Abstract
Objectives
Documentation of semilunar valve growth in fetal transposition of the great arteries (TGA) and the relationship between neo‐aortic root (NAoR) dilatation, a cause for postoperative reinterventions after the arterial switch operation (ASO), and pulmonary valve (PV) annulus dimensions prenatally.
Methods
This retrospective multicenter observational study included TGA fetuses suitable for ASO. Semilunar valve annuli pre‐ASO and NAoR diameters (post‐ASO) were measured. Trends in annulus diameters were analyzed using a linear mixed‐effects model and compared with normal values. Prenatal semilunar valve Z‐scores were correlated with NAoR diameters post‐ASO.
Results
We included 137 TGA fetuses (35.8% with significant ventricular septal defects [VSDs]). One hundred twenty‐one underwent ASO. Fetal TGA‐PV diameters were significantly larger than control aortic valve (AoV) and PV annuli from 23 and 27 weeks, respectively, especially when a VSD was present. Fetal TGA‐AoV annuli were significantly larger than control AoV and PV annuli from 26 and 30 weeks, respectively.
Z‐scores of fetal TGA‐PV and NAoR diameter at last follow‐up correlated significantly (P < .001 at 26‐30 wk).
Conclusion
Fetal TGA semilunar valve annuli are larger than control annuli, especially when there is a significant VSD. Factors besides postoperative hemodynamics, including fetal anatomy, PV Z‐score, prenatal flow, connective tissue properties, and genetics, may influence the risk for late reintervention in these fetuses.
Short abstract
What's already known about this topic?
Transposition of the great arteries (TGA) is treated with the arterial switch operation (ASO). Neo‐aortic root (NAoR) dilatation is seen postoperatively and may require late reintervention.
What does this study add?
The first serial data on semilunar valve size in fetal TGA in a large cohort are provided.
TGA semilunar valve diameters are larger than in normal fetal hearts, giving a larger left ventricular outflow tract.
The prenatal pulmonary valve size may predispose to NAoR dilatation post‐ASO.
What's already known about this topic?
Transposition of the great arteries (TGA) is treated with the arterial switch operation (ASO). Neo‐aortic root (NAoR) dilatation is seen postoperatively and may require late reintervention. (26)
What does this study add?
The first serial data on semilunar valve size in fetal TGA in a large cohort are provided.
TGA semilunar valve diameters are larger than in normal fetal hearts, giving a larger left ventricular outflow tract.
The prenatal pulmonary valve size may predispose to NAoR dilatation post‐ASO. (44)
1. INTRODUCTION
D‐loop transposition of the great arteries (TGA) is a frequently seen cyanotic cardiac anomaly, occurring in 1 in 3.500 to 5.000 live births per year.1 The pulmonary and systemic circulations are in parallel not series, and blood with a higher saturation is ejected into the pulmonary circulation instead of the systemic circulation.2, 3 Historically, low prenatal TGA detection rates have contributed to a relatively limited knowledge of fetal TGA pathophysiology.4 With the inclusion of additional views in prenatal screening protocols, the prenatal detection rate of TGA is improving.5 Fetal cardiologists are required to discuss long‐term postoperative outcomes of TGA more frequently.
Currently, the arterial switch operation (ASO) is the preferred intervention for d‐TGA and Taussig‐Bing anomaly (TBA) worldwide with an operative mortality less than 5%.6, 7, 8 Despite this encouraging figure, some troubling complications do occur.8 Problems with coronary perfusion and peripheral pulmonary stenosis may occur in the short term. The growth of the anastomoses of the great arteries and the competence of the native pulmonary valve (PV) and root, now functioning as the neo‐aortic valve and root (NAoR), in the systemic circulation are important in the long term.6, 7, 8 NAoR dilatation has been reported,8, 9, 10, 11, 12, 13, 14, 15, 16, 17 even in the first year post‐ASO,9, 10, 11 and the root continues to grow excessively,15, 16, 17 at a rate >4 times normal.9 The resulting aortic valve (AoV) regurgitation and aneurysm formation may require a reintervention.15, 16, 17 The 25‐year reintervention‐free survival for the neo‐aortic valve or root after ASO is reported to be 95%.15
The underlying etiology of NAoR dilatation is not completely understood and is probably multifactorial. Prior pulmonary artery banding, presence of a ventricular septal defect (VSD), TBA, gender, the technique used for the transfer of the coronary buttons, and the length of follow‐up have been implicated.11, 12, 13, 14, 15, 16, 17, 18, 19, 20
The study aim was to document the natural growth of the semilunar valves in fetuses with TGA and to compare it with normal semilunar valve growth. We also wished to determine if NAoR dilatation post‐ASO is related to the prenatal semilunar valve growth. This knowledge could be important to understand the pathophysiology of fetal TGA and the long‐term post‐ASO complications.
2. METHODS
2.1. Study population
All fetuses diagnosed between 2000 and 2017 with d‐TGA or TBA (TGA without pulmonary stenosis with a large VSD and overriding of the PV < 50%) suitable for ASO at four academic centers in the Netherlands (Amsterdam University Medical Centers [Academic Medical Center and VU Medical Center], Leiden University Medical Center, and the University Medical Center Groningen) were included in this observational study.
The patients were divided into two subgroups: (a) TGA with a hemodynamically significant VSD and (b) TGA with an intact interventricular septum (IVS).
The study was approved by the Medical Ethics Committee of the Academic Medical Centre, Amsterdam University Medical Centers, Amsterdam; however, such approval is legally not required for retrospective studies in the Netherlands.
2.2. Clinical data
The collected clinical data included sex, gestational age at prenatal follow‐ups, prenatal and final diagnosis after birth, gestational age at birth, birth weight, surgical procedures performed, and outcome (alive or dead). The age, weight, and height of the patients at 1‐year post‐ASO and/or last follow‐up moment were also collected.
2.3. Echocardiographic measurements
The diameters of the semilunar valve annuli at each prenatal follow‐up visit and on the first postnatal day were measured in millimeters. The prenatal and pre‐ASO measurements were made with the valves open using outflow tract views and compared with the normal data of Vigneswaran et al21 and Schneider et al22 as Vigneswaran et al measured the annuli with the valves closed and Schneider et al with the valves open.
Fetal and pre‐ASO Z‐scores for the PV and AoV annular diameters were calculated from Vigneswaran et al21 and Schneider et al.22
Measurements post‐ASO included the neo‐aortic annulus, NAoR at the level of the sinuses, and sino‐tubular (ST) junction at the follow‐up closest to 1‐year post‐ASO and at the last follow‐up visit. The measurements were made with the valves open from inner‐edge to inner‐edge (Figure 1). The Haycock formula was used to calculate the body surface area,23 and the Z‐scores were determined according to a pediatric reference dataset.24
Figure 1.
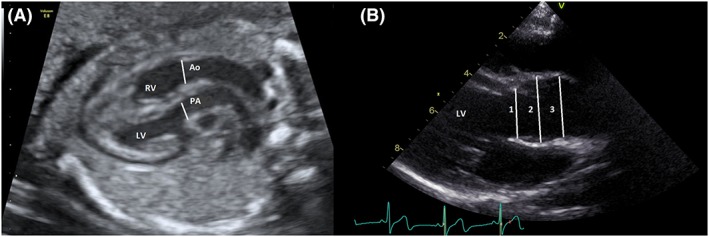
A, Measurements were made prenatally at the level of the semilunar valve annulus with the valve open. B, Post arterial switch measurements of the aortic root were made in the long axis view with the neo‐aortic valve open; 1 = annulus, 2 = neo‐aortic root, and 3 = sino‐tubular junction. Ao, aorta; LV, left ventricle; PA, pulmonary artery; RV, right ventricle [Colour figure can be viewed at http://wileyonlinelibrary.com]
Measurements were performed by two observers (S.‐A.C. and R.v.d.P.) using stored images. The prenatal images were recorded using Voluson E8 and E10 ultrasound machines (General Electric). The postnatal echo images were recorded on ViVid 7 echomachines (General Electric) and measured using the EchoPac workstation.
2.4. Statistical analysis
2.4.1. Population characteristics
Statistical analysis was performed using IBM SPSS Statistics 23.0 (SPSS Inc, Chicago, Illinois) and R version 3.3.2.25 Clinical characteristics and echocardiographic parameters were presented as a number (percentage) for categorical variables, mean (standard deviation) for continuous variables with an approximately symmetric distribution, and median (inter‐quartiles) for continuous data with a skewed distribution. Comparisons between baseline characteristics for TGA fetuses with IVS and VSD were performed using the chi‐squared test for categorical variables and for continuous variables, the Welch approximate t‐test or the Mann‐Whitney U‐test, as appropriate.
2.4.2. Prenatal trends in PV and AoV annular diameters
A repeated measurements analysis with a linear mixed‐effects model was used to assess average age trends in PV and AoV annular diameters, using all available prenatal echocardiograms per fetus and the first postnatal echocardiogram. This was also done for the Z‐scores of the PV and AoV annular diameters, using only the fetal data. A mixed‐effects model takes account of repeated measurements per fetus over time during which the number and timing of the measurements may vary per fetus.26 Natural cubic splines were modeled to evaluate the variations in time. Knots were placed at five fixed quintiles of the predictor's distribution as suggested by Stone and Koo.27 The intercept was allowed to differ per fetus and was assumed to follow a multivariate normal distribution (random effect). Since the slope and the intercept of both semilunar valve models had a correlation of 1.0, no random effect for the slope was used. Control lines for PV and AoV annular diameters were plotted on the basis of regression equations described by Vigneswaran et al21 and Schneider et al.22
Several comparisons were performed for the PV and AoV annular diameters: (a) the average trend in PV annular diameters of the TGA fetuses compared with the trend in PV annular diameters of controls and (b) with the AoV annulus of the controls. Further, (c) the average trend in AoV annulus of the TGA fetuses was compared with the trend in PV annulus of the controls and (d) the AoV annulus of controls. The average trends in (e) the PV and AoV annuli of the TGA fetuses were additionally allowed to differ by the presence or absence of a VSD as well as (f) by the presence of TBA.
A sensitivity analysis was also performed for the TGA‐PV annulus trend where only measurements made during fetal life were included.
For the Z‐scores of the PV and AoV annular diameters, the average trend was analyzed and the trends were allowed to differ by the presence or absence of a VSD.
Sampling uncertainty was quantified via 95% confidence intervals and P values. A P value < 0.05 was considered statistically significant.
2.4.3. Correlation between prenatal and postnatal measurements
Correlations between the Z‐scores of the prenatal semilunar valve annuli and the neo‐aortic dimensions 1‐year post‐ASO and last outpatient follow‐up were performed, stratified for gestational age: 18 to 22, 26 to 30, and 32 to 36 weeks' gestation. Each group included only one measurement of PV and AoV annulus per fetus. Correlation between prenatal and postnatal measurements was tested by the Spearman correlation coefficient (Rho).
2.4.4. Prediction of NAoR dilatation post‐ASO
Receiver‐operating characteristic (ROC) analyses were performed, and the area under the curves (AUCs) were determined for NAoR dilatation (defined as Z‐score > +2) at 1‐year post‐ASO or the last post‐ASO follow‐up visit for all gestational age groups (ie, 8‐22, 26‐30, and 32‐36 wk' gestation), based on the fetal PV and AoV Z‐score of one measurement per age group. An AUC > 0.700 was considered to be of a good predictive value, and of these values, an optimal cutoff value was defined (Youden index).
A logistic regression analysis was additionally performed to stratify the predictive value for sex.
3. RESULTS
3.1. Population characteristics
One hundred forty‐nine fetuses were identified with a prenatal diagnosis of TGA suitable for ASO. There were no fetal echocardiograms available for revision in 12 (8.1%). One hundred thirty‐seven fetuses were included in the analyses of whom 90 (65.7%) were male and 46 were female. The gender of one fetus, where the pregnancy was interrupted, was not recorded. Eighty‐eight fetuses (64.2%) had an IVS and 49 (35.8%) a significant VSD of which 12 (8.8%) had a TBA. In total, 15 fetuses (11%) died (Figure 2). One hundred twenty‐one underwent an ASO. There were three post‐ASO deaths related to coronary perfusion problems giving an operative mortality of 2.5%. Table 1 shows the baseline characteristics of the included fetuses.
Figure 2.
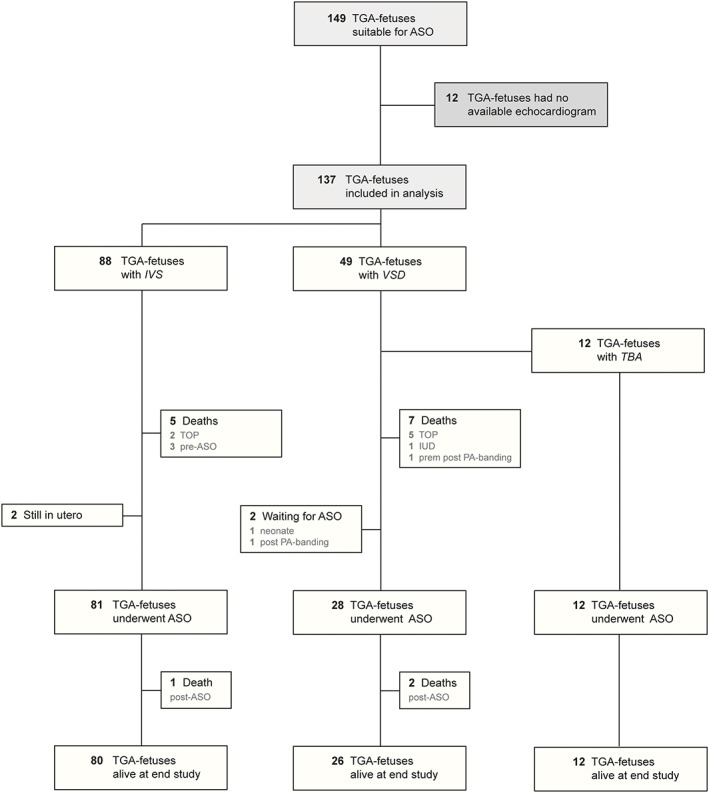
Flow diagram of included patients. ASO, arterial switch operation; IUD, intra‐uterine death; IVS, intact inter‐ventricular septum; TBA, Taussig‐Bing anatomy; TGA, transposition of the great arteries; TOP, termination of pregnancy; VSD, ventricular septal defect [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Baseline characteristics
| All (n = 137) | IVS (n = 88) | VSD (n = 49) | P Value IVS vs VSD | |
|---|---|---|---|---|
| Male | 90 (65.7%) | 63 (71.6%) | 27 (55.1%) | .0001 |
| Female | 46 (33.6%) | 25 (28.4%) | 21 (42.9%) | .55 |
|
Number of echocardiograms Median (IQR) |
5 (4−7) | 6 (4−7) | 5 (4−6) | .007 |
|
Gestation age at birth, wk Mean ± standard deviation |
39.1 ± 1.2 (n = 126) | 39.2 ± 0.9 (n = 83) | 39.0 ± 1.5 (n = 43) | .43 |
|
Birth weight, kg Mean ± standard deviation |
3.359 ± 0.442 (n = 126) | 3.380 ± 0.438 (n = 83) | 3.320 ± 0.454 (n = 43) | .47 |
| Balloon atrial septostomy | 65 (51.6%) (n = 126) | 49 (59%) (n = 83) | 16 (37.2%) (n = 43) | P = .03 |
| Deaths | 15 (10.9%) | 6 (6.8%) | 9 (18.4%) | .07 |
Significant results are shown in bold (P <.05). Abbreviations: IVS, interventricular septum; IQR, inter‐quartile range; VSD, ventricular septal defect.
There were 384 fetal and 119 postnatal echocardiograms analyzed giving pre‐ASO data from 503 echocardiograms. The median number of included echocardiograms was 5 (range 1‐10) per patient. Follow‐up data post‐ASO were available from 119 fetuses. The median follow‐up was 2.9 years (range 1 mo‐14.3 y).
3.2. Trends in PV annular diameters
A statistically significant trend in PV annular diameter (P < .0001) was seen in the TGA fetuses through gestation. The average trend in PV annular diameter in TGA fetuses compared with controls was different from 27 weeks' gestation, with the PV annuli being larger in the TGA fetuses (Figures 3A and S1). In late gestation, the PV annular growth rate reduced (change in slope of curve). The sensitivity analysis with the exclusion of the first postnatal measurements showed a subtler reduction in PV annular growth rate in late gestation (Figure S2).
Figure 3.
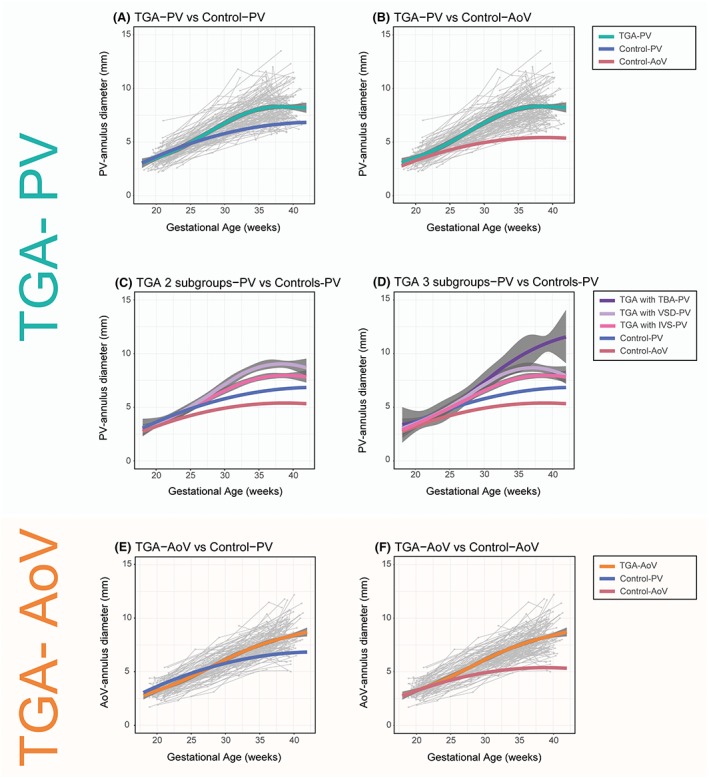
Average trends in PV annular diameters in TGA fetuses (TGA‐PV): (A) versus trends in controls PV annular diameter (Control‐PV); (B) versus AoV annular diameter (Control‐AoV); (C) TGA with an IVS (TGA with IVS‐PV) and a VSD (TGA with VSD‐PV); and (D) with the TGA‐VSD fetuses further stratified on the basis of a TBA or non‐TBA anatomy (TGA with TBA‐PV). Average trend in AoV annular diameters in TGA fetuses (TGA‐AoV): (E) versus trends in controls of PV annular diameter (Control‐PV); and (F) AoV annular diameter (Control‐AoV). Trends in PV and AoV annular diameters in controls are shown (Control‐PV and Control‐AoV). Grey dots are individual measurements, and grey lines are individual trends. The 95% confidence intervals for both the fitted model and the control line as described by Vigneswaran et al21 are shown in blue (pulmonary valve) and red (aortic valve). Note that the 95% confidence intervals for the controls are smaller than the thickness of the line. AoV, aortic valve; IVS, intact inter‐ventricular septum; PV, pulmonary valve; TBA, Taussig‐Bing anomaly; TGA, transposition of the great arteries; VSD, ventricular septal defect [Colour figure can be viewed at http://wileyonlinelibrary.com]
When compared with the AoV annular diameter of the controls (Figures 3B and S1), TGA fetuses had a larger PV annular diameter from 23 weeks' gestation onwards.
There was a statistically significant difference in the PV annular diameter of TGA fetuses with and without a VSD (P = .005) (Figure 3C). TGA fetuses with VSD had, on average, larger PV annular dimensions. When the TGA‐VSD fetuses were further stratified on the basis of a TBA or non‐TBA anatomy, there was a statistically significant difference in the trend in PV annular diameter between the subgroups (P < .0001) (Figure 3D).
3.3. Trends in AoV annular diameters
A statistically significant trend was seen in the AoV annular diameter in the TGA fetuses (P < .0001). There was a significant difference between the average trend in AoV annulus in TGA fetuses compared with the PV annular diameter of the controls from 30 weeks' gestation (Figure 3E). TGA fetuses had a significantly larger AoV annular diameter compared with the AoV annular diameter of the controls from 26 weeks' gestation onwards (Figure 3F).
3.4. Prenatal trends in Z‐score of PV and AoV annular diameters
A statistically significant trend was seen in the semilunar valve Z‐scores in the TGA fetuses (P < .0001 for both valves). The trends in the Z‐scores of the PV and AoV annular diameters were not different for the presence or absence of a VSD (P = .052 and P = .078, respectively) (Figures 4 and S3).
Figure 4.
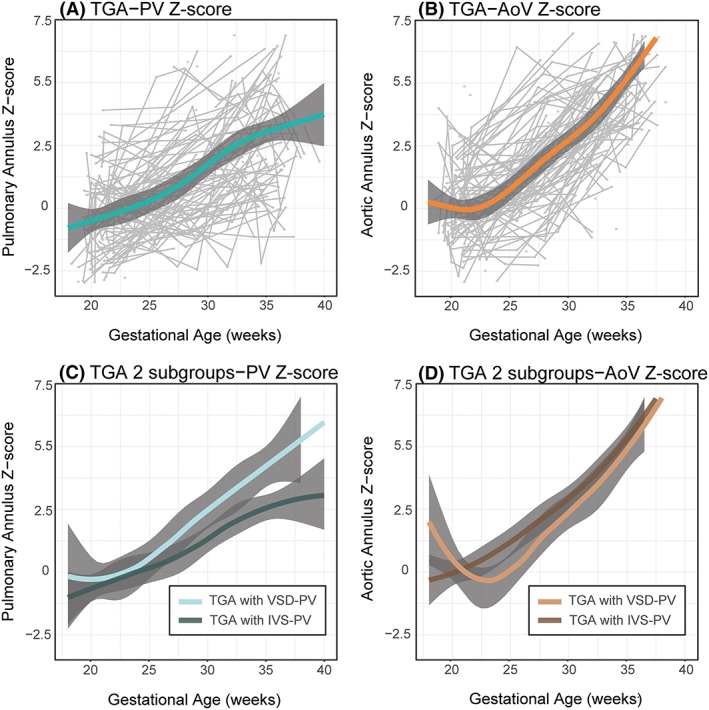
Average trend in TGA fetal (A) PV annular diameter Z‐score (TGA‐PV Z‐score) and (B) AoV annular diameter Z‐score (TGA‐AoV Z‐score). Grey dots are individual measurements, and grey lines are individual trends. Both trends were stratified on the basis of the presence (TGA with VSD) or absence (TGA with IVS) of a VSD (C) and (D) respectively. AoV, aortic valve; IVS, intact intra‐ventricular septum; PV, pulmonary valve; TGA, transposition of the great arteries; VSD, ventricular septal defect. Z‐scores based on data from Vigneswaran et al21 [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.5. Correlation between prenatal and postnatal measurements
All correlations are documented in Table 2. Most importantly, at 26 to 30 weeks gestation, Z‐scores of the PV and AoV annular diameters correlated with the Z‐score of the NAoR measurement at the last follow‐up visit (r = −0.49, P < .001 and r = −0.32, P = .008, respectively).
Table 2.
Comparisons between Z‐scores of semilunar valve annulus and left ventricular outflow tract measurements at 1 y and last follow‐up
| Comparisons | Rho | P Value | Comparisons | Rho | P Value | ||
|---|---|---|---|---|---|---|---|
| 18−22 wk | |||||||
| 1 y FU | Last FU | ||||||
| Z‐fPV vs Z‐AoV | −0.18 | .142 | Z‐fPV vs Z‐oV | −0.10 | .370 | ||
| Z‐fPV vs Z‐NAoR | −0.27 | .027 | Z‐fPV vs Z‐NAoR | −0.29 | .009 | ||
| Z‐fPV vs Z‐STjunction | −0.09 | .491 | Z‐fPV vs Z‐STjunction | 0.01 | .949 | ||
| Z‐fAoV vs Z‐AoV | −0.35 | .002 | Z‐fAoV vs Z‐AoV | −0.21 | .062 | ||
| Z‐fAoV vs Z‐NAoR | −0.16 | .171 | Z‐fAoV vs Z‐NAoR | −0.12 | .338 | ||
| Z‐fAoV vs Z‐STjunction | −0.09 | .501 | Z‐fAoV vs Z‐STjunction | −0.03 | .794 | ||
| 26−30 wk | |||||||
| 1 y FU | Last FU | ||||||
| Z‐fPV vs Z‐AoV | −0.31 | .020 | Z‐fPV vs Z‐AoV | −0.32 | .008 | ||
| Z‐fPV vs Z‐NAoR | −0.28 | .035 | Z‐fPV vs Z‐NAoR | −0.49 | <.001 | ||
| Z‐fPV vs Z‐STjunction | −0.20 | .150 | Z‐fPV vs Z‐STjunction | −0.39 | .019 | ||
| Z‐fAoV vs Z‐AoV | −0.31 | .019 | Z‐fAoV vs Z‐AoV | −0.29 | .016 | ||
| Z‐fAoV vs Z‐NAoR | −0.13 | .333 | Z‐fAoV vs Z‐NAoR | −0.35 | .003 | ||
| Z‐fAoV vs Z‐STjunction | −0.08 | .574 | Z‐fAoV vs Z‐STjunction | −0.10 | .441 | ||
| 32−36 wk | |||||||
| 1 y FU | Last FU | ||||||
| Z‐fPV vs Z‐AoV | −0.24 | .054 | Z‐fPV vs Z‐AoV | −0.27 | .024 | ||
| Z‐fPV vs Z‐NAoR | −0.24 | .063 | Z‐fPV vs Z‐NAoR | −0.28 | .019 | ||
| Z‐fPV vs Z‐STjunction | −0.06 | .658 | Z‐fPV vs Z‐STjunction | −0.11 | .394 | ||
| Z‐fAoV vs Z‐AoV | −0.11 | .410 | Z‐fAoV vs Z‐AoV | −0.24 | .056 | ||
| Z‐fAoV vs Z‐NAoR | −0.21 | .112 | Z‐fAoV vs Z‐NAoR | −0.17 | .164 | ||
| Z‐fAoV vs Z‐STjunction | −0.17 | .228 | Z‐fAoV vs Z‐STjunction | −0.02 | .877 | ||
Note. Significant results are shown in bold (P < .05). Fetal Z‐scores based on Vigneswaran et al.21
Abbreviations: FU, follow‐up; Z‐AoV, Z‐score of neo‐aortic valve annulus diameter post arterial switch operation; Z‐fAoV, Z‐score of the fetal aortic valve annulus diameter; Z‐fPV, Z‐score of the fetal pulmonary valve annulus diameter; Z‐NAoR, Z‐score of the neo‐aortic root; Z‐STjunction, Z‐score of the sino‐tubular junction.
3.6. Prediction of NAoR dilatation post‐ASO
Only the PV annulus Z‐score at 26 to 30 weeks' gestation was considered to be of a predictive value for NAoR dilatation at the last follow‐up visit (AUC = 0.761) (Table S1). The optimal cutoff value to predict NAoR dilatation at the last follow‐up visit post‐ASO was a PV annulus Z‐score of −0.04 at 26 to 30 weeks' gestation (sensitivity 73%, specificity 69%) (Figure S4).
There was no statistically significant difference between males and females in the prediction of NAoR dilatation at last follow‐up visit post‐ASO based on the PV annulus Z‐score at 26 to 30 weeks' gestation (P = .395).
4. DISCUSSION
4.1. Main findings
This study has shown that the semilunar valves are larger in TGA fetuses compared with fetuses with normal hearts. PV annular diameters in the TGA fetuses were significantly larger than controls from 27 weeks' gestation, especially when a VSD was present. They were also significantly larger than control AoV annular diameters from 23 weeks' gestation. The Z‐scores of the PV annulus and NAoR diameter at last follow‐up post‐ASO correlated significantly, but only the PV annulus Z‐score at 26 to 30 weeks' gestation was considered to be of a predictive value for NAoR dilatation at the last follow‐up visit (AUC = 0.761).
Additionally, the AoV annular diameters were significantly larger in TGA fetuses than controls from 26 weeks' gestation and larger than control PV annular measurements from 30 weeks' gestation.
4.2. Flow and growth of the semilunar valves
The size of a vessel can be related to the flow through it,28, 29 growing larger with more flow. The PV annulus is usually larger than the AoV annulus in a normal fetal heart. This correlates with the larger proportion of the combined cardiac output (CCO) ejected by the right ventricle compared with the left ventricle, which increases with gestation to about 66% for the right ventricle.3, 30, 31, 32 In the fetus, oxygenated blood from the venous duct is preferentially streamed across the oval fossa into the left atrium and left ventricle. In TGA, this blood, with a higher oxygen saturation, is ejected into the pulmonary artery and may cause pulmonary vasodilatation and potential ductal constriction. Increased pulmonary blood flow, pulmonary venous return to the left atrium and left ventricular filling then follows.2, 3, 5 The percentage of the CCO ejected by the left ventricle increases as a consequence to about 50%.2, 3, 5
So, if only flow determines growth, the PV annular diameter in fetal TGA hearts should be smaller than control PV annuli but larger than control AoV annuli. However, we found the diameters of fetal TGA‐PV annuli to be significantly larger than controls from 27 weeks and also larger than the control AoV annuli from 23 weeks onwards. A previous study has also shown PV Z‐scores smaller than the AoV Z‐scores (−0.65 vs +1.13), in TGA fetuses with IVS,4 in concordance with our findings. Furthermore, the fetal TGA‐AoV annuli were not smaller than control PV annuli as may be expected if only flow determines annular growth.
Our findings suggest that other factors besides flow also play a role in the growth of the semilunar valve annuli in fetal TGA. Lalezari et al33 have shown that the amount of collagen was diminished in unoperated neonatal TGA hearts and the anchorage and embedding of both arterial roots in the myocardium was less extensive. This deficiency in support for the great vessels possibly contributes to the increased size of the semilunar valves. Furthermore, the pulmonary artery in unoperated TGA hearts showed a clear trend in loss of actin‐positive smooth muscle cells with age,34 which may further contribute to NAoR dilatation post‐ASO.
4.3. Effect of a VSD
The enlargement of the PV annulus was especially marked when there was a significant VSD from about 30 weeks' gestation. In TBA, the PV annular diameter is larger than the AoV annulus as the subpulmonary VSD allows a proportion of the right ventricular output, in addition to the left ventricular output, to cross the PV, encouraging growth. Larger PV annular diameters in non‐TBA TGA hearts with a significant VSD were also found, suggesting a net right to left shunt across the fetal VSD and increased flow over the PV, coinciding with the fall in pulmonary resistance that is seen in the fetus around 28 weeks' gestation.31, 32
4.4. Late gestational changes in PV annular growth
We found a reduced growth rate in the PV annulus at the end of gestation. A corresponding leveling in the prenatal PV‐Z‐score was seen from 35 weeks onwards. Godfrey et al have also described a significant reduction in the PV annular Z‐score in fetal TGA between the second and third trimesters.4 The reason for this is not entirely clear. After 30 weeks, the proportion of the CCO passing through the lungs and oval fossa is stable in normal fetal hearts, but the pulmonary vascular resistance increases.32 In TGA fetuses, the pulmonary venous return may be increased elevating left atrial pressure and encouraging restriction of the oval foramen,2 reducing right to left shunting at atrial level, left ventricular filling and flow over the PV in later gestation with possible reduced growth of the PV as a result. When the postnatal measurements were not included in our model, the late gestational trend for reduction in PV annular growth was less evident.
4.5. Neo‐aortic root
The long‐term prognosis of the ASO depends on the adequate growth of the repaired arteries and the adaption of the neo‐aortic valve.6, 8, 9 We have shown that the size of the PV annulus at 26 to 30 weeks correlated with the size of the NAoR at last follow‐up visit post‐ASO and that the PV annulus in fetal TGA is already larger than a normal AoV annulus. However, this correlation was not strong enough to be clinically useful for a prenatal prediction of NAoR dilatation post‐ASO. Measuring the PV annulus at 28 weeks' gestation will not influence the immediate postnatal management of the baby. In countries where there are constraints on prenatal echo facilities, the emphasis should rather lie on the initial diagnosis of the TGA around 20 weeks of gestation with the use of the outflow tract views. A follow‐up echo in the last 2 weeks of pregnancy is also important to evaluate the oval fossa size and risk for a postnatal Rashkind procedure and pulmonary hypertension.35
Hourihan et al also observed that the NAoR and PV annulus are already larger than in normal infants before the ASO.8 An increased risk of NAoR dilation in TBA and TGA with VSD has previously been recognized,13, 16, 18 highlighting the role of a significant VSD in the pathophysiology of NAoR dilatation. This is clinically relevant as the reintervention rate is higher in TGA patients with a VSD compared with those with an IVS.14, 17 Furthermore, the NAoR growth rate post‐ASO is ≥4 times the normal rate.9 The resultant valve regurgitation may require reintervention.7, 15, 16, 17
4.6. Strengths and limitations
This was a multicenter study of one of the largest cohorts of fetal TGA reported to date and presents serial data. As with all retrospective studies, it has limitations. Not all cases had stored data that could be analyzed. The fetal echocardiograms were made by different observers, and there was no predetermined protocol, which may have biased the results. However, two researchers performed all the measurements. We did not have our own control group and used the normal data of Vigneswaran et al21 based on a cohort of more than 7000 fetuses. As the semilunar valves were measured in de closed position by Vigneswaran et al21 and they had no measurement data after 36 weeks' gestation, we also plotted our cohort against the normal data of Schneider et al22 where the semilunar valves were measured in the open position in 130 fetuses. The trends and our conclusions using the different control data were similar.
4.7. Conclusions
In conclusion, fetal TGA semilunar valve annuli are larger than normal, especially when there is a significant VSD. This research adds to our understanding of the pathophysiology of fetal TGA and the long‐term complications post‐ASO.
CONFLICT OF INTEREST
None declared.
Supporting information
Table S1. Area under curve for prediction of neo‐aortic root dilatation (Z >+2) based on the fetal semilunar valve annular diameter.
Figure S1. Average trends in PV‐annular diameters in TGA‐fetuses (TGA‐PV):
(A) versus trends in controls PV‐annular diameter (Control‐PV);
(B) versus AoV‐annular diameter (Control‐AoV);
(C) TGA with an IVS (TGA with IVS‐PV) and a VSD (TGA with VSD‐PV); and (D) with the TGA‐VSD fetuses further stratified on the basis of a TBA or non‐TBA anatomy (TGA with TBA‐PV).
Average trend in AoV‐annular diameters in TGA‐fetuses (TGA‐AoV):
(E) versus trends in controls of PV‐annular diameter (Control‐PV); and
(F) AoV‐annular diameter (Control‐AoV).
Trends in PV‐ and AoV‐annular diameters in controls are shown (Control‐PV and Control‐AoV).
Figure S2. Sensitivity analysis
Figure S3. Average trend in TGA‐fetal (A) PV‐annular diameter Z‐score (TGA‐PV Z‐score) and (B) AoV‐annular diameter Z‐score (TGA‐AoV Z‐score).
Figure S4. ROC for the prediction of NAoR dilatation post ASO at the last follow‐up visit postnatally based on the PV‐annular Z‐score at 26‐30 weeks gestation. Fetal Z‐scores based on Vingeswaran et al.21
van der Palen RLF, van der Zee C, Vink AS, et al. Transposition of the great arteries: Fetal pulmonary valve growth and postoperative neo‐aortic root dilatation. Prenatal Diagnosis. 2019;39:1054–1063. 10.1002/pd.5539
Roel L. F. van der Palen and Carlijn van der Zee shared first authorship.
ISUOG and AEPC
The copyright line for this article was changed on 26 September 2019 after original online publication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Samanek M, Slavik Z, Zborilova B, Hroboňová V, Voříšková M, Škovránek J. Prevalence, treatment, and outcome of heart disease in live‐born children: a prospective analysis of 91,823 live‐born children. Pediatr Cardiol. 1989;10(4):205–211. [DOI] [PubMed] [Google Scholar]
- 2. Rudolph AM. Congenital cardiovascular malformations and the fetal circulation. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F132–F136. [DOI] [PubMed] [Google Scholar]
- 3. Rudolph AM. Congenital Diseases of the Heart: Clinical‐Physiological Considerations. 3rd ed. Chichester, UK: Wiley‐Blackwell; 2009. [Google Scholar]
- 4. Godfrey ME, Friedman KG, Drogosz M, Rudolph AM, Tworetzky W. Cardiac output and blood flow redistribution in the fetus with D‐loop transposition of the great arteries and intact ventricular septum: insights into the pathophysiology. Ultrasound Obstet Gynecol. 2017;50(5):612–617. 10.1002/uog.17370 [DOI] [PubMed] [Google Scholar]
- 5. Everwijn SMP, van Nisselrooij AEL, Rozendaal L, et al. The effect of the introduction of the three‐vessel view on the detection rate of transposition of the great arteries and tetralogy of Fallot. Prenat Diagn. 2018;38(12):951–957. 10.1002/pd.5347. Epub 2018 Sep 11 [DOI] [PubMed] [Google Scholar]
- 6. Rodrigues C, Cerejo R. Arterial switch: how to predict reoperation. Rev Port Cir Cardiotorac Vasc. 2017;24:124. [PubMed] [Google Scholar]
- 7. de Koning WB, van Osch‐Gevers M, Ten Harkel AD, et al. Follow‐up outcomes 10 years after arterial switch operation for transposition of the great arteries: comparison of cardiological health status and health‐related quality of life to those of the a normal reference population. Eur J Pediatr. 2008;167(9):995–1004. [DOI] [PubMed] [Google Scholar]
- 8. Hourihan M, Colan SD, Wernovsky G, Maheswari U, Mayer JE Jr, Sanders SP. Growth of the aortic anastomosis, annulus, and root after the arterial switch procedure performed in infancy. Circulation. 1993;88(2):615–620. [DOI] [PubMed] [Google Scholar]
- 9. van der Bom T, van der Palen RL, Bouma BJ, et al. Persistent neo‐aortic growth during adulthood in patients after an arterial switch operation. Heart. 2014;100:1360–1365. [DOI] [PubMed] [Google Scholar]
- 10. Marino BS, Wernovsky G, McElhinney DB, et al. Neo‐aortic valvar function after the arterial switch. Cardiol Young. 2006;16(5):481–489. [DOI] [PubMed] [Google Scholar]
- 11. van der Palen RL, van der Bom T, Dekker A, et al. Progression of aortic root dilatation and aortic valve regurgitation after the arterial switch operation. Heart. 2019;1–9. 10.1136/heartjnl-2019-315157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McMahon CJ, Ravekes WJ, Smith EO, et al. Risk factors for neo‐aortic root enlargement and aortic regurgitation following arterial switch operation. Pediatr Cardiol. 2004;25(4):329‐335. [DOI] [PubMed] [Google Scholar]
- 13. Michalak KW, Moll JA, Moll M, et al. The neoaortic root in children with transposition of the great arteries after an arterial switch operation. Eur J Cardiothorac Surg. 2013;43(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 14. Co‐Vu JG, Ginde S, Bartz PJ, Frommelt PC, Tweddell JS, Earing MG. Long‐term outcomes of the neoaorta after arterial switch operation for transposition of the great arteries. Ann Thorac Surg. 2013;95(5):1654–1659. [DOI] [PubMed] [Google Scholar]
- 15. Koolbergen DR, Manshanden JS, Yazdanbakhsh AP, et al. Reoperation for neoaortic root pathology after the arterial switch operation. Eur J Cardiothorac Surg. 2014;46(3):474–479. [DOI] [PubMed] [Google Scholar]
- 16. Raju V, Burkhart HM, Durham LA, et al. Reoperation after arterial switch operation: a 27‐year experience. Ann Thorac Surg. 2013;95(6):2105–2113. [DOI] [PubMed] [Google Scholar]
- 17. Fricke TA, d'Udekem Y, Richardson M, et al. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg. 2012;94(1):139–145. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz ML, Gauvreau K, del Nido P, Mayer JE, Colan SD. Long‐term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation. 2004;110(11 Suppl 1):II128–II132. [DOI] [PubMed] [Google Scholar]
- 19. Baruteau AE, Vergnat M, Kalfa D, et al. Long‐term outcomes of the arterial switch operation for transposition of the great arteries and ventricular septal defect and/or arch obstruction. Interact Cardiovasc Thorac Surg. 2016;23(2):240–246. [DOI] [PubMed] [Google Scholar]
- 20. Formigari R, Toscano A, Giardini A, et al. Prevalence and predictors of neoaortic regurgitation after arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 2003. Dec;126(6):1753–1759. [DOI] [PubMed] [Google Scholar]
- 21. Vigneswaran TV, Akolekar R, Syngelaki A, et al. Cardiac outflow tracts from 13 to 36 weeks gestation. A single‐center study of over 7000 cases. Circ Cardiovasc Imaging. 2018;11:e007575 10.1161/CIRCIMAGING.118.007575 [DOI] [PubMed] [Google Scholar]
- 22. Schneider C, McCrindle BW, Carvalho JS, Hornberger LK, McCarthy KP, Daubeney PE. Development of Z‐scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26(6):599–605. [DOI] [PubMed] [Google Scholar]
- 23. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height‐weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–66. [DOI] [PubMed] [Google Scholar]
- 24. Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21(8):922–934. [DOI] [PubMed] [Google Scholar]
- 25. R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [computer program]. [Google Scholar]
- 26. R package nlme [computer program]. Version 3.1‐120.
- 27. Stone CJ, Koo CY. Additive splines in statistics In: Proceedings of the Statistical Computing Section ASA. Washington: American Statistical Association; 1985. [Google Scholar]
- 28. Lev M. Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complex. Lab Invest. 1952;1:61–70. [PubMed] [Google Scholar]
- 29. Fishman NH, Hof RB, Rudolph AM. Models of congenital heart disease in foetal lambs. Circulation. 1978;58(2):354–364. [DOI] [PubMed] [Google Scholar]
- 30. Clur SA, Oude Rengerink K, Mol BW, Ottenkamp J, Bilardo CM. Fetal cardiac function between 11 and 35 weeks' gestation and nuchal translucency thickness. Ultrasound Obstet Gynecol. 2011;37(1):48–56. 10.1002/uog.8807. Erratum in: Ultrasound Obstet Gynecol 2011;38:486 [DOI] [PubMed] [Google Scholar]
- 31. Kiserud T, Acharya G. The fetal circulation. Prenat Diagn. 2004;34:49–59. [DOI] [PubMed] [Google Scholar]
- 32. Rasanen J, Wood DC, Weiner S, Ludomirski A, Huhta JC. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation. 1996;94(5):1068–1073. 10.1161/01.CIR.94.5.1068 [DOI] [PubMed] [Google Scholar]
- 33. Lalezari S, Mahtab EAF, Bartelings MM, Wisse LJ, Hazekamp MG, Gittenberger‐de Groot AC. The outflow tract in transposition of the great arteries; an anatomic and morphologic study. Ann Thorac Surg. 2009;88(4):1300–1305. [DOI] [PubMed] [Google Scholar]
- 34. Lalezari S, Hazekamp MG, Bartelings MM, Schoof PH, Gittenberger‐de Groot AC. Pulmonary artery remodeling in transposition of the great arteries: relevance for neoaortic root dilatation. J Thorac Cardiovasc Surg. 2003;126(4):1053–1060. [DOI] [PubMed] [Google Scholar]
- 35. Sarris GE, Balmer C, Bonou P, et al. Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. The task force on transposition of the great arteries of the European Association for Cardio‐Thoracic Surgery (EACTS) and the Association for European Paediatric and Congenital Cardiology (AEPC). Eur J Cardiothorac Surg. 2017;51(1):e1–e32. 10.1093/ejcts/ezw360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Area under curve for prediction of neo‐aortic root dilatation (Z >+2) based on the fetal semilunar valve annular diameter.
Figure S1. Average trends in PV‐annular diameters in TGA‐fetuses (TGA‐PV):
(A) versus trends in controls PV‐annular diameter (Control‐PV);
(B) versus AoV‐annular diameter (Control‐AoV);
(C) TGA with an IVS (TGA with IVS‐PV) and a VSD (TGA with VSD‐PV); and (D) with the TGA‐VSD fetuses further stratified on the basis of a TBA or non‐TBA anatomy (TGA with TBA‐PV).
Average trend in AoV‐annular diameters in TGA‐fetuses (TGA‐AoV):
(E) versus trends in controls of PV‐annular diameter (Control‐PV); and
(F) AoV‐annular diameter (Control‐AoV).
Trends in PV‐ and AoV‐annular diameters in controls are shown (Control‐PV and Control‐AoV).
Figure S2. Sensitivity analysis
Figure S3. Average trend in TGA‐fetal (A) PV‐annular diameter Z‐score (TGA‐PV Z‐score) and (B) AoV‐annular diameter Z‐score (TGA‐AoV Z‐score).
Figure S4. ROC for the prediction of NAoR dilatation post ASO at the last follow‐up visit postnatally based on the PV‐annular Z‐score at 26‐30 weeks gestation. Fetal Z‐scores based on Vingeswaran et al.21
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


