumin [transthyretin]).
Safety Assessment
Safety outcomes assessed included evaluation of all AEs, treatment‐related AEs, clinical laboratory tests (hematology, biochemistry, and urinalysis), 12‐lead electrocardiography, and vital signs. Version 4.03 of the National Cancer Institute Common Terminology Criteria for Adverse Events was used for toxicity and AE reporting.28
Statistical Analysis
Previous Japanese25 and non‐Japanese24 studies showed that the threshold and expected responder rates for the current study were estimated to be 30.7% and 54.9%, respectively, based on data from the placebo and 100 mg ANAM arms.
The planned sample size was calculated based on the probability (>90%) that the lower limit of the Clopper‐Pearson 95% CI for the expected responder rate (54.9%) would exceed the threshold rate (30.7%), which was the placebo responder rate in 5 previous ANAM studies. Assuming <10% of patients would be excluded from the primary analysis set, a planned sample size of 50 was considered to be sufficient to assess the primary endpoint.
All eligible patients who received at least 1 dose of the study drug comprised the safety analysis set (SAF). The full analysis set (FAS) included all patients in the SAF who had undergone a minimum of 1 efficacy assessment after treatment was initiated.
The objective of the primary analysis was to calculate the proportion of patients who maintained or gained LBM measured by DEXA and to estimate the 95% CI using the Clopper‐Pearson method. The primary endpoint criteria required the lower 95% CI value for LBM responders to be higher than the threshold responder rate of 30.7%. The least square (LS) mean change in LBM at week 12 was calculated using a model‐based analysis for LBM, body weight, and QoL‐ACD. The LS mean change at each time point was calculated using a model‐based analysis for each endpoint. Descriptive summary values were calculated for quantitative variables, including demographics, patient characteristics, and baseline values. All statistical analyses were performed by qualified statisticians at Ono Pharmaceutical Company Ltd using SAS Version 9.3 software (SAS Institute Inc).
Results
Patient Characteristics
Fifty patients were enrolled in this study, with 49 patients included in the FAS and SAF (Fig. 1). Thirty‐one patients completed the study.
Figure 1.
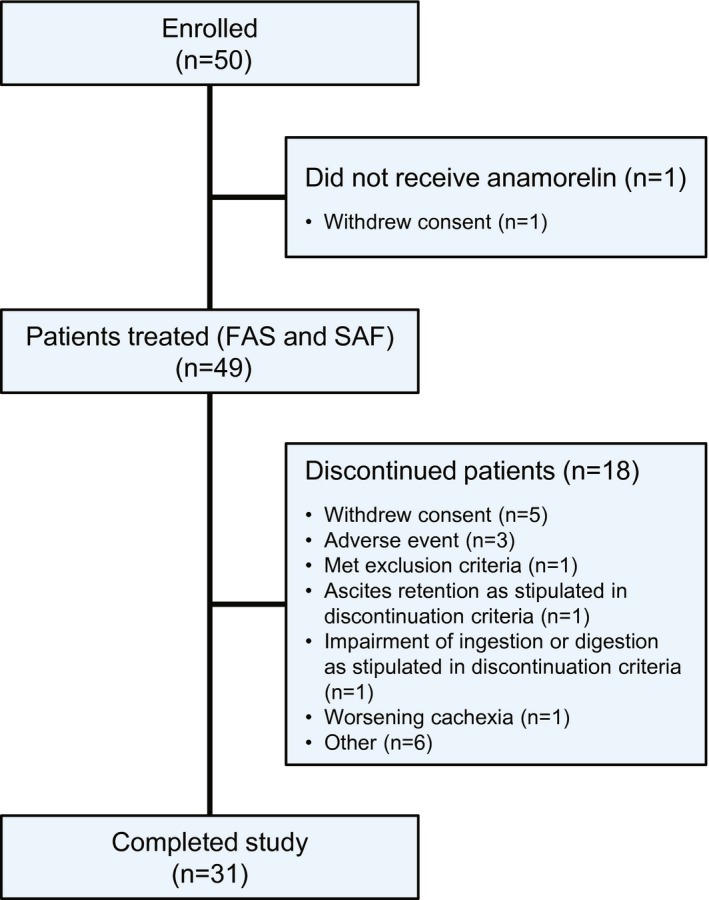
Patient disposition is illustrated. FAS indicates, full analysis set; SAF, safety analysis set.
Patient baseline demographic data are shown in Table 1. In brief, there were 20 (40%) females and 30 males (60%) with a median age of 66.5 years (range, 49‐88 years) and a median body mass index of 18.95 kg/m2 (range, 13.9‐29.6 kg/m2). Patients were diagnosed with colorectal cancer (n = 40), gastric cancer (n = 5), or pancreatic cancer (n = 5) and had an Eastern Cooperative Oncology Group performance status of 0 (n = 11), 1 (n = 33), 2 (n = 5), or unknown (n = 1).
Table 1.
Patient Demographics and Characteristics at Baseline
| Parameter | ANAM 100 mg (N = 50)a |
|---|---|
| Sex, no. (%) | |
| Men | 30 (60.0) |
| Women | 20 (40.0) |
| Age: Median [range], y | 66.5 [49‐88] |
| Weight: Median [range], kg | 49.20 [36.0‐77.1] |
| BMI: Median [range], kg/m2 | 18.95 [13.9‐29.6] |
| Tumor type, no. (%) | |
| Colorectal | 40 (80.0) |
| Gastric | 5 (10.0) |
| Pancreatic | 5 (10.0) |
| Body weight loss, no. (%) | |
| 5%‐10% | 26 (53.1) |
| >10% | 23 (46.9) |
| Unknown | 1 |
| ECOG PS, no. (%) | |
| 0 | 11 (22.4) |
| 1 | 33 (67.3) |
| 2 | 5 (10.2) |
| Unknown | 1 |
| Disease status, no. (%) | |
| Locally advanced unresectable | 4 (8.0) |
| Metastatic | 24 (48.0) |
| Recurrence after surgery | 22 (44.0) |
| Concomitant cancer therapy, no. (%) | |
| Yes | 41 (82.0) |
| No | 9 (18.0) |
| No. of previous treatment regimens, no. (%) | |
| 1 | 14 (28.0) |
| 2 | 16 (32.0) |
| ≥3 | 20 (40.0) |
Abbreviations: ANAM, anamorelin; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status.
All enrolled patients were included.
Lean Body Mass
During the study, ANAM treatment maintained or increased LBM over time in 31 of 49 patients, and the LBM responder rate was 63.3% (95% CI, 48.3%‐76.6%). The lower limit of the 95% CI for the responder rate exceeded the threshold rate (30.7%).
The LS mean changes in LBM from baseline to week 12 are shown in Figure 2. The percent change for each patient in LBM from baseline to the final evaluation point, defined as week 12 of the treatment period or the last time point with available measurements for patients who discontinued, is shown in Figure 3. The LS mean ± SE change at week 12 was 1.89 ± 0.36 kg from baseline. When stratified by tumor type, 24 of 39 patients (61.5%) with colorectal tumors, 2 of 5 (40%) with gastric tumors, and 5 of 5 (100%) with pancreatic tumors were LBM responders.
Figure 2.
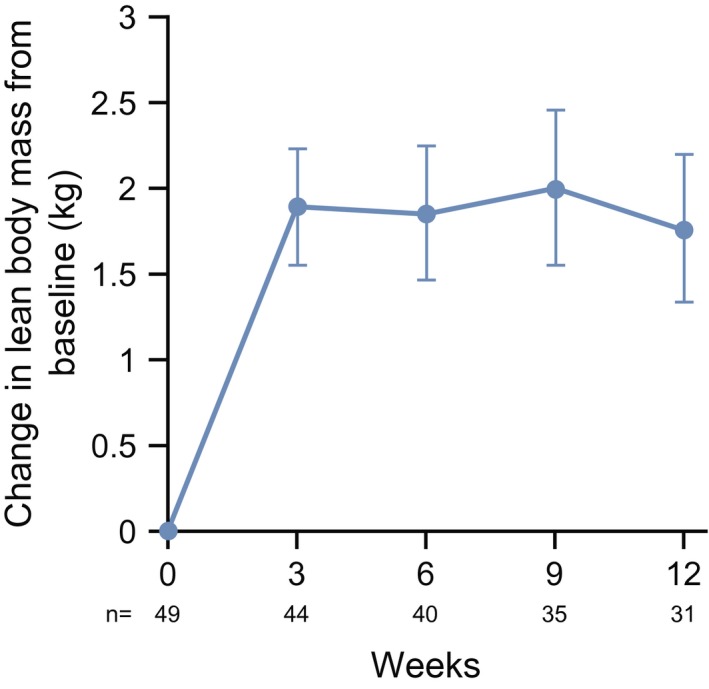
Changes in lean body mass from baseline to week 12 are illustrated. Data points are the least square mean ± SE.
Figure 3.
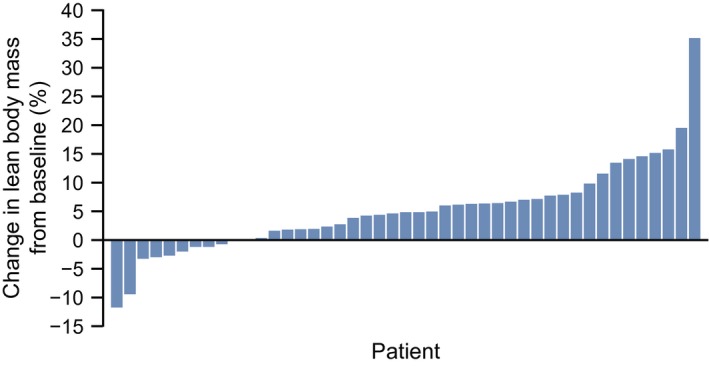
The percentage change in lean body mass is illustrated for each patient from baseline to the final evaluation point during the 12‐week treatment period. Data for 45 patients are shown (lean body mass was evaluated only at baseline in 4 patients).
Secondary Endpoints
The LS mean changes in body weight from baseline to week 12 are shown in Figure 4. The LS mean ± SE change at week 12 was 1.41 ± 0.61 kg from baseline.
Figure 4.
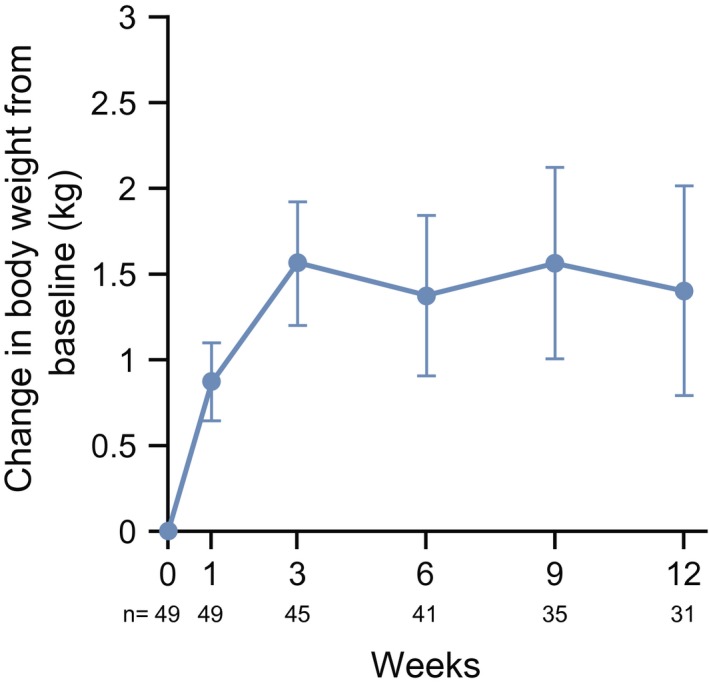
Changes in body weight from baseline to week 12 are illustrated. Data points are the least square mean ± SE.
The LS mean change in patients' answers to appetite‐related questions (8, 9, and 11) in the QoL‐ACD is shown in Figure 5A‐C and clearly demonstrates that 100 mg ANAM improved appetite over time. No apparent change in the QoL‐ACD total scores was observed (data not shown).
Figure 5.
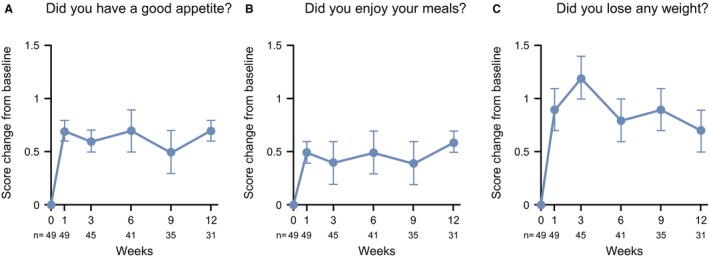
Changes in scores on the quality‐of‐life questionnaire for patients who were treated with anticancer drugs (QoL‐ACD) are illustrated for appetite‐related questions from baseline to week 12. (A) Did you have a good appetite? (B) Did you enjoy your meals? (C) Did you lose any weight? Data points are the least square mean ± SE.
The LS mean changes in IGF‐1, IGFBP‐3, and transthyretin levels from baseline are shown in Figure 6A‐C. IGF‐1, IGFBP‐3, and transthyretin all increased after the administration of ANAM, and the mean levels were higher than baseline at all evaluation points except for transthyretin at week 12.
Figure 6.
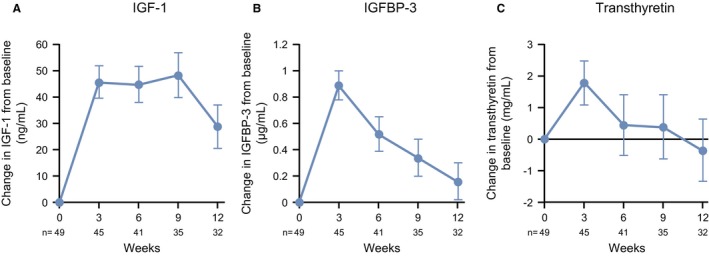
Changes in biomarkers are illustrated from baseline to week 12, including (A) insulin‐like growth factor‐1 (IGF‐1), (B) insulin‐like growth factor binding protein‐3 (IGFBP‐3), and (C) transthyretin. Data points are the least square mean ± SE.
Safety
AEs occurred in 79.6% of patients, of which 10.2% were serious AEs and 10.2% resulted in discontinuation. Six patients died during the study period; however, the cause of death was judged to be the natural course of cancer in all six patients.
Treatment‐related AEs were observed in 42.9% of patients, of which 4.1% were serious and 10.2% resulted in discontinuation. The most common treatment‐related AEs (≥5%) were increased γ‐glutamyl transpeptidase (8.2%), diabetes mellitus (6.1%), hyperglycemia (6.1%), and prolonged QRS complex (6.1%) (Table 2). Grade 3 treatment‐related AEs included diabetes mellitus (6.1%) and increased γ‐glutamyl transpeptidase (2%), and decreased lymphocyte counts (2%).
Table 2.
Most Common Treatment‐Related Adverse Events: Safety Analysis Set, N = 49
| Event | No. (%) | |
|---|---|---|
| All Grades | Grade 3 | |
| All events | 21 (42.9) | 5 (10.2) |
| Metabolism and nutrition disorders | ||
| Type 2 diabetes mellitus/diabetes mellitus | 3 (6.1) | 3 (6.1) |
| Hyperglycemia | 3 (6.1) | |
| Glucose tolerance impaired | 1 (2.0) | |
| Investigations | ||
| γ‐GTP increased | 4 (8.2) | 1 (2.0) |
| Lymphocyte count decreased | 1 (2.0) | 1 (2.0) |
| ALP increased | 1 (2.0) | |
| Abnormal ECG | ||
| QRS complex prolonged | 3 (6.1) | |
| PR prolongation | 1 (2.0) | |
| Atrioventricular block first degree | 1 (2.0) | |
| Supraventricular extrasystoles | 1 (2.0) | |
| General disorders | ||
| Fatigue | 2 (4.1) | |
| Asthenia | 1 (2.0) | |
| Face edema | 1 (2.0) | |
| Thirst | 1 (2.0) | |
| Other | ||
| Endocrine disorder | 1 (2.0) | |
| Vision blurred | 1 (2.0) | |
| Nasopharyngitis | 1 (2.0) | |
| Hyperhidrosis | 1 (2.0) | |
| Hypertension | 1 (2.0) | |
Abbreviations: ALP, alkaline phosphatase; ECG, electrocardiogram; γ‐GTP, gamma glutamyl transpeptidase.
Discussion
This is the first study to evaluate ANAM in Japanese patients with advanced gastrointestinal cancer and cancer cachexia. Patients with gastrointestinal cancers suffer from impaired food intake and absorption of nourishment, and a high percentage of patients experience more weight loss than patients with other types of cancer.29
The lower limit of the 95% CI for the proportion of responders who maintained or increased LBM in response to ANAM treatment surpassed the threshold for demonstrating improvement (30.7%); therefore, the primary endpoint was achieved. The responder rate and actual changes in LBM were comparable to those observed in previous studies conducted in cachexic patients with advanced NSCLC.25, 26 The changes in the degree of anorexia and biomarkers, including IGF‐1, IGFBP‐3, and transthyretin, also showed patterns similar to those in previous reports.25, 26
ANAM rapidly increased LBM and body weight and improved anorexia. Moreover, patients' overall nutritional state improved, as indicated by the observed increases in transthyretin. These results suggest that the observed gains in weight were caused not only by increased protein synthesis but also by increased appetite, which led to increased food intake and, in turn, improved nutritional status overall.
Ghrelin receptor agonists increase the secretion of GH from the arcuate nucleus of the hypothalamus, which stimulates the release of IGF‐1 from the liver.30 Both nonclinical31 and clinical studies17, 22 have shown that ANAM also promotes the secretion of GH and IGF‐1 but does not promote tumor growth.
GH and IGF‐1 both play important roles in anabolic pathways, which increase body weight primarily through increases in LBM rather than increasing stored fat.32, 33 In this regard, previous studies have shown that ghrelin activation can enhance muscle protein synthesis and thus may prevent muscle atrophy.34 Therefore, ghrelin agonists may help improve physical function in patients with cancer cachexia.35 However, there has been no consistent evidence of an improvement in motor function in the cancer cachexia clinical studies conducted to date.17, 23, 36, 37
A more appropriate parameter for evaluating motor function in patients with cancer cachexia has not been established, despite observations of increased muscle mass. This may be because of the lack of a correlation between muscle mass and motor function in patients with chronic inflammatory disorders.37, 38, 39 Furthermore, it would be a reasonable expectation that concurrent rehabilitation and appropriate daily exercise would be mandatory requirements to improve motor function, as even healthy people would experience diminished motor function if they remained at rest throughout the day without partaking in load‐bearing activity. However, in general, rehabilitation has not been applied to patients enrolled in clinical studies of this area. Cancer cachexia is characterized by a decrease in muscle tissue, and the guidelines stipulate that treatment should maintain or increase body weight and LBM.2 Therefore, LBM, and not motor function, was selected as the primary endpoint in this study. Because cancer cachexia is a syndrome caused by multiple factors, including loss of general activity because of fatigue and poor oral intake, if the study design had included improvement in muscle function as a co‐primary endpoint, then it should have included daily burden to muscle by rehabilitation and exercise. This comprehensive approach to investigating cancer cachexia syndrome is being carried out in Japan40 and internationally.41 It is hoped that the results will provide insights into the potential benefits of multimodal intervention for the management of cancer cachexia.
This study showed that ANAM administration was well tolerated and that any treatment‐related AEs were mostly mild in severity. The occurrence of grade 3 diabetes mellitus in this study (n = 3 patients) is believed to be consistent with the action of ghrelin.42 The development of diabetes mellitus was also reported in both the ROMANA‐1 and ROMANA‐2 studies (Safety and Efficacy of Anamorelin HCl in Patients With Non–Small Cell Lung Cancer‐Cachexia).23 It is hypothesized that, in addition to the action of ghrelin, an increase in food intake because of increases in appetite may have been a factor in these metabolic AEs. However, these AEs were controlled with the administration of an oral hypoglycemic drug or insulin.
Limitations
Limitations of this study include the small sample size, especially for patients with gastric (n = 5) and pancreatic (n = 5) cancers, and the absence of a functional endpoint (eg, handgrip strength, which may not correlate well with muscle mass).43 In addition, this study was limited by the open‐label and single‐arm design. However, no placebo was included in this study because previous studies have shown that patients with cancer cachexia receiving placebo appeared to have decreases in LBM; therefore, any increases observed in weight‐related endpoints would be because of the study drug ANAM.22, 23 Although this study has limited generalizability because of the inclusion of only Japanese patients, increased LBM, body weight, and appetite have been consistently confirmed in both Japanese phase 2 studies25, 26 and non‐Japanese phase 3 studies.23, 24 Therefore, it may be possible to apply this study's data to populations of non‐Japanese patients with gastrointestinal cancer.
Conclusions
ANAM increased LBM, improved anorexia, and increased transthyretin, a marker of nutritional status, in patients with gastrointestinal cancer and cachexia. Patients who have advanced gastrointestinal cancer are more prone to weight loss because of impaired food intake and absorption of nutrients compared with patients who have NSCLC. However, the results of this study are consistent with the efficacy and safety profiles of ANAM in patients with NSCLC and cachexia. Thus, ANAM can be considered an important treatment option for cancer cachexia syndrome in combination with other modalities, including nutritional support, rehabilitation and exercise, and treatment to suppress inflammatory processes.
Funding Support
This work was funded by Ono Pharmaceutical Company Ltd (Osaka, Japan).
Conflict of Interest Disclosures
Junji Furuse reports grants from Ono Pharmaceutical Company Ltd during the conduct of the study; grants from Janssen, OncoTherapy Science Inc, and NanoCarrier; grants and personal fees from Taiho Pharmaceutical, Yakult Honsha, Eli Lilly Japan, Chugai Pharmaceutical, Eisai, Ono Pharmaceutical Company Ltd, Daiichi Sankyo, Merck Serono, Bayer, Novartis, Sumitomo Dainippon, MSD, AstraZeneca, Mochida, Takeda, Astellas Pharma, Shionogi & Company, Kyowa Hakko Kirin, Zeria, J‐Pharma, and Bristol‐Myers Squibb; and personal fees from Mitsubishi Tanabe, Sawai, Pfizer, EA Pharma, Fujifilm, Sanofi, Sandoz, Otsuka, Boehringer Ingelheim, Shire, and Teijin outside the submitted work. Toshimi Takano reports grants from Ono Pharmaceutical Company Ltd during the conduct of the study; grants and personal fees from Daiichi Sankyo, Kyowa Hakko Kirin, and Eisai; and grants from Novartis, Chugai Pharmaceutical, Taiho Pharmaceutical, Takeda, MSD, and Merck Serono outside the submitted work. Ken Furuya reports grants, personal fees, and nonfinancial support from Ono Pharmaceutical Company Ltd during the conduct of the study. Hideo Baba reports grants, personal fees, and nonfinancial support from Ono Pharmaceutical Company Ltd during the conduct of the study; grants, personal fees, and nonfinancial support from Taiho Pharmaceutical and Eli Lilly Japan; grants from Covidien Japan, Astellas Pharma, Toyama Chemical, Ono Pharmaceutical Company Ltd, Shin Nippon Biomedical Laboratories, and Novartis Pharma; grants and nonfinancial support from Chugai Pharmaceutical, Yakult Honsha, Shionogi & Company, Merck Serono, Johnson & Johnson, Takeda Pharmaceutical; and nonfinancial support from MIYARISAN Pharmaceutical Company, Olympus Corporation, and Kaken Pharmaceutical outside the submitted work. Satoshi Hamauchi, Yoshinori Munemoto, Manabu Takeuchi, Yasuhiro Choda, and Takashi Higashiguchi report grants from Ono Pharmaceutical Company Ltd during the conduct of the study. Kei Muro reports grants and personal fees from Ono Pharmaceutical Company Ltd during the conduct of the study; grants from MSD, Daiichi Sankyo, Shionogi & Company, Kyowa Hakko Kirin, Gilead Sciences, Sanofi, Pfizer, and Merck Serono; and personal fees from Chugai Pharmaceutical, Taiho Pharmaceutical, Takeda Pharmaceutical, Bayer, and Eli Lilly Japan outside the submitted work. Koichi Takayama reports personal fees from Ono Pharmaceutical Company Ltd during the conduct of the study; grants and personal fees from Ono Pharmaceutical Company Ltd, Boehringer Ingelheim, and Chugai‐Roche; grants from Taiho Pharmaceutical Company; and personal fees from AstraZeneca, MSD‐Merck, Eli Lilly Japan, Daiichi Sankyo, and Pfizer outside the submitted work. Shusuke Oyama, Toru Takiguchi and Naoyuki Komura are employees of Ono Pharmaceutical Company Ltd, and report personal fees from the company during the conduct of the study. Kazuo Tamura reports personal fees from Ono Pharmaceutical Company Ltd during the conduct of the study and personal fees from Eisai and Kyowa Hakko Kirin outside the submitted work. Tateaki Naito made no disclosures.
Author Contributions
Satoshi Hamauchi: Investigation, writing–original draft, and writing–review and editing. Junji Furuse: Conceptualization, investigation, project administration, supervision, and writing–review and editing. Toshimi Takano: Investigation and writing–review and editing. Yoshinori Munemoto: Investigation and writing–review and editing. Ken Furuya: Investigation and writing–review and editing. Hideo Baba: Investigation and writing–review and editing. Manabu Takeuchi: Investigation and writing–review and editing. Yasuhiro Choda: Investigation and writing–review and editing. Takashi Higashiguchi: Conceptualization and writing–review and editing. Tateaki Naito: Conceptualization and writing–review and editing. Kei Muro: Conceptualization, project administration, supervision, and writing–review and editing. Koichi Takayama: Conceptualization, project administration, supervision, and writing–review and editing. Shusuke Oyama: Conceptualization, formal analysis, software, writing–original draft, and writing–review and editing. Toru Takiguchi: Conceptualization, project administration, writing–original draft, and writing–review and editing. Naoyuki Komura: Conceptualization, project administration, writing–original draft, and writing–review and editing. Kazuo Tamura: Conceptualization, project administration, supervision, and writing–review and editing.
Presented in part at the 2018 Japanese Society of Medical Oncology (JSMO) Annual Meeting; July 19‐21, 2018; Hyogo, Japan.
We thank all of the participating patients, their families, and health care professionals who made this study possible. We also thank Clare Cox, PhD, and James Graham, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by Ono Pharmaceutical Company Ltd, through EMC K.K., in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
References
- 1. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical Practice Guidelines on Cancer Cachexia in Advanced Cancer Patients With a Focus on Refractory Cachexia. Aachen, Germany: Department of Palliative Medicine/European Palliative Care Research Collaborative; 2010. [Google Scholar]
- 3. Lasheen W, Walsh D. The cancer anorexia‐cachexia syndrome: myth or reality? Support Care Cancer. 2010;18:265‐272. [DOI] [PubMed] [Google Scholar]
- 4. von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. J Am Med Dir Assoc. 2014;15:866‐872. [DOI] [PubMed] [Google Scholar]
- 5. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491‐497. [DOI] [PubMed] [Google Scholar]
- 6. Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315:1219‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takayama K, Atagi S, Imamura F, et al. Quality of life and survival survey of cancer cachexia in advanced non‐small cell lung cancer patients—Japan nutrition and QOL survey in patients with advanced non‐small cell lung cancer study. Support Care Cancer. 2016;24:3473‐3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R. Systematic review of the treatment of cancer‐associated anorexia and weight loss. J Clin Oncol. 2005;23:8500‐8511. [DOI] [PubMed] [Google Scholar]
- 9. Tuca A, Jimenez‐Fonseca P, Gascon P. Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol. 2013;88:625‐636. [DOI] [PubMed] [Google Scholar]
- 10. Konishi M, Ishida J, Springer J, Anker SD, von Haehling S. Cachexia research in Japan: facts and numbers on prevalence, incidence and clinical impact. J Cachexia Sarcopenia Muscle. 2016;7:515‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. Body‐composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol. 1993;11:152‐154. [DOI] [PubMed] [Google Scholar]
- 12. Ruiz Garcia V, Lopez‐Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort‐Marti S. Megestrol acetate for treatment of anorexia‐cachexia syndrome. Cochrane Database Syst Rev. 2013;3:CD004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol. 2017;18:192‐201. [DOI] [PubMed] [Google Scholar]
- 14. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495‐522. [DOI] [PubMed] [Google Scholar]
- 15. Satou M, Nakamura Y, Ando H, Sugimoto H. Understanding the functional significance of ghrelin processing and degradation. Peptides. 2011;32:2183‐2190. [DOI] [PubMed] [Google Scholar]
- 16. Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo). 2013;2013:518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia JM, Polvino WJ. Effect on body weight and safety of RC‐1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo‐controlled, multiple‐dose study in healthy volunteers. Oncologist. 2007;12:594‐600. [DOI] [PubMed] [Google Scholar]
- 18. DeBoer MD. Ghrelin and cachexia: will treatment with GHSR‐1a agonists make a difference for patients suffering from chronic wasting syndromes? Mol Cell Endocrinol. 2011;340:97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Argiles JM, Stemmler B. The potential of ghrelin in the treatment of cancer cachexia. Expert Opin Biol Ther. 2013;13:67‐76. [DOI] [PubMed] [Google Scholar]
- 20. Kumor K, Polvino W. Biological activity of RC‐1291, a novel oral ghrelin mimetic for cancer anorexia/cachexia: results from a phase I randomized, double‐blind, placebo‐controlled trial in healthy volunteers [abstract]. Support Care Cancer. 2006;14:593. [Google Scholar]
- 21. Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer‐related cachexia: a multicenter, randomized, double‐blind, crossover, pilot study. Support Care Cancer. 2013;21:129‐137. [DOI] [PubMed] [Google Scholar]
- 22. Garcia JM, Boccia RV, Graham CD, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo‐controlled, double‐blind trials. Lancet Oncol. 2015;16:108‐116. [DOI] [PubMed] [Google Scholar]
- 23. Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol. 2016;17:519‐531. [DOI] [PubMed] [Google Scholar]
- 24. Temel JS, Currow DC, Fearon K, Yan Y, Friend J, Abernethy AP. Phase III trials of anamorelin in patients with advanced non‐small cell lung cancer (NSCLC) and cachexia (ROMANA 1 and 2) [abstract]. J Clin Oncol. 2015;33(15 suppl):9500. [Google Scholar]
- 25. Takayama K, Katakami N, Yokoyama T, et al. Anamorelin (ONO‐7643) in Japanese patients with non‐small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer. 2016;24:3495‐3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO‐7643) for the treatment of patients with non‐small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer. 2018;124:606‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793‐799. [DOI] [PubMed] [Google Scholar]
- 28. National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. 2009. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed October 3, 2018.
- 29. Hill A, Kiss N, Hodgson B, Crowe TC, Walsh AD. Associations between nutritional status, weight loss, radiotherapy treatment toxicity and treatment outcomes in gastrointestinal cancer patients. Clin Nutr. 2011;30:92‐98. [DOI] [PubMed] [Google Scholar]
- 30. Khatib N, Gaidhane S, Gaidhane AM, et al. Ghrelin: ghrelin as a regulatory peptide in growth hormone secretion. J Clin Diagn Res. 2014;8:MC13‐MC17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Northrup R, Kuroda K, Duus EM, et al. Effect of ghrelin and anamorelin (ONO‐7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer. 2013;21:2409‐2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152‐177. [DOI] [PubMed] [Google Scholar]
- 33. Velloso CP. Regulation of muscle mass by growth hormone and IGF‐I. Br J Pharmacol. 2008;154:557‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porporato PE, Filigheddu N, Reano S, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Invest. 2013;123:611‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen JA, Splenser A, Guillory B, et al. Ghrelin prevents tumour‐ and cisplatin‐induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle. 2015;6:132‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abernethy A, Temel J, Currow D, Gleich L, Friend J. Anamorelin HCl for the treatment of anorexia‐cachexia in lung cancer: study design and baseline characteristics of patients in the phase III clinical trial ROMANA 2 (HT‐ANAM‐302). Abstracts of the 7th Cachexia Conference, Kobe/Osaka, Japan, December 9‐11, 2013 [abstract]. J Cachexia Sarcopenia Muscle. 2013;4:5‐02. [Google Scholar]
- 37. Dobs AS, Boccia RV, Croot CC, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double‐blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813‐1818. [DOI] [PubMed] [Google Scholar]
- 39. Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population‐based, cross‐sectional study of older adults in the United States. BMC Geriatr. 2013;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naito T, Mitsunaga S, Miura S, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non‐small‐cell lung cancer. J Cachexia Sarcopenia Muscle. 2019;10:73‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solheim TS, Laird BJA, Balstad TR, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:778‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heppner KM, Tong J. Mechanisms in endocrinology: regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. Eur J Endocrinol. 2014;171:R21‐R32. [DOI] [PubMed] [Google Scholar]
- 43. Ramage MI, Skipworth RJE. The relationship between muscle mass and function in cancer cachexia: smoke and mirrors? Curr Opin Support Palliat Care. 2018;12:439‐444. [DOI] [PubMed] [Google Scholar]


