In a recently published article in the Annals of Neurology, Bakshi and colleagues determined the concentration of the antioxidant urate in serum samples from ~1,500 individuals with or without LRRK2 mutations that were affected or unaffected by Parkinson disease (PD).1 In 3 independent cohorts, they detected significantly lower levels of urate in manifesting (LRRK2+/PD+) compared to nonmanifesting (LRRK2+/PD−) LRRK2 mutation carriers and speculated that the elevated PD risk in the LRRK2+/PD+ group may be due to increased LRRK2 activity,2 which can interfere with urate‐sensitive pathways including Nrf2 antioxidant signaling.1 There is evidence that Nrf2 counteracts mitochondrial damage by triggering the expression of mitochondrial transcription factor A (TFAM).3 TFAM functions as a mitochondrial transcription factor, but the protein is equally involved in mitochondrial DNA (mtDNA) replication and packaging of the mitochondrial genome into nucleoids.4
In search of a penetrance biomarker for LRRK2‐associated PD, we explored the link between mtDNA integrity and disease progression. Employing a high‐throughput multiplex real‐time polymerase chain reaction assay,4 we assessed the levels of mitochondrial major arc deletions in fibroblasts from manifesting (LRRK2+/PD+; n = 10, mean age ± standard deviation [SD] = 66.0 ± 12.5 years) and nonmanifesting carriers (LRRK2+/PD−; n = 21, mean age ± SD = 58.5 ± 15.4 years) of the G2019S mutation and healthy mutation‐negative controls (n = 10, mean age ± SD = 58.3 ± 14.5 years). mtDNA deletion levels were derived from the MT‐ND4:MT‐ND1 ratio. Analysis of variance (ANOVA) testing, regression models, and investigation of sensitivity with receiver operating characteristic (ROC) curves were constructed using statistical analysis software (JMP 14; SAS Institute, Cary, NC). The mean values for MT‐ND4:MT‐ND1 differed significantly between the 3 groups (1‐way ANOVA: F = 13.26, p < 0.0001), with the highest deletion levels in manifesting G2019S mutation carriers (significance levels after Bonferroni correction for multiple testing: controls vs LRRK2+/PD−, p = 0.0047; controls vs LRRK2+/PD+, p < 0.0001; LRRK2+/PD− vs LRRK2+/PD+, p = 0.0355). The levels of somatic mtDNA deletions in LRRK2 mutation carriers were associated with PD status even after adjusting for age in a logistic regression model, where the disease status is the dependent variable and the covariates are the MT‐ND4:MT‐ND1 ratio and age (LRRK2+/PD+ vs LRRK2+/PD−, odds ratio = 2.40, 95% confidence interval = 1.05–5.16, p = 0.014). The sensitivity of the MT‐ND4:MT‐ND1 ratio as a biological marker was assessed using covariant‐adjusted ROC analysis. The resulting areas under the curves (>0.75) indicated good discrimination for all investigated comparisons: (1) controls versus all individuals with LRRK2 G2019S, (2) LRRK2+/PD+ versus all unaffected individuals, and (3) LRRK2+/PD+ versus LRRK2+/PD− (Fig).
Figure 1.
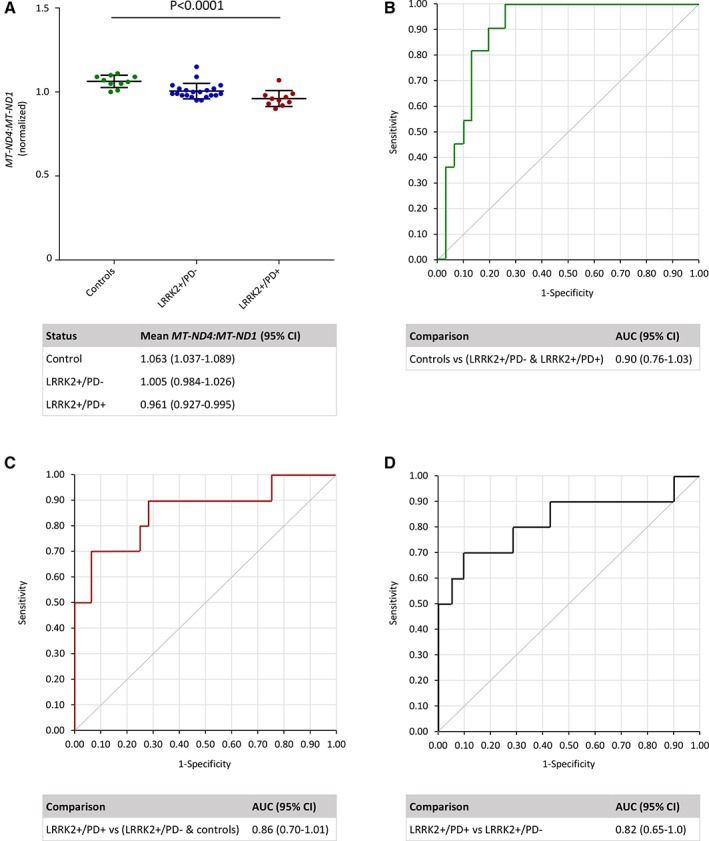
Analysis of mtDNA major arc deletions in controls, and nonmanifesting (LRRK2+/PD−) and manifesting carriers (LRRK2+/PD+) of the LRRK2 G2019S mutation. (A) MT‐ND4:MT‐ND1 ratios differed significantly between the control, LRRK2+/PD−, and LRRK2+/PD+ groups, as determined by 1‐way analysis of variance (p < 0.0001). Dots indicate mean values per person derived from 3 independent experiments. In addition, group mean values with standard deviations and 95% confidence intervals (CIs) are shown. A logistic regression model was used to predict the outcome of Parkinson disease (PD) status in LRRK2 mutation carriers with MT‐ND4:MT‐ND1 ratios, adjusted by age (LRRK2+/PD− vs LRRK2+/PD+, odds ratio = 2.40, 95% CI = 1.05–5.16, p = 0.014). (B–D) Receiver operating characteristic curves. Areas under the curve (AUCs) and 95% CI are given for different group comparisons as indicated in the tables.
Increased levels of reactive oxygen species resulting from reduced urate concentrations in manifesting G2019S mutation carriers may also be the cause of the mtDNA phenotype5 observed in this study. Our finding of increased mtDNA deletions in LRRK2+/PD+ compared to LRRK2+/PD− individuals supports a link between LRRK2 kinase activity,2 urate‐mediated Nrf2 signaling, and oxidative stress in the progression of LRRK2‐associated PD.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
A.G. was supported by grants from the Luxembourg National Research Fund (FNR) in the ATTRACT (Model IPD, FNR9631103) and INTER programs (ProtectMove, FNR11250962). A.G., P.S., I.R.K., and C.K. received funding from the German Research Foundation (DFG) within the framework of the research unit ProtectMove (GR 3731/5‐1, SE 2608/2‐1, KO 2250/7‐1, FOR 2488/1). J.T. is an Alexander von Humboldt fellow and receives funding from the Canadian Institutes of Health Research and the Joachim Herz Foundation. This project was supported by the high‐throughput/high‐content screening platform at the Luxembourg Center for Systems Biomedicine (LCSB).
Correction added on November 25, 2019 after first online publication: the copyright line has been revised.
References
- 1. Bakshi R, Macklin EA, Logan R, et al. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann Neurol 2019;85:593–599. [DOI] [PubMed] [Google Scholar]
- 2. Fraser KB, Moehle MS, Alcalay RN, et al. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology 2016;86:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu KLH, Wu CW, Chao YM, et al. Impaired Nrf2 regulation of mitochondrial biogenesis in rostral ventrolateral medulla on hypertension induced by systemic inflammation. Free Radic Biol Med 2016;97:58–74. [DOI] [PubMed] [Google Scholar]
- 4. Grunewald A, Rygiel KA, Hepplewhite PD, et al. Mitochondrial DNA depletion in respiratory chain‐deficient Parkinson disease neurons. Ann Neurol 2016;79:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zsurka G, Peeva V, Kotlyar A, Kunz WS. Is there still any role for oxidative stress in mitochondrial DNA‐dependent aging? Genes (Basel) 2018;9(4). pii: E175. [DOI] [PMC free article] [PubMed] [Google Scholar]


