Abstract
BACKGROUND
Universal pathogen inactivation of platelet concentrates (PCs) using amotosalen/ultraviolet A with 7‐day storage was implemented in Switzerland in 2011. Routine‐use data were analyzed at the University Hospital Basel, Switzerland.
STUDY DESIGN
A retrospective two‐cohort study of patient and PC characteristics, component usage, patient outcomes, count increments (CIs), and adverse events were analyzed for two consecutive 5‐year periods with either 0‐ to 5‐day‐old conventional PC (C‐PC) (n = 14,181) or 0‐ to 7‐day‐old pathogen‐inactivated PC (PI‐PC) (n = 22,579).
RESULTS
In both periods, PCs were issued for transfusion on a “first in, first out” basis. With 7‐day PI‐PC, wastage was reduced from 8.7% to 1.5%; 16.6% of transfused PI‐PCs were more than 5 days old. Transfusion of PI‐PC more than 5 days old compared with 5 days old or less did not increase platelet and RBC use on the same or next day as an indirect measure of hemostasis and did not increase transfusion reactions. Mean corrected count increments (CCIs) for PI‐PC stored for 5 days or less were 22.6% lower than for C‐PC (p < 0.001), and declined with increasing storage duration for both, although the correlation was weak (r2 = 0.005‐0.014). Mean number of PCs used per patient and duration of PC support were not different for hematology/oncology, allogeneic and autologous hematopoietic stem cell transplant (HSCT), and general medical/surgical patients, who used the majority (~92.0%) of PI‐PCs. Five‐year treatment‐related mortality in allogeneic HSCT was unchanged in the PI‐PC period.
CONCLUSIONS
PI‐PCs with 7‐day storage reduced wastage and did not increase PC or red blood cell utilization or adverse reactions compared with fresh PI‐PC or a historical control group, demonstrating preserved efficacy and safety.
ABBREVIATIONS
- BC
buffy coat
- CCIs
corrected count increments
- CIs
count increments
- C‐PC
conventional platelet concentrate
- CVS
cardiovascular surgery
- EBMT
European Group for Blood and Marrow Transplantation
- HSCT
hematopoietic stem cell transplant
- IPTAS
Italian Platelet Technology Assessment Study
- PAS
platelet additive solution
- PCs
platelet concentrates
- PI‐PC
pathogen‐inactivated platelet concentrate
- TRM
treatment‐related mortality
The Swiss Red Cross Blood Transfusion Services introduced universal treatment of all platelet concentrates (PCs) with amotosalen/ultraviolet A pathogen inactivation (INTERCEPT Blood System for Platelets, Cerus BV) by November 2011.1, 2 Implementation was in response to emerging infectious threats, unacceptable septic transfusion reaction rates reported to the Swissmedic hemovigilance program, and the recent death of a pediatric patient attributed to a bacterially contaminated PC.1 The Regional Blood Transfusion Service in Basel fully implemented pathogen inactivation of PCs in January 2011. Prior to licensure of INTERCEPT by Swissmedic in 2009, a study evaluated the efficacy and safety of pathogen‐inactivated PCs (PI‐PCs) demonstrating effective posttransfusion count increments (CIs).3 A second study in hematology/oncology patients demonstrated acceptable CIs compared to irradiated conventional PC (C‐PC).4 In these studies, as well as in routine use, PI‐PCs were stored for up to 7 days. Subsequently, the University Hospital Basel initiated a study to summarize the clinical impact of 5 years of routine use data to specifically look at potential effects of PI‐PC with storage duration of more than 5 days.
Following CE mark approval in 2005, INTERCEPT PI‐PCs stored for more than 5 days have been transfused in Sweden, Spain, Belgium, Iceland, Portugal, and Switzerland. In April 2018, the French health authority, Agence Nationale de Sécurité du Médicament et des Produits de Santé, approved shelf life extension to 7 days. Pathogen inactivation with INTERCEPT provides an effective solution to reduce the risk from current and emerging pathogen contamination of PC, effectively reduces the risk of bacterial sepsis, and replaces irradiation to prevent transfusion‐associated graft‐versus‐host disease.5 Extension of storage to 7 days may reduce wastage and improve logistics and availability, but has been posited to expose patients to less effective platelet hemostasis.
We hypothesized that routine use data can address the question of whether older PCs maintain hemostasis, as indirectly assessed by the need for additional PCs and/or red blood cell (RBC) transfusions on the day of or the day following transfusion of PCs older than 5 days. We analyzed 10 years of routine‐use clinical data from a large academic center where pretransfusion and 1‐ to 4‐hour posttransfusion platelet counts were commonly ordered as the standard of care.
METHODS
Platelet concentrate production
The Swiss Red Cross Blood Transfusion Service, Basel, commenced manufacture of all PCs with the INTERCEPT Blood System for Platelets in January 2011 with documentation of platelet dose after production.1, 2 PCs are resuspended in approximately 65% platelet additive solution (PAS) (InterSol, Fenwal Europe) and contain 2.4 × 1011 platelets/unit or greater with 100% leukoreduction (leukocyte count <1 × 106/PC).1 With pathogen inactivation, 65% to 75% of PCs are now collected by apheresis and 25% to 35% are prepared from whole blood buffy coat (BC) concentrates and are not gamma irradiated. During the PI‐PC period, approximately 13.4% (a mixture of BC and apheresis PCs) were obtained after INTERCEPT treatment from other Swiss regional blood centers to accommodate an increase in the number of patients transfused in Basel. Before pathogen inactivation, all PCs were 100% apheresis in a platelet additive solution (T‐Sol), approximately 70% were collected on an AMICUS apheresis collection system (Fenwal Europe) and approximately 30% on an TRIMA Accel automated blood collection system (TerumoBCT); the PCs were stored for 5 days, were not screened for bacteria, and were universally gamma irradiated to prevent transfusion‐associated graft‐versus‐host disease.
Pathogen inactivation treatment
INTERCEPT large‐volume and dual‐storage processing kits were used. PCs containing 2.5–8.0 × 1011 platelets were treated with amotosalen (nominal final concentration, 150 μM) and 3 J/cm2 ultraviolet A illumination according to the manufacturer's instructions, followed by incubation in a compound absorption device for 6 to 16 hours. Apheresis components were treated on the day of collection (Day 0) or on the next day (Day 1), and products were released into inventory the next day on receipt of the infectious disease testing results. For BC PCs, INTERCEPT treatment was performed immediately after BC pool preparation on the day after collection, and the unit was released into inventory after incubation on a compound absorption device for 6 to 16 hours. PI‐PCs were stored for up to 7 days before transfusion.6
Patient data and study design
Patients were transfused according to hospital guidelines and the prescription of treating physicians. In hematology/oncology and autologous or allogeneic HSCT patients, the transfusion threshold was 10 × 109/L in nonbleeding, nonfebrile patients or 20 × 109/L in patients with active graft‐versus‐host disease or with a fever.3
A retrospective observational, two‐period study of the effect of storage duration of PI‐PCs on platelet and RBC utilization was performed comparing routine use data for C‐PCs for 5 years before (January 1, 2006, to January 9, 2011) and for PI‐PCs for 5 years after (January 10, 2011, to May 17, 2016) introduction of pathogen inactivation. All patients receiving PC transfusions in the observation period in Basel were included, regardless of age, underlying disease, or indication for PC transfusion (prophylactic or therapeutic), as documented in the hospital and blood center computer systems. Hematology/oncology and HSCT patients were primarily transfused for prophylactic indications, while patients undergoing cardiovascular surgery (CVS) and general medical/surgical patients mostly received therapeutic transfusions to treat bleeding. Data were prospectively collected and obtained from the electronic records of the Regional Blood Transfusion Service, which serves as the University Hospital transfusion laboratory. Patient and donor identifying information were anonymized, and institutional review board approval was obtained from the Ethical Committee of Northern and Central Switzerland (approval number 2019‐00744).
Data analysis
Due to substantial differences in transfused patient populations, indications for transfusion and PC characteristics between the two periods, a per‐patient analysis broken out by transfusion indication was used to evaluate PC utilization. The patient diagnostic groups (Table 1) were defined according to a hierarchy based on the indication for PC transfusion for all patients who received at least one PC in the following clinical categories: allogeneic HSCT; autologous HSCT; hematology/oncology; CVS; or “other” medical and surgical conditions (including where an indication was unspecified).
Table 1.
Patient and PC transfusion characteristics at University Hospital Basel, Switzerland, from 2006 to 2016
| Period | Conventional PCs (01/02/2006‐01/09/2011) | INTERCEPT PCs (01/10/2011‐5/17/2016) | p value |
|---|---|---|---|
| Patients* | 2,036 | 2,809 | |
| Patient age (y) | |||
| Mean (SD) | 58.3 (20.5) | 59.7 (19.9) | <0.001 |
| Age distribution | |||
| < 1 mo | 29 (1.4%) | 35 (1.2%) | |
| 1 mo‐18 y | 103 (5.1%) | 131 (4.6%) | |
| 19‐64 y | 967 (47.5%) | 1,264 (45.0%) | |
| ≥65 y | 937 (46.0%) | 1,378 (49.1%) | |
| Male (%) | 61.1% | 64.1% | <0.043 |
| Patient diagnosis | |||
| Heme/oncology | 439 (21.6%) | 551 (19.6%) | <0.001 |
| HSCT, allogeneic | 248 (12.2%) | 367 (13.1%) | |
| HSCT, autologous | 44 (2.2%) | 50 (1.8%) | |
| CVS | 416 (20.4%) | 749 (26.7%) | |
| Other medical/surgical | 889 (43.7%) | 1,092 (38.9%) | |
| Days on study† | |||
| Mean (SD) | 54.8 (157.2) | 64.6 (188.0) | 0.050 |
| Median | 1.0 | 1.0 | |
| Range | 1–1,533 | 1–1,835 | |
| Days of platelet support‡ | |||
| Mean (SD) | 9.4 (21.8) | 9.6 (26.6) | 0.789 |
| Median | 1.0 | 1.0 | |
| Days PC transfusion occurred | |||
| Mean (SD) | 6.0 (13.0) | 6.4 (14.2) | 0.322 |
| Median | 1.0 | 1.0 | |
| Platelets transfused | 14,181 | 22,579 | |
| Platelets per patient | |||
| Mean (SD) | 70 (15.7) | 8.0 (18.2) | 0.028 |
| Median | 2.0 | 2.0 | |
111 patients received both conventional and INTERCEPT PCs and are counted in both groups.
Interval between the patients' first and last PC transfusion.
Days of PC support = sum of periods of PC support, where a period is defined as interval between the first platelet transfusion and all subsequent platelet transfusions with less than 5 days between platelet transfusions.
CVS = cardiovascular surgery; HSCT = hematopoietic stem cell transplant; PC = platelet concentrate; SD = standard deviation.
Days of platelet transfusion support and the intertransfusion interval were calculated only during periods of active platelet transfusion support, defined as all PC transfusions during any period without a hiatus of 5 days. An interval of more than 5 days defined platelet transfusion independence. One‐hour corrected count increments (CCIs) were calculated as follows: (post platelet count – pre platelet count in 109/L) × (body surface area in m2) / (platelet dose transfused × 1011).
Data were summarized descriptively by mean, standard deviation, median, and range for continuous data or by frequencies and proportions (%) for categorical data, using computer software (SAS version 9.4, SAS Institute). All C‐PCs and PI‐PCs administered to a patient in each of the two study periods were included in the data analysis, and data were summarized on a per‐transfusion and a per‐patient basis. Differences between the two treatment periods were assessed by two‐sided t test (with unequal variances) for continuous variables, Fisher's exact test for binary data, Cochran–Mantel–Haenszel test with the row mean scores differ statistic for ordinal data, or Cochran–Mantel–Haenszel test with general association for nonordinal data. In addition, the Wilcoxon rank‐sum test was used for continuous variables that had very skewed distributions (e.g., per‐patient PC use and days of platelet support). The Kaplan–Meier estimator was used to assess treatment related mortality. The log‐rank test was used to compare among groups. Statistical significance was set at a p value (two‐sided) of 0.05 or less.
RESULTS
The University Hospital Basel is a 770‐bed tertiary‐care academic hospital. During the PI‐PC period, 38% more patients were transfused (2809 vs. 2036 patients), with proportionately more patients in the allogeneic HSCT and CVS groups (Table 1). The age distribution was different between the two periods, with slightly more patients 65 years old or greater (49% for PI‐PC vs. 46% for C‐PC) and more males (64.1% vs. 61.2%; p = 0.043) transfused during the PI‐PC period.
Although the median time on study (time from first to last PC transfusion reported regardless of periods of platelet transfusion independence) was 1 day for both observation periods, many patients in the PI‐PC period were observed for a longer time (up to 1835 days vs. 1533 days in the C‐PC period), providing information on longitudinal exposure. The overall duration of PC transfusion support per patient (mean, 9.6 days vs. 9.4 days; p = 0.789, excluding periods of PC transfusion independence), and days on which PC transfusion occurred (mean, 6.4 days vs. 6.0 days; p = 0.322) were not different in the two periods. The overall number of PCs used (22,579 PC vs. 14,181 PC) was 59% higher during the PI‐PC period. With the changes in the patient populations, medical practice, and primary indications for transfusion, a higher mean number of PCs were used per patient (8.0 vs. 7.0/patient: p = 0.028) during the PI‐PC period, although the median exposure was 2.0 PCs/patient in both periods.
PCs were issued for transfusion on a “first in, first out” basis for up to 5 days during the C‐PC period, with 1359 (8.7%) PCs discarded due to expiration. In contrast, during the 7‐day storage PI‐PC period, only 355 (1.5%) PCs expired (Table 2). As expected, average storage duration was higher during the PI‐PC period (4.2 vs. 3.4 days; p < 0.001), and 16.6% of all PI‐PCs were more than 5 days old at the time of transfusion (Fig. 1 and Table 2). The mean platelet dose measured at time of PC treatment was higher for PI‐PCs (3.0 vs. 2.8 × 1011; p < 0.001), and PI‐PCs were more likely to be recipient ABO compatible (74.4% vs. 67.2% ABO compatible; p < 0.001). The number of PCs issued to every patient diagnostic group was higher in the PI‐PC period, with proportionately more used to support patients undergoing allogeneic HSCT and CVS (Table 2).
Table 2.
Characteristics of platelet components transfused at University Hospital Basel, Switzerland
| Period | Conventional PCs (01/02/2006‐01/09/2011) | INTERCEPT PCs (01/10/2011‐5/17/2016) | p value |
|---|---|---|---|
| Platelet age (days) | |||
| Mean (SD) | 3.4 (1.2) | 4.2 (1.4) | <0.001 |
| Median | 3.0 | 4.0 | |
| Platelet dose (×1011) | |||
| Mean (SD) | 2.8 (0.7) | 3.0 (0.4) | <0.001 |
| Apheresis/whole blood buffy coat PCs (%) | 100% | 85.5%/14.5% | |
| Maximum routine storage | 5 days | 7 days | |
| >5 days old | 162 (1.1%)† , ‡ | 3,750 (16.6%)‡ | |
| Outdates | 1,359 (8.7%) | 355 (1.5%) | <0.001 |
| ABO compatible* | 67.2% | 74.4% | <0.001 |
| Patient diagnosis | |||
| Hematology/oncology | 4,638 (32.7%) | 6,069 (26.8%) | <0.001 |
| HSCT, allogeneic | 6,201 (43.7%) | 10,639 (47.1%) | |
| HSCT, autologous | 328 (2.3%) | 778 (3.4%) | |
| CVS | 682 (4.8%) | 1,819 (8.1%) | |
| Other medical/surgical | 2,332 (16.4%) | 3,283 (14.5%) | |
ABO identical or minor mismatched.
Used in a clinical study.
Percentage of all transfused PC shown. Percentage > 5 days of PC with known storage duration was 1.2% for C‐PCs and 18.3% for PR‐PCs.
CVS = cardiovascular surgery; HSCT = hematopoietic stem cell transplant; PC = platelet concentrate.
Figure 1.

Platelet storage age distribution before and after adoption of PI‐PCs. [Color figure can be viewed at http://wileyonlinelibrary.com]
Per‐patient platelet use and outcomes in hematology/oncology and HSCT patients
Platelet use was analyzed by patient diagnosis representing the major hospital consumers of PCs. During the PI‐PC period, hematology/oncology (non‐HSCT), allogeneic HSCT, and autologous HSCT patients comprised 34.5% of the transfusion recipients and used 77.3% of the PI‐PCs. These groups did not require more PCs per patient comparing the two periods: hematology/oncology (mean, 11.1 vs.10.7 PCs/patient: p = 0.763), allogeneic HSCT (mean, 27.8 vs. 23.9 PCs/patient: p = 0.111), autologous HSCT (mean, 9.0 vs. 7.4 PCs/patient: p = 0.247), or an increased mean or median duration of PC support (Table 3) during the PI‐PC period. This was despite significantly shorter intervals (p < 0.048) between transfusions and lower CCIs (p < 0.001) (Table 3). Furthermore, there were no differences in the proportion of hematology/oncology, allogeneic HSCT, or autologous HSCT patients who received RBCs or the number of RBCs transfused per patient (Table 3).
Table 3.
Platelet utilization outcomes by clinical indication. Wilcoxon rank‐sum test p values are shown in parentheses
| Mean values (median) | Heme/Oncology | Allogeneic HSCT | Autologous HSCT | Cardiovascular Surgery (CVS) | Other Med/Surgical | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | INTERCEPT | p value | Control | INTERCEPT | p value | Control | INTERCEPT | p value | Control | INTERCEPT | p value | Control | INTERCEPT | p value | |
| Patients | 355 | 441 | 277 | 411 | 110 | 130 | 414 | 748 | 880 | 1079 | |||||
| Platelets | 3,806 | 4,911 | 6,628 | 11,416 | 816 | 1,173 | 677 | 1,817 | 2,254 | 3,262 | |||||
| Mean plts/pt (median) | 10.7 (4.0) | 11.1 (4.0) | 0.763 | 23.9 (13.0) | 27.8 (18.0) | 0.111 (0.001) | 7.4 (3.5) | 9.0 (4.0) | 0.247 | 1.6 (1.0) | 2.4 (2.0) | <0.001 | 2.6 (1.0) | 3.0 (1.0) | 0.119 |
| Days of platelet support (median) | 16.9 (5.0) | 16.5 (5.0) | 0.842 | 31.7 (17.0) | 31.3 (20.0) | 0.880 (>0.05) | 11.4 (4.5) | 11.5 (5.0) | 0.980 | 1.4 (1.0) | 1.6 (1.0) | 0.011 | 2.9 (1.0) | 3.9 (1.0) | 0.179 |
| Number of cycles (median) | 2.8 (1.0) | 2.8 (1.0) | 0.895 | 2.7 (2.0) | 2.9 (2.0) | 0.340 | 2.3 (1.5) | 2.3 (1.5) | 0934 | 1.0 (1.0) | 1.1 (1.0) | 0.077 | 1.3 (1.0) | 1.4 (1.0) | 0.073 |
| Days with platelet transfusion (median) | 9.5 (3.0) | 9.4 (3.0) | 0.928 | 20.4 (12.0) | 21.8 (15.0) | 0.446 | 6.9 (3.0) | 7.9 (4.0) | 0.38 1 | 1.3 (1.0) | 1.4 (1.0) | 0.003 | 2.2 (1.0) | 2.5 (1.0) | 0.184 |
| Any RBCs transfused | 84.2% | 85.7% | 0.617 | 94.9% | 94.9% | 1.000 | 73.6% | 70.8% | 0.666 | 92.0% | 82.2% | <0.001 | 35.6% | 41.3% | 0.010 |
| Number of RBCs (median) | 12.6 (6.0) | 11.2 (6.0) | 0.245 | 18.7 (10.0) | 19.3 (13.0) | 0.721 | 7.5 (2.0) | 7.0 (2.0) | 0.774 | 9.7 (6.0) | 8.5 (4.0) | 0.136 | 3.5 (0.0) | 4.1 (0.0) | 0.151 |
| Count before transfusion (×109/L) (median) | 17.4 (15.0) | 14.7 (12.0) | <0.001 | 18.7 (16.0) | 14.2 (13.0) | <0.001 | 16.5 (15.0) | 13.4 (13.0) | <0.001 | 90.7 (67.0) | 81.0 (69.0) | 0.176 | 40.5 (18.0) | 27.8 (13.0) | <0.001 |
| Interval (median) | 1.9 (1.9) | 1.7 (1.6) | 0.048 | 1.5 (1.4) | 1.3 (1.2) | <0.001 | 1.8 (1.6) | 1.3 (1.0) | <0.001 | 0.5 (0.0) | 0.4 (0.0) | 0.220 | 1.2 (1.0) | 1.0 (0.9) | 0.106 |
| CI (×109/L) (median) | 17.6 (15.0) | 15.7 (13.0) | <0.001 | 19.2 (16.0) | 13.4 (11.0) | <0.001 | 20.3 (17.0) | 12.5 (11.0) | <0.001 | 22.8 (22.0) | 22.3 (20.0) | 0.876 | 18.1 (14.0) | 14.2 (10.0) | 0.004 |
| Proportion of PCs with CCI data | 52.8% | 63.2% | 64.4% | 78.1% | 52.2% | 70.7% | 11.8% | 11.1% | 13.8% | 33.5% | |||||
| CCI (×103) (median) | 9.8 (9.1) | 8.7 (7.5) | <0.001 | 11.1 (9.8) | 8.0 (6.8) | <0.001 | 10.4 (9.5) | 7.2 (6.6) | <0.001 | 13.8 (12.7) | 13.5 (12.7) | 0.910 | 10.1 (7.7) | 7.6 (6.0) | 0.002 |
| CCI ≥ 5.0 × 103 | 74.1% | 68.9% | <0.001 | 77.6% | 63.8% | <0.001 | 77.5% | 62.2% | <0.001 | 70.0% | 75.2% | 0.371 | 61.6% | 58.2% | 0.295 |
CCI = corrected count increment; CI = count increment; PC = platelet concentrate.
Patients undergoing allogeneic HSCT were noted to receive a significantly higher median number of PI‐PCs (median, 18.0 vs. 13.0; p = 0.001 by the Wilcoxon rank‐sum test). These patients tended to have a higher European Group for Blood and Marrow Transplantation (EBMT) risk score (p = 0.05) (Fig. 3) during the PI‐PC period, indicative of increased transplantation risk.7 Increasing EBMT risk score is reported to correlate with higher overall 5‐year treatment‐related mortality (TRM), but the 5‐year TRM during the PI‐PC period was not higher (p = 0.135) than that of the C‐PC period; indeed, it appeared to be lower in the early posttransplant time during which patients received most platelet transfusions (Fig. 3).
Figure 3.
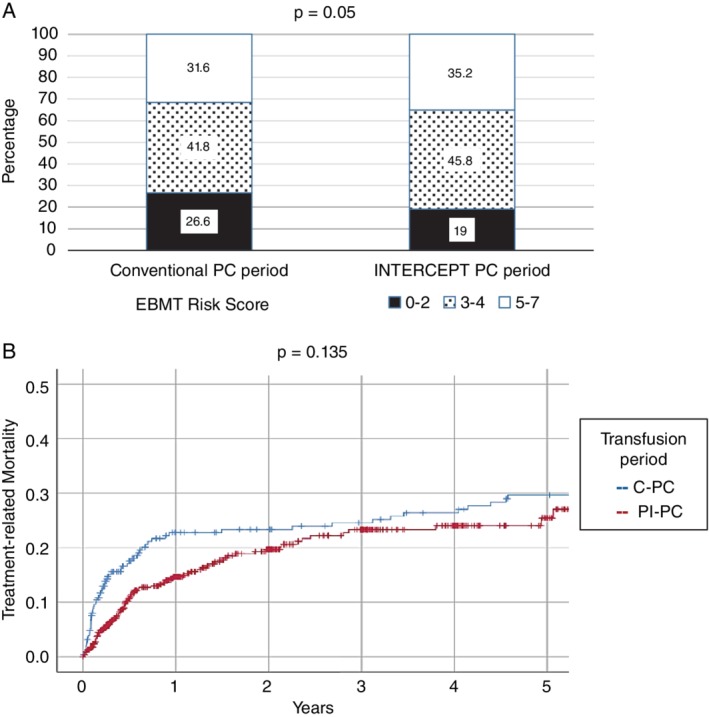
(A) EBMT risk score proportions during the 2006 to 2011 Conventional PC and 2011 to 2016 INTERCEPT PC periods. (B) Five‐year TRM according to PC support period (2006‐2011 C‐PC vs. 2011‐2016 INTERCEPT PC period). Data in (B) is shown as a cumulative (1 – survival) plot with censured data designated as (+) on each graph.
Per patient platelet use in CVS and “other” patients
During the PI‐PC period, 8.0% of the transfused PCs were given to 748 patients undergoing CVS (mean, 2.4 PCs/patient). This was a statistically significant increase compared to the previous period (1.6 PCs/patient: p < 0.001), and the duration of PC support was also increased (mean, 1.6 vs. 1.4 days; p = 0.011). In contrast, the proportion of patients transfused with PI‐PCs who also required RBC transfusion was smaller (82.2% vs. 92.0%; p < 0.001), although the number of RBC components transfused were not different (Table 3).
Patients of the “other” group, a mix of medical and surgical diagnoses other than cardiovascular, were transfused with a median of one PC during a median of 1 day of PC support during both periods. Numerically, this was the largest group of PI‐PC recipients (1079/2809 [38.4%] patients), but they were issued only 14.5% of the PI‐PCs. This heterogeneous group did not require more PCs per patient (3.0 vs. 2.6 PCs/patient; p = 0.119) or a longer PI‐PC support (3.9 vs. 2.9 days; p = 0.179) with PI‐PC. However, a larger proportion of patients required RBC transfusions (41.3% vs. 35.6%; p = 0.010), although the number of RBC transfusions per patient was similar (Table 3).
Response to PC transfusion
One‐hour CCI data were available for 14,138/22,579 (62.6%) platelet transfusions during the PI‐PC period and 7093/14,181 (50.0%) during the C‐PC period (Table 3). Relatively few CCI data (approx. 11% during both periods) were recorded for patients undergoing CVS, as most transfusions occurred during surgery. All patient subgroups, except patients undergoing CVS, were transfused at significantly (p < 0.001) lower pretransfusion platelet counts during the PI‐PC period, indicating transition to lower transfusion trigger values. With PI‐PC, mean intertransfusion intervals were shorter, and CI and CCI responses were significantly lower (p < 0.001; Table 3), although CCI values (Fig. 2) varied considerably, with substantial overlap between the PI‐PC and C‐PC increments. Considering all patients, CCI values were 22.6% lower when comparing PI‐PCs and C‐PCs each stored 5 days or less (8.2 × 103 vs. 10.6 × 103), and 24.3% lower when comparing all transfusions (8.1 × 103 vs. 10.7 × 103, respectively).
Figure 2.
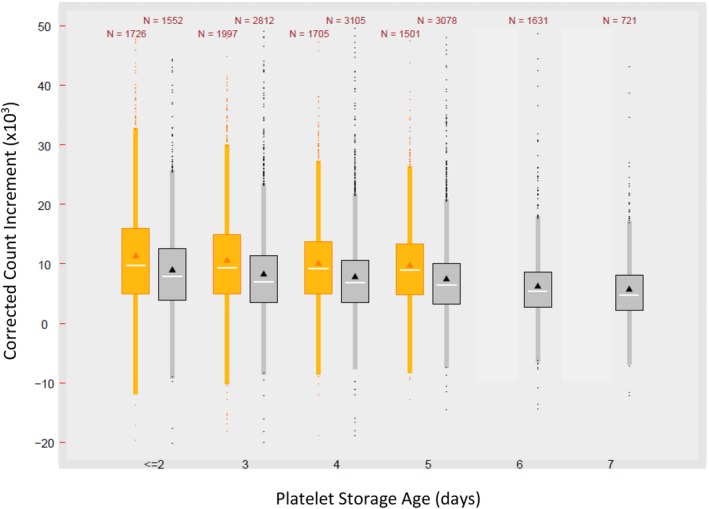
CCI by PC storage duration during the C‐PC (2006‐2011) (orange) and the PI‐PC (2011‐2016) periods (gray). Box plots show the mean (triangle), median (line), 25th and 75th percentiles (upper and lower box limits), and quarter ± 1.5 interquartile range (whiskers). Outliers are also plotted.
Patients undergoing CVS were the notable exception, although CCI data were available in only 202 of 1817 (11.1%) PI‐PC and 80 of 677 (11.8%) C‐PC transfusions. Pretransfusion platelet counts were considerably higher (mean pretransfusion count, 81.0 × 109/L) compared with all other PI‐PC recipients (Table 3). Mean CCIs with PI‐PCs were also markedly higher in patients undergoing CVS (13.5 × 103) than in other populations (7.2–8.7 × 103) and were similar to those of C‐PCs (mean, 13.8 × 103: p = 0.910; median, 12.7 × 103 for each period).
The impact of 7‐day storage on platelet and RBC use
Evaluation of mean CCIs for all transfusion episodes relative to day of transfusion revealed a pattern of decrease with increasing PC storage during both periods, although responses were broad and widely overlapping (Fig. 2). Using regression analysis, the mean CCI declined an average of 0.52 × 103 per day with C‐PC and an average of 0.61 × 103 per day with PI‐PCs, but with overall very little correlation between day of transfusion and CCI (r2 = 0.005 and 0.014, respectively). A similar impact on mean CCI with increasing PC storage was seen in each patient subgroup, with the possible exception of CVS, where only limited data are available.
The effect of reduced mean CCI responses with older PCs was evaluated for impact on clinical bleeding by evaluating the need for subsequent transfusions after the initial PC transfusion as an indirect measure of hemostasis. PI‐PC storage duration had no effect on the proportion of index PCs that required the transfusion of a second PC (Fig. 4a) or of RBCs on the same or the next calendar day (Fig. 4b). Indeed, older PCs tended to require fewer additional PC transfusions on the same day and fewer RBC transfusions on the same or next day. Similar trends were evident in all patient subgroups.
Figure 4.
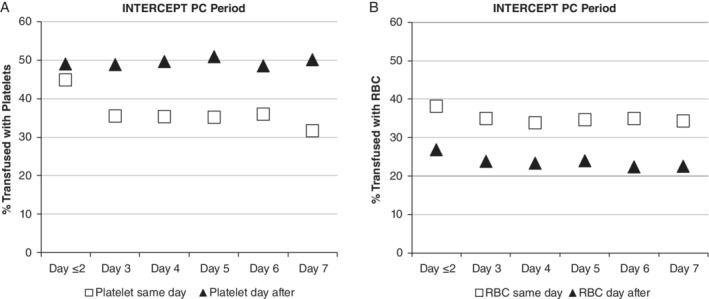
The proportion (%) of index PC transfusions that were associated with one or more (A) additional PCs or (B) RBCs transfused on the same day (open squares) or the next day (solid triangles).
Transfusion reactions
The rate of reported adverse events imputed to PCs was 0.90% in the C‐PC and 1.60% in the PI‐PC periods, and to RBCs was 0.30% in the C‐PC to 0.91% in the PI‐PC periods (Table 4). The frequency of reporting adverse events associated with both PC and RBC components increased in the second study period and was mainly due to an increase in reported febrile nonhemolytic transfusion reactions to both PCs (3.6 fold increase) and RBCs (4.4 fold increase). Allergic reactions to PI‐PCs were significantly lower (0.22% vs. 0.43%: odds ratio, 0.55; 95% confidence interval, 0.35‐0.74), while the rate with RBCs was unchanged. With PI‐PCs, no difference was seen in the incidence of transfusion reactions for PCs aged 5 days or less (266/16,766 [1.6%] of PC transfusions) versus more than 5 days old (62/3,750 [1.7%] of PC transfusions; p = 0.768) (not shown).
Table 4.
Transfusion reactions reported for PCs and RBCs in patients who received PCs during the C‐PC and PI‐PC transfusion periods, assessed per transfusion
| Platelets | Red Blood Cells | |||||
|---|---|---|---|---|---|---|
| Conventional platelet period | INTERCEPT platelet period | OR (95% confidence interval) | Conventional platelet period | INTERCEPT platelet period | OR (95% confidence interval) | |
| Dates | (01/02/2006‐01/09/2011) | (01/10/2011‐1/17/2016) | (01/02/2006‐01/09/2011) | (01/10/2011‐1/17/2016) | ||
| Number of transfusions | 14,181 | 22,579 | 23,018 | 30,382 | ||
| Total reactions | 128 (0.90%) | 361 (1.60%) | 1.78 (1.46‐2.18) | 68 (0.30%) | 276 (0.91%) | 3.10 (2.37‐4.03) |
| Febrile nonhemolytic | 54 (0.38%) | 304 (1.35%) | 3.57 (2.67‐4.77) | 44 (0.19%) | 254 (0.84%) | 4.40 (3.20‐6.06) |
| Anaphylactic | 8 (0.06%) | 4 (0.02%) | NS | 1 (<0.01%) | 3 (0.01%) | NS |
| Mild allergic | 61 (0.43%) | 50 (0.22%) | 0.55 (0.35‐0.74) | 11 (0.05%) | 16 (0.05%) | NS |
| TACO | 1 (0.01%) | 2 (0.01%) | NS | 5 (0.02%) | 2 (0.01%) | NS |
| TRALI | 1 (0.01%) | 1 (0.01%) | NS | 0 (0.0%) | 0 (0.0%) | NS |
| Septic | 3 (0.02%) | 0 (0.0%) | NS | 2 (0.01%) | 0 (0.0%) | NS |
| Acute hemolytic | 0 (0.0%) | 0 (0.0%) | NS | 5 (0.02%) | 1 (0.01%) | NS |
NS = not significant; TACO = transfusion‐associated circulatory overload; TRALI = transfusion‐related acute lung injury.
DISCUSSION
Swissmedic, the Swiss regulatory agency for therapeutic products, approved the use of the INTERCEPT Blood System for Platelets in 2009 and recommended universal implementation in 2011, based primarily on the risk of bacterial sepsis.1 Between 2005 and 2009 approximately 120,000 conventional PCs stored for up to 5 days were issued, and 16 febrile/septic reactions (including three fatal reactions) were documented by the mandatory but passive Swiss hemovigilance system.8 Swissmedic estimated that the bacterial contamination rate was 1:8000 PCs; that 1:14,000 PCs would cause a life‐threatening transfusion reaction; and 1:40,000 PC transfusions would result in death from sepsis.1 With the simultaneous introduction of pathogen inactivation and 7‐day storage, Swissmedic sought to reduce the risk of sepsis with PCs as well as a general decrease in number and severity of transfusion reactions.1, 9, 10 They proposed that the costs of PC treatment would be offset by the expansion of double‐dose apheresis collection, use of more whole blood–derived PCs, reduced wastage with 7‐day storage,11 reduced cytomegalovirus testing, and replacement of gamma irradiation. In 2018, Swissmedic published data indicating that from inception of pathogen inactivation 205,574 PI‐PCs had been issued with no transfusion‐transmitted bacterial infections reported to their passive hemovigilance system, corresponding to 20 transfusion‐transmitted infections avoided, including four fatalities.1, 8 Furthermore, a decline of 66% for life‐threatening and fatal reactions and of 26% for all high‐imputability transfusion reactions was observed, correlating with fewer allergic transfusion reactions.1 No reports of increased bleeding or clinical observations of ineffectiveness of PI‐PCs were received, and they reported that “the increase of PC requirements in daily clinical practice has turned out markedly less steep than expected based on data originating from clinical trials and does not exceed the increases observed in other European countries where the majority of PC transfusions are covered by C‐PCs.”12, 13
The Regional Blood Transfusion Service, Swiss Red Cross, Basel introduced universal PI‐PCs with 7‐day storage in January 2011 following validation studies.3, 4 The average PC dose increased by approximately 7% to 3.0 × 1011 platelets with a minimum dose of 2.4 × 1011 platelets, due to the collection of greater platelet doses with apheresis to compensate for the expected platelet loss inherent to the pathogen inactivation processing.
The adoption of PI‐PCs did not lead to an increase in average per‐patient platelet use or duration of PC support in hematology/oncology, allogeneic HSCT, and autologous HSCT patients who used the majority (approx. 77.3%) of PCs over an extended period of observation, and despite significantly lower CI and CCI responses and shorter intertransfusion intervals. In patients undergoing allogeneic HSCT, an increase in the median number of PI‐PCs per patient (median, 18.0 vs. 13.0; p = 0.001) and an increase in the median days of PC support (median, 20.0 vs. 17.0 days; p > 0.05) compared with the C‐PC period7 were observed concurrently with an overall higher transplantation risk as assessed by the EBMT score (p = 0.05; Fig. 3). Besides the selection of patients of older age and with more severe disease, there were also differences in the allogeneic HSCT treatment protocols and in stem cell donor selection (e.g., more transplants from haploidentical and unrelated stem cells donors) between the two periods. The 5‐year TRM was not increased in the PI‐PC period, despite higher EBMT risk and lower CCIs. Overall, both the number and the frequency of RBC transfusions in hematology/oncology patients and patients undergoing autologous and allogeneic HSCT were similar in the two study periods. Taken together, these data suggest that PI‐PCs were equivalent to C‐PCs in maintaining hemostasis. Moreover, there was no increase in the overall average number of patient/donor exposures per day of transfusion support associated with a heightened risk of alloimmunization.
During the PI‐PC period, both a higher average number of PC use per patient (2.4 PI‐PCs/patient vs. 1.6 C‐PCs/ patient; p < 0.001) and a longer duration of support (1.6 days vs. 1.4 days, respectively; p = 0.011) were observed in the CVS subgroup. Patients undergoing CVS were transfused at a substantially higher pretransfusion platelet counts (mean, 81.0 × 109/L during the PI‐PC period) than other patient subgroups, while CCIs were similar to C‐PCs (p = 0.910). However, a smaller proportion of patients undergoing CVS required transfusion with RBCs (82.2% vs. 92.0% during the C‐PC period; p < 0.001). In our opinion, the increased need for PC transfusion in this patient group does not indicate a lack of PI‐PC efficacy, but is rather explained with a change in medical practice. Relevant changes included patient blood management practices, with platelets transfused based on viscoelastic hemostatic assays (e.g., rotational thromboelastography), the use of coagulation factor concentrates, the adoption of new emergency transfusion protocols (e.g., transfusion of 4:4:1 ratios of RBCs, plasma, and platelets14 for massive bleeding), and, most importantly, the increasing number of surgical interventions in patients with potent antiplatelet drugs for which there were no reversal strategies (e.g., prasugrel).
Regarding PC inventory issues and component characteristics, wastage due to expiration was substantially reduced after the implementation of pathogen inactivation, while, as expected, the median age of transfused PCs increased from 3.0 days to 4.0 days, with 16.6% of all PC >5‐day old at issue for transfusion.
As to transfusion reactions, although the overall number of reports was higher, there were no differences noted in reported rate with PI‐PCs stored for 5 days or less versus those stored for more than 5 days, supporting the safety of older PI‐PCs. The higher number of hemovigilance reports during the PI‐PC period indicates a greater awareness toward the adverse events of transfusions, in particular regarding febrile reactions (most being febrile nonhemolytic transfusion reactions), rather than an increase in actual reaction rates, as witnessed by the concurrent increase in reported reactions with RBC transfusions.
Transfusion of PCs with longer storage duration resulted in lower mean CIs and CCIs, as well as decreased mean intertransfusion intervals,15 posing the potential risk of hemostatic failure. We observed a decline in mean CCI responses with increasing platelet age with both C‐PCs (−0.52 × 103 per day) and PI‐PC (−0.61 × 103 per day), but regression analysis showed very little overall correlation (r2 = 0.005 and 0.014, respectively), suggesting that clinical factors also significantly influence CCI results. Indeed, we detected no evidence that older platelets resulted in hemostatic failure, as indirectly indicated by the need for additional platelet or RBC transfusions on the same day or the day after an index 6‐ to 7‐day‐old PI‐PC transfusion. The observed trend to fewer PC and RBC transfusions on the same day and fewer RBC transfusions on the following day supports the hemostatic efficacy of 7‐day stored PI‐PCs and highlights the need to interpret CCI results in the clinical context of the single patient.
A recent meta‐analysis of 10 randomized controlled clinical trials comparing INTERCEPT‐treated PCs with C‐PCs concluded that participants who received PI‐PC transfusions required more PCs with a shorter interval between transfusions and a lower 24‐hour CCI.16 The finding of increased platelet usage with PI‐PC in clinical trials has not been replicated in routine practice at our institution and in other published reports at three hospitals in different countries, where considerably more patients were exposed to platelet transfusion for a variety of indications, including hematology/oncology and general medical and surgical conditions.9, 10, 17, 18, 19 These different findings may be attributed to the clinical trial conditions where physicians may be required to transfuse according to monitored platelet trigger values and protocol compliance rather than by clinical bleeding risk assessment. The same meta‐analysis16 found no difference between INTERCEPT PI‐PCs and standard platelets in the incidence of clinically significant bleeding (World Health Organization Grade 2 or higher), severe bleeding (World Health Organization Grade 3 or higher), all‐cause mortality, or serious adverse events. Recently, a randomized trial by Garban et al.20 compared the use of C‐PCs suspended in plasma or PAS, and PI‐PCs suspended in PAS. The authors observed more frequent transfusions of lower‐dose PI‐PCs but found no statistically significant difference in the total number of platelets transfused per patient and in the incidence of Grade 2 or higher bleeding among the three study arms. Noninferiority for bleeding was not demonstrated when PI‐PCs suspended in PAS were compared with C‐PCs suspended in plasma, but was achieved comparing PI‐PCs with C‐PCs suspended in PAS, although the study was designed with a relatively low statistical power (80%) for demonstrating noninferiority.21
In the Cochrane meta‐analysis, participants who received PI‐PC transfusions had an increased risk of platelet refractoriness, generally defined as two successive 1‐hour CCIs below 5 × 103.22, 23, 24, 25, 26 In our analysis, CCI responses varied broadly for a given platelet dose with considerable overlap between C‐PCs and PI‐PCs at all storage durations. Since the CCIs for PI‐PCs stored for up to 7 days were 24.3% lower than those of C‐PCs stored for up to 5 days, and because mean CCIs declined with increasing platelet age, it is more likely that older 7‐day storage PI‐PC may result in a CCI of less than 5 × 103 than younger, fresh PC. Furthermore, blood bank first in, first out practices dictate that when PC are in good supply, consecutive PC issued may be near the end of storage, increasing the likelihood of observing lower CCI response despite clinical evidence of adequate hemostasis. These findings emphasize the need to diagnose CCI‐based refractoriness using fresh PC.
The Cochrane meta‐analysis also reported a higher risk of refractoriness due to platelet alloimmunization with INTERCEPT‐PC,16 an effect not seen in an earlier meta‐analysis from the same authors.27 The later analysis included preliminary data that did not actually address alloimmunization from the Italian Platelet Technology Assessment Study (IPTAS).28, 29 The final IPTAS study, however, reported that INTERCEPT PCs provided a threefold reduction of risk of alloimmunization to the human leukocyte antigen Class I antigens that are associated with immune refractoriness to PC transfusions.26, 30 Therefore, in our opinion, the conclusions of the most recent Cochrane meta‐analysis should be taken with caution with respect to immune‐based refractoriness to INTERCEPT PCs.
Limitations of our study include the retrospective nature of the analysis, the influence of relevant changes in medical practice and patient populations between the two study periods, and the incomplete determination of CI data, especially in patients transfused for non–hematology/oncology conditions. On the other hand, our results provide information on longitudinal real‐life experience with PI‐PCs, which can be considered the major strength of this analysis. Our data are consistent with those of several other studies, indicating that PI‐PCs stored longer than 5 days retain clinical efficacy and, more specifically, that although average CCIs and CIs decreased with increasing platelet age, 7‐day PCs provided adequate intervals to the next transfusion without evidence of hemostatic failure.5, 6, 9, 10, 17, 18, 19, 31, 32
In conclusion, cumulative data from more than 5 years of experience confirms clinical efficacy and safety of PI‐PCs, including that of components stored up to 7 days, for the broad indications for platelet transfusion support, including hematology/oncology, CVS, and general medical and surgical patients.
CONFLICTS OF INTEREST
LI, AB, AH, JP, DB, DAT, RM, and AP have disclosed no conflicts of interest. RJB, LC, JSL, JI, and DT are employees of Cerus Corporation, a manufacturer of pathogen inactivation technologies.
REFERENCES
- 1. Jutzi M, Mansouri Taleghani B, Rueesch M, et al. Nationwide implementation of pathogen inactivation for all platelet concentrates in Switzerland. Transfus Med Hemother 2018;45:151‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruesch M, Jutzi M, Stoller R., 2 years experience with pathogen inactivation for all platelet concentrates in Switzerland. 2014. [cited 2014 Dec 1]. Available from: https://www.swissmedic.ch/marktueberwachung.
- 3. Infanti L, Stebler C, Job S, et al. Pathogen‐inactivation of platelet components with the INTERCEPT Blood System: a cohort study. Transfus Apher Sci 2011;45:175‐81. [DOI] [PubMed] [Google Scholar]
- 4. Sigle JP, Infanti L, Studt JD, et al. Comparison of transfusion efficacy of amotosalen‐based pathogen‐reduced platelet components and gamma‐irradiated platelet components. Transfusion 2013;53:1788‐97. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin RJ, Braschler T, Weingand T, et al. Hemovigilance monitoring of platelet septic reactions with effective bacterial protection systems. Transfusion 2017;57:2946‐57. [DOI] [PubMed] [Google Scholar]
- 6. Lozano M, Knutson F, Tardivel R, et al. A multi‐centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion. Br J Haematol 2011;153:393‐401. [DOI] [PubMed] [Google Scholar]
- 7. O'Meara A, Holbro A, Meyer SC, et al. Forty years of haematopoietic stem cell transplantation: a review of the Basel experience. Swiss Med Wkly 2014;144:1‐8. [DOI] [PubMed] [Google Scholar]
- 8. Swissmedic . Hemovigilance annual reports. 2003. ‐2017. [cited 2019 Feb 19]. Available from: http://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktueberwachung/haemovigilance/publications.html.
- 9. Osselaer JC, Cazenave JP, Lambermont M, et al. An active haemovigilance programme characterizing the safety profile of 7437 platelet transfusions prepared with amotosalen photochemical treatment. Vox Sang 2008;94:315‐23. [DOI] [PubMed] [Google Scholar]
- 10. Osselaer JC, Messe N, Hervig T, et al. A prospective observational cohort safety study of 5106 platelet transfusions with components prepared with photochemical pathogen inactivation treatment. Transfusion 2008;48:1061‐71. [DOI] [PubMed] [Google Scholar]
- 11. Dumont LJ, Kleinman S, Murphy JR, et al. Screening of single‐donor apheresis platelets for bacterial contamination: the PASSPORT study results. Transfusion 2010;50:589‐99. [DOI] [PubMed] [Google Scholar]
- 12. Cid J, Escolar G, Lozano M. Therapeutic efficacy of platelet components treated with amotosalen and ultraviolet A pathogen inactivation method: results of a meta‐analysis of randomized controlled trials. Vox Sang 2012;103:322‐30. [DOI] [PubMed] [Google Scholar]
- 13. Vamvakas EC. Meta‐analysis of the studies of bleeding complications of platelets pathogen‐reduced with the Intercept system. Vox Sang 2012;102:302‐16. [DOI] [PubMed] [Google Scholar]
- 14. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Triulzi DJ, Assmann SF, Strauss RG, et al. The impact of platelet transfusion characteristics on posttransfusion platelet increments and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood 2012;119:5553‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev 2017;7:CD009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amato M, Schennach H, Astl M, et al. Impact of platelet pathogen inactivation on blood component utilization and patient safety in a large Austrian Regional Medical Centre. Vox Sang 2017;112:47‐55. [DOI] [PubMed] [Google Scholar]
- 18. Cazenave JP, Waller C, Kientz D, et al. An active hemovigilance program characterizing the safety profile of 7483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment. Transfusion 2010;50:1210‐9. [DOI] [PubMed] [Google Scholar]
- 19. Osselaer JC, Doyen C, Defoin L, et al. Universal adoption of pathogen inactivation of platelet components: impact on platelet and red blood cell component use. Transfusion 2009;49:1412‐22. [DOI] [PubMed] [Google Scholar]
- 20. Garban F, Guyard A, Labussiere H, et al. Comparison of the hemostatic efficacy of pathogen‐reduced platelets vs untreated platelets in patients with thrombocytopenia and malignant hematologic diseases: a randomized clinical trial. JAMA Oncol 2018;4:468‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamin RJ, Katz L, Gammon RR, et al. The argument(s) for lowering the US minimum required content of apheresis platelet components. Transfusion 2019;59:779‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCullough J, Vesole DH, Benjamin RJ, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood 2004;104:1534‐41. [DOI] [PubMed] [Google Scholar]
- 23. Janetzko K, Cazenave JP, Kluter H, et al. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion 2005;45:1443‐52. [DOI] [PubMed] [Google Scholar]
- 24. van Rhenen D, Gulliksson H, Cazenave JP, et al. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood 2003;101:2426‐33. [DOI] [PubMed] [Google Scholar]
- 25. Cazenave JP, Isola H, Waller C, et al. Use of additive solutions and pathogen inactivation treatment of platelet components in a regional blood center: impact on patient outcomes and component utilization during a 3‐year period. Transfusion 2011;51:622‐9. [DOI] [PubMed] [Google Scholar]
- 26. Rebulla P, Vaglio S, Beccaria F, et al. Clinical effectiveness of platelets in additive solution treated with two commercial pathogen‐reduction technologies. Transfusion 2017;57:1171‐83. [DOI] [PubMed] [Google Scholar]
- 27. Butler C, Doree C, Estcourt LJ, et al. Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev 2013;7:CD009072. [DOI] [PubMed] [Google Scholar]
- 28. Rebulla P, Vaglio S, Aprili G, et al. Clinical efficacy and safety of platelets in additive solution treated with two commercial pathogen reduction technologies. Transfusion 2015;55(Suppl 3):3A Abstract no. P2‐030A. [DOI] [PubMed] [Google Scholar]
- 29. Rebulla P, Grazzini G, Liumbruno GM, et al. Pathogen inactivated platelets and prevention of immunological adverse reactions: the Italian Platelet Technology Assessment Study (IPTAS). Blood Transfusion 2009;7(Suppl 1: Abstract No. LE08), s19‐s21. 10.2450/2009.0013-09. [DOI] [Google Scholar]
- 30. Norris PJ, Kaidarova Z, Maiorana E, et al. Ultraviolet light‐based pathogen inactivation and alloimmunization after platelet transfusion: results from a randomized trial. Transfusion 2018;58:1210‐7. [DOI] [PubMed] [Google Scholar]
- 31. Knutson F, Osselaer J, Pierelli L, et al. A prospective, active haemovigilance study with combined cohort analysis of 19 175 transfusions of platelet components prepared with amotosalen‐UVA photochemical treatment. Vox Sang 2015;109:343‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nussbaumer W, Amato M, Schennach H, et al. Patient outcomes and amotosalen/UVA‐treated platelet utilization in massively transfused patients. Vox Sang 2017;112:(3):249‐56. [DOI] [PubMed] [Google Scholar]


