Abstract
Aim
To investigate potential cost savings associated with the use of real‐time continuous glucose monitoring (RT‐CGM) throughout pregnancy in women with Type 1 diabetes.
Methods
A budget impact model was developed to estimate, from the perspective of National Health Service England, the total costs of managing pregnancy and delivery in women with Type 1 diabetes using self‐monitoring of blood glucose (SMBG) with and without RT‐CGM. It was assumed that the entire modelled cohort (n = 1441) would use RT‐CGM from 10 to 38 weeks’ gestation (7 months). Data on pregnancy and neonatal complication rates and related costs were derived from published literature, national tariffs, and device manufacturers.
Results
The cost of glucose monitoring was £588 with SMBG alone and £1820 with RT‐CGM. The total annual costs of managing pregnancy and delivery in women with Type 1 diabetes were £23 725 648 with SMBG alone, and £14 165 187 with SMBG and RT‐CGM; indicating potential cost savings of approximately £9 560 461 from using RT‐CGM. The principal drivers of cost savings were the daily cost of neonatal intensive care unit (NICU) admissions (£3743) and the shorter duration of NICU stay (mean 6.6 vs. 9.1 days respectively). Sensitivity analyses showed that RT‐CGM remained cost saving, albeit to lesser extents, across a range of NICU costs and durations of hospital stay, and with varying numbers of daily SMBG measurements.
Conclusions
Routine use of RT‐CGM by pregnant women with Type 1 diabetes, would result in substantial cost savings, mainly through reductions in NICU admissions and shorter duration of NICU care.
What's new?
Real‐time continuous glucose monitoring (RT‐CGM) improves neonatal health outcomes, with fewer large for gestational age infants, fewer neonatal intensive care unit (NICU) admissions and a shorter neonatal length of hospital stay.
It is not known whether the costs of implementing RT‐CGM into National Health Service England antenatal care, would be offset by the reduction in neonatal complications.
The approximately threefold higher costs of RT‐CGM use, compared with self‐monitoring of blood glucose (£1820 vs. £588), are offset by substantial cost savings, mainly through reductions in NICU admissions and a shorter duration of NICU stay.
What's new?
Real‐time continuous glucose monitoring (RT‐CGM) improves neonatal health outcomes, with fewer large for gestational age infants, fewer neonatal intensive care unit (NICU) admissions and a shorter neonatal length of hospital stay.
It is not known whether the costs of implementing RT‐CGM into National Health Service England antenatal care, would be offset by the reduction in neonatal complications.
The approximately threefold higher costs of RT‐CGM use, compared with self‐monitoring of blood glucose (£1820 vs. £588), are offset by substantial cost savings, mainly through reductions in NICU admissions and a shorter duration of NICU stay.
Introduction
Type 1 diabetes during pregnancy is associated with increased risks of adverse outcomes such as pre‐eclampsia, premature delivery, perinatal morbidity and admission to a neonatal intensive care unit (NICU) 1, 2, 3, 4, which are at least partly attributable to suboptimal glycaemic control as measured by maternal glycated haemoglobin (HbA1c) levels 5. For this reason, the UK National Institute for Health and Care Excellence (NICE) has recommended that glycaemic control should be optimized before and during pregnancy in women with Type 1 diabetes, with self‐monitoring of blood glucose (SMBG), at least four, and up to 10 times daily 6, 7. Despite frequent glucose monitoring, optimal glucose control is often difficult to achieve due to pregnancy‐related changes in insulin sensitivity and day‐to‐day variations in insulin pharmacokinetics with advancing gestation 8, 9, 10.
Real‐time continuous glucose monitoring (RT‐CGM) offers the potential to improve glycaemic control, compared with SMBG because it provides real‐time data on changing glucose concentrations, thereby enabling users to take appropriate action in response to glucose fluctuations 11, 12. The potential value of this approach has been demonstrated in the Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial (CONCEPTT), in which the use of RT‐CGM, in addition to SMBG, resulted in improvements in time in glycaemic target ranges during the second and third trimesters. This was accompanied by improved neonatal outcomes such as fewer large for gestational age infants, fewer NICU admissions > 24 h, less neonatal hypoglycaemia, and a shorter duration of hospitalization among infants of mothers using SMBG and RT‐CGM 11. Importantly, the treatment effect of RT‐CGM was comparable in women receiving insulin pump therapy, and in those receiving multiple daily injections (MDI). This is consistent with the experience of RT‐CGM users outside pregnancy, and suggests that the potential benefits of RT‐CGM are applicable to a broad population of people with Type 1 diabetes 13.
Because RT‐CGM and insulin delivery technologies are expensive, it is important to demonstrate the budgetary impact of these advancing technologies in clinical practice. Such evidence can be obtained through the use of budget impact models, which estimate the affordability of an intervention in a specific population over a short‐term time horizon 14. Our aim was to develop a budget impact model to estimate the costs and potential cost savings associated with the introduction of RT‐CGM in pregnant women with Type 1 diabetes.
Methods
A model was developed to estimate, from the perspective of National Health Service (NHS) England, the costs associated with the use of RT‐CGM by pregnant women with Type 1 diabetes. It assumes that RT‐CGM is used throughout pregnancy for ~ 28 weeks (from 10 to 38 weeks’ gestation), and that neonates not admitted to a NICU stayed on a normal postnatal ward (Fig. 1). The model was constructed in Microsoft Excel, v1808 (Microsoft Corp, Redmond, WA, USA), and is available from the authors.
Figure 1.
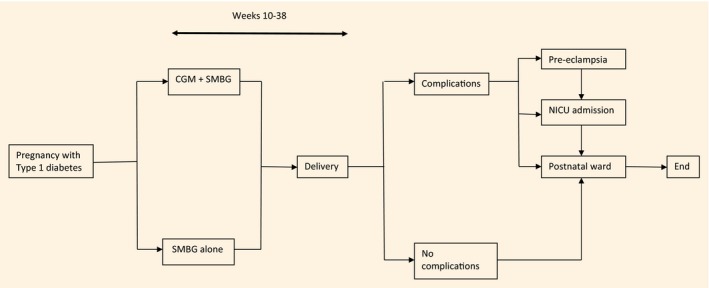
Model design. CGM: continuous glucose monitoring; NICU: neonatal intensive care unit; SMBG: self‐monitoring of blood glucose.
Model inputs
Model inputs are summarized in Table 1. Based on data from the 2014–2016 UK National Pregnancy Diabetes Audit, indicating 4323 pregnant women with Type 1 diabetes over 3 years, we estimated that there were on average 1441 women per year throughout England 18. Data on rates of complications (pre‐eclampsia and NICU admission), durations of hospitalization or NICU stay, and frequency of glucose monitoring by RT‐CGM or SMBG, were derived from CONCEPTT 11 and NICE guidance for the management of diabetes during pregnancy 6, 7. The indications for NICU admission and country‐to‐country NICU admission data were assessed post hoc after peer review.
Table 1.
Budget impact model inputs
| Complication rates | RT‐CGM + SMBG | SMBG alone | Source |
|---|---|---|---|
| Admission to NICU > 24 h (%) | 27 | 43 | CONCEPTT 11 |
| Mean length of stay in NICU (days) | 6.6 | 9.1 | CONCEPTT ‡ |
| Proportion of neonates admitted to NICU who also had a postnatal ward stay (%) | 57 | 42 | CONCEPTT‡ |
| Number of days neonates admitted to NICU also had on a postnatal ward | 222 | 260 | |
| Mean duration of postnatal ward care pre‐ or post‐NICU admission (days) | 4.1 | 6.4 | CONCEPTT‡ |
| Mean duration of hospitalization in neonates not admitted to NICU (days) | 3 | 3 | CONCEPTT‡ |
| Pre‐eclampsia (%) | 9 | 18 | CONCEPTT 11 |
| Costs | Cost (£) | Source | |
| NICU stay (24 h) | 3743 | Published data 15 | |
| Neonatal (non‐NICU) bed stay (24 h) | 347 | NHS National Tariff 16 | |
| Incremental cost of delivery with complications (pre‐eclampsia) | 1400 | NHS National Tariff/NICE guidance 6, 16 | |
| RT‐CGM with SMBG | |||
| RT‐CGM costs | |||
| Transmitter (replaced annually) | £350 | Manufacturer's data* | |
| Sensor unit cost | £52.50 | Manufacturer's data | |
| Number of sensors per month | 4 | CONCEPTT 11 | |
| Number of sensors per pregnancy† | 28 | ||
| Total sensor cost per pregnancy | £1470 | ||
| Total RT‐CGM cost per pregnancy | £1820 | ||
| SMBG costs | |||
| Mean number of fingerstick measurements per day | 4 | NICE guidance 6, 7 | |
| Cost per glucose strip | £0.30 | British National Formulary 17 | |
| Cost per day | £1.20 | ||
| Cost per month | £33.60 | ||
| Cost per pregnancy | £235.20 | ||
| SMBG costs | |||
| Mean number of fingerstick measurements per day | 10 | ||
| Cost per glucose strip | £0.30 | British National Formulary 17 | |
| Cost per day | £3 | ||
| Cost per month | £84 | ||
| Cost per pregnancy | £588 |
RT‐CGM, real‐time continuous glucose monitoring; SMBG, self‐monitoring of blood glucose; NICU, neonatal intensive care unit; CONCEPTT, Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial; NICE, National Institute for Health and Care Excellence.
Manufacturer's data provided by Medtronic Ltd (Watford, UK).
Assumed that RT‐CGM is used for 7 months (from 10 to 38 weeks’ gestation) per pregnancy based on CONCEPTT 11.
Unpublished CONCEPTT data (provided by HR Murphy and DS Feig).
Neonates admitted to a NICU also had a stay in the postnatal ward, either before or after NICU admission. The duration of these stays was recorded, and if this was less than 24 h the corresponding cost of the postnatal ward admission was not included in the cost calculation; hence, this calculation can be considered conservative. Based on data from CONCEPTT, it was assumed that women would use a mean of four CGM sensors per month, giving a total of 28 sensors between 10 and 38 weeks’ gestation. In addition, based on the NICE guidelines on pregnancy (NG3) and management of Type 1 diabetes (NG17) 6, 7, it was assumed that women would make an average of 10 fingerstick measurements per day if they were using SMBG alone, and four if they were using SMBG together with RT‐CGM.
Costs of managing complications and glucose monitoring were derived from the 2018/2019 NHS National Tariffs 16, NICE guidance 6, 7, a published clinical trial of glycaemic control in paediatric intensive care units 15, and commercial data from Medtronic Ltd (Watford, UK). NICE data show that the mean costs of normal and complicated deliveries are £1957 and £3357 respectively, and hence the incremental cost of a complicated pregnancy, compared with normal pregnancy is £1400 19. Because the NICE guidance states that women with pre‐eclampsia undergo deliveries with complications and comorbidities 7, this incremental cost was multiplied by the proportion of women with pre‐eclampsia. Costs associated with the management of diabetes (e.g. costs of insulin therapy) were not included in the model which focuses on glucose monitoring rather than mode of insulin delivery. All costs are reported as 2018 GBP (£).
Sensitivity analyses
The base case analysis assumed that 18% of deliveries would be complicated by pre‐eclampsia 11, the mean cost of NICU was £3743 per day, and the mean duration of NICU care when RT‐CGM was used with SMBG, compared with SMBG alone was 6.6 vs. 9.1 days, respectively (unpublished CONCEPTT data). A number of sensitivity analyses were performed to determine the cost impact of varying different inputs. One‐way analyses explored the impact of varying the proportion of complicated deliveries from 18% to 32%, and of varying the daily cost of NICU care from £3743 to £2400 or £3800. Two‐way analyses investigated the potential cost impact of using between four and 12 blood glucose strips per day, and of durations of normal postnatal ward hospitalization (excluding NICU) of between 1 and 6 days. It is now possible to use RT‐CGM without SMBG, so the possibility of RT‐CGM use with zero to four SMBG was assessed post hoc, after peer review.
Results
In the modelled population (n = 1441), the total annual costs of glucose monitoring and the management of pregnancies and deliveries in women with Type 1 diabetes were £23 725 648 when glucose monitoring was performed by SMBG alone (Table S1). These costs decreased to £14 165 187 when it was assumed that the entire modelled cohort used RT‐CGM together with SMBG during pregnancy (Fig. 2). Hence, the potential cost‐saving resulting from RT‐CGM use was approximately £9 560 461. The principal drivers of this saving were the daily cost of NICU care (£3743) and the shorter duration of NICU care when RT‐CGM was used with SMBG, compared with SMBG alone (6.6 vs. 9.1 days, respectively).
Figure 2.
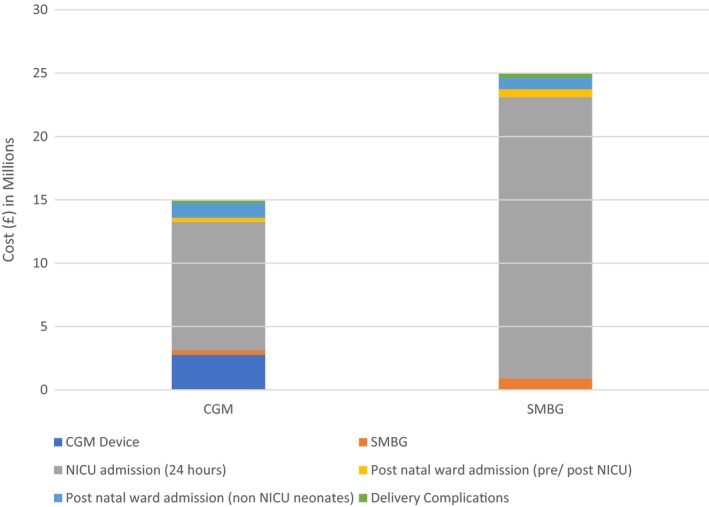
Modelled cost of type 1 diabetes in pregnancy (10‐38 weeks gestation) with real‐time continuous glucose monitoring (RT‐CGM) and self‐monitoring of blood glucose (SMBG), compared to SMBG alone.
The main reasons for NICU admission were preterm delivery (63%), neonatal hypoglycaemia treated with intravenous dextrose (56%), neonatal hyperbilirubinemia (54%) and respiratory distress (26%), with comparable indications for NICU admission when RT‐CGM was used with SMBG, compared with SMBG alone. The UK sites had the highest proportion of NICU admissions (63%), followed by Canada (34%) with only one or none in Spain, Italy, Ireland and the USA (Table S2).
The impact of changes in complication rates and NICU costs on the potential cost savings achievable with RT‐CGM was examined in sensitivity analyses. In the base case analysis, it was assumed that, in the absence of RT‐CGM, 18% of deliveries would be complicated by pre‐eclampsia. Increasing this proportion resulted in a progressive increase in the potential savings achievable with RT‐CGM, which reached £9 842 896 with a complication rate of 32%. Further analysis showed that RT‐CGM was still cost‐saving when the daily cost of NICU care was reduced from the base case value of £3743 to £2400 (potential saving £5 444 736), and that the savings increased to £9 735 141 when the daily cost was increased to £3800 (Fig. S1).
Further sensitivity analyses examined the impact of SMBG strip use and length of non‐NICU postnatal ward stay. The potential savings associated with RT‐CGM use increased from approximately £9.1 million to £9.7 million when the mean number of daily fingerstick measurements in the SMBG group was varied between 4 and 12, respectively (Table S3). Furthermore, RT‐CGM remained cost‐saving, albeit to lesser extents, when the number of SMBG measurements in the RT‐CGM users was increased from four to seven. In addition, greater cost savings were depicted when the number of SMBG measurements in the RT‐CGM users was reduced to zero demonstrating the potential savings of newer CGM systems with reduced and/or no need for additional SMBG tests.
Similarly, decreasing the duration of postnatal (non‐NICU) ward hospitalization from 3 days to 1 day, among RT‐CGM users, increased the potential savings achievable (Fig. S2). The maximum potential saving was £11 145 546 when duration of non‐NICU postnatal ward admission increased from 3 to 6 days in SMBG users and decreased from 3 days to 1 day among RT‐CGM users (Table S4).
Discussion
This study has shown that the routine use of RT‐CGM by pregnant women with Type 1 diabetes could produce savings to the NHS of approximately £9.6 million, mainly through reductions in NICU admissions and a shorter duration of NICU stay. Furthermore, RT‐CGM remained cost saving, albeit to lesser extents, across a range of NICU daily costs, durations of NICU stay and varied number of daily SMBG measurements. Our model highlights the impact of NICU admissions on the total costs associated with the management of Type 1 diabetes during pregnancy. By contrast, the costs of postnatal ward admissions, in infants not admitted to NICU, and before or after NICU admission, account for smaller proportions of the total costs.
In this budgetary impact model, the cost of RT‐CGM use from 10 to 38 weeks’ gestation was approximately threefold higher than that of SMBG alone (£1820 vs. £588 respectively), with the assumption that 10 SMBG measurements would be made per day in SMBG users 7. Nevertheless, sensitivity analyses showed that RT‐CGM still remained cost‐saving, when SMBG measurements were reduced to less than four per day.
Furthermore, the observed savings may be underestimates because we conservatively assumed that only 18% of pregnancies would be impacted by the additional costs associated with a complicated delivery (£3357 for complicated and £1957 for normal delivery 19). Additional obstetric morbidities such as hypertensive disorders of pregnancy (any gestational hypertension, worsening of pre‐existing hypertension) as well as maternal morbidity relating to large for gestational age birthweight (postpartum haemorrhage and perineal trauma) were not included with the incremental complicated delivery costs.
Data on the cost‐effectiveness of RT‐CGM during pregnancy are scarce 20, 21. A recent systematic review 21 identified only two studies that directly compared CGM with capillary glucose monitoring 22, 23, neither of which included cost data. It is noteworthy that in CONCEPTT, the numbers needed to treat with CGM to prevent one neonatal complication were low; six for NICU admissions and large for gestational age, and eight for neonatal hyperglycaemia 11. This suggests that the potential cost savings seen in the present analysis are achievable. Furthermore, more than 50% of pregnant women in CONCEPTT were using multiple daily injections 11, and hence the costs of insulin treatment would have been lower than with pump therapy. By contrast, in the Juvenile Diabetes Research Foundation study, ~ 90% of adults with Type 1 diabetes, were using insulin pump therapy 24. Importantly, the clinical efficacy of RT‐CGM in women using insulin pump therapy and multiple daily injections was comparable, although rates of NICU admission > 24 h were higher among insulin pump users 25. However, the costs of insulin therapy were not included in our model, so we cannot draw conclusions about the potential costs of RT‐CGM in women using pumps or multiple daily injections.
Because Type 1 diabetes during pregnancy is associated with increased risks of serious pregnancy complications such as congenital abnormalities, stillbirth and neonatal mortality, it imposes particular clinical, societal and financial burdens on healthcare systems 26. Large for gestational age remains the most common complication, affecting half of all infants born to mothers with Type 1 diabetes, and increases risk for obstetric complications including shoulder dystocia, instrumental and/or operative delivery and postpartum haemorrhage 27. These costs are considered only in the duration of NICU and postnatal hospitalization. Recent data confirm that the risk of adolescent obesity is 1.5 times higher in infants born large for gestational age 28, suggesting that the acceleration of BMI and sustained obesity persist throughout childhood and adolescence. The longer‐term costs associated with childhood overweight and obesity attributable to large for gestational age birthweight in Type 1 diabetes pregnancy are unknown.
Strengths of the present study include the use of outcome data from a multicentre randomized controlled trial, robust sensitivity analyses and the use of contemporary National Diabetes Pregnancy data in the model. Approximately two‐thirds of NICU admissions occurred in the UK, making these data representative of the factors affecting NICU admission in the NHS. As well, the model inputs have been varied to reflect different scenarios of SMBG use and NICU costs, with RT‐CGM found to be consistently cost saving. The reductions in large for gestational age neonates, neonatal hypoglycaemia and NICU admissions in RT‐CGM users were generalizable across 31 centres from the UK, Canada, Spain, Italy, Ireland and the USA, so although there is no reason to assume that the potential for cost savings would vary substantially in different healthcare settings, they may be most applicable in settings with high NICU admission rates. The study has additional limitations. The modelled population is restricted to England, which may limit the generalizability of our findings, although pregnancy outcome data are comparable with studies from other Northern European, Canadian and USA healthcare settings 1, 2, 4, 5, 29, 30. A further potential limitation is that costs associated with the treatment of diabetes, such as diabetes educator time and costs of insulin therapy, were excluded from the model. As a result, it is not possible to determine whether, or to what extent, these costs affect the RT‐CGM cost savings. Furthermore, the RT‐CGM used during CONCEPTT has been superseded by newer CGM systems with a longer sensor lifespan. Recent improvements in sensor accuracy and reduced need for pre‐meal SMBG and/or additional calibration tests, also mean that the current costs of glucose monitoring with modern CGM devices may now be lower.
The results of this study have important implications for clinicians and policy‐makers. Current NICE guidance recommends that women with diabetes should aim to achieve an HbA1c level of < 48 mmol/mol (<6.5%) 6, but achieving this level of control throughout pregnancy is often difficult. It was achieved by only 40% of women with Type 1 diabetes in England and Wales, with substantial variability across different maternity clinics 26. By contrast, the NICE target HbA1c was achieved by 66% of women in CONCEPTT, with no heterogeneity across differing baseline maternal HbA1c levels or across countries. Pregnant women are often among the early adopters of advanced diabetes technologies, with data from the US T1D Exchange clinic registry participants suggesting that approximately one‐third used CGM and three‐quarters used insulin pump therapy 29. The Belgian healthcare authorities have authorized reimbursement of RT‐CGM for insulin pump users with Type 1 diabetes treated in selected specialized diabetes centres. Initial data from over 500 users including 66 women who were pregnant and/or planning pregnancy suggested potential for sustained improvements in glucose control for up to 12 months 30. Inclusion of diabetes technology use (both RT‐CGM and insulin pump therapy) as a key metrics in national and international Diabetes Pregnancy data sets is needed to determine whether the clinical and cost‐effectiveness demonstrated in CONCEPTT can be translated into real‐world NHS clinical settings.
In conclusion, our results suggest that the higher costs of RT‐CGM, compared with SMBG alone, are offset by savings in NICU care. The cost savings associated with RT‐CGM use are achieved mainly through reductions in NICU admission rates, and in the shorter length of NICU stay. This is an important message for clinicians and healthcare providers, given that 40% of infants born to mothers with Type 1 diabetes are admitted to NICU 26. Routine use of RT‐CGM by pregnant women with Type 1 diabetes would result in substantial cost savings to the NHS, and probably to other healthcare systems. Recent improvements in sensor accuracy and duration mean that current RT‐CGM use may result in more substantial cost savings.
Ethical approval
The clinical study protocol was approved by the Health Research Authority, East of England Research Ethics Committee (12/EE/0310) for all UK sites and at each individual centre for all other sites. All participants provided written informed consent.
Funding sources
Funding for the development of the economic model reported here was provided by Medtronic. CONCEPTT was funded by JDRF grants #17‐2011‐533, and grants under the JDRF Canadian Clinical Trial Network (CCTN), a public–private partnership including JDRF and FedDev Ontario and supported by JDRF #80‐2010‐585. Medtronic supplied the CGM sensors and CGM systems at a reduced cost. HRM is supported by Tommy's charity.
Competing interests
DSF reports advisory/speaker fees from Medtronic, Novo Nordisk and Dexcom. HRM reports personal fees from NovoNordisk, Roche, Medtronic, Abbott Diabetes Care, outside the submitted work. HRM sits on the Medtronic European Scientific Advisory Board. AS is a full‐time employee of Medtronic. SDP is a full‐time employee of Medtronic, and owns stocks in the company.
Author contributions
AS, SDP and HRM conceived and designed the study. AS, DSF, JJS, HRM collected the data. AS analysed the data. AS and HRM prepared the manuscript, which all authors critically reviewed. All authors have given final approval of the version to be published. HRM is the guarantor of this work, had full access to all the study data and takes responsibility for the integrity of the data.
Supporting information
Doc. S1. CONCEPTT Collaborative Group.):
Figure S1. Tornado plot showing results of one‐way sensitivity analyses of the impact of varying neonatal intensive care unit costs from £2400 to £3800.
Figure S2. Results of two‐way sensitivity analyses showing impact of varying duration of postnatal ward care from 1 to 6 days for both cohorts.
Table S1. Costs of management of Type 1 diabetes pregnancies and deliveries, when glucose monitoring was performed with and without real‐time continuous glucose monitoring.
Table S2. Diagnoses of neonates admitted to the neonatal intensive care unit.
Table S3. Sensitivity analyses of the impact of changes in the daily number of SMBG measurements on the potential cost savings with RT‐CGM use.
Table S4. Sensitivity analyses of the impact of changes in the duration of neonatal hospital admission (postnatal ward without NICU) on the potential cost savings with RT‐CGM use.
Acknowledgements
Medical writing and submission support was provided by Michael Shaw (MScript Ltd, Hove, UK), and funded by Medtronic. The authors would like to thank all the women with Type 1 diabetes who participated. We also acknowledge the invaluable support from the 31 clinical care teams and the Clinical Trials Services/Centre for Mother, Infant, and Child Research team at Sunnybrook Research Institute, Toronto, Canada in particular Sonya Mergler, Kathryn Mangoff, Minhee Myung and Gail Klein.
Diabet. Med. 36, 1652–1658 (2019)
References
- 1. Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 2004; 328: 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feig DS, Hwee J, Shah BR, Booth GL, Bierman AS, Lipscombe LL. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population‐based study in Ontario, Canada, 1996–2010. Diabetes Care 2014; 37: 1590–1596. [DOI] [PubMed] [Google Scholar]
- 3. Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D et al Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ 2006; 333: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population‐based study. Diabetes Care 2009; 32: 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen DM, Korsholm L, Ovesen P, Beck‐Nielsen H, Moelsted‐Pedersen L, Westergaard JG et al Peri‐conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care 2009; 32: 1046–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence . Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. NICE guideline 3. Available at https://www.nice.org.uk/guidance/ng3/ Last accessed 24 April 2019. [PubMed]
- 7. National Institute for Health and Care Excellence . Type 1 Diabetes in Adults: Diagnosis and Management. NICE guideline 17. Available at https://www.nice.org.uk/guidance/ng17/ Last accessed 24 April 2019.
- 8. Garcia‐Patterson A, Gich I, Amini SB, Catalano PM, de Leiva A, Corcoy R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia 2010; 53: 446–451. [DOI] [PubMed] [Google Scholar]
- 9. Goudie RJ, Lunn D, Hovorka R, Murphy HR. Pharmacokinetics of insulin aspart in pregnant women with type 1 diabetes: every day is different. Diabetes Care 2014; 37: e121–122. [DOI] [PubMed] [Google Scholar]
- 10. Murphy HR, Elleri D, Allen JM, Harris J, Simmons D, Rayman G et al Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012; 55: 282–293. [DOI] [PubMed] [Google Scholar]
- 11. Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF et al Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017; 390: 2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy HR. Intensive glycemic treatment during type 1 diabetes pregnancy: a story of (mostly) sweet success!. Diabetes Care 2018; 41: 1563–1571. [DOI] [PubMed] [Google Scholar]
- 13. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S et al Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017; 317: 371–378. [DOI] [PubMed] [Google Scholar]
- 14. Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M et al Budget impact analysis‐principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014; 17: 5–14. [DOI] [PubMed] [Google Scholar]
- 15. Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J et al A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014; 370: 107–118. [DOI] [PubMed] [Google Scholar]
- 16. NHS England and NHS Improvement . National Tariff Payment System 2017/18 and 2018/19. Available at https://improvement.nhs.uk/resources/national-tariff-1719/ Last accessed 24 April 2019.
- 17. National Institute for Health and Care Excellence . British National Formulary. London: NICE, 2019. [Google Scholar]
- 18. NHS Digital . National Pregnancy in Diabetes Audit 2014–16. Available at https://files.digital.nhs.uk/publication/7/s/national_pregnancy_in_diabetes_2016_report.pdf Last accessed 24 April 2019.
- 19. National Institute for Health and Care Excellence . Costing Statement: Diabetes in Pregnancy; Implementing the NICE Guideline on Diabetes in Pregnancy (NG3). Available at https://www.nice.org.uk/guidance/ng3/resources/costing-statement-pdf-3782989 Last accessed 24 April 2019.
- 20. Farrar D, Campbell MD. Does continuous glucose monitoring during pregnancy improve glycaemic and health outcomes in women with type 1 diabetes?‐what the CONCEPTT trial adds. Ann Transl Med 2018; 6: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moy FM, Ray A, Buckley BS. West HM. Techniques of monitoring blood glucose during pregnancy for women with pre‐existing diabetes. Cochrane Database of Systematic Reviews 2017; (6) CD009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K et al Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008; 337: a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real‐time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 2013; 36: 1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R et al Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 25. Feig DS, Corcoy R, Donovan LE, Murphy KE, Barrett JFR, Sanchez JJ et al Pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care 2018; 41: 2471–2479. [DOI] [PubMed] [Google Scholar]
- 26. Murphy HR, Bell R, Cartwright C, Curnow P, Maresh M, Morgan M et al Improved pregnancy outcomes in women with type 1 and type 2 diabetes but substantial clinic‐to‐clinic variations: a prospective nationwide study. Diabetologia 2017; 60: 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mackin ST, Nelson SM, Kerssens JJ, Wood R, Wild S, Colhoun HM et al Diabetes and pregnancy: national trends over a 15 year period. Diabetologia 2018; 61: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E et al Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med 2018; 379: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 29. Polsky S, Wu M, Bode BW, DuBose SN, Goland RS, Maahs DM et al Diabetes technology use among pregnant and nonpregnant women with T1D in the T1D Exchange. Diabetes Technol Ther 2018; 20: 517–523. [DOI] [PubMed] [Google Scholar]
- 30. Charleer S, Mathieu C, Nobels F, De Block C, Radermecker RP, Hermans MP et al Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real‐world study. J Clin Endocrinol Metab 2018; 103: 1224–1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doc. S1. CONCEPTT Collaborative Group.):
Figure S1. Tornado plot showing results of one‐way sensitivity analyses of the impact of varying neonatal intensive care unit costs from £2400 to £3800.
Figure S2. Results of two‐way sensitivity analyses showing impact of varying duration of postnatal ward care from 1 to 6 days for both cohorts.
Table S1. Costs of management of Type 1 diabetes pregnancies and deliveries, when glucose monitoring was performed with and without real‐time continuous glucose monitoring.
Table S2. Diagnoses of neonates admitted to the neonatal intensive care unit.
Table S3. Sensitivity analyses of the impact of changes in the daily number of SMBG measurements on the potential cost savings with RT‐CGM use.
Table S4. Sensitivity analyses of the impact of changes in the duration of neonatal hospital admission (postnatal ward without NICU) on the potential cost savings with RT‐CGM use.


