Abstract
Melanoma of unknown primary (MUP) may have a different biology to melanoma of known primary, but clinical trials of novel therapies (e.g., immune checkpoint or BRAF/MEK inhibitors) have not reported the outcomes in this population. We therefore evaluated the overall survival (OS) among patients with MUP in the era of novel therapy. Data for stage III or IV MUP were extracted from a nationwide database for the period 2003–2016, with classification based on the eighth edition of the American Joint Committee on Cancer criteria. The population was divided into pre‐ (2003–2010) and post‐ (2011–2016) novel therapy eras. Also, OS in the post‐novel era was compared between patients with stage IV MUP by whether they received novel therapy. In total, 2028 of 65,110 patients (3.1%) were diagnosed with MUP. Metastatic sites were known in 1919 of 2028 patients, and most had stage IV disease (53.8%). For patients with stage III MUP, the 5‐year OS rates were 48.5% and 50.2% in the pre‐ and post‐novel eras, respectively (p = 0.948). For those with stage IV MUP, the median OS durations were unchanged in the pre‐novel era and post‐novel era when novel therapy was not used (both 4 months); however, OS improved to 11 months when novel therapy was used in the post‐novel era (p < 0.001). In conclusion, more than half of the patients with MUP are diagnosed with stage IV and the introduction of novel therapy appears to have significantly improved the OS of these patients.
Keywords: melanoma, unknown primary, immunotherapy, targeted therapy
Short abstract
What's new?
Melanoma of unknown primary (MUP) site may have a different biology to melanoma of known primary, but clinical trials of novel therapies (e.g., immune checkpoint or BRAF/MEK inhibitors) have not reported the outcomes in this population. Knowledge about outcomes could however aid clinical management of patients with MUP. In this nationwide study from 2003 to 2016, the authors show that the introduction of novel therapy has significantly improved the overall survival for patients with stage IV melanoma of unknown primary, who represented more than half of the patients diagnosed with MUP in the Netherlands.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CNS
central nervous system
- MKP
melanoma known primary
- MUP
melanoma unknown primary
- N/A
not applicable
- NCR
Netherlands Cancer Registry
- NOS
not otherwise specified
- nr
not reached
- OS
overall survival
Introduction
There is a continuing upward trend in melanoma incidence in many European countries, including the Netherlands.1 Approximately 3% of patients who newly present with melanoma are diagnosed with melanoma of unknown primary (MUP).2, 3 According to the eighth edition of the American Joint Committee on Cancer (AJCC) staging criteria, patients presenting with melanoma metastases in the (sub)cutis, soft tissue, and/or lymph nodes, without a detectable primary tumour, are diagnosed with stage III disease; by contrast, patients presenting with distant metastases, including visceral metastases, are diagnosed with stage IV disease.4
Previous research has demonstrated improved survival in patients with stage III and IV MUP compared to stage‐matched patients with melanoma of known primary (MKP).3, 5, 6, 7, 8 Therefore, it is conceivable that there is a difference in biology between MUP and MKP. It has also been hypothesised that primary melanomas remain undetected due to immune‐mediated spontaneous regression.9, 10 These research findings may have implications for patients with MUP in the current era of targeted therapy and immunotherapy.
Until 2011, treatment regimens for patients with advanced melanoma (unresectable stage IIIC or stage IV) usually consisted of chemotherapy (e.g., dacarbazine) and were of limited therapeutic benefit.11 Over the past decade, however, the introduction of novel therapies has dramatically improved survival.12 Since 2011, seven systemic novel therapies have been approved by the Food and Drug Administration and subsequently by the European Medicines Agency and the Dutch Medicines Evaluation Board for the treatment of advanced melanoma, and these are broadly grouped into immune checkpoint inhibitors (immunotherapy) and BRAF and/or MEK inhibitors (targeted therapy). The CTLA‐4 blocking antibody ipilimumab was the first immune checkpoint inhibitor to be approved in 2011, followed by the BRAF inhibitors vemurafenib (2012) and dabrafenib (2013) and the MEK inhibitor trametinib (2014). The PD‐1 blocking antibodies nivolumab and pembrolizumab were then approved in 2015, along with the combined BRAF/MEK inhibitors dabrafenib plus trametinib and vemurafenib plus cobimetinib. The combination of ipilimumab and nivolumab was approved in 2016. Of note, immunotherapy is available for all patients with advanced melanoma, irrespective of mutation status, whereas only patients with BRAF‐mutated melanomas are eligible for targeted therapy. Even though BRAF/MEK inhibitors are considered targeted therapies, yet these agents also appear to induce immune responses in melanoma.13
While clinical trials of novel therapy have included patients with MUP, the outcomes in these patients have not been reported specifically.14 Given the potential difference in biology compared to MKP, knowing the outcomes during novel therapy for patients with MUP could aid clinical decision‐making. In the current study, we aimed to investigate the incidence, presentation, and treatment of MUP in the Netherlands and to assess overall survival (OS) associated with MUP in the era of novel therapy.
Patients and Methods
Study design and population
This was an exploratory, population‐based observational study of all patients diagnosed with MUP between 2003 and 2016 for whom data were recorded in the Netherlands Cancer Registry (NCR).15 The NCR is embedded in the Netherlands Comprehensive Cancer Organisation and is linked annually to the Municipal Personal Records database to retrieve information on vital status. Cancer registration in the Netherlands is based on notification of all new malignancies by the nationwide automated pathological archive. The national registry of hospital discharge diagnoses is an additional source of patient identification, accounting for up to 8% of new cases. After notification, trained and certified registrars from the NCR retrieve the patient's medical records and check all diagnoses. Besides details on patient and primary tumour characteristics, the NCR records details on the morphology, topography, and location of metastases for all newly diagnosed malignancies according to the classifications in the International Classification of Diseases‐Oncology.16 The quality of the collected data is of a high standard thanks to computerised consistency checks and the reliance on trained and certified registrars who only obtain registration if they achieve a correctness score of ≥95%. Although details of first‐line treatment are recorded, details of secondary treatment, disease recurrence, and/or progression are not recorded.
Prior to this study, vital status had last been updated on 1st January 2018. Patients diagnosed with MUP were identified by morphological codes 872–879 (nevi and melanoma) in combination with topographical code C80.9 (unknown primary site).16 Data on year of diagnosis, age, gender, number and location of involved lymph nodes, location and number of metastases, first‐line treatment, and vital status were retrieved. First‐line treatment was categorised as follows: no therapy; local therapy, including surgical excision, radiation therapy, radiofrequency ablation, and isolated limb perfusion; chemotherapy, with/without concurrent local therapy; and novel therapy, including immunotherapy and/or targeted therapy, with/without concurrent local therapy and/or chemotherapy. Immunotherapy consisted of immune checkpoint inhibition with CTLA‐4 blockade (ipilimumab), PD‐1 blockade (either nivolumab or pembrolizumab), or combined CTLA‐4 and PD‐1 blockade (ipilimumab and nivolumab). Targeted therapy consisted of BRAF‐inhibition (vemurafenib or dabrafenib), MEK‐inhibition (trametinib) or combined BRAF and MEK inhibition (dabrafenib and trametinib or vemurafenib and cobimetinib).
Staging
Classification was based on the eight edition of the AJCC criteria: stage IIIB was diagnosed if there was one involved lymph node or cutaneous/subcutaneous metastasis; stage IIIC, if there was more than one involved lymph node or involved lymph node(s) plus cutaneous/subcutaneous metastasis; stage IV–M1a, if there was distant metastasis to skin, soft tissue (including muscle), and/or non‐regional lymph node(s); stage IV–M1b, if there was metastasis distant to the lungs, with or without concurrent M1a sites; stage IV–M1c, if there was metastasis distant to visceral sites, excluding the central nervous system (CNS), with or without concurrent M1a or M1b sites; and stage IV–M1d, if there was metastasis distant to the CNS with or without concurrent M1a, M1b, or M1c sites.4 If the number of involved lymph nodes was unknown, classification was based on the number of involved lymph node basins, with the presence of one nodal basin regarded as stage IIIB and the presence of more than one nodal basin regarded as stage IIIC. Involvement of intra‐thoracic or intra‐abdominal nodes was considered non‐regional nodal metastasis. In principle, parotid gland or submandibular gland involvement, and cervical, axillary, inguinal, or pelvic nodal involvement were each taken to indicate regional nodal metastasis, while breast involvement was considered to indicate regional soft tissue metastasis, unless anatomic distribution made regional coherence implausible (e.g., cervical nodal metastasis with a subcutaneous lesion on the arm). If the site of metastasis was unclear (C76: other and ill‐defined sites; or C80: unknown site), disease was classified based on other available information (e.g., involvement of lymph nodes, lungs, and an ‘overlapping lesion of ill‐defined sites’ were regarded as stage IV–M1b). If no other information was available, we labelled patients as ‘not otherwise specified (NOS)’ for the demographic analysis and excluded them from further analysis.
Patients with stage IV MUP were categorised according to the number of involved metastatic sites (≤2 versus >2). The following locations were regarded as distinct sites: cutis; subcutis/soft tissue; lymph nodes; pulmonary tract; heart/mediastinum; liver; gallbladder; pancreas; adrenal gland; spleen; upper gastrointestinal tract, including the oesophagus, stomach, and duodenum; lower gastrointestinal tract, including the small intestine, colon, sigmoid, rectum, and anus; retroperitoneum; peritoneum; urogenital; bone; head and neck, including tongue, tonsils, and (para)thyroid glands; and the CNS.
Statistical analysis
To evaluate the impact of novel therapy on OS, the population was divided into two eras: 2003–2010 (pre‐novel therapy era) and 2011–2016 (post‐novel therapy era). For the latter era, patients with stage IV MUP were further categorised into those who received novel therapy as a first‐line treatment (novel therapy group) and those who did not (no novel therapy group). In the novel therapy group, we also compared OS between patients who received first‐line therapy in 2011–2012, 2013–2014, and 2015–2016 and between those who received first‐line immunotherapy and those who received first‐line targeted therapy. Patients who received both agents as first‐line treatment were excluded from this analysis.
The proportion of patients with MUP relative to all newly diagnosed melanomas was determined. Univariable analysis consisted of Mann–Whitney U or Kruskal–Wallis tests for continuous variables and chi‐square or Fisher exact tests for categorical variables, as appropriate. Where data were missing or unknown, an ‘unknown’ subcategory was created for analysis. OS was calculated from the date of diagnosis to the date of last follow‐up or death. The Kaplan–Meier method was used to estimate survival, and differences between groups were assessed by the log‐rank test. The median follow‐up duration among survivors was calculated from the date of diagnosis to the date of last follow‐up using the reversed Kaplan–Meier method (deaths were censored). Multivariable cox regression analysis was performed to identify whether type of novel therapy was an independent prognostic factor for OS. Statistical analyses were performed using IBM SPSS for Windows, Version 24 (IBM Corp., Armonk, NY). Two‐sided p values of <0.05 were considered statistically significant.
Results
Patient characteristics
During the study period, 2028 of 65,110 patients (3.1%) were diagnosed with MUP in the Netherlands (Fig. 1 a). Information on the metastatic site was available for 1919 of these patients (94.6%), with most presenting with visceral metastasis (n = 999; 51.2%), followed by nodal involvement alone (n = 594; 31.0%), (sub)cutaneous involvement alone (n = 243; 12.7%), (sub)cutaneous and nodal involvement (n = 50; 2.6%), and distant (sub)cutaneous and/or nodal involvement (n = 33; 1.7%). Nodal metastasis was predominantly located in the axilla, affecting 253 of 644 patients (39.3%). The distributions of nodal and (sub)cutaneous metastases are illustrated in Figure S1, Supporting Information.
Figure 1.
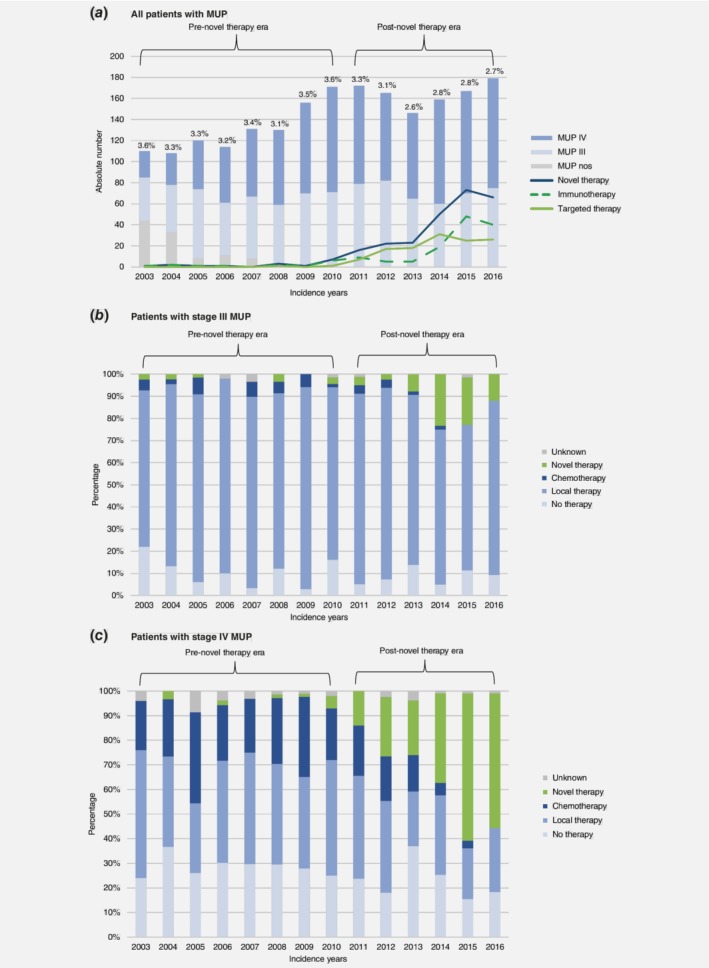
Overview of patients diagnosed with MUP in the Netherlands and corresponding changes in first‐line treatment use between 2003 and 2016. (a) the bar graph represents the absolute number of Dutch patients diagnosed with MUP during the study period, including those classified as stage III, stage IV, or not otherwise defined according to the eighth edition of the American Joint Committee on Cancer staging criteria. The curves show the numbers receiving novel therapy each year, while the percentages show the total percentages with MUP relative to all primary diagnoses of melanoma in the Netherlands for that year; (b) overview of first‐line treatment for patients in the Netherlands with stage III MUP; and (c) overview of first‐line treatment for patients in the Netherlands with stage IV MUP. Abbreviations: MUP, melanoma unknown primary. [Color figure can be viewed at http://wileyonlinelibrary.com]
Comparison by disease stage
According to the eight edition of the AJCC criteria, 887 patients had stage III disease (46.2%) and 1,032 patients had stage IV disease (53.8%) (Tables 1 and 2). The proportion of patients with MUP who presented with stage IV disease increased from 37.9% to 58.1% between 2003 and 2016. In patients with stage III and IV disease, the median follow‐up durations were 85 months (interquartile range [IQR] 51–126 months) and 47 months (IQR 30–85 months), respectively.
Table 1.
Patient, tumour, and treatment characteristics by era among patients with stage III MUP
| Pre‐novel therapy era 2003–2010 | Post‐novel therapy era 2011–2016 | ||
|---|---|---|---|
| Characteristics | (n = 456) | (n = 431) | p Value1 |
| Age2 | 62 (48–72) | 64 (53–74) | 0.0093 |
| Gender | 0.686 | ||
| Male | 252 (55.3) | 244 (56.6) | |
| Female | 204 (44.7) | 187 (43.4) | |
| Substage III | 0.897 | ||
| IIIB | 389 (85.3) | 369 (85.6) | |
| IIIC | 67 (14.7) | 62 (14.4) | |
| Presentation stage III | 0.324 | ||
| (Sub)cutaneous only | 115 (25.2) | 128 (29.7) | |
| Nodal only | 315 (69.1) | 279 (64.7) | |
| (Sub)cutaneous + nodal | 26 (5.7) | 24 (5.6) | |
| Treatment strategy | <0.001 | ||
| No therapy | 46 (10.1) | 37 (8.6) | |
| Local therapy | 379 (83.1) | 336 (78.0) | |
| Chemotherapy | 20 (4.4) | 8 (1.9) | |
| Novel therapy | 7 (1.5) | 48 (11.1) | |
| Unknown | |||
| Novel therapy type | 0.142 | ||
| Immunotherapy | 6 (85.7) | 22 (45.8) | |
| Targeted therapy | 1 (14.3) | 25 (52.1) | |
| Both | 0 | 1 (2.1) |
Values in parentheses are percentages, unless otherwise indicated. Abbreviations: MUP, metastasis of unknown primary.
Chi‐square test;
Values are median (interquartile range);
Mann–Whitney U test.
Table 2.
Patient, tumour, and treatment characteristics by era and therapy type among patients with stage IV MUP
| Pre‐novel therapy era 2003–2010 | Post‐novel therapy era 2011–2016 | |||
|---|---|---|---|---|
| Characteristics | (n = 475) | No novel (n = 355) | Novel (n = 202) | p Value1 |
| Age2 | 60 (49–71) | 65 (55–73) | 62 (51–69) | 0.0093 |
| Gender | 0.561 | |||
| Male | 287 (60.4) | 224 (63.1) | 130 (64.4) | |
| Female | 188 (39.6) | 131 (36.9) | 72 (35.6) | |
| Substage IV | 0.043 | |||
| IV–M1a | 20 (4.2) | 9 (2.5) | 4 (2.0) | |
| IV–M1b | 84 (17.7) | 55 (15.5) | 20 (9.9) | |
| IV–M1c | 215 (45.3) | 150 (42.3) | 99 (49.0) | |
| IV–M1d | 156 (32.8) | 141 (39.7) | 79 (39.1) | |
| Metastatic sites stage IV | <0.001 | |||
| ≤2 sites | 352 (74.1) | 268 (75.5) | 96 (47.5) | |
| >2 sites | 123 (25.9) | 87 (24.5) | 106 (52.5) | |
| Treatment strategy | n/a | |||
| No therapy | 134 (28.2) | 126 (35.5) | 0 | |
| Local therapy | 196 (41.3) | 167 (47.0) | 0 | |
| Chemotherapy | 123 (25.9) | 54 (15.2) | 0 | |
| Novel therapy | 9 (1.9) | 0 | 202 (100) | |
| Unknown | 13 (2.7) | 8 (2.3) | 0 | |
| Novel therapy type | 0.0664 | |||
| Immunotherapy | 8 (88.9) | 0 | 94 (46.5) | |
| Targeted therapy | 1 (11.1) | 0 | 99 (49.0) | |
| Both | 0 | 0 | 9 (4.5) | |
Values in parentheses are percentages unless indicated otherwise. Abbreviations: MUP, metastasis of unknown primary.
Chi‐square test;
Values are median (interquartile range);
Kruskal–Wallis test;
Fisher's exact test.
In the post‐novel therapy era, 41 of 431 patients with stage III MUP (9.5%) and 202 of 557 patients with stage IV MUP (36.3%) received novel therapy first‐line. Patients with stage III MUP were typically treated with local therapies throughout the study (715 of 887 patients, 80.6%). Among patients with stage IV MUP, chemotherapy was used as a first‐line treatment in 123 of 475 patients (25.9%) in the pre‐novel therapy era and in 54 of 557 patients (9.7%) in the post‐novel therapy era; its use was not recorded at all for 2016. The details of first‐line treatment, by stage, are depicted for each year in Figures 1 b and 1 c.
Survival
For patients with stage III MUP, the 5‐year OS rates in the pre‐ and post‐novel therapy eras were 48.5% (standard error, 2.3%) and 50.2% (standard error, 2.8%), respectively (p = 0.948) (Fig. 2 a). For patients with stage IV MUP, the median OS durations were 4 months (IQR 2–11) in the pre‐novel therapy era and 4 months (IQR 2–16) in the post‐novel era when not using novel therapy; however, this improved to 11 months (IQR 6–31) in the post‐novel era when using novel therapy (p < 0.001) (Fig. 2 b). When this latter era was subdivided by year into 2011–2012, 2013–2014, and 2015–2016, the median OS durations were 8 months (IQR 6–14), 8 months (IQR 5–18), and 16 months (IQR 6–31), respectively (p < 0.001) (Fig. 2 c). In all subgroups, the median OS for patients with stage IV MUP was superior for those receiving novel therapy compared to those not receiving novel therapy (Table 3).
Figure 2.
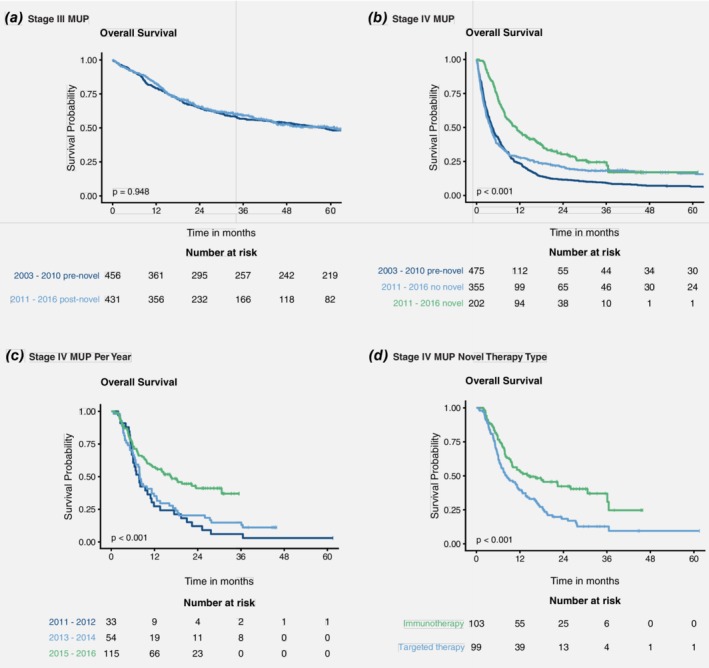
Overall survival rates by stage, treatment era, and treatment type. Overall survival rates are shown for the following patient groups: (a) patients with stage III MUP in the pre‐ and post‐novel therapy eras; (b) patients with stage IV MUP in the pre‐ and post‐novel therapy eras, including those with and without novel therapy in the post‐novel era; (c) patients with stage IV MUP who received novel therapy in 2011–2012, 2013–2014, and 2015–2016 in the post‐novel therapy era; and (d) patients with stage IV MUP who received immunotherapy or targeted therapy first‐line in the post‐novel era. Abbreviations: MUP, melanoma unknown primary. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Median OS compared between patients with stage IV MUP by whether they received novel therapy
| Novel therapy era, 2011–2016 | |||
|---|---|---|---|
| Subgroups | No novel therapy | Novel therapy | p Value1 |
| Age | |||
| ≤65 years | 4 (2–42) | 10 (6–37) | 0.022 |
| >65 years | 4 (2–9) | 12 (6–31) | <0.001 |
| Metastatic sites | |||
| ≤2 sites | 5 (2–23) | 12 (6–nr) | 0.001 |
| >2 sites | 3 (1–6) | 10 (5–31) | <0.001 |
| CNS metastasis | |||
| No | 4 (2–20) | 14 (6–37) | <0.001 |
| Yes | 4 (2–14) | 8 (5–20) | 0.021 |
All data are shown in months. Values in parentheses are the interquartile ranges. Abbreviations: CNS, central nervous system; nr, not reached; MUP, metastasis of unknown primary; OS, overall survival.
Log‐rank test.
For nine patients, both immunotherapy and targeted therapy were recorded as first‐line therapy in the post‐novel era, so these patients were excluded from further analysis. When patients with stage IV MUP received first‐line treatment with immunotherapy or targeted therapy, the median OS rates were 18 months (IQR 7 to ‘not reached’) and 8 months (IQR 5–18), respectively (p < 0.001) (Fig. 2 d). Multivariable analysis of the factors affecting OS indicated that first‐line immunotherapy remained a more favourable prognostic factor compared to first‐line targeted therapy when adjusted for year (2011–2012, 2013–2014, and 2015–2016), gender, age, number of metastatic sites (≤ 2 or > 2), and the presence of CNS metastasis (no or yes) (Table 4).
Table 4.
Multivariable analysis for OS in patients with stage IV MUP receiving first‐line novel therapy (n = 193)
| Variables | Hazard ratio (95% CI) | p Value |
|---|---|---|
| Incidence years | ||
| 2011–2012 | Reference | |
| 2013–2014 | 0.85 (0.53–1.36) | 0.491 |
| 2015–2016 | 0.44 (0.27–0.72) | 0.001 |
| Age | 1.01 (0.99–1.02) | 0.269 |
| Gender | ||
| Male | Reference | |
| Female | 0.92 (0.64–1.31) | 0.632 |
| Metastatic sites | ||
| ≤2 sites | Reference | |
| >2 sites | 1.31 (0.92–1.86) | 0.135 |
| CNS metastasis | ||
| No | Reference | |
| Yes | 1.63 (1.14–2.33) | 0.008 |
| First‐line novel therapy | ||
| Targeted therapy | Reference | |
| Immunotherapy | 0.64 (0.44–0.94) | 0.021 |
Abbreviations: CNS, central nervous system; MUP, melanoma unknown primary; OS, overall survival.
Discussion
The absolute number of Dutch patients diagnosed with MUP increased between 2003 and 2016, but the relative percentage compared to all newly diagnosed melanomas remained stable at approximately 3%. More than half of the patients presented with visceral metastases during this period and were diagnosed with stage IV MUP. However, 2006 was associated with a change in disease incidence, with stage III MUP being most common before 2006 and the relative incidence of stage IV MUP increasing to be higher in the subsequent years. This stage migration might be explained by the increased use of whole‐body positron emission tomography, which in turn, might have increased the early detection of visceral metastases. In contrast to this result, previous studies have shown that patients with MUP tend to present with nodal involvement alone and less often with visceral metastasis.17, 18 This inconsistency may be explained by issues with the earlier studies, which used small sample sizes (65–88 patients) and data from single specialist centres for melanoma care. In contrast, we used a representative large Dutch nationwide database.
Currently, monotherapy with PD‐1 blockade (pembrolizumab or nivolumab) or PD‐1 blockade combined with CTLA‐4 blockade (nivolumab and ipilimumab) are the preferred options for immunotherapy. The combination of BRAF and MEK inhibitors (dabrafenib and trametinib or vemurafenib and cobimetinib) are the preferred options for targeted therapy. In clinical trials, these immunotherapy and targeted therapy approaches have reported median OS durations that exceed 30 months and 20 months, respectively.19 Although there are no specific treatment recommendations for patients with MUP, physicians tend to apply similar strategies for patients with stage‐matched MKP.20 This approach is supported by the results of a large study into the molecular characterisation of patients diagnosed with MUP, in which it was shown that the clinical behaviours and molecular patterns of BRAF/NRAS alterations were similar between patients with MUP and stage‐matched MKP.21
The percentage of Dutch patients diagnosed with stage IV MUP who were primarily treated with novel therapy increased considerably during the study period. Consistent with the respective approval dates, targeted therapies (approved 2012–2015) were prescribed at higher rates early in the novel therapy era, whereas immunotherapies were prescribed at higher rates later in that era (approved 2011 and 2015–2016). Parallel to this, the percentage of patients receiving chemotherapy decreased over time and was no longer prescribed by 2016. Together with the observed stage migration, these changes may have contributed to the significantly improved OS from 4 to 11 months among patients with stage IV MUP. Median OS durations even increased to 16 months for patients receiving first‐line novel therapy in 2015–2016. Indeed, improvement was observed in all subgroups, including those presumed to have worse outcomes (e.g., >2 metastatic sites or CNS metastasis). Patients who received first‐line immunotherapy also showed a superior OS compared to those who received first‐line targeted therapy, even after adjustment for year of presentation, age and gender, number of metastatic sites, and presence of CNS metastasis.
Given the hypothesis that MUP is a distinct biologic entity and the current exploratory results, one might suggest that immunotherapy may be considered the preferred first‐line treatment for patients with stage IV MUP. However, the possibility of selection bias combined with the retrospective design denotes that such a conclusion should be interpreted with caution. Unfortunately, it was not possible to adjust for other potential confounding factors, such as the serum lactate dehydrogenase level, performance status, or BRAF mutation status because these factors were not recorded in the NCR. The optimal first‐line treatment strategy for patients with BRAF‐mutated melanoma has yet to be determined, as no randomised clinical trial has compared immunotherapy with targeted therapy for these patients.22
We observed no benefit in OS for patients with stage III MUP after the introduction of novel therapies. This was expected because, in principle, only patients with unresectable stage IIIC were eligible to receive novel therapy. Very few patients with stage III MUP were registered in the NCR as having received novel therapy, with some of these patients even classified with stage IIIB disease. This could be explained by the likely underestimation of stage IIIC disease in our study, resulting from a lack of information about the number of involved lymph nodes in a considerable number of patients. Currently, the effective novel therapies are introduced in the adjuvant setting as routine care.23, 24, 25, 26, 27 It is therefore likely that the survival of patients with stage III disease will significantly improve in the near future.
In this large population‐based study, we reported the OS for patients with MUP since the introduction of immunotherapy and targeted therapy. A recent small, single‐center study has also evaluated survival in patients with MUP who received novel therapy and showed that 23 patients had a median OS of 9 months after initiating immunotherapy.14 In an accompanying systematic review, the authors only identified three other papers reporting on patients with MUP who received immunotherapy (a phase II study, a retrospective cohort study, and one case report) and seven papers concerning targeted therapy (six case reports and one small prospective observational study reporting on one patient). The phase II single‐arm study reported a median OS duration of 9.9 months for patients with MUP who received ipilimumab.28 Other studies have reported on the survival in patients with stage III and IV MUP, but they have not specifically addressed treatment with novel agents.2, 5, 29
There are several limitations that must be considered when interpreting our data. Notably, the retrospective design is associated with inherent biases and must not be discounted. One example is selection bias. To partly overcome this issue, we divided the stage IV MUP population into three groups: pre‐novel therapy era, 2011–2016 no novel therapy and 2011–2016 novel therapy. This division takes into account that patients in the pre‐novel therapy era did not receive first‐line novel therapy due to unavailability whereas patients in the 2011–2016 no novel therapy group did not receive first‐line novel therapy due to unknown other reasons. In addition, patients in the 2011–2016 no novel therapy group might have received novel therapy as a second‐line treatment, which might also have influenced overall survival. Another limitation is the possible misclassification of MUP, because there was no record of how many patients met the exclusion criteria proposed in 1963 by Das Gupta.30 These include prior orbital exenteration or enucleation; evidence of a scar in the area of a positive lymph node; or prior skin excision, electrodessication, cauterization, or other surgical manipulation of a mole, freckle, birthmark, paronychia, or skin blemish. However, although we do not know the misclassification rate, we assume that it will have been low because of the high quality of recorded data and the observation that previous authors have reported a comparable incidence (approximately 3%).2, 3 A final limitation is that the NCR does not register disease recurrence and/or progression and only records the primary treatment, meaning that we are unaware of later changes in who received novel therapy.
In conclusion, this nation‐wide study showed that the incidence of MUP was approximately 3% and that this has remained largely unchanged over time. Throughout the study period, more than half of the patients with MUP presented with distant metastases and were diagnosed with stage IV MUP. We observed marked improvements in OS associated with the use of targeted therapy and immunotherapy in patients with stage IV MUP. These findings are highly relevant to clinical practice given the greater availability of novel therapy, and we anticipate continued improvements in OS should these trends persist, even among patients traditionally expected to have worse outcomes. To better understand the aetiology of MUP, additional (confirmatory) studies are needed that report on this particular type of melanoma in the era of novel therapies, and preferably that compare outcomes between patients with MUP and stage‐matched MKP during novel therapy.
Supporting information
Figure S1 Anatomic distribution of (sub)cutaneous tissue and lymph node involvement in patients with stage III MUP
Involved (sub)cutaneous tissue and lymph nodes are shown by green triangles and blue circles, respectively. Abbreviations: MUP, melanoma unknown primary.
Acknowledgements
We thank Dr Robert Sykes (http://www.doctored.org.uk) for providing editorial services. No funding to declare for this study.
Conflict of interest: D Verver: consultancy/advisory board for Amgen, unrelated to this work. AAM van der Veldt: honoraria for MSD, BMS, Pfizer, Eisai, Roche, Novartis, Sanofi, and Ipsen; consultancy for MSD, BMS, Pfizer, Eisai, Roche, Novartis, Sanofi, and Ipsen; all unrelated to this work. ACJ van Akkooi: consultancy/advisory role for Amgen, Bristol‐Meyers Squibb, Novartis, MSD‐Merck, Merck‐Pfizer, and Roche; funding from Amgen and Novartis; all unrelated to this work. DJ Grunhagen: honoraria for Amgen, BMS, and Abbvie, all unrelated to this work. All other authors declare that they have no other potential or real competing interests.
The findings of this study have been presented at the European Organization for Research and Treatment of Cancer (EORTC)‐melanoma group annual fall meeting in Mallorca on October 4, 2018 and in the form of a poster at the European Society of Surgical Oncology (ESSO) congress in Budapest on the October 10, 2018.
References
- 1. Sacchetto L, Zanetti R, Comber H, et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer 2018;92:108–18. [DOI] [PubMed] [Google Scholar]
- 2. Kamposioras K, Pentheroudakis G, Pectasides D, et al. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol 2011;78:112–26. [DOI] [PubMed] [Google Scholar]
- 3. de Waal AC, Aben KK, van Rossum MM, et al. Melanoma of unknown primary origin: a population‐based study in The Netherlands. Eur J Cancer 2013;49:676–83. [DOI] [PubMed] [Google Scholar]
- 4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin 2017;67:472–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bae JM, Choi YY, Kim DS, et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta‐analysis of observational studies. J Am Acad Dermatol 2015;72:59–70. [DOI] [PubMed] [Google Scholar]
- 6. Prens SP, van der Ploeg AP, van Akkooi AC, et al. Outcome after therapeutic lymph node dissection in patients with unknown primary melanoma site. Ann Surg Oncol 2011;18:3586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Ploeg AP, Haydu LE, Spillane AJ, et al. Melanoma patients with an unknown primary tumor site have a better outcome than those with a known primary following therapeutic lymph node dissection for macroscopic (clinically palpable) nodal disease. Ann Surg Oncol 2014;21:3108–16. [DOI] [PubMed] [Google Scholar]
- 8. Lee CC, Faries MB, Wanek LA, et al. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol 2009;27:3489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott JF, Gerstenblith MR. Melanoma of unknown primary In: Scott JF, Gerstenblith MR, eds Noncutaneous Melanoma. Brisbane: Codon Publications, 2018. [PubMed] [Google Scholar]
- 10. Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology 1992;20:315–22. [DOI] [PubMed] [Google Scholar]
- 11. Eigentler TK, Caroli UM, Radny P, et al. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 2003;4:748–59. [DOI] [PubMed] [Google Scholar]
- 12. Luke JJ, Flaherty KT, Ribas A, et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–82. [DOI] [PubMed] [Google Scholar]
- 13. Ascierto PA, Dummer R. Immunological effects of BRAF+MEK inhibition. Oncoimmunology 2018;7:e1468955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Utter K, Goldman C, Weiss SA, et al. Treatment outcomes for metastatic melanoma of unknown primary in the new era: a single‐institution study and review of the literature. Oncology 2017;93:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Netherlands Comprehensive Cancer Registration (IKNL) . Cijfers over Kanker. Nederlandse Kankerregistratie 2018;8:2017. [Google Scholar]
- 16. Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology (ICD‐O) ‐ 3rd edition, 1st Revision. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 17. Katz KA, Jonasch E, Hodi FS, et al. Melanoma of unknown primary: experience at Massachusetts General Hospital and Dana‐Farber Cancer Institute. Melanoma Res 2005;15:77–82. [DOI] [PubMed] [Google Scholar]
- 18. Savoia P, Fava P, Osella‐Abate S, et al. Melanoma of unknown primary site: a 33‐year experience at the Turin melanoma Centre. Melanoma Res 2010;20:227–32. [PubMed] [Google Scholar]
- 19. Rozeman EA, Dekker TJA, Haanen J, et al. Advanced melanoma: current treatment options, biomarkers, and future perspectives. Am J Clin Dermatol 2018;19:303–17. [DOI] [PubMed] [Google Scholar]
- 20. Ribero S, Pampena R, Bataille V, et al. Unknown primary melanoma: worldwide survey on clinical management. Dermatology 2016;232:704–7. [DOI] [PubMed] [Google Scholar]
- 21. Gos A, Jurkowska M, van Akkooi A, et al. Molecular characterization and patient outcome of melanoma nodal metastases and an unknown primary site. Ann Surg Oncol 2014;21:4317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ugurel S, Rohmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies‐update 2017. Eur J Cancer 2017;83:247–57. [DOI] [PubMed] [Google Scholar]
- 23. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 24. Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF‐mutated melanoma. N Engl J Med 2017;377:1813–23. [DOI] [PubMed] [Google Scholar]
- 25. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–801. [DOI] [PubMed] [Google Scholar]
- 26. Eggermont AM, Chiarion‐Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with Ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hauschild A, Dummer R, Schadendorf D, et al. Longer follow‐up confirms relapse‐free survival benefit with adjuvant Dabrafenib plus Trametinib in patients with resected BRAF V600‐mutant stage III melanoma. J Clin Oncol 2018;36:3441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zimmer L, Eigentler TK, Kiecker F, et al. Open‐label, multicenter, single‐arm phase II DeCOG‐study of ipilimumab in pretreated patients with different subtypes of metastatic melanoma. J Transl Med 2015;13:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott JF, Conic RZ, Thompson CL, et al. Stage IV melanoma of unknown primary: a population‐based study in the United States from 1973 to 2014. J Am Acad Dermatol 2018;79:258–265.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dasgupta T, Bowden L, Berg JW. Malignant melanoma of unknown primary origin. Surg Gynecol Obstet 1963;117:341–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Anatomic distribution of (sub)cutaneous tissue and lymph node involvement in patients with stage III MUP
Involved (sub)cutaneous tissue and lymph nodes are shown by green triangles and blue circles, respectively. Abbreviations: MUP, melanoma unknown primary.


