Abstract
Severe injury and hemorrhagic shock (HS) result in multiple changes to hematopoietic differentiation, which contribute to the development of immunosuppression and multiple organ failure (MOF). Understanding the changes that take place during the acute injury phase may help predict which patients will develop MOF and provide potential targets for therapy. Obtaining bone marrow from humans during the acute injury phase is difficult so published data are largely derived from peripheral blood samples, which infer bone marrow changes that reflect the sustained inflammatory response. This preliminary and opportunistic study investigated leucopoietic changes in rat bone marrow 6 h following traumatic injury and HS. Terminally anesthetized male Porton Wistar rats were allocated randomly to receive a sham operation (cannulation with no injury) or femoral fracture and HS. Bone marrow cells were flushed from rat femurs and immunophenotypically stained with specific antibody panels for lymphoid (CD45R, CD127, CD90, and IgM) or myeloid (CD11b, CD45, and RP‐1) lineages. Subsequently, cell populations were fluorescence‐activated cell sorted for morphological assessment. Stage‐specific cell populations were identified using a limited number of antibodies, and leucopoietic changes were determined 6 h following trauma and HS. Myeloid subpopulations could be identified by varying levels CD11b expression, CD45, and RP‐1. Trauma and HS resulted in a significant reduction in total CD11b + myeloid cells including both immature (RP‐1(−)) and mature (RP‐1+) granulocytes. Multiple B‐cell lymphoid subsets were identified. The total percentage of CD90+ subsets remained unchanged following trauma and HS, but there was a reduction in the numbers of maturing CD90(−) cells suggesting movement into the periphery. © 2019 The Authors. Cytometry Part A published by Wiley Periodicals, Inc. on behalf of International Society for Advancement of Cytometry.
Keywords: bone marrow, blunt trauma, hemorrhagic shock (HS), flow cytometry, hematopoietic progenitor cells (HPC), granulocytes, monocytes, lymphocytes
Traumatic injury and hemorrhagic shock (HS) continues to be a leading cause of mortality and morbidity in patients of all ages 1. One of the fundamental problems in the weeks following trauma is severe inflammation and immune dysregulation, which not only renders the host susceptible to infection, but leads to tissue damage and ultimately multiple organ failure (MOF) 2, 3, 4. The bone marrow is responsible for generating the immune, stromal, and endothelial cells involved in a posttraumatic response 5. Any sustained inflammatory condition, for example, following trauma, undoubtedly has an impact upon bone marrow function and hematopoietic stem/progenitor cell proliferation and differentiation. A greater understanding of how hematopoietic differentiation is altered following severe trauma may indicate which patients are more likely to become immunosuppressed and enter into MOF and/or provide potential targets for treatment.
Hematopoietic stem cells (HSC) reside in stromal niches in the bone marrow and, in response to the relevant cues, generate a large, but diverse, population of mature functional blood cells. These maturing cells move from the bone marrow into the peripheral blood to replace lost or damaged cells and maintain immune function 6. HSC differentiate into multipotent progenitor cells, which become lineage restricted with proliferation and maturation 7, 8. This is accompanied by a change in morphological appearance and a phenotypic change in their surface expression of proteins, which aids in identification 9, 10. With the exception of a very small number of immature progenitor cells that move between the bone marrow and periphery to aid in repair, HSC and hematopoietic progenitor cells (HPC) are not found within the peripheral circulation under normal circumstances 11.
Hematopoietic cells can be loosely divided into two main lineages: myeloid, which generates granulocytes, monocytes/macrophages, erythrocytes and platelets; and lymphoid, which generates B cells that mature in the bone marrow and T cells that mature in the Thymus 8. The initial granulocyte precursors are virtually indistinguishable from monoblasts both morphologically and immunophenotypically but differentiation results in a series of morphological and immunophenotypical changes, which can be identified 12. The bone marrow holds a reserve pool of more mature (RP‐1+ in rat) granulocytes that can be rapidly mobilized into the peripheral circulation in response to infection or trauma‐induced stress 13. Once this reserve has been diminished immature, and potentially dysfunctional, granulocytes are released in an attempt to maintain immune function 14. Immature CD45R+ (B220), CD90+ and IL‐7 receptor alpha (CD127) expressing pro‐B lymphocytes reside in specific IL‐7 expressing niches within the bone marrow that support proliferation 6. These proliferative niches are likely to increase in number in response to infection and trauma‐induced stress 13. Larger mitotically active pre‐B cells differentiate into smaller more independent cells that lose CD127 expression and go on to become newly generated IgM‐expressing B cells 6.
Severe injuries, including HS, result in changes to lineage maturation and movement of immature nonfunctional progenitor cells into the peripheral circulation 15, 16, 17, 18. Multiparameter flow cytometry can be used to identify and quantify hematopoietic cells at different stages of differentiation using forward scatter (FSC: measure of cell size), side scatter (SSC: a measure of granularity), and antibody panels directed against lineage‐specific cell surface antibodies 19. In humans, blunt force trauma has been reported to suppress bone marrow activity with a reduction in myeloid colony formation 17, 20 and CD34+ HSC/HPCs 21. However, obtaining human bone marrow during the acute injury phase is difficult so most of the bone marrow data published infers changes reflected during the sustained systemic inflammatory response, or from the peripheral blood response. Identifying changes in differentiation within each lineage during the acute injury phase have been demonstrated 21 and could be a useful tool to identify potential trauma patients who may be likely to develop bone marrow failure as part of MOF.
We hypothesized that trauma and HS would result in changes in the profile of bone marrow hematopoietic progenitors compared to those obtained after anesthesia alone. This opportunistic study used multiparameter flow cytometry to investigate leucopoiesis in rat bone marrow during the acute phase response to injury and HS. It focuses on both myeloid and lymphoid changes that take place downstream of the early blast‐stage progenitors. Cell populations were confirmed by morphological assessment following fluorescence activated cell sorting.
Materials and Methods
Legislation
This study was performed under the Authority of Animals (Scientific Procedures) Act 1986 and was subject to local Ethical Review at the Defense, Science, and Technology Laboratories (DSTL), Porton Down and at Swansea University Medical School. This bone marrow investigation was an opportunistic study as part of another ethically approved study investigating a drug intervention to reduce inflammatory consequences posttrauma.
Animals and Anesthesia
Male Porton Wistar rats (n = 18; 6–8 weeks, weight 223–260 g) were kept in standard conditions (temperature 20–24°C, humidity 45–65% with 12 h light/dark cycles) and fed LabDiet EURodetn Diet 22% ad libitum with free access to water. The study was conducted under terminal anesthesia (animals remained anesthetized throughout the procedure and were killed humanely at the end of the study with an overdose of anesthetic). Anesthesia was induced with isoflurane in 100% oxygen in an induction chamber. Arterial oxygen saturation was monitored by pulse oximetry (Starr Life Sciences, Oakmont, PA) and body temperature maintained at 38.5°C using a thermostatically controlled under‐body heating blanket (Harvard Apparatus, Holliston, MA). Surgical anesthesia was maintained using alfaxalone (10 mg/ml, Alfaxan®, Jurox (UK) Limited, Crawley, UK) given by continuous intravenous infusion (PHD Ultra Infusion Pump, Harvard Apparatus, UK) via a 22G cannula (BD Instye™) in lateral tail vein. The jugular vein was cannulated with a 3 french cannula (Solomon Scientific). Arterial blood pressure was monitored continuously via an implanted femoral artery cannula (2F Solomon Scientific) using a strain gauge manometer (Sensonor 840, SensoNor a.s., Norway) and data acquisition system (MacLab 16/sp, ADInstruments, UK). Anesthetized rats were allowed to stabilize for 30 min prior to injury.
Trauma Model
Anesthetized rats were allocated randomly to receive a sham operation (cannulation with no injury; n = 6) or subjected to femoral fracture followed by HS (n = 12). After stabilization following anesthesia, the right femur was approached via a skin incision and blunt dissection in preparation for femoral fracture using bone cutters. The femur was fractured and 3 min later hemorrhage commenced. A target volume of 30% of the animal's estimated blood volume (2% per minute) was taken from the femoral artery catheter into syringes containing anticoagulant citrate phosphate dextrose, which was stored at room temperature. The mean arterial blood pressure was maintained at 40–45 mm Hg with either removal of blood or administration of 0.9% saline. At 90 min resuscitation, whole autologous blood was commenced to a target mean arterial pressure of 70–80 mm Hg followed by an infusion of colloid (GelofusinTM) at 8 ml/kg/h for the reminder of the study. Six hours following injury, all animals were killed humanely with an over dose of anesthetic (Euthatal, Merial Animal Health Ltd, Harlow, UK). Immediately after postmortem, one femur from each animal was excised and put into DMEM (Gibco) and stored at 4–8°C overnight prior to transport to Swansea University on wet ice. Approximately 20 h elapsed between the femurs being recovered and the bone marrow extraction.
Antibodies and Reagents
Immunophenotypical staining was used to identify the different myeloid and lymphoid subpopulations during leucopoiesis in rat bone marrow (Fig. 1).
Figure 1.
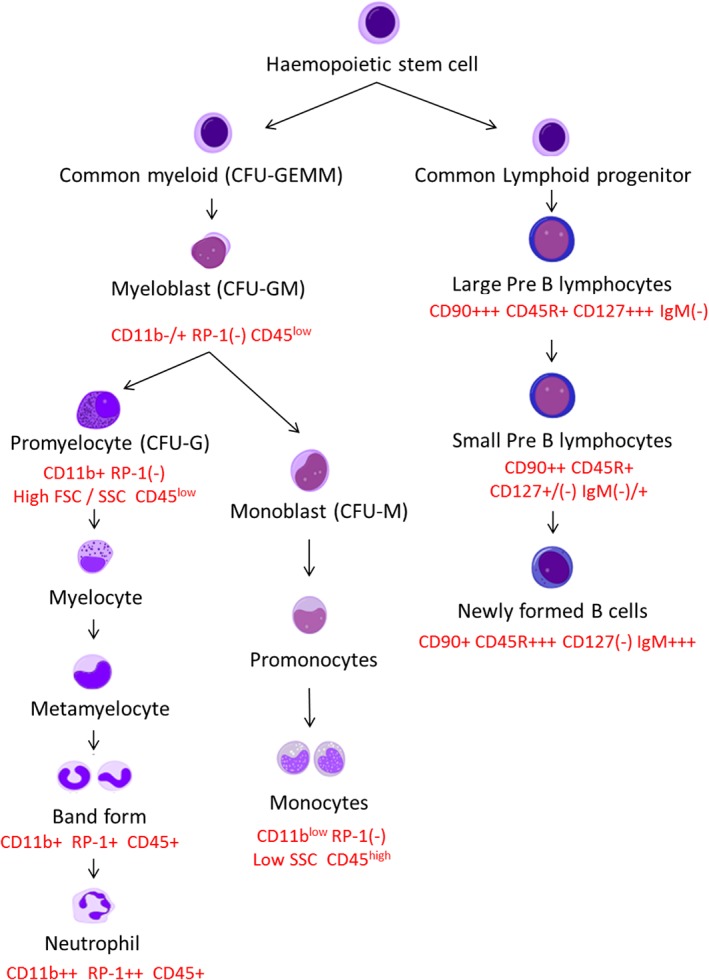
Simplified schematic diagram showing myeloid and lymphoid haemopoietic differentiation with CD nomenclature for flow cytometry identification in rat bone marrow. [Color figure can be viewed at http://wileyonlinelibrary.com]
Myeloid antibody markers: Anti‐rat CD45‐FITC (OX‐1) is a leukocyte‐specific antigen expressed on all hematopoietic cells except erythrocytes. Granulocyte‐PE (RP‐1) is expressed on neutrophils during development and CD11b‐APC (WT‐5) specifically binds to the α subunit of Mac‐1 expressed on all myeloid cells. These antibodies were obtained from BD Biosciences (Oxford, UK).
Lymphoid antibody markers: CD90 (Thy1.1)‐APC (HIS51) is an early differentiation marker. CD45R (B220)‐FITC (HIS24) is expressed on developing B lymphocytes, and IgM‐PE (HIS40) is expressed on the more mature B cells during development. These antibodies were purchased from eBioscience (Thermo Fisher Scientific, UK). CD127 (rIL‐7Rα)‐PE is expressed on pre‐B cells during early development, which along with purified rat IgG was obtained from R&D Systems, UK.
EasyLyse lysis buffer was obtained from Dako (Agilent Technologies) UK. Alpha‐Minimal Essential Media (Alpha‐MEM) with GlutaMAX supplement and Fetal Bovine Serum (FBS) was obtained from Gibco (Thermo Fisher Scientific) UK.
Isolating Bone Marrow Cells
The in‐tact femurs were cleared of all soft tissue and the femoral head and epiphysis were removed to allow for bone marrow collection. Bone marrow cells were isolated by flushing each femur from both ends, firstly with approximately 5 ml FBS and then using 10 ml Alpha‐MEM with a 21‐gauge needle and syringe. The cells were washed and resuspended in Alpha‐MEM 10% FCS. A total nucleated cell count was determined using the Countess automated cell counter (Thermo Fisher Scientific, UK).
Flow Cytometry
One million cells/100 μl were incubated with 10 μl purified Rat IgG per flow cytometry tube for 15 min at room temperature. The cells were subsequently incubated on ice for 30 min with preoptimized concentrations of antibodies to identify myeloid (2.5 μg CD45 (OX‐1)‐FITC, 1 μg Granulocyte (RP‐1)‐PE, & 1 μg CD11b (WT‐5)‐APC) or lymphoid populations (2.5 μg CD45R (HIS24)‐FITC, 0.2 μg IgM (HIS40)‐PE, 0.5 μg IL‐7Rα/CD127‐PE & 0.06 μg CD90 (HIS51)‐APC). Nonspecific isotype‐matched controls were included as control. Red blood cells were lysed by incubating for 15 min in the dark at room temperature with 3 ml EasyLyse. The cells were washed in FACS buffer (PBS with 0.2% bovine serum albumin and 0.05% sodium azide), fixed in 0.1% paraformaldehyde, and the data were acquired on a flow cytometer (FACS Aria I, BD Biosciences) within 24 h. Single‐stained samples were used for automated compensation matrices. The data were analyzed using Kaluza® software (Beckman Coulter).
Myeloid populations were analyzed in 6 surgical control (no injury) rats and in 12 injured (femoral fracture and HS) rats. Thirty thousand events were acquired. Debris, Eosinophils, and smaller Lymphocyte and blast populations were excluded by gating (Fig. 2 Gate A). All gated cells expressing the myeloid marker CD11b were analyzed for CD45 and RP‐1 expression.
Figure 2.
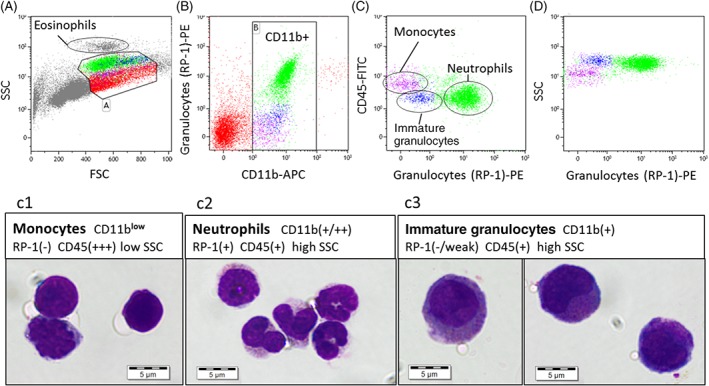
One million rat bone marrow cells were stained with CD11b‐APC, granulocyte (RP‐1)‐PE, and CD45‐FITC. Thirty thousand events were taken, and the data were analyzed using Kaluza. Debris, eosinophils, blasts, and some lymphocytes were excluded from the analysis (A Gate A). CD11b + myeloid cells were gated (B Gate B). RP‐1+ neutrophils were identified (C). Using SSC and CD45, it was possible to split the RP‐1(−) cells into two sub‐populations: Monocytes (low SSC and high CD45 expression) and immature granulocytes (high SSC and low CD45 expression) (C,D). The monocytes were identified immediately below the RP‐1+ granulocytes on the FSC versus SSC plot (A). The immature granulocytes were larger than the RP‐1+ neutrophils with a higher FSC (A). The CD11b + sub‐populations were isolated using Fluorescent Activated Cell Sorting and used to prepare cytospins. The cytospins were stained with May‐Grunwald Giemsa. More than 90% of RP‐1+ events stained for band form to polymorphonuclear neutrophils (C2). More than 90% of the RP‐1(−), low CD45, high SSC, high FSC cells stained for the larger immature granulocytes, which had oval or crescent moon nuclei and prominent cytoplasmic granulation (C3). The morphology of the RP‐1(−), high CD45, low SSC sub‐population was more variable (C1). Cells and nuclei were more irregular in shape with a high nuclear to cytoplasmic ratio. This population likely consists of a mixture of immature to mature monocytes. There may also be some overlap with the immature granulocytes with some cells appearing to have some granulation in the cytoplasm. [Color figure can be viewed at http://wileyonlinelibrary.com]
Lymphoid populations were analyzed in five surgical control rats and eight injured rats. Hundred thousand events were acquired. Debris, Granulocytes, and Eosinophils were largely excluded by gating (Fig. 4 Gate A). Various lymphoid populations were selected based on CD127 and CD45R positivity and FSC v SSC expression (Fig. 4 Gate B).
Flow Sorting and Cytospin Preparation
Following flow cytometric analysis, randomly selected samples were chosen for fluorescence‐activated cell sorting (FACS Aria I, BD Biosciences) and cytospin preparation. The myeloid cell populations were sorted using the FACS Aria I and used to prepare cytospins for morphological assessment. A high pressure sort (70 psi) with the 70‐μm nozzle was used. Myeloid populations (Fig. 2) were sorted (up to 10,000 events) into flow cytometry tubes using a flow rate of approximately 2000 events/s. Each sorted cell population was made up to 50 μl in FACS buffer and transferred to cytospin chambers attached to labeled microscope slides. The cytospins were centrifuged for 3 min at 1000 rpm (Cytospin3, Thermo Shandon); air dried and stained using an automated Hematology slide preparation unit (Sysmex SP‐10), which fixes the slides in Methanol and stains using May Grunwald and Giemsa (tcs biosciences, UK). Slides were air dried and DPX mounted. The cytospins were viewed at 100x magnification under oil.
Statistical Analysis
Data are represented as median +/− percentiles (box) and min‐max range (whiskers). All data were subjected to nonparametric statistics due to the small sample size, including Mann–Whitney U test for bivariate analysis, with P < 0.05 deemed to be statistically significant. The graphics and data were analyzed using Statistica 6 (StatSoft).
Results
Characterizing Myeloid Populations
Rat bone marrow‐derived cells were analyzed using FSC, SSC, CD11b (WT‐5), Granulocyte (RP‐1), and CD45 (OX‐1). Using the FSC and SSC plot eosinophils, smaller lymphocytes, blast populations, possible doublets and debris were excluded from the analysis (Fig. 2A, Gate A) to focus on characterizing neutrophils and monocytes. The myeloid cells were gated on CD11b (Fig. 2B, Gate B). Maturing Neutrophils‐stained positively for the granulocyte marker RP‐1 (Fig. 2B,C), which alongside CD11b expression, increased in fluorescent intensity with maturity (Fig. 2B Gate B). Two granulocyte (RP‐1) negative subpopulations were identified within the CD11b + myeloid population (Fig. 2C). One RP‐1(−) subpopulation showed high expression for CD45 (CD45+++; Fig. 2C) with low SSC (Fig. 2D). The other RP‐1(−) sub‐population had a similar SSC and CD45 expression to RP‐1+ neutrophils but were larger in size (higher FSC, Fig. 2A). These populations were isolated using flow sorting, and cytospins were used to characterize their morphology (Fig. 2C1‐C3).
The RP‐1 marker is expressed on band form and mature neutrophils (Fig. 2 C2). The segmentation of the nuclei is not as pronounced in rat as it is in human, and the rat neutrophils are smaller at approximately 5 μm in diameter. Granulation can be observed within the cytoplasm accounting for the high SSC. The RP‐1(−) subpopulation with high SSC and lower CD45 expression are immature granulocytes (Fig. 2 C3). These cells were much larger than the mature neutrophils at approximately 10 μm in diameter, accounting for the larger FSC and are granular in nature (SSC expression). Promyelocytes and myelocytes were identified with round to oval nuclei as well as metamyelocytes that had a more‐indented nuclei. Their cytoplasm stained much darker than the RP‐1+ neutrophils from coarse granulation. They stained positively for CD11b expression but had not yet developed the RP‐1 marker on the surface of their cells. The other RP‐1(−) subpopulation with high CD45 expression and low SSC were more variable in nature. These were identified as monocytes (Fig. 2 C1). They were between 5 and 10 μm in diameter with a high nuclear to cytoplasmic ratio, which was convoluted and irregular in shape. The cells were darkly stained and vacuolar but were agranular accounting for the low SSC. There was some contamination of immature granulocytes within this population following flow sorting.
Effect of Trauma and HS on Myeloid Populations
Trauma and HS had a significant impact on the myeloid population within rat bone marrow. The total percentage of CD11b + myeloid cells (Fig. 3A), RP‐1+ neutrophils (Fig. 3B), and immature RP‐1(−) neutrophils (Fig. 3C) were all significantly reduced 6 h following trauma in rat bone marrow. The population of RP‐1(−) CD45+++ monocytes were not affected 6 h following trauma (Fig. 3D). The proportion of neutrophils, monocytes, and immature granulocytes within the CD11b + myeloid population remained unchanged following trauma.
Figure 3.
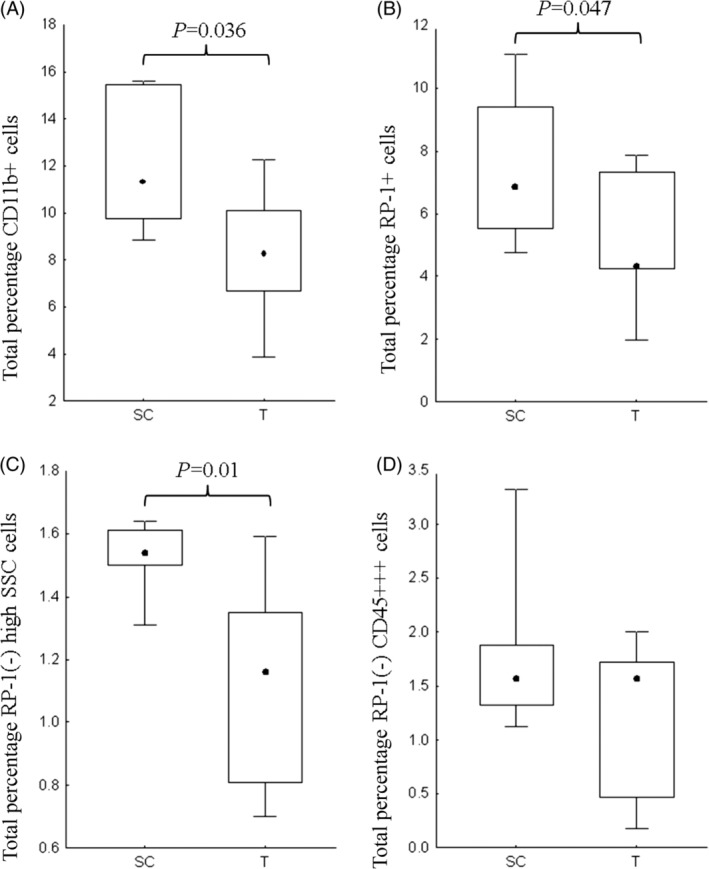
The total percentage of CD11b + myeloid cells (A), RP‐1+ neutrophils (B), and immature RP‐1(−) high SSC/FSC granulocytes (C) were significantly reduced in rat bone marrow 6 h posttrauma (T) in comparison to the surgical control (SC) population. There was no difference in the total percentage monocytes (D) following trauma. Data graphically represented as median +/− percentiles (box) and min‐max (whisker). Significance denoted as P < 0.05.
Characterizing B‐Cell Lymphopoiesis
Rat bone marrow‐derived cells were analyzed using FSC, SSC, CD45R (HIS24), CD90 (HIS51), CD127 (rIL‐7Rα), and IgM (HIS40). Using the FSC and SSC plot Eosinophils, Neutrophils, possible doublets and debris were largely excluded from the analysis (Fig. 4, Gate A) to focus on characterizing Lymphocytes. Monocyte contamination was excluded using CD45R.
Figure 4.
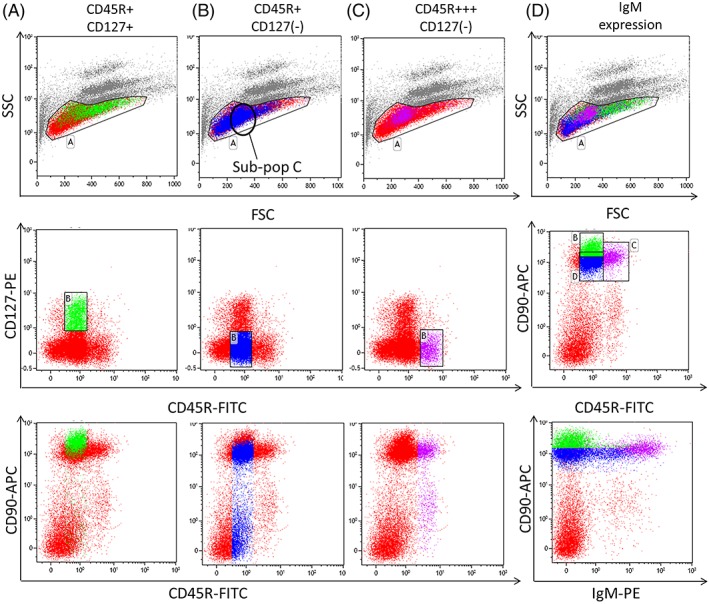
One million rat bone marrow cells were stained with CD45R‐FITC, CD127‐PE, CD90‐APC or CD45R‐FITC, IgM‐PE, CD90‐APC. Up to 100,000 events were taken, and the data analyzed using Kaluza. Debris, eosinophils, and neutrophils and blasts were excluded from the analysis (Gate A). Three populations were identified using CD45R and CD127: A) CD45R+ CD127+, B) CD45R+ CD127(−), and C) CD45R+++ CD127(−). CD45R+ CD127+ (A) lymphocytes were larger (higher FSC) than the CD45R+ CD127(−) (B) cells; stained intensely for CD90 and CD127 and was negative for IgM. CD90 expression reduced with the loss of CD127. With differentiation CD45R+ lymphocytes lost CD127 expression and increased their CD45R (C) and IgM positivity (D). CD45R+ CD127(−) (B) cells represented a heterogeneous population, whereas the CD45R+++ CD127(−) (C) lymphocytes identified one specific population on the FSC v SSC plot (Gate A). [Color figure can be viewed at http://wileyonlinelibrary.com]
Three populations were characterized using CD45R and CD127: CD45R+ CD127+ (Fig. 4A), CD45R+ CD127(−) (Fig. 4B) and CD45R+++ CD127(−) (Fig. 4C). The CD45R+ CD127+ (Fig. 4A) population were larger in size and stained intensely for CD90. CD90 expression reduced with the loss of CD127. The CD45R+ CD127(−) population (Fig. 4B) was diverse and varied in size (FSC), granularity (SSC), and CD90 expression, therefore, this population was further split using FSC and SSC for statistical analysis (Fig. 5B). The CD45R+++ CD127(−) population (Fig. 4C) formed 1 clear population on the FSC SSC plot (Gate A) and were variable for CD90 expression.
Figure 5.
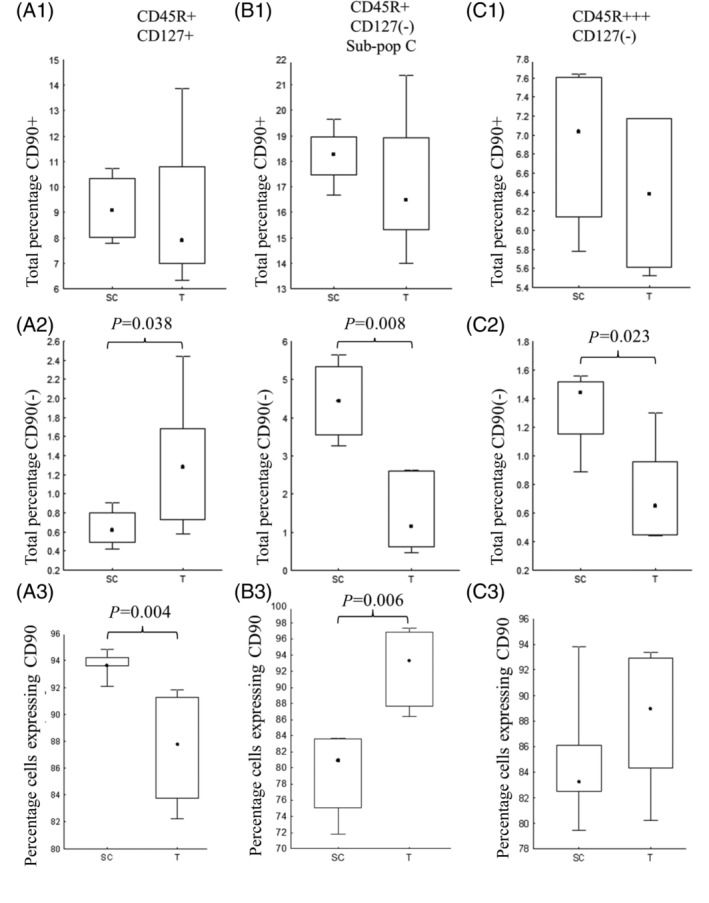
The total percentage of CD45R+ CD127+ CD90+ (A1), CD45R+ CD127(−) CD90+ (B1), and CD45R+++ CD127(−) CD90+ (C1) cells within rat bone marrow remained unchanged 6 h following trauma and HS. The analysis of CD45R+ CD127(−) (B) was restricted to sub‐population C (Fig. 4) due to the heterogeneous nature of the population. The gated percentage of CD90+ cells within CD45R+ CD127(−) (A3) population was significantly reduced following trauma and HS, while the gated percentage of CD90+ cells within the more mature CD45R+ CD127(−) population was significantly increased (B3). Trauma and HS resulted in a significant reduction in the total percentage of CD45R+ CD127(−) CD90(−) (B2) and CD45R+++ CD127(−) CD90(−) (C2) populations. Data graphically represented as median +/− percentiles (box) and min‐max (whisker). Significance denoted as P < 0.05.
IgM was used as a marker of maturity. IgM positivity increased with CD45R expression so CD45R+++ cells were strongly positive for IgM (Fig. 4D). Cells staining intensely for CD90 were IgM(−) and correlated with CD127+ cells. The variable CD45R+ CD127(−) population showed an increasing expression of IgM with maturation.
Effect of Trauma and HS on B‐Cell Lymphopoiesis
The CD45R+ CD127(−) subset (Fig. 5B) was subdivided according to FSC/SSC for analysis due to its heterogeneous expression. No significant differences were observed between the SC and Trauma groups in the subpopulations with low FSC/SSC or large FSC/SSC (data not shown). Subpopulation C (Fig. 4B) was used for statistical analysis. Looking at the bone marrow as a whole, trauma and HS had little impact on the total percentage of CD45R+ CD127+ (Fig. 5 A1), CD45R CD127(−) (Fig. 5 B1) or CD45R+++ CD127(−) (Fig. 5 C1) cells expressing CD90 in comparison with the surgical control group. Trauma and HS, however, resulted in a significant reduction in the total percentage of cells with weak or no expression of CD90 (Fig. 5 B2,C2). These were very small populations but may represent movement out of the bone marrow into the periphery.
Looking more specifically at the populations identified, the CD45R+ CD127+ subset showed a significantly reduced proportion of CD90+ cells following trauma and HS (Fig. 5 A3). In contrast, both the CD45R+ CD127(−) (Fig. 5 B3) and CD45R+++ CD127(−) (Fig. 5 C3) populations showed an increase in the gated percentage of CD90+ cells.
Discussion
This study identified subpopulations of cells at different levels of maturity within rat bone marrow using a limited number of antibodies. It also established an acute phase response to trauma demonstrating a rapid change in bone marrow lineage differentiation to meet the innate requirements of the periphery. We have previously shown in humans a depletion of the CD34+ hematopoietic progenitors in the bone marrow one to two weeks following major trauma, which was specifically narrowed down to the less primitive CD34+ CD38bright fraction 21. This may be related to an increased demand due to sustained inflammation, particularly in extremis patients, to generate more lineage‐restricted progenitors and maturing immune cells with depletion of pluripotential progenitors. We sought to understand those changes during the acute phase response to trauma but obtaining bone marrow from humans within hours of trauma is difficult. This opportunistic study in rats enabled us to study hematopoietic changes in bone marrow within 6 h of blunt force trauma and HS. The study was limited by the lack of compatibility of cell surface markers in comparison to human, preventing a direct comparison, and from a lack of availability of rat‐specific antibodies, which can limit the ability to elucidate different lineage strands. However, we successfully identified different stage‐specific leucopoiesis with a small number of cell surface markers and established changes during the acute phase response to trauma.
Myeloid cells were identified using CD11b (WT‐5), which forms an integrin molecule with CD18 to mediate leukocyte adhesion, migration, and innate immune function. It is commonly expressed on granulocytes, monocytes, and natural killer cells at different stages of maturity and can be found on a subset of B‐lymphocytes 22. In this study, no lymphocytes were demonstrated morphologically within the myeloid gates (Fig. 2), and there was no coexpression of CD45R and CD11b (data not shown). Myeloblasts, progenitors for both the granulocytic and monocytic lineages (Fig. 1), are CD11b(−) and indistinguishable immunophenotypically between lineages 12. CD11b, therefore, identified the lineage‐specific myeloid subpopulations. Rat granulocytes comprised of eosinophils, which were eliminated from the analysis based on their high SSC (Fig. 2), and the more abundant RP‐1+ neutrophils. RP‐1 was used in combination with CD45 and SSC to separate the mature neutrophils from RP‐1(−) immature granulocytes. An intermediate CD45 expression and high SSC were used to discriminate between immature RP‐1(−) granulocytes and CD45bright monocytes or lymphocytes 12. Morphologically all RP‐1+ cells were mature polymorphonuclear neutrophils or band form (Fig. 2 c2), while >90% of RP‐1(−) CD45 intermediate cells with high SSC were larger immature granulocytes (myelocytes to metamyelocytes). RP‐1 proved to be a good specific granulocyte marker in comparison to HIS48, which showed cross‐reactivity with monocytes (data not shown) 23.
B‐lineage lymphocytes were identified based on their expression of CD45R (B220), a marker consistently present on B‐lineage cells in mice 24 and rats 25. Differentiation was quantitated on the level of CD45R expression, which increased with maturity, and the presence of CD127 (large pre‐B) or IgM (newly formed and maturing cells). CD45R+++ expressing cells are reported to be terminally differentiated lymphocytes while CD90, a Thy‐1 membrane glycoprotein, is found on immature B lymphocytes in rat 25. CD127+ cells stained intensely for CD90 and a loss of CD127 expression was reflected by a reduction in CD90 with differentiation. CD127 is part of the IL‐7 receptor, expressed from early small pro‐B cell to the larger pre‐B stage and is downregulated with differentiation to the small pre‐B cell stage 26. This corresponds to the larger (high FSC) cells staining intensely for CD90 and CD127 and cell size diminishing as the expression of these markers reduced. The immature CD127+ cells are reliant on IL‐7, secreted by stromal cells, for proliferation and differentiation. Mouse knock‐out models have demonstrated that early B and T‐cell development is blocked in the absence of IL‐7Rα 27, therefore CD127, in the presence of CD45R, is a good marker for early B cell development.
Within the CD45R+ CD127+ gate (Fig. 4A), there were a small number of cells that weakly expressed CD45R and CD127 and were CD90 low or negative. It is unclear what these cells were but they were increased following trauma and HS. Could these CD127weak CD45Rweak cells form an early subset of B cell development prior to acquiring CD90 expression? CD127 is one of the earliest markers of B‐cell lineage commitment, prior to developing B‐cell lineage markers such as CD19 28, 29.
Both CD45R+ CD127(−) and CD45R+++ CD127(−) populations were subdivided into CD90+ and CD90(−) subsets (Fig. 4). The CD45R+++ CD127(−) CD90+ population clearly identified as newly formed B cells and expressed high levels of IgM 30, 31. The CD45R+ CD127(−) population (Fig. 4B) was heterogeneous in terms of size, granularity, and CD90 expression, indicating the inclusion of cells at different stages of differentiation. To further delineate this population, more lineage‐specific antibodies and fluorescent channels would be needed. For a more defined analysis, FSC/SSC was used to separate the population into three subpopulations. Subpopulation C (Fig. 4) is likely developing into newly formed B cells. These cells have similar FSC/SSC expression profiles and express equivalent levels of CD90 but have not developed high levels of CD45R and IgM expression. Newly formed B cells give rise to follicular B cells, which characterize as CD45Rbright, CD90(−) IgM(low) 30, 31 These cells can be identified in Figure 4 and are significantly lost from the bone marrow (Fig. 5 B2,C2), presumably into the periphery, following trauma and HS.
Traumatic injuries and HS lead to complex multisystem abnormalities that interplay with one another 4. Understanding the acute phase response to trauma in humans is difficult so rat trauma and HS models offer a prime alternative. They closely mimic that of the human response to trauma and are useful to investigate systemic changes and potential immunomodulatory treatments 32. A HS period of 90 min and subsequent resuscitation aligns with that of humans following severe injuries and is sufficient to mount a substantial systemic response 33.
It is well known that neutrophils are the primary effector cell in a systemic inflammatory response following trauma or sepsis, which results in a loss of natural immunity and causes tissue damage 34, 35, 36. Within the first hour following injury, there is a flux of neutrophils from the marginal pools along the endothelium and the bone marrow into the circulation 14, which remains high depending on the severity of injury and subsequent surgical interventions 17, 37. This study showed a marked reduction in bone marrow CD11b + myeloid cells within 6 h of trauma and HS, which suggests movement out into the periphery 14, 20. Both mature RP‐1+ and immature RP‐1(−) granulocytes were significantly reduced, but monocytes appeared unaffected by 6 h. This may be because monocytes respond later to injury, but they are also suppressed following trauma, particularly in patients who have a poor prognosis 38, 39. The movement of immature cells into the periphery following injury has important consequences if those cells are unable to provide the same natural immunity as mature cells 40. Myeloid‐derived suppressor cells (MDSC) are a population of granulocytic/monocytic precursors that accumulate systemically in response to tumors, traumatic stress, or infection to suppress T‐cell responses in mice or humans 41, 42, 43. This study identified a reduction in the total percentage of immature RP‐1(−) granulocytes from rat bone marrow following trauma and HS but identification of MDSCs in rat have proved challenging and this study did not demonstrate MDSC function. Dolen et al. 44 identified mature multilobed RP‐1+ HIS‐48+ granulocytic cells that could suppress CD4+ T‐cell proliferation in tumor‐bearing rats. Mobilization of RP‐1(−) progenitors into the peripheral circulation likely augments repair after trauma 16 and prevents immune‐mediated damage through suppression of T cell responses 41. If this persists for prolonged periods, bone marrow suppression and ultimately failure will result.
Lymphocyte counts have been reported to be increased during the first 12 h following trauma in humans 14. Rats have a reverse differential in the peripheral blood with approximately 10–20% neutrophils and 70–80% lymphocytes 45, 46, so it is possible there is a greater impact on lymphocyte egression and function in rats than in humans. Trauma and HS had no impact on the numbers of CD90+ developing B lymphocytes within rat bone marrow, but there was a significant reduction in the total numbers of maturing CD127(−) CD90(−) cells. This was offset by an increase in the gated percentage of CD90+ cells, suggesting movement of the more mature CD90(−) populations into the periphery. In contrast, the early CD127+ population showed an increase in the total percentage of CD90(−) cells with a concomitant decrease in the gated percentage of CD90+ cells. This may suggest movement between compartments within the bone marrow to push differentiation and maintain homeostasis 6 h following trauma and HS.
The extent of adaptation within the bone marrow is likely related to the severity of traumatic injury. The sympathetic nervous system, which regulates the body's response to trauma, can directly interact with immune cells through adrenergic receptors 15, 47. Trauma, HS, and subsequent release of proinflammatory cytokines can result in a sustained increase in circulatory norepinephrine, which can suppress the growth and differentiation of hematopoietic progenitors, particularly erythroid progenitors, and facilitate their mobilization out of the bone marrow 48, 49, 50. Blocking the adrenergic receptors in Sprague–Dawley rats was shown to restore progenitor function 15, 51. Despite the effect on early progenitors, the use of vasopressors, such as norepinephrine, in the management of trauma patients with HS remains controversial with some trauma centers continuing to use them. Further randomized control studies are required to determine any benefit 52.
Identifying a reduction of hematopoietic progenitors within the bone marrow and increases in immature cells within the peripheral circulation may provide indicators of bone marrow dysfunction. Admittedly these findings in rat may not be directly transferable to human, but previous human studies have identified impaired progenitor growth in the bone marrow 21 and release of immature granulocytes into the peripheral circulation 14, 20. There is the potential for cellular therapies, which may provide many benefits posttrauma, for example, transfused HSCs and/or mesenchymal stromal cells may have regeneration properties, reduce inflammation and help prevent bone marrow failure 53, 54, 55. There may also be the potential to use therapeutic drugs such as Erythropoietin, which besides driving erythropoiesis, has shown antiinflammatory, cytoprotective, and antiapoptotic benefits 56, 57.
This was a preliminary study, which identified stage‐specific differentiation in rat bone marrow using a limited number of fluorescence channels and cell‐surface markers and elucidated changes within the bone marrow within 6 h of trauma and HS. Both the myeloid and lymphoid compartments were significantly modified in the acute phase response to trauma and HS, and the proliferation/differentiation of early progenitors was suppressed. Later stage immune cells were mobilized into the peripheral circulation to maintain the inflammatory response, while early progenitors may have been lost to the circulation to aid in repair and prevent immune‐mediated damage by T cells. Further work needs to be done, using more fluorescent channels and cell surface markers, to draw out the CD45R+ CD127(−) mixed population and determine the impact of trauma on MDSC in rat bone marrow and peripheral blood.
Supporting information
MIFlowCyt: MIFlowCyt‐Compliant Items
References
- 1. Fulop A, Turoczi Z, Garbaisz D, Harsanyi L, Szijarto A. Experimental models of hemorrhagic shock: A review. Eur Surg Res 2013;50:57–70. [DOI] [PubMed] [Google Scholar]
- 2. Bochicchio GV, Napolitano LM, Joshi M, Knorr K, Tracy JK, Ilahi O, Scalea TM. Persistent systemic inflammatory response syndrome is predictive of nosocomial infection in trauma. J Trauma 2002;53:245–250. [DOI] [PubMed] [Google Scholar]
- 3. Malone DL, Kuhls D, Napolitano LM, McCarter R, Scalea T. Back to basics: Validation of the admission systemic inflammatory response syndrome score in predicting outcome in trauma. J Trauma 2001;51:458–463. [DOI] [PubMed] [Google Scholar]
- 4. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, Koenderman L, Kubes P, Lilford RJ. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014;384:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laing AJ, Dillon JP, Condon ET, Street JT, Wang JH, McGuinness AJ, Redmond HP. Mobilization of endothelial precursor cells: Systemic vascular response to musculoskeletal trauma. J Orthop Res 2007;25:44–50. [DOI] [PubMed] [Google Scholar]
- 6. Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 2004;20:707–718. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993;81:2844–2853. [PubMed] [Google Scholar]
- 8. Seita J, Weissman IL. Hematopoietic stem cell: Self‐renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2010;2:640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parekh C, Crooks GM. Critical differences in hematopoiesis and lymphoid development between humans and mice. J Clin Immunol 2013;33:711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulich TR, del Castillo J. The hematopoietic and mature blood cells of the rat: Their morphology and the kinetics of circulating leukocytes in control rats. Exp Hematol 1991;19:639–648. [PubMed] [Google Scholar]
- 11. Xiang M, Yuan Y, Fan L, Li Y, Li A, Yin L, Scott MJ, Xiao G, Billiar TR, Wilson MA, et al. Role of macrophages in mobilization of hematopoietic progenitor cells from bone marrow after hemorrhagic shock. Shock 2012;37:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Lochem EG, van der Velden VH, Wind HK, te Marvelde JG, Westerdaal NA, van Dongen JJ. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: Reference patterns for age‐related changes and disease‐induced shifts. Cytometry B Clin Cytom 2004;60:1–13. [DOI] [PubMed] [Google Scholar]
- 13. Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. Bone marrow and the control of immunity. Cell Mol Immunol 2012;9:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazeldine J, Naumann DN, Toman E, Davies D, Bishop JRB, Su Z, Hampson P, Dinsdale RJ, Crombie N, Duggal NA, et al. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med 2017;14:e1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elhassan IO, Hannoush EJ, Sifri ZC, Jones E, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta‐blockade prevents hematopoietic progenitor cell suppression after hemorrhagic shock. Surg Infect (Larchmt) 2011;12:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badami CD, Livingston DH, Sifri ZC, Caputo FJ, Bonilla L, Mohr AM, Deitch EA. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma 2007;63:596–600. [DOI] [PubMed] [Google Scholar]
- 17. Moore FA, Peterson VM, Moore EE, Rundus C, Poggetti R. Inadequate granulopoiesis after major torso trauma: A hematopoietic regulatory paradox. Surgery 1990;108:667–674. [PubMed] [Google Scholar]
- 18. Shah S, Ulm J, Sifri ZC, Mohr AM, Livingston DH. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma 2009;67:315–321. [DOI] [PubMed] [Google Scholar]
- 19. Zamir E, Geiger B, Cohen N, Kam Z, Katz BZ. Resolving and classifying haematopoietic bone‐marrow cell populations by multi‐dimensional analysis of flow‐cytometry data. Br J Haematol 2005;129:420–431. [DOI] [PubMed] [Google Scholar]
- 20. Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg 2003;238:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Francis WR, Bodger OG, Pallister I. Altered leucocyte progenitor profile in human bone marrow from patients with major trauma during the recovery phase. Br J Surg 2012;99:1591–1599. [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Jiang X, Liu R, Wang L, Qian T, Zheng Y, Deng Y, Huang E, Xu F, Wang JY, et al. B cells expressing CD11b effectively inhibit CD4+ T‐cell responses and ameliorate experimental autoimmune hepatitis in mice. Hepatology 2015;62:1563–1575. [DOI] [PubMed] [Google Scholar]
- 23. Ysebaert DK, de Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, de Broe ME. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant 2000;15:1562–1574. [DOI] [PubMed] [Google Scholar]
- 24. Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro‐B and pre‐pro‐B cell stages in normal mouse bone marrow. J Exp Med 1991;173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kakiuchi S, Ohara S, Ogata S, Miura D, Kasahara Y, Izawa Y. Flow cytometric analyses on lineage‐specific cell surface antigens of rat bone marrow to seek potential myelotoxic biomarkers: Status after repeated dose of 5‐fluorouracil. J Toxicol Sci 2004;29:101–111. [DOI] [PubMed] [Google Scholar]
- 26. Sasson SC, Smith S, Seddiki N, Zaunders JJ, Bryant A, Koelsch KK, Weatherall C, Munier ML, McGinley C, Yeung J, et al. IL‐7 receptor is expressed on adult pre‐B‐cell acute lymphoblastic leukemia and other B‐cell derived neoplasms and correlates with expression of proliferation and survival markers. Cytokine 2010;50:58–68. [DOI] [PubMed] [Google Scholar]
- 27. Ryan DH, Nuccie BL, Ritterman I, Liesveld JL, Abboud CN, Insel RA. Expression of interleukin‐7 receptor by lineage‐negative human bone marrow progenitors with enhanced lymphoid proliferative potential and B‐lineage differentiation capacity. Blood 1997;89:929–940. [PubMed] [Google Scholar]
- 28. Mansson R, Zandi S, Anderson K, Martensson IL, Jacobsen SEW, Bryder D, Sigvardsson M. B‐lineage commitment prior to surface expression of B220 and CD19 on hematopoietic progenitor cells. Blood 2008;112:1048–1055. [DOI] [PubMed] [Google Scholar]
- 29. de Andres B et al. The first 3 days of B‐cell development in the mouse embryo. Blood 2002;100:4074–4081. [DOI] [PubMed] [Google Scholar]
- 30. Milicevic NM, Nohroudi K, Milicevic Z, Hedrich HJ, Westermann J. T cells are required for the peripheral phase of B‐cell maturation. Immunology 2005;116:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FG. The origin of marginal zone B cells in the rat. Eur J Immunol 1999;29:1522–1531. [DOI] [PubMed] [Google Scholar]
- 32. Weckbach S et al. A new experimental polytrauma model in rats: Molecular characterization of the early inflammatory response. Mediators Inflamm 2012;2012:890816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ronn T, Lendemans S, de Groot H, Petrat F. A new model of severe hemorrhagic shock in rats. Comp Med 2011;61:419–426. [PMC free article] [PubMed] [Google Scholar]
- 34. Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006;368:157–169. [DOI] [PubMed] [Google Scholar]
- 35. Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury 2014;45:1824–1833. [DOI] [PubMed] [Google Scholar]
- 36. Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care 2016;20:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grzelak I, Olszewski WL, Zaleska M, Ziolkowska A, Durlik M, Lagiewska B, Muszynski M, Rowinski W. Surgical trauma evokes a rise in the frequency of hematopoietic progenitor cells and cytokine levels in blood circulation. Eur Surg Res 1998;30:198–204. [DOI] [PubMed] [Google Scholar]
- 38. Galbraith N, Walker S, Galandiuk S, Gardner S, Polk HC Jr. The significance and challenges of monocyte impairment: For the ill patient and the surgeon. Surg Infect (Larchmt) 2016;17:303–312. [DOI] [PubMed] [Google Scholar]
- 39. Ditschkowski M, Kreuzfelder E, Rebmann V, Ferencik S, Majetschak M, Schmid EN, Obertacke U, Hirche H, Schade UF, Grosse‐Wilde H. HLA‐DR expression and soluble HLA‐DR levels in septic patients after trauma. Ann Surg 1999;229:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drifte G, Dunn‐Siegrist I, Tissieres P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med 2013;41:820–832. [DOI] [PubMed] [Google Scholar]
- 41. Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid‐derived suppressor cells: Similarities and differences. Cell Mol Life Sci 2013;70:3813–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathias B, Delmas AL, Ozrazgat‐Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, et al. Human myeloid‐derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg 2017;265:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dolen Y, Gunaydin G, Esendagli G, Guc D. Granulocytic subset of myeloid derived suppressor cells in rats with mammary carcinoma. Cell Immunol 2015;295:29–35. [DOI] [PubMed] [Google Scholar]
- 45. Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K, Panthong A. Acute and subacute toxicities of the ethanol extract from the rhizomes of Cyperus rotundus Linn. Mahidol Univ J Pharmaceut Sci 2005;32:15–22. [Google Scholar]
- 46. Faas MM, Moes H, van der Schaaf G, de Leij LF, Heineman MJ. Total white blood cell counts and LPS‐induced TNF alpha production by monocytes of pregnant, pseudopregnant and cyclic rats. J Reprod Immunol 2003;59:39–52. [DOI] [PubMed] [Google Scholar]
- 47. Millar JK, Kannan KB, Loftus TJ, Alamo IG, Plazas J, Efron PA, Mohr AM. Persistent injury‐associated anemia: The role of the bone marrow microenvironment. J Surg Res 2017;214:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL‐6. J Trauma 2005;59:884–889. [DOI] [PubMed] [Google Scholar]
- 49. Kumar M, Bhoi S. Impaired hematopoietic progenitor cells in trauma hemorrhagic shock. J Clin Orthop Trauma 2016;7:282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alamo IG, Kannan KB, Ramos H, Loftus TJ, Efron PA, Mohr AM. Clonidine reduces norepinephrine and improves bone marrow function in a rodent model of lung contusion, hemorrhagic shock, and chronic stress. Surgery 2017;161:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beiermeister KA, Keck BM, Sifri ZC, ElHassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Hematopoietic progenitor cell mobilization is mediated through beta‐2 and beta‐3 receptors after injury. J Trauma 2010;69:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gupta B, Garg N, Ramachandran R. Vasopressors: Do they have any role in hemorrhagic shock? J Anaesthesiol Clin Pharmacol 2017;33:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 2010;121:2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kumar M, Bhoi S, Galwankar S. Hematopoietic stem cells: Can it be a therapeutic option for the hematopoietic failure in patients with trauma‐hemorrhagic shock? J Emerg Trauma Shock 2016;9:51–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pati S, Rasmussen TE. Cellular therapies in trauma and critical care medicine: Looking towards the future. PLoS Med 2017;14:e1002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thiemermann C. Beneficial effects of erythropoietin in preclinical models of shock and organ failure. Crit Care 2007;11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007;357:965–976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MIFlowCyt: MIFlowCyt‐Compliant Items


