Abstract
Objective
Cannabidiol (CBD) has been approved by the US Food and Drug Administration (FDA) to treat intractable childhood epilepsies, such as Dravet syndrome and Lennox‐Gastaut syndrome. However, the intrinsic anticonvulsant activity of CBD has been questioned due to a pharmacokinetic interaction between CBD and a first‐line medication, clobazam. This recognized interaction has led to speculation that the anticonvulsant efficacy of CBD may simply reflect CBD augmenting clobazam exposure. The present study aimed to address the nature of the interaction between CBD and clobazam.
Methods
We examined whether CBD inhibits human CYP3A4 and CYP2C19 mediated metabolism of clobazam and N‐desmethylclobazam (N‐CLB), respectively, and performed studies assessing the effects of CBD on brain and plasma pharmacokinetics of clobazam in mice. We then used the Scn1a +/− mouse model of Dravet syndrome to examine how CBD and clobazam interact. We compared anticonvulsant effects of CBD‐clobazam combination therapy to monotherapy against thermally‐induced seizures, spontaneous seizures and mortality in Scn1a +/− mice. In addition, we used Xenopus oocytes expressing γ‐aminobutyric acid (GABA)A receptors to investigate the activity of GABAA receptors when treated with CBD and clobazam together.
Results
CBD potently inhibited CYP3A4 mediated metabolism of clobazam and CYP2C19 mediated metabolism of N‐CLB. Combination CBD‐clobazam treatment resulted in greater anticonvulsant efficacy in Scn1a +/− mice, but only when an anticonvulsant dose of CBD was used. It is important to note that a sub‐anticonvulsant dose of CBD did not promote greater anticonvulsant effects despite increasing plasma clobazam concentrations. In addition, we delineated a novel pharmacodynamic mechanism where CBD and clobazam together enhanced inhibitory GABAA receptor activation.
Significance
Our study highlights the involvement of both pharmacodynamic and pharmacokinetic interactions between CBD and clobazam that may contribute to its efficacy in Dravet syndrome.
Keywords: cannabidiol, cannabis, clobazam, Dravet syndrome, epilepsy
Key Points.
We explored pharmacodynamic and pharmacokinetic interactions between cannabidiol (CBD) and clobazam in a mouse model of Dravet syndrome
Co‐treatment of CBD and clobazam had greater anticonvulsant efficacy, but only when an anticonvulsant dose of CBD was used
A sub‐anticonvulsant dose of CBD did not potentiate the effects of clobazam despite the presence of a pharmacokinetic interaction
We report a novel pharmacodynamic mechanism where CBD and clobazam together enhanced inhibitory γ‐aminobutyric acid (GABA)A receptor activation
Both pharmacodynamic and pharmacokinetic mechanisms likely contribute to the anticonvulsant efficacy of CBD and clobazam co‐treatment
1. INTRODUCTION
Cannabidiol (CBD), a major constituent of hemp cannabis varieties, is of increasing therapeutic interest due to the global rise of medicinal cannabis. Recently, the US Food and Drug Administration (FDA) approved a purified preparation of CBD, Epidiolex, for the treatment of two severe, intractable childhood epilepsies: Dravet syndrome and Lennox‐Gastaut syndrome. The approval was supported by four Phase III randomized controlled trials (RCTs), where CBD, given as an adjunct to conventional antiepileptic drugs (AEDs), significantly reduced seizure frequency in patients with childhood epilepsy.1, 2, 3, 4
Dravet syndrome is a rare pediatric encephalopathy that typically presents during infancy with seizures provoked by fever, subsequently progressing to pleomorphic afebrile seizure types.5, 6 Children with Dravet syndrome exhibit behavioral disturbances and an increased risk for sudden unexplained death in epilepsy (SUDEP).7 Thus, the introduction of CBD in the management of Dravet syndrome offers new hope for improved therapeutic outcomes for patients afflicted by this devastating condition.8 The adjunctive nature of CBD use in Dravet syndrome means that it is administered concomitantly with other AEDs. In the recent clinical trials, patients were taking a median of three conventional AEDs, the most common being clobazam used in 66% of patients.1
A drug‐drug interaction between CBD and clobazam has been identified, where CBD increased plasma concentrations of both clobazam and its active metabolite, N‐desmethylclobazam (N‐CLB).9, 10, 11 Two key studies reported that CBD increased the steady‐state concentrations of clobazam and N‐CLB in children with refractory epilepsy, by approximately 60% and 500%, respectively.9, 11 The pharmacokinetic interaction between CBD and clobazam has led to repeated suggestions that the anticonvulsant efficacy of CBD may be overstated and its efficacy is merely explained by CBD enhancing clobazam and N‐CLB exposure.12, 13, 14, 15 The existing clinical studies were not specifically designed to test this hypothesis, which would require a group given CBD without any concomitant AEDs. Furthermore, the issue has not been solved by subgroup analyses of CBD efficacy in patients taking clobazam vs those who were not, as such analyses are confounded by a range of factors, including CBD having interactions with other AEDs that patients may have been administered.15, 16 For instance, stiripentol, which was frequently coadministered in the Devinsky et al (2016) trial (49% in the CBD group), is also well established to increase plasma clobazam and N‐CLB concentrations.17, 18
Preclinical research may provide the level of precision and control required to illuminate the nature of the interaction between CBD and clobazam in treating Dravet syndrome. The present study aimed to achieve this using a variety of in vitro and in vivo approaches, including use of a genetic mouse model of Dravet syndrome. De novo heterozygous loss‐of‐function SCN1A mutations are found in more than 80% of Dravet syndrome patients,19 and heterozygous null Scn1a +/− mice recapitulate the characteristic phenotypes of Dravet syndrome, including febrile seizures, spontaneous seizures, and premature death.20 In the current study, we examined how CBD and clobazam interacted against thermally‐ induced seizures, spontaneous seizures, and mortality in the Scn1a +/− mice. In addition, we examined CBD and clobazam pharmacokinetic interactions in vivo using wildtype mice and in vitro using human recombinant enzymes, to characterize the effect of CBD on cytochrome P450 (CYP)–mediated metabolism of clobazam.
Our group has recently reported that CBD is a positive allosteric modulator of γ‐aminobutyric acid (GABA)A receptors,21 similar to the well‐known action of benzodiazepine drugs such as clobazam. It is, therefore, possible that a pharmacodynamic interaction occurs, where co‐application of CBD further enhances the activity of GABAA receptors beyond the actions of clobazam and N‐CLB alone. This was investigated using Xenopus oocytes expressing GABAA receptors.
2. METHODS
All animal care and procedures were approved by the University of Sydney Animal Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. In vitro and in vivo pharmacokinetic experiments were conducted as described in Supporting Information (SI) Methods. Scn1a +/− mice were generated and used for hyperthermia‐induced and spontaneous seizure experiments as described previously in SI Methods.20, 22 GABAA recordings were conducted as described previously and methods are presented in SI Methods.23, 24
3. RESULTS
3.1. CBD inhibits clobazam metabolism and alters pharmacokinetic parameters of clobazam and N‐CLB in mice
CBD inhibits CYP3A4 and CYP2C19, the isoforms responsible for the metabolism of clobazam and N‐CLB, respectively. We characterized interactions between CBD and clobazam and N‐CLB in vitro using SupersomesTM expressing human CYP3A4 or CYP2C19. CBD inhibited CYP3A4‐mediated metabolism of clobazam to N‐CLB with a K i value of 139 nmol/L (95% confidence interval [95% CI] 103‐187 nmol/L; Figure 1A). N‐CLB hydroxylation via CYP2C19 was less potently inhibited (K i 2.6 μmol/L) by CBD (95% CI 1.7‐4.2 μmol/L; Figure 1B, Table S1).
Figure 1.
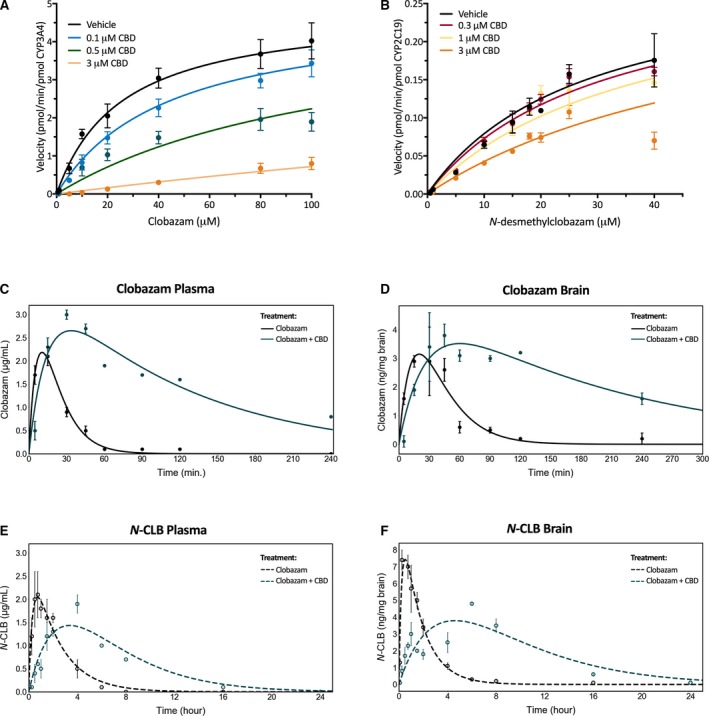
Cannabidiol (CBD) inhibits the metabolism of clobazam and its active metabolite, N‐desmethylclobazam (N‐ CLB). Substrate‐velocity curves of (A) CYP3A4‐mediated metabolism of clobazam and (B) CYP2C19‐mediated metabolism of N‐ CLB in the presence of varying concentrations of CBD. Error bars represent standard error of the mean (SEM), with n = 3. Clobazam (5 mg/kg, i.p. injection) was administered in the absence (black) and presence (green‐blue) of 12 mg/kg CBD (i.p.). Concentration‐time curves for clobazam (solid lines) in mouse (C) plasma and (D) brain and N‐ CLB (dashed lines) in (E) plasma and (F) brain. Error bars represent SEM, with n = 4 per time point. Curves represent fit of data using a two‐compartment model
We then determined the effect of CBD on the pharmacokinetic parameters of clobazam and N‐CLB in vivo. Wildtype mice were administered clobazam with or without CBD and pharmacokinetic profiles were generated for clobazam and N‐CLB in plasma and brain (Figure 1C,D). The dose of CBD (12 mg/kg) used was chosen to produce a plasma concentration (411 ± 87 ng/mL) consistent with those observed in patients with childhood epilepsy (100‐800 ng/mL).9 CBD treatment caused a significant drug‐drug interaction, significantly delaying T max values, prolonging the half‐lives (t ½), and increasing the total clobazam and N‐CLB exposure (AUC, area under the curve) (Table 1). Consistent with CBD potently inhibiting clobazam metabolism by CYP3A4, CBD prolonged the t½ of clobazam by 3.5‐5‐fold in brain and plasma, respectively (plasma: 15 minutes vs 78 minutes; brain: 24 vs 81 minutes). The prolonged t½ of clobazam resulted in approximately a 6 times greater drug exposure (plasma: 50 vs 310 μg min/mL; brain: 127 vs 820 ng min/mg brain). In addition, the longer t½ of clobazam delayed N‐CLB reaching C max (longer T max). Although there was no difference in the t½ of N‐CLB in either plasma or brain, there was greater drug exposure with CBD co‐medication (plasma: 351 vs 584 μg min/mL; brain: 778 vs 2103 ng min/mg brain) resulting from the delayed T max values. Notably, the brain‐to‐plasma ratio of N‐CLB increased from 2.2 to 3.6 when CBD was administered adjunctive to clobazam, suggesting that CBD may be inhibiting the efflux of N‐CLB from the brain.
Table 1.
Pharmacokinetics of clobazam and metabolite (N‐desmethylclobazam) in mouse plasma and brain samples
| Treatment | Clobazam | N‐desmethylclobazam | ||
|---|---|---|---|---|
| Clobazam | Clobazam + CBD | Clobazam | Clobazam + CBD | |
| Plasma | ||||
| C max (μg/mL) | 2.1 ± 0.2 | 2.1 ± 0.5 | 3.0 ± 0.4 | 1.9 ± 0.2 |
| t max (min) | 15 | 45 | 30 | 240 |
| t ½ (95% CI) (min) | 15 (10‐21) | 78 (61‐105) | 123 (89‐198) | 189 (155‐242) |
| AUC∞ (μg min/mL) | 50 | 310 | 351 | 584 |
| Brain | ||||
| C max (ng/mg brain) | 2.9 ± 1.2 | 7.4 ± 0.6 | 3.8 ± 0.2 | 4.8 ± 0.1 |
| t max (min) | 15 | 15 | 45 | 360 |
| t ½ (95% CI) (min) | 24 (17‐44) | 81 (70‐96) | 166 (112‐321) | 171 (133‐241) |
| AUC∞ (ng min/mg brain) | 127 | 820 | 778 | 2103 |
| Brain/plasma ratio | 2.5 | 2.6 | 2.2 | 3.6 |
95% Confidence Interval (95% CI) shown italicized in parentheses.
3.2. CBD and clobazam coadministration improves survival of Scn1a +/− mice
We then determined whether combination CBD and clobazam treatment produced a greater anticonvulsant effect against spontaneous seizures and improved survival. Dravet syndrome typically presents with febrile seizures that progress to other afebrile seizure types including spontaneous generalized tonic‐clonic seizures (GTCS).5 In addition, patients with Dravet syndrome have a reduced lifespan. We used Scn1a +/− mice primed with a hyperthermia‐induced seizure trigger to model this seizure progression and determined whether CBD increased the anticonvulsant effects of clobazam against spontaneous seizures and survival. A single thermal GTCS was induced at postnatal day 18 (P18) and subsequent spontaneous GTCS frequency and survival were measured. Treatment with both drugs supplemented in chow began after thermally‐induced GTCS. The dose of CBD used (3500 mg/kg chow) resulted in a steady‐state plasma concentration of 598 ± 165 ng/mL (Table 2), consistent with CBD levels observed in patients.9
Table 2.
Plasma concentrations from spontaneous seizure experiments
| Dose (mg/kg chow) | Clobazam (μg/mL) | N‐CLB (μg/mL) | CBD (ng/mL) | |
|---|---|---|---|---|
| Clobazam | 80 | 0.0 ± 0.0 | 0.4 ± 0.1 | n/a |
| Clobazam + CBD | 80 + 3500 | 0.0 ± 0.0 | 1.6 ± 0.2 | 482 ± 83 |
| CBD | 3500 | n/a | n/a | 598 ± 165 |
Clobazam treatment was anticonvulsant against spontaneous seizures, with only 28% of clobazam‐treated mice experiencing GTCS compared to 82% of untreated controls (P = 0.0012; Figure 2A). CBD treatment alone had no effect on spontaneous seizures (P = 0.1605; Figure 2A). CBD and clobazam co‐treatment lowered the proportion (16%) of mice experiencing spontaneous GTCS compared to controls (P < 0.0001) or clobazam alone (28%), although the latter comparison failed to reach statistical significance (P = 0.3760).
Figure 2.
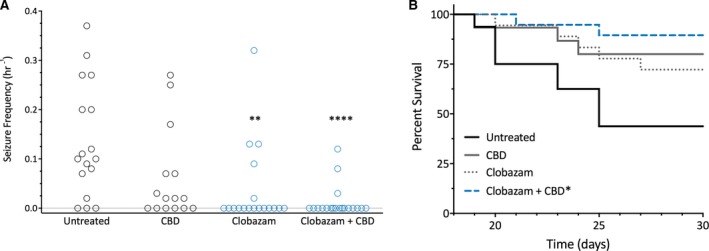
Cannabidiol (CBD) and clobazam combination therapy improved survival of Scn1a +/− mice. A, Spontaneous generalized tonic‐clonic seizure (GTCS) frequency of individual untreated and drug‐treated mice. Treatment began after the induction of a single hyperthermia‐induced seizure and spontaneous GTCS were subsequently quantified over a 60‐h recording period. Clobazam in both the absence and presence of CBD significantly reduced the proportion of mice experiencing GTCS (blue, open symbols), with n = 15‐19 per group (**P < 0.005, ****P < 0.0001; Fisher's exact test). B, Survival curves comparing untreated and drug‐treated mice. Clobazam in the presence of CBD significantly improved survival (blue line), with n = 15‐19 per group (**P < 0.005; log‐rank Mantel‐Cox)
Despite having an anticonvulsant effect against spontaneous seizures, clobazam monotherapy did not significantly improve survival compared to controls (P = 0.0745). Although there was a trend toward improved survival, CBD monotherapy did not significantly improve survival (P = 0.0526; Figure 2B). However, the combination of CBD and clobazam significantly improved survival compared to controls (P = 0.0033). Subchronic co‐treatment of CBD and clobazam again resulted in a drug‐drug interaction, as CBD increased the plasma levels of N‐CLB by fourfold compared to controls (0.4 ± 0.1 μg/mL vs 1.6 ± 0.2 μg/mL, P = 0.0136; Table 2).
3.3. CBD augments the anticonvulsant action of clobazam against thermally induced seizures
Next we assessed whether CBD could increase the anticonvulsant effects of clobazam against hyperthermia‐induced seizures, a model of febrile seizures that occur in Dravet syndrome. We first examined whether CBD, co‐administered at the same dose (12 mg/kg) that produced a significant augmentation of plasma and brain clobazam concentrations in our earlier pharmacokinetic studies, modulated the effects of a range of clobazam doses against hyperthermia‐induced seizures (0.1–10 mg/kg). Clobazam treatment dose‐dependently increased the body temperature threshold for GTCS compared to vehicle (Figure 3A). Co‐treatment with CBD (12 mg/kg) did not improve seizure response compared to clobazam monotherapy despite CBD significantly increasing plasma concentrations of clobazam (F 1,66 = 12.79, P = 0.0007; see Figure 3A,B, respectively). CBD plasma concentrations were not different across groups and were within the range found in patients with intractable epilepsy (Figure S1A).
Figure 3.
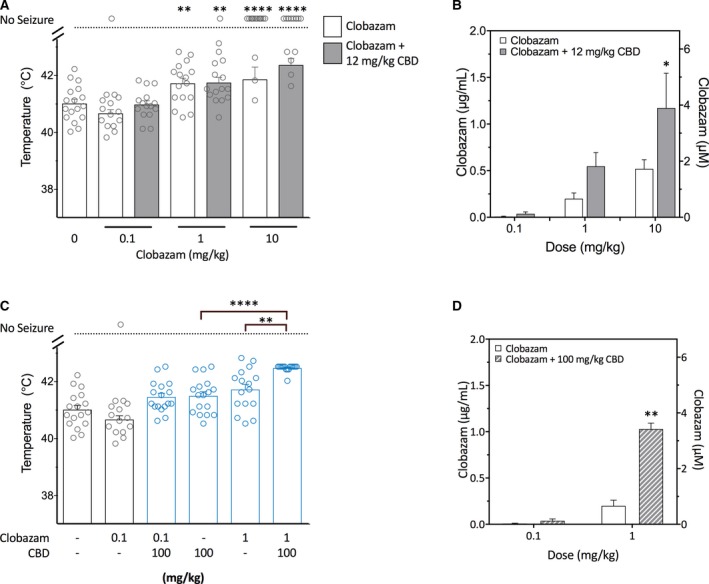
Increased anticonvulsant effect of co‐administered clobazam and cannabidiol (CBD) on hyperthermia‐induced seizures in Scn1a +/− mice. CBD (12 mg/kg) did not increase the anticonvulsant effects of clobazam on hyperthermia‐induced seizures in Scn1a +/− mice despite the presence of a pharmacokinetic interaction. A, Temperature threshold for generalized tonic‐clonic seizures (GTCS) in individual mice treated with varying doses of clobazam in the absence (white bars) or presence (gray bars) of CBD. Clobazam treatment significantly increased body temperature of GTCS compared to vehicle. Concurrent CBD treatment did not affect the response of clobazam to thermally‐induced seizures. Error bars represent standard error of the mean (SEM), with n = 13‐17 per treatment group (**P < 0.005, ****P < 0.0001 compared to vehicle; log‐rank Mantel‐Cox). B, Average clobazam plasma concentrations in Scn1a +/− mice treated with clobazam only (white bars) or clobazam and CBD (12 mg/kg; grey bars). Combination treatment resulted in significantly higher plasma clobazam concentrations (P < 0.001; two‐way analysis of variance [ANOVA] followed by Sidak's post hoc; *P < 0.05). Error bars represent SEM, with n = 5‐8 per group. C, Threshold temperature of individual mice for GTCS induced by hyperthermia following acute treatment with clobazam (0.1 or 1 mg/kg) or CBD at a higher, anticonvulsant dose (100 mg/kg) administered individually or as a combination. Low‐dose clobazam (0.1 mg/kg) had no effect of GTCS threshold. All other treatments resulted in a significantly improved response to thermal seizure induction compared to vehicle (blue, open symbols, P < 0.05). Combination clobazam (1 mg/kg) and CBD (100 mg/kg) treatment was significantly more effective than either treatment alone, with n = 16‐17 per group (**P < 0.005, ****P < 0.0001; log‐rank Mantel‐Cox). [Note: vehicle and clobazam alone are replotted from 3A for clarity.] D, Average clobazam plasma concentrations in Scn1a +/− mice from the hyperthermia‐induced seizure experiment treated with clobazam (0.1 or 1 mg/kg) alone (white bars) or clobazam and CBD (100 mg/kg; gray‐striped bars). Combination treatment resulted in significantly higher clobazam levels (P < 0.001; two‐way ANOVA followed by Sidak's post hoc; **P < 0.005). Error bars represent SEM, with n = 5‐7 per group
Because 12 mg/kg CBD administered alone did not have an anticonvulsant effect on hyperthermia‐induced seizures (Figure S1B), we sought to determine whether CBD could improve the anticonvulsant effects of clobazam when administered at a dose that has intrinsic anticonvulsant effects. Clobazam (1 mg/kg) and CBD (100 mg/kg) administered individually both significantly elevated the body temperature threshold for GTCS compared to vehicle‐treated mice (P = 0.0037 and P = 0.0408, respectively; Figure 3C). A combination of clobazam and CBD treatment significantly raised the thermal threshold further than either drug administered alone (P = 0.0036 vs clobazam; P < 0.0001 vs CBD or vehicle). A pharmacokinetic interaction was observed, as plasma clobazam levels were significantly elevated with CBD co‐administration (Figure 3D). CBD (100 mg/kg) co‐administered with a lower dose of clobazam (0.1 mg/kg), which itself was ineffective, had no additional benefit relative to 100 mg/kg CBD alone. Collectively, these results suggest a pharmacodynamic interaction in that intrinsic anticonvulsant doses of both clobazam and CBD are necessary to see a greater anticonvulsant effect of the combination therapy against hyperthermia‐induced seizures.
3.4. Co‐incubation of CBD and clobazam confer a greater potentiation of GABA‐evoked currents through GABAA receptors than each administered alone
Because clobazam and CBD are both positive allosteric modulators of GABAA receptors through different binding sites,21, 25 we explored whether CBD and clobazam might act cooperatively to potentiate the inhibitory effects of GABA at these receptors. We first compared the modulation of GABAA receptors by CBD and clobazam individually using two‐electrode voltage‐clamp electrophysiology on Xenopus oocytes expressing concatenated GABAA receptors (γ2‐β2‐α1‐β2‐α1). Concentration‐response curves of clobazam and CBD modulation of 15 μmol/L GABA‐evoked currents were generated (Figure 4A). CBD alone maximally enhanced the GABA‐evoked currents by 106% with a half maximal effective concentration (EC50) of 2.4 μmol/L. Clobazam was more efficacious and potent than CBD, modulating with a maximum of 369% with an EC50 of 662 nmol/L. We next determined whether CBD enhances the potentiation of GABA‐evoked currents by clobazam. CBD (10 μmol/L), clobazam (10 μmol/L), N‐CLB (10 μmol/L) and their combinations were applied to oocytes together with 15 μmol/L GABA. CBD, clobazam and N‐CLB alone all potentiated the GABA currents (Figure 4C). When CBD was combined with clobazam, the response was further increased compared to either drug alone (289 ± 54% compared to 204 ± 33%, P = 0.0489 vs clobazam and 82 ± 11%, P = 0.016 vs CBD). Similarly, the combination of CBD and N‐CLB significantly increased the GABA currents observed compared to either compound alone (230 ± 41% compared to 161 ± 28%, P = 0.023 vs N‐CLB; P = 0.0186 vs CBD). We then sought to determine whether the potentiation observed with CBD and clobazam co‐treatment resulted from synergistic action by generating concentration‐response curves of CBD modulating GABA‐evoked currents in the presence and absence of clobazam (Figure S2). These results suggest that, although CBD and clobazam modulate GABAA receptors to a greater extent when combined, they do not act in a synergistic manner.
Figure 4.
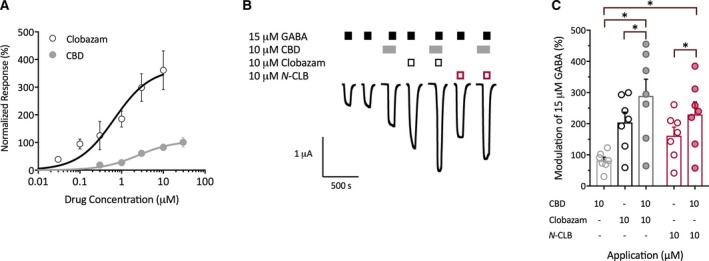
Interaction between cannabidiol (CBD) and clobazam at α1β2γ2 γ‐aminobutyric acid (GABA)A receptors. A, Concentration‐response curves for clobazam (black) and CBD (gray) at GABAA receptors. Clobazam and CBD modulate currents evoked by 15 μmol/L GABA. Data are shown as mean ± standard error of the mean (SEM) fit to the Hill equation, with n = 4 per group. B, Representative trace of currents evoked by GABA applied alone or in the presence of CBD, clobazam, or N‐desmethylclobazam (N‐ CLB) individually or in combination in oocytes expressing the GABAA receptor. C, Paired modulation of 15 μmol/L GABA by CBD (gray, open symbols), clobazam (black, open symbols), N‐ CLB (red, open symbols), CBD and clobazam (gray, closed circles) and CBD and N‐ CLB (red, closed circles). Error bars represent SEM, with n = 7 (*P < 0.05; paired one‐way analysis of variance (ANOVA) followed by Dunnett's post hoc test)
4. DISCUSSION
There is an urgent need to introduce better treatment options for intractable epilepsies, particularly for Dravet syndrome, since less than 30% of patients respond to first‐line treatments.26, 27, 28 CBD has been shown to attenuate seizures in a mouse model of Dravet syndrome and reduce seizure frequency and severity in various acute seizure models.29, 30, 31, 32, 33 Following several successful Phase III trials, CBD has been approved by the FDA and offers a novel therapeutic option for patients with Dravet syndrome. Despite abundant evidence detailing the anticonvulsant efficacy of CBD, several commentators question CBD's intrinsic anticonvulsant activity and have attributed the positive trial outcomes to a pharmacokinetic interaction, where CBD simply increases the plasma levels and consequent efficacy of clobazam and its active metabolite, N‐CLB.12, 13, 14, 15
In the present study we used various in vivo and in vitro approaches to explore pharmacodynamic and pharmacokinetic interactions between CBD and clobazam. We provide direct in vitro evidence that CBD potently inhibits the metabolism of clobazam and N‐CLB mediated by human CYP3A4 and CYP2C19, respectively, and reproduce a robust pharmacokinetic interaction between CBD and clobazam in mice. In a mouse model of Dravet syndrome, we demonstrate that CBD and clobazam coadministration promotes a greater anticonvulsant effect than each of the drugs alone against thermally induced seizures. The increased anticonvulsant efficacy of combined CBD and clobazam required a CBD dose with intrinsic anticonvulsant activity. Notably we show that coadministration of a lower, subthreshold dose of CBD did not enhance the anticonvulsant effects of clobazam despite CBD significantly increasing plasma clobazam concentrations. This highlights that the propensity of CBD to increase plasma clobazam concentrations does not necessarily lead to greater anticonvulsant effects. In addition, we observed a novel benefit of combining CBD and clobazam, where subchronic treatment with the combination significantly improved survival of Scn1a +/− mice. Finally, we describe a novel pharmacodynamic interaction that might explain the magnified anticonvulsant effects of combination therapy, in which CBD and clobazam cooperatively enhance inhibitory currents at GABAA receptors.
Clobazam and N‐CLB are both positive allosteric modulators (PAMs) of the GABAA receptor, enhancing inhibitory function of GABAergic interneurons.34, 35, 36 Because NaV1.1 channels are expressed predominantly on GABAergic interneurons, loss‐of‐function SCN1A mutations found in Dravet syndrome patients impair interneuron function leading to neuronal hyperexcitability. This explains why increasing inhibitory tone with PAMs of GABAA receptors yields beneficial therapeutic effects.37, 38 Here we confirm that at concentrations relevant to its anticonvulsant effects, CBD is a weak PAM of the GABAA receptor.21 In addition, co‐application of CBD and clobazam or CBD and N‐CLB, potentiated GABA‐evoked inhibitory currents to a greater degree than any of the compounds alone. Our prior work suggests that the binding site of CBD at GABAA receptors is distinct from the classical benzodiazepine binding site; hence, our results imply augmented GABA signaling via simultaneous positive allosteric modulation of the receptor by CBD and clobazam at distinct binding sites.21 These results provide the first evidence of a pharmacodynamic interaction between CBD and clobazam that could contribute to improved outcomes in patients with Dravet syndrome.
The additional anticonvulsant benefit conferred through combining CBD and clobazam may not simply be explained by an interaction on GABAA receptor signaling, as the anticonvulsant mechanism of CBD is likely multimodal, involving several pharmacologic targets, including G‐protein‐coupled receptor 55 (GPR55).29, 39, 40, 41, 42 GPR55 is antagonized by CBD, which is thought to play a preeminent role in the anticonvulsant effects of CBD. Kaplan et al (2017) showed that CBD restored inhibitory interneuron function in hippocampal slice cultures of Scn1a +/− mice that was attributed to GPR55 antagonism.29 CBD and clobazam co‐medication may then improve the management of Dravet syndrome via CBD having a distinct mechanism of action to clobazam.
Our results reaffirm that CBD monotherapy is anticonvulsant in mouse models of Dravet syndrome, as CBD (100 mg/kg) increased the body temperature threshold at which Scn1a +/− mice experienced a GTCS. CBD was effective at 100 mg/kg (i.p.), which approximates doses found effective in human trials (oral 20 mg/kg) based on interspecies scaling and consideration of the low oral bioavailability of CBD. However, plasma concentrations following 100 mg/kg CBD, i.p. (~1800 ng/mL) in our experimental mice exceed those observed in human studies (100‐800 ng/mL).9 Kaplan et al29 showed CBD reduced the duration and severity of febrile seizures at 100 and 200 mg/kg in a different Scn1a +/− mouse line. We could not, however, resolve an anticonvulsant effect of CBD monotherapy on spontaneous seizures. Kaplan et al showed that twice daily injections of 100 mg/kg CBD for eight days reduced spontaneous seizure frequency.29 Although plasma CBD concentrations were not measured in the earlier study, we would predict them to be similar to those seen in mice dosed with 100 mg/kg CBD in our hyperthermia experiments (~1800 ng/mL). We anticipate that if our dose were increased to achieve plasma CBD concentrations equivalent to those estimated for Kaplan et al we would also see a reduction in spontaneous GTCS frequency with CBD monotherapy and likely an increase in the anticonvulsant action of clobazam. Unfortunately, mice have an aversion to the taste of chow containing enough CBD to attain those plasma concentrations.
Clobazam is metabolized by CYP3A4 to its long‐acting active metabolite N‐CLB, which is inactivated by CYP2C19. CBD inhibits both CYP3A4 and CYP2C19, suggesting that the CYP450 pathway mediates the drug‐drug interaction observed clinically between clobazam and CBD.9, 11, 43, 44 Here we provide unprecedented evidence that CBD potently inhibits the metabolism of clobazam (K i 139 nmol/L) and, to a lesser extent, N‐CLB (K i 2.6 μmol/L). The potency of CYP450 inhibition by CBD is clearly substrate‐dependent; by comparison, CBD inhibits both CYP3A4‐mediated metabolism of diltiazem and CYP2C19‐mediated metabolism of mephenytoin with K i values of 1 and 0.79 μmol/L, respectively.43, 45 The K i values reported here for clobazam and N‐CLB are relevant to the plasma CBD concentrations observed in patients with epilepsy (318‐2544 nmol/L; 100‐800 ng/mL),9 and this CYP‐mediated interaction likely accounts for the drug‐drug interaction observed in vivo, as it is well established that genetic and pharmacologic disruption of CYP3A4 and CYP2C19 in humans profoundly affects plasma concentrations of clobazam and N‐CLB.9, 17, 18, 46, 47 Accordingly, we found that CBD greatly enhanced overall clobazam and N‐CLB exposure in plasma and brain of mice, consistent with human studies.
The combination of CBD and clobazam produced an anticonvulsant effect against hyperthermia‐induced seizures that was greater than that observed with either CBD or clobazam alone. This increased effect appeared to require a dose of CBD with intrinsic anticonvulsant activity, as an ineffective subthreshold dose of CBD (12 mg/kg) failed to augment the anticonvulsant effects of clobazam against thermally‐induced seizures in Scn1a +/− mice. This was despite the lower dose causing a highly significant pharmacokinetic interaction, increasing clobazam concentrations. This suggests that the pharmacokinetic interaction between clobazam and CBD alone is either not sufficient to augment anticonvulsant effects or must obtain a critical level of magnitude before a potentiation of anticonvulsant effects are obtained.
Unfortunately, our in vivo studies could not assess synergy. Assessing synergy of two combined drugs is best achieved using isobolographic analysis, which requires the determination of doses that produce 50% of maximal response (ED50). We have not been able to establish the ED50 of CBD on seizures in Scn1a +/− mice, as CBD does not produce a classical dose‐response relationship in this model.29
Our results showing efficacy of CBD and clobazam combination therapy against thermally‐induced seizures were supported by the combination also improving other epilepsy‐relevant phenotypes observed in Scn1a +/− mice. Treatment with clobazam and CBD resulted in a lower proportion of mice experiencing spontaneous seizures than with clobazam alone (16% vs 28%), although this difference fell short of statistical significance most likely due to the floor effect imposed by the efficacy of clobazam alone. In the same experiment, the survival of Scn1a +/− mice was best obtained with CBD and clobazam combination treatment, as monotherapies did not produce significant survival advantages despite clear trends. The effect of CBD and clobazam on mortality in patients with Dravet syndrome is not an endpoint that clinical studies are designed to examine with SUDEP occurring in 10%‐18% of patients48 over periods of many years. Our results here suggest a novel benefit of CBD and clobazam combination therapy on premature death, a devastating aspect of the Dravet syndrome phenotype.
This preclinical study supports the view that the clinical efficacy of CBD may not simply be explained by CBD increasing plasma concentrations of clobazam and N‐CLB. Collectively, the pharmacokinetic interaction between CBD and clobazam, the pharmacodynamic interaction at GABAA receptors and the multimodal actions of CBD targeting complementary anticonvulsant pathways are all likely to contribute to the improved management of Dravet syndrome with CBD treatment.
CONFLICTS OF INTEREST
Associate Professor Jonathon Arnold is Deputy Academic Director of the Lambert Initiative. He has served as an expert witness in various medicolegal cases involving cannabis and recently served as a temporary advisor to the World Health Organization (WHO) on their review of cannabis and the cannabinoids. His research is also funded by the NHMRC. Professor Iain McGregor is academic director of the Lambert Initiative and an NHMRC Principal Research Fellow, and he receives research funding from the Australian Research Council and NHMRC. He is involved in an NHMRC‐funded clinical trial using the cannabis extract, nabiximols (Sativex). He has served as an expert witness in various medicolegal cases involving cannabis and cannabinoids. A/Prof Arnold, Prof McGregor and Dr Lyndsey Anderson hold a patent on a novel anticonvulsant therapy for epilepsy. The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded center for medicinal cannabis research at the University of Sydney and the Australian National Health and Medical Research Council (NHMRC). The authors gratefully acknowledge Barry and Joy Lambert for their continued support of the Lambert Initiative for Cannabinoid Therapeutics. In addition, we thank Katelyn Lambert for inspiring our work on novel cannabinoid therapies for childhood epilepsy.
Anderson LL, Absalom NL, Abelev SV, et al. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia. 2019;60:2224–2234. 10.1111/epi.16355
REFERENCES
- 1. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug resistant seizure in the Dravet Syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 2. Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 3. Greenwood SM, VanLandingham KE, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut Syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 4. Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52:3–9. [DOI] [PubMed] [Google Scholar]
- 6. Dravet C, Oguni H. Dravet syndrome (severe myoclonic epilepsy in infancy) In: Dulac O, Lassonde M, Sarnat HB. editors. Handbook of clinical neurology. Amsterdam: Elsevier; 2013,111:627–33 [DOI] [PubMed] [Google Scholar]
- 7. Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64:69–74. [DOI] [PubMed] [Google Scholar]
- 8. Chen JW, Borgelt LM, Blackmer AB. Cannabidiol: a new hope for patients with Dravet or Lennox‐Gastaut Syndromes. Ann Pharmacother. 2019;53:603–11. [DOI] [PubMed] [Google Scholar]
- 9. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug‐drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51. [DOI] [PubMed] [Google Scholar]
- 10. Hess EJ, Moody KA, Geffrey AL, Pollack SF, Skirvin LA, Bruno PL, et al. Cannabidiol as a new treatment for drug‐resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1617–24. [DOI] [PubMed] [Google Scholar]
- 11. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP, UAB CBD Program . Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58:1586–92. [DOI] [PubMed] [Google Scholar]
- 12. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen KA, Farrar M, Cardamone M, Gill D, Smith R, Cowell CT, et al. Cannabidiol for treating drug‐resistant epilepsy in children: the New South Wales experience. Med J Aust. 2018;209:217–21. [DOI] [PubMed] [Google Scholar]
- 14. Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at Last? J Epilepsy Res. 2018;7:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devinsky O, Cross JH, Wright S. Trial of cannabidiol for drug‐resistant seizures in the Dravet Syndrome. N Engl J Med. 2017;377:699–700. [DOI] [PubMed] [Google Scholar]
- 16. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol. 2016;15:270–8. [DOI] [PubMed] [Google Scholar]
- 17. Giraud C, Treluyer J‐M, Rey E, Chiron C, Vincent J, Pons G, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34:608–11. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto Y, Takahashi Y, Imai K, Miyakawa K, Nishimura S, Kasai R, et al. Influence of CYP2C19 Polymorphism and concomitant antiepileptic drugs on serum clobazam and N‐Desmethyl clobazam concentrations in patients with epilepsy. Ther Drug Monit. 2013;35:305–12. [DOI] [PubMed] [Google Scholar]
- 19. Marini C, Scheffer IE, Nabbout R, Suls A, DeJonghe P, Zara F, et al. The genetics of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):24–9. [DOI] [PubMed] [Google Scholar]
- 20. Hawkins NA, Anderson LL, Gertler TS, Laux L, George AL Jr, Kearney JA. Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Ann Clin Transl Neurol. 2017;4:326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bakas T, van Nieuwenhuijzen PS, Devenish SO, McGregor IS, Arnold JC, Chebib M. The direct actions of cannabidiol and 2‐arachidonoyl glycerol at GABAAreceptors. Pharmacol Res. 2017;119:358–70. [DOI] [PubMed] [Google Scholar]
- 22. Hawkins NA, Zachwieja NJ, Miller AR, Anderson LL, Kearney JA. Fine mapping of a Dravet Syndrome modifier locus on mouse chromosome 5 and candidate gene analysis by RNA‐Seq. PLoS Genet. 2016;12:e1006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao VWY, Chua HC, Kowal NM, Chebib M, Balle T, Ahring PK. Concatenated γ‐aminobutyric acid type A receptors revisited: finding order in chaos. J Gen Physiol. 2019;151:798–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chua HC, Absalom NL, Hanrahan JR, Viswas R, Chebib M. The direct actions of GABA, 2’‐methoxy‐6‐methylflavone and general anaesthetics at β3γ2L GABAA receptors: evidence for receptors with different subunit stoichiometries. PLoS ONE. 2015;10:10848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Griffin CE, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system‐mediated effects. Ochsner J. 2013;13:214–23. [PMC free article] [PubMed] [Google Scholar]
- 26. Shi X‐Y, Tomonoh Y, Wang W‐Z, Ishii A, Higurashi N, Kurahashi H, et al. Efficacy of antiepileptic drugs for the treatment of Dravet syndrome with different genotypes. Brain Dev. 2016;38:40–6. [DOI] [PubMed] [Google Scholar]
- 27. Wallace A, Wirrell E, Kenney‐Jung DL. Pharmacotherapy for Dravet Syndrome. Pediatr Drugs. 2016;18:197–208. [DOI] [PubMed] [Google Scholar]
- 28. Wirrell EC, Laux L, Donner E, Jette N, Knupp K, Meskis MA, et al. Optimizing the diagnosis and management of Dravet Syndrome: recommendations from a North American Consensus Panel. Pediatr Neurol. 2017;68(18–34):e3. [DOI] [PubMed] [Google Scholar]
- 29. Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci. 2017;114:11229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiu P, Olsen DM, Borys HK, Karler R, Turkanis SA. The influence of cannabidiol and Δ9‐tetrahydrocannabinol on cobalt epilepsy in rats. Epilepsia. 1979;20:365–75. [DOI] [PubMed] [Google Scholar]
- 31. Jones NA, Glyn SE, Akiyama S, Hill TD, Hill AJ, Weston SE, et al. Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52. [DOI] [PubMed] [Google Scholar]
- 32. Lindamood C 3rd, Colasanti BK. Effects of delta 9‐tetrahydrocannabinol and cannabidiol on sodium‐dependent high affinity choline uptake in the rat hippocampus. J Pharmacol Exp Ther. 1980;213:216–21. [PubMed] [Google Scholar]
- 33. Karler R, Cely W, Turkanis SA. The anticonvulsant activity of cannabidiol and cannabinol. Life Sci. 1973;13:1527–31. [DOI] [PubMed] [Google Scholar]
- 34. Nakajima H. A pharmacological profile of clobazam (Mystan), a new antiepileptic drug. Nihon Yakurigaku Zasshi. 2001;118:117–22. [DOI] [PubMed] [Google Scholar]
- 35. Sankar R. GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5‐benzodiazepine clobazam. CNS Drugs. 2012;26:229–44. [DOI] [PubMed] [Google Scholar]
- 36. Jensen HS, Nichol K, Lee D, Ebert B. Clobazam and its active metabolite N‐desmethylclobazam display significantly greater affinities for α2‐ versus α1‐GABAA–receptor complexes. PLoS ONE. 2014;9:e88456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamakawa K. Molecular basis of severe myoclonic epilepsy in infancy. Brain Develop. 2009;31:401–4. [DOI] [PubMed] [Google Scholar]
- 38. Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin‐ and parvalbumin‐expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci. 2014;111:E3139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anavi‐Goffer S, Baillie G, Irving AJ, Ross RA. Modulation of L‐α‐lysophosphatidylinositol/GPR55 MAP kinase signalling by cannabinoids. J Biol Chem. 2011;27:303–10. [Google Scholar]
- 40. Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–41. [DOI] [PubMed] [Google Scholar]
- 42. Patel RR, Barbosa C, Brustovetsky T, Cummins TR. Aberrant epilepsy‐associated mutant Na v 1.6 sodium channel activity can be targeted with cannabidiol. Brain. 2016;139:2164–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–70. [DOI] [PubMed] [Google Scholar]
- 44. Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–8. [DOI] [PubMed] [Google Scholar]
- 45. Yamaori S, Ebisawa J, Okushima Y, Yamamoto I, Watanabe K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88:730–6. [DOI] [PubMed] [Google Scholar]
- 46. Walzer M, Bekersky I, Blum RA, Tolbert D. Pharmacokinetic drug interactions between clobazam and drugs metabolized by cytochrome P450 isoenzymes. Pharmacotherapy. 2012;32:340–53. [DOI] [PubMed] [Google Scholar]
- 47. Seo T, Nagata R, Ishitsu T, Murata T, Takaishi C, Hori M, et al. Impact of CYP2C19 polymorphisms on the efficacy of clobazam therapy. Pharmacogenomics. 2008;9:527–37. [DOI] [PubMed] [Google Scholar]
- 48. Genton P, Velizarova R, Dravet C. Dravet syndrome: the long‐term outcome. Epilepsia. 2011;52(Suppl 2):44–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


