Dear Editor, There is a variety of outcome reporting in the clinical research on peripheral vascular malformations,1, 2, 3 including capillary, venous, lymphatic, arteriovenous and combined malformations. Without harmonization of outcome measures, treatments cannot be properly compared. This hampers the development of evidence‐based treatment guidelines, urgently needed for these challenging congenital conditions. The mission of the Outcome measures for VAscular MAlformations (OVAMA) project is to uniform outcome reporting in clinical research.
To evaluate treatment efficacy, the first step is deciding on what to measure. In a previous study, we developed a core domain set (CDS) for peripheral vascular malformations, excluding capillary malformations.4 A CDS is a minimum set of outcome domains that should be measured when evaluating treatment outcomes in health conditions.5 This international consensus project, involving 167 physician and 134 patient/parent contributors, consisted of a three‐round e‐Delphi study and an online consensus meeting. For some domains consensus was not achieved, specifically ‘recurrence’, ‘appearance’, ‘radiological imaging’ and ‘lymphatic fluid leakage’.4 A face‐to‐face consensus meeting was organized to establish the final CDS.
As an addendum to the previous study, this letter describes the conclusions of this face‐to‐face meeting and reports the final CDS for peripheral vascular malformations.
The meeting was chaired by the then coordinator of the OVAMA project, and was held at the International Society for the Study of Vascular Anomalies (ISSVA) conference (28 May to 1 June 2018) in Amsterdam, the Netherlands. All previous study participants (n = 301)4 were invited to join. Participants included 26 experts of the OVAMA Consensus Group; 85% represented various medical specialities (surgery, otolaryngology, paediatrics, paediatric haematology/oncology, radiology and dermatology) and 15% were patient organization representatives.
An overview of the e‐Delphi and online consensus meeting results was sent to all participants beforehand, and printed summaries were provided. The undecided domains were then separately discussed by the whole group and a final consensus reached. In order to reach different results than in the online consensus meeting, group unanimity on including/dropping/changing each outcome domain was required before proceeding to the next. The final CDS is presented in Figure 1.
Figure 1.
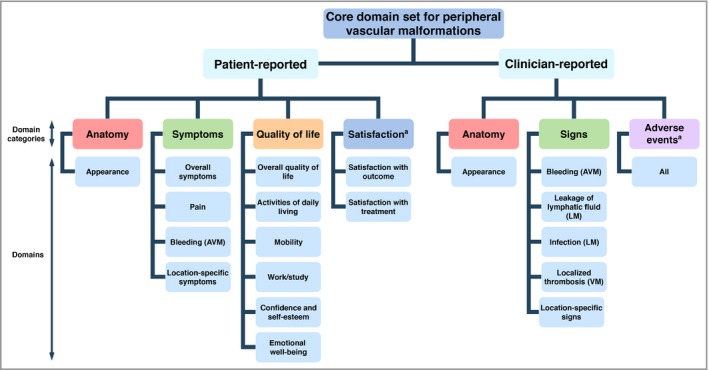
Core domain set for peripheral vascular malformations. Domain categories and domains were based on the classification as reported in Appendix S2 of the previously published core outcome set development study (Horbach SER, van der Horst C, Blei F et al. Development of an international core outcome set for peripheral vascular malformations: the OVAMA project. Br J Dermatol 2018; 178: 473–81). aOnly relevant at follow‐up. AVM, only for arteriovenous malformations, LM, only for lymphatic malformations, VM, only for venous malformations.
The provisionally included domain ‘recurrence’ was excluded from the final CDS, as participants agreed that it was a reflection of other domains rather than a distinct domain.
The domain ‘appearance’, defined as the visible anatomical characteristics of the vascular malformation such as size, colour and texture, was excluded from the e‐Delphi study; however, it was considered essential during the online consensus meeting. Participants noted during the e‐Delphi study that ‘appearance’ may be confused with ‘body image’. As ‘appearance’ often initiates treatment, participants suggested that it should be incorporated in the final CDS. As it was considered relevant from both the patient's and the clinician's perspective, ‘appearance’ was included as a patient‐reported and clinician‐reported core domain.
‘Radiological imaging’ was found to be the instrument by which the radiological characteristics are evaluated, so this domain was changed to ‘radiological characteristics’. Because follow‐up radiological imaging is not routinely performed in all cases, the domain ‘radiological characteristics’ was not considered obligatory, and was hence excluded from the final CDS. However, if radiological imaging is performed before and after treatment, it should be reported.
The group concluded that diagnosing ‘lymphatic fluid leakage’ requires medical knowledge that cannot be expected of patients. Consequently, it was moved from the patient‐reported ‘symptoms’ to the ‘clinician‐reported ‘signs’ in the final CDS.
The general opinion of the group was that the domain categories ‘patient satisfaction’ and ‘adverse events’ should be included in the final CDS, but were only relevant after treatment has started, and therefore should only be measured at follow‐up.
No other domains or discussion points were left unresolved.
With this face‐to‐face consensus meeting, we successfully finalized the CDS for clinical research in peripheral vascular malformations (Fig. 1).
As measurement of these domains does not require invasive or costly techniques, measurement of the relatively high number of domains is still considered feasible. By including many international experts in the field and patients, this process ensured a diversity of perspectives. The face‐to‐face set‐up and information provided beforehand enabled in‐depth discussion and enhanced participant engagement. An unavoidable limitation was that only stakeholders present at the ISSVA conference were able to participate.
This project represents a significant step towards meaningful assessment and comparison of treatments for peripheral vascular malformations. The next step towards uniform outcome reporting is determining how to measure these core domains, i.e. developing a core outcome measurement set. This project, involving an appraisal of available instruments and development of a new disease‐specific instrument, is currently being carried out by the OVAMA Steering Group.
Supporting information
Appendix S1 OVAMA SteeringGroup collaborators.
S.E.R. Horbach was OVAMA project coordinator during this study.
A full list of the OVAMA Steering Group collaborators is provided in Appendix S1 (see Supporting Information).
Funding sources: none.
Conflicts of interest: none to declare.
References
- 1. Horbach SE, Lokhorst MM, Saeed P et al Sclerotherapy for low‐flow vascular malformations of the head and neck: a systematic review of sclerosing agents. J Plast Reconstr Aesthet Surg 2016; 69:295–304. [DOI] [PubMed] [Google Scholar]
- 2. Horbach SE, Rigter IM, Smitt JH et al Intralesional bleomycin injections for vascular malformations: a systematic review and meta‐analysis. Plast Reconstr Surg 2016; 137:244–56. [DOI] [PubMed] [Google Scholar]
- 3. Langbroek GB, Horbach SE, van der Vleuten CJ et al Compression therapy for congenital low‐flow vascular malformations of the extremities: a systematic review. Phlebology 2018; 33:5–13. [DOI] [PubMed] [Google Scholar]
- 4. Horbach SER, van der Horst C, Blei F et al Development of an international core outcome set for peripheral vascular malformations: the OVAMA project. Br J Dermatol 2018; 178:473–81. [DOI] [PubMed] [Google Scholar]
- 5. Boers M, Kirwan JR, Wells G et al Developing core outcome measurement sets for clinical trials: OMERACT filter 2·0. J Clin Epidemiol 2014; 67:745–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 OVAMA SteeringGroup collaborators.


