Abstract
Background
While the importance of patients’ quality of life (QoL) in chronic cardiac or pulmonary disease is uncontroversial, the burden of an acute pulmonary embolism (PE) on QoL has received little attention thus far.
Objectives
We aimed to validate the German PEmb‐QoL questionnaire, identify associations between QoL and clinical/functional parameters, and investigate the prognostic relevance of QoL for long‐term survival in survivors of an acute PE episode.
Patients/Methods
Patients were invited for a clinical follow‐up visit including assessment of QoL using the German PEmb‐QoL questionnaire 6 months after an objectively confirmed PE at a single center. Internal consistency reliability, construct‐related validity, and regressions between PEmb‐QoL and clinical patient‐characteristics were assessed using standard scale construction techniques.
Results
Overall, 101 patients [median age, 69 ([interquartile range] IQR 57‐75) years; women, 48.5%] were examined 208 (IQR 185‐242) days after PE. Internal consistency reliability and construct‐related validity of the PEmb‐QoL questionnaire were acceptable. As many as 47.0% of patients reported dyspnea, 27.5% had right ventricular (RV) dysfunction on transthoracic echocardiography (TTE), and 25.3% were diagnosed with post‐PE impairment (PPEI) at 6‐month follow‐up. Furthermore, 15.9% of patients were diagnosed with depression 6 months after an acute PE. The QoL was affected by dyspnea, preexisting pulmonary disease, and PPEI, and a reduced QoL was associated with an increased risk for long‐term mortality after an observation period of 3.6 years.
Conclusions
The German PEmb‐QoL questionnaire is a reliable instrument for assessing QoL 6 months after PE. The QoL was affected by dyspnea, preexisting pulmonary disease, and PPEI and was associated with long‐term mortality.
Essentials.
The burden of a PE on QoL has received little attention. The PEmb‐QoL questionnaire is a reliable instrument for assessing QoL in PE survivors.
QoL is affected by dyspnea, pre‐existing pulmonary disease and post‐PE impairment and reduced QoL associated with an increased risk of long‐term mortality.
1. INTRODUCTION
The long‐term course of PE is complicated by several adverse events including recurrent venous thromboembolism (VTE), development of chronic thromboembolic pulmonary hypertension (CTEPH), and major bleeding due to anticoagulant therapy.1, 2, 3 Furthermore, methodically heterogeneous studies demonstrated that residual perfusion defects can be detected in up to 50% of the patients after 6 months using ventilation‐perfusion lung scan4, 5, 6 and that approximately 50% of patients suffer from persisting symptoms and cardiopulmonary functional limitations up to 1 year after the initial PE.3, 7, 8, 9 While the long‐term hemodynamic and functional consequences of an acute PE have received increasing attention during the past years, the burden of an acute PE on patients’ psychological‐emotional well‐being and QoL has remained insufficiently studied thus far.10, 11 Health‐related crises such as an acute PE can result in emotional distress and negative emotional reactions, which may manifest as anxiety, anger, depression, and posttraumatic stress disorder.10 In patients with chronic heart failure and pulmonary artery hypertension, a reduced QoL has been shown to be associated with poor prognosis12, 13 and thus has become a treatment target in chronic heart failure.12, 14, 15
Although QoL can be assessed using generic questionnaires such as the 36‐item Short Form Health Survey, disease‐specific questionnaires promise higher sensitivity in detecting and quantifying disease‐specific changes in QoL.16 The first disease‐specific QoL questionnaire for patients with PE, the so‐called Pulmonary Embolism‐Quality of Life (PEmb‐QoL) questionnaire, was developed in 200917 and consists of nine questions with a total of 38 items summarized in six categories related to activities and emotional/social complaints. Subsequently, the PEmb‐QoL was validated in different cohorts and languages.16, 18, 19, 20, 21 However, limitations of these studies encompass the inclusion of patients at various time points after the initial PE event and the lack of investigating association between QoL and clinical parameters or prognosis. Thus, we aimed (i) to validate the German version of the disease‐specific PEmb‐QoL questionnaire in survivors of acute PE 6 months after the initial event, (ii) to identify associations between QoL and clinical and functional parameters, and (iii) to investigate the prognostic relevance of QoL for long‐term survival.
2. METHODS
2.1. Study design
Consecutive patients aged ≥18 years with objectively confirmed acute PE diagnosed at the University Medical Center, Goettingen, Germany (January 2011‐August 2013) were included in an ongoing prospective cohort study (Pulmonary Embolism Registry of Goettingen). The study protocol has been described in detail previously.22 All treatment decisions were made by the physicians caring for the patients according to current guidelines and were not influenced by the study protocol at any time. The study was conducted in accordance with the amended Declaration of Helsinki and the study protocol was approved by the local independent ethics committee. All patients provided written informed consent.
At baseline, detailed information on initial presentation including symptoms, findings from risk stratification, comorbidities, and VTE risk factors; treatment and in‐hospital outcomes were obtained using a standardized case report form. Details on diagnostic procedures, definitions used for risk stratification, and laboratory measurements are provided in the Data S1. An adverse in‐hospital outcome was defined as PE‐related death, cardiopulmonary resuscitation, mechanical ventilation, or need for catecholamine administration.
Patients were invited to participate in a 6‐month routine follow‐up visit at the outpatient clinic of the Clinic of Cardiology and Pulmonology of the University Medical Center, Goettingen, Germany. Exclusion criteria and study flow are shown in Figure 1. At the follow‐up visit, information on adverse events [including recurrent VTE, clinically relevant bleeding (defined according to the International Society of Thrombosis and Hemostasis)23 new diagnosis of depression, cancer, and CTEPH (confirmed according to current guideline recommendations)24] were recorded. Additionally, all patients received a conventional 12‐lead electrocardiogram, TTE, physical examination, and laboratory testing and were asked to complete the German version of the PEmb‐QoL questionnaire and questions related to their socioeconomic status (Figure S1, Table S1). Long‐term survival status was assessed by contacting the responsible registration office at various time points (generally at 2‐year intervals).
Figure 1.
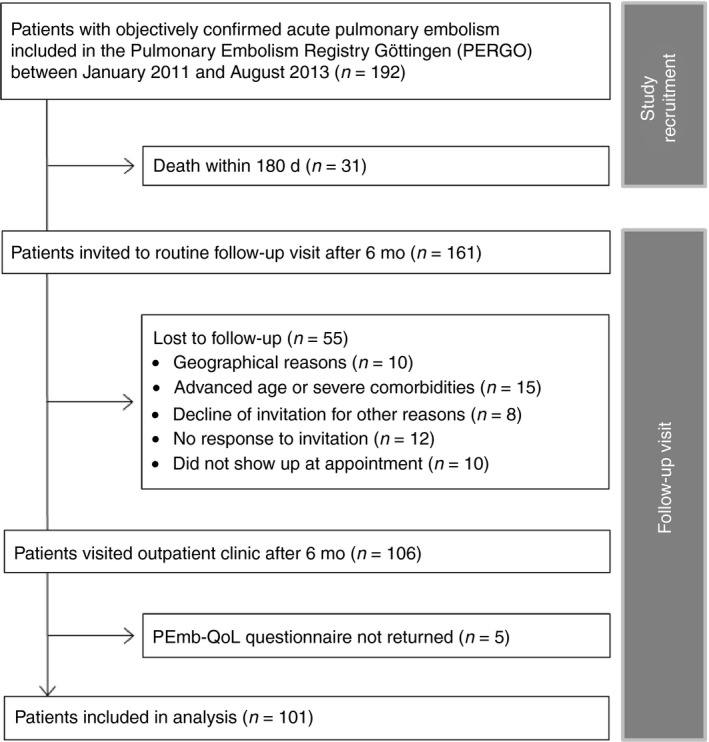
Flow diagram of exclusion criteria. PE, pulmonary embolism; PEmb‐QoL, pulmonary embolism‐quality of life; PERGO, Pulmonary Embolism Registry Goettingen
2.2. PEmb‐QoL questionnaire
The disease‐specific PEmb‐QoL questionnaire16, 17, 18, 19, 20 consists of nine questions with a total of 38 included items summarized in six categories (dimensions): frequency of complaints (FO, 8 items), activities of daily living limitations (AD, 13 items), work‐related problems (WR, 4 items), social limitations (SL, 1 item), intensity of complaints (IO, 2 items), and emotional complaints (EC, 10 items). Two additional questions (questions 2 and 3) are descriptive only and not scored in the dimensions. The minimum score is 1 (indicating no complaints) and thus, higher scores are associated with a more complaints and decreased QoL.
The German PEmb‐QoL questionnaire (Figure S1) is a linguistically validated translation of the Dutch PEmb‐QoL questionnaire16, 17 provided by the MAPI institute (Lyon, France). The validation process included forward and backward translation, clinicians’ review, and cognitive interview of five patients with PE. For the present study, question 3 (referring to the change of symptoms over time) was adapted to the 6‐month follow‐up period.
2.3. Study outcomes at 6‐month follow‐up
Study outcomes at 6‐month follow‐up include (i) severe dyspnea [defined as New York Heart Association (NYHA) class III/IV], (ii) depression (defined as confirmed diagnosis by a physician or administration of antidepressive drugs), (iii) RV dysfunction on TTE, and (iv) PPEI.
Right ventricular dysfunction on TTE was defined according to Rudski et al25 and Konstantinides et al26 as the presence of one or more of the following parameters: RV basal diameter >4.2 cm, tricuspid annular plane systolic excursion <1.6 cm, elevated right atrial pressure based on the absence of an inspiratory collapse of the inferior vena cava, tricuspid regurgitant velocity ≥2.8 m/s. and presence of pericardial effusion.
Post‐pulmonary embolism impairment was defined as a combination of one or more echocardiographic sign(s) of RV dysfunction plus presence of one or more clinical/laboratory sign(s) including peripheral edema, dyspnea (NYHA class ≥ II), or an elevated sex‐specific and age‐adjusted N‐terminal pro brain natriuretic peptide level (Table S2).26
2.4. Statistical analyses
Dichotomous and nominal variables are presented as absolute numbers and proportions and were compared using McNemar's test. Continuous variables are presented as median and IQR and were compared using Wilcoxon rank sum test.
The PEmb‐QoL scores were calculated for each dimension by summing up the item values divided by the number of items. Calculation of the “activities of daily living limitations” (AD) score was dependent on the answer for question 4a; if the answer was “I do not work,” the item sum was divided by 12, otherwise by 13 items. In the case of missing items, no score for the respective dimension was calculated.27 Quality criteria of the PEmb‐QoL questionnaire that were tested comprised outcome measures validity, reliability with internal consistency, and reproducibility and interpretability.28, 29 The detailed statistical approach is provided in the Data S1. Briefly, to evaluate construct‐related validity, we assessed the associations of single items with proposed PEmb‐QoL dimensions using linear regression models with single items as outcomes and scores as predictors adjusted for age and sex (Table S3). We further used linear regression models to evaluate the impact of patients’ baseline characteristics (adjusted for sex and age, Table S4), socioeconomic status (Table S5), and increasing NYHA classes (Table S6) on PEmb‐QoL dimensions. The results are presented as beta coefficients (β) with 95% confidence intervals (CIs). To evaluate internal consistency reliability, we calculated Cronbach's α for each PEmb‐QoL dimension (Table 2).28, 29, 30 For examination of floor and ceiling effects, we presented the proportion of participants with minimal and maximal possible scores of single dimensions (Table 2).16, 18, 19, 20
Univariate logistic regression models were used to assess the impact of study outcomes (Table 3) and socioeconomic status on PEmb‐QoL dimensions (Table S5) and the impact of patients’ baseline characteristics on clinical outcomes at 6‐month follow‐up (Table S7). The results were presented as odds ratios with 95% CIs. Cox regression models (univariate and adjusted for sex and age) were used to evaluate the prognostic impact of PEmb‐QoL dimensions and study outcomes on long‐term mortality (Table 4). The results were presented as hazard ratios with 95% CIs.
All analyses were explorative analyses. No adjustments for multiple testing were conducted. P values were provided for descriptive reasons only and should be interpreted with caution and in connection with the effect estimates. We used SPSS (versions 21.0‐23.0) and R (version 3.0.3) for the statistical analyses.
3. RESULTS
3.1. Patients’ characteristics
Overall, 192 patients were enrolled in the Pulmonary Embolism Registry Goettingen between January 2011 and August 2013; of those, 31 patients (16.1%) died within the first 180 days. Thus, 161 patients were invited to the routine clinical follow‐up visit after 6 months. After applying the exclusion criteria shown in Figure 1, 101 patients [62.7%; median age, 69 (IQR, 57‐75) years; 48.5% women] completed the 6‐month follow‐up visit and were included in the present analysis.
Patients’ characteristics at baseline (time of initial PE) are shown in Table 1, left column. The majority of patients (61.4%) had an unprovoked PE event, 14.0% had chronic heart failure, and 13.0% chronic pulmonary disease. A TTE within 48 h after admission was performed in 68 (67.3%) patients; of those, 49.3% of patients were diagnosed with RV dysfunction. According to the 2014 European Society of Cardiology guideline algorithm, 9 patients (8.9%) were classified as high‐risk, 28 (27.7%) as intermediate‐high‐risk, 52 (51.5%) as intermediate‐low‐risk, and 12 (11.9%) as low‐risk. Overall, 11 patients (10.9%) received systemic thrombolysis; of those, 4 patients received early systemic thrombolysis (within 24 h) and 7 patients were included in the PEITHO study and randomized to either single‐bolus tenecteplase or placebo.31 Nine patients (8.9%) had an adverse in‐hospital outcome. Median in‐hospital stay was 9 days (IQR, 6‐13 days).
Table 1.
Patients’ characteristics at baseline (time of PE event) and 6‐month follow‐up
| All study patients (n = 101) | Baseline (admission for acute PE event) | Follow‐up (6 months after PE event) | P value |
|---|---|---|---|
| Age at PE event (years) | 69.0 (56.5‐75.0) | ‐ | ‐ |
| Male sex | 52 (51.5%) | ‐ | ‐ |
| BMI (kg/m2) | 28.1 (25.4‐31.4) (n = 100) | 28.4 (25.0‐31.3) (n = 97) | .229 |
| Risk factors for VTE | |||
| Previous deep vein thrombosis | 29 (29.0%) (n = 100) | ‐ | ‐ |
| Previous pulmonary embolism | 12 (12.0%) (n = 100) | ‐ | ‐ |
| Thrombophilia | 5 (5.0%) (n = 100) | ‐ | ‐ |
| Trauma/surgerya | 15 (14.9%) | ‐ | ‐ |
| Immobilization or recent long travela | 19 (19.0%) | ‐ | ‐ |
| Pregnancy/postpartum periodb | 0 (0%) | ‐ | ‐ |
| Cancerc | 11 (11.0%) (n = 100) | 17 (17.0%) (n = 100) | .031 |
| Comorbidities | |||
| Chronic (left) heart failure | 14 (14.0%) (n = 100) | ‐ | ‐ |
| Heart failure with reduced LVEF (<40%) (HFrEF) | 3 (21.4%) | ||
| Heart failure with midrange reduced LVEF (40%‐49%) (HFmrEF) | 5 (35.7%) | ||
| Heart failure with preserved LVEF (≥50%) (HFpEF) | 4 (28.6%) | ||
| LVEF unkown | 2 (14.3%) | ||
| Coronary artery disease | 18 (18.0%) (n = 100) | ‐ | ‐ |
| Peripheral artery disease | 7 (8.5%) (n = 82) | ‐ | ‐ |
| Arterial hypertension | 65 (64.4%) | ‐ | ‐ |
| Diabetes mellitus | 12 (11.9%) | ‐ | ‐ |
| Hyperlipidemia | 30 (29.7%) | ‐ | ‐ |
| Previous stroke | 5 (5.0%) | ‐ | ‐ |
| Chronic pulmonary diseased | 13 (13.0%) (n = 100) | ‐ | ‐ |
| Renal insufficiencye | 10 (10.6%) (n = 94) | ‐ | ‐ |
| Depressionf | 9 (10.2%) (n = 88) | 14 (15.9%) (n = 88) | .063 |
| Symptoms | |||
| Acute onset of symptoms (<24 h before admission) | 46 (45.5%) | ‐ | ‐ |
| Dyspnea (NYHA ≥II) | 94 (93.1%) | 47 (47.0%) (n = 100) | <.001 |
| NYHA class II | ‐ | 28 (28.0%) (n = 100) | ‐ |
| NYHA class III | ‐ | 18 (18.0%) (n = 100) | ‐ |
| NYHA class IV | ‐ | 1 (1.0%) (n = 100) | ‐ |
| Chest pain | 59 (59.0%) (n = 100) | 20 (20.6%) (n = 97) | <.001 |
| Syncope | 14 (14.0%) (n = 100) | ‐ | ‐ |
| Leg swelling or leg pain | 31 (31.0%) (n = 100) | 37 (38.5%) (n = 96) | .568 |
| Vital signs | |||
| Systolic blood pressure (mm Hg) < 100 mm Hg | 12 (12.0%) (n = 100) | 0 (0%) (n = 85) | Not calculable |
| Tachycardia (heart rate ≥ 100 bpm) | 36 (36.0%) (n = 100) | 1 (1.1%) (n = 90) | <.001 |
| Hypoxiaf | 23 (27.7%) (n = 83) | ‐ | ‐ |
| Transthoracic echocardiography | |||
| RV > LV | 24 (44.4%) (n = 54) | 3 (3.6%) (n = 84) | <.001 |
| RV D1 > 4.2 cm | ‐ | 9 (26.5%) (n = 34) | ‐ |
| Paradoxical septal movement | 19 (43.2%) (n = 44) | 2 (2.3%) (n = 87) | <.001 |
| Absence of the inspiratory collapse of the IVC | 21 (40.4%) (n = 52) | 2 (2.7%) (n = 75) | <.001 |
| TAPSE <1.6 cm | ‐ | 2 (3.1%) (n = 65) | ‐ |
| TR jet velocity ≥2.8 m/s | 16 (50.0%) (n = 32) | 17 (25.4%) (n = 67) | .219 |
| Systolic PA pressure >50 mm Hg | 14 (33.3%) (n = 42) | 7 (10.9%) (n = 64) | .125 |
| Reduced LV EF (<50%) | 10 (18.2%) (n = 55) | 7 (7.7%) (n = 91) | .016 |
| Electrocardiogram | |||
| SIQIII‐type pattern | 33 (34.7%) (n = 95) | 4 (4.4%) (n = 90) | <.001 |
| Negative T‐waves in leads V1 to V3 | 38 (40.0%) (n = 95) | 9 (10.0%) (n = 90) | <.001 |
| Incomplete or complete RBBB | 17 (17.9%) (n = 95) | 16 (17.8%) (n = 90) | .554 |
| Laboratory biomarkers | |||
| hsTnT ≥14 pg/mL | 52 (62.7%) (n = 83) | 15 (24.2%) (n = 62) | <.001 |
| NT‐proBNP ≥600 pg/mL | 43 (55.8%) (n = 77) | 7 (11.3%) (n = 62) | <.001 |
Abbreviations: BMI, body mass index; EF, ejection fraction; hsTnT, high sensitive troponin T; IVC, inferior vena cava; LV, left ventricle; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary artery; PE, pulmonary embolism; RBBB, right bundle branch block; RV, right ventricle; RVD1, right ventricle diameter 1; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitant; VTE, venous thromboembolism.
Within 4 weeks prior to PE index event.
Postpartum period of within 6 weeks prior to PE index event.
Active or within 6 months prior to PE event.
Chronic obstructive pulmonary disease, asthma, and interstitial lung disease.
Glomerular filtration rate of ≤60 mL/min/1.73 m2.
O2 saturation of <90% or partial pressure of O2 <60 mm Hg (8 kPa) in arterial blood gas analysis.
The median time to the follow‐up visit was 208 (IQR, 185‐242) days. At the time of follow‐up, 85.1% of the patients still received therapeutic anticoagulation, no patient was diagnosed with recurrent VTE, 2 (2.0%, n = 100) had developed clinically relevant bleeding (excluding bleeding events occurring during the in‐hospital stay), 13 (13.4%, n = 97) were diagnosed with postthrombotic syndrome, and 4 (4.3%, n = 94) with CTEPH. As shown in Table 1, right column, 6.0% (n = 100) of patients had a new diagnosis of cancer.
3.2. Validation of the PEmb‐QoL questionnaire: Psychometric characteristics
Overall, 86.1% of the patients completed the PEmb‐QoL questionnaire in adequate quality for analyses. Median PEmb‐QoL scores of the six dimensions were 1.4 (IQR, 1.0‐1.8; maximum 5 points) for “frequency of complaints” (FO), 1.3 (1.1‐2.0; maximum 3 points) for “activities of daily living limitations” (AD), 1.0 (1.0‐1.8; maximum 2 points) for “work‐related problems” (WR), 1.0 (1.0‐2.0; maximum 5 points) for “social limitations” (SL), 2.0 (1.0‐3.0; maximum 6 points) for “intensity of complaints” (IO), and 1.8 (1.3‐2.7; maximum 6 points) for “emotional complaints” (EC) (Figure 2). For “work‐related problems” (WR) and “social limitations” (SL), the median was equal to the lowest possible score, indicating that the usability of these two dimensions might be limited. All dimensions revealed distinct floor effects, ranging from 12.0% for “emotional complaints” to as high as 59.2% for “social limitations.” Ceiling effects were especially observed in “work‐related problems” (Table 2).
Figure 2.
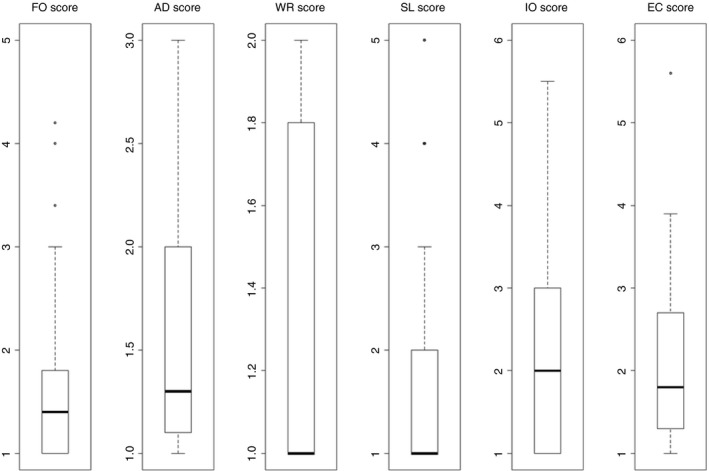
PEmb‐QoL dimension scores. Higher scores indicate decreased QoL. AD, activities of daily living limitations; EC, emotional complaints; FO, frequency of complaints; IO, intensity of complaints; SL, social limitations; WR, work‐related problems
Table 2.
Internal validation of the PEmb‐QoL questionnaire
| Dimensions | Missing % ( n ) | Cronbach's α | Floor effects % ( n / N ) | Ceiling effects % ( n / N ) |
|---|---|---|---|---|
| Frequency of complaints (FO) | 6.9% (7) | 0.77 | 25.5% (24/94) | 0% (0/94) |
| Activities of daily living limitations (AD) | 23.8% (24) | 0.90 | 22.1% (17/77) | 3.9% (3/77) |
| Work‐related problems (WR) | 10.9% (11) | 0.91 | 55.6% (50/90) | 21.1% (19/90) |
| Social limitations (SL) | 3.0% (3) | ‐ | 59.2% (58/98) | 2.0% (2/98) |
| Intensity of complaints (IO) | 2.0% (2) | 0.54 | 32.3% (32/99) | 0% (0/99) |
| Emotional complaints (EC) | 8.9% (9) | 0.86 | 12.0% (11/92) | 0% (0/92) |
Construct‐related validity was evaluated using linear regression models, which showed an acceptable association of single items with the proposed scores (Table S3). Three items (9.e, 9.h, and 9.i) assigned to “emotional complaints” revealed their strongest associations with other scores (“work‐related problems” and “activities of daily living limitations,” respectively) and three items (1.h, 7 and 8) assigned to “intensity of complaints” showed strong associations with “work‐related problems.” In summary, these findings support good construct‐related validity of the PEmb‐QoL questionnaire.
Most patients’ baseline characteristics (Table S4) and self‐reported socioeconomic status (Table S5) had only minor impact on PEmb‐QoL scores except pulmonary disease, which was associated with increased “activities of daily living limitations” and “work‐related problems” scores.
Cronbach's α coefficients for the different dimensions were in the acceptable range, except for the “intensity of complaints” score (0.54) (Table 2). Therefore, the reliability of the PEmb‐QoL questionnaire was mainly confirmed.
3.3. Study outcomes
As shown in Table 1, right column, as many as 47.0% of patients reported persisting dyspnea (NYHA class ≥II) at the time of follow‐up; of those, 19 patients (40.4%) were in NYHA class III/IV. Severe dyspnea (NYHA class III/IV) was associated with obesity, duration of hospital stay at baseline, and chronic pulmonary diseases (Table S7). Furthermore, 14 patients (15.9%) were diagnosed with depression (compared to 10.2% at the time of PE, P = .063). Depression at follow‐up was associated with female sex and the duration of hospital stay at baseline (Table S7). Although a higher number of patients was diagnosed with cancer and depression at follow‐up compared to baseline (Table 1), depression at follow‐up was not more prevalent in the patients with cancer compared to those without (5.9% vs. 18.1%, P = .253).
Overall, 91 patients (90.1%) underwent TTE at 6‐month follow‐up; of those, 25 (27.5%) were diagnosed with echocardiographic signs of RV dysfunction and 23 (25.3%) with PPEI. Surprisingly, acute RV dysfunction at the PE index event was neither predictive of RV dysfunction or PPEI nor of severe dyspnea (NYHA class III/IV) at follow‐up (Table S7). An adverse in‐hospital outcome was associated with PPEI (Table S7).
3.4. Association of study outcomes and QoL
While PEmb‐QoL dimensions were not affected by depression, cancer, and RV dysfunction on TTE at follow‐up, PPEI was associated with higher scores in “work‐related problems” [odds ratio, 3.4 (95% CI, 1.1‐10.8); P = .041] (Table 3). Severe dyspnea (NYHA class III/IV) affected all PEmb‐QoL dimensions except “emotional complaints” (Table 3) and increasing NYHA classes were strongly related to all PEmb‐QoL dimensions (Table S6).
Table 3.
Prognostic impact of study outcomes on PEmb‐QoL dimensions
| Study outcomes | Dyspnea (NYHA class III/IV) (19/101 [18.8%]) | RV dysfunction on TTE (25/91 [27.5%]) | Post‐PE impairment (23/91 [25.2%]) | Depression (14/89 [15.7%]) | Cancer (17/100 [17.0%]) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Frequency of complaints (FO) | 5.2 (2.0‐13.3) | .001 | 1.3 (0.6‐2.6) | .500 | 1.5 (0.7‐3.0) | .283 | 1.1 (0.5‐2.5) | .820 | 1.0 (0.4‐2.2) | .933 |
| Activities of daily living limitations (AD) | 10.6 (3.0‐36.9) | <.001 | 1.1 (0.5‐2.7) | .793 | 1.4 (0.6‐3.6) | .423 | 2.6 (0.7‐9.2) | .134 | 2.1 (0.8‐5.6) | .150 |
| Work‐related problems (WR) | 49.0 (7.2‐332.5) | <.001 | 2.5 (0.8‐7.7) | .109 | 3.4 (1.1‐10.8) | .041 | 1.7 (0. 4‐7.1) | .501 | 1.3 (0.4‐4.6) | .670 |
| Social limitations (SL) | 3.7 (2.1‐6.6) | <.001 | 1.1 (0.7‐1.7) | .660 | 1.2 (0.8‐1.8) | .416 | 1.0 (0.6‐1.7) | .979 | 1.0 (0. 6‐1.6) | .910 |
| Intensity of complaints (IO) | 4.5 (2.3‐9.0) | <.001 | 1.2 (0.8‐1.8) | .451 | 1.3 (0.8‐1.9) | .244 | 1.5 (0.9‐2.4) | .112 | 1.0 (0.7‐1.7) | .875 |
| Emotional complaints (EC) | 1.4 (0.8‐2.7) | .242 | 0.9 (0.5‐1.5) | .678 | 1.0 (0.5‐1.7) | .862 | 1.3 (0.7‐2.4) | .467 | 1.1 (0.6‐2.1) | .671 |
Abbreviations: CI, confidence interval; NYHA, New York Heart Association; OR, odds ratio; PE, pulmonary embolism; PEmb‐QoL, pulmonary embolism‐quality of life; RV, right ventricular; TTE, transthoracic echocardiography.
Long‐term survival status was available for all patients and 12 patients (11.9%) died during the observation period of 3.6 (IQR, 3.1‐4.5) years (range: 276‐1888 days). Interestingly, the PEmb‐QoL dimensions “activities of daily living limitations,” “work‐related problems,” “social limitations,” and “emotional complaints” were associated with an increased risk for long‐term mortality (Table 4, left column). In multivariable Cox regression analyses adjusted for sex and age, patients with poorer QoL in “activities of daily living limitations” and “work‐related problems” showed a more than four‐fold increased risk for long‐term mortality (Table 4, right column). In contrast, all clinical study outcomes at the 6‐month follow‐up, including PPEI and RV dysfunction on TTE, had no relevant impact on long‐term survival.
Table 4.
Prognostic value of the PEmb‐QoL dimensions and study outcomes at 6‐month follow‐up with regard to long‐term mortality
| Univariate Cox regression | Adjusted for age and sex | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| PEmb‐QoL dimensions | ||||
| Frequency of complaints (FO) | 1.2 (0.5‐2.9) | .707 | 1.2 (0.5‐2.9) | .669 |
| Activities of daily living limitations (AD) | 5.4 (1.9‐15.0) | .001 | 5.3 (1.6‐17.8) | .007 |
| Work‐related problems (WR) | 4.5 (1.2‐16.8) | .024 | 4.2 (1.1‐16.4) | .038 |
| Social limitations (SL) | 1.7 (1.1‐2.7) | .010 | 1.7 (1.1‐2.6) | .022 |
| Intensity of complaints (IO) | 1.5 (0.9‐2.4) | .106 | 1.4 (0.8‐2.3) | .146 |
| Emotional complaints (EC) | 1.8 (1.0‐3.3) | .038 | 1.8 (1.0‐3.3) | .048 |
| Study outcomes at the 6‐mo follow‐up | ||||
| Dyspnea (NYHA class III/IV) | 1.5 (0.7‐3.0) | .309 | 1.4 (0.7‐2.9) | .364 |
| RV dysfunction on TTE | 1.1 (0.3‐4.3) | .850 | 1.0 (0.3‐4.0) | .956 |
| Post‐PE impairment | 1.3 (0.3‐4.8) | .742 | 1.2 (0.3‐4.5) | .809 |
| Depression | 1.3 (0.3‐6.0) | .775 | 1.0 (0.2‐5.2) | .963 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NYHA, New York Heart Association; PE, pulmonary embolism; PEmb‐QoL, pulmonary embolism‐quality of life; RV, right ventricular; TTE, transthoracic echocardiography.
4. DISCUSSION
The main study findings can be summarized as follows: (i) the German PEmb‐QoL questionnaire is a valid and reliable instrument for assessing disease‐specific QoL 6 months after an acute PE; (ii) as many as 47.0% of patients presented with dyspnea, 27.5% with RV dysfunction on TTE, and 25.3% with PPEI at 6‐month follow‐up; (iii) 15.9% were diagnosed with depression 6 months after an acute PE; (iv) QoL was affected by dyspnea and PPEI; and (v) reduced QoL was associated with an increased risk for long‐term mortality.
4.1. Validation of the German PEmb‐QoL questionnaire
The results of this present validation study indicate that the German PEmb‐QoL questionnaire is a valid and reliable tool for assessing disease‐specific QoL 6 months after an acute PE. However, some weaknesses were identified: Although its construct‐related validity was acceptable (Table S3) and its criterion‐related validity was previously confirmed by other studies16, 18, 19, 20 (and therefore not tested in the present study again), the internal consistency measured by Cronbach's α coefficients was in an acceptable range for all dimensions except for “intensity of complaints.” Nevertheless, the reliability of the questionnaire was widely confirmed. Of note, the reproducibility as the test‐retest stability of the PEmb‐QoL questionnaire was not tested again because its stability was already confirmed in several previous studies.16, 18, 19, 20 Although Klok et al.16 reported only small floor and ceiling effects, we identified, similarly to previous studies,18, 19, 20 distinct floor and ceiling effects of up to 59.2% (Table 2). Thus, the ability of the PEmb‐QoL questionnaire to detect small changes in QoL of mildly affected patients might be limited especially in the dimensions “work‐related problems” and “social limitations.” However, since QoL is operationalized as the absence of subjective impairments, 53.0% of our patients reported that they never experienced lung symptoms and 22.1% negated the presence of any lung problems at follow‐up visit. Hence, the prevalence of distinct floor effects in our study seems plausible and unavoidable rather than a major limitation.
4.2. Symptoms and functional limitations 6 months after acute PE
It is increasingly acknowledged that a (single) PE event can cause restricting and profound alterations such as persisting symptoms and cardiopulmonary functional limitations over the long term.3, 8 In accordance with the literature, in the present study, every second patient suffered from persisting symptoms 6 months after PE: as many as 47.0% of patients reported dyspnea (NYHA class ≥II), which was severe (NYHA class III/IV) in 19.0% of all patients. Unexpectedly, and probably because of the small sample size, although higher NYHA classes have been shown to be associated with increased mortality in patients with chronic heart failure,32 in the present study, severe dyspnea at 6‐month follow‐up had no prognostic impact with regard to long‐term mortality (Table 4). Furthermore, every fourth patient was diagnosed with RV dysfunction on TTE at 6‐month follow‐up. In comparison, RV dysfunction on TTE was observed by Stevinson et al9 in 16.5% of 109 previously healthy (free of cardiopulmonary diseases or other disabling processes) patients 6 months after a first‐time PE, by Golpe et al33 in 17.8% of 95 initially hemodynamic stable PE patients after 6 months, by Samaranayake et al34 in 14.3% of 42 patients with “submassive” PE after a mean follow‐up of 7.6 months, and by Held et al35 in 11.5% of 130 symptomatic patients 6 months after newly diagnosed PE. The higher prevalence in our study might be related to different definitions of RV dysfunction in the respective studies. Consistent with our observation that RV dysfunction on TTE at 6‐month follow‐up was no predictor of long‐term mortality (Table 4), Golpe et al.33 as well as Samaranayake et al.34 reported that RV dysfunction at follow‐up was not associated with long‐term mortality after a mean of 2.8 ± 1.1 years and after 1 year, respectively.
Since RV dysfunction on TTE constitutes an unspecific finding that may also be related to underlying comorbidities such as cardiopulmonary diseases, a number of attempts have been undertaken in the past to define long‐term sequelae of PE more precisely. For example, the term “post‐PE syndrome” has been introduced and proposed to be defined as (i) persistence or worsening of cardiac or pulmonary function in combination with (ii) deterioration of the clinical symptoms, functional status, or patients’ quality of life (iii) in the absence of an obvious alternative explanation.3 However, diagnostic criteria and thresholds to define “post‐PE syndrome” are not available thus far. The ongoing “FOllow‐up after aCUte pulmonary emboliSm” (FOCUS) study aims to determine the incidence of CTEPH and persisting or progressive functional and/or hemodynamic PPEI over a 2‐year follow‐up period after an index episode of acute symptomatic PE.26 For this purpose, a definition of PPEI was introduced by the FOCUS steering committee26 and was used in slight modification in the present study. Overall, in the present study, 25.3% of patients were diagnosed with PPEI at 6‐month follow‐up. In comparison, in the Evaluation of Long‐Term Outcomes after PE (ELOPE) study, following 100 patients 1, 3, 6, and 12 months after acute PE at five Canadian hospitals between 2010 and 2013, 46.5% of patients had functional limitations (defined as peak oxygen consumption <80% on cardiopulmonary exercise testing) 1 year after the PE event.7 The authors concluded that deconditioning might have contributed to this notably high prevalence. Data from Germany indicate that 35.3% of symptomatic patients with a normal echocardiogram have functional limitation on cardiopulmonary exercise testing 3 months after an episode of acute PE.35
Assuming that persisting or progressive functional and/or hemodynamic impairment after acute PE is an early indicator of the subsequent development of CTEPH, PPEI has been defined as a coprimary outcome in the FOCUS study.26 Indeed, a recent cohort study demonstrated that patients fulfilling all diagnostic criteria of CTEPH despite elevation of mean pulmonary artery pressure (a condition called “chronic thromboembolic disease”) showed similar objective functional impairment using cardiopulmonary exercise testing compared to patients with CTEPH.36 These assumptions become of clinical importance if aiming to define who, when, and how patients should be followed after acute PE. At present, current guidelines concordantly state that screening for CTEPH in asymptomatic survivors of PE is not recommended.2, 24 However, the present study findings and the increasing body of evidence may indicate that, even in the absence of “typical” symptoms, patients with PPEI might benefit from closer follow‐up.37
4.3. Associations between QoL and study outcomes
In the present study, every sixth patient was diagnosed with depression at 6‐month follow‐up. While depression was associated with female sex and a longer duration of in‐hospital stay, no higher prevalence was observed in cancer patients. Interestingly, and in contrast to previous studies using more “global” questionnaires,38, 39 depression and cancer did not affect QoL assessed by the PEmb‐QoL questionnaire in the present analysis (Table 3). Thus, the PEmb‐QoL questionnaire appears less useful to detect depressive episodes. Since an acute PE may result in emotional distress reactions reaching from anxiety to posttraumatic stress disorder, screening for depression using validated methods (for example, the Hospital Anxiety and Depression Scale40) should be included in routine follow‐up of PE patients.
As shown in Figure 2, patients’ QoL 6 months after PE was especially reduced in the PEmb‐QoL dimensions “activities of daily living limitations,” “intensity of complaints,” and “emotional complaints.” The strongest association with PEmb‐QoL dimensions was observed for dyspnea: increasing severity of dyspnea did affect all PEmb‐QoL dimensions (Table S6); the only baseline variable affecting PEmb‐QoL dimensions was preexisting pulmonary disease (Table S4). This observation is not surprising since all questions in the PEmb‐QoL refer to “lung problems.”16 Post‐pulmonary embolism incident was the only other outcome related to QoL (Table 3); however, only the dimension “work‐related problems” appeared to be affected [median score in patients with PPEI 1.5 (95% CI 1.0‐2.0) compared to 1.0 (95% CI 1.0‐1.7) in patients without PPEI, P = .055]. Study design and patients’ characteristics may substantially affect the interpretation of study findings related to QoL. In the present analysis, most patients’ baseline characteristics and self‐reported socioeconomic status had only minor impact on PEmb‐QoL dimensions (Tables S4 and S5), as reported previously.16 In contrast to previous studies with large follow‐up periods of >6 years,16, 20 the present cohort consists of consecutive patients who underwent follow‐up examination after 208 (IQR, 185‐242) days, allowing for analysis of a homogeneous study population. Since previous studies assessed QoL in median >2 years after a PE event,16, 18, 27 comparability might be limited.
This is the first study demonstrating that a reduced QoL in survivors of an acute PE episode was associated with an increased risk for long‐term mortality after a median observation period of 3.6 years (Table 4). Highest odds ratio was observed for the PEmb‐QoL dimension “activities of daily living limitations” [odds ratio 5.3 (95% CI 1.6‐17.8)], while other clinical outcomes were not identified as predictors of long‐term mortality. This finding is in accordance with studies investigating patients with heart failure, stroke, and coronary artery disease.12, 41, 42 At first sight, it appears confusing that on the one hand most of the PEmb‐QoL dimensions were influenced by dyspnea and a reduced QoL was associated with a reduced probability of long‐term survival, while on the other hand severe dyspnea at 6‐month follow‐up had no impact on long‐term survival. However, this discrepancy might be explained by the fact that the PEmb‐QoL questionnaire contains questions about physical capacity (daily living and working) that might reflect the overall patients’ condition and, in case of a low score in these dimensions, frailty. Thus, by providing information on “overall physical condition,” it appears less surprising that the prognostic performance of the PEmb‐QoL questionnaire with regard to long‐term mortality is superior to that of dyspnea.
4.4. Limitations
Although the number of patients included in the present study was comparable to previous studies investigating quality of life after acute PE,7, 16, 19, 20 the small sample size constitutes a potential limitation of our study.
5. CONCLUSION
The results of the present study indicate that dyspnea, RV dysfunction on TTE, PPEI, and reduced QoL are frequent findings in survivors of PE 6 months after the acute event. Quality of life is associated with an increased risk of long‐term mortality and can reliably be assessed by the German PEmb‐QoL questionnaire. Thus, assessment of presence and severity of symptoms, functional limitations, QoL, and screening for depression after an episode of acute PE should be implemented in future outcome trials in short‐term and long‐term management of acute PE.
ADDENDUM
K. Keller analyzed the data and drafted and revised the manuscript critically for important intellectual content. C. Tesche acquired and analyzed the data and revised the manuscript critically for important intellectual content. A. Gerhold‐Ay analyzed the data and revised the manuscript critically for important intellectual content. S. Nickels analyzed the data and revised the manuscript critically for important intellectual content. F. A. Klok interpreted the data and revised the manuscript critically for important intellectual content. L. Rappold acquired and interpreted the data and revised the manuscript critically for important intellectual content. G. Hasenfuß interpreted the data and revised the manuscript critically for important intellectual content. C. Dellas acquired and interpreted the data and revised the manuscript critically for important intellectual content. S. Konstantinides interpreted the data and revised the manuscript critically for important intellectual content. M. Lankeit designed and supervised the study, acquired and analyzed the data, and drafted and revised the manuscript critically for important intellectual content. All authors gave final approval of the version of the manuscript to be published and agreed to be accountable for all aspects of the work.
CONFLICT OF INTERESTS
None of the authors reports a relationship with industry or other relevant entities – financial or otherwise – that might pose a conflict of interest in connection with the submitted article. The following authors report financial activities outside the submitted work: F. A. Klok reports research grants from the Dutch Heart Foundation, Dutch Thrombosis Association, Actelion, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi‐Sankyo, and MSD. G. Hasenfuß reports having received consultancy and lecture honoraria from AstraZeneca, Corvia, Impulse Dynamics, Novartis, Berlin Chemie, Servier, and Vifor Pharma and editor honoraria from Springer International Publishing AG. S. V. Konstantinides reports having received consultancy and lecture honoraria from Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, MSD, and Pfizer‐Bristol‐Myers Squibb; and institutional grants from Actelion, Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, and Pfizer‐Bristol‐Myers Squibb. M. Lankeit reports having received consultancy and lecture honoraria from Actelion, Bayer, Daiichi‐Sankyo, MSD, and Pfizer‐Bristol‐Myers Squibb; and research funding from BRAHMS–Thermo Fisher Scientific. The other authors report no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Dr. F. Schulze, Prof. Dr. L. Lüthje, Dr. C. Buck, and Prof. Dr. F. Edelmann (Clinic of Cardiology and Pulmonology, University Medical Center, Goettingen, Germany) for assistance in performing the 6‐month follow‐up visits. We thank V. Seeber, A. Ullmann, and A. Schulz (Center for Thrombosis and Hemostasis, University Medical Center, Mainz, Germany) for statistical assistance and Prof. Dr. H. Binder [Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), Mainz, Germany] for senior statistical advice.
Keller K, Tesche C, Gerhold‐Ay A, et al. Quality of life and functional limitations after pulmonary embolism and its prognostic relevance. J Thromb Haemost. 2019;17:1923–1934. 10.1111/jth.14589
Funding information
This study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and BMBF 01EO1503; institutional grant to the Center for Thrombosis and Hemostasis of the University Medical Center Mainz, Germany). The authors are responsible for the contents of this publication.
Manuscript handled by: Sabine Eichinger
Final decision: Sabine Eichinger 24 July 2019
REFERENCES
- 1. Fanikos J, Piazza G, Zayaruzny M, Goldhaber SZ. Long‐term complications of medical patients with hospital‐acquired venous thromboembolism. Thromb Haemost. 2009;102:688‐693. [DOI] [PubMed] [Google Scholar]
- 2. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033‐3069, 69a‐69k. [DOI] [PubMed] [Google Scholar]
- 3. Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post‐PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28:221‐226. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez O, Helley D, Couchon S, et al. Perfusion defects after pulmonary embolism: risk factors and clinical significance. J Thromb Haemost. 2010;8:1248‐1255. [DOI] [PubMed] [Google Scholar]
- 5. Cosmi B, Nijkeuter M, Valentino M, Huisman MV, Barozzi L, Palareti G. Residual emboli on lung perfusion scan or multidetector computed tomography after a first episode of acute pulmonary embolism. Intern Emerg Med. 2011;6:521‐528. [DOI] [PubMed] [Google Scholar]
- 6. Pesavento R, Filippi L, Palla A, et al. Impact of residual pulmonary obstruction on the long‐term outcome of patients with pulmonary embolism. Eur Respir J. 2017;49:1601980. [DOI] [PubMed] [Google Scholar]
- 7. Kahn SR, Hirsch AM, Akaberi A, et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE prospective cohort study. Chest. 2017;151:1058‐1068. [DOI] [PubMed] [Google Scholar]
- 8. Sista AK, Miller LE, Kahn SR, Kline JA. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: systematic review with meta‐analysis. Vasc Med. 2017;22:37‐43. [DOI] [PubMed] [Google Scholar]
- 9. Stevinson BG, Hernandez‐Nino J, Rose G, Kline JA. Echocardiographic and functional cardiopulmonary problems 6 months after first‐time pulmonary embolism in previously healthy patients. Eur Heart J. 2007;28:2517‐2524. [DOI] [PubMed] [Google Scholar]
- 10. Noble S, Lewis R, Whithers J, Lewis S, Bennett P. Long‐term psychological consequences of symptomatic pulmonary embolism: a qualitative study. BMJ Open. 2014;4:e004561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klok FA, van Kralingen KW, van Dijk AP, et al. Quality of life in long‐term survivors of acute pulmonary embolism. Chest. 2010;138:1432‐1440. [DOI] [PubMed] [Google Scholar]
- 12. O'Loughlin C, Murphy NF, Conlon C, O'Donovan A, Ledwidge M, McDonald K. Quality of life predicts outcome in a heart failure disease management program. Int J Cardiol. 2010;139:60‐67. [DOI] [PubMed] [Google Scholar]
- 13. Mathai SC, Suber T, Khair RM, Kolb TM, Damico RL, Hassoun PM. Health‐related quality of life and survival in pulmonary arterial hypertension. Ann Am Thorac Soc. 2016;13:31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross‐sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867‐1876. [DOI] [PubMed] [Google Scholar]
- 15. Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klok FA, Cohn DM, Middeldorp S, et al. Quality of life after pulmonary embolism: validation of the PEmb‐QoL Questionnaire. J Thromb Haemost. 2010;8:523‐532. [DOI] [PubMed] [Google Scholar]
- 17. Cohn DM, Nelis EA, Busweiler LA, Kaptein AA, Middeldorp S. Quality of life after pulmonary embolism: the development of the PEmb‐QoL questionnaire. J Thromb Haemost. 2009;7:1044‐1046. [DOI] [PubMed] [Google Scholar]
- 18. Tavoly M, Jelsness‐Jorgensen LP, Wik HS, Roaldsnes C, Sandset PM, Ghanima W. Quality of life after pulmonary embolism: first cross‐cultural evaluation of the pulmonary embolism quality‐of‐life (PEmb‐QoL) questionnaire in a Norwegian cohort. Qual Life Res. 2015;24:417‐425. [DOI] [PubMed] [Google Scholar]
- 19. Rochat M, Mean M, Limacher A, et al. Quality of life after pulmonary embolism: validation of the French version of the PEmb‐QoL questionnaire. Health Qual Life Outcomes. 2014;12:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frey PM, Mean M, Limacher A, et al. Quality of life after pulmonary embolism: prospective validation of the German version of the PEmb‐QoL questionnaire. Thromb Res. 2015;135:1087‐1092. [DOI] [PubMed] [Google Scholar]
- 21. Sun X, Li J, Shi J. Validating the Chinese version of the PEmb‐QoL questionnaire: a measure for quality of life assessment after pulmonary embolism. Thromb Res. 2018;166:86‐91. [DOI] [PubMed] [Google Scholar]
- 22. Klok FA, Tesche C, Rappold L, et al. External validation of a simple non‐invasive algorithm to rule out chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2015;135:796‐801. [DOI] [PubMed] [Google Scholar]
- 23. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of A . Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119‐2126. [DOI] [PubMed] [Google Scholar]
- 24. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67‐119. [DOI] [PubMed] [Google Scholar]
- 25. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685‐713. [DOI] [PubMed] [Google Scholar]
- 26. Konstantinides SV, Barco S, Rosenkranz S, et al. Late outcomes after acute pulmonary embolism: rationale and design of FOCUS, a prospective observational multicenter cohort study. J Thromb Thrombolysis. 2016;42:600‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Es J, den Exter PL, Kaptein AA, et al. Quality of life after pulmonary embolism as assessed with SF‐36 and PEmb‐QoL. Thromb Res. 2013;132:500‐505. [DOI] [PubMed] [Google Scholar]
- 28. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34‐42. [DOI] [PubMed] [Google Scholar]
- 29. Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality‐of‐life instruments: attributes and review criteria. Qual Life Res. 2002;11:193‐205. [DOI] [PubMed] [Google Scholar]
- 30. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297‐334. [Google Scholar]
- 31. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate‐risk pulmonary embolism. N Engl J Med. 2014;370:1402‐1411. [DOI] [PubMed] [Google Scholar]
- 32. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Golpe R, Testa‐Fernandez A, Perez‐de‐Llano LA, et al. Long‐term clinical outcome of patients with persistent right ventricle dysfunction or pulmonary hypertension after acute pulmonary embolism. Eur J Echocardiogr. 2011;12:756‐761. [DOI] [PubMed] [Google Scholar]
- 34. Samaranayake CB, Royle G, Jackson S, Yap E. Right ventricular dysfunction and pulmonary hypertension following sub‐massive pulmonary embolism. Clin Respir J. 2017;11:867‐874. [DOI] [PubMed] [Google Scholar]
- 35. Held M, Hesse A, Gott F, et al. A symptom‐related monitoring program following pulmonary embolism for the early detection of CTEPH: a prospective observational registry study. BMC Pulm Med. 2014;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Held M, Kolb P, Grun M, et al. Functional characterization of patients with chronic thromboembolic disease. Respiration. 2016;91:503‐509. [DOI] [PubMed] [Google Scholar]
- 37. Ende‐Verhaar YM, Huisman MV, Klok FA. To screen or not to screen for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2017;151:1‐7. [DOI] [PubMed] [Google Scholar]
- 38. Quinten C, Coens C, Ghislain I, et al. The effects of age on health‐related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 involving 6024 cancer patients. Eur J Cancer. 2015;51:2808‐2819. [DOI] [PubMed] [Google Scholar]
- 39. Sivertsen H, Bjorklof GH, Engedal K, Selbaek G, Helvik AS. Depression and quality of life in older persons: a review. Dement Geriatr Cogn Disord. 2015;40:311‐339. [DOI] [PubMed] [Google Scholar]
- 40. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 41. Ayis S, Wellwood I, Rudd AG, McKevitt C, Parkin D, Wolfe CD. Variations in Health‐Related Quality of Life (HRQoL) and survival 1 year after stroke: five European population‐based registers. BMJ Open. 2015;5:e007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long‐term outcome in outpatients with coronary disease. Circulation. 2002;106:43‐49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


