Abstract
Type 2 diabetes mellitus (T2DM) is the most common cause of chronic kidney disease (CKD), and when it causes CKD it is collectively referred to as diabetic kidney disease. One of the newer therapies for managing hyperglycemia is the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) drug class. This review summarizes the effects of GLP‐1RAs in patients with T2DM with CKD and evidence for renoprotection with GLP‐1RAs using data from observational studies, prospective clinical trials, post hoc analyses, and meta‐analyses. Evidence from some preclinical studies was also reviewed. Taken together, subgroup analyses of patients with varying degrees of renal function demonstrated that glycemic control with GLP‐1RAs was not markedly less effective in patients with mild or moderate renal impairment vs that in patients with normal function. GLP‐1RAs were associated with improvements in some cardiorenal risk factors, including systolic blood pressure and body weight. Furthermore, several large cardiovascular outcome studies showed reduced risks of composite renal outcomes, mostly driven by a reduction in macroalbuminuria, suggesting potential renoprotective effects of GLP‐1RAs. In conclusion, GLP‐1RAs effectively reduced hyperglycemia in patients with mild or moderately impaired kidney function in the limited number of studies to date. GLP‐1RAs may be considered in combination with other glucose‐lowering medications because of their ability to lower glucose in a glucose‐dependent manner, lowering their risk for hypoglycemia, while improving some cardiorenal risk factors. Potential renoprotective effects of GLP‐1RAs, and their renal mechanisms of action, warrant further investigation.
Keywords: chronic kidney disease, diabetic kidney disease, glucagon‐like peptide‐1 receptor agonist, type 2 diabetes mellitus, visceral insulin resistance adiposity syndrome
Highlights
Diabetic kidney disease is a common comorbidity of type 2 diabetes mellitus.
In studies investigating the effect of renal function on the efficacy of treatment with glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), these agents improved glycemic control in patients with mild to moderately impaired kidney function, without significant differences compared with patients with normal renal function.
GLP‐1RAs were associated with a lower incidence of diabetic nephropathy, mostly driven by a reduction in albuminuria, compared with placebo in several large cardiovascular outcome trials.
摘要
2型糖尿病(type 2 diabetes mellitus, T2DM)是慢性肾脏病(chronic kidney disease, CKD)最常见的病因, 当引起CKD时统称为糖尿病肾病。胰高血糖素样肽‐1受体激动剂(glucagon‐like peptide‐1 receptor agonist, GLP‐1RA)是一类较新的降糖药物。本综述利用来自观察性研究、前瞻性临床试验、事后分析以及meta分析的数据, 总结了GLP‐1RAs在T2DM合并CKD患者中的疗效及GLP‐1RAs肾脏保护的证据。同时还回顾了一些来自临床前研究的证据。分析显示, 不同程度肾功能患者的亚组分析表明, 与肾功能正常的患者相比较, 轻度或中度肾功能受损患者使用GLP‐1Ras治疗的降糖疗效并未明显降低。GLP‐1RAs可改善一些心肾危险因素, 包括收缩压与体重。此外, 几项大型心血管结局研究结果显示, 复合肾脏结局的风险降低, 这主要是由于大量白蛋白尿减少所致, 表明GLP‐1RAs具有潜在的肾脏保护作用。总之, 迄今为止有限的几项研究显示GLP‐1RAs可有效降低轻度或中度肾功能受损患者的血糖水平。目前认为GLP‐1RAs可与其他降糖药物联合使用, 因其降糖作用呈葡萄糖依赖性, 可降低发生低血糖的风险, 同时还可改善一些心肾危险因素。GLP‐1RAs的潜在肾脏保护作用及其作用机制值得进一步研究。
Keywords: 慢性肾脏病变, 糖尿病肾病, 胰高血糖素样肽‐1受体激动剂, 2型糖尿病, 内脏胰岛素抵抗肥胖综合征。
1. INTRODUCTION
Kidney disease is a common comorbidity in patients with type 2 diabetes mellitus (T2DM). According to National Health and Nutrition Examination Survey data from 2007‐2012, the age‐adjusted prevalence of chronic kidney disease (CKD) in patients with T2DM was 38.3%, with 18.5% having normal renal function or mild renal impairment (estimated glomerular filtration rate [eGFR] ≥90 or 60 to 89 mL/min/1.73 m2, respectively, with urinary albumin‐to‐creatinine ratio [UACR] ≥30 mg/g), 16.7% having moderate renal impairment (eGFR 30‐59 mL/min/1.73 m2), and 3.1% having severe renal impairment or end‐stage renal disease (ESRD; eGFR 15 to 29 or <15 mL/min/1.73 m2, respectively).1 Diabetic kidney disease (DKD), a pathognomonic microvascular complication of diabetes, is characterized by albuminuria, impaired GFR, or both, and is associated with molecular, physiologic, and structural changes to the kidney induced by hyperglycemia.2, 3 People with T2DM typically have high blood pressure (BP) and are usually overweight or obese; hyperglycemia, hypertension, and obesity are all risk factors for the development and progression of CKD.4, 5, 6 This triad of clinical findings is driven by an excess of visceral fat and is frequently referred to as “the metabolic syndrome,” although the term “visceral insulin resistance adiposity syndrome (VIRAS)” is more descriptive.7
Landmark studies have shown that poor glycemic control increases the risk of developing microvascular and macrovascular complications, while good glycemic control reduces the risk of these complications.8, 9 Thus, one of the treatment goals for T2DM is reduction of hyperglycemia to delay onset of and slow progression of microvascular complications, including nephropathy.3, 10, 11 Recent research efforts have increasingly focused on the renal effects of glucose‐lowering therapies, with the greatest benefits for renal outcomes reported for sodium‐glucose cotransporter‐2 inhibitors and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs).12
GLP‐1RAs are a class of glucose‐lowering agents that have been shown to reduce hyperglycemia in a glucose‐dependent manner and are associated with weight loss and improvement in some other cardiorenal risk factors, including BP and lipid levels, in patients with T2DM.13, 14, 15, 16 GLP‐1RAs are generally well tolerated, with a low risk of hypoglycemia when not taken with concomitant insulin or sulfonylurea,17, 18, 19, 20, 21, 22, 23 but are associated with an increased frequency of gastrointestinal adverse events (AEs) such as nausea, vomiting, and diarrhea.24 Gastrointestinal symptoms associated with GLP‐1RAs occur early in the course of therapy and generally lessen over time.25, 26, 27 Hydration is important for all patients with diabetes, particularly those with DKD, given that severe vomiting without commensurate fluid replacement can lead to hypovolemia and acutely worsening renal function.26, 27 Postmarketing cases of acute kidney failure associated with GLP‐1RA use have been reported,28 resulting in warnings and precautions in the prescribing information for their use in patients with impaired renal function.
Because renal impairment and T2DM are often comorbid conditions, a need exists for effective glucose‐lowering therapies in patients with renal impairment. GLP‐1RAs vary in their primary mechanism of metabolism and elimination, and while some agents undergo renal clearance, GLP‐1RAs are not nephrotoxic. However, impaired renal function would be expected to affect the pharmacokinetics of renally eliminated GLP‐1RAs, potentially increasing drug exposure and the risk of AEs. GLP‐1RAs currently available in the United States include exenatide twice daily (bid), exenatide once weekly (qw), lixisenatide once daily (qd), liraglutide qd, dulaglutide qw, and semaglutide qw; albiglutide qw has been withdrawn from the market. Exenatide undergoes renal elimination and generalized proteolysis; exenatide qw is not recommended for patients with an eGFR below 45 mL/min/1.73 m2.18, 19 Lixisenatide is eliminated through glomerular filtration, followed by tubular reabsorption and subsequent metabolic degradation; patients treated with lixisenatide who have mild, moderate, or severe renal impairment should be monitored for changes in renal function and for gastrointestinal AEs.21 The “glutides”: liraglutide, dulaglutide, and semaglutide are human GLP‐1 analogs eliminated by general proteolysis pathways by mechanisms other than renal elimination but should be used with caution in patients with renal impairment, particularly during treatment initiation or dose escalation, as adverse gastrointestinal reactions associated with GLP‐1RAs can increase the risk of developing volume depletion and worsen renal function.20, 22, 23
Given the relationship between diabetes and kidney disease, the objective of this review is to summarize the efficacy of GLP‐1RAs and their effects on renal outcomes in patients with T2DM and renal impairment. To identify studies reporting effects of GLP‐1RAs in patients with renal impairment, the US National Library of Medicine PubMed database was searched for combinations of relevant terms including “exenatide,” “lixisenatide,” “liraglutide,” “dulaglutide,” “semaglutide,” “glucagon‐like peptide‐1 receptor agonists,” “kidney,” “renal,” “nephropathy,” and “diabetic kidney disease.” The search was limited to English‐language publications. Articles were manually searched to identify studies reporting the efficacy of GLP‐1RAs in patients with varying degrees of renal function and effects of GLP‐1RAs on renal outcomes, with additional studies identified within the reference lists of resultant articles.
2. RENAL EFFECTS OF GLP‐1RAS
Experiments evaluating exenatide and liraglutide in animal model systems have demonstrated improvements in glomerular hyperfiltration, albuminuria, oxidative stress, and histologic features indicative of DKD, suggesting a role for exenatide and liraglutide in protecting renal function (Table 1).29, 30, 31, 32, 33, 34 These effects may extend across the GLP‐1RA class; although to date no preclinical studies directly examining renoprotective effects with other GLP‐1RAs are known. Importantly, preclinical studies have generated hypotheses regarding potential renoprotective effects of GLP‐1RAs. While clinical studies on renoprotection are limited, several suggest that GLP‐1RAs may promote improved kidney function in humans (Table 2). In addition, clinical studies including subgroup analyses stratified by renal function have examined the effect of impaired renal function on the efficacy of GLP‐1RAs in patients with T2DM.
Table 1.
Effects of GLP‐1RAs on renal parameters in preclinical studies
| First author | Study design | Main renal outcomes of treatment |
|---|---|---|
| Kodera et al29 | Exendin‐4 10 μg/kg for 8 weeks in a streptozotocin‐induced rat model of diabetes | Reduced albuminuria, glomerular hyperfiltration, glomerular hypertrophy, mesangial matrix expansion, and ICAM‐1 and type IV collagen |
| Ojima et al30 | Exendin‐4 1.5 μg/kg for 2 weeks in a streptozotocin‐induced rat model of diabetes | Inhibited expression of the AGE receptor, which mediates oxidative stress pathways; reduced albuminuria; and improved histopathologic changes in the kidney |
| Park et al31 | Exendin‐4 0.5‐1.0 nmol/kg for 8 weeks in a db/db mouse model of diabetes | Reduced albuminuria, glomerular hypertrophy, mesangial matrix expansion, TGF‐β1 expression, type IV collagen accumulation, infiltrating macrophages, and apoptosis |
| Rieg et al32 | Exendin‐4 10 μg/kg (acute) in a db/db mouse model of diabetes | Induced diuresis and natriuresis due to increased GFR in wild‐type mice; effects on natriuresis were preserved in db/db mice |
| Hendarto et al33 | Liraglutide 0.3 mg/kg/12 h for 4 weeks in a streptozotocin‐induced rat model of diabetes | Reduced oxidative stress markers, TGF‐β1, fibronectin in renal tissues, and albuminuria |
| Zhao et al34 | Liraglutide applied to HK‐2 cells and liraglutide 0.3 mg/kg/12 h for 5 weeks in a streptozotocin‐induced rat model of diabetes | Attenuated high glucose‐induced toxicity in HK‐2 cells; inhibited glomerular hypertrophy and attenuated high glucose‐induced autophagy in diabetic rats |
Abbreviations: AGE, advanced glycation end products; GFR, glomerular filtration rate; HK‐2, human renal tubular epithelial cell line; ICAM‐1, intercellular adhesion molecule 1; TGF‐β1, transforming growth factor β1.
Table 2.
Effects of GLP‐1RAs on renal parameters in clinical studies
| First author (study name; http://clinicaltrials.gov identifier) | Study design | Main renal outcomes of treatment |
|---|---|---|
| Short‐term clinical studies | ||
| Zhang et al35 | 16‐week, randomized, comparator‐controlled study of exenatide 10 μg bid vs glimepiride 1‐4 mg qd in patients with T2DM and microalbuminuria | Exenatide bid resulted in reductions from baseline in 24‐h urinary albumin (−38.0%), urinary TGF‐β1 (−37.3%), and type IV collagen (−25.3%; P < .01 for all); there were no significant reductions with glimepiride |
| von Scholten et al (NCT02545738)36 | 12‐week, randomized, placebo‐controlled, crossover trial of liraglutide 1.8 mg qd in patients with T2DM and persistent albuminuria who were receiving RAS blockers | Liraglutide reduced UAER vs placebo (−32%; P = .017) |
| Tuttle et al (pooled analysis of NCT01149421; NCT01064687; NCT00734474; NCT01075282; NCT01191268; and NCT01126580)37 | Pooled analysis of 26‐week results from six phase 2/3 studies of dulaglutide 0.75‐1.5 mg qw vs placebo, active comparators, or insulin glargine in patients with T2DM | Dulaglutide decreased median percent changes in UACR vs placebo (−16.7% vs −10.0%; P = .043), active comparators (−20.0% vs −12.5%; P = .054), and insulin glargine (−20.0% vs −9.4%; P = .100) |
| Long‐term clinical studies | ||
| Zavattaro et al38 | 12‐month observational study of liraglutide 0.6‐1.8 mg qd in patients with T2DM | eGFR reached the normal range for 7 of 41 patients with baseline eGFR <90 mL/min/1.73 m2 |
| Imamura et al39 | 12‐month observational study of liraglutide 0.3‐0.9 mg qd in patients with T2DM and diabetic kidney disease | Liraglutide reduced proteinuria from 2.53 to 1.47 g/g creatinine and reduced the rate of eGFR decline from 6.6 to 0.3 mL/min/1.73 m2 per year |
| Desai et al40 | 3‐year observational study of exenatide and liraglutide compared with other glucose‐lowering drugs in patients with T2DM | GLP‐1RA treatment decreased albuminuria (−39.6 mg/g; P < .0001) vs other glucose‐lowering drugs, which were associated with an increase in albuminuria (+5.6 mg/g) |
| Tuttle et al (AWARD‐7; NCT01621178)41 | 52‐week, randomized, open‐label trial of dulaglutide 1.5 mg and dulaglutide 0.75 mg compared with insulin glargine, each added to insulin lispro, in patients with T2DM and stage 3‐4 CKD | eGFR was higher with dulaglutide 1.5 mg (34.0 mL/min/1.73 m2; P = .005) and dulaglutide 0.75 mg (33.8 mL/min/1.73 m2; P = .009) vs insulin glargine (31.3 mL/min/1.73 m2); reductions in UACR were −22.5% for dulaglutide 1.5 mg, −20.1% for dulaglutide 0.75 mg, and −13.0% for insulin glargine |
| Cardiovascular outcome trials | ||
| Pfeffer et al (ELIXA; NCT01147250)42 Muskiet et al43 | Randomized, placebo‐controlled, event‐driven study (median 25 months) of lixisenatide 10‐20 μg qd in patients with T2DM and a recent acute coronary syndrome |
Lixisenatide resulted in a smaller increase in UACR vs placebo (+24% vs +34%; P = .004) New‐onset macroalbuminuria was lower with lixisenatide vs placebo (6.5% vs 7.7%; HR, 0.81 [95% CI, 0.66‐0.99]; P = .0404)a |
| Holman et al (EXSCEL; NCT01144338)44 Bethel et al45 | Randomized, placebo‐controlled, event‐driven study (median 3.2 years) of exenatide qw 2 mg in patients with T2DM and with or without previous CV disease | The renal composite outcome was lower with exenatide qw vs placebo (5.8% vs 6.5%; HR, 0.85 [95% CI, 0.73‐0.98]; P = .027)b , c |
| Marso et al (LEADER; NCT01179048)46 Mann et al47 | Randomized, placebo‐controlled, event‐driven study (median 3.8 years) of liraglutide 1.8 mg qd in patients with T2DM and established CV disease or CV risk factors |
Nephropathy was lower with liraglutide vs placebo (5.7% vs 7.2%; HR, 0.78 [95% CI, 0.67‐0.92]; P = .003)d Results driven by 26% decrease in new‐onset macroalbuminuria |
| Marso et al (SUSTAIN‐6; NCT01720446)48 | 104‐week, randomized, placebo‐controlled study of semaglutide 0.5‐1.0 mg qw in patients with T2DM and established CV disease or CV risk factors |
New or worsening nephropathy was lower with semaglutide vs placebo (3.8% vs 6.1%; HR, 0.64 [95% CI, 0.46‐0.88]; P = .005)d Results driven by 46% decrease in new‐onset macroalbuminuria |
| Gerstein et al (REWIND; NCT01394952)49 Gerstein et al50 | Randomized, placebo‐controlled, event‐driven study (median 5.4 years) of dulaglutide 1.5 mg qw in patients with T2DM and established CV disease or CV risk factors |
The renal composite outcome was lower with dulaglutide vs placebo (17.1% vs 19.6%; HR, 0.85 [95% CI, 0.77‐0.93]; P = .0004)e Results driven by 23% decrease in new‐onset macroalbuminuria |
Abbreviations: AGE, advanced glycation end products; bid, twice daily; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ELIXA, Evaluation of Lixisenatide in Acute Coronary Syndrome; EXSCEL, Exenatide Study of Cardiovascular Event Lowering; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; HR, hazard ratio; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; qd, once daily; qw, once weekly; RAS, renin‐angiotensin system; REWIND, Researching Cardiovascular Events with a Weekly Incretin in Diabetes; SUSTAIN, Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes; T2DM, type 2 diabetes mellitus; TGF‐β1, transforming growth factor β1; UACR, urinary albumin‐to‐creatinine ratio; UAER, urinary albumin excretion rate.
Adjusted for baseline HbA1c.
40% eGFR decline, renal replacement, renal death, or new macroalbuminuria.
Adjusted for age, sex, ethnicity, race, region, duration of diabetes, history of CV event, insulin use, baseline HbA1c, eGFR, and body mass index.
New‐onset macroalbuminuria, persistent doubling of serum creatinine level and creatinine clearance <45 mL/min/1.73 m2, continuous renal replacement therapy, or death due to renal disease.
New macroalbuminuria, sustained ≥30% decline in eGFR, or chronic renal replacement therapy.
2.1. Exenatide
Several studies have examined the effect of renal function on the efficacy of exenatide treatment. A post hoc analysis of a randomized controlled trial (RCT) compared the effects of exenatide qw formulated for autoinjection with exenatide bid by renal function status.51 As renal function decreased, the glycemic effect of exenatide bid increased (glycated hemoglobin [HbA1c] reductions of −0.7%, −1.3%, and −1.4% [−7.5, −14.6, and −15.2 mmol/mol] for the eGFR subgroups ≥90, 60‐89, and 30‐59 mL/min/1.73 m2, respectively), while there was no effect on body weight. In contrast, renal impairment had no effect on HbA1c reductions associated with exenatide qw for autoinjection (−1.5%, −1.4%, and −1.4% [−16.7, −15.0, and −15.2 mmol/mol] for eGFR subgroups ≥90, 60‐89, and 30‐59 mL/min/1.73 m2, respectively), but greater weight loss was observed as renal function decreased. These findings suggest impaired renal function may increase exposure, and thereby glycemic response, to exenatide bid. Renal function status showed no clear effect on the safety profile of either formulation.
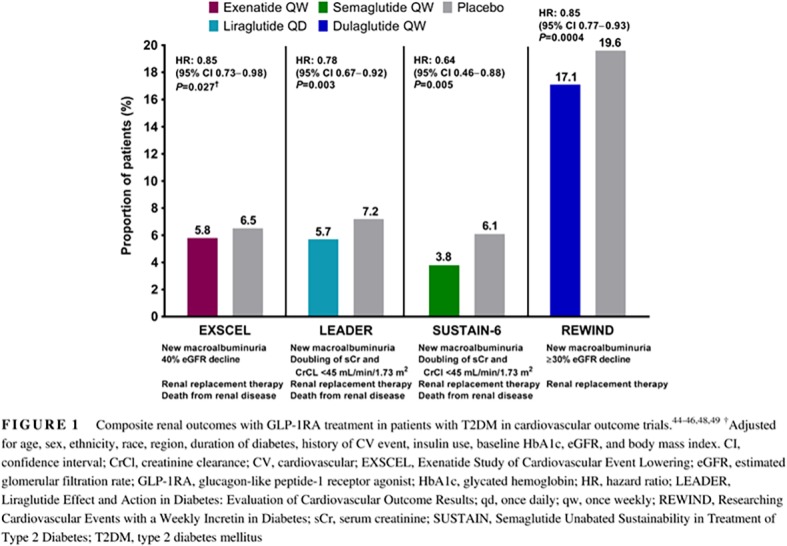
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) examined the effects of exenatide qw compared with placebo on cardiovascular outcomes in patients with T2DM who had a wide range of cardiovascular risk (N = 14 752); in addition, ~22% of patients had moderate renal impairment (eGFR 30‐59 mL/min/1.73 m2), and 0.1% had severe renal impairment (eGFR <30 mL/min/1.73 m2).44 A subanalysis of the primary outcome of major adverse cardiovascular events (MACE; first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) in prespecified renal function subgroups demonstrated no significant treatment interaction, suggesting no effect modification by renal function status. In the total population, exenatide qw showed improvement in terms of overall difference from placebo for some cardiorenal risk factors, including reductions in systolic BP (SBP; −1.57 mmHg; P < .001) and body weight (−1.27 kg; P < .001). Furthermore, a composite renal outcome consisting of 40% eGFR decline, renal replacement, renal death, or new macroalbuminuria (UACR >300 mg/g) was significantly reduced with exenatide qw vs that with placebo in an analysis adjusted for age, sex, ethnicity, race, region, duration of diabetes, history of cardiovascular event, insulin use, baseline HbA1c, eGFR, and body mass index (5.8% vs 6.5%; adjusted hazard ratio [HR], 0.85 [95% confidence interval (CI), 0.73‐0.98]; P = .027) (Figure 1).45
Figure 1.
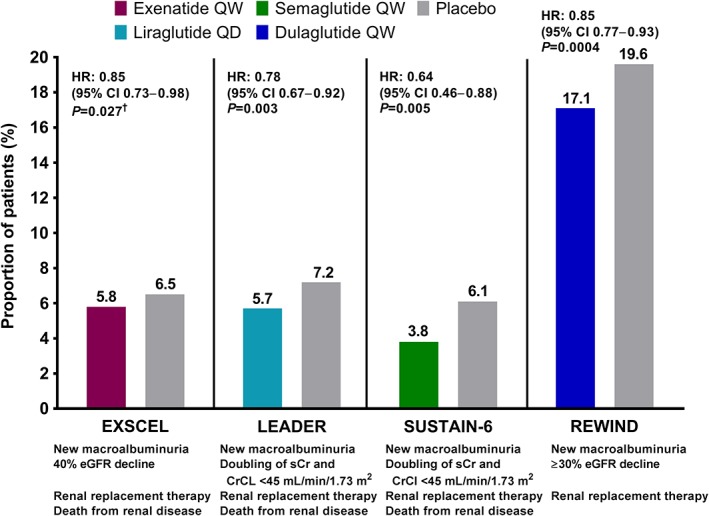
Composite renal outcomes with GLP‐1RA treatment in patients with T2DM in cardiovascular outcome trials.44, 45, 46, 48, 49 †Adjusted for age, sex, ethnicity, race, region, duration of diabetes, history of CV event, insulin use, baseline HbA1c, eGFR, and body mass index. CI, confidence interval; CrCl, creatinine clearance; CV, cardiovascular; EXSCEL, Exenatide Study of Cardiovascular Event Lowering; eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; HR, hazard ratio; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; qd, once daily; qw, once weekly; REWIND, Researching Cardiovascular Events with a Weekly Incretin in Diabetes; sCr, serum creatinine; SUSTAIN, Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes; T2DM, type 2 diabetes mellitus
The effect of exenatide on renal fibrosing factors has also been examined in patients with T2DM and renal impairment. Transforming growth factor β1 (TGF‐β1) and type IV collagen both contribute to extracellular matrix accumulation in DKD. In a small study (N = 31) of patients with T2DM and microalbuminuria (defined as urinary albumin 30‐300 mg/24 hours), after 16 weeks, exenatide bid significantly reduced 24‐hour urinary albumin (−38.0%), urinary TGF‐β1 (−37.3%), and type IV collagen (−25.3%; P < .01 for all), whereas the glimepiride‐treated group had no significant reductions in these measurements.35 Exenatide also resulted in a small, nonsignificant reduction in SBP vs glimepiride. However, neutral effects of exenatide bid on renal function have also been observed. A post hoc analysis of 54 patients without overt nephropathy treated with exenatide bid or insulin glargine for 52 weeks found no significant change from baseline in creatinine clearance or albuminuria (urinary albumin excretion and UACR) among exenatide‐treated patients.52
An observational study examined renal outcomes with glucose‐lowering treatments among 466 patients studied sequentially over 3 years, 275 of whom were treated with a GLP‐1RA (exenatide or liraglutide).40 GLP‐1RA‐treated patients had a mean decrease in albuminuria (−39.6 mg/g; P < .0001) compared with a mean increase in albuminuria (+5.6 mg/g) in patients treated with unspecified glucose‐lowering drugs. Among those with macroalbuminuria at baseline, greater proportions of GLP‐1RA‐treated patients developed microalbuminuria (UACR 30‐300 mg/g; 23%) or normoalbuminuria (UACR <30 mg/g; 2.8%) compared with those receiving unspecified glucose‐lowering therapies (microalbuminuria, 12.3%; normoalbuminuria, 0%; P = .0005). SBP was also lower among patients receiving GLP‐1RAs (by 3 mmHg).
2.2. Lixisenatide
A post hoc meta‐analysis of nine RCTs that examined lixisenatide in patients with normal renal function or with mild or moderate renal impairment found no difference in efficacy on the basis of renal status (end‐of‐study placebo‐adjusted differences in HbA1c of −0.52%, −0.50%, and − 0.85% [−5.7, −5.5, and −9.3 mmol/mol] for creatinine clearance subgroups ≥90, 60‐89, or 30‐59 mL/min, respectively).53 However, a higher incidence of gastrointestinal AEs occurred with mild renal impairment vs the incidence with normal renal function.
In the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) study (N = 6068), which examined cardiovascular outcomes with lixisenatide treatment in patients with T2DM who had a recent acute coronary syndrome, 23% of patients had eGFR 30 to 60 mL/min/1.73 m2 and 0.1% had eGFR <30 mL/min/1.73 m2.42 A subgroup analysis of the primary outcome (time to event for composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina) demonstrated no significant interactions in prespecified renal function subgroups. In the total population, lixisenatide showed improvement in terms of average difference from placebo across all visits for some cardiorenal risk factors, including modest reductions in SBP (−0.8 mmHg; P = .001) and body weight (−0.7 kg; P < .001). In addition, lixisenatide resulted in a smaller increase in the UACR vs placebo (+24% vs +34%; P = .004) after 108 weeks of treatment. Subgroup analyses demonstrated significant reductions in UACR with lixisenatide vs those with placebo among patients with macroalbuminuria (UACR >300 mg/g) at baseline (treatment difference for percent change from baseline: −39.18%; P = .0070). Further, lixisenatide showed a reduced risk of progression to macroalbuminuria compared with placebo when adjusted for baseline HbA1c (6.5% vs 7.7%; adjusted HR, 0.81 [95% CI, 0.66‐0.99]; P = .0404).43
2.3. Liraglutide
Multiple studies have examined the glycemic effects of liraglutide in patients with T2DM and renal impairment. A 26‐week RCT investigating liraglutide 1.8 mg (n = 140) vs placebo (n = 139) in patients with moderate renal impairment (eGFR 30‐59 mL/min/1.73 m2) demonstrated significant HbA1c reductions with liraglutide (−1.05% vs −0.38% with placebo [−11.5 vs −4.2 mmol/mol]; P < .0001), with no effect on renal function as measured by UACR or eGFR.54 In a separate RCT of 24 patients with dialysis‐dependent ESRD, liraglutide reduced HbA1c at 12 weeks (−0.5% [−5.5 mmol/mol]) from baseline (6.7% [50 mmol/mol]), but not significantly vs placebo.55 Nausea and vomiting occurred more frequently with liraglutide than placebo in this subpopulation. In a meta‐analysis of six 26‐week RCTs of liraglutide, patients with normal, mild, or moderate/severe renal impairment had similar mean HbA1c reductions (liraglutide 1.2 mg: −1.29%, −1.40%, and −1.34% [−14.1, −15.3, and −14.6 mmol/mol], respectively; liraglutide 1.8 mg: −1.40%, −1.34%, and −1.39% [−15.3, −14.6, and −15.2 mmol/mol], respectively), although the number of patients in the moderate/severe group was too small to draw firm conclusions in this subpopulation.56 There was a trend toward increased nausea in patients with moderate or severe renal impairment.
The effects of liraglutide on renal measurements have also been examined in patients with T2DM and impaired renal function. In a 12‐month longitudinal study of liraglutide (N = 84), eGFR reached the normal range (≥90 mL/min using the Chronic Kidney Disease‐Epidemiology Collaboration equation) in 7 of 41 patients with baseline eGFR <90 mL/min.38 Furthermore, three of five patients with baseline microalbuminuria returned to normal albuminuria. Among 23 patients with DKD who had received renin‐angiotensin system blockers, 12‐month treatment with liraglutide significantly decreased proteinuria from 2.53 to 1.47 g/g creatinine and reduced the rate of eGFR decline from 6.6 to 0.3 mL/min/1.73 m2 per year.39 In a small randomized controlled crossover trial (N = 32), treatment with liraglutide for 12 weeks significantly reduced the urinary albumin excretion rate vs placebo (−32%; P = .017) in patients with persistent albuminuria (UACR ≥30 mg/g) and eGFR ≥30 mL/min/1.73 m2 who were receiving stable renin‐angiotensin system‐blocking treatment, further suggesting a renoprotective role for liraglutide.36
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial (N = 9340), which included ~23% of patients with moderate or severe renal impairment, studied cardiovascular outcomes during treatment with liraglutide vs those with placebo.46 A prespecified subgroup analysis comparing the primary outcome of MACE in patients with moderate or severe renal impairment (eGFR <60 mL/min/1.73 m2) vs patients with eGFR ≥60 mL/min/1.73 m2 showed a greater benefit of liraglutide in the moderate or severe renal impairment group (P = .01). However, a sensitivity analysis showed no clinically meaningful treatment interaction based on renal function. The LEADER trial also showed a beneficial effect of GLP‐1RAs on some renal outcomes. The incidence of nephropathy (defined as new‐onset macroalbuminuria or a doubling of serum creatinine level and eGFR ≤45 mL/min/1.73 m2, the need for continuous renal replacement therapy, or death from renal disease) was lower with liraglutide vs that with placebo (5.7% vs 7.2%; HR, 0.78 [95% CI, 0.67‐0.92]; P = .003) (Figure 1).46 This result was driven by a 26% reduction of new‐onset persistent macroalbuminuria.47 Placebo‐subtracted reductions in cardiorenal risk factors, including SBP (−1.2 mmHg) and body weight (−2.3 kg) at 36 months, were also observed.46
2.4. Dulaglutide
A pooled analysis of renal effects and safety in nine phase 2 and 3 studies of dulaglutide included 6005 patients, of whom 4.4% had persistent eGFR <60 mL/min/1.73 m2, 3.0% had persistent macroalbuminuria, and 7.1% had eGFR <60 mL/min/1.73 m2 and/or macroalbuminuria.37 Renal end points were evaluated in six of the trials, while renal safety was evaluated in all nine trials. At 26 weeks, dulaglutide slightly decreased albuminuria, reflected by larger reductions in median percent change in UACR vs placebo (−16.7% vs −10.0%; P = .043), active comparators (−20.0% vs −12.5%; P = .054), and insulin glargine (−20.0% vs −9.4%; P = .100). No significant differences in eGFR were observed with dulaglutide vs those with other treatments. In addition, dulaglutide was not associated with AEs indicative of potential acute renal failure.37
The AWARD‐7 RCT investigated the effects of dulaglutide 1.5 mg, dulaglutide 0.75 mg, or insulin glargine, each added to insulin lispro, in 576 patients with T2DM and moderate‐to‐severe CKD (stages 3‐4).41 After 52 weeks, similar HbA1c reductions from baseline were observed with dulaglutide 1.5 mg (−1.1% [−12.0 mmol/mol]), dulaglutide 0.75 mg (−1.1% [−12.0 mmol/mol]), and insulin glargine (−1.0% [−10.9 mmol/mol]; P < .0001 for all), while dulaglutide was associated with greater weight loss and a lower rate of hypoglycemia.
Dulaglutide was also associated with a reduced decline in eGFR (a secondary outcome of the trial) compared with insulin glargine.41 After 52 weeks, eGFR was higher with dulaglutide 1.5 mg (34.0 mL/min/1.73 m2; P = .005 vs insulin glargine) and dulaglutide 0.75 mg (33.8 mL/min/1.73 m2; P = .009 vs insulin glargine) compared with insulin glargine (31.3 mL/min/1.73 m2). Reductions in the secondary outcome of UACR were numerically, although not significantly, greater with dulaglutide (dulaglutide 1.5 mg, −22.5%; dulaglutide 0.75 mg, −20.1%; insulin glargine, −13.0%). Thus, while glycemic efficacy was similar with dulaglutide and insulin glargine, dulaglutide demonstrated additional potential renal benefits.
The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial (N = 9901), which included ~22% of patients with eGFR <60 mL/min/1.73 m2, studied cardiovascular outcomes with dulaglutide vs placebo among patients with T2DM who had a previous cardiovascular event or cardiovascular risk factors.49 The incidence of a composite renal outcome, comprising UACR >33.9 mg/mmol in those with a lower baseline concentration, sustained ≥30% decline in eGFR, or chronic renal replacement therapy, was lower with dulaglutide vs placebo (17.1% vs 19.6%; HR, 0.85 [95% CI, 0.77‐0.93]; P = .0004) (Figure 1). This result was driven by a 23% reduction of new‐onset macroalbuminuria.50 Placebo‐subtracted reductions in cardiorenal risk factors, including SBP (−1.70 mmHg; P < .0001) and body weight (−1.46 kg; P < .0001), were also observed.49
2.5. Semaglutide
In the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN)‐6 study (N = 3297), a cardiovascular outcome trial, 25%, 3%, and 0.4% of patients had eGFR 30‐59, 15‐29, and <15 mL/min/1.73 m2, respectively.48 An analysis of the primary outcome of MACE by renal function subgroup showed no significant treatment interaction. The SUSTAIN‐6 study also had a prespecified secondary outcome of new or worsening nephropathy (defined as new‐onset persistent macroalbuminuria, persistent doubling of serum creatinine level, and creatinine clearance <45 mL/min/1.73 m2 [per Modification of Diet in Renal Disease criteria], the need for continuous renal replacement therapy, or death due to renal disease). A smaller proportion of semaglutide‐treated patients experienced new or worsening nephropathy vs that with placebo (3.8% vs 6.1%; HR, 0.64 [95% CI, 0.46‐0.88]; P = .005) (Figure 1). This result was driven by a 46% reduction in macroalbuminuria.
2.6. Effect of GLP‐1RAs on renal outcomes across cardiovascular outcome trials
A recent meta‐analysis of cardiovascular outcomes trials, including EXSCEL, ELIXA, LEADER, and SUSTAIN‐6, examined the effect of GLP‐1RAs on progression of kidney disease.57 GLP‐1RAs were associated with an 18% reduction in the risk of a broad composite renal outcome consisting of new‐onset macroalbuminuria, worsening of eGFR, ESRD, or death due to renal causes compared with placebo (HR, 0.82 [95% CI, 0.75‐0.89]; P < .001). The reduction in risk was driven primarily by a reduction in macroalbuminuria, as excluding this outcome from the analysis resulted in a nonsignificant risk reduction. These results suggest that GLP‐1RAs reduce renal events mainly by reducing macroalbuminuria.
3. MECHANISMS OF ACTION
Renal benefits of GLP‐1RAs may be attributable to favorable effects on cardiorenal risk factors, including improved glucose control, BP lowering, and weight loss. In addition, GLP‐1RAs may have direct renal effects, as GLP‐1 receptors are expressed in the kidney.58
The mechanism of action of GLP‐1 in the kidney is not completely understood, but may involve both neural and nonneural pathways.59 A gut‐renal axis is possible, with regulatory linkages through the gastrointestinal tract, central nervous system, and kidney (Figure 2).2 The main physiologic effect of GLP‐1 on the kidney may possibly be to reduce prandial intraglomerular pressure to reduce macronutrient loss in the glomerular filtrate. This would allow increased time for macronutrient uptake by other tissues without having to expend energy to transport macronutrients back into the body through the proximal tubule or overwhelming the proximal tubule reuptake system for macronutrients, such as glucose, amino acids, and free fatty acids. It may do so by decreasing sympathetic activity at the glomerulus through the central nervous system or by direct effects on the mesangium and renal interstitium.
Figure 2.
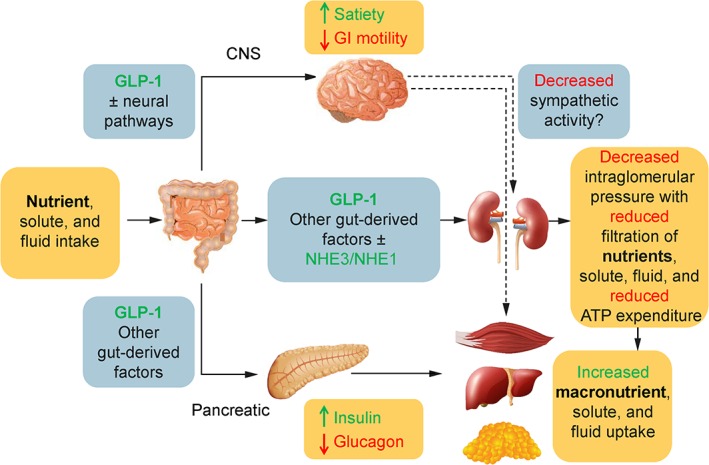
The GLP‐1 gut‐renal axis. The role of GLP‐1 is to facilitate macronutrient storage through multiple pathways. Two of the pathways shown—the CNS and direct pathways—may affect the kidneys by decreasing intraglomerular pressure. This potentially may result in decreased nutrient loss or energy expenditure needed to reabsorb nutrients such as glucose or amino acids. GLP‐1 works through the pancreatic pathway to increase macronutrient storage in the liver, skeletal muscle, and fat by increasing insulin and decreasing glucagon levels in a glucose‐dependent manner. The dotted lines represent proposed mechanisms whereby the brain, potentially through the autonomic nervous system, may reduce sympathetic activity, insulin resistance, and intraglomerular pressure. Green text indicates an increase; red text indicates a reduction. ATP, adenosine triphosphate; CNS, central nervous system; GI, gastrointestinal; GLP‐1, glucagon‐like peptide‐1; NHE, sodium‐hydrogen exchanger
Several studies have reported GLP‐1RA‐induced natriuresis in healthy subjects and in patients with T2DM,60, 61, 62, 63 possibly resulting from decreased activity of the sodium‐hydrogen exchanger 3 (NHE3). GLP‐1 receptor activation has been shown to inhibit activity of NHE3 in the proximal tubule, which would increase distal tubular sodium transport in the kidney to the macula densa, resulting in tubular glomerular feedback with a reduction in intraglomerular pressure, hyperfiltration, and renin‐angiotensin system activity.2, 58, 64 Reducing intraglomerular pressure would be expected to have an antiproteinuric effect in the diabetic kidney and help preserve kidney function.
4. CONCLUSIONS
DKD is a common comorbidity of T2DM; therefore, glucose‐lowering treatments that are efficacious, do not increase hypoglycemia, and may have additional benefits for the kidney are of interest. In the limited number of studies to date investigating the effect of renal function on the efficacy of GLP‐1RA treatment, GLP‐1RAs improved glycemic control in patients with mild to moderately impaired kidney function, without significant differences compared with patients with normal renal function.
Hyperglycemia, obesity, and hypertension all contribute to the development of kidney and heart disease,4 and the multiple effects of GLP‐1RAs for improving glycemic control, body weight, and BP may be beneficial for delaying the onset or progression of DKD. However, GLP‐1RAs may potentially have direct effects on the kidney as well.
In animal models, GLP‐1RAs may have a renoprotective effect, as demonstrated by improvement in some renal function measures and histologic features. In addition, these agents were associated with a lower incidence of diabetic nephropathy and/or albuminuria compared with placebo in several large clinical studies. These observations should be the basis for continued research efforts into the long‐term effects of GLP‐1RAs on kidney function and mechanistic studies examining how GLP‐1RAs affect the kidney, potentially through the gut‐renal axis.
For now, GLP‐1RAs should be considered in combination with other complementary glucose‐lowering medications in patients with CKD, due to their safety and ability to lower glucose in a glucose‐dependent manner.
DISCLOSURE
Dr Sloan has served as a consultant, researcher, and speaker for AbbVie, AstraZeneca, Boehringer Ingelheim‐Lilly, Janssen, Merck, Novo Nordisk, Pfizer, and Salix.
ACKNOWLEDGEMENTS
The development of this manuscript was supported by AstraZeneca. Elizabeth Strickland, PhD, CMPP, and Amanda Sheldon, PhD, CMPP, of inScience Communications, Springer Healthcare (Philadelphia, PA, USA), provided medical writing support in accordance with Good Publication Practice guidelines http://annals.org/aim/article/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3, funded by AstraZeneca.
Sloan LA. Review of glucagon‐like peptide‐1 receptor agonists for the treatment of type 2 diabetes mellitus in patients with chronic kidney disease and their renal effects. Journal of Diabetes. 2019;11:938–948. 10.1111/1753-0407.12969
Funding information AstraZeneca
REFERENCES
- 1. Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns‐NHANES 2007‐2012. BMJ Open Diabetes Res Care. 2016;4:e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muskiet MH, Smits MM, Morsink LM, Diamant M. The gut‐renal axis: do incretin‐based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10:88‐103. [DOI] [PubMed] [Google Scholar]
- 3. Gnudi L, Karalliedde J. Beat it early: putative renoprotective haemodynamic effects of oral hypoglycaemic agents. Nephrol Dial Transplant. 2016;31:1036‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maric‐Bilkan C. Obesity and diabetic kidney disease. Med Clin North Am. 2013;97:59‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(suppl 2):64‐78. [DOI] [PubMed] [Google Scholar]
- 6. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864‐2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidson JA, Sloan L. Fixed‐dose combination of canagliflozin and metformin for the treatment of type 2 diabetes: an overview. Adv Ther. 2017;34:41‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 10. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 11. National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850‐886. [DOI] [PubMed] [Google Scholar]
- 12. Scheen AJ. Effects of glucose‐lowering agents on surrogate endpoints and hard clinical renal outcomes in patients with type 2 diabetes. Diabetes Metab. 2019;45:110‐121. [DOI] [PubMed] [Google Scholar]
- 13. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a systematic review and mixed‐treatment comparison analysis. Diabetes Obes Metab. 2017;19:524‐536. [DOI] [PubMed] [Google Scholar]
- 14. Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon‐like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta‐analysis. PLoS One. 2015;10:e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun F, Wu S, Guo S, et al. Impact of GLP‐1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Res Clin Pract. 2015;110:26‐37. [DOI] [PubMed] [Google Scholar]
- 16. Sun F, Wu S, Wang J, et al. Effect of glucagon‐like peptide‐1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta‐analysis. Clin Ther. 2015;37:225‐241. e8. [DOI] [PubMed] [Google Scholar]
- 17. Byetta® (Exenatide): US Prescribing Information. Wilmington, DE: AstraZeneca; 2018. [Google Scholar]
- 18. Bydureon® (Exenatide Extended Release): US Prescribing Information. Wilmington, DE: AstraZeneca; 2019. [Google Scholar]
- 19. Bydureon® BCise™ (Exenatide Extended Release): US Prescribing Information. Wilmington, DE: AstraZeneca; 2019. [Google Scholar]
- 20. Victoza® (Liraglutide): US Prescribing Information. Plainsboro, NJ: Novo Nordisk Inc.; 2017. [Google Scholar]
- 21. Adlyxin® (Lixisenatide): US Prescribing Information. Bridgewater, NJ: sanofi‐aventis U.S. LLC; 2016. [Google Scholar]
- 22. Trulicity® (Dulaglutide): US Prescribing Information. Indianapolis, IN: Eli Lilly and Company; 2019. [Google Scholar]
- 23. Ozempic® (Semaglutide): US Prescribing Information. Plainsboro, NJ: NovoNordisk; 2017. [Google Scholar]
- 24. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336‐347. [DOI] [PubMed] [Google Scholar]
- 25. Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon‐like peptide‐1 receptor agonists: incidence and consequences. Diabetes. 2017;19:672‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacConell L, Gurney K, Malloy J, Zhou M, Kolterman O. Safety and tolerability of exenatide once weekly in patients with type 2 diabetes: an integrated analysis of 4,328 patients. Diabetes Metab Syndr Obes. 2015;8:241‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo‐controlled and comparator‐controlled clinical trials. Diabetes Metab Syndr Obes. 2012;5:29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filippatos TD, Elisaf MS. Effects of glucagon‐like peptide‐1 receptor agonists on renal function. World J Diabetes. 2013;4:190‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kodera R, Shikata K, Kataoka HU, et al. Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965‐978. [DOI] [PubMed] [Google Scholar]
- 30. Ojima A, Ishibashi Y, Matsui T, et al. Glucagon‐like peptide‐1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin‐induced diabetic rats by blocking advanced glycation end product‐induced protein arginine methyltranferase‐1 expression. Am J Pathol. 2013;182:132‐141. [DOI] [PubMed] [Google Scholar]
- 31. Park CW, Kim HW, Ko SH, et al. Long‐term treatment of glucagon‐like peptide‐1 analog exendin‐4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18:1227‐1238. [DOI] [PubMed] [Google Scholar]
- 32. Rieg T, Gerasimova M, Murray F, et al. Natriuretic effect by exendin‐4, but not the DPP‐4 inhibitor alogliptin, is mediated via the GLP‐1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol. 2012;303:F963‐F971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hendarto H, Inoguchi T, Maeda Y, et al. GLP‐1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin‐induced diabetic rats via protein kinase A‐mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012;61:1422‐1434. [DOI] [PubMed] [Google Scholar]
- 34. Zhao X, Liu G, Shen H, et al. Liraglutide inhibits autophagy and apoptosis induced by high glucose through GLP‐1R in renal tubular epithelial cells. Int J Mol Med. 2015;35:684‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang H, Zhang X, Hu C, Lu W. Exenatide reduces urinary transforming growth factor‐beta1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press Res. 2012;35:483‐488. [DOI] [PubMed] [Google Scholar]
- 36. von Scholten BJ, Persson F, Rosenlund S, et al. The effect of liraglutide on renal function: a randomized clinical trial. Diabetes Obes Metab. 2017;19:239‐247. [DOI] [PubMed] [Google Scholar]
- 37. Tuttle KR, McKinney TD, Davidson JA, Anglin G, Harper KD, Botros FT. Effects of once‐weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials. Diabetes Obes Metab. 2017;19:436‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zavattaro M, Caputo M, Sama MT, et al. One‐year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine. 2015;50:620‐626. [DOI] [PubMed] [Google Scholar]
- 39. Imamura S, Hirai K, Hirai A. The glucagon‐like peptide‐1 receptor agonist, liraglutide, attenuates the progression of overt diabetic nephropathy in type 2 diabetic patients. Tohoku J Exp Med. 2013;231:57‐61. [DOI] [PubMed] [Google Scholar]
- 40. Desai A, Nithi A, Kunduru D, et al. GLP‐1 receptor agonists reverse albuminuria. Paper presented at: Endocrine Society's 98th Annual Meeting and Expo (ENDO) 2016; April 1–4, 2016; Boston, MA.
- 41. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605‐617. [DOI] [PubMed] [Google Scholar]
- 42. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 43. Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2018;6:859‐869. [DOI] [PubMed] [Google Scholar]
- 44. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bethel MA, Mentz RJ, Merrill P, et al. Renal outcomes in the exenatide study of cardiovascular event lowering (EXSCEL) [abstract]. Diabetes. 2018;67(suppl 1):A138. [Google Scholar]
- 46. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 48. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 49. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 50. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo‐controlled trial. Lancet. 2019;394:131‐138. 10.1016/s0140-6736(19)31150-x. [DOI] [PubMed] [Google Scholar]
- 51. Wysham CH, Hardy E, Wang H, Rosenstock J. Impact of renal function on efficacy and safety of autoinjected exenatide once‐weekly suspension vs. exenatide twice‐daily in type 2 diabetes [abstract]. Diabetes. 2017;66((suppl 1)):A300. [Google Scholar]
- 52. Muskiet MHA, Bunck MC, Heine RJ, et al. Exenatide twice‐daily does not affect renal function or albuminuria compared to titrated insulin glargine in patients with type 2 diabetes mellitus: a post‐hoc analysis of a 52‐week randomised trial. Diabetes Res Clin Pract. 2019;153:14‐22. [DOI] [PubMed] [Google Scholar]
- 53. Hanefeld M, Arteaga JM, Leiter LA, et al. Efficacy and safety of lixisenatide in patients with type 2 diabetes and renal impairment. Diabetes Obes Metab. 2017;19:1594‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add‐on to glucose‐lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA‐RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222‐230. [DOI] [PubMed] [Google Scholar]
- 55. Idorn T, Knop FK, Jorgensen MB, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and end‐stage renal disease: an investigator‐initiated, placebo‐controlled, double‐blind, parallel‐group, randomized trial. Diabetes Care. 2016;39:206‐213. [DOI] [PubMed] [Google Scholar]
- 56. Davidson JA, Brett J, Falahati A, Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocr Pract. 2011;17:345‐355. [DOI] [PubMed] [Google Scholar]
- 57. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose co‐transporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: a systematic review and meta‐analysis of cardiovascular outcomes trials. Circulation. 2019;139:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 58. Muskiet MHA, Tonneijck L, Smits MM, et al. GLP‐1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13:605‐628. [DOI] [PubMed] [Google Scholar]
- 59. Skov J. Effects of GLP‐1 in the kidney. Rev Endocr Metab Disord. 2014;15:197‐207. [DOI] [PubMed] [Google Scholar]
- 60. Muskiet MH, Tonneijck L, Smits MM, et al. Acute renal haemodynamic effects of glucagon‐like peptide‐1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab. 2016;18:178‐185. [DOI] [PubMed] [Google Scholar]
- 61. Skov J, Pedersen M, Holst JJ, et al. Short‐term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2016;18:581‐589. [DOI] [PubMed] [Google Scholar]
- 62. Tonneijck L, Muskiet MHA, Smits MM, et al. Postprandial renal haemodynamic effect of lixisenatide vs once‐daily insulin glulisine in patients with type 2 diabetes on insulin‐glargine: an 8‐week, randomised, open‐label trial. Diabetes Obes Metab. 2017;19:1669‐1680. [DOI] [PubMed] [Google Scholar]
- 63. Tonneijck L, Smits MM, Muskiet MH, et al. Acute renal effects of the GLP‐1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double‐blind, placebo‐controlled trial. Diabetologia. 2016;59:1412‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Packer M. Activation and inhibition of sodium‐hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017;136:1548‐1559. [DOI] [PubMed] [Google Scholar]


