Abstract
Objective
To prospectively assess the efficacy, general safety, and joint safety of fasinumab, an anti–nerve growth factor monoclonal antibody, in osteoarthritis (OA) hip and/or knee pain.
Methods
Patients with moderate‐to‐severe OA pain (knee or hip) and history of inadequate response or intolerance to analgesics were randomized to receive fasinumab (at 1 mg, 3 mg, 6 mg, or 9 mg) or placebo every 4 weeks over 16 weeks and were followed up to week 36. Efficacy end points were the change from baseline to week 16 in the pain and physical function subscale scores of the Western Ontario and McMaster Universities OA Index (WOMAC), and patient global assessment (PGA) of OA. Joints were monitored at scheduled assessments (by plain film radiography and magnetic resonance imaging) during treatment and follow‐up, and if prompted, at the time of active joint symptoms.
Results
Of the 421 patients randomized, 342 completed the 36‐week study. All doses of fasinumab yielded statistically significant and clinically important reductions in pain compared to placebo (least squares mean difference in WOMAC pain subscale scores at week 16 ranging −0.78 to −1.40), without any clear dose dependence. Physical function and PGA scores improved in parallel. Treatment‐emergent adverse event rates were 17% with fasinumab and 10% with placebo, and 4% and 1% of patients, respectively, discontinued treatment. Arthropathies (25 in total, 7% of fasinumab‐treated patients and 1% of placebo‐treated patients) occurred in a dose‐dependent manner, with 2 occurring in patients receiving the lowest dose of fasinumab and 10 in patients receiving the highest dose. Most of the arthropathies (16 of 25) were discovered with scheduled radiographs and not based on symptoms. Destructive arthropathy (in 1 of 337 treated patients) occurred in 1 patient who was receiving 6 mg fasimumab.
Conclusion
Fasinumab provided improvements in OA pain and function, even in those benefitting little from previous analgesics. The observed benefit‐to‐risk relationship favors further clinical development to explore the lowest doses of fasinumab in patients with knee or hip OA.
Introduction
Nerve growth factor (NGF), a neurotrophin released by injured or inflamed tissue, mediates peripheral pain by binding to its receptors, tropomyosin receptor kinase A and p75, on nociceptive neurons 1. Although strongly expressed on nociceptive neurons, the tissue distribution of these receptors is broader and includes bone and cartilage as well as other non‐neuronal tissues 1. Biologic agents that specifically block NGF to treat pain may obviate many of the side effects of currently used analgesic medications, such as opioids and nonsteroidal antiinflammatory drugs (NSAIDs), which rely on different mechanisms of action 2, 3. This new therapeutic could benefit patients experiencing pain from osteoarthritis (OA), a progressive, chronic disease characterized by joint breakdown and functional loss 3. However, NGF‐directed therapies exhibit their own unique side effect profile in OA, which includes alterations in peripheral sensation and development of arthropathies 3, 4, 5.
Fasinumab is a recombinant, fully human, IgG4 anti‐NGF monoclonal antibody that binds selectively to NGF without affecting signaling via other neurotrophins, such as neurotrophin 3 and brain‐derived neurotrophic factor 6. In a proof‐of‐concept study involving 217 patients with OA knee pain, fasinumab (administered intravenously on days 1 and 57 of the 24‐week study at 0.03, 0.1, and 0.3 mg/kg—corresponding to approximate doses of 2 mg, 7 mg, and 20 mg, respectively, per administration) was generally well tolerated and, compared to placebo, significantly reduced walking knee pain and improved the Western Ontario and McMaster Universities OA Index (WOMAC) subscale scores for pain and function at the 8‐ or 16‐week assessments 6. In that study, the 2 highest doses provided generally greater benefits than the lowest dose. Based on these results, the doses of fasinumab selected for further study ranged from 1 mg to 9 mg subcutaneously every 4 weeks.
The current study assessed the efficacy and safety of fasinumab in patients with moderate‐to‐severe knee and/or hip OA pain who had an inadequate response or intolerance to standard‐of‐care analgesic therapies, including NSAIDs, acetaminophen, or opioids. To better understand the benefits and risks of this new therapeutic agent, this study was designed to evaluate pain relief and functional benefit while closely monitoring side effects, including symptomatic and clinically silent joint changes. Extensive radiographic monitoring of the joints was performed at baseline and over the course of the trial, supplemented by additional imaging prompted by any clinically meaningful change in joint symptoms. Findings were adjudicated by a blinded committee of expert bone radiologists. This was the first study with an anti‐NGF antibody to prospectively conduct regular radiologic evaluation of major joints over the course of a clinical study in all patients, while assessing clinical joint symptoms.
Patients and Methods
Patients
Eligible patients were ages 40–80 years, had OA of the knee and/or hip based on the American College of Rheumatology OA classification criteria 7, 8, with radiologic confirmation of the diagnosis based on a Kellgren/Lawrence (K/L) severity grade of ≥2 on a scale of 0–4 9, and demonstrated moderate‐to‐severe OA pain, defined as a WOMAC pain subscale score of ≥4 both at screening (index joint being selected according to the worst pain and K/L score) while receiving the usual analgesic medications, and at randomization, 7 days after withdrawal of the analgesic therapy. Eligible patients had a history of inadequate pain relief with, or intolerance to, acetaminophen, ≥1 oral NSAID, and ≥1 opioid (or unwillingness to use opioids) and required regular analgesic use for OA pain (average of 4 days/week during the 4 weeks prior to screening). Patients were excluded if they had a history of other joint diseases, index joint trauma within 30 days of screening, active fibromyalgia, another moderate‐to‐severe pain condition, or a body mass index (BMI) of >39 kg/m2.
Study design
This phase IIb/III double‐blind, placebo‐controlled study was conducted at 61 sites in the United States. Patients were randomized (1:1:1:1:1) to receive fasinumab at 1 mg, 3 mg, 6 mg, or 9 mg or placebo, administered subcutaneously every 4 weeks for a total of 4 doses, with the last dose at 12 weeks. The primary efficacy analysis was conducted at 16 weeks (see Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41012/abstract), and follow‐up was carried out until week 36.
Random number generation with SAS software was used to assign treatment, via a centralized Interactive Voice‐Web Response System. Patients were stratified by index joint (knee or hip) and K/L score (grades 2 or 3 versus grade 4) using block sizes of 5. The investigational product (IP) was provided to sites in 1‐ml vials (6 mg/ml fasinumab or placebo, in a blinded manner). The site study pharmacist (or designee) prepared the volume for each patient and administered it without knowledge as to whether the IP was fasinumab or placebo. All other site personnel involved in assessment of patients were blinded with regard to treatment assignment. Efficacy and safety assessments were performed through week 36.
Patients were required to stop analgesic medications at a prerandomization visit, 7 days before randomization. Pain scores were obtained before and after withdrawal of previous analgesics. Although these scores had to meet a pain threshold (pain score of ≥4 points on a 0–10 scale), there was no requirement for pain flare.
From the time of the prerandomization visit and continuing through week 20, patients could take rescue analgesics (1–2 tablets of acetaminophen at 325 mg every 4–6 hours) as needed for intolerable pain (maximum of 2,600 mg per day), which had to be discontinued ≥48 hours prior to the start of each study visit through week 16. Patients could receive opioids after the week 16 visit, if needed, but were not allowed to take any NSAIDs (oral or topical, except aspirin ≤100 mg/day for cardiac prophylaxis) until ≥16 weeks after the last dose of study drug (week 28).
An independent data monitoring committee periodically reviewed all unblinded data and made recommendations to the sponsor as to the conduct of the study, in accordance with the ethics principles outlined in the Declaration of Helsinki and consistent with International Conference on Harmonisation Guidelines for Good Clinical Practice and applicable regulatory requirements. The study committees included the independent data monitoring committee and a joint adjudication committee, as described in Supplementary Methods (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41012/abstract). Informed consent was obtained from all patients prior to enrollment.
Efficacy end points
The primary efficacy end point was change from baseline to week 16 in the WOMAC pain subscale score (scale of 0–10), which represented the average score in response to 5 questions assessing joint pain while walking, using stairs, at rest in bed, sitting or lying, and standing (minimal clinically important difference [MCID] 0.75 [10]).
Secondary efficacy end points were change from baseline to week 16 in the WOMAC physical function subscale score (scale of 0–10, average score in response to 17 questions; MCID 0.67 [10]) and patient global assessment (PGA) of OA (scale of 1–5, with 5 being worst [11]).
Exploratory efficacy end points included the following: daily and weekly walking index joint pain scores on a Numeric Rating Scale (NRS) (scale of 0–10, with 0 = no pain; MCID ~1 point [12]); the percentage of patients who responded to treatment according to ≥30% and ≥50% reductions at week 16 in the WOMAC pain and physical function subscale scores; and rate of treatment response on the Outcome Measures in Rheumatology (OMERACT)–Osteoarthritis Research Society International (OARSI) responder index 13. An additional post hoc exploratory analysis was performed to assess the response to fasinumab according to the occurrence of pain flare after discontinuation of a prior analgesic, defined by thresholds of change in the score from screening to randomization of −1, −1.5, and −2 points on the 10‐point WOMAC pain subscale.
Safety end points
Safety was evaluated based on the frequency of treatment‐emergent adverse events (TEAEs), AEs of special interest (adjudicated arthropathy and sympathetic nervous system dysfunction), and laboratory tests. Joint safety was monitored in all patients via plain radiographs of the shoulders, hips, and knees at screening, at the end of the treatment period (week 16), and at study end (week 36). Imaging was also conducted at any time for worsening joint pain that was assessed as inconsistent with the patient's normal OA pain. Magnetic resonance imaging (MRI) was performed at baseline, 16 weeks, and 36 weeks on the index and contralateral joints, and on any joint with a K/L score at baseline of ≥3. Additional MRIs were performed if follow‐up radiographs exhibited important interval changes.
Based on reports of joint AEs that were previously described in clinical trials with anti‐NGF antibodies 3, 4, 5, the incidence of adjudicated arthropathy, an umbrella term for rapidly progressive OA type 1 (RPOA‐1), RPOA‐2, subchondral insufficiency fracture, and primary osteonecrosis, was determined during this study. RPOA‐1 was defined as joint space narrowing exceeding prespecified thresholds. For a baseline joint space width (JSW) of ≥2 mm, the reduction had to be ≥2 mm or 50% (whichever was greater). For joints with a baseline JSW of <2 mm, a reduction in JSW of 0 qualified as RPOA‐1. For the hips, thresholds were similar, except that the criteria centered on the baseline JSW and change in JSW of 1.5 mm. MRI was used to confirm cartilage loss in RPOA‐1. RPOA‐2 was defined as changes in bone structure on plain film radiography or MRI.
Primary osteonecrosis was defined as a focal circumscribed or extended region of mottled radiolucency without evidence of subchondral collapse or bone fragmentation, as confirmed by MRI. Subchondral insufficiency fracture was defined as subchondral radiolucency, a possible sclerotic linear component, and articular surface flattening without significant collapse or fragmentation, as confirmed by MRI. Based on imaging studies, all suspected arthropathies were adjudicated by an independent blinded committee of musculoskeletal radiologists with formal training on reading methodology, using training set image data.
In addition, patients were monitored for sympathetic nervous system dysfunction using prespecified criteria, including an autonomic dysfunction questionnaire and thresholds for positional changes in blood pressure or heart rate.
Statistical analysis
The statistical analysis plan was designed prior to the study start and finalized prior to database lock and unblinding. A sample size of 375 randomized patients was required in balanced allocation to the 5 treatment arms in order to detect a difference of 1.1 in the primary end points (active treatment versus placebo), with an assumed SD of 2.3 and Type 1 error rate of 0.05, providing statistical power of at least 83%. A combination of the Hochberg procedure 14 and gatekeeping method was used to address multiplicity, by applying the Hochberg method to test first the 6‐mg and 9‐mg doses versus placebo, at a significance level of 0.05, followed by sequential testing of the 3‐mg and 1‐mg doses versus placebo, each at a significance level of 0.05.
Efficacy variables were analyzed using a mixed‐effects model repeated‐measures (MMRM) approach. The model included randomization strata, baseline score, treatment, and treatment‐by‐visit interaction. Data from all patients were used in the primary efficacy analysis according to intent‐to‐treat principles, using the MMRM approach and with no imputation for missing data. Least squares (LS) mean values for the change from baseline to week 16, as well as LS mean differences between fasinumab doses and placebo, with their corresponding SEs, P values, and 95% confidence intervals, were obtained by MMRM.
Results
Disposition of the patients
The study was conducted from May 2015 through July 2016, during which 1,214 patients with knee and/or hip OA were screened, and 421 were randomized to receive either fasinumab (n = 338) or placebo (n = 83) (Figure 1), with follow‐up through week 36. During the study, 419 patients received ≥1 dose of study medication (1 patient randomized to receive placebo and 1 randomized to receive 9 mg fasinumab discontinued before drug administration). A total of 342 patients completed the entire 36‐week study (n = 294 in the fasinumab group [87%] and n = 67 in the placebo group [81%]).
Figure 1.
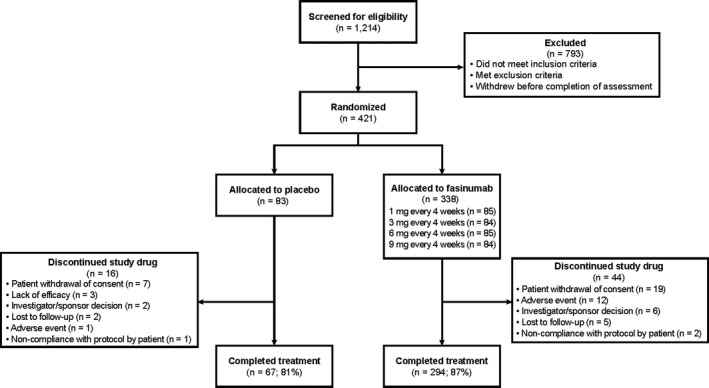
Disposition of the patients.
Demographic and baseline clinical characteristics of the patients
Demographics and baseline clinical characteristics were generally balanced across the treatment groups (Table 1). Most patients (66%) had K/L radiologic OA severity scores of 3 or 4. The index joint was mostly the knee (~88% of patients). Patients who were enrolled had a history of inadequate pain relief with acetaminophen (99%), NSAIDs (98%), and opioids (45%), with 2.6%, 8.3%, and 19% of patients, respectively, reporting intolerance to these drugs. Furthermore, 64% of patients were unwilling to take opioids. Of those who took opioids in the past, nearly 100% reported having tried both strong (e.g., hydrocodone) and weak (e.g., tramadol) opioids.
Table 1.
Patient demographics and baseline clinical characteristics (full analysis set)a
| Characteristic | Placebo (n = 83) | Fasinumab | Total (n = 421) | ||||
|---|---|---|---|---|---|---|---|
| 1 mg (n = 85) | 3 mg (n = 84) | 6 mg (n = 85) | 9 mg (n = 84) | Combined (n = 338) | |||
| Age, mean ± SD years | 60.1 ± 7.2 | 60.7 ± 8.9 | 60.7 ± 8.9 | 60.1 ± 7.9 | 61.5 ± 7.8 | 60.6 ± 8.1 | 60.6 ± 8.1 |
| Female, no. (%) | 54 (65.1) | 59 (69.4) | 54 (64.3) | 51 (60.0) | 54 (64.3) | 218 (64.5) | 272 (64.6) |
| White, no. (%) | 65 (78.3) | 64 (75.3) | 61 (72.6) | 61 (71.8) | 67 (79.8) | 253 (74.9) | 318 (75.5) |
| BMI, mean ± SD kg/m2 | 31.8 ± 4.5 | 30.6 ± 5.0 | 30.9 ± 4.7 | 30.5 ± 4.9 | 31.8 ± 5.0 | 30.95 ± 4.9 | 31.12 ± 4.9 |
| Index joint, no. (%) | |||||||
| Hip | 9 (10.8) | 10 (11.8) | 10 (11.9) | 11 (12.9) | 10 (11.9) | 41 (12.1) | 50 (11.9) |
| Knee | 74 (90.2) | 75 (88.2) | 74 (88.1) | 74 (87.1) | 74 (88.1) | 297 (88.1) | 371 (88.1) |
| K/L score, no. (%) | |||||||
| 1 | 0 | 0 | 0 | 0 | 1 (1.2) | 1 (0.3) | 1 (0.2) |
| 2 | 23 (27.7) | 31 (36.5) | 30 (35.7) | 30 (35.3) | 28 (33.3) | 119 (35.2) | 142 (33.7) |
| 3 | 26 (31.3) | 20 (23.5) | 21 (25.0) | 20 (23.5) | 21 (25.0) | 82 (24.3) | 108 (25.7) |
| 4 | 34 (41.0) | 34 (40.0) | 33 (39.3) | 35 (41.2) | 34 (40.5) | 136 (40.2) | 170 (40.4) |
BMI = body mass index; K/L = Kellgren/Lawrence (radiologic severity score).
Efficacy
Significantly greater reductions in WOMAC pain subscale scores were observed from baseline to week 16 with all 4 dose levels of fasinumab compared to placebo. The LS mean difference in WOMAC pain scores in the active treatment groups compared to placebo ranged from −0.78 to −1.40, without any clear dependence on dose level (Table 2 and Figure 2). Pain subscale score reductions, evident by week 2, were maintained throughout 16 weeks of treatment (Figure 2A). A per‐protocol analysis provided similar results (data not shown). During the follow‐up (after week 16), pain scores in all fasinumab dose groups returned toward baseline levels, although not fully. A subgroup analysis of the WOMAC pain subscale scores stratified by age, sex, race, K/L score, index joint, weight, and BMI demonstrated results that were generally consistent with the overall results (see Supplementary Figures 2A–E, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41012/abstract). In addition, all 4 doses of fasinumab yielded statistically significant and clinically meaningful improvements from baseline to week 16 in the WOMAC physical function subscale scores as compared to placebo (Figure 2B and Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41012/abstract), paralleling changes in the WOMAC pain subscale scores.
Table 2.
Change from baseline to week 16 in WOMAC pain subscale scores (full analysis set)a
| Placebo (n = 83) | Fasinumab | ||||
|---|---|---|---|---|---|
| 1 mg (n = 85) | 3 mg (n = 84) | 6 mg (n = 85) | 9 mg (n = 84) | ||
| Baseline | |||||
| No. of patients | 83 | 85 | 84 | 85 | 84 |
| WOMAC pain score | |||||
| Mean ± SD | 6.4 ± 1.7 | 6.3 ± 1.6 | 6.4 ± 1.6 | 6.1 ± 1.4 | 6.5 ± 1.5 |
| Median (range) | 6.4 (1.4, 10.0) | 6.2 (3.0, 9.4) | 6.2 (3.0, 10.0) | 6.2 (2.0, 9.6) | 6.6 (3.6, 10.0) |
| Week 16 | |||||
| No. of patients | 71 | 75 | 78 | 77 | 79 |
| WOMAC pain score | |||||
| Mean ± SD | 3.9 ± 2.6 | 2.8 ± 2.2 | 2.9 ± 2.31 | 3.2 ± 2.4 | 2.7 ± 2.5 |
| Median (range) | 4.2 (0.0, 9.0) | 2.4 (0.0, 8.2) | 2.4 (0.0, 7.6) | 2.8 (0.0, 8.0) | 2.2 (0.0, 10.0) |
| Change from baseline | |||||
| No. of patients | 71 | 75 | 78 | 77 | 79 |
| WOMAC score change | |||||
| Mean ± SD | −2.4 ± 2.4 | −3.5 ± 2.1 | −3.4 ± 2.4 | −3.1 ± 2.3 | −3.8 ± 2.5 |
| Median (range) | −2.2 (−8.6, 2.2) | −3.2 (−8.4, 0.2) | −3.5 (−7.8, 2.6) | −3.4 (−7.6, 1.6) | −3.8 (−8.8, 1.2) |
| LS mean ± SE | −2.3 ± 0.29 | −3.4 ± 0.3 | −3.3 ± 0.3 | −3.0 ± 0.3 | −3.7 ± 0.3 |
| 95% CI | −2.8, −1.7 | −3.9, −2.8 | −3.9, −2.8 | −3.6, −2.5 | −4.2, −3.1 |
| Difference vs. placebo | |||||
| LS mean ± SE | – | −1.1 ± 0.4 | −1.1 ± 0.4 | −0.8 ± 0.4 | −1.4 ± 0.4 |
| 95% CI | – | −1.8, −0.4 | −1.8, −0.4 | −1.5, −0.1 | −2.1, −0.7 |
| P | – | 0.0025 | 0.0029 | 0.0304 | 0.0001 |
Analyses were based on a mixed‐effects model repeated‐measures approach. The prespecified time for assessment of the primary efficacy end point of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale scores was 16 weeks. Change from baseline (versus placebo) was significant for all doses of fasinumab at weeks 2, 4, 8, and 12 (nominal P ≤ 0.05). Post hoc analysis, difference versus placebo for fasinumab doses of 1 mg, 3 mg, 6 mg, and 9 mg: at week 20, −0.61, −0.92, −0.67, and −1.02, respectively (P < 0.05 for 3 mg and 9 mg versus placebo); at week 36, −0.24, 0.21, 0.33, and 1.00, respectively (P < 0.05 only for 9‐mg dose versus placebo). LS = least squares; 95% CI = 95% confidence interval.
Figure 2.

Change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain (A) and physical function (B) subscale scores by visit, and change from baseline in WOMAC pain subscale score in patients exhibiting pain flare (C) compared to those not exhibiting pain flare (D) upon withdrawal of a prior analgesic (full analysis set). Pain and physical function subscales were each normalized to a scale of 0–10, as described in Patients and Methods.
Across all doses, fasinumab reduced PGA scores (week 16 versus baseline) as compared to placebo, with reductions that were statistically significant with the 1‐mg and 9‐mg doses (>30% improvement in PGA scores; P = 0.0132 and P = 0.008, respectively). PGA scores returned to baseline levels during follow‐up.
Treatment with fasinumab also resulted in clinical benefit across most of the exploratory end points, although our study was not specifically powered for these comparisons. Statistically significant reductions in the NRS walking pain score were noted by week 2 and were maintained over the 16‐week treatment period across all fasinumab doses (see Supplementary Figure 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41012/abstract).
In responder analyses, substantial treatment effects, defined as ≥30% improvement from baseline in WOMAC pain and physical function subscale scores, were observed with fasinumab compared to placebo. A greater proportion of patients receiving fasinumab achieved ≥30% improvement in both the WOMAC pain score (63.5–73.8% of fasinumab‐treated patients versus 47% of patients receiving placebo) and physical function score (61.2–71.4% of fasinumab‐treated patients versus 44.6% of patients receiving placebo). Similar results were demonstrated when the responder analyses were based on ≥50% improvement thresholds, yielding statistically significant differences in all 4 fasinumab dose groups compared to placebo at week 16.
In the responder analysis based on the OMERACT–OARSI responder index, greater proportions of patients receiving fasinumab exhibited clinically meaningful treatment responses compared to those receiving placebo (72.9%, 72.6%, 63.5%, and 78.6% of patients treated with fasinumab at 1 mg, 3 mg, 6 mg, and 9 mg, respectively, versus 51.8% of patients receiving placebo; all P < 0.01 except for the comparison of the 6‐mg dose to placebo).
WOMAC pain scores were also assessed in patients with and those without pain flare upon withdrawal of a prior analgesic. The proportion of patients who experienced a pain flare after analgesic withdrawal (pain score ≥1 on a scale of 0–10) was ~25% across doses. Patients with pain flare had worse mean pain scores at baseline compared to those without flare. Improvements in the mean pain scores at week 16 with fasinumab ranged from −1.12 to −1.81 in patients with pain flare compared to change in mean pain scores ranging from −0.87 to −1.14 in those without pain flare (Figures 2C and D). Patients with a pain flare, compared to those without pain flare, who were randomized to receive placebo had higher baseline pain scores (mean 6.96 versus 6.22), and showed more improvement in pain scores at week 16 (LS mean difference in score −3.68 versus −2.19). Similar trends were noted in analyses using higher pain thresholds. Greater treatment effects were observed in patients with higher baseline pain scores or who exhibited greater worsening of pain on withdrawal of the prior analgesic therapy.
Safety
The duration of treatment was similar between the placebo and pooled fasinumab groups (mean ± SD 101 ± 26 days with placebo versus 105 ± 20 days with fasinumab). Furthermore, the duration of observation was similar between the groups (mean ± SD 219 ± 75 days with placebo and 236 ± 54 days with fasinumab).
During the 16‐week treatment period, the incidence of TEAEs was 62% with fasinumab and 55% with placebo (Table 3). Nervous system and musculoskeletal symptoms were more frequent following treatment with fasinumab (17% and 19%, respectively) than with placebo (9% and 17%, respectively). The pooled fasinumab group, as compared to the placebo group, had a higher incidence of paresthesia (3% versus 0%) and arthralgia (8% versus 2%). Of the 12 paresthesia events in 10 patients, 10 were mild, 2 were moderate, and none were severe; ~50% of these events resolved by the study end. There was 1 event of carpal tunnel syndrome. Preexisting neuropathies were an exclusion criterion. There was no indication of sympathetic nervous system dysfunction.
Table 3.
TEAEs reported in >3% of patients during the treatment and follow‐up periods, by system organ class (safety analysis set)a
| Placebo (n = 82) | Fasinumab | |||||
|---|---|---|---|---|---|---|
| 1 mg (n = 85) | 3 mg (n = 84) | 6 mg (n = 85) | 9 mg (n = 83) | Combined (n = 337) | ||
| Treatment period | ||||||
| ≥1 TEAE | 45 (54.9) | 54 (63.5) | 52 (61.9) | 55 (64.7) | 48 (57.8) | 209 (62.0) |
| Infections and infestations | 13 (15.9) | 18 (21.2) | 17 (20.2) | 16 (18.8) | 21 (25.3) | 72 (21.4) |
| Upper respiratory tract infection | 1 (1.2) | 5 (5.9) | 3 (3.6) | 3 (3.5) | 7 (8.4) | 18 (5.3) |
| Urinary tract infection | 3 (3.7) | 5 (5.9) | 1 (1.2) | 4 (4.7) | 3 (3.6) | 13 (3.9) |
| Sinusitis | 3 (3.7) | 1 (1.2) | 2 (2.4) | 2 (2.4) | 4 (4.8) | 9 (2.7) |
| Musculoskeletal and connective tissue disorders | 14 (17.1) | 16 (18.8) | 15 (17.9) | 18 (21.2) | 14 (16.9) | 63 (18.7) |
| Arthralgia | 2 (2.4) | 9 (10.6) | 5 (6.0) | 8 (9.4) | 5 (6.0) | 27 (8.0) |
| Back pain | 2 (2.4) | 4 (4.7) | 1 (1.2) | 2 (2.4) | 3 (3.6) | 10 (3.0) |
| Joint swelling | 0 | 1 (1.2) | 6 (7.1) | 3 (3.5) | 0 | 10 (3.0) |
| Pain in extremity | 3 (3.7) | 3 (3.5) | 1 (1.2) | 2 (2.4) | 4 (4.8) | 10 (3.0) |
| Musculoskeletal pain | 4 (4.9) | 0 | 1 (1.2) | 2 (2.4) | 1 (1.2) | 4 (1.2) |
| Myalgia | 0 | 1 (1.2) | 0 | 2 (2.4) | 0 | 3 (0.9) |
| Osteoarthritis | 0 | 0 | 0 | 0 | 3 (3.6) | 3 (0.9) |
| Nervous system disorders | 7 (8.5) | 15 (17.6) | 14 (16.7) | 15 (17.6) | 13 (15.7) | 57 (16.9) |
| Headache | 5 (6.1) | 7 (8.2) | 2 (2.4) | 4 (4.7) | 4 (4.8) | 17 (5.0) |
| Paresthesia | 0 | 2 (2.4) | 4 (4.8) | 0 | 4 (4.8) | 10 (3.0) |
| Dizziness | 2 (2.4) | 3 (3.5) | 2 (2.4) | 3 (3.5) | 1 (1.2) | 9 (2.7) |
| Hypoesthesia | 1 (1.2) | 2 (2.4) | 3 (3.6) | 2 (2.4) | 1 (1.2) | 8 (2.4) |
| Gastrointestinal disorders | 7 (8.5) | 9 (10.6) | 12 (14.3) | 9 (10.6) | 5 (6.0) | 35 (10.4) |
| Nausea | 3 (3.7) | 6 (7.1) | 1 (1.2) | 2 (2.4) | 1 (1.2) | 10 (3.0) |
| Diarrhea | 3 (3.7) | 1 (1.2) | 1 (1.2) | 4 (4.7) | 2 (2.4) | 8 (2.4) |
| Dry mouth | 1 (1.2) | 3 (3.5) | 4 (4.8) | 1 (1.2) | 0 | 8 (2.4) |
| Vomiting | 0 | 1 (1.2) | 3 (3.6) | 1 (1.2) | 2 (2.4) | 7 (2.1) |
| Vascular disorders | 8 (9.8) | 6 (7.1) | 4 (4.8) | 5 (5.9) | 4 (4.8) | 19 (5.6) |
| Orthostatic hypotension | 3 (3.7) | 3 (3.5) | 3 (3.6) | 3 (3.5) | 2 (2.4) | 11 (3.3) |
| Hypertension | 4 (4.9) | 1 (1.2) | 1 (1.2) | 2 (2.4) | 1 (1.2) | 5 (1.5) |
| Skin and subcutaneous tissue disorders | 1 (1.2) | 6 (7.1) | 4 (4.8) | 2 (2.4) | 5 (6.0) | 17 (5.0) |
| Rash | 0 | 1 (1.2) | 2 (2.4) | 1 (1.2) | 4 (4.8) | 8 (2.4) |
| Follow‐up period | ||||||
| ≥1 posttreatment AE | 31 (37.8) | 36 (42.4) | 38 (45.2) | 42 (49.4) | 44 (53.0) | 160 (47.5) |
| Musculoskeletal and connective tissue disorders | 10 (12.2) | 15 (17.6) | 20 (23.8) | 25 (29.4) | 23 (27.7) | 83 (24.6) |
| Arthralgia | 5 (6.1) | 3 (3.5) | 11 (13.1) | 12 (14.1) | 9 (10.8) | 35 (10.4) |
| Rapidly progressive OA | 0 | 2 (2.4) | 2 (2.4) | 5 (5.9) | 7 (8.4) | 16 (4.7) |
| OA | 0 | 3 (3.5) | 4 (4.8) | 3 (3.5) | 4 (4.8) | 14 (4.2) |
| Musculoskeletal pain | 2 (2.4) | 2 (2.4) | 5 (6.0) | 4 (4.7) | 1 (1.2) | 12 (3.6) |
| Joint swelling | 1 (1.2) | 1 (1.2) | 2 (2.4) | 4 (4.7) | 1 (1.2) | 8 (2.4) |
| Pain in extremity | 1 (1.2) | 1 (1.2) | 1 (1.2) | 3 (3.5) | 2 (2.4) | 7 (2.1) |
| Infections and infestations | 7 (8.5) | 11 (12.9) | 15 (17.9) | 8 (9.4) | 12 (14.5) | 46 (13.6) |
| Upper respiratory tract infection | 0 | 2 (2.4) | 7 (8.3) | 3 (3.5) | 0 | 12 (3.6) |
| Urinary tract infection | 2 (2.4) | 3 (3.5) | 2 (2.4) | 0 | 1 (1.2) | 6 (1.8) |
| Bronchitis | 1 (1.2) | 0 | 1 (1.2) | 0 | 3 (3.6) | 4 (1.2) |
| Vascular disorders | 4 (4.9) | 2 (2.4) | 5 (6.0) | 3 (3.5) | 5 (6.0) | 15 (4.5) |
| Orthostatic hypotension | 4 (4.9) | 2 (2.4) | 4 (4.8) | 0 | 4 (4.8) | 10 (3.0) |
Adverse events (AEs) were defined according to the Medical Dictionary of Regulatory Activities (version 18.0) with system organ class preferred terms. A patient who reported ≥2 treatment‐emergent AEs (TEAEs) with the same preferred term was counted only once for that term. A patient who reported ≥2 TEAEs with different preferred terms within the same system organ class was counted only once in that system organ class. Values are the number (%) of patients. OA = osteoarthritis.
During treatment, in the pooled fasinumab dose groups compared to the placebo group, there were variably higher rates of infections (21% versus 16%; generally of the respiratory tract) and a decreased rate of vascular disorders (6% versus 10%; mostly hypertension). In follow‐up, these imbalances were less evident. Across all treatment groups, most AEs were mild‐to‐moderate in severity. The incidence of serious TEAEs during treatment was low (2% with placebo versus 1% with fasinumab). There was no apparent fasinumab dose relationship in terms of the proportion of patients with serious TEAEs. A low proportion of patients discontinued therapy due to TEAEs (4% [n = 14] in the fasinumab group and 1% [n = 1] in the placebo group). No group exhibited a predominant cause of discontinuation, as the reasons for discontinuation spanned musculoskeletal, nervous system, skin, and subcutaneous tissue disorders, with incidence rates of 0–2% for each.
During the 20‐week follow‐up, the incidence of TEAEs was higher in the combined fasinumab group than in the placebo group (48% versus 38%) (Table 3), although the incidence of serious TEAEs was similar between the groups (6% of fasinumab‐treated patients versus 5% of placebo‐treated patients), varying across the fasinumab dose groups.
Due to the historical interest in arthropathy associated with anti‐NGF treatment, careful analyses were performed to detect both symptomatic arthropathy and arthropathies detected via routine radiologic surveillance without requiring presence of symptoms. Adjudicated arthropathies were detected in 23 patients (5%) overall, involving 25 joints (13 index joints and 12 non‐index joints) in 7% of patients in the combined fasinumab group and 1% of patients in the placebo group (Table 4).
Table 4.
Adjudicated arthropathies and total joint replacements (safety analysis set)a
| Placebo (n = 82) | Fasinumab | |||||
|---|---|---|---|---|---|---|
| 1 mg (n = 85) | 3 mg (n = 84) | 6 mg (n = 85) | 9 mg (n = 83) | Combined (n = 337) | ||
| Arthropathiesb | ||||||
| No. of arthropathies | 1 | 2 | 4 | 6 | 12 | 24 |
| Patients with ≥1 arthropathy | 1 (1.2) | 2 (2.4) | 4 (4.8) | 6 (7.1) | 10 (12.0) | 22 (6.5) |
| RPOAc | 0 | 2 (2.4) | 2 (2.4) | 5 (5.9) | 7 (8.4) | 16 (4.7) |
| Subchondral insufficiency fracture | 1 (1.2) | 0 | 2 (2.4) | 1 (1.2) | 3 (3.6) | 6 (1.8) |
| Joint replacements | ||||||
| No. of joint replacements | 4 | 3 | 4 | 4 | 3 | 14 |
| Patients with ≥1 joint replacementd | 3 (3.7) | 3 (3.5) | 3 (3.6) | 4 (4.7) | 3 (3.6) | 13 (3.9) |
| No. of joint replacements per 1,000 patient‐yearse | 81.2 | 56.5 | 73.8 | 72.7 | 53.8 | 64.2 |
| Joint replaced | ||||||
| Knee | 3 | 3 | 4 | 3 | 2 | 12 |
| Hip | 0 | 0 | 0 | 1 | 1 | 2 |
| Shoulder | 1 | 0 | 0 | 0 | 0 | 0 |
Except where indicated otherwise, values are the number (%) of patients.
Arthropathies include those during the treatment and follow‐up periods combined, detected on scheduled and unscheduled radiographic assessments.
Of the rapidly progressive osteoarthritis (RPOA) events, 2 patients had RPOA‐2 (1 in the 6 mg fasinumab group and 1 in the 9 mg fasinumab group) and 14 patients (16 events) had RPOA‐1 (across fasinumab doses). Two patients (both in the 9 mg fasinumab group) had bilateral RPOA‐1.
Two patients had bilateral joint replacements.
The corresponding 95% confidence intervals (95% CIs) were as follows: for placebo, 95% CI 63.5, 98.9; for 1 mg fasinumab, 95% CI 41.8, 71.2; for 3 mg fasinumab, 95% CI 57.0, 90.6; for 6 mg fasinumab, 95% CI 56.0, 89.4; for 9 mg fasinumab, 95% CI 39.4, 68.2; for fasinumab doses combined, 95% CI 48.5, 79.9.
An increase in arthropathies according to fasinumab dose and time was observed during the study. There was a clear dependence on the fasinumab dose, with 1 arthropathy observed in the placebo group compared to 2 arthropathies in patients receiving the 1‐mg dose, 4 in patients receiving the 3‐mg dose, 6 in patients receiving the 6‐mg dose, and 12 in patients receiving the 9‐mg dose. Arthropathies consisted of RPOA in 5% of patients in the fasinumab group (with joint space narrowing [RPOA‐1] in 14 patients; with bony changes [RPOA‐2] in 2 patients) compared to none in the placebo group. Subchondral insufficiency fracture occurred in 1.8% of patients in the combined fasinumab group and 1.2% in the placebo group. Most arthropathies (16 of the 25) were discovered by a scheduled radiologic assessment. The incidence of arthropathy prompted by symptoms (9 of 25), outside of routine assessment, was low (1 in the placebo group, 1 in the 1‐mg dose group, 3 in the 3‐mg dose group, 2 in the 6‐mg dose group, and 2 in the 9‐mg dose group, involving subchondral insufficiency fracture in 5 patients, RPOA‐1 in 3 patients, and RPOA‐2 in 1 patient). No primary osteonecrosis was observed.
Because MRI is superior to radiography for observing changes in joint structure, its use for monitoring resulted in detection of subtle changes in bone structure, with variable degrees of severity, that were not evident on radiography. To highlight those cases that were most severe, reflecting the level of destructive joint changes for this class of drug as outlined by the 2012 Arthritis Advisory Committee 15, a blinded post hoc analysis was performed on all adjudicated arthropathies to identify destructive arthropathy, which was defined as abnormal bone fragmentation, destruction, or fracture during the study, including near‐total or total collapse of an articular surface, and subluxation/malalignment, all inconsistent with radiographic findings in advanced OA, and readily observed on radiography. Destructive arthropathy was identified in only 1 patient, who was receiving 6 mg fasinumab (<0.3% of the 337 fasinumab‐treated patients).
Overall, 16 patients (4%) underwent 18 joint replacements, predominantly involving the knees (15 with knee replacement, 2 with hip replacement, and 1 with shoulder joint replacement). Joint replacements occurred at an incidence of 3–4 per group (Table 4), with no evidence of either drug or dose dependence.
Routine monitoring of laboratory test findings revealed no significant changes, except in the levels of alkaline phosphatase (ALP), which increased in a time‐ and dose‐dependent manner during the trial, although group mean values remained within the normal range (see Supplementary Figure 4, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41012/abstract). In 3 patients (1 receiving placebo and 2 receiving 6 mg fasinumab), the study‐defined cutoff for a significant ALP elevation (1.5× the upper limit of normal) was exceeded. All 3 of these patients had ALP levels that were above normal limits at baseline, which, over the follow‐up, increased by 48.9%, 74.2%, and 19.4%, respectively. The mean increase in ALP levels, likely being of bone origin given the lack of liver enzyme changes, were marginally greater (7–9 units) in those who developed arthropathy compared to those without arthropathy, which occurred in only the highest 2 fasinumab dose groups. During the follow‐up, ALP values largely resolved across the groups by week 36, although they had not returned fully to baseline levels. There were no deaths during the study.
Discussion
In this phase IIb/III study involving patients with moderate‐to‐severe OA, fasinumab was superior to placebo for improving pain and physical function. Patients receiving fasinumab, as compared to those receiving placebo, demonstrated statistically significant and clinically important reductions in WOMAC pain and physical function subscale scores. The placebo‐adjusted group mean improvements in the WOMAC pain scores ranged from −0.78 to −1.40 points, exceeding the MCID 10. Responder analyses (thresholds of ≥30% and ≥50% reductions in WOMAC pain and physical function subscale scores) confirmed that a substantially greater proportion of patients receiving fasinumab achieved improvements compared to placebo. Furthermore, notable relief in the severity of walking pain was achieved within 7 days of initiation of fasinumab therapy across all 4 doses, as evidenced by the NRS walking pain scores. Overall, efficacy was observed at all doses of fasinumab. No obvious dose–response relationship was observed to suggest having met or exceeded the threshold for maximal response.
Improvements in pain and function with fasinumab should be placed in the context of published data with regard to analgesic treatments. A recent meta‐analysis across 17 trials in OA showed that acetaminophen, the analgesic of first choice for OA pain, provided very modest pain relief, with an improvement of ~0.4 points from a baseline pain score of 6 points on the 10‐point WOMAC pain subscale 16. In another analysis 17, the effect size of acetaminophen versus placebo (pain score reduction −0.09) was substantially lower than that of any of the studied NSAIDs (reductions ranging from −0.39 to −0.49 with naproxen, ibuprofen, and diclofenac). Effect sizes with celecoxib, the only selective cyclooxygenase 2 inhibitor available in the United States, with pain score reductions ranging from −0.11 to −0.34 across dosages, were lower than that seen with nonspecific NSAIDs. A comprehensive meta‐analysis comparing opioids to NSAIDs in OA showed little difference in pain relief between these categories of analgesics 18. A more recent review arrived at similar conclusions 19. In our study, patients receiving fasinumab averaged an improvement of >3.4 points on the 10‐point WOMAC pain subscale score (−3.5, −3.4, −3.1, and −3.8 points with the 1‐mg, 3‐mg, 6‐mg, and 9‐mg doses, respectively), representing a 50–58% improvement from baseline and yielding effect sizes of −0.46, −0.45, −0.32, and −0.59 points across fasinumab doses of 1 mg, 3 mg, 6 mg, and 9 mg, respectively. For some of these doses, the effect size was substantially greater than has been reported with acetaminophen, opioids, and some NSAIDs.
Evaluation of effect size must take into consideration trial design. Most OA analgesic trials enrolled patients who had experienced pain flare upon withdrawal of a prior analgesic therapy, with flare being identified by an increase of ≥10 points on the WOMAC 0–100 pain scale (or 1 point on a 0–10 scale) 20, 21. In contrast, our study enrolled patients who had OA knee and/or hip pain both at screening and at baseline, but pain flare was not an enrollment criterion, and therefore patients were enrolled whose pain may not have been adequately treated with a prior analgesic. Most patients did not exhibit pain flare after analgesic withdrawal. In the subgroup of patients who did experience a pain flare, treatment responses were greater than in those who did not, consistent with the greater treatment effect observed in OA trials employing a study design that required flare 19. One group reported that pain and functional scores in study designs that did not require pain flare may underestimate treatment effects by 37–50% 20, although another study, using a different methodology, did not observe such a difference 21. Clinical trials assessing the efficacy of fasinumab compared to NSAIDs have been initiated.
With respect to other anti‐NGF antibodies, a knee OA proof‐of‐concept study compared tanezumab, a humanized IgG2 anti‐NGF monoclonal antibody administered intravenously at a dose of 10–200 μg/kg on days 1 and 56, to placebo, employing a study design that required pain flare (defined as WOMAC pain score worsening of ≥10 points) 22. Improvements in pain scores from baseline were demonstrated in a relatively flat dose response at the lowest 3 doses, with the best efficacy observed at the highest 2 doses. In subsequent phase III studies of tanezumab (at doses ranging from 2.5 to 10 mg given intravenously every 8 weeks) in patients with knee OA 23 and hip OA 24, with a study design requiring flare, these efficacy results were largely recapitulated.
Fasinumab was generally well tolerated in the present study. Although the rate of TEAEs was higher with fasinumab than with placebo, there were few discontinuations attributable to TEAEs. ALP elevations in treated patients may reflect the effect of fasinumab on anabolic bone metabolism or, perhaps, could have been the result of increases in physical activity (not quantified in this study) after relief of pain, stimulating bone formation (25, 26, 27). Although study populations may vary across clinical trials, the proportions of patients with nervous system and musculoskeletal disorders, which was higher with fasinumab than with placebo, appeared to be consistent with the rates previously reported with tanezumab 5, and may be related to the inhibition of NGF, although few patients discontinued treatment and there were no cases of sympathetic nerve dysfunction.
Despite the promise of efficacy seen with anti‐NGF agents, enthusiasm for this class of drugs has been tempered by observations of treatment‐associated arthropathies. To our knowledge, this is the first study of an NGF inhibitor to incorporate routine, prospective, intense radiologic joint assessment using both radiographs and MRI, to comprehensively document the occurrence of nonsymptomatic, as well as symptomatic, arthropathies. Fasinumab was associated with a greater rate of adjudicated arthropathies, which were clearly dose dependent, compared to placebo, with most being nonsymptomatic and dominated by joint space narrowing (RPOA‐1). There were no reports of osteonecrosis, and the rates of joint replacements were comparable across treatment arms.
The rate of destructive arthropathy reported in our study was small (1 of 338 patients randomized to receive fasinumab at 6 mg) and difficult to compare to rates reported in studies with the other NGF inhibitors, in which joint events of this type were observed largely upon retrospective assessment of radiographs performed to assess symptoms and after patient referral for joint replacement 28, 29, 30, 31. Insights into the etiology of the arthropathies observed in this trial await additional analysis of specific bone and other biomarkers, and qualitative and quantitative assessments of physical activity. Data on the efficacy and safety of fasinumab over longer treatment periods will emerge from larger, ongoing clinical trials (see http://www.clinicaltrials.gov).
In conclusion, in this phase IIb/III study involving >400 patients with knee and/or hip OA, fasinumab demonstrated a substantial degree of analgesia in patients with moderate‐to‐severe pain from OA, without clear evidence of dependence on dose level for efficacy, even in patients who had not experienced benefits with prior analgesics, a group previously excluded in most other pain studies in OA. This represents an important, previously unaddressed patient population. Fasinumab was well tolerated by most patients, with a clear dose‐dependent increase in joint‐related abnormalities. The observation that the efficacy of lower doses was similar to that of higher doses but was associated with lower rates of arthropathy demands that future studies explore the benefit versus risk at these lower doses of fasinumab.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Geba had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Gao, Maloney, Stahl, Yancopoulos, Geba.
Acquisition of data
Dakin, Gao, Maloney, Kivitz, Schnitzer, Stahl, Yancopoulos, Geba.
Analysis and interpretation of data
Dakin, DiMartino, Gao, Maloney, Kivitz, Schnitzer, Stahl, Yancopoulos, Geba.
Role of the Study Sponsor
This study was sponsored by Regeneron Pharmaceuticals, Inc. StemScientific (part of UDG Healthcare, plc, Tarrytown, New York) provided editorial assistance that was funded by Regeneron Pharmaceuticals, Inc. All of the authors were involved in the final study design, manuscript development, data analysis, and interpretation of the results, and approved its final form for publication.
Supporting information
Acknowledgment
We thank Dr. Catherine Stehman‐Breen for contributions to the early design and conduct of the trial.
ClinicalTrials.gov identifier: NCT02447276.
Supported by Regeneron Pharmaceuticals, Inc.
Drs. Dakin and DiMartino contributed equally to this work.
Drs. Dakin, DiMartino, Gao, Maloney, Stahl, Yancopoulos, and Geba own stock in Regeneron Pharmaceuticals, Inc. Dr. Kivitz has received consulting fees from AbbVie, Astellas Pharma, Regeneron, Pfizer, Sanofi, and Genentech (less than $10,000 each). Dr. Schnitzer has received consulting fees from Regeneron (less than $10,000).
References
- 1. Denk F, Bennett DL, McMahon SB. Nerve growth factor and pain mechanisms. Annu Rev Neurosci 2017;40:307–25. [DOI] [PubMed] [Google Scholar]
- 2. Chang DS, Hsu E, Hottinger DG, Cohen SP. Anti‐nerve growth factor in pain management: current evidence. J Pain Res 2016;9:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller RE, Malfait AM, Block JA. Current status of nerve growth factor antibodies for the treatment of osteoarthritis pain. Clin Exp Rheumatol 2017;35 Suppl 107:85–7. [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar V, Mahal BA. NGF: the TrkA to successful pain treatment. J Pain Res 2012;5:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hochberg MC. Serious joint‐related adverse events in randomized controlled trials of anti‐nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015;23 Suppl 1:S18–21. [DOI] [PubMed] [Google Scholar]
- 6. Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double‐blind, placebo‐controlled exploratory study in osteoarthritis of the knee. Pain 2014;155:1245–52. [DOI] [PubMed] [Google Scholar]
- 7. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 8. Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991;34:505–14. [DOI] [PubMed] [Google Scholar]
- 9. Kellgren JH, Lawrence JS. Radiological assessment of osteo‐arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF‐36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum 2001;45:384–91. [DOI] [PubMed] [Google Scholar]
- 11. Strand V, Kellman A. Outcome measures in osteoarthritis: randomized clinical trials. Curr Rheumatol Rep 2004;6:20–30. [DOI] [PubMed] [Google Scholar]
- 12. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–91. [DOI] [PubMed] [Google Scholar]
- 13. Pham T, Van Der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT‐OARSI set of responder criteria. J Rheumatol 2003;30:1648–54. [PubMed] [Google Scholar]
- 14. Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika 1988;75:800–2. [Google Scholar]
- 15. US Food and Drug Administration, Center for Drug Evaluation and Research . Arthritis Advisory Committee (AAC) meeting. 2012. URL: https://wayback.archive-it.org/7993/20170404145630/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM307880.pdf. p. 178–207.
- 16. Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta‐analysis of randomized placebo‐controlled trials. BMJ 2015;350:h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stam WB, Jansen JP, Taylor SD. Efficacy of etoricoxib, celecoxib, lumiracoxib, non‐selective NSAIDs, and acetaminophen in osteoarthritis: a mixed treatment comparison. Open Rheumatol J 2012;6:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non‐steroidal anti‐inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage 2016;24:962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berthelot JM, Darrieutort‐Lafitte C, Le Goff B, Maugars Y. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine 2015;82:397–401. [DOI] [PubMed] [Google Scholar]
- 20. Trijau S, Avouac J, Escalas C, Gossec L, Dougados M. Influence of flare design on symptomatic efficacy of non‐steroidal anti‐inflammatory drugs in osteoarthritis: a meta‐analysis of randomized placebo‐controlled trials. Osteoarthritis Cartilage 2010;18:1012–8. [DOI] [PubMed] [Google Scholar]
- 21. Smith TO, Zou K, Abdullah N, Chen X, Kingsbury SR, Doherty M, et al. Does flare trial design affect the effect size of non‐steroidal anti‐inflammatory drugs in symptomatic osteoarthritis? A systematic review and meta‐analysis. Ann Rhem Dis 2016;75:1971–8. [DOI] [PubMed] [Google Scholar]
- 22. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363:1521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double blind, placebo‐controlled phase III trial. J Pain 2012;13:790–8. [DOI] [PubMed] [Google Scholar]
- 24. Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double‐blind, placebo‐controlled phase III trial. Arthritis Rheum 2013;65:1795–803. [DOI] [PubMed] [Google Scholar]
- 25. Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site‐specific and load‐dependent. J Bone Miner Res 1996;11:218–25. [DOI] [PubMed] [Google Scholar]
- 26. Heinonen A, Kannus P, Sievänen H, Oja P, Pasanen M, Rinne M, et al. Randomized controlled trial of effect of high‐impact exercise on selected risk factors for osteoporotic fractures. Lancet 1996;348:1343–7. [DOI] [PubMed] [Google Scholar]
- 27. Rudberg A, Magnusson P, Larsson L, Joborn H. Serum isoforms of bone alkaline phosphastase increase during physical exercise in women. Calcif Tissue Int 2000;66:342–7. [DOI] [PubMed] [Google Scholar]
- 28. Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 2015;23 Suppl 1:S8–17. [DOI] [PubMed] [Google Scholar]
- 29. Pfizer . Tanezumab Arthritis Advisory Committee briefing document. 2012. URL: https://wayback.archive-it.org/7993/20170405210034/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM295205.pdf.
- 30. Janssen Research & Development, LLC . Advisory Committee briefing document: JNJ‐42160443 (fulranumab). URL: https://wayback.archive-it.org/7993/20170405210031/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM295204.pdf.
- 31. Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, et al. When is osteonecrosis not osteonecrosis? Adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol 2016;68:382–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


