Abstract
Introduction
The ProTWIN trial previously showed no beneficial effect of treatment with a cervical pessary vs usual care to prevent preterm birth in women with a multiple pregnancy. However, in women with a midtrimester short cervix (<38 mm), pessary did reduce the composite outcome of neonatal morbidity and mortality. This follow‐up study evaluates the long‐term outcomes of all children born to mothers who participated in the ProTWIN trial at 4 years of age.
Material and methods
Parents received the Ages and Stages Questionnaire, Strength and Difficulties Questionnaire and a health questionnaire. All questionnaires were reported separately and as a combined outcome (abnormal child outcome). A linear mixed effects model was used to adjust for correlated data in twins and correction for confounders was performed. In exploratory analysis, a composite outcome of death or survival with abnormal child outcome was used by combining extrapolated data on child outcome with survival data. All data were analyzed for the total group and the subgroup of women with midtrimester short cervix.
Results
Of the original 813 women of the ProTWIN trial, we approached 579, of whom 258 participated (45%) in follow‐up. We received questionnaires of 514 children (281 pessary vs 233 control), with 119 children in the subgroup of women with midtrimester short cervix. An abnormal child outcome was found in 23% in the pessary group vs 16% in the control group (odds ratio 1.58; 95% confidence interval 0.94‐2.65). In exploratory analysis with extrapolated data on child outcome (n = 815), no difference in abnormal child outcome was seen between the pessary and control group. In the subgroup of women with a short cervix (n = 268), this composite outcome indicated a favorable outcome for children born to mothers with pessary.
Conclusions
In women with a multiple pregnancy, the use of a cervical pessary did not improve development, behavior or physical outcomes of the surviving children at age 4.
Keywords: behavior, child, development, follow‐up, multiple pregnancy, pessary, preterm birth
Abbreviations
- ASQ
Ages and Stages Questionnaire
- CI
confidence interval
- NNH
number needed to harm
- NNT
number needed to treat
- SDQ
Strength and Difficulties Questionnaire
- SD
standard deviation
Key message.
In offspring of women with a multiple pregnancy randomized to cervical pessary or no intervention, cervical pessary did not improve child outcome in surviving children at age 4.
1. INTRODUCTION
Prematurity, defined as birth prior to 37 weeks of gestation, is the leading cause of perinatal morbidity, mortality and long‐term neurodevelopmental impairment, and is seen more often in women with a multiple pregnancy than in singletons (48.1% vs 6.0%).1
Several interventions are known for their use in preterm birth prevention such as progesterone, pessary and cerclage. Evaluations of these interventions in women with a multiple pregnancy show no benefit of progesterone and a potentially harmful effect of cerclage.2 Studies evaluating the use of a cervical pessary are still ongoing. Although some pessary studies show promising results,3, 4 none of these studies reports long‐term childhood outcomes. The importance of long‐term outcomes related to agents given to pregnant women has been demonstrated by previous studies, showing that a short‐term benefit can have unexpected long‐term effects on children which may not be apparent at birth.5, 6, 7, 8
We previously reported the results of the ProTWIN trial, showing no beneficial effect of a cervical pessary in the short‐term in unselected women with an asymptomatic multiple pregnancy. In women with a midtrimester short cervix (cervical length <38 mm), a significant reduction in a composite of neonatal morbidity and mortality was seen in the pessary group (10% vs 24%, relative risk 0.42 [95% confidence interval {CI} .19‐0.91]). A 3‐year follow‐up of the ProTWIN trial, analyzing children born to mothers with a midtrimester short cervix, showed a significant reduction in the composite outcome of death or survival with a neurodevelopmental disability (10% vs 29%, odds ratio [OR] 0.26 [95% CI 0.09‐0.75]).9 However, this 3‐year follow‐up study did not report on all participants of the ProTWIN trial.
The aim of this follow‐up study was to compare developmental, behavioral and physical outcomes of surviving children at age 4 born to all mothers in the ProTWIN trial, comparing the cervical pessary group with the control group. In addition, these outcomes are studied in the subgroup of women with short cervix (<38 mm). Finally, the composite outcome of death or abnormal developmental and/or behavioral and/or physical outcome at age 4 is explored in the offspring of all randomized women of the ProTWIN trial, as well as in the subgroup of women with a short cervix.
2. MATERIAL AND METHODS
We performed a follow‐up study of the ProTWIN trial, a multicenter randomized controlled clinical trial (NTR1858) conducted in 40 hospitals in the Netherlands. Protocol and initial results of this study have been described in detail elsewhere.10, 11 In short, the ProTWIN trial randomized women with an asymptomatic multiple pregnancy between pessary (n = 403) and usual care (n = 410). If assigned to the intervention group, a cervical pessary was inserted between 16 and 20 weeks of gestation; women in the control group received care as usual. Cervical length was measured at baseline using transvaginal sonography.11 Following the guideline of the Dutch Society for Obstetrics and Gynecology, no progesterone was administered.12
2.1. Follow‐up assessment
We aimed to evaluate all children born in the ProTWIN trial who were alive at discharge at a corrected age of 4 years, calculated from the expected date of delivery. Research nurses in participating centers crosschecked medical records of all participating children of the original trial to track the possible occurrence of death of one or both children before contacting women. Mothers of whom at least one of the children was alive were contacted by telephone 3 months prior to the corrected age of 4 years. After consent, questionnaires were sent and filled out when the child was the corrected age of 4. Parents were asked to complete a paper version of the Ages and Stages Questionnaire 48 months (ASQ),13 the Strengths & Difficulties Questionnaire (SDQ)14 and a general health questionnaire. All mothers provided written informed consent.
2.2. Ages and Stages Questionnaire
The ASQ is a developmental screening tool that covers five domains of child development, including communication, gross and fine motor development, problem‐solving and personal‐social skills. Each domain is assessed by six questions on developmental milestones.13 A validated Dutch translation of the ASQ 48 months was used. The construction of a binary ASQ developmental delay score was based on a comparison with the mean score and standard deviation (SD) of the Dutch reference group. Scores of 1 SD below the mean of the ASQ normative data in two or more domains, or 2 SD below the normative mean in at least one domain were considered delayed.13, 15
2.3. Strengths & Difficulties Questionnaire
The SDQ is a questionnaire screening for behavioral problems in children. It consists of 25 items, grouped in five subscales: emotional problems, conduct problems, hyperactivity, peer problems and pro‐social behavior. A total difficulties score can be calculated summing the first four subscales, leaving out pro‐social behavior.16 The validated Dutch translation of the SDQ 4‐17 years was used. Total difficulties score was coded according to the Dutch SDQ manual (0‐10 normal, 11‐14 borderline and ≥15 abnormal) with mean values based upon data validated in the Netherlands.14 In the analysis, the total difficulties score was treated as a binary variable, with a cut‐off score of 15 to define abnormal.14, 17
2.4. Demographic and Health Questionnaire
A separate questionnaire was used to address demographic variables (for example, family composition, education of both parents, use of daycare, bilingualism and position within the family) and healthcare use of the children (for example visits to healthcare providers, medication use in the past and present, hospital admission and surgery) until 4 years of age.
2.5. Abnormal child outcome
The three assessment instruments were combined in a binary outcome (abnormal child outcome). A child was classified as abnormal when it showed a delayed ASQ developmental score, an abnormal SDQ total difficulties score or the presence of a physical problem (defined as ≥3 hospital admissions and/or ≥3 surgeries between discharge after birth and age 4).
We also studied a composite outcome integrating mortality data (stillbirth, death until 6 weeks after the expected term date and death before the age of 4 years) and abnormal child outcome at age 4 as defined above.
2.6. Statistical analyses
Abnormal cut‐off scores and mean scores of ASQ and SDQ questionnaires were analyzed according to randomization in the ProTWIN trial for all surviving children that could be assessed at 4 years of age and in the subgroup of women with a cervical length <38 mm. Differences concerning maternal characteristics and neonatal short‐term outcomes between pessary and control group of mothers and children that participated in the follow‐up were calculated with t test, Mann‐Whitney U test, Chi‐square test or Fisher's exact test when appropriate. Also differences in characteristics between follow‐up participants and participants lost to follow‐up were calculated. A two‐sided P‐value <0.05 indicated statistical significance. A generalized linear mixed effects model (GLMM) was used to adjust for correlations between children of the same mother and confounders.18 Through a Directed Acyclic Graph (DAG), we visualized potential confounders: due to the randomization process no confounders were found (Figure S1). However, prior to using DAG, we considered the influence of each potential confounder on the determinant‐outcome association individually, using 10% difference as a cut‐off for identification of confounders. In this analysis, the following variables were listed as potential confounders: parental education, smoking during pregnancy, ethnicity, bilingualism, whether the twins were the oldest children in the family, daycare participation and breastfeeding ≥6 months. A power calculation before the start of the follow‐up study showed that data of 225 children in each group would provide 80% power to detect a difference of 13% to 5% in delayed ASQ scores between the two groups with a two‐sided α of .05.
Results of the general health questionnaire were clustered.9 We also performed an exploratory analysis evaluating the effect of pessary on a composite outcome of death or survival with abnormal child outcome in the offspring of all randomized women of the ProTWIN study and in the subgroup of women with cervical length <38 mm. Because baseline characteristics of children that participated in the follow‐up, besides ethnicity, did not differ significantly from children that were lost to follow‐up (Table S1) we calculated a simple case extrapolation scenario, assuming children lost to follow‐up were showing the same percentage of disability as the group that was followed‐up. Furthermore, we also explored a best and worst case scenario in both groups, assuming all children lost to follow‐up had either a normal child outcome (best case) or abnormal child outcome (worst case).
These extrapolated data were analyzed using a generalized linear mixed model calculating an OR. We adjusted the 95% CI by using the standard error of the assessed group of children at age 4 instead of the SE of the whole group that included extrapolated data. This was done to avoid misleading narrow confidence intervals due to the increase in patient numbers in the extrapolated data. Additionally, number needed to treat (NNT) or number needed to harm (NNH) and 95% CI were calculated. All statistical analyses were conducted in IBM SPSS version 21 (IBM Corp., Armonk, NY, USA).
2.7. Ethical approval
Ethical approval for this follow‐up assessment was given by the Medical Ethics Committee of the Academic Medical Center in Amsterdam (NL46768.018.13).
3. RESULTS
In the original ProTWIN trial, 813 women were randomly assigned to pessary (403 women, 815 children) or care as usual (410 women, 829 children) (Figure 1). In total, 75 children (34 in the pessary group and 41 in the control group) died due to stillbirth or death until 6 weeks after the expected term date. During the 4‐year follow‐up period, six children died (2 in the pessary group vs 4 in the control group). The risk of death was 36/815 in the pessary group and 45/829 in the control group (relative risk 0.87, 95% CI 0.58‐1.31).
Figure 1.
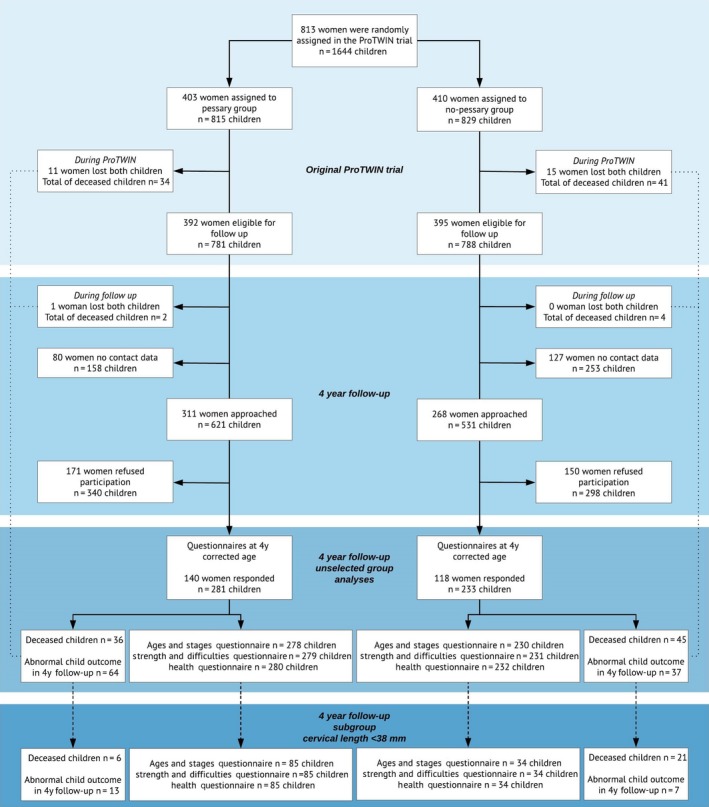
Flowchart of participants in the ProTWIN trial (unselected and subgroup of women with short cervix), starting from randomization of women with a multiple pregnancy until the 4‐y follow‐up of the children [Color figure can be viewed at https://wileyonlinelibrary.com]
Of the 813 women, 27 women lost both children, resulting in 786 women with 1563 surviving children. Of these, 207 women with 411 children were lost to follow‐up due to missing contact data. A total of 579 women (311 pessary vs 268 control) with 1152 children (621 pessary vs 531 control) were approached. Of the approached women, 321 refused participation (171 pessary vs 150 control) due to lack of time (n = 13, 4%), not interested (n = 21, 7%) or no reason provided (n = 287, 89%). Eventually, questionnaires were received from 258 mothers, reporting on 514 children (45% of women approached, 281 pessary vs 233 control). For the subgroup of women with a cervical length of <38 mm, questionnaires from 61 mothers were received, reporting on 119 children (85 pessary vs 34 control) (Figure 1).
Mothers participating in follow‐up were more often of European origin compared with women that were lost to follow‐up (96% vs 88%, P < 0.001) (Table S1). No other differences in maternal or neonatal characteristics between these two groups were found. When comparing maternal and neonatal characteristics of mothers and children participating in follow‐up, no differences were seen between the pessary and control groups. The mean age of children assessed for follow‐up was 3.98 (SD 0.19) years in the pessary group compared with 3.96 (SD .21) years in the control group (P = 0.198) (Table 1).
Table 1.
ProTWIN maternal baseline characteristics, pregnancy outcomes and child neonatal and sociodemographic characteristics of the women participating in the 4‐y follow‐up study
| Maternal characteristics at entry of the ProTWIN trial | n/na | Pessary group (n = 140) | Control group (n = 118) | P value |
|---|---|---|---|---|
| Median (IQR) maternal age at randomization | 140/118 | 32 (29‐36) | 33 (30‐37) | 0.471 |
| Nulliparity, n (%) | 140/118 | 87 (62.1) | 66 (55.9) | 0.312 |
| Smoking during pregnancy, n (%) | 139/114 | 4 (2.9) | 5 (4.4) | 0.735 |
| Previous preterm delivery, n (%)b | 51/52 | 10 (19.6) | 7 (13.5) | 0.438 |
| Parental education, n (%)c | ||||
| High | 134/115 | 102 (76.1) | 89 (77.4) | 0.908 |
| Middle | 20 (14.9) | 15 (13.0) | ||
| Low | 12 (9.0) | 11 (9.6) | ||
| Ethnic origin European, n (%) | 140/118 | 132 (94.3) | 106 (89.8) | 0.234 |
| Monochorionic pregnancy, n (%) | 139/118 | 33 (23.7) | 30 (25.4) | 0.755 |
| Triplet pregnancy, n (%) | 140/118 | 5 (3.6) | 2 (1.7) | 0.459 |
| Cervical length (mm), median (IQR) | ||||
| Unselected group | 140/118 | 43 (37‐48.75) | 43 (39‐49) | 0.268 |
| Subgroup cervical length <38 mm | 43/18 | 35 (33‐37) | 34 (32‐35.25) | 0.100 |
| Maternal pregnancy outcomes ProTWIN trial | ||||
| Pregnancy duration in weeks median (IQR) | 140/118 | 36.6 (34.4‐37.6) | 36.3 (34.2‐37.4) | 0.417 |
| <28 wk, n (%) | 2 (1.4) | 2 (1.7) | >0.999 | |
| <32 wk, n (%) | 14 (10.0) | 13 (11.0) | 0.790 | |
| <37 wk, n (%) | 71 (50.7) | 62 (52.5) | 0.770 | |
| PPROM, n (%) | 125/99 | 16 (12.8) | 12 (12.1) | 0.879 |
| Tocolytic drug, n (%) | 140/118 | 23 (16.4) | 21 (17.8) | 0.771 |
| Corticosteroids, n (%) | 131/113 | 33 (25.2) | 35 (31.0) | 0.315 |
| Neonatal characteristics of the ProTWIN trial | n/n | Pessary group (n = 281) | Control group (n = 233) | P value |
|---|---|---|---|---|
| Male gender, n (%) | 281/233 | 138 (49.1) | 122 (52.4) | 0.463 |
| Composite primary outcome of the ProTWIN trial, n (%)d | 279/233 | 15 (5.4) | 15 (6.4) | 0.611 |
| Congenital anomalies, n (%) | 277/233 | 9 (3.2) | 9 (3.9) | 0.811 |
| Birthweight, n (%) | ||||
| <2500 g | 280/233 | 147 (52.5) | 134 (57.5) | 0.256 |
| <1500 g | 32 (11.4) | 22 (9.4) | 0.465 | |
| Social background of the children at 4 y of age | ||||
| Age at follow‐up, mean (SD) | 281/233 | 3.98 (0.19) | 3.96 (0.21) | 0.198 |
| Living in two parent family, n (%)e | 275/233 | 269 (97.8) | 230 (98.7) | 0.517 |
| Twins are eldest of the siblings, n (%) | 277/233 | 169 (61.0) | 128 (54.9) | 0.166 |
| Dutch primary language spoken at home, n (%) | 275/231 | 269 (97.8) | 229 (99.1) | 0.300 |
| Bilingual, n (%) | 277/233 | 40 (14.4) | 34 (14.6) | 0.961 |
| Daycare, n (%) | 277/233 | 256 (92.4) | 217 (93.1) | 0.757 |
| Breastfed in the first 6 mo, n (%)f | 276/230 | 61 (22.1) | 42 (18.3) | 0.258 |
Number of analyzed mothers or children, without missing data. Pessary group/control group.
Previous preterm delivery, excluding all nulliparous women.
Parental education: “low level” = total years post elementary schooling <6, if at least one of the parents has a low level of education (but not if one parent is highly educated); “middle level” = total years post elementary schooling 6‐8, if both parents have a middle level of education; “high level” = total years post elementary schooling >8, if one of the parents is highly educated.26 Parental education at the time of follow‐up.
Composite outcome of the ProTWIN trial: stillbirth, PVL grade ≥2, RDS grade ≤2, BPD, IVH grade 2B or worse, NEC, proven sepsis, neonatal death until 6 weeks after expected term date.11
Living in two parent family: Children living with one or two biological parents, new marriage and de facto relationship.
Breastfed in the first 6 mo: breastfeeding for at least 6 mo, with or without infant formula.
3.1. Ages and Stages Questionnaires, Strength and Difficulties Questionnaires and General Health outcomes
No significant differences were found in delayed or mean ASQ scores, abnormal SDQ scores and physical problems between the pessary and control groups (Tables 2 and S2). No differences were seen in the individual SDQ subscales (results not shown) or the individual items of physical problems (ie, use of healthcare, medication and number of hospital admissions or surgeries) (Table S3). When abnormal scores of ASQ, SDQ and physical problems were combined, 64 (23%) pessary children vs 37 (16%) control children were found to have an abnormal child outcome (OR 1.58 [95% CI 0.94‐2.65]). In the subgroup of women with a cervical length <38 mm, an abnormal child outcome was found for 13 (15%) in the pessary and 7 (21%) in the control children (OR 0.73 [95% CI 0.22‐2.48]; Table 2).
Table 2.
Outcomes of Ages and Stages Questionnaire (ASQ), Strengths and Difficulties Questionnaire (SDQ) and physical outcomes in children at 4‐y follow‐up between pessary and control group. Results shown for unselected group of mothers and in the subgroup of cervical length <38 mm with generalized linear mixed effects model, unadjusted and adjusted for relevant confounders
| Unselected group | n/na | Pessary group (n = 281) | Control group (n = 233) | OR unadjusted for confounders (95% CI) | OR adjusted for confounders (95% CI)e |
|---|---|---|---|---|---|
| ASQ, delayed, n (%)b | 277/229 | 41 (14.8) | 23 (10) | 1.54 (0.83‐2.85) | 1.44 (0.77‐2.70)1 |
| SDQ, abnormal, n (%)b | 279/229 | 19 (6.8) | 10 (4.4) | 1.37 (0.66‐2.82) | 1.31 (0.64‐2.71)2 |
| Physical problemc | 277/229 | 12 (4.3) | 6 (2.6) | 1.28 (0.57‐2.91) | —3 |
| Abnormal child outcomed | 281/233 | 64 (22.9) | 37 (15.9) | 1.58 (0.94‐2.65) | —4 |
| Cervical length <38 mm | n/n | Pessary group (n = 85) | Control group (n = 34) | OR unadjusted for confounders (95% CI) | OR adjusted for confounders (95% CI)e |
|---|---|---|---|---|---|
| ASQ, delayed, n (%)b | 85/34 | 9 (10.6) | 3 (8.8) | 1.26 (0.27‐5.96) | 1.62 (0.34‐7.64)5 |
| SDQ, abnormal, n (%)b | 85/34 | 4 (4.7) | 0 | 2.08 (0.22‐19.34) | 1.92 (0.20‐18.27)6 |
| Physical problemc | 85/34 | 2 (2.4) | 4 (11.8) | 0.33 (0.07‐1.47) | 0.42 (0.09‐2.03)7 |
| Abnormal child outcomed | 85/34 | 13 (15.3) | 7 (20.6) | 0.73 (0.22‐2.48) | 0.95 (0.24‐3.69)8 |
Number of analyzed children, without missing data. Pessary group/control group.
Normal or delayed ASQ scores were based on the mean score and SD of the Dutch reference group. Scores of 1 SD below the mean in two or more domains, or 2 SD below the mean in at least one domain were considered delayed.15 SDQ scores were coded (normal, borderline and abnormal) according to the SDQ manual with mean values based upon data validated in a Dutch population.14, 16 Total difficulties score was calculated summing up the first four subscales, excluding pro‐social behavior.
Defined as ≥3 hospital admissions or ≥3 surgeries in the past 4 years.
Delayed ASQ score or abnormal SDQ total difficulties score or a physical problem (as defined above).
- Breastfeeding more than 6 mo (with or without infant formula);
- Ethnicity
- No significant confounders with beta difference >10%;
- No significant confounders with beta difference >10%
- Breastfeeding more than 6 mo (with or without infant formula), use of daycare and ethnicity;
- Parental education and use of daycare;
- Daycare and breastfeeding more than 6 mo (with or without infant formula);
- Daycare and breastfeeding more than 6 mo (with or without infant formula).
3.2. A composite outcome of death or abnormal child outcome
We calculated the composite outcome of death or abnormal child outcome for all randomized women in the ProTWIN trial. In the pessary group, 36 of 815 children died. Extrapolation of the data for the 498 surviving children without follow‐up data, based on the percentage abnormal child outcome found in the measured follow‐up children, resulted in a composite death or abnormal outcome in 214 children (26%) of the 815 children. In the control group (n = 829), 45 children died. Extrapolation of the abnormal child outcome for the 551 surviving children without follow‐up data resulted in a composite death or abnormal outcome in 170 children (21%) of the 829 children (OR 1.38 [95% CI 0.94‐2.03]) (Table 3), with a NNH of 18 [95% CI 10.2‐60.0]. In the extrapolated data of the subgroup of children born to mothers with a cervical length <38 mm, the composite outcome occurred in 29 (19%) pessary children vs 40 (36%) control children (OR 0.40 [95% CI 0.17‐0.96]) (Table 3), with a NNT in this subgroup of 6 [95% CI 3.5‐14.8]. However, these results should be interpreted with caution, since an extrapolation technique is used to substitute for 67% of the children that were lost to follow‐up. When exploring best and worst case scenarios, a consistent beneficial effect of pessary was seen in the subgroup of women with short cervix (Table S4).
Table 3.
Exploratory analysis; Composite outcome of death or survival with abnormal child outcome in the offspring of all randomized women of the ProTWIN trial (all cervical lengths included) and in the subgroup of women with a cervical length <38 mm group, analyzed with generalized linear mixed effects model
| Unselected group | Pessary (n = 815) | Control (n = 829) | OR unadjusted for confounders (95% CI) | NNT or NNH (95% CI) |
|---|---|---|---|---|
| Simple case scenario—composite outcome (%)a | 214 (26.3) | 170 (20.5) | 1.38 (0.94‐2.03) | 18 (10.2‐60.0)b |
| Subgroup CL <38 mm | Pessary (n = 157) | Control (n = 111) | ||
| Simple case scenario—composite outcome (%)a | 29 (18.5) | 40 (36.0) | 0.40 (0.17‐0.96) | 6 (3.5‐14.8)c |
Death or survival with any developmental problem; all deceased children (stillborn, death until 6 wk after the expected term date and death before the age of 4 y) and children with an abnormal score in the ASQ or SDQ total difficulties or a physical problem (≥3 admissions to the hospital or ≥3 surgeries), assuming that the children that were lost to follow‐up were showing the same percentage of disability as the group that was followed‐up.
NNH, number needed to harm.
NNT, number needed to treat.
4. DISCUSSION
In this follow‐up study, we assessed child outcomes at 4 years of age in children born to mothers randomized to treatment with a cervical pessary vs usual care. We found no beneficial effect of a pessary on development, behavior or physical outcomes (abnormal child outcome) in surviving children, irrespective of cervical length. When we combined death or abnormal child outcome in an exploratory analysis, we found no improvement in this composite outcome in unselected women. However, in women with a short midtrimester cervical length, the use of a pessary did reduce the risk of a composite outcome of death or abnormal child development in exploratory analysis.
In this follow‐up study, we were able to collect data on a broad range of child outcomes (development, behavior and physical) 4 years after child birth. Furthermore, the ProTWIN trial is the first study to report on the long‐term effects in children born to all mothers treated with a cervical pessary in their pregnancy to prevent preterm delivery. To date, two other randomized controlled trials have been published evaluating the use of a pessary in women with a twin pregnancy3, 19 and four trials in singletons as prevention of preterm birth strategy.20, 21, 22, 23 These trials have not (yet) reported long‐term follow‐up.
A limitation of this study is the high rate of loss to follow‐up, with a difference in follow‐up rate between the pessary and control group in all eligible women (36% vs 30% in pessary and control, respectively) but not between randomization groups in the women approached (45% vs 44%, pessary and control, respectively). In the 4 years after randomization, many families moved at least once, and their addresses and phone numbers could not be retrieved despite several efforts. Furthermore, due to logistic reasons, one center that initially recruited 30 women did not collaborate in the follow‐up study. We expect that these data will be missing at random and will not contribute to substantial bias. However, women who refused participation (55% of those approached) can have caused an attrition bias. With respect to the baseline characteristics, we found that a higher proportion of highly educated mothers participated in the follow‐up study (69%) compared with the original trial (61%); however, this difference was not significant. This may have influenced the results by giving more favorable ASQ and SDQ scores in this follow‐up sample, as high education of parents is known to be associated with better cognitive and behavioral outcomes.24 Furthermore, mothers participating in follow‐up were more often of European origin compared with women who were lost to follow‐up (96% vs 88%).
Another drawback of the loss to follow‐up in this study was the inability to define the denominator of the rate of developmental problems (whereas the denominator of mortality was known, since we had information about the survival status at 4 years of age of all offspring). Because of the magnitude of this missing information, multiple imputation techniques were considered inadequate. Instead, we could only do exploratory analyses evaluating several scenarios with the data extrapolated to all women randomized in the original ProTWIN trial and to a subgroup of women with a short midtrimester cervical length.
Another limitation is the sole use of questionnaires as screening tool for developmental delay and behavioral problems instead of additional physical developmental tests or behavioral observations. Questionnaires provide less detailed and accurate information but do give information from parents who know their children well and are in addition less time‐consuming for both parents and children. Furthermore, the ASQ and SDQ are widely used in follow‐up studies and validated in several countries, including the Netherlands, with norms based on a Dutch population.
Unfortunately, we cannot draw an affirmative conclusion for the use of a pessary in women with a multiple pregnancy concerning the long‐term outcomes of their children. We experienced a high loss to follow‐up and therefore decided to use an exploratory extrapolating technique to impute missing outcomes.
The original ProTWIN trial showed a reduced short‐term neonatal mortality and morbidity with the use of a cervical pessary in women with a short cervix (<38 mm). The 3‐year follow‐up study of the ProTWIN trial, evaluating Bayley developmental scores in children born to mothers with a short cervical length only, showed no signs of harm caused by the pessary: surviving children had comparable neurodevelopmental outcomes. Translating these results into clinical practice combining the risk of death and an abnormal developmental outcome, six to eight women with a short cervix need to be treated with a cervical pessary to prevent one child from death or survival with neurodevelopmental disability.9 In our 4‐year follow‐up, the NNT in the subgroup with a short cervix is 6 and is therefore consistent with the previously reported NNT.
Unfortunately, long‐term follow‐up is not very common in obstetric studies25 and data of other trials evaluating the long‐term effect of pessary is lacking. Worldwide, more trials on the use of a pessary are in progress (for example, trials registered with numbers NCT02901626, NCT02901626, NCT02901626, NCT02518594, NCT02328989, NTR4414, IRAS ID 156783, NCT02235181, NCT00735137) evaluating not only the use of a pessary but also the use of progestogens and cerclage. Our data can hopefully contribute to future meta‐analysis on long‐term follow‐up of pessary interventions.
5. CONCLUSION
In women with a multiple pregnancy, the use of a cervical pessary did not improve development, behavior or physical outcomes of the surviving children at age 4. Exploratory analysis with simple imputation technique showed promising results for children born to women with a short cervix; however, no conclusions can be drawn from this analysis. A follow‐up study on a randomized trial evaluating the use of pessary in women with a short midtrimester cervix is needed to confirm our results.
CONFLICT OF INTEREST
B.W.M. reports consultancies for ObsEva, Merck and Guerbet. The other authors report no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We like to thank the research nurses of the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynecology for their help in finding contact details of the parents.
Simons NE, van de Beek C, van der Lee JH, et al. Child outcomes after placement of a cervical pessary in women with a multiple pregnancy: A 4‐year follow‐up of the ProTWIN trial. Acta Obstet Gynecol Scand. 2019;98:1292‐1300. 10.1111/aogs.13630
Funding information
The original ProTWIN trial was funded by ZonMW (20031004) and registered at the Netherlands Trial Registry (NTR1858). The follow‐up study was funded by the Academic Medical Center. B.W.M. is supported by a NHMRC Practitioner Fellowship (GNT1082548).
REFERENCES
- 1. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray SRR, Stock SJJ, Cowan S, Cooper ESS, Norman JEE. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynaecol. 2018;20(1):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goya M, De La Calle M, Pratcorona L, et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: a multicenter randomized controlled trial (PECEP‐Twins). Am J Obstet Gynecol. 2016;214(2):145‐152. [DOI] [PubMed] [Google Scholar]
- 4. Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in twin pregnancies with short cervical length: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med. 2017;30(24):2918‐2925. [DOI] [PubMed] [Google Scholar]
- 5. Senekjian EKK, Potkul RKK, Frey K, Herbst ALL. Infertility among daughters either exposed or not exposed to diethylstilbestrol. Am J Obstet Gynecol. 1988;158(3 Pt 1):493‐498. [DOI] [PubMed] [Google Scholar]
- 6. Crowther CAA, Hiller JEE, Haslam RRR, Robinson JSS. Australian Collaborative Trial of Antenatal Thyrotropin‐Releasing Hormone: adverse effects at 12‐month follow‐up. ACTOBAT Study Group. Pediatrics. 1997;99(3):311‐317. [DOI] [PubMed] [Google Scholar]
- 7. Kenyon S, Pike K, Jones DRR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7‐year follow‐up of the ORACLE II trial. Lancet. 2008;372(9646):1319‐1327. [DOI] [PubMed] [Google Scholar]
- 8. Norman JEE, Marlow N, Messow CMM, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double‐blind trial. Lancet. 2016;387(10033):2106‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van‘t Hooft J, van der Lee JH, Opmeer BC, et al. Pessary for preterm birth prevention in twin pregnancy with short cervix: 3‐year follow‐up study. Ultrasound Obstet Gynecol. 2018;51(5):621‐628. [DOI] [PubMed] [Google Scholar]
- 10. Hegeman MA, Bekedam DJJ, Bloemenkamp KWW, et al. Pessaries in multiple pregnancy as a prevention of preterm birth: the ProTwin Trial. BMC Pregnancy Childbirth. 2009;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): a multicentre, open‐label randomised controlled trial. Lancet. 2013;382(9901):1341‐1349. [DOI] [PubMed] [Google Scholar]
- 12. Derks JB. Vandenbussche FPHA. NVOG Meerlingzwangerschap Versie 3.0. 2011:16.
- 13. Squires J, Twombly E, Bricker D, Potter L. Ages and Stages Questionnaires – Third Edition In: ASQ‐3 User's Guide [Internet]. 2009:3‐6. http://agesandstages.com/wp-content/uploads/2015/02/asq3_technical_report.pdf Accessed February 01, 2014. [Google Scholar]
- 14. Theunissen MHC, de Wolff MS, van Grieken A, Mieloo C. Handleiding voor het gebruik van de Strengths and Difficulties Questionnaire binnen de Jeugdgezondheidszorg. Leiden: TNO; 2016. [Google Scholar]
- 15. Kerstjens JMM, Bos AFF, ten Vergert EMJMJ, de Meer G, Butcher PRR, Reijneveld SAA. Support for the global feasibility of the Ages and Stages Questionnaire as developmental screener. Early Hum Dev. 2009;85(7):443‐447. [DOI] [PubMed] [Google Scholar]
- 16. SDQinfo.com . Scoring the Strengths & Difficulties Questionnaire for age 4‐17. 2014;(August):17‐9.
- 17. Van Widenfelt BMM, Goedhart AWW, Treffers PDADA, Goodman R. Dutch version of the Strengths and Difficulties Questionnaire (SDQ). Eur Child Adolesc Psychiatry. 2003;12(6):281‐289. [DOI] [PubMed] [Google Scholar]
- 18. Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? BJOG. 2004;111(3):213‐219. [DOI] [PubMed] [Google Scholar]
- 19. Nicolaides KHH, Syngelaki A, Poon LCC, et al. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: a randomized controlled trial. Am J Obstet Gynecol. 2016;214(1):3.e1‐3.e9. [DOI] [PubMed] [Google Scholar]
- 20. Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open‐label randomised controlled trial. Lancet. 2012;379(9828):1800‐1806. [DOI] [PubMed] [Google Scholar]
- 21. Hui SAA, Chor CMM, Lau TKK, Lao TTT, Leung TYY. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30(4):283‐288. [DOI] [PubMed] [Google Scholar]
- 22. Nicolaides KHH, Syngelaki A, Poon LCC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374(11):1044‐1052. [DOI] [PubMed] [Google Scholar]
- 23. Saccone G, Maruotti GMM, Giudicepietro A, et al. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length a randomized clinical trial. JAMA. 2017;318(23):2317‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cserjesi R, Van Braeckel KNJA, Timmerman M, et al. Patterns of functioning and predictive factors in children born moderately preterm or at term. Dev Med Child Neurol. 2012;54(8):710‐715. [DOI] [PubMed] [Google Scholar]
- 25. Teune MJJ, Van Wassenaer AGG, Malin GLL, et al. Long‐term child follow‐up after large obstetric randomised controlled trials for the evaluation of perinatal interventions: a systematic review of the literature. BJOG. 2013;120(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 26. Potharst ES, van Wassenaer AG, Houtzager BA, van Hus JWP, Last BF, Kok JH. High incidence of multi‐domain disabilities in very preterm children at five years of age. J Pediatr. 2011;159(1):79‐85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


