Abstract
Background
This review is one in a series of Cochrane Reviews of interventions for shoulder disorders.
Objectives
To synthesise the available evidence regarding the benefits and harms of rotator cuff repair with or without subacromial decompression in the treatment of rotator cuff tears of the shoulder.
Search methods
We searched the CENTRAL, MEDLINE, Embase, Clinicaltrials.gov and WHO ICRTP registry unrestricted by date or language until 8 January 2019.
Selection criteria
Randomised controlled trials (RCTs) including adults with full‐thickness rotator cuff tears and assessing the effect of rotator cuff repair compared to placebo, no treatment, or any other treatment were included. As there were no trials comparing surgery with placebo, the primary comparison was rotator cuff repair with or without subacromial decompression versus non‐operative treatment (exercises with or without glucocorticoid injection). Other comparisons were rotator cuff repair and acromioplasty versus rotator cuff repair alone, and rotator cuff repair and subacromial decompression versus subacromial decompression alone. Major outcomes were mean pain, shoulder function, quality of life, participant‐rated global assessment of treatment success, adverse events and serious adverse events. The primary endpoint for this review was one year.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane.
Main results
We included nine trials with 1007 participants. Three trials compared rotator cuff repair with subacromial decompression followed by exercises with exercise alone. These trials included 339 participants with full‐thickness rotator cuff tears diagnosed with magnetic resonance imaging (MRI) or ultrasound examination. One of the three trials also provided up to three glucocorticoid injections in the exercise group. All surgery groups received tendon repair with subacromial decompression and the postoperative exercises were similar to the exercises provided for the non‐operative groups. Five trials (526 participants) compared repair with acromioplasty versus repair alone; and one trial (142 participants) compared repair with subacromial decompression versus subacromial decompression alone.
The mean age of trial participants ranged between 56 and 68 years, and females comprised 29% to 56% of the participants. Symptom duration varied from a mean of 10 months up to 28 months. Two trials excluded tears with traumatic onset of symptoms. One trial defined a minimum duration of symptoms of six months and required a trial of conservative therapy before inclusion. The trials included mainly repairable full‐thickness supraspinatus tears, six trials specifically excluded tears involving the subscapularis tendon.
All trials were at risk of bias for several criteria, most notably due to lack of participant and personnel blinding, but also for other reasons such as unclearly reported methods of random sequence generation or allocation concealment (six trials), incomplete outcome data (three trials), selective reporting (six trials), and other biases (six trials).
Our main comparison was rotator cuff repair with or without subacromial decompression versus non‐operative treatment. We identified three trials for this comparison, that compared rotator cuff repair with subacromial decompression followed by exercises with exercise alone with or without glucocorticoid injections, and results are reported here for the 12 month follow up.
At one year, moderate‐certainty evidence (downgraded for bias) from 3 trials with 258 participants indicates that surgery probably provides little or no improvement in pain; mean pain (range 0 to 10, higher scores indicate more pain) was 1.6 points with non‐operative treatment and 0.87 points better (0.43 better to 1.30 better) with surgery. Mean function (zero to 100, higher score indicating better outcome) was 72 points with non‐operative treatment and 6 points better (2.43 better to 9.54 better) with surgery (3 trials; 269 participants), low‐certainty evidence (downgraded for bias and imprecision). Participant‐rated global success rate was 48/55 after non‐operative treatment and 52/55 after surgery corresponding to risk ratio (RR) 1.08, 95% confidence interval (CI) 0.96 to 1.22; low‐certainty evidence (downgraded for bias and imprecision). Health‐related quality of life was 57.5 points (SF‐36 mental component score, 0 to 100, higher score indicating better quality of life) with non‐operative treatment and 1.3 points worse (4.5 worse to 1.9 better) with surgery (1 trial; 103 participants), low‐certainty evidence (downgraded for bias and imprecision).
We were unable to estimate the risk of adverse events and serious adverse events as only one event was reported across the trials (very low‐certainty evidence; downgraded once due to bias and twice due to very serious imprecision).
Authors' conclusions
At the moment, we are uncertain whether rotator cuff repair surgery provides clinically meaningful benefits to people with symptomatic tears; it may provide little or no clinically important benefits with respect to pain, function, overall quality of life or participant‐rated global assessment of treatment success when compared with non‐operative treatment. Surgery may not improve shoulder pain or function compared with exercises, with or without glucocorticoid injections.
The trials included have methodology concerns and none included a placebo control. They included participants with mostly small degenerative tears involving the supraspinatus tendon and the conclusions of this review may not be applicable to traumatic tears, large tears involving the subscapularis tendon or young people. Furthermore, the trials did not assess if surgery could prevent arthritic changes in long‐term follow‐up. Further well‐designed trials in this area that include a placebo‐surgery control group and long follow‐up are needed to further increase certainty about the effects of surgery for rotator cuff tears.
Plain language summary
Does repair of torn rotator cuff tendons work?
Review question
To assess the effect of surgical repair of rotator cuff tendons on shoulder pain, function and other outcomes in adults with full‐thickness rotator cuff tears compared with non‐surgical management.
Background
The rotator cuff is a group of tendons that move the shoulder joint. Some people have pain in their shoulder related to wear and tear of the rotator cuff tendons. The weakening of the tendon is thought to be caused by aging and mechanical wear. Eventually, the process may result in a tear of the tendons.
Rotator cuff tears can cause pain and impair arm function but asymptomatic tears also occur. For people with symptomatic tears, non‐operative management including pain medicines (simple analgesia and anti‐inflammatories), glucocorticoid injections and physical therapies do not always result in satisfactory outcomes.
Surgery is usually considered when other treatments fail. Surgery includes removing part of the bone to broaden the tendon passage (subacromial decompression) and repair of the torn tendons. Sometimes the surgeons cannot repair the tendon due to the size of the tear or degeneration of the muscle, and in these cases only subacromial decompression may be performed. Most rotator cuff surgery is now performed arthroscopically (surgical instruments are inserted through small key holes to perform surgery) or through small incisions (mini‐open approach).
Study characteristics
This Cochrane Review is current to January 2019. We found nine trials with 1007 participants. Participants mean age was 56 to 68 years, and females comprised 29% to 56% of the participants. The participants had symptoms for several months or years and were diagnosed with a full‐thickness tear with magnetic resonance imaging or ultrasound examination. Studies were conducted in Finland, Norway, Canada, USA, France, the Netherlands, Italy and South Korea. Our primary analysis included three trials with 339 participants who received either surgery (tendon repair and removal of bone from undersurface of acromion) or non‐operative therapy (exercises with or without glucocorticoid injection). Three studies received funding however none of them reported using the funds directly for these trials.
Key results
Compared with non‐operative treatment, surgery resulted in little or no benefit in people with rotator cuff tears for up to one year.
Pain (lower scores mean less pain) Improved by 9% (4% better to 13% better) or 0.9 points on a zero to 10 scale • People who had non‐operative treatment rated their pain as 1.6 points • People who had surgery rated their pain as 0.7 points. Function (0 to 100; higher scores mean better function)Improved by 6% (2% better to 10% better) or 6 points on a zero to 100 scale • People who had non‐operative treatment scored 72 points • People who had surgery scored 78 points
Participant‐rate global treatment success (participants satisfied with the outcome) 7% more people rated their treatment a success (4% fewer to 13% more), or seven more people out of 100. • 48/55 (873/1000) of people considered treatment as successful with non‐operative treatment • 51/54 (943/1000) of people considered treatment as successful with surgery
Overall quality of life (higher scores mean better quality of life)Worsened 1% (4% worse to 2% better) or 1.3 points on a zero to 100 scale • People who had non‐operative treatment rated their quality of life 58 • People who had surgery (subacromial decompression) rated their quality of life 57
Adverse events • One adverse event (frozen shoulder) was reported in the trials in exercise group. Thus, we are unable to estimate comparative risk.
Serious adverse events • No serious adverse events were reported in the trials.
Quality of the evidence
As compared with non‐operative treatment, moderate‐certainty evidence (downgraded due to risk of bias) indicates that surgery (rotator cuff repair with or without subacromial decompression) probably provides little or no benefit in pain and low‐certainty evidence indicates that it may provide little or no improvement in function, participant‐rated global treatment success or overall quality of life (downgraded due to bias and imprecision) in people with rotator cuff tears. Due to only one reported adverse event across the trials, we cannot estimate if there is higher risk for adverse events after either treatment (very low‐certainty evidence).
Summary of findings
Summary of findings for the main comparison. Surgery compared to non‐operative treatment for people with full thickness rotator cuff tears.
| Surgery compared to non‐operative treatment for people with full thickness rotator cuff tears | ||||||
| Patient or population: people with full‐thickness rotator cuff tears Setting: hospital Intervention: subacromial decompression and rotator cuff repair Comparison: non‐operative treatment (exercises with or without glucocorticoid injection) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐operative treatment | Risk with surgery | |||||
| Pain (VAS) from 0 to 10, 0 is no pain follow‐up 12 months | The mean pain was 1.61 | MD 0.87 better (0.43 better to 1.30 better) | ‐ | 258 (3 RCTs) | ⊕⊕⊕⊝ Moderate2 | Surgery provides probably little or no benefit; absolute difference 9% better (4% better to 13% better)3; relative difference 16% better (8% better to 25% better)4 |
| Functional outcome (Constant score) from 0 to 100, 100 is best) follow‐up 12 months | The mean function was 72 points1 | MD 5.98 better (2.43 better to 9.54 better) | ‐ | 269 (3 RCTs) | ⊕⊕⊝⊝ Low2,5 | Surgery may have little or no effect; absolute difference 6% better (2% better to 10% better)3; relative difference 16% better (6% better to 25% better)3 |
| Participant‐rated global assessment of treatment success at 12 months | 873 per 1,000 | 943 per 1,000 (838 to 1,000) | RR 1.08 (0.96 to 1.22) | 110 (1 RCT) | ⊕⊕⊝⊝ Low2,5 | Number of participants reporting success may not differ; absolute difference 7% better (4% worse to 13% better); relative difference 8% better (4% worse to 22% better)4 |
| Health‐related quality of life (SF‐36 mental component) from 0‐100; 100 is best) follow‐up 12 months |
The mean health‐related quality of life was 57.5 points1 | MD 1.39 worse (4.49 worse to 1.89 better) | ‐ | 103 (1 RCT) | ⊕⊕⊕⊝ Low2,5 | Surgery may have little or no effect; absolute difference 1% worse (4% worse to 2% better)3; relative difference 2% worse (8% worse to 3% better)3 |
| Adverse events | One frozen shoulder | No events | No reliable estimate | 103 (1 RCT) | ⊕⊝⊝⊝ Very low2,5,6 | We are uncertain about the risks of adverse events |
| Serious adverse events | No events | No events | No reliable estimate | ⊕⊝⊝⊝ Very low2,5,6 | We are uncertain about the risks of serious adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited, The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Median value in the exercise groups at one year.
2 Downgraded one level due to risk of bias, due to potential for performance and detection biases.
3 We assumed the clinically important improvement was 1.5 points or 15% absolute improvement (Hao 2018) for pain; 8.3 points or 8% absolute improvement (Hao 2018) for function; and 10 points or 10% absolute improvement for health‐related quality of life.
4 Relative difference calculated relative to baseline in control group (i.e. absolute change (mean difference) divided by mean at baseline in the non‐operative group from Moosmayer 2010 (values were: 5.3 points on 0 to 10 point VAS pain; 38.4 points on 0 to 100 point Constant score and 57.3 in 0 to 100 point SF‐36 mental component score), and expressed as percentage for continuous outcomes. Relative difference calculated as 1‐RR and expressed as percentage for dichotomous outcomes.
5 Downgraded one level due to imprecision, as 95% CI included both a clinically important effect and a clinically unimportant effect, or had low event rates.
6 Downgraded again due to very serious imprecision (no events were reported and we are unable to estimate the risk)
Background
This Cochrane Review is one of an updated series of Cochrane Reviews of interventions for shoulder disorders. The original review on all interventions for shoulder pain (Green 1998) has been split into a series of reviews that examine interventions for different shoulder disorders separately. The last Cochrane Review on surgery for rotator cuff disease was published in 2008, Issue 1(assessed as up to date to 3 September 2006) (Coghlan 2008). For this update, we have split the surgery for rotator cuff disease review into three reviews: 1) surgical repair for full thickness rotator cuff tears (the topic of this review); 2) subacromial decompression surgery for rotator cuff disease (Karjalainen 2019); and 3) surgery for calcific rotator cuff tendinitis.
Estimates of the lifetime and monthly prevalence of shoulder pain in the general population varies between 6.7% and 66.7% and 18% to 31%, respectively (Luime 2004). Shoulder pain is the third most common musculoskeletal complaint presenting to primary care (Rekola 1993). The direct annual healthcare expenses attributable to shoulder disorders was estimated to be $7 billion in the USA in 2000 (Johnson 2004). Rotator cuff disorders are the most common underlying cause, with estimates varying between 65% and 85% depending upon the setting and age of the study population (Chard 1991; Östor 2005; Vecchio 1995).
Rotator cuff repairs (with or without subacromial decompression) are increasingly performed for rotator cuff disorders; for example, a UK study reported a ten‐fold increase in people undergoing rotator cuff repair and subacromial decompression between 2004 and 2010 (age‐adjusted incidence from 1.4/100,000 people to 13.7/100,000 people) (Judge 2014).
Description of the condition
Rotator cuff disease is one of the most common causes of shoulder pain and its incidence is expected to grow as the population ages (Gomoll 2004). A wide range of conditions are included under the umbrella term of rotator cuff disease, including rotator cuff tendinopathy, subacromial bursal pathology, and partial‐thickness or full‐thickness rotator cuff tears.
Patients with symptomatic rotator cuff tears present with shoulder pain, loss of strength, or limitation, or both in active range of motion. The pain typically interferes with sleep. Small tears may limit the function only, due to pain but large tears result in imbalance in the joint kinematics and thus limit the reach and strength of the hand (Greenspoon 2015; Yamaguchi 2000).
Data on the utility of patient history and physical examination in the diagnosis of a rotator cuff tear are limited (Hermans 2013; Jain 2013). Imaging modalities such as ultrasound and magnetic resonance imaging (MRI) have high sensitivity and specificity in the structural diagnosis of full‐thickness rotator cuff tears (Dinnes 2003). Based upon a Cochrane Diagnostic Review, both tests are equally accurate for detection of full‐thickness tears in people with shoulder pain for whom surgery is being considered (Lenza 2013). Both MRI and ultrasound may have poor sensitivity for detecting partial‐thickness tears, and the sensitivity of ultrasound may be much lower than that of MRI. However the strength of evidence for all test comparisons was limited because most studies were small, heterogeneous and methodologically flawed, and there were few comparative studies.
Although rotator cuff tear may occur in young people with trauma (e.g. acute shoulder dislocation), typically tears present in middle‐aged or elderly people and cannot always be attributed to a precipitating event or trauma (Gombera 2014; Reilly 2006). Instead, many findings suggest that rotator cuff tears are a result of several biological and mechanical factors. Histological studies show vascular, cellular and tendon matrix changes typical of degenerative tendon disorders (Hegedus 2010); increasing age, high body mass index (BMI), hypertension and smoking are risk factors (Sayampanathan 2017); and genetic and familial factors have been found to predispose to the condition (Dabija 2017). The tears typically occur at the supraspinatus insertion, which comes into contact with the acromion in shoulder flexion (Neer 1983), supporting a mechanical aetiology; and the anatomical type (hook shaped) of the acromion has also been found to predict presence of tear (Morelli 2019).
The evidence of an association between pathology and pain is conflicting. People with tears suffer from pain more often compared with people without tears (Yamaguchi 2006); symptomatic tears are bigger compared with asymptomatic tears and progression in size of the tear predicts increased symptoms (Mall 2010; Moosmayer 2009; Yamaguchi 2006); and asymptomatic tears become symptomatic in follow‐up in 50% of the cases (Mall 2010; Moosmayer 2009; Yamaguchi 2006). However, several findings suggest that the tear itself cannot explain the symptoms alone. The prevalence of asymptomatic abnormalities is high and increases with age (e.g. 4% to 7% in people aged under 50 and up to 56% in people aged 80 and older) (Teunis 2014); more than half of the patients remain asymptomatic, often people who also show no progression in the tear diameter (Mall 2010; Moosmayer 2013); and the severity of the tear does not correlate with symptom severity (Curry 2015; Dunn 2014).
Intensity of pain is associated with gender (higher risk in women); fatty degeneration of the muscle; presence of inflammation and hyperplasia of the tendon; and inflammation, necrosis, hypertropia, oedema, or high concentration of substance P in the subacromial bursa (Chillemi 2016; Gotoh 1998). Furthermore, a large tear may cause imbalance in the forces moving the shoulder joint, which may further aggravate the pathology and symptoms (Nam 2012; Yamaguchi 2000). Once a full‐thickness tear develops, it usually does not heal spontaneously (Yamaguchi 2001). Large tears start to affect the strength of the arm and eventually may result in the development of painful osteoarthritis (Rugg 2018), but we have no experimental data that repair would prevent arthritic changes.
Description of the intervention
Surgery for rotator cuff tears is increasingly performed mini‐invasively, either through a small incision or arthroscopically. Often, the bursa is removed first, followed by removal of bone from the anteroinferior surface of acromion and release of the acromioclavicular ligament. The torn tendon is re‐inserted into its normal attachment in the humerus using sutures and bone anchors. Less invasive surgery may in theory result in less morbidity and shorter recovery time enabling earlier return to work or sport compared with open procedures (Hata 2001). The evidence from systematic reviews suggest that the final outcomes are comparable between mini‐open and arthroscopic surgery (Huang 2016; Ji 2015).
Subacromial decompression (removal of the bursa and bone from the undersurface of the acromion) is often performed in conjunction with the tendon repair on the premise that it removes any impingement on the repaired tendon and thus improves the outcomes of the repair.
Large tears in the older population have inferior healing capacity (Mall 2014, Nho 2007), and it has been suggested that these tears could be treated by performing subacromial decompression only (Kempf 1999). Partial tears may be debrided or repaired directly or by first completing the tear (Franceschi 2011).
Patients typically wear a sling for three to six weeks after surgery and undergo postoperative rehabilitation for up to six months after the repair (Hertling 1990; Millett 2006; van der Meijden 2012). The principles of postoperative physical therapy are comparable to those for physical therapy alone except that the regimen is usually adjusted due to postoperative pain and to protect the integrity of repair in the early postoperative period.
A previous systematic review found evidence that increasing age, larger tear size, and additional biceps tendon procedure or acromioclavicular resection have a negative influence on cuff integrity at follow‐up, while being on workers' compensation has a negative influence on functional outcome after surgery (Lambers Heerspink 2014). Greater rotator cuff muscle fatty degeneration also correlates with poor functional outcome and higher repair failure rate because severely degenerated muscles may not function even if the tendon is repaired (Chaudhury 2012).
Potential risks of surgery include complications related to the surgery or anaesthesia such as pulmonary embolism, surgical site infection, postoperative adhesive capsulitis (or frozen shoulder), injury to peripheral nerve, chronic ongoing pain, and failed rotator cuff repair (re‐tear) (Hill 2017; Shields 2015).
Non‐operative treatment includes physical therapies such as muscle strengthening, scapular stabilisation, and stretching and flexibility exercises (Bennell 2007; Hertling 1990; Kuhn 2009; Misamore 1995; Page 2016a), glucocorticoid injection, non‐steroidal anti‐inflammatory drugs (NSAIDs), acupuncture, iontophoresis, phonophoresis, transcutaneous electrical nerve stimulation (TENS), pulsed electromagnetic field (PEMF), topical glyceral trinitrate and ultrasound (Buchbinder 2003; Buchbinder 2011; Cumpston 2009; Engebretsen 2009; Gialanella 2011; Green 2005; Page 2016b; Pedowitz 2012). The benefits of many of these treatments have not been established in high‐quality randomised placebo‐controlled trials.
How the intervention might work
The mechanistic theory suggests that the tear is caused by repetitive compressive and shearing forces subjected to the tendon. Subacromial decompression aims to remove the bursa (which may or may not be inflamed) and bone from the anterior/lateral undersurface of the acromion to reduce compressive forces on the rotator cuff, which is assumed to halt the pathological process. The repair of the tendon is believed to restore normal kinematics of the joint thus improving function of the shoulder. It is also believed that restoring normal tendon function may prevent the progression of arthritic changes in the shoulder joint.
Why it is important to do this review
Rotator cuff disease has substantial economic and quality of life implications for the patient and healthcare systems. Surgery, performed increasingly, exposes participants to risks, while in the absence of placebo‐controlled trials, the benefits are unclear and improvements can also occur in the absence of surgery .
Our 2008 Cochrane review identified 14 randomised controlled trials (RCTs) involving 829 participants (Coghlan 2008). Two trials included participants with rotator cuff tear and none of them compared surgery with placebo‐surgery, non‐surgical treatment, or no treatment. Since then, additional RCTs assessing the benefits and harms of surgery for rotator cuff tears when compared with exercise therapy have been published (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010). Therefore an updated review of the available evidence is timely.
Objectives
To synthesise the available evidence regarding the benefits and harms of rotator cuff repair with or without subacromial decompression in the treatment of rotator cuff tears of the shoulder.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that were described as randomised controlled trials (RCTs) and planned to include trials using quasi‐randomised methods of participant allocation, with no language or publication status restrictions.
Types of participants
We included trials that enrolled adults (as defined in the trials) with rotator cuff tears, confirmed by clinical history, physical examination, and imaging (magnetic resonance imaging (MRI), ultrasound or arthrogram). Trials including participants with impingement without any tears of the tendon were excluded unless participants with an intact rotator cuff were in a minority (defined as < 20%). Studies of adults undergoing surgery for benign or malignant tumours, adhesive capsulitis, shoulder instability, joint replacement or fractures were excluded.
Types of interventions
Rotator cuff repair, with or without subacromial decompression (open or arthroscopic bursectomy or acromioplasty, or both) or debridement of tear versus placebo, non‐operative treatment, or no treatment were included. For this update as the benefit of surgical repair over placebo, or non‐surgical treatment is not yet established, we excluded studies comparing one type of repair technique to another. Studies only assessing different surgical devices (such as comparing two types of suture materials or techniques) or biologics were also excluded unless they were compared with placebo‐surgery or non‐surgical treatment.
Comparisons could include the following.
Rotator cuff repair with or without subacromial decompression or debridement versus placebo surgery
Rotator cuff repair with or without subacromial decompression or debridement versus non‐operative treatment including physical therapy, exercises, pharmacologic interventions such as NSAIDs or glucocorticoid or other injections, or combinations of these.
Rotator cuff repair with or without subacromial decompression or debridement versus 'wait and see' or no or delayed treatment
Rotator cuff repair with acromioplasty versus rotator cuff repair alone. In this comparison both groups had subacromial bursectomy (which is a usual component of subacromial decompression) while one group also received an acromioplasty.
Rotator cuff repair with subacromial decompression versus subacromial decompression alone.
Types of outcome measures
We ensured that the outcomes in our review were consistent with The Outcome Measures in Rheumatology (OMERACT) draft core domain set for clinical trials of shoulder disorders (Buchbinder 2017).
Major outcomes
We included the following outcomes.
Overall pain (mean or mean change measured by visual analogue scale (VAS), numeric or categorical rating scale). If trials did not measure overall pain, we planned to include other pain measures highest on the following hierarchy: unspecified pain, pain with activity, night or rest pain.
Physical function. Where trialists reported outcome data for more than one function scale, we extracted data on the scale that was highest on the following pre‐defined list: 1) Constant Murley Score ; 2) Shoulder Pain and Disability Index (SPADI); 3) Oxford Shoulder Score (OSS); 4) American Shoulder and Elbow Surgeons Standardized Form (ASES‐SF; 5) the University of California at Los Angeles (UCLA) Shoulder Score; 6) Disabilities of the Arm, Shoulder and Hand (DASH); 7) Shoulder Disability Questionnaire (SDQ); 8) any other shoulder function scale. These questionnaires generally include several domains such as pain, function, range of motion and strength, and provide a shoulder‐specific composite score. Our hierarchy was based upon the most commonly used scores used in trials assessing surgery, given that there is a paucity of research to inform us which measure is the gold standard (Page 2015).
Participant‐rated global assessment of treatment success as defined by the trialists (e.g. proportion of participants with significant overall improvement).
Health‐related quality of life (HRQoL) measured by generic tools (such as mental component score of the Short Form‐36 (SF‐36) or SF‐12 or the EQ‐5D, 15D) or disease‐specific quality of life tools.
Number of participants experiencing adverse events (including, infections, postoperative shoulder stiffness or adhesive capsulitis (frozen shoulder) or adverse events as defined by the authors of included trials.
Number of participants experiencing a serious adverse event. We defined serious harms as death, bleeding (uncontrolled or requiring transfusion), cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, cerebrovascular accident, acute renal failure, unplanned intubation, ventilator > 48 hours, deep infection (surgical site or organ/space), sepsis, septic shock, wound dehiscence, pulmonary embolism, deep vein thrombosis, peripheral nerve injury.
Minor outcomes
Participation (recreation and work).
Treatment failure (Incidence of full‐thickness tear at follow‐up). For the surgery versus non‐operative treatment, we report the incidences for repair group only as we could not identify comparable measures of treatment failure in the non‐operative groups. Cross‐overs were not compared as cross‐over could only occur in non‐operative groups.
Timing of outcome assessment
We extracted outcome measures at the following time points.
Up to six months.
Up to 12 months.
Two years or more, up to five years.
We extracted the latest time point within the time frame if there were multiple time points at which outcomes were measured (i.e. if a study reported outcomes at six weeks and four months and 12 months, we extracted outcomes at four months (to six‐month analysis), and 12 months. The primary time point was 12 months.
Search methods for identification of studies
Electronic searches
This current review update includes studies published between March 2006 and 9th January 2019. We searched the following databases for randomised or quasi‐randomised trials.
OVID MEDLINE, 2006 to 9th January, 2019 Appendix 1;
OVID EMBASE, 2006 to 9th January, 2019 Appendix 2;
Cochrane Controlled Trials Register (via Cochrane Library) to 9th January, 2019 Appendix 3;
Clinicaltrials.gov, for ongoing trials to 11th February, 2019 Appendix 4.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/) for ongoing trials to 11th February, 2019 Appendix 5.
Searching other resources
We also reviewed the reference lists of the included trials and any relevant review articles retrieved from the electronic searches, to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
Four review authors in pairs (TK, JH, NBJ and CP) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups:
possibly relevant ‐ trials that met the inclusion criteria and trials from which it was not possible to determine whether they met the criteria either from their title or abstract;
excluded ‐ those clearly not meeting the inclusion criteria.
If a title or abstract suggested that the trial was eligible for inclusion,or we could not tell, we obtained a full‐text version of the article and four review authors (TK, JH, NBJ and CP) in pairs independently assessed it to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a third author (RB).
Data extraction and management
Four review authors working in pairs (TK, JH, NBJ, CP) independently extracted the following data from the included trials.
Trial characteristics, including design, country, sample size calculation, primary analysis, source of funding, and trial registration status (with registration number recorded if available).
Number of participants, inclusion/exclusion criteria, participant characteristics, including age, sex, duration of symptoms, outcomes at baseline and details regarding the cuff tear if present.
Intervention characteristics for each treatment group, and use of co‐interventions.
Outcomes reported, including the measurement instrument used and timing of outcome assessment.
When additional data were required, we contacted the trial authors to obtain this. Where data were imputed or calculated (e.g. standard deviations calculated from standard errors, P values, confidence intervals, imputed from graphs, from standard deviations in other trials), we reported this in the Characteristics of included studies table, notes. Any disagreements and issues were resolved by consultation with RB.
To prevent selective inclusion of data based on the results, we used the following a priori defined decision rules to select data from trials.
Where trialists reported both final values and change from baseline values for the same outcome, we extracted final values.
Where trialists reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values.
Where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
We used a priori hierarchies (see Types of outcome measures) to choose the outcome for each domain if the trial measured one outcome with several instruments.
When trialists had used different scales, we transformed the scales to match the most commonly used instrument scale before pooling (and reversed the scale if needed to make it comparable to the most commonly used instrument).
Assessment of risk of bias in included studies
Two review authors (TK, RJ) assessed the risk of bias of each included trial and resolved any disagreements by consensus, or consultation with RB where necessary.
We assessed the following methodological domains, as recommended by Cochrane (Higgins 2011):
sequence generation;
allocation sequence concealment;
blinding of participants and study personnel;
blinding of outcome assessment (assessed separately for self reported and objectively assessed outcomes);
incomplete outcome data;
selective outcome reporting;
other potential source of bias: in this bias we judged whether the number of cross‐overs from placebo or from exercise therapy to surgery might bias the analysis
Each item was rated as being at “Low risk”, “Unclear risk” or “High risk” of bias. We resolved any discrepancies through discussion or adjudication by a third review author (RB).
Measures of treatment effect
We used the Cochrane software, Review Manager 5.3 to perform data analysis. For dichotomous outcomes, we expressed the difference as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we expressed results as mean differences (MD) with 95% CIs when the same measurement tool was used across studies.
Where different measures were used for same outcome or concept, we planned to use the most common outcome measure as an index outcome measure and use standardised mean difference (SMD) as the summary estimate. To facilitate interpretation, we planned to back‐transform SMDs to a typical scale (e.g. 0 to 100 for function) by multiplying the SMD by a typical among‐person standard deviation (e.g. the standard deviation (SD) of the control group at baseline from the most representative trial; as per Chapter 12 of theCochrane Handbook).
We performed back‐translation in Analysis 2.2 (repair with acromioplasty versus repair alone; functional outcome) using SD of 17.1 from Abrams 2014, and in (Analysis 3.1) (repair with subacromial decompression versus decompression alone; pain) using SD of 2 from Kukkonen 2014.
2.2. Analysis.
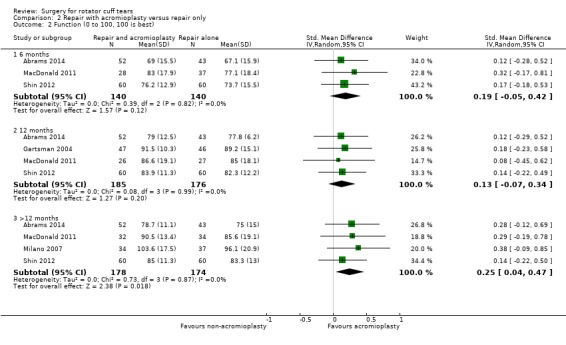
Comparison 2 Repair with acromioplasty versus repair only, Outcome 2 Function (0 to 100, 100 is best).
3.1. Analysis.
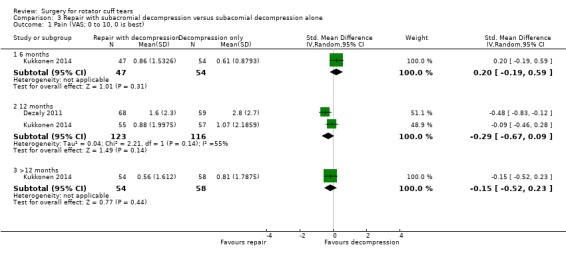
Comparison 3 Repair with subacromial decompression versus subacomial decompression alone, Outcome 1 Pain (VAS; 0 to 10, 0 is best).
In the Comments column of the 'Summary of findings' table, we reported the absolute percent difference, the relative per cent change from baseline, and for outcomes that show a clinically important difference between treatment groups, we reported the number needed‐to‐treat for an additional beneficial outcome (NNTB), or number needed‐to‐treat for an additional harmful outcome (NNTH). For dichotomous outcomes, we planned to calculate the NNTB or NNTH from the control group event rate and the relative risk using the Visual Rx NNT calculator (Cates 2008). As there were no clinically important differences in the analyses, we did not calculate the NNTB for dichotomous measures. For dichotomous outcomes, the absolute difference was calculated from the difference in the risks between the intervention and control group using GRADEpro (GRADEpro GDT 2015) and expressed as a percentage. The relative per cent change was calculated as the Risk Ratio‐1 and expressed as a percentage.
For continuous outcomes, we calculated absolute per cent difference by dividing the MD by the scale of the measure, and expressed as percentage. The relative difference was calculated as the absolute benefit (MD) divided by the baseline mean of the control group, and expressed as a percentage.
Unit of analysis issues
Where multiple trial arms are reported in a single trial, we included only the relevant arms, but reported that there were multiple trial arms in the 'Characteristics of included studies' table. For studies containing more than two intervention groups, making multiple pair‐wise comparisons between all possible pairs of intervention groups possible, we included the same group of participants only once in the meta‐analysis.
If we had identified cross‐over trials, we planned to extract data from the first phase of the trial to avoid potential carry‐over effects. If we had identified cluster‐randomised trials that did not adjust for potential unit of analysis issues, we would note this and assess the effect of including studies with potential unit of analysis issues in a sensitivity analysis,
The unit of analysis was the participant for all trials.
Dealing with missing data
When required, we contacted trial authors to obtain data that were missing from the trial reports. For continuous outcomes (pain and disability), we calculated the weight of the trial using the number of patients analysed at that time point. If the number of patients analysed was not presented for each time point, we used the number of randomised patients in each group at baseline. For dichotomous outcomes, we used the final data for the events reported in each trial.
For continuous outcomes with no SD reported, we calculated SDs from standard errors (SEs), 95% confidence intervals (CIs) or P values. We planned to impute SDs when we could not obtain any measurement of variance from the trial reports or by contacting the authors. Where data were imputed or calculated (e.g. SDs calculated from SEs, 95% CIs or P values, or imputed from graphs or from SDs in other trials), we reported this in the Characteristics of included studies table.
Assessment of heterogeneity
We assessed clinical diversity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the I2 statistic. We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess small‐study effects, we planned to generate funnel plots for meta‐analyses including at least 10 trials of varying size. If asymmetry in the funnel plot was detected, we planned to review the characteristics of the trials to assess whether the asymmetry was likely due to publication bias or other factors such as methodological or clinical heterogeneity of the trials (Sterne 2011).
To assess outcome reporting bias, we compared the outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications; if trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial publications (Dwan 2011; Kirkham 2010).
Data synthesis
When we compared similar interventions, we pooled outcomes using the random‐effects model as a default based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analysis (including pain and function).
For repair versus non‐operative treatment comparison:
with and without subacromial decompression.
For repair with acromioplasty versus repair without acromioplasty comparison:
type of acromion (I, II and III).
Sensitivity analysis
We planned the following sensitivity analyses for primary comparison:
removing trials with potential for selection and detection biases;
removing trials including with traumatic onset of symptoms.
We performed the sensitivity analyses for the outcomes of pain and function at primary time point (12 months).
We also planned a sensitivity analysis to assess the impact of including studies with imputed SDs for the outcomes of pain and function.
GRADE and 'Summary of findings' tables
We presented the six major outcomes (pain, function, global assessment of success, health‐related quality of life, adverse events, serious adverse events) of the review in 'Summary of findings' tables which summarise the certainty of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes as recommended by Cochrane (Schünemann 2011a). The 'Summary of findings' table includes an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 2011b).
We planned one 'Summary of findings' table (surgery versus non‐operative treatment).
Two review authors (TK and RJ) assessed the certainty of the evidence as high, moderate, low, or very low using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence which contribute data to the meta‐analyses for the prespecified outcomes (Schünemann 2011b). We used GRADEpro software to prepare the SoF tables (GRADEpro GDT 2015). Decisions to downgrade the certainty of evidence are justified in the footnotes.
We used the following minimal important difference (MID) values when interpreting the importance of differences between the groups: pain (visual analogue scale (VAS) or numeric rating scale (NRS); 0 to 10): 1.5 points (Hao 2018; Tashjian 2009); function (Constant score; 0 to 100) 8.3 points (Hao 2018); American Shoulder and Elbow Surgeons Shoulder (ASES) score (0 to 100): 21.9 points (Gagnier 2018).
Results
Description of studies
Results of the search
Only one (Gartsman 2004) of the 14 trials included in the previous Cochrane Review (Coghlan 2008) met the inclusion criteria for this updated review due to the restriction in scope that occurred as a result of splitting the original review. Ten trials from the earlier review were not eligible for this update because they compared one type of surgery with another, and three trials were excluded because they compared surgery with exercise therapy in people without full‐thickness rotator cuff tears (Coghlan 2008).
The results of the updated search are shown in Figure 1. The updated search returned 3862 records. After removing duplicates and screening titles and abstracts for eligibility, we retrieved 24 unique studies. From these, we included eight new RCTs (Abrams 2014; Dezaly 2011; Kukkonen 2014; Lambers Heerspink 2015; MacDonald 2011; Milano 2007; Moosmayer 2010; Shin 2012), as well as retaining the one study from the old review (Gartsman 2004).
1.
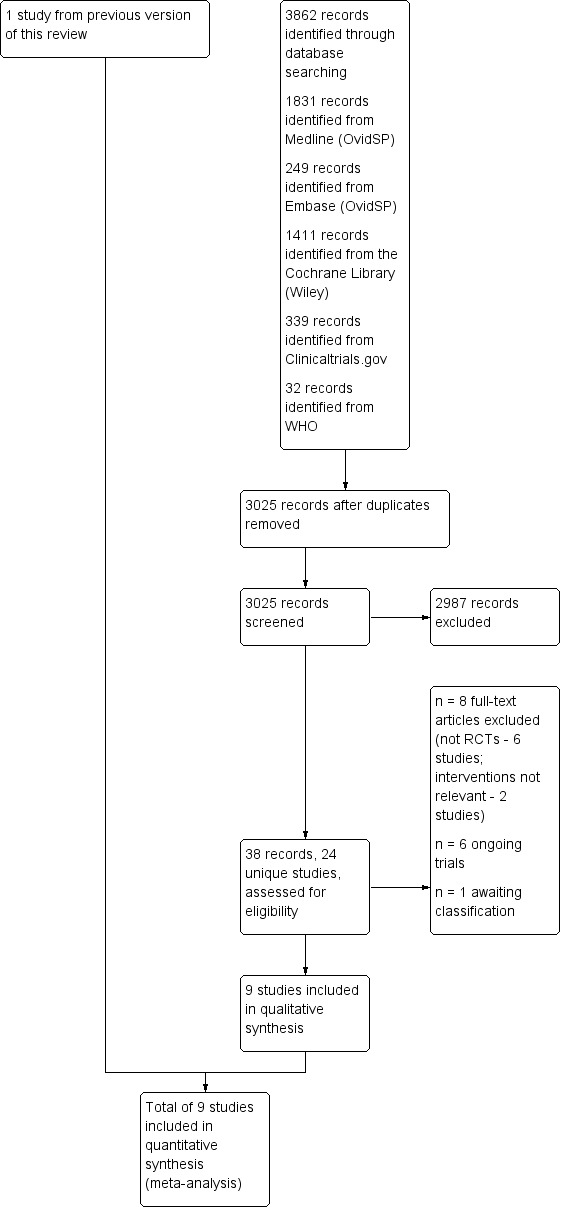
Study flow diagram.
We excluded eight studies, the reasons are given in Characteristics of excluded studies (Berth 2010; Flurin 2013; Franceschi 2013; Franceschi 2015; Heuberer 2016; Maillot 2018; Mardani‐Kivi 2016; Shin 2012a). We identified six ongoing trials meeting the inclusion criteria and their characteristics are presented in Table 5 and Characteristics of ongoing studies (NCT00695981; NCT01498198; NCT02059473; NCT02885714; NCT03183466; NCT03295994). We identified another trial that was presented in a congress but as the full results were not available and the authors did not respond to email queries at the time of submission of the review, this study remains as awaiting classification (Lhee 2013).
1. Characteristics of ongoing studies.
| Trial registration number | Principle Investigator/s and Country | Comparator/s | Main selection criteria | Registration date | Recruitment commenced | StatusMay 2018 | Planned sample size |
| NCT00695981 | J Paloneva Finland | Physiotherapy | Rotator cuff tear not responding to a minimum of 3‐month conservative therapy. Age >35 | 6/2008 | 6/2008 | Active, not recruiting. | 100 |
| NCT01498198 | P. MacDonald, US | 6 weeks physiotherapy and cross‐over to surgery if no response | > 50% partial tear or < 1cm full‐thickness tear and works compensation claim. | 12/2011 | not provided | Not yet recruiting | 144 |
| NCT02059473 | H. Bjornsson, Sweden | Physiotherapy | Traumatic full thickness rotator cuff tear | 2/2014 | not provided | Recruiting, recruitment status unknown | 50 |
| NCT02885714 | V. Äärimaa Finland, Sweden, Norway | Placebo‐surgery | Rotator cuff tear related to trauma 45 to 70 years | 8/2016 | 12/2016 | Recruiting | 200 |
| NCT03295994 | H Koudelkova US | Physiotherapy | Non‐traumatic rotator cuff tear 50 to 84 years | 9/2017 | 3/2018 | Recruiting | 700 |
| NCT03183466 | Samsung Medical Center | Debridement | Subscapularis tear | 6/2017 | 1/2011 | Active, not recruiting | 80 |
Included studies
A full description of all included trials is provided in the table of Characteristics of included studies. A summary of trial and participant characteristics is provided in Table 6.
2. Baseline demographic and clinical characteristics of the trial participants.
| Trial | Country | Groups (number randomised) | Mean age, yrs | Mean symptom duration in months | Mean pain (0 to 10, higher is worse) | Mean shoulder specific score (0 to 100, higher is better) | Mean HRQoL | Subscapularis tear N |
| 1. Surgery (repair with or without subacromial decompression) versus non‐operative treatment | ||||||||
| Kukkonen 2014 | Finland | Surgery (60) | 65 | 28 | 2.6 | 58a | Not measured | 0 (excluded) |
| Exercise (60) | 65 | 26 | 2.7 | 58a | ||||
| Lambers Heerspink 2015 | Netherlands | Surgery (25) | 61 | 12.5 | 6.7 | 56a | Not measured | 1 (4%) |
| Exercise and glucocorticoid injection (31) | 61 | 12 | 6.3 | 57a | 4 (13%) | |||
| Moosmayer 2010 | Norway | Surgery (52) | 59 | 12 | 5.6 | 35a | 54 | 1 (2%; > 25 % tears excluded) |
| Exercise (51) | 61 | 10 | 5.3 | 38a | 57 | 1 (2%; > 25 % tears excluded) | ||
| 2. Repair with acromioplasty versus repair only | ||||||||
| Abrams 2014 | US | Repair with acromioplasty (65) | 60 | Not reported | 4.4 | 52a | Not measured | Not reported (mean of 1.3 tendons involved) |
| Repair (49) | 58 | 3.8 | 48a | Not reported (mean of 1.4 tendons involved) | ||||
| Gartsman 2004 | US | Repair with acromioplasty (47) | 59 | Not reported | Not measured | 31b | Not measured | 0 (excluded) |
| Repair (46) | 60 | 31b | ||||||
| MacDonald 2011 | Canada | Repair with acromioplasty (41) |
56 | Minimum of 6 months | Not measured | 45b | 37 (WORC) | Not reported |
| Repair (45) | 57 | 44b | 35 (WORC) | |||||
| Milano 2007 | Italy | Repair with acromioplasty (40) |
61 | Not reported | Not reported | aNot reported | Not measured | 5 (13) |
| Repair (40) | 60 | 4 (10) | ||||||
| Shin 2012 | South Korea | Repair with acromioplasty (75) |
58 | 14 | 5.5 | 58a | Not measured | 21 (28%) |
| Repair (75) | 56 | 17 | 5.5 | 57a | 23 (31%) | |||
| 3. Repair with subacromial decompression versus decompression only | ||||||||
| Dezaly 2011 | France | Repair with subacromial decompression (70) | 68 | Not reported | 3.7 | 44%c | Not measured | 0 (excluded) |
| Subacromial decompression (60) | 68 | 44%c | ||||||
| Kukkonen 2014 | Finland | Repair with subacromial decompression (60) | 65 | 28 | 2.6 | 58a | Not measured | 0 (excluded) |
| Subacromial decompression (60) | 65 | 28 | 2.5 | 60a | ||||
a Constant score
b American Shoulder and Elbow Surgery (ASES) score
c Weighted Constant score (reported as percentage by authors)
Trial design, setting and characteristics
All participants were recruited from people referred to hospital orthopaedic outpatient clinic due to their shoulder pain.
Three trials compared rotator cuff repair and subacromial decompression versus non‐operative treatment (279 participants) (Kukkonen 2014; Lambers Heerspink 2015, Moosmayer 2010). One of the trials included a third arm of subacromial decompression alone (Kukkonen 2014).
Five trials (Abrams 2014; Gartsman 2004; MacDonald 2011; Milano 2007; Shin 2012) compared rotator cuff repair and acromioplasty with rotator cuff repair alone, with both groups receiving bursectomy (526 participants). The acromioplasty included removing bone from under surface of acromion and release of the acromioclavicular ligament.
As well as Kukkonen 2014, one other trial compared rotator cuff repair and subacromial decompression with subacromial decompression alone (Dezaly 2011) (250 participants).
The key clinical characteristics of studies and participants are presented in Table 6.
All included trials followed up the participants for at least one year. The longest follow‐up point was 92 months (Abrams 2014).
We identified trial registration for only four of the nine included trials (Kukkonen 2014; Lambers Heerspink 2015; MacDonald 2011Moosmayer 2010).
Three trials disclosed receiving funding from non‐commercial sources. Lambers Heerspink 2015 received funding from Anna Fonds (Nederland Orthopedisch Research en Educatie Fonds); MacDonald 2011 from the Alexander Gibson Fund of University of Manitoba; and Moosmayer 2010 from the South‐Eastern Norway Regional Health Authority. All three reported that the funding source had no role in the execution of the trial. Kukkonen 2014 declared that the authors did not have any financial relationship with any entity in the biomedical arena that could be perceived to influence or have the potential to influence the report in the 36 months prior to submission of the work. Milano 2007, Dezaly 2011 and Shin 2012 reported that the authors had no potential conflict of interests. In Abrams 2014, four of the authors disclosed potential financial conflicts of interest with the medical industry. Gartsman 2004 did not report whether or not there were any potential conflicts of interest.
Trial participants
The mean age of the participants in the three trials in the primary comparison varied between 59 and 65 years (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010). Only one trial limited inclusion by age (over 55 years) (Kukkonen 2014). Two trials excluded tears with traumatic onset of symptoms (Kukkonen 2014; Lambers Heerspink 2015), whereas Moosmayer 2010 included 59/103 (57%) people who presented with a traumatic event in a shoulder with preceding episodes of symptoms.
The mean age of the participants in the five trials that compared rotator cuff repair and acromioplasty versus rotator cuff repair alone varied between 55 and 61 years (Abrams 2014; Gartsman 2004; MacDonald 2011; Milano 2007; Shin 2012). Females comprised 29% to 56% of the participants.
For the two trials that compared repair and subacromial decompression versus decompression alone, mean ages varied between 65 and 68 years (Dezaly 2011; Kukkonen 2014). Dezaly 2011 limited inclusion to people over 60 year of age and Kukkonen 2014 limited inclusion to people over 55 year of age.
Only MacDonald 2011 defined a minimum symptom duration or required failure to respond to a period of conservative therapy before inclusion (six months). Symptom duration varied between 10 and 28 months. At baseline, the mean pain varied between and 2.5 and 6.3 (range 0 to 10, higher score indicates greater pain). The mean function varied between 31 to 45 in the American Shoulder and Elbow Surgeons (ASES) score and 58 to 60 in the Constant score (both 0 to 100 scales with higher scores indicating better function). Mean health‐related quality of life varied between 54 and 57 in the SF‐36 mental component score (0 to 100, higher is better) in Moosmayer 2010 and 35 and 37 in the Western Ontario Rotator Cuff (WORC) score (range 0 to 100, higher is better) in MacDonald 2011.
All trials excluded participants with partial or irreparable tears (Table 6). Some trials explicitly excluded tears of the subscapularis tendon (Dezaly 2011; Gartsman 2004; Kukkonen 2014) and some trials limited inclusion according the size of the tears (under 4 cm in MacDonald 2011 or 3 cm in Moosmayer 2010 and Shin 2012).
Lambers Heerspink 2015 excluded 2/25 (8%) patients during surgery because they did not have a full‐thickness tear and 2/25 (8%) because the tear was deemed irreparable. These post‐allocation exclusions were performed only in the surgical arm. The authors included them in the intention‐to‐treat (ITT) analysis as last observation carried forward (LOCF) , but the follow‐up time for these four participants was unclear.
Four trials (Dezaly 2011; Kukkonen 2014; Milano 2007; Shin 2012) defined fatty degeneration of rotator cuff muscles preoperatively according to the Goutallier classification (0 to 4, higher indicates worse degeneration) (Goutallier 1994). The other trials included mainly participants with Goutallier grade < 3 except Milano 2007 who included 35% participants with grade 3 or 4 fatty degeneration (Characteristics of included studies).
Interventions
Details of the interventions in each trial are presented in the table of Characteristics of included studies. The deviations from the protocol, co‐interventions and re‐operations are presented in Table 7.
3. Deviations from protocol and side interventions.
| Study | Group (N) | Did not receive allocated treatment | Crossed over | Re‐operated | Additional interventions |
| 1. Surgery (repair with or without subacromial decompression) versus non‐operative treatment | |||||
| Kukkonen 2014 | Exercise therapy (60) | 0 | 4 (7%) by one year 7 (12%) by 2 years | 0 | Not described if any were allowed |
| Subacromial decompression (60) | 0 | 1 (2%; to repair of cuff) | 1 (2%) | 7 (12%) distal clavicle resection 29 (51%) biceps tenotomy |
|
| Subacromial decompression and rotator cuff repair (60) | 5 (8%) | N/A | 0 | 8 (15%) distal clavicle resection 23 (42%) biceps tenotomy |
|
| Lambers Heerspink 2015 | Subacromial decompression and rotator cuff repair (25) | 0 | N/A | 0 | Biceps procedure was planned for participants with irreparable tear, not clearly reported if any were performed (two participants) |
| Exercise therapy (31) | 0 | 3 (10%) | 0 | 1 to 3 glucocorticoid injections | |
| Moosmayer 2010 | Subacromial decompression and rotator cuff repair (52) | 1 (2%) | N/A | 0 | 18 (35%) biceps tenodesis |
| Exercise therapy (51) | 0 | 9 (18%) by 1 year 12 (24%) by 5 years | 0 | No supplementary treatments | |
| 2. Repair with acromioplasty versus repair only | |||||
| Abrams 2014 | Repair with acromioplasty (65) | 0 | 0 | 1 re‐repair | 23 (43%) biceps tenotomy 3 (6%) distal clavicle resection |
| Repair (49) | 0 | 0 | 3 re‐repairs 1 capsular release and biceps tenotomy |
17 (40%) biceps tenotomy 2 (5%) distal clavicle resection |
|
| Gartsman 2004 | Repair with acromioplasty (47) | 0 | 0 | 0 | 0 (excluded) |
| Repair (46) | 0 | 0 | 0 | ||
| MacDonald 2011 | Repair with acromioplasty (41) | 2 (5%) | 0 | 0 (0%) 0 | 0 |
| Repair (45) | 1 (2%) | 3 (7%) | 1 re‐repair + acromioplasty 2 acromioplasty |
0 | |
| Milano 2007 | Repair with acromioplasty (40) | 0 | 0 | 0 | 13 (33%) biceps tendon procedure |
| Repair (40) | 0 | 0 | 0 | 20 (50%) biceps tendon procedures | |
| Shin 2012 | Repair with acromioplasty (75) | 0 | 0 | 2 capsular release 1 bursectomy |
12 (20%) biceps tendon procedures a |
| Repair (75) | 0 | 0 | 0 | 17 (28%) biceps tendon procedures a | |
| 3. Repair with subacromial decompression versus subacromial decompression only | |||||
| Dezaly 2011 | Repair with subacromial decompression (70) | 0 | 0 | 0 | 70 (100%) biceps tenotomy |
| Subacromial decompression (60) | 0 | 0 | 0 | 60 (100%) biceps tenotomy | |
| Kukkonen 2014 | Subacromial decompression and rotator cuff repair (60) | 5 (8%) | 0 | 0 | 8 (15%) distal clavicle resection 23 (42%) biceps tenotomy |
| Subacromial decompression (60) | 1 (2%) | 1 (2%) | 1 repair | 7 (12%)distal clavicle resection 29 (51%) biceps tenotomy |
|
a Authors report additional procedures for 60 participants who were not lost to follow‐up.
Surgery was performed by orthopedic surgeons in all trials and performed arthroscopically in seven trials (Abrams 2014; Dezaly 2011; Gartsman 2004; Kukkonen 2014; MacDonald 2011; Milano 2007; Shin 2012). Moosmayer 2010 performed nine mini‐open and 42 open procedures, and Lambers Heerspink 2015 performed all operative procedures via a mini‐open approach. In the primary comparison, all surgery groups received acromioplasty and likely bursectomy although bursectomy was not explicitly reported in Moosmayer 2010.
Six trials (Abrams 2014; Dezaly 2011; Gartsman 2004; Kukkonen 2014; MacDonald 2011; Shin 2012) used bone anchors to repair the tendon; one trial (Milano 2007) bone anchors, side‐to‐side repair, or combined technique; one trial (Lambers Heerspink 2015) either side‐to‐side repairs or bone anchors; and one (Moosmayer 2010) used bone tunnels for repair. Of the trials performing arthroscopic repair, Abrams 2014, Dezaly 2011, Kukkonen 2014, and Shin 2012 described using both single‐row and double‐row techniques, MacDonald 2011 single‐row technique, and Gartsman 2004 and Milano 2007 did not describe whether they used a single‐ of double‐row technique.
In all trials, surgery was followed by a period of sling with or without passive range of motion exercises (three weeks in Kukkonen 2014 and Milano 2007; four weeks in Dezaly 2011 and Shin 2012; six weeks in Abrams 2014, Gartsman 2004, Lambers Heerspink 2015, and Moosmayer 2010). This was followed by active home rehabilitation supervised by a physiotherapist (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010).
Participants in the non‐operative groups were prescribed exercises directed to active strengthening and correction of balance and humeroscapular kinematics. Kukkonen 2014 scheduled 10 visits, Moosmayer 2010 two visits per week for 12 weeks, and Lambers Heerspink 2015 did not specify the number of visits. Lambers Heerspink 2015 also provided one to three glucocorticoid injections prior to the exercise therapy in the non‐operative group and allowed use of analgesia. Moosmayer 2010 did not allow analgesia or injections, and Kukkonen 2014 did not report whether they used any co‐interventions in the exercise group. All three trials that included a non‐operative group allowed cross‐over to surgery if symptoms were not adequately controlled.
Outcomes
Pain
Pain was reported on a 0 to 10 scale (higher score indicates more pain) in five trials (Abrams 2014; Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010; Shin 2012), and in a 0 to 15 scale (higher score indicates less pain ) in one trial (Constant sub scale) (Dezaly 2011). None of the studies specifically measured night or other types of pain.
Function
Six trials measured function using the Constant score (Abrams 2014; Kukkonen 2014; Lambers Heerspink 2015; Milano 2007; Moosmayer 2010; Shin 2012) and four used the ASES (Abrams 2014; Gartsman 2004; MacDonald 2011; Moosmayer 2010) (both scales are 0 to 100 with higher scores indicating better function). In addition, Abrams 2014 and Lambers Heerspink 2015 also measured function using the Simple Shoulder Test (SST) score. Abrams 2014 also included the The University of California at Los Angeles (UCLA) shoulder score (0 to 35, higher is better), and Moosmayer 2010 included the physical component score of the 36‐item Short Form Health Survey (SF‐36).
Participant‐rated global assessment of treatment success
Three trials measured participant‐rated global treatment success (Kukkonen 2014; Moosmayer 2010; Shin 2012). Kukkonen 2014 asked participants whether the shoulder was better or worse compared with its preoperative state and if the patients were satisfied or dissatisfied with the treatment outcome (yes/no). Moosmayer 2010 and Shin 2012 assessed global satisfaction using a VAS scale (0 to 10, higher score indicates better satisfaction).
Health‐related quality of life (HRQoL)
Moosmayer 2010 measured SF‐36 and reported both mental and physical component scores at one year but only the physical component score at two and five years.
Adverse event
Kukkonen 2014Moosmayer 2010 and Shin 2012 reported adverse events by group. MacDonald 2011 reported that adverse events were provided in an appendix but we could only find data for re‐operations. Dezaly 2011 reported complications for all participants combined and did not report these by treatment group.
Serious adverse events
None of the studies reported observing serious adverse events.
Treatment failure
Five trials reported on tendon integrity according to imaging in follow‐up (Dezaly 2011; Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010; Shin 2012). In the primary comparison, the non‐operative group could not develop re‐tears, and thus we did not compare any events. In repair with acromioplasty versus repair only, we defined treatment failure as incidence of tear at follow‐up.
Excluded studies
Eight trials were excluded after retrieving the full text. Six studies were not randomised trials (Berth 2010; Flurin 2013; Franceschi 2015; Heuberer 2016; Maillot 2018; Mardani‐Kivi 2016), and two studies compared two different surgical repair techniques (Franceschi 2013; Shin 2012a) (Characteristics of excluded studies).
Risk of bias in included studies
A summary of the 'Risk of bias' assessment for each included trial is presented in Figure 2 and details by domain are provided in the Characteristics of included studies table.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
All trials were at high risk of bias most notably due to lack of participant and personnel blinding.
Allocation
Three trials reported adequate random sequence generation and allocation concealment, and were deemed to be at low risk of selection bias (MacDonald 2011; Milano 2007; Moosmayer 2010).Other trials reported using randomisation but did not describe their method of sequence generation or allocation concealment and we assessed the selection bias as unclear (Abrams 2014; Dezaly 2011; Gartsman 2004; Kukkonen 2014; Lambers Heerspink 2015; Shin 2012).
Blinding
The participants and personnel were aware of the treatment allocation across all three trials in the primary comparison and therefore were deemed to be at high risk of performance and detection bias (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010).
MacDonald 2011 blinded both personnel and participants, while both Abrams 2014 and Gartsman 2004 blinded personnel but it was unclear if participants were blinded. Neither Milano 2007 nor Shin 2012 reported whether or not participants and personnel were blinded and Dezaly 2011 did not blind participants or personnel.
We assigned a high risk of bias when participants were not blinded even when trialists had blinded outcome assessors, because if the participants were aware of the allocation that could affect their responses to questionnaires or strength or mobility testing. For radiological outcomes the radiologist probably could not be reliably blinded to allocation (metal anchors and signs of removed bone probably visible in the imaging).
Incomplete outcome data
Risk of attrition bias was high in Lambers Heerspink 2015 and unclear in MacDonald 2011 and Shin 2012 due to relatively large but balanced loss to follow‐up without reported reasons. The risk of attrition bias was low in the remaining trials (Abrams 2014; Dezaly 2011; Gartsman 2004; Kukkonen 2014; Milano 2007; Moosmayer 2010).
Lambers Heerspink 2015 lost 6/25 (24%) to follow‐up in the exercise group, and 5/25 (20%) were lost or excluded post‐allocation in the surgery group; two participants had large tears which could not be repaired and two had no full‐thickness tears despite positive MRI. In ITT analysis data for these participants were 'last observation carried forward' and since the post‐allocation exclusions did not occur in the exercise group, bias could affect the outcomes in either direction.
Selective reporting
Risk of reporting bias was low in MacDonald 2011; Moosmayer 2010 and Shin 2012. Moosmayer 2010 reported all outcomes specified in the ClinicalTrial registry except for SF‐36 mental component scores at five‐year follow‐up where only the physical component scores were reported. Shin 2012 omitted one month outcomes in their report but as we deemed this time point clinically irrelevant (and we did not report outcomes at this time point in the review), we assigned Shin 2012 a low risk for reporting bias. MacDonald 2011 reported all pre‐specified outcomes and the adverse events were reported in ClinicalTrials.gov.
We deemed three trials to be at unclear risk of reporting bias (Gartsman 2004; Kukkonen 2014; Milano 2007). Kukkonen 2014 specified only Constant score in the trials registry and asked satisfaction with two questions but only reported one. Thus, we assigned unclear risk of bias. Gartsman 2004 reported only ASES score and due to no protocol or registration being available, we could not determine if they measured any other outcomes. Milano 2007 did not report adverse events and no protocol was available.
We assigned high risk of bias for three trials (Abrams 2014; Dezaly 2011; Lambers Heerspink 2015). Abrams 2014 reported adverse events incompletely and defined SF‐12 as an outcome in the methods but did report any results. Lambers Heerspink 2015 reported collecting outcomes at six weeks, three months, six months and 12 months, but only reported 12‐month results. Dezaly 2011 did not report adverse events by group and did not report Constant score pain at four years although they reported it at one year (the trial did not have other pain outcomes).
Other potential sources of bias
We assigned high risk of other potential sources of bias to three trials Abrams 2014; Dezaly 2011; Moosmayer 2010).
In Moosmayer 2010, 9/51(18%) had surgery in the exercise group by 12 months and 12/51 (24%) by five years. This cohort was analysed in the non‐operative group as allocated. This could mask the potential benefit of surgery. Abrams 2014 had imbalance in the proportion of participants receiving allocated treatment due to exclusion of more participants in the non‐acromioplasty group. Dezaly 2011 had imbalance in the proportion of participants receiving postoperative supervised physical therapy.
Three trials were at unclear risk of other potential sources of bias (Kukkonen 2014; Lambers Heerspink 2015; Milano 2007). In Kukkonen 2014, an additional acromioclavicular resection was performed in seven participants (12%) in the decompression group and eight participants (15%) in the repair group. An additional biceps tenotomy was performed in 29 participants (51%) in the subacromial decompression group and in 23 participants (42%) in the subacromial decompression and repair group. Twelve per cent of participants in the non‐operative therapy also crossed over to surgery. The cross‐overs and co‐interventions could have biased the results in either direction. In Milano 2007, the authors reported Constant scores over 100 points although the scale of the measure is 0 to 100. The reason is unclear, but it is likely that the method of calculation was similar in both groups.
Effects of interventions
See: Table 1
1. Surgery versus non‐operative treatment
Benefits
Pain
We pooled data from all three trials. Two trials reported pain at six months (Kukkonen 2014; Moosmayer 2010); three trials at 12 months (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010); and two trials at >12 months (Kukkonen 2014 at two years; Moosmayer 2010 at five years). Statistical heterogeneity was substantial to low: I2 = 71% at six months; I2 = 8% at 12 months and I2 = 0% at >12 months.
Moderate‐certainty evidence (downgraded for bias) at 12 months indicates that surgery (repair with subacromial decompression) probably provides no important improvement in pain when compared with non‐operative treatment. The 95% confidence intervals (CIs) excluded an important difference (Analysis 1.1) and included both the clinically important improvement of 1.5 points on a 0 to 10 pain scale, and no improvement.
1.1. Analysis.
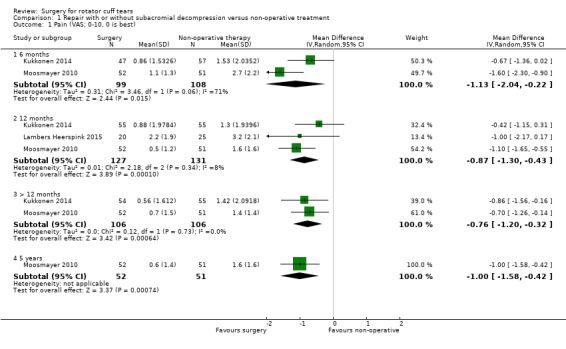
Comparison 1 Repair with or without subacromial decompression versus non‐operative treatment, Outcome 1 Pain (VAS; 0‐10, 0 is best).
At six months, the mean pain (0 to 10, lower is better) was 2.1 with non‐operative treatment and 1.13 points better (95% CI 0.22 better to 2.04 better; 207 participants) with surgery; at 12 months, the mean pain was 1.6 with non‐operative treatment and 0.87 points better (95% CI 0.43 better to 1.30 better; 3 trials, 258 participants) an absolute improvement of 9% (4% better to 13% better) and a relative improvement of 16% (8% better to 25% better) with surgery; and at >12 months, the mean pain was 1.4 points after non‐operative treatment and 0.76 points better (95% CI 0.32 better to 1.20 better; 212 participants) with surgery. At five years, the mean pain was 1.6 with non‐operative treatment and 1 point better (95% CI 1.58 better to 0.42 better) with surgery.
Function
Two trials reported function at six months (Kukkonen 2014; Moosmayer 2010); three trials at 12 months (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010); and two trials at >12 months (Kukkonen 2014; Moosmayer 2010) at two years; and one trial (Moosmayer 2010) at five years). Statistical heterogeneity was unimportant at all time points (I2 = 0%)
Compared with non‐operative treatment, low‐certainty evidence indicates surgery (repair with subacromial decompression) may have little or no effect on function at 12 months. The evidence was downgraded two steps, once for bias and once for imprecision – the 95% CIs overlap minimal important difference in favour of surgery at this time point (Analysis 1.2).
1.2. Analysis.
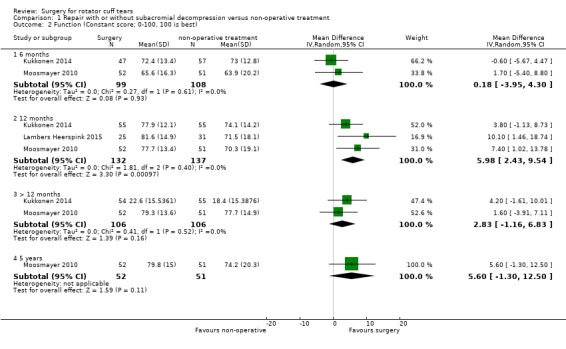
Comparison 1 Repair with or without subacromial decompression versus non‐operative treatment, Outcome 2 Function (Constant score; 0‐100, 100 is best).
At six months, the mean function (0 to 100, higher is better) was 68 points with non‐operative treatment and 0.18 points worse (95% CI 3.95 worse to 4.30 better; 207 participants) with surgery; at 12 months, the mean function was 72 points with non‐operative treatment and 5.98 points better (95% CI 2.43 better to 9.54 better; 269 participants), an absolute improvement of 6% (2% better to 10% better), and a relative improvement of 16% (6% better to 25% better) with surgery; at >12 months, the mean function was 75 points with non‐operative treatment and 2.83 points better (95% CI 1.16 worse to 6.83 better; 212 participants) with surgery. At five years, the mean function was 74.2 with non‐operative treatment and 5.60 points better (95% CI 1.30 worse to 12.50 better). Thus, assuming an improvement of 8.3 points on a 0 to 100 point scale or 8% absolute improvement is clinically important, there was no clincally important difference in function at six months and 12 months, but at five years, the difference included both no effect and a clinically important improvement.
8.3 points or 8% absolute improvement (Hao 2018) for function; and 10 points or 10% absolute improvement for health‐related quality of life and include both the minimal important difference and no difference
Participant‐rated global assessment of treatment success
Kukkonen 2014 measured treatment success using a binary outcome, Moosmayer 2010 used a VAS scale and Lambers Heerspink 2015 did not measure this outcome.
Low‐certainty evidence (downgraded for bias and imprecision) from one trial indicates that surgery may not improve participant‐rated treatment success at one year. 52/55(95%) in the surgery group reported treatment success compared with 48/55 (87%) in the exercise group; RR 1.08, 95% CI 0.96 to 1.22, an absolute improvement of 7% (4% worse to 13% better) and relative improvement of 8% (4% worse to 22% better). At >12 months (surgery 51/54 (95%), exercise: 49/54 (89%) ; RR 1.06, 95% CI 0.95 to 1.19 (Analysis 1.3).
1.3. Analysis.
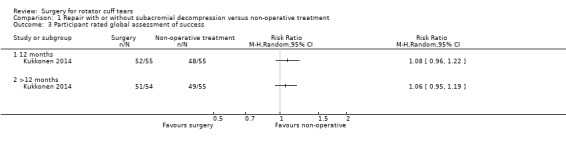
Comparison 1 Repair with or without subacromial decompression versus non‐operative treatment, Outcome 3 Participant rated global assessment of success.
In Moosmayer 2010, mean satisfaction (0 to 10, higher is better) was 7.2 with exercise and 9 with surgery at 12 months (MD 2.8; 95% CI not reported). At five years, the VAS for satisfaction was 8.3 with exercise and 9.2 with surgery (MD 1; 95% CI 0.1 to 1.8).
Health‐related quality of life
Moosmayer 2010 reported health‐related quality of life at six, 12 and >12 months.
Low‐certainty evidence (downgraded for bias and imprecision) indicates that surgery may have little or no effect on health‐related quality of life, and the confidence intervals did not include a clinically important improvement of 10 points on a 0 to 100 scale, or absolute improvement of 10%, at any time point.
At six months, mean health‐related quality of life (0 to 100, higher is better) was 57.6 points with non‐operative treatment and 0.10 points worse (95% CI 3.29 worse to 3.09 better; 103 participants) with surgery; at 12 months, 57.5 points with non‐operative treatment and 1.3 points worse (95% CI 4.49 worse to 1.89 better; 103 participants) with surgery, an absolute difference 1% worse (4% worse to 2% better) and relative difference 2% worse (8% worse to 3% better). At >12 months, the mean health‐related quality of life was 49 points with non‐operative treatment and 0.60 points worse (95% CI 3.05 worse to 4.25 better; 103 participants) with surgery.
Treatment failures
The trials in this comparison examined the continuity of rotator cuff using ultrasound or MRI in the surgery groups. Kukkonen 2014 and Moosmayer 2010 also followed the participants in the exercise group. (Table 8).
4. Re‐tears after repair.
| Study | Imaging | Time from repair | Partial‐thickness tear n (%) | Full‐thickness tear n (%) |
| Dezaly 2011 | Ultrasound | 1 year | 22/68 (32%)a | |
| Kukkonen 2014 | MRI | 2 years | Not reported | 15/49 (31%) |
| Lambers Heerspink 2015 | MRI | 1 year | 14/19 (74%)a | |
| Moosmayer 2010 | MRI | 1 years | 6/59 (10%)b | 5/59 (8%)b |
| Ultrasound | 5 years | 7/60(12%)b | 8/60 (13%)b | |
| Shin 2012 | MRI, CT, or ultrasound | 3 yearsc | Not reported | 22/120 (18%) |
a The authors did not specify if the tears at follow‐up were full or partial thickness.
b The total number includes participants who crossed over from exercise group to repair during follow‐up
c Mean 35 months, range 24 to 54 months
MRI: magnetic resonance imaging
Kukkonen 2014 performed MRI at two years and found a full‐thickness tear in 15/49 (31%) participants who received rotator cuff repair, 41/51 (80%) in the non‐operative group and 40/48 (83%) in the subacromial decompression only group (tears were not repaired in the latter two groups).
In Lambers Heerspink 2015 participants in the surgical repair group had an MRI at one year and 14/19 (74%) had a re‐tear. However, they did not report if the re‐tears were full or partial thickness.
Moosmayer 2010 found that 4/50 (8%) participants in the repair group had a full‐thickness re‐tear and 6/50 (12%) had a partial‐thickness re‐tear on MRI at one year. At five years, 60/64 participants who had their tear repaired (this number includes participants that crossed over from exercise therapy) had an ultrasound. A full‐thickness re‐tear was found in eight participants (13%) and a partial‐thickness re‐tear in seven (12%) participants.
In the non‐operative groups, 16/142 (11%) were dissatisfied with their treatment outcome and crossed over to surgery by 12 months. At five years, the cumulative number of cross‐overs was 22/142 (15%) (Table 7) (Kukkonen 2014; Lambers Heerspink 2015; Moosmayer 2010).
Participation
None of the studies measured participation in work or recreation.
Harms
Adverse events
We could not estimate the risk of adverse events. Moosmayer 2010 reported one humerus fracture due to falling in the surgery group and one participant was diagnosed with polymyalgia rheumatica four months after inclusion in the exercise group. We deemed these events unrelated to the received treatments. Kukkonen 2014 observed no treatment‐related complications in any of the groups. Lambers Heerspink 2015 reported one frozen shoulder in the non‐operative treatment group.
Serious adverse events
We could not estimate the risk of serious adverse events because none of the trials reported serious adverse events.
Sensitivity analysis
Excluding data from the single trial that included participants with traumatic onset of symptoms (Moosmayer 2010) did not appreciably alter the results for pain or function at 12 months. For pain, the MD was ‐0.87 points (95% CI ‐1.30 to ‐0.43) including data from Moosmayer 2010 and ‐0.5 (95% CI ‐1.2 to 0.04) excluding its data. The 95% confidence intervals exclude important differences in both analyses. For function, the MD was 5.98 points (95% CI 2.43 to 9.54) including data from Moosmayer 2010, and MD 5.91 points (95% CI 0.08 to 11.74) excluding its data (data not shown in forest plots or tables). The 95% CIs do not exclude an important difference in favour of surgery in the analysis that excluded Moosmayer 2010.
Excluding data from trials with unclear or high risk of selection bias left only one trial in this comparison (Moosmayer 2010). For pain the MD was ‐1.10 ( 95% CI ‐1.65 to ‐0.55) in favour of surgery. The 95% CIs do not exclude an important difference. For function the MD was 7.40 (95% CI 1.02 to 13.78) in favour of surgery. Similar to the pain outcome, the 95% CIs do not exclude the possibility of an important between‐group difference.
2. Repair with acromioplasty versus repair alone
Benefits
Pain
Moderate‐certainty evidence (downgraded due bias) from two trials indicates that acromioplasty probably does not improve pain when performed in conjunction with repair of rotator cuff tears. At six months, statistical heterogeneity was considerable (I2 = 77%) but the confidence intervals (two trials) at that time were overlapping. At 12 months, I2 = 0%, and at >12 months I2 = 46% (Analysis 2.1).
2.1. Analysis.
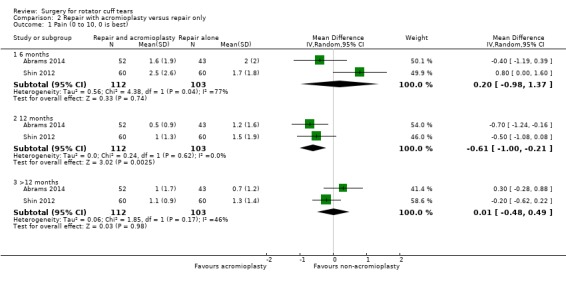
Comparison 2 Repair with acromioplasty versus repair only, Outcome 1 Pain (0 to 10, 0 is best).
At six months, the mean pain (zero to 10 scale) was 1.9 points with repair and 0.20 points worse (95% CI 0.98 better to 1.37 worse; 215 participants) with repair and acromioplasty. At 12 months, the mean pain was 1.4 points with repair and 0.61 points better (95% CI 0.21 better to 1.00 better; 215 participants) with repair and acromioplasty. At >12 months, the mean pain was 1.0 point with repair and 0.01 point worse (95% CI 0.49 worse to 0.48 better; 215 participants).
Function
Moderate‐certainty evidence (downgraded due bias) indicates that acromioplasty probably provides no or little benefit in function when performed in conjunction with repair of rotator cuff tears. The statistical heterogeneity was unimportant at all time points (I2 = 0%) (Analysis 2.2).
At six months, SMD was 0.19 (95% CI ‐0.05 to 0.42; 280 participants, 3 trials). When back‐transformed to Constant score (0 to 100, higher is better), the mean function was 73.7 points with repair and 3.2 points better (95% CI 0.9 worse to 7.2 better) with repair and acromioplasty. At 12 months, SMD was 0.13 (95% CI ‐0.07 to 0.34; 361 participants; 4 studies). When back‐transformed to Constant score, the mean function was 83.7 points with repair and 2.2 points better (95% CI 1.2 worse to 5.8 better) with repair and acromioplasty. At >12 months, SMD was 0.25 (95% CI 0.04 to 0.47; 352 participants; 4 studies). When back‐transformed to Constant score, the mean function was 84.4 points with repair and 4.2 points better (95% CI 0.7 better to 8.0 better) with repair and acromioplasty.
Participant‐rated global assessment of treatment success
Shin 2012 assessed global satisfaction with VAS (0 to 10, higher is better). In repair with acromioplasty group the mean satisfaction was 8.4 and in the repair only group it was 8.3.
Health‐related quality of life
None of the studies reported this outcome.
Treatment failures
Low‐quality evidence (downgraded twice for imprecision due to wide confidence intervals and low rate of events) from one trial (Shin 2012) indicates that acromioplasty may not protect from re‐tears or non‐healing when performed in conjunction with repair (Analysis 2.3).
2.3. Analysis.
Comparison 2 Repair with acromioplasty versus repair only, Outcome 3 Repair failure.
Full‐thickness tear (non‐healing or re‐tear) was observed in 10/60 (17%) in the repair with acromioplasty group and 12/60 (20%) participants in the repair only group corresponding to a RR of 0.83 (95% CI 0.39 to 1.78) (Analysis 2.3).
Re‐operations were performed in 4/294 (1.4%) participants in the repair and acromioplasty group and 7/291 (2.4%) participants in the repair alone group. Abrams 2014 performed one re‐repair in the repair with acromioplasty group and three re‐repairs and one capsulotomy in the repair alone group. MacDonald 2011 offered re‐operation to 4/45 participants in the repair alone group due to ongoing pain; two had acromioplasty, one had re‐repair and acromioplasty and one participant declined further surgery. Shin 2012 performed three re‐operations in the repair and acromioplasty group: two arthroscopic capsular releases and an arthroscopic bursectomy. Gartsman 2004 did not report any re‐operations (but did not declare that there were none either).
We did not include re‐operations in Analysis 2.3, as not all operations were performed due to failed healing of the repair and the exact indications for re‐operations were not always clear.
Participation
None of the studies reportedthis outcome.
Harms
Adverse events
Two studies reported their adverse events (MacDonald 2011; Shin 2012) and it is unclear if the other trials measured them as this was not reported.
MacDonald 2011 reported there were no events in either group. Shin 2012 reported 1/60 (2%) participant in the repair group developed stiffness of the operated shoulder and received physical therapy and glucocorticoid injection. In the repair with acromioplasty group, 3/60 (5%) developed stiffness and all three participants underwent a secondary procedure due to stiffness. We are uncertain of the risk estimates (RR 3.00; 95% CI 0.32 to 28.03; very low‐certainty evidence, downgraded once for bias and twice for imprecision) (Analysis 2.4).
2.4. Analysis.
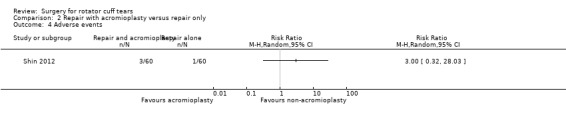
Comparison 2 Repair with acromioplasty versus repair only, Outcome 4 Adverse events.
Serious adverse events
None of the studies reported serious adverse events.
Subgroup analysis
Two trials reported ASES score at two years and one trial reported pain per acromion type. We planned to do the analysis at the one‐year time point but as MacDonald 2011 did not report data regarding function for type I acromion, we used two years data in the subgroup analysis for both outcomes. The type of acromion did not modify the treatment effect for pain or function (Analysis 2.6; Analysis 2.5).
2.6. Analysis.
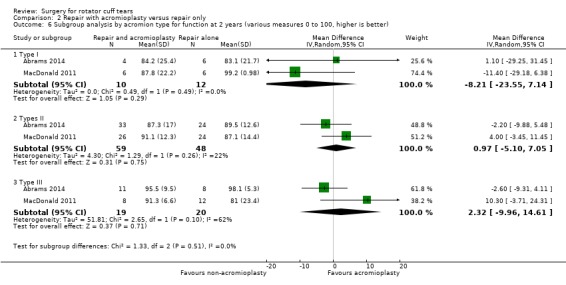
Comparison 2 Repair with acromioplasty versus repair only, Outcome 6 Subgroup analysis by acromion type for function at 2 years (various measures 0 to 100, higher is better).
2.5. Analysis.
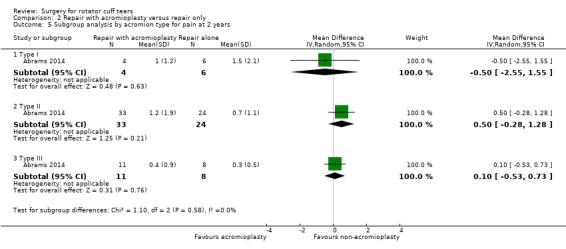
Comparison 2 Repair with acromioplasty versus repair only, Outcome 5 Subgroup analysis by acromion type for pain at 2 years.
3. Rotator cuff repair with subacromial decompression versus decompression alone
Benefits
Pain
Low‐certainty evidence (downgraded due to bias and imprecision) indicates that repair and subacromial decompression may provide little or no benefit in pain when compared with subacromial decompression alone. At 12 months, the statistical heterogeneity was substantial (I2= 55%) but there were only two trials and the confidence intervals overlapped (Analysis 3.1).
For clarity, the forest plot and tables show SMD due to different measures in the trials, but in time points with only one trial we used MD as a summary measure.
At six months, the mean pain (0 to 10 scale, 0 indicates no pain) was 0.61 points with subacromial decompression and 0.25 points worse (95% CI 0.75 worse to 0.25 better; 101 participants = 101, one study) with repair and subacromial decompression (SMD 0.20 ;95% CI ‐0.19 to 0.59) . At one year, the SMD was ‐0.29 (95% CI ‐0.67 to 0.09; 239 participants; 2 studies). When back‐transformed to VAS, the mean pain was 1.5 points with subacromial decompression and 0.58 points better (95% CI 0.18 worse to 1.34 better) with repair and subacromial decompression. At >12 months, the pain was 0.81 points with subacromial decompression and 0.25 points better (95% CI 0.38 worse to 0.88 better; 112 participants; 1 study) with repair and subacromial decompression (SMD ‐0.15 ; 95%CI ‐0.52 to 0.23).
Function
Adding acromioplasty to repair may provide little or no benefit with respect to function (low‐certainty evidence; downgraded once for bias and a second time for imprecision, as the 95% CIs do not exclude the possibility of benefit with repair).
At six months, the mean function (0 to 100, higher is better) was 74.8 points with decompression alone and 2.40 points worse (95% CI 7.36 worse to 2.56 better; 101 participants; 1 study) with repair and decompression. At 12 months, the mean function was 73 points with decompression alone and 4.12 points better (95% CI 2.03 worse to 10.27 better; 239 participants; 2 studies) with repair and decompression. At >12 months, the mean function was 76.6 with decompression alone and 4.09 points better (95% CI 0.89 better to 7.30 better; 214 participants; 2 studies). The statistical heterogeneity was considerable at 12 months (I2= 80%) but the confidence interval were overlapping (Analysis 3.2). At > 12 months, the statistical heterogeneity was unimportant (I2 = 0%).
3.2. Analysis.
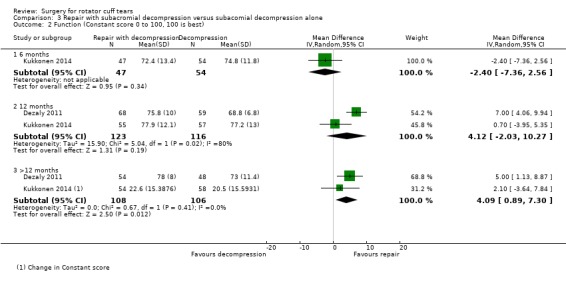
Comparison 3 Repair with subacromial decompression versus subacomial decompression alone, Outcome 2 Function (Constant score 0 to 100, 100 is best).
Participant‐rated global assessment of success
Low‐certainty evidence (downgraded for bias and imprecision) from two studies indicates that repair and subacromial decompression may not result in higher global success compared with decompression alone. The statistical heterogeneity was substantial at one year (I2 = 64%; 2 studies) but the confidence intervals overlapped (Analysis 3.3).
3.3. Analysis.
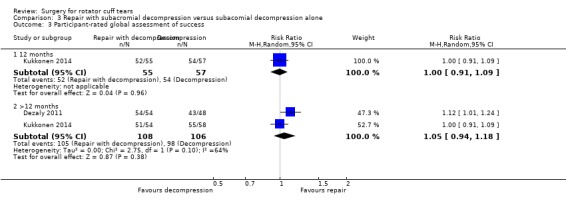
Comparison 3 Repair with subacromial decompression versus subacomial decompression alone, Outcome 3 Participant‐rated global assessment of success.
At 12 months, 54/57 (95%) participants reported success with decompression alone and 52/55 (95%) reported success with repair and subacromial decompression. This corresponds with a RR of 1.00 (95% CI 0.91 to 1.09). At > 12 months, the success rates were: 98/106 (92%) for decompression alone and 105/108 (97%) for repair and subacromial decompression. This corresponds with a RR of 1.05 (95% CI 0.94 to 1.18)
Health‐related quality of life
No studies in this comparison measured health‐related quality of life.
Treatment failures
We did not compare treatment failures as there were no comparable events reported; only participants in the repair group could have re‐tear (in decompression groups, no repair was attempted). In the repair group, 46/68 (68%) participants had a healed cuff on ultrasound examination at one year (Table 8).
Participation
None of the studies reported on this outcome.
Harms
Adverse events
We could not estimate the comparative risks for adverse events. Kukkonen 2014 reported no treatment‐related complications and Dezaly 2011 observed 10 complications (9% of all recruited or 7.9% of all followed‐up) in the two groups without specifying which group they belonged (three stiff shoulders, three transient brachial plexus neurapraxia and four anchor migrations).
Serious adverse events
The studies in this comparison did not report serious adverse events.
Discussion
Summary of main results
Surgery versus non‐operative treatment
In people with full‐thickness tears, repair with subacromial decompression may provide little or no benefit when compared with exercise with or without glucocorticoid injections. We are uncertain about the risks of adverse events. We included three trials with 339 randomised participants in this comparison.
For pain, we found moderate‐certainty evidence (downgraded for bias); for function, global treatment success or health‐related quality of life low‐quality evidence (downgraded for bias and imprecision); and for adverse events very low‐quality evidence (downgraded due to bias and serious imprecision).
Due to a lack of participant blinding, the trials were all at high risk of performance and detection biases. These biases can overestimate the effects of interventions (Wood 2008; Savovic 2012). The estimates for most outcomes were imprecise due to small numbers of participants or events or wide confidence intervals around the effect estimates (Table 1).
We are uncertain about the comparative risks of adverse events and serious adverse events as only one event was reported. Two trials reported that the participants had no adverse events (Kukkonen 2014; Moosmayer 2010), while one trial reported a single adverse event of a frozen shoulder (Lambers Heerspink 2015). A considerable proportion of participants (ranging from 8% to 31%) who underwent rotator cuff repair were found to have a full‐thickness tear within one to five years following surgery (Table 8).
We could not estimate the risk of adverse events from the trials. Observational data from a surgical registry indicates that the risk of serious adverse events such as deep infection, pulmonary embolism, or death is likely less than 1% (Hill 2017; Shields 2015; Karjalainen 2019).
Repair with acromioplasty versus repair alone
Adding acromioplasty to repair to reduce mechanical wear did not appear to alter the outcomes.
Moderate‐certainty evidence from five trials with 526 included participants indicates that performing acromioplasty in conjunction with rotator cuff repair probably provides little or no improvement in pain or shoulder function when compared with repairing the tendon without performing acromioplasty. Sensitivity analysis suggested that the type of acromion did not seem to have an impact on the treatment effect.
Low‐certainty evidence suggest that acromioplasty may provide little or no effect in health‐related quality of life or incidence of tears (non‐healing or re‐tear) at follow‐up.
We are uncertain about the risks of adverse events and serious adverse events (very low‐certainty evidence downgraded once for bias and twice for very serious imprecision). One trial reported adverse events while it was unclear if the remaining four trials measured adverse and serious adverse events (very low‐certainty evidence).
Global assessment of treatment success was not measured in any trial.
Repair with subacromial decompression versus decompression alone
Repair of rotator cuff may offer little or no benefit in outcomes when compared with performing subacromial decompression alone.
Low‐certainty evidence from two trials with 142 participants indicates that rotator cuff repair may not improve pain, function or global treatment success when compared to subacromial decompression alone.
We were unable to estimate the comparative risks of adverse or serious adverse events as one trial reported that no adverse events occurred (Kukkonen 2014), and the second trial did not report the events for both groups (Dezaly 2011).
Neither trial measured health‐related quality of life and we did not compare incidence of tears at follow‐up as the other group did not receive repair.
Overall completeness and applicability of evidence
Surgery versus non‐operative treatment
We did not identify any placebo‐controlled trials of surgical repair for rotator cuff tears and all included trials comparing surgery with non‐operative treatment were at high risk of bias mainly due to lack of participant and personnel blinding and may have therefore overestimated the benefits of surgery.
The trials in the primary comparison included participants from three countries with typical clinical history and imaging findings consistent with rotator cuff tear. Trials included similar participants in terms of age and symptom duration. Participants included in Kukkonen 2014 had less severe pain at baseline, and function was most impaired in participants included in Moosmayer 2010. The trials report whether the pain measure was overall or pain with activity or another measure and this could account for some of the baseline difference. In Moosmayer 2010, approximately half of the participants had traumatic onset of symptoms. One trial (Lambers Heerspink 2015), did not limit the extent of the tear to the supraspinatus tendon. Across the trials, the majority of tears were small and non‐traumatic and mean age ranged from 59 to 65 years. Thus the conclusions of this review may not extend to all traumatic tears in younger populations or large tears involving several tendons or severely impairing function, or both.
There was diversity in the co‐interventions between studies as well as between treatment groups in the same trials. Biceps tendon debridement, tenotomy or tenodesis is sometimes performed in addition to rotator cuff repair if pathology is observed during the surgery. In the included trials, these procedures were performed in up to 51% of participants in surgery groups in Kukkonen 2014, but it is unclear if any participants undergoing repair received additional biceps procedures in Lambers Heerspink 2015. This variation in additional procedures is likely to reflect normal clinical practice. It is unclear if the variation in biceps procedures affected the results within the trials, but it did not appear to result in statistical heterogeneity in the pooled data.
A systematic review estimated that additional co‐interventions may predict poorer outcome of surgery (Lambers Heerspink 2014), but this finding may be related to the condition rather than the procedure. Biceps tendon pathology likely does not bias treatment estimates in either direction as the incidence is likely to be equal in both groups in properly randomised trials.
Sixteen per cent of all participants in the non‐operative groups crossed over to surgery presumably because they were dissatisfied with the outcome of non‐operative treatment. We cannot draw any conclusions from these trials about whether or not surgery provides benefits in this population, given that we cannot predict their outcome if they had remained in the non‐operative group.
Repair with acromioplasty versus repair alone
The participants in the trials likely reflect the diversity seen in practice in terms of size and extent of rotator cuff tears. Abrams 2014 and Shin 2012 excluded participants with isolated subscapularis tears and Shin 2012 also excluded tears larger than three centimetres. Gartsman 2004 excluded participants with tears involving two or more tendons, while MacDonald 2011 limited inclusion to tears smaller than four centimetres. As the statistical heterogeneity was unimportant, this diversity did not seem to affect the outcomes.
Repair with subacromial decompression versus subacromial decompression alone
In practice, decompression without repair could sometimes be offered to older people with large rotator cuff tears as repair is often unsuccessful in this age group (Nho 2009). However, as the benefits of subacromial decompression over non‐operative treatment or placebo have not been established, the value of either intervention remains unclear. The two trials in this comparison included participants with mean age of 65 to 68 years and the conclusions may not be applicable to a younger population.
Quality of the evidence
Repair with or without subacromial decompression versus non‐operative treatment
We graded the evidence as moderate certainty for pain (downgraded due to bias most notably due to lack of blinding leading to potential for performance and detection biases). We did not downgrade for imprecision, as the 95% confidence intervals did not include the clinically important difference (1.5 points on a 10‐point scale, or 10% absolute difference between groups) and the optimal information size criterion was likely met, i.e. the number of participants included in the analysis exceeds the number generated by a conventional sample size calculation for a single adequately powered trial. For function, health‐related quality of life, and global assessment of treatment success, the 95% confidence intervals included both a clinically important effect and a clinically unimportant effect, thus we downgraded the evidence to low certainty, for imprecision as well as for bias. As surgery was not found to provide important benefits, it is likely that future placebo‐controlled trials would increase the precision of the effect estimates, and potentially upgrade the certainty of the conclusions.
We are uncertain of the risk of adverse events as there was only one event reported in the included trials and we could not calculate estimates for the comparative risks.
As there were only three trials in this comparison we could not get precise estimates for statistical heterogeneity;the I2 was mostly low in all other comparisons except pain at six months (I2 = 71%), which may reflect the small number of trials ‐ only two measured outcomes at this time point, rather than true statistical heterogeneity.
Although we identified only a few studies for this comparison and three ongoing trials, we believe this reflects a paucity of research in this topic rather than publication bias, and thus did not downgrade any outcome for publication bias. We also did not downgrade for indirectness, as the populations, interventions and outcomes in the trials reflect those of the review.
Repair with acromioplasty versus repair only
We graded the evidence moderate certainty for pain and function (downgraded for risk of bias caused by lack of blinding or attrition). For health‐related quality of life, we further downgraded the evidence to low certainty due to imprecision as the 95% CI did not exclude an important benefit of repair.
For adverse events, we downgraded the evidence to low certainty due to low number of events.
We could not estimate risks of serious adverse events as none were reported in the included trials.
We did not suspect publication bias or indirectness for any outcome in this comparison.
Repair with subacromial decompression versus decompression only
The evidence was low certainty for pain, function and global assessment of success. We downgraded once for bias and a second time for imprecision; the two trials in the comparison did not blind the participants and the risk for selection bias was unclear for both trials; furthermore, the 95% confidence intervals did not exclude important differences. The certainty regarding adverse events was downgraded one more time to very low as no events were reported in Kukkonen 2014 and Dezaly 2011 reported complications for the whole cohort and not by group.
Similar to the other comparisons, we did not downgrade any outcome for publication bias or indirectness.
Potential biases in the review process
To the best of our knowledge, we identified all relevant trials meeting our inclusion/exclusion criteria through searching all major databases without language restrictions. We used two independent assessors for article screening, selection and for 'Risk of bias' judgement assessment. None of the review authors has been involved with the conduct of the included trials.
We identified one ongoing placebo‐controlled trial (NCT02885714) and four open‐label trials (NCT00695981; NCT01498198; NCT02059473; NCT03295994) comparing surgery with exercise therapy for full‐thickness tears. The results of these trials, once available, may not affect the estimate of treatment effects reported in the primary comparison of this review, but are likely to increase their precision. Furthermore, one study awaiting classification (Lhee 2013) and one ongoing trial (NCT03183466) are comparing repair with arthroscopic debridement in people with subscapularis tears. These trials will yield estimates for an eligible comparison which is not present in the current review (repair versus debridement of isolated subscapularis tear).
Agreements and disagreements with other studies or reviews
We identified one systematic review and two meta‐analyses assessing the benefits of surgery compared with non‐operative treatment of full‐thickness rotator cuff repairs (Piper 2018; Ryosa 2017; Seida 2010). Two systematic reviews included the same three trials as our review (Piper 2018; Ryosa 2017). Our review is in agreement with the these reviews. One systematic review Seida 2010 also included trials comparing one type of surgery versus other types of surgery.
Three systematic reviews assessed the value of acromioplasty in conjunction with rotator cuff repairs. Song 2016 included all the same trials as this review; Familiari 2015 included all the same trials except Shin 2012; and Chahal 2012 included Abrams 2014, Gartsman 2004, MacDonald 2011, and Milano 2007. Their conclusions are also largely in agreement with this review. Song 2016 found a statistically significant improvement in the American Shoulder and Elbow Surgeons (ASES) score in the acromioplasty group but the difference was clinically unimportant.
Authors' conclusions
Implications for practice.
Moderate‐ to low‐quality evidence in this review indicates that we are uncertain if rotator cuff repair surgery provides any clinically meaningful benefits in people with symptomatic rotator cuff tears. Well‐designed and rigorous placebo‐controlled trials in this area are lacking. This is similar to the conclusions in the early version of this review, notwithstanding that there was only one available study in this population at the time the original review was published (Coghlan 2008).
The risk for adverse or serious events associated with surgery is probably low. Due to low event rates, we could not obtain a reliable estimate of the risk of these events from the trials in this review. However, as serious adverse events such as infections, deep venous thrombosis, pneumonia, peripheral nerve damage and death following arthroscopic shoulder surgery have been reported in observational studies (Hill 2017; Shields 2015), the potential risks must be weighed against the limited evidence of benefits.
Participants in the trials experienced mild pain and impaired function in the follow‐up regardless of the treatment. People with symptomatic rotator cuff tears will likely experience further pain and limitation of function, but at the moment, we are uncertain whether rotator cuff repair surgery provides clinically meaningful benefits; it may provide little or no clinically important benefits with respect to pain, function, overall quality of life or participant‐rated global assessment of treatment success when compared with exercises, with or without glucocorticoid injections. Furthermore, a substantial portion of people may have a re‐tear within one to five years following surgery.
Acromioplasty in conjunction with surgical repair probably offers little or no benefits in pain and function and may not result in better treatment success or decrease the risk of re‐tears or failure to heal.
The conclusions of this review may not apply to traumatic tears in a younger population.
Implications for research.
Since there were no placebo‐controlled trials in this review and unblinded trials are prone to overestimate the benefits (Savovic 2012), further unbiased research may find smaller effect sizes for surgery and affect the estimates of this review. Future trials should preferably include a placebo control and participants and hospital personnel should be blinded to treatment allocation to reduce potential detection and performance biases. Trials should include a long‐term follow‐up (>10 years) to assess if the repair of the tendons could prevent the development of shoulder joint arthritis and subsequent need for arthroplasty.
At the end of follow‐up, participants in the non‐operative treatment groups had little pain (mean pain was 1.7 after a year, and 1.4 at two years), so it seems unlikely that trials would find a clinically important improvement in pain at these time points. To show a transient benefit, the improvement should manifest early, which may not occur after surgery. Functional scores following non‐operative interventions were also not much worse than those without shoulder disorders based upon normative population values at the end of follow‐up: 73 at one year and 78 at two years (normative values: 81 to 83 points in women and 86 to 91 in men over 50; minimal important difference (MID) 8.3 points) (Yian 2005) This implies that finding clinically important differences may be difficult.
Trials should clearly define the inclusion criteria regarding the extent of tear and history of trauma and ideally include sufficient samples to allow analyses in both traumatic and non‐traumatic onset of symptoms. Authors should also consider assessing the effect of surgery in younger populations as we identified no studies in this population.
While there is a subgroup of participants who will be dissatisfied with non‐operative treatment, it is uncertain whether or not they would be better off with surgery. Further rigorous research in this subpopulation may be warranted.
We will update this review once results from rotator cuff repair versus placebo‐surgery are available. If surgery is shown to have a clinically meaningful benefit over placebo, then future reviews may include comparisons of different types of repair techniques.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2019 | Amended | Typing error fixed |
History
Review first published: Issue 12, 2019
| Date | Event | Description |
|---|---|---|
| 8 January 2019 | New search has been performed | Update of the review Subacromial decompression surgery for rotator cuff disease, divided into Surgery for calcifying tendinopathy of the shoulder and this review. |
| 8 January 2019 | New citation required and conclusions have changed | 8 trials added for this comparison |
| 1 December 2008 | Amended | Converted to new review format. CMSG ID: C083‐R |
Acknowledgements
Tamara Rader, Knowledge Translation Specialist, Cochrane Musculoskeletal Group, for designing and executing the search strategy for the previous review.
Simeon King, Vanderbilt University School of Medicine, Nashville, TN, US for contributions to earlier work on the update in study selection.
We are grateful to Gustavo Zanoli (Milan, Italy), Mario Lenza (Sao Paulo, Brazil) and Mohit Patralekh for their comments during the peer‐review process.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 shoulder/ (11615)
2 rotator cuff/ (5502)
3 1 or 2 (16578)
4 calcium/ (257302)
5 exp bursitis/ (4455)
6 4 or 5 (261746)
7 3 and 6 (732)
8 shoulder pain/ (4075)
9 shoulder impingement syndrome/ (1581)
10 rotator cuff injuries/ (4533)
11 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (13224)
12 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain)).tw. (12827)
13 or/7‐12 (22497)
14 exp Surgical Procedures, Operative/ (2847798)
15 su.fs. (1819530)
16 (surger$ or surgical$ or operat$).tw. (1944943)
17 decompress$.tw. (34248)
18 bursectom$.tw. (574)
19 acromioplast$.tw. (463)
20 (calcium adj remov$).tw. (301)
21 debrid$.tw. (19906)
22 ARTHROSCOPY/ (20572)
23 arthroscop$.tw. (21931)
24 or/14‐23 (4145221)
25 13 and 24 (11569)
26 randomized controlled trial.pt. (458491)
27 controlled clinical trial.pt. (92309)
28 randomized.ab. (357908)
29 placebo.ab. (171901)
30 drug therapy.fs. (2009606)
31 randomly.ab. (248101)
32 trial.ab. (371145)
33 groups.ab. (1551065)
34 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 (3870583)
35 exp animals/ not humans.sh. (4446637)
36 34 not 35 (3302687)
37 25 and 36 (2282)
38 limit 37 to yr="2006 ‐Current" (1692)
Appendix 2. Embase (Ovid) search strategy
1 shoulder/ (30424)
2 rotator cuff/ (5433)
3 1 or 2 (33875)
4 calcium/ (273333)
5 exp bursitis/ (4560)
6 4 or 5 (277860)
7 3 and 6 (675)
8 shoulder pain/ (13768)
9 exp shoulder impingement syndrome/ (2426)
10 exp rotator cuff injury/ (9234)
11 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (18601)
12 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain)).tw. (19693)
13 or/7‐12 (37559)
14 exp surgery/ (4329852)
15 su.fs. (1974350)
16 (surger$ or surgical$ or operat$).tw. (2921649)
17 decompress$.tw. (50428)
18 bursectom$.tw. (697)
19 acromioplast$.tw. (613)
20 (calcium adj remov$).tw. (369)
21 debridement/ (34049)
22 debrid$.tw. (28473)
23 shoulder arthroscopy/ (1647)
24 arthroscop$.tw. (32269)
25 or/14‐24 (5736356)
26 13 and 25 (19708)
27 random$.tw. (1294111)
28 factorial$.tw. (32556)
29 crossover$.tw. (65747)
30 cross over.tw. (28947)
31 cross‐over.tw. (28947)
32 placebo$.tw. (272731)
33 (doubl$ adj blind$).tw. (188634)
34 (singl$ adj blind$).tw. (21007)
35 assign$.tw. (335768)
36 allocat$.tw. (126659)
37 volunteer$.tw. (231815)
38 crossover procedure/ (55181)
39 double blind procedure/ (148991)
40 randomized controlled trial/ (499001)
41 single blind procedure/ (31120)
42 or/27‐41 (1997713)
43 26 and 42 (2117)
44 limit 43 to exclude medline journals (219)
45 limit 44 to yr="2006 ‐Current" (202)
Appendix 3. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Shoulder] this term only
#2 MeSH descriptor: [Rotator Cuff] this term only
#3 #1 or #2
#4 MeSH descriptor: [Calcium] this term only
#5 MeSH descriptor: [Bursitis] 1 tree(s) exploded
#6 #4 or #5
#7 #3 and #6
#8 MeSH descriptor: [Shoulder Pain] this term only
#9 MeSH descriptor: [Shoulder Impingement Syndrome] this term only
#10 MeSH descriptor: [Rotator Cuff Injuries] this term only
#11 rotator cuff:ti,ab or supraspinatus:ti,ab or infraspinatus:ti,ab or subscapular*:ti,ab or teres:ti,ab
#12 ((shoulder*:ti,ab or subacromial:ti,ab or rotator cuff:ti,ab) near/5 (tendon*:ti,ab or tendin*:ti,ab or bursitis:ti,ab or calcium:ti,ab or calcif*:ti,ab or impinge*:ti,ab or tear*:ti,ab or pain:ti,ab))
#13 #7 or #8 or #9 or #10 or #11 or #12
#14 MeSH descriptor: [Surgical Procedures, Operative] explode all trees
#15 (surger*:ti,ab or surgical*:ti,ab or operat*:ti,ab)
#16 decompress*:ti,ab
#17 bursectom*:ti,ab
#18 acromioplast*:ti,ab
#19 (calcium:ti,ab next remov*:ti,ab)
#20 debrid*:ti,ab
#21 MeSH descriptor: [Arthroscopy] this term only
#22 arthroscop*:ti,ab
#23 #14 or #15 or #16 or #17 or #18 or #19 or #22
#24 #13 and #23 Publication Year from 2006 to 2018
Appendix 4. Clinicaltrials.gov
Rotator cuff tear or rotator cuff rupture or impingement in Condition
Appendix 5. WHO ITCRP
Rotator cuff tear AND
surg* or decompress* or bursectom* acromioplast* or debrid* or arthroscop* or repair or suture
(without synonyms)
Data and analyses
Comparison 1. Repair with or without subacromial decompression versus non‐operative treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS; 0‐10, 0 is best) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 207 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.04, ‐0.22] |
| 1.2 12 months | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.30, ‐0.43] |
| 1.3 > 12 months | 2 | 212 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.20, ‐0.32] |
| 1.4 5 years | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.58, ‐0.42] |
| 2 Function (Constant score; 0‐100, 100 is best) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 207 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐3.95, 4.30] |
| 2.2 12 months | 3 | 269 | Mean Difference (IV, Random, 95% CI) | 5.98 [2.43, 9.54] |
| 2.3 > 12 months | 2 | 212 | Mean Difference (IV, Random, 95% CI) | 2.83 [‐1.16, 6.83] |
| 2.4 5 years | 1 | 103 | Mean Difference (IV, Random, 95% CI) | 5.60 [‐1.30, 12.50] |
| 3 Participant rated global assessment of success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 >12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Health‐related quality of life (SF‐36 mental component, 0‐100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 > 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 5 years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.4. Analysis.
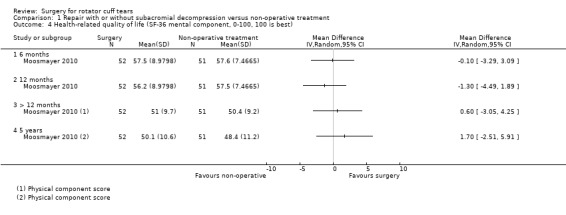
Comparison 1 Repair with or without subacromial decompression versus non‐operative treatment, Outcome 4 Health‐related quality of life (SF‐36 mental component, 0‐100, 100 is best).
Comparison 2. Repair with acromioplasty versus repair only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (0 to 10, 0 is best) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.98, 1.37] |
| 1.2 12 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.00, ‐0.21] |
| 1.3 >12 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.48, 0.49] |
| 2 Function (0 to 100, 100 is best) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 3 | 280 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.05, 0.42] |
| 2.2 12 months | 4 | 361 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.07, 0.34] |
| 2.3 >12 months | 4 | 352 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.04, 0.47] |
| 3 Repair failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Subgroup analysis by acromion type for pain at 2 years | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Type I | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.55, 1.55] |
| 5.2 Type II | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.28, 1.28] |
| 5.3 Type III | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.53, 0.73] |
| 6 Subgroup analysis by acromion type for function at 2 years (various measures 0 to 100, higher is better) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Type I | 2 | 22 | Mean Difference (IV, Random, 95% CI) | ‐8.21 [‐23.55, 7.14] |
| 6.2 Types II | 2 | 107 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐5.10, 7.05] |
| 6.3 Type III | 2 | 39 | Mean Difference (IV, Random, 95% CI) | 2.32 [‐9.96, 14.61] |
Comparison 3. Repair with subacromial decompression versus subacomial decompression alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS; 0 to 10, 0 is best) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 months | 1 | 101 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.19, 0.59] |
| 1.2 12 months | 2 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.67, 0.09] |
| 1.3 >12 months | 1 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.52, 0.23] |
| 2 Function (Constant score 0 to 100, 100 is best) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐7.36, 2.56] |
| 2.2 12 months | 2 | 239 | Mean Difference (IV, Random, 95% CI) | 4.12 [‐2.03, 10.27] |
| 2.3 >12 months | 2 | 214 | Mean Difference (IV, Random, 95% CI) | 4.09 [0.89, 7.30] |
| 3 Participant‐rated global assessment of success | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 12 months | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.09] |
| 3.2 >12 months | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrams 2014.
| Methods | Design: single‐centre, parallel, two‐arm, randomised controlled trial Setting: one orthopedic centre in the USA Timing: October 2007 and January 2011 Interventions: rotator cuff repair with acromioplasty versus rotator cuff repair without acromioplasty Sample size: a post hoc power analysis was conducted before enrolment closure based on previous study, which reported ASES scores at 2‐year follow‐up in similar comparison and found a 95% confidence interval of ‐13.0 to 3.2 points for the score difference. Using the reported SD and MID of 10 in the ASES score, and group size of 45 patients each, we would be powered at 81.1% to detect a difference. The authors set a goal of 50 patients in each of the groups for the current investigation to account for attrition. Analysis: per protocol analysis (the authors excluded participants after randomisation if they did not have full‐thickness tears in the operation) | |
| Participants |
Number of participants 156 assessed for eligibility 42 excluded; 21 for not meeting inclusion criteria, 15 refused to participate, 6 for other reasons 114 randomised (65 to repair and subacromial decompression (SAD) and 49 to repair) At 6, 12 and 24 months data available for 52 in repair and SAD and for 43 in repair group Inclusion criteria
Exclusion criteria
Baseline data Rotator cuff repair with acromioplasty Mean (SD) age 59.6 (8.2) Number (%) females 15 (29) Number (%) smokers 2 (4) Number (%) diabetes 5 (10) Number (%) workers compensation 8 (15) Number (%) acute tears 27 (52) Number (SD) of involved tendons 1.4 (0.6) Mean (SD) size of tear, mm 25.8 (10.8) Mean (SD) retraction of tendon, mm 12.3 (11.9) Mean (SD) ASES score 48.8 (18.2) Mean (SD) SST score 5.2 (2.6) Mean (SD) UCLA score 10.7 (2.9) Mean (SD) VAS for pain 4.4 (2.3) Mean (SD) Constant score 51.9 (17.2) Rotator cuff repair without acromioplasty Mean (SD) age 58.0 (8.0) Number (%) females 16 (37) Number (%) smokers 4 (9) Number (%) diabetes 3 (7) Number (%) workers compensation 7 (16.3) Number (%) acute tears 24 (56) Number (SD) of involved tendons 1.3 (0.5) Mean (SD) size of tear, mm 25.8 (8.5) Mean (SD) retraction of tendon, mm 12.5 (10.4) Mean (SD) ASES score 55.1 (19.1) Mean (SD) SST score 5.1 (3.0) Mean (SD) UCLA score 11.8 (2.8) Mean (SD) VAS for pain 3.8 (2.5) Mean (SD) Constant score 48.3 (17.1) |
|
| Interventions | All repairs were performed arthroscopically by four trained surgeons. Repair with acromioplasty group Preferred suture passing techniques typically included use of a curved shuttling device through the posterior portal while viewing from lateral. Details including type and number of anchors used as well as repair configuration were recorded. Those in the acromioplasty group underwent release of the coracoacromial ligament and flattening of the anterior inferior surface of the acromion. This was performed with a combination of shaver and electrocautery use to remove bursal tissue and define the lateral border and undersurface of the acromion. A motorised bur was then used to remove bone until the undersurface of the acromion was flat when viewed from the lateral portal using a posterior cutting block technique. Rotator cuff repair was performed in standard fashion by use of a combination of suture passing devices. Extensive releases were not performed with the exception of rotator interval releases to assist with reduction of retracted tears. Repair without acromioplasty group Repair was performed similarly as in repair and SAD group. Extension of bursectomy not described Both groups All patients were discharged on the day of the surgery. Physical therapy was standardised for both groups; it was instituted approximately 1 to 2 weeks after surgery, after the first postoperative visit, and focused on passive motion only. Sling immobilisation when patients were not performing physical therapy or a home exercise program was continued for 6 weeks after surgery. Active range of motion was begun at 6 weeks, and strengthening was deferred until 12 weeks postoperatively. |
|
| Outcomes | Outcomes were assessed at 6 months, 12 months and two years.
Outcomes:
Outcomes used in this review
|
|
| Source of funding | Several authors report receiving funding from medical industry but the authors do not report any source of funding for this particular work. | |
| Notes |
Trial registration: N/A Data analysis: we used data from long‐term (mean of 92 months) follow‐up in repair failure analysis (Analysis 2.3). Withdrawals: in repair and SAD group, 13/65 (20%) were lost to follow‐up. In repair‐only group 6/49 (12%) were lost to follow‐up Re‐operations: in repair and SAD group, 1 re‐repair. In repair‐only group 3 re‐repairs and 1 capsular release and biceps tenotomy. Adverse events: the authors did not report if there were any adverse events besides re‐repairs. Serious adverse events: the authors did not report if there were any serious adverse events or not. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised to acromioplasty or non‐acromioplasty groups via a sealed envelope" No further safe guards were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not reported if the hospital staff or the study personnel were blinded to the intervention. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Low risk | Quote: "Assignments were not disclosed to the patient". The postoperative treatment and data collection were similar in both groups. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | Unclear risk | Trial authors did not report this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/49 (12%) in repair versus 13/65 (20%) in repair with acromioplasty group were lost to follow‐up with reasons not reported. Not likely to bias the results significantly |
| Selective reporting (reporting bias) | High risk | Protocol not available, adverse events incompletely reported. SF‐12 measured but not reported. |
| Other bias | High risk | There was imbalance in the treatment received. Quote "An increased number of patients who were enrolled in the non‐acromioplasty group were examined intraoperatively and excluded for a lack of full‐thickness rotator cuff tear" It is unclear if this could bias the outcomes in favour of acromioplasty. |
Dezaly 2011.
| Methods | Design: a single‐centre randomised trial between February 2007 and July 2008. Setting: hospital in France Timing: from February 2007 to July 2008 Interventions: rotator cuff repair with subacromial decompression and biceps tenotomy versus subacromial decompression and biceps tenotomy Sample size: the minimum number of patients required per group was 54 to achieve 0.90 power and an expected 10‐point difference in weighted Constant score,allowing for 10% loss to follow‐up. Analysis: at one year ITT analysis; at four years, authors report per‐protocol analysis (excluding one participant from subacromial decompression and biceps tenotomy group who had received later rotator cuff repair) | |
| Participants |
Number of participants Number screened not reported 142 met inclusion criteria 12 excluded due to irreducible retracted rotator‐cuff tear or spontaneous long‐head biceps tear 130 randomised (70 for repair with subacromial decompression and biceps tenotomy group and 60 subacromial decompression and biceps tenotomy group) Data available for 127 (68 (97%) in repair with subacromial decompression and biceps tenotomy group and 59 (98%) in subacromial decompression and biceps tenotomy group) at one year Data available for 102 (54 (77%) in repair with subacromial decompression and biceps tenotomy group and 48 (80%) in subacromial decompression and biceps tenotomy group at four years. Inclusion criteria
Exclusion criteria
Baseline data Repair with subacromial decompression and biceps tenotomy group Mean (SD) age, years 67.5 (4.6) Number (%) females 38 (56) Number (%) dominant 41 (76) Constant score 44 (12.1) Constant pain sub scale 5.4 (2.8) Number (%) retracted tear 18 (27) Subacromial decompression and biceps tenotomy group Mean (SD) age, years 68.1 (5.6) Number (%) females 31 (53) Number (%) dominant 33 (67) Constant score 43.5 (12.3) Constant pain sub scale 5.5 (2.8) Number (%) retracted tear 9 (15) |
|
| Interventions | All the procedures were performed under arthroscopy in lateral decubitus with axial traction. Rotator cuff repair with subacromial decompression and biceps tenotomy group The first step consisted of glenohumeral and subacromial exploration to assess lesion size (distal,intermediate or retracted) and confirm reparability, and check that there was no spontaneous long‐head biceps tear or subscapularis extension. Acromioplasty and biceps long‐head tenotomy were performed in all cases. In the repair group, metallic suture anchors were used systematically, with single‐row suture in 24 cases and double‐row in 44 after rasping the greater tuberosity. Subacromial decompression and biceps tenotomy group After arthroscopy, acromioplasty and biceps long‐head tenotomy were performed for all participants. Both groups Postoperative course in both groups comprised early self‐rehabilitation with partial immobilisation in a simple sling for four weeks. |
|
| Outcomes | The outcomes were assessed at one and four years. Primary outcome 'Weighted' Constant score (at one year, value adjusted for age and sex. At 4 years absolute score; in both time points scale is 0 to 100; higher indicates better) Secondary outcome Satisfaction questionnaire with four response options at four‐year follow‐up only (very satisfied, satisfied,somewhat satisfied, dissatisfied) Tendon healing by ultrasound (healed versus failed) Adverse events Acromiohumeral distance at four years follow‐up Outcomes used in this review Mean pain; Constant score pain sub scale Mean function; Constant score Global assessment of satisfaction; satisfaction questionnaire Failure; proportion of failed repairs in ultrasound examination |
|
| Source of funding | Source of funding not declared. The authors report no conflicts of interest. | |
| Notes |
Trial registration: no registration Data analysis: we reversed Constant pain subscale (Analysis 2.1) to lower is better. Adjusted values (for age and sex) reported for Constant score at one year. Adverse events were reported for all participants (7.9%) and not by group. The authors did not respond to email. Withdrawals: at the one‐year follow‐up, two participants were lost and one had died (two from repair group and one from ASD group). At mean of four years follow‐up, six participants had died and 21 had been lost to follow‐up. The authors did not report reasons per group. Cross overs: none Adverse events: 10 patients had at least one postoperative complication. No revision surgery was performed. Authors did not report the adverse event per group. Three participants had spontaneously reversible neurapraxia of the brachial plexus.Three showed painful postoperative stiffness due to adhesive capsulitis; In four cases (in repair group),metal anchor migration was found on X‐ray at one month postoperatively. Serious adverse events: authors did not report serious adverse events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On the day before surgery, randomisation was performed" Random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"On the day before surgery, randomisation was performed and the patient was informed as to which technique had been attributed". The authors did not attempt blinding |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Quote:"The patient was informed as to which technique had been attributed (on the day before surgery)" The authors did not attempt blinding. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | Unclear risk | As the repairs were performed using metal anchors, the assessor would know, which intervention the participant had. The radiological outcomes were not included in the analysis as re‐tears were measured only in repair group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/70 (3%) in repair group and 1/60 (2%) in subacromial decompression group were lost to follow‐up at one year and 16/70 (23%) in repair group and 12/60 (20%) subacromial decompression group were lost to follow‐up at four years. Reasons not reported per group except data from one participant from the decompression group were excluded due to later surgery (repair of rotator cuff) |
| Selective reporting (reporting bias) | High risk | No protocol. The authors report adverse event for the whole data set and not per group. Quote: "The complication rate was not different between the two treatment groups". Constant score pain sub scale not reported at four years. |
| Other bias | High risk | Imbalance in participants receiving postoperative physical therapy; in subacromial decompression (and tenotomy) group 58% and in repair group 85% received physical therapy. This may overestimate the benefits of repair group. |
Gartsman 2004.
| Methods | Design: single‐centre, parallel, two‐arm, randomised controlled trial Setting: USA Timing: not reported Interventions: rotator cuff repair + subacromial decompression versus rotator cuff repair only Sample size: one goal of the study was to test the null hypothesis that the two group means would be equal after undergoing treatment. The criterion for significance was set at .05, and the test was 2‐tailed. With a sample size of 45 and 45 for the two groups, the study had a power of 99.5% to yield a statistically significant result should a true difference exist between groups. The authors assumed that the mean difference between groups would be 7.0 (corresponding to a mean of (87.0 vs 80.0), and the common within‐group SD would be 10.0. This effect was selected as the smallest effect that would be important to detect. N = 93. Analysis: ITT analysis. | |
| Participants |
Number of participants 93 randomised (47 to rotator cuff repair and SAD and 46 to repair only) Inclusion criteria
Exclusion criteria
Baseline data Subacromial decompression and repair group Mean (range) age 59.3 (39 to 81) Tear length (range), mm 20.1 (10 to 25) ASES score (range) 31.1 (20 to 46.7) Repair only group Mean (range) age 60 (37 to 79) Tear length (range), mm 22.5 (15 to 51) ASES score (range) 31.0 (18.3 to 41.7) |
|
| Interventions | The operations were performed arthroscopically using suture anchors Rotator cuff repair and subacromial decompression group Details not described Rotator cuff repair group Details not described Both groups All patients were managed postoperatively in a similar fashion. Continuous passive motion was used for the first 2 weeks after surgery. Two weeks after surgery, the patients were examined in the clinic, and passive range of motion was continued at home with patients using a dowel to move the shoulder in elevation and external rotation. We examined them 6 weeks after surgery, at which time we discontinued the sling and instructed patients with regard to active range‐of‐motion exercises. We next examined the patients 3 months after surgery and instructed them to start exercises with surgical tubing for muscle strengthening. |
|
| Outcomes | Quote:"The patients were seen at 6 months and again at 1 year after surgery. Minimum follow‐up was 1 year (mean (SD) 15.6 (3.3) months)" Primary outcome ASES score (0 to 100, higher score indicates better function) Outcome used in this review ASES score at mean of 15.6 months |
|
| Source of funding | Not reported | |
| Notes |
Trial registration: N/A Data analysis: postoperative ASES questionnaire appears not to have been administered at any particular time point (mean 15 months). This data used in 12‐month function analysis. Adjusted (for tear length) values reported and used. Withdrawals: no reported withdrawals Re‐operations: the authors did not report if there were re‐operations or not. Adverse events: the authors did not report if there were any adverse events or not. Serious adverse events: the authors did not report if there were any serious adverse events or not. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table used for sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Once we repaired the rotator cuff tear, the circulating nurse consulted a random‐number table to place the patient in either group 1 or group 2. We then performed an arthroscopic subacromial decompression on group 1 patients" It is unclear if the random number list was concealed from the study personnel and the participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were unaware of their treatment. The authors did not report if the personnel was blinded but state that participants were treated similarly postoperatively. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Low risk | Quote "Patients did not know to which group they had been assigned" |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | Unclear risk | The authors do not report this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. It is unclear if adverse events were measured. |
| Other bias | Low risk | None apparent |
Kukkonen 2014.
| Methods | Design: multi‐centre, parallel, three‐arm, randomised controlled trial Setting: 2 tertiary and 1 secondary care hospitals in Finland Timing: October 2007 and December 2012 Interventions: rotator cuff repair + subacromial decompression + exercises versus subacromial decompression + exercises versus exercises. Sample size: the power calculations were based on the assumed statistical behaviour of the Constant score. The mean score value at baseline was assumed to be 50 (SD 10). The score in the best treatment group at follow‐up was assumed to be 70, and in the worst treatment group 60.25. The correlation between the measurements during the follow‐up was estimated to be 0.40 to 0.50 (SD 20). By analysis of variance (ANOVA), with alpha = 0.05 and power = 85%, statistical significance could be reached with 51 participants per treatment group. The expected drop‐out rate was set at 15%, so the number of participants per group was 60. Analysis: per protocol analysis. The authors excluded participants with no full‐thickness tear after allocation and data from these participants were not analysed at follow‐up time points. Participants who crossed over, were analysed as allocated. | |
| Participants |
Number of participants
370 screened clinically, 271 scanned with MRI
180 eligible
180 randomised (60 repair, decompression and exercises, 60 to decompression + exercises and 60 to exercises alone)
174 received treatment as randomised
At 3 months, data available for 170 (55/59 (93%) for repair, decompression and exercises, 58/59 (98%) for decompression and exercises, and 57/58 (98%) for exercises alone) At 6 months, data available for 158 (47/59 (80%) for repair, decompression and exercises, 54/59 (92%) for decompression and exercises and 57/58 (98%) for exercises alone At 12 months, data available for 167 (55/59 (93%) for repair, decompression and exercises, 57/59 (97%) for decompression and exercises, and 55/58 (95%) for exercises alone) Inclusion criteria
Exclusion criteria
Baseline data Repair, decompression and exercise group Mean (SD) age 65: (6.0) years Number (%) females: 26 (47) Number (%) participants with right side affected n (%): 36 (65) Number (%) participants working: 23 (42) Number (%) participants on sick leave: 1 (2) Number (%) participants retired: 30 (55) Number (%) smokers: 8 (15) Number (%) participants with prior corticosteroid injection: 31 (56) Mean (SD) duration of symptoms: 28 (9.5) months Mean (95% CI) Constant score: 58 (54.1‐61.9) Mean (95% CI) pain (VAS) 2.6 (2.0 to 3.2) Mean (SD) tear size in mm: 8.5 (4.0) Number (%) participants with biceps pathology present: 16 (29) Number (%) participants with OA changes in GH‐joint: 23 (42) Number (%) participants with OA in AC‐joint: 51 (93) Number (%) participants with grade II fatty degeneration 26/55 (47) Number (%) participants with grade III fatty degeneration 2/55 (4) Number (%) participants with muscle atrophy 2/57(4) Decompression + exercise group Mean (SD) age 65 (5.1) years Number (%)females: 28 (49) Number (%) participants with right side affected: 33 (58) Number (%) participants working: 10 (18) Number (%) participants on sick leave: 1(2) Number (%) participants retired: 42 (74) Number (%) smokers: 5 (9) Number (%) participants with prior corticosteroid injection: 33 (58) Mean (SD) duration of symptoms: 28 (9.7) months Mean (95% CI) Constant score: 59.6 (55.8‐63.4) Mean (95% CI) pain (VAS) 2.5 (1.8 to 3.2) Mean (SD) tear size in mm: 9.3 (5.3) Number (%) participants with biceps pathology present: 21 (37) Number (%) participants with OA changes in GH‐joint: 28 (49) Number (%) participants with OA in AC‐joint: 52 (89) Number (%) participants with grade II fatty degeneration 26/57 (46) Number (%) participants with grade III fatty degeneration 5/57 (9) Number (%) participants with muscle atrophy 5/60 (8) Exercise group Mean (SD) age: 65 (5.8) years Number (%) females: 31 (56) Number (%) participants with right side affected: 41 (75) Number (%) participants working: 17 (31) Number (%) participants with on sick leave: 5 (9) Number (%) participants retired: 32 (58) Number (%) smokers: 10 (18) Number (%) participants with prior corticosteroid injection: 39 (71) Mean duration of symptoms: 26 (9.9) months Mean Constant score (95% CI): 57.8 (53.9‐61.7) Mean (SD) tear size in mm: 9.6 (5.2) Number (%) participants with biceps pathology present: 16 (29) Number (%) participants with OA changes in GH‐joint: 19 (35) Number (%) participants with OA in AC‐joint: 48 (87) Number (%) participants with grade 2 fatty degeneration 29/55 (53) Number (%) participants with grade 3 fatty degeneration 1/55 (2) Mean (95% CI) pain (VAS) 2.7 (1.9 to 3.5) Number (%) participants with muscle atrophy 1/60 (2) |
|
| Interventions | All operations were performed arthroscopically in a standardised manner by four experienced shoulder surgeons. A physiotherapist trained in shoulder rehabilitation gave the patient written information and guided the patient in how to perform a standardised training exercise protocol at home. Exercise group A physiotherapist trained in shoulder rehabilitation gave the patient written information and guidance for exercises to be conducted at home. The exercise protocol was standardised and started with exercises aimed at improving glenohumeral motion and active scapular retraction for the first six weeks. Then static and dynamic exercises for the scapular and glenohumeral musculature were gradually increased from six weeks to 12 weeks, after which the participant increased resistance and strength training up to six months. In addition to written instructions the patient was referred for ten sessions of physiotherapy in an outpatient health care facility where their progress was monitored. Subacromial decompression group Subacromial debridement and an arthroscopic acromioplasty were carried out by smoothing the inferior surface of the acromion from a postero‐anterior direction. The sagittal size of the supraspinatus tear was measured with a probe. In addition, 6 mm of the acromioclavicular (AC) joint was resected if it had been painful before surgery and if there were severe degenerative changes in the AC joint on the MRI. If the long head of the biceps tendon was unstable or frayed, a biceps tenotomy was also performed. After the operation a physiotherapist gave the patient guidance on how to exercise to improve free glenohumeral motion and how to retract the scapula actively. After three weeks the physiotherapist controlled the progress of rehabilitation and gave the patient written information for movement and gradual resistance exercises to be conducted at home and sessions of physiotherapy, as for exercise group. Subacromial decompression and rotator cuff repair group Subacromial debridement and acromioplasty were performed arthroscopically. The sagittal size of the supraspinatus tear was measured with a probe. The rotator cuff was repaired anatomically using standard titanium bone anchors with non‐absorbable sutures (Corkscrew FT II; Arthrex Inc., Naples, Florida or Twinfix; Smith‐Nephew, Andover, Massachusetts). AC resection and/or biceps tenotomy were per‐ formed, if indicated. After the operation the arm was immobilised in a sling for three weeks after which the rehabilitation followed the same regime as exercise group. |
|
| Outcomes | Outcomes were assessed at 3 months, 6 months and 12 months
Primary outcome:
Secondary outcomes:
Outcomes used in this review
|
|
| Source of funding | The authors report no conflicts of interest that could have affected the report. | |
| Notes |
Trial registration: NCT01116518 Comparison: we included the following comparisons: repair with subacromial decompression versus non‐operative treatment, repair with subacromial decompression versus decompression alone. We excluded subacromial decompression versus non‐operative treatment as it did not meet inclusion criteria. Data analysis: for function at 6 months, we extracted the Constant score from figure; at 12 months the Constant score was reported; at 24 months, the change from baseline was reported (Analysis 1.2). For pain, we extracted values from figure (Analysis 1.1). Withdrawals: in exercise group, 3/60 (5%); in subacromial decompression group 1/57(2%); in subacromial decompression and repair group 5/60 (8%). Data missing: 2/60 (3%) in exercise; 2/60 (3%) in subacromial decompression; 0/60(0%) in decompression and repair group at 12 months. The authors excluded participants who withdrew or had missing data. Cross overs: in exercise group 4/60 (7%) had rotator cuff repair and in subacromial decompression group 1/60 (2%) had rotator cuff repair by 12 months. Their data were analysed in their allocated groups. Two participants were excluded after allocation because they were found to have intact supraspinatus tendons at surgery. Adverse events: no treatment related complications in any of the groups Serious adverse events: the authors did not report if there were any serious adverse events or not. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"After consent, the study nurse randomised the patients into one of the three treatment groups using sequentially numbered, opaque, sealed envelopes. The randomisation process was stratified according to participating hospital". No further information was given about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"After randomisation, the patient and the treating physician were openly informed of the treatment group." Participants and personnel were not blinded. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were not blinded. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | High risk | Constant scores were recorded by an independent study nurse at each time point. It is not stated if this nurse was blinded to the allocation. All MRI images were re‐evaluated at the end of follow‐up by two musculoskeletal radiologists (KTM and EKJT) blinded to patient data but they could not have been reliably blinded to treatment intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: in exercise group, 3/60 (5%); in subacromial decompression (ASD) group 1/57(2%); in ASD and repair group 5/60 (8%). Data missing at 6 months:1/60 (2%) in exercise; 5/60 (8%) in ASD group; 8/60 (13%) in ASD and repair group. Data missing at 12 months: 2/60 (3%) in exercise; 2/60 (3%) in ASD; 0/60 (0%) in ASD and repair group. Data missing at 24 months: 2/60 (3%) in exercise group; 1/60 (2%) in ASD group; 1/60 (2%) in ASD and repair group. The authors have excluded participants who withdrew or had missing data at each time point. Small numbers dropped out and reasons for dropout reported and unlikely to influence results. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not reported and unclear if they were measured. Global satisfaction partially reported. |
| Other bias | Unclear risk | By one year, 4/60(7%) and by two years, 7/60 (12%) participants from the exercise group had rotator cuff repair during follow‐up but were analysed according to their allocated treatment groups. One participant from the subacromial decompression had rotator cuff repair during follow‐up. All participants were analysed according to their allocated treatment groups. An additional AC resection was performed in 7/57 (12%) in decompression group and 8/55 (15%) in decompression and repair group. An additional biceps tenotomy was performed in 29/57 (51%) in subacromial decompression group and in 23/55 (42%) in subacromial decompression and repair group. The cross overs and side interventions could bias the results regarding surgery versus non‐operative treatment but unclear if to an important level. |
Lambers Heerspink 2015.
| Methods | Design: multi‐centre, parallel, two‐arm, open‐label, randomised controlled trial Setting: 1 University and 2 regional hospitals in the Netherlands Timing: January 2009 until December 2012 Interventions: open rotator cuff repair, subacromial decompression and physical therapy versus up to three glucocorticoid injections, physical therapy (different to the surgical group) and analgesia (NSAODs, paracetamol or tramadol) Sample size: Quote "Sample size calculation was performed; primary out‐ come measure was the Constant‐Murley Score, whereby 10 points was considered a clinically relevant difference between the two groups, with a standard deviation of 20, alpha set on 5% and power on 80%. This resulted in a required number of 49 patients in each group. Assuming a dropout rate of 10%, two groups of 54 patients will have to be included." Final sample size 25 in surgery and 31 in non‐operative group. Analysis: per‐protocol analysis was the primary analysis with an ITT analysis last observation carried forward as a secondary analysis. | |
| Participants |
Number of participants
92 Screened
36 excluded (27 not meeting inclusion; 6 declined to participate; other reasons 3)
56 randomised (25 to surgery; 31 to exercises and injections)
Data available for 45 (20 (80%) in surgery; 25 (81%) in exercises and injections group) at 12 months. Inclusion criteria:
Exclusion criteria:
Baseline data Surgery group Mean (SD) age: 60.8 (7.2) years Number (%) females: 10 (40) Number (%) participants with right side affected: 12 (48) Number (%) participants with right hand dominance: 20 (80) Mean (SD) Constant score: 55.6 (18.4) Mean (SD) DSST score 5.5: (2.3) Mean (SSDD) pain on VAS: 6.7 (1.7) Mean (SD) VAS disability: 6.2 (1.7) Duration of symptoms (median): 12.5 months Exercise therapy group Mean (SD) age: 60.5 (7.0) years Number (%) females: 11 (35) Number (%) participants with right side: 20 (65) Number (%) participants with right‐hand dominance: 26 (84) Mean (SD) Constant score: 56.9 (15) DSST score: 6.1 (2.7) Mean (SD) pain in VAS: 6.3 (1.3) Mean (SD) disability in VAS: 5.8 (2.1) Duration of symptoms (median): 12 months |
|
| Interventions | Two experienced surgeons performed the operations Surgery (subacromial decompression and rotator cuff repair) Surgery was scheduled within 6 weeks of inclusion and was done with the patient under general anaesthesia, supplemented with an interscalene brachial plexus block. Operation was performed in beach chair position using an anterolateral mini‐open approach. Coracoacromial ligament was detached from its insertion, and the subacromial bursa was excised. The anteroinferior part of the acromion was removed. The footprint of the rotator cuff on the greater tuberosity was debrided, and a bleeding bony bed was created. Side‐to‐side repair and repair augmented with bone anchors were performed depending on the shape of the rupture. A side‐to‐side repair was performed in 6 patients. The deltoid muscle was reattached to the acromion by transosseous refixation. Postoperative therapy was 6 weeks of sling and passive motion exercises, then active motion until strengthening started at 3 months. Exercise treatment with glucocorticoid injection Participants underwent a standardised physical therapy protocol for the conservative treatment of rotator cuff tears. In addition to explaining the cause of the symptoms and the rehabilitation protocol, the physiotherapist advised about activities of daily living. Passive glenohumeral and scapulothoracic movements were performed, and static and dynamic exercises were started. Poor posture was corrected. In weeks 4 to 6, exercises were gradually increased, and deltoid training was started. In weeks 6 to 12, rehabilitation was aimed at further optimisation of mobility and strength regeneration of the remaining cuff and deltoid. Physical therapy was continued until patients reached an optimum range of motion and an improvement in strength was achieved. Patients in the exercise group were given an glucocorticoid injection in the subacromial space by a posterior approach before commencing exercises. If the first injection gave no pain relief, a second infiltration was performed under radiologic or ultrasound guidance. The number of subacromial infiltrations was limited to a maximum of three. Co‐interventions Analgesic medication with non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol, or tramadol was also offered to the participants in the exercise group as needed. It is not reported if the surgery group were given any injections or analgesics during follow‐up. |
|
| Outcomes | Outcomes were assessed at 6 weeks, 3 months, 6 months, and 12 months after surgery for the surgical group and after inclusion for the exercises + injection group
Primary outcome:
Secondary outcomes:
Outcomes used in this analysis
|
|
| Source of funding | This study received a grant from Anna Fonds (Nederland Orthopedisch Research en Educatie Fonds). There was no involvement in data collection, data analysis, the preparation, or editing of the manuscript by Anna Fonds. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article. | |
| Notes |
Trial registration: the Nethelands Trial Register NTR2343 Data analysis: trialist report only 12‐month data from per protocol analysis and Constant score from ITT‐analysis. Regrading function(Analysis 1.2), we used values from ITT‐analysis and regarding pain (Analysis 1.1) we used values from per protocol analysis. Withdrawals: in exercise group, 6/31 (19%) were lost (3 discontinued intervention, 1 died and 2 moved away. In surgery group 5/25 (20%) excluded (4 due to failed surgery or intact cuff and 1 moved away) Cross‐overs: three patients in the exercise group received surgery and were the same three participants reported to have discontinued the intervention. Adverse events: unclear if measured and not reported. One frozen shoulder in exercise group mentioned in discussion. Serious adverse events: unclear if measured. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Prefilled opaque sealed envelopes held the randomisation codes but no information was given about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were not blinded. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | High risk | Blinding of outcome assessor is not reported. Assessment by MRI at one year would not have been blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the exercise group, 6 (24%) were lost to follow‐up (3 discontinued intervention, 1 died and 2 moved away) In the surgery group, 5/25(20%) were lost or excluded post allocation (4 had large tears which could not be repaired and 1 participant moved away). |
| Selective reporting (reporting bias) | High risk | Pre‐specified outcomes were not reported at 6 weeks, 3 months, and 6 months. Only 12 month data were reported. |
| Other bias | Unclear risk | The trialists terminated recruitment early because the recruitment took so long time due to eligible participants declining participation in the trial and therefore the planned 70 participants/group was not achieved. |
MacDonald 2011.
| Methods | Design: multi‐centre, double‐blind, randomised, 1:1 parallel group trial Setting: two orthopaedic surgery facilities in Canada Timing: June 2003 to February 2011 (including all follow‐up examinations to twenty‐four months after surgery). Interventions: rotator cuff repair + subacromial decompression versus rotator cuff repair only Sample size: sample size calculation was based on estimates of the expected mean and standard deviation of the WORC score determined in a pilot study, 63% and 16%, respectively, and a difference in WORC score of 25% was considered clinically relevant. To achieve 80% power to detect a significant difference, with alpha at 0.05, 74 patients were required in the trial. The sample size was then inflated to 86 patients to account for an expected loss to follow‐up of 15%. Analysis: ITT analysis | |
| Participants |
Number of participants 102 assessed for eligibility 86 randomised (45 to repair only and 41 to repair and subacromial decompression; 44 received intervention in repair only and 39 in repair and decompression ) At three months, data available (ASES) for 34/45 (96%) in repair only and 24/41 (98%) repair and decompression group At six months, data available for 37/45 (82%) in repair only and 28/41 (68%) repair and decompression group At 12 months, data available for 27/45 (60%) in repair only and 26/41 (63%) repair and decompression group. At 18 months, data available for 22/45 (49%) in repair only and 22/41 (54%) repair and decompression group. At 24 months, data available for 34/45 (75%) in repair only and 32/41 (78%) repair and decompression group. Inclusion criteria
Exclusion criteria
Patients with a Workers’ Compensation claim or an unwillingness to be followed for the duration of the study. Baseline data All Mean (SD) age 56.8 (8.8) Number (%) female 30 (35) Rotator cuff repair group Mean (SD) age 57.1 (9.5) Number (%) female 15 (33) Mean (SD) WORC score 34.5(15.7) Mean (SD) ASES score 44(18.0) Rotator cuff repair and subacromial decompression group Mean (SD) age 56.4 (8.1) Number (%) female 15 (36) Mean (SD) WORC score 36.8 (21.1) Mean (SD) ASES score 45.2 (21.4) |
|
| Interventions | All surgical procedures were performed by one of two fellowship‐trained shoulder surgeons. Surgery was performed arthroscopically with the patient in the lateral decubitus position and under general anaesthesia Repair only group Arthroscope was inserted through a standard posterior portal. An anterior portal was established anterior to the acromioclavicular joint, and a lateral portal was made slightly inferior to the lateral border of the acromion. Instrumentation was done through these two portals. Assessment of the glenohumeral joint was performed with special attention paid to the labrum, biceps tendon, humeral head, and glenohumeral ligaments. Any frayed tissue in these regions was debrided, and the arthroscope was then redirected to the subacromial space. Any bursal tissue that obscured visualisation of the rotator cuff was removed. A tear in the tendon was visualised and adhesions removed from retracted tendons until full excursion was possible. The characteristics of the tear and its suitability for repair and inclusion in the study were assessed. Next, a soft‐tissue shaver or electrical ablation device was used to remove scar tissue between the supraspinatus tendon and the acromion or deltoid fascia.If additional release was necessary, the superior articular capsule was incised along the glenoid margin to allow increased excursion of the supraspinatus. Interval releases were performed as necessary but were limited to the rotator interval. This involved a release of the superior glenohumeral ligament and/or coracohumeral ligament to the glenoid rim to facilitate re‐approximation of the tendon to the insertion site. A cancellous osseous bed was prepared at the site of the proposed attachment of the tendon between the articular cartilage of the head of the humerus and the greater tuberosity. A burr was used to remove a thin layer of cortical bone, and at least one bone anchor loaded with braided, non‐absorbable suture was placed lateral to the cancellous bone surface in a single‐row configuration. Sutures were passed through the supraspinatus tendon approximately 5 mm from the site of the tear. The number of suture anchors varied with the length of the tear. Traction was placed on each suture in the margin of the tendon to reduce the tendon to its repair site and to allow tying of the suture without excessive tension with the upper extremity in the adducted position. If this could not be accomplished, the tendon was repaired by attaching it medial to its anatomical location. After all of the repair sutures were tied, the traction suture was removed and the incisions were closed with absorbable sutures and adhesive tape. Participant in this group did not undergo either the division of the coracoacromial ligament or partial resection of the acromion. Rotator cuff repair and subacromial decompression Acromioplasty was performed before tendon repair by first releasing the coracoacromial ligament off the anterior undersurface of the acromion by use of an arthroscopic soft‐tissue shaving resector and then thinning the inferior surface of the acromion, with a motorised burr, until it was flat. A motorised arthroscopic burr was then again used to smooth the acromial undersurface and check for ridges, and any inferior osteophytes were resected. Those in the ACR group did not undergo either the division of the coracoacromial ligament or partial resection of the acromion. After acromioplasty, the cuff repair was performed following the same principles as in repair only group. Both groups Patients were discharged on the same day as the surgery. Passive or active‐assisted shoulder range‐of‐motion exercises began one week after surgery. Active shoulder motion began at eight weeks postoperatively with strengthening exercises and reintegration into normal activities at twelve weeks postoperatively. Patients returned to the clinic at two weeks and at six to eight weeks postoperatively for wound check only, and at three, six, 12, 18, and 24 months postoperatively for study follow‐up. |
|
| Outcomes | Patients completed both outcome measures preoperatively and at three, six, 12, 18, and 24 months after surgery. The primary outcome WORC index ( 0% to 100%, higher indicates better quality of life) Secondary outcomes ASES score (0 to 100, higher indicates better) re‐operation rate Shoulder Range of Motion (at 24 months) Upper Extremity Strength Grading (at 24 months) Outcomes in this review Mean function; ASES score |
|
| Source of funding | The funding source for this study was the Alexander Gibson Fund from the University of Manitoba. It supported the expenses for a study coordinator. statistical support, and supplies (e.g. postage, courier, patient files, and questionnaires). The funding source did not play a role in this investigation. Financial disclosure quote: "None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty‐six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work." |
|
| Notes |
Trial registration: NCT00290888 Data analysis: The authors report WORC in a zero to 100 scale (total score divided by 21). The authors did not systematically perform imaging to participants and thus we did not use re‐operation data in Analysis 2.3 (One re‐repair in the repair alone group) Withdrawals: reasons for loss in follow‐up not given. Cross‐overs: four (9%) of 45 participants in the repair only group were offered further surgery due to ongoing pain. No re‐operations in repair and decompression group. Adverse events: zero in both groups(reported in ClinicalTrials.gov) Serious adverse events: zero in both groups(reported in ClinicalTrials.gov) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment was based on a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Allocation of patients was conducted with use of a series of opaque envelopes containing group assignment. Patient assignment to the study groups was concealed from the researcher who enrolled and assessed the patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"The randomisation envelope was opened following intraoperative inspection of the shoulder. The surgeon was not blinded to the treatment allocation, but the patient and the research assistant performing follow‐up evaluations were blinded" Other personnel blinding not described. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Low risk | The participant was blinded. Both groups received similar postoperative rehabilitation regimen. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | Low risk | Not measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At three months, data missing (ASES) for 9/45 (20%) in repair only and 17/41 (41%) repair and decompression group At six months, data missing for 8/45 (17%) in repair only and 13/41 (31%) repair and decompression group At 12 months, data missing for 18/45 (40%) in repair only and 15/41 (37%) repair and decompression group. At 18 months, data missing for 23/45 (51%) in repair only and 19/41 (46%) repair and decompression group. At 24 months, data missing for 11/45 (24%) in repair only and 9/41 (22%) repair and decompression group. Missing data are not in balance at 6 months. High proportion of missing data at primary time point of this review (12 months). Reasons not reported. |
| Selective reporting (reporting bias) | Low risk | Range of motion and strength grading of the shoulder not reported. Not outcome in this review so does not bias the results. |
| Other bias | Low risk | None apparent. |
Milano 2007.
| Methods | Design: a single‐centre randomised (1:1) controlled trial Setting: a university hospital in Italy Timing: not reported Interventions: rotator cuff repair with acromioplasty versus rotator cuff repair alone. Sample size: authors defined type I error equal to 0.05; power of 0.80, and assumed effect size for the DASH and Constant score 0.7, which gave 32 participants per group. To allow loss of follow‐up, the authors recruited 80 participants Analysis:nNot reported but it appears that authors performed ITT analysis. | |
| Participants |
Number of participants Number screened not reported 80 participants recruited (40 to repair with acromioplasty and 40 to repair alone) Data available for 34 (85%) in repair with acromioplasty group and 37 (93%) in repair alone group Inclusion criteria
Exclusion criteria
Baseline data Repair with acromioplasty Mean (SD) Age, years 61 (7.0) Number (%) females 14 (41) Number (%) dominant shoulder involved 23 (68) Mean (SD) area, mm2 398 (464) Number (%) with fatty degeneration grade I 9 (26%); grade II 12 (35%); grade III 7 (21%); grade IV 6 (18%) Number (%) subscapularis tear 5 (15) Number (%) acromion type 2: 24 (71); type 3: 10 (29) Repair without acromioplasty Mean (SD) Age, years 59.7 (9.7) Number (%) females 18 (49) Number (%) dominant shoulder involved 24 (65) Mean (SD) area, mm2 356 (423) Number (%) with fatty degeneration grade I 7 (19%); grade II 15 (41%); grade III 11 (30%); grade IV 4 (11%) Number (%) subscapularis tear 4 (11%) Number (%) acromion type 2: 24 (65); type 3: 13 (35) |
|
| Interventions | The surgeons performed operations arthroscopically Rotator cuff repair with acromioplasty The authors performed arthroscopic rotator cuff repair and subacromial decompression, consisting of anterior‐inferior acromioplasty, release of the coracoacromial ligament, and subacromial bursectomy. Rotator cuff repairs were performed with 3 different techniques, according to tear pattern: 1) tendon‐to‐bone repair with metal suture anchors (Corkscrew, 5.0 mm; Arthrex, Naples, FL) loaded with doubled polyester braided No. 2 sutures in the crescent‐shaped tears; 2) side‐to‐side repair with polyester braided No. 2 sutures in the more retracted U‐ and V‐shaped tears, and 3) a combined repair technique (tendon to bone and side to side) in the other cases. Pathology of the long head of the biceps (LHB) was treated by shaving when the lesion involved less than 25% of the tendon; more severe biceps lesions or tendon instability was treated depending on the patient’s age20: in patients aged over 50 years, we performed a biceps tenotomy; in the other cases we performed a tenodesis with 2 metal suture anchors (Corkscrew, 5.0 mm). Rotator cuff repair without acromioplasty Only subacromial bursectomy was performed, in addition to rotator cuff repair. The repair technique was same as in the other group. Both groups After surgery, a sling was applied to the operative limb and was maintained for 3 weeks; after this period, all patients underwent the following rehabilitation program: First phase (fourth through eighth week after surgery): range‐of‐motion exercise program (passive, active‐assisted, and active). Second phase (ninth through twelfth week after surgery): muscle‐strengthening program by closed kinetic chain exercises for rotator cuff, subscapularis, biceps, deltoid, pectoralis major, and scapular stabilisers. Third phase (thirteenth through sixteenth week after surgery): open kinetic chain exercises, proprioceptive and plyometric exercises, and postural rehabilitation of the kinetic chain (lumbopelvic, thoracolumbar, and scapulothoracic regions). No differences in the rehabilitation program were considered according to the extent of rotator cuff tear or involvement of the biceps and subscapularis. |
|
| Outcomes | The outcomes were assessed at two years. Authors also reported examining participants every two weeks for the first three months and then once per month until the sixth month after surgery but probably this examination did not include outcome assessment Primary outcome Not reported Outcomes Constant score (scale the authors used is unclear, higher indicates better function) Disabilities for Arm, Shoulder and Hand (DASH) score (0 to 100,. higher score indicates worse function) DASH work (0 to 100, higher score indicates worse disability) Outcomes used in this review Mean function; Constant score |
|
| Source of funding | Source of funding not reported. The authors report no conflicts of interests. | |
| Notes |
Trial registration: No registration found Data analysis: The authors report Constant score over 100 (103) for acromioplasty group. We assume the error is systematic and applies to the other group also and thus to the difference between the groups. Withdrawals: The authors report three drop outs in repair with acromioplasty group and six in repair only group but in table 1 it seems that the numbers are vice versa. We assumed the table gives the correct numbers. Cross overs or re‐operations: No cross overs or re‐operations reported Adverse events: Not reported, unclear if measured. Serious adverse events: Not reported, unclear if measured. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomization was performed with statistical software through a random selection of 50% of cases" |
| Allocation concealment (selection bias) | Low risk | Quote:"The randomisation list was kept by an independent researcher (not involved in the study), and the code for assignment of each patient to one of the two groups was revealed to the surgeon at the time of surgery." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The blinding of participants and personnel not described. Probably no blinding. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Unclear risk | The blinding of participants or assessors not described. Probably authors did not attempt blinding. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | Unclear risk | The authors did not report this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/40 (15%) participants were lost to follow‐up in repair with acromioplasty and 3/40 (8%) participants in repair only group. The authors did not disclose reasons. Likely does not bias the results. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Authors report all outcomes defined in the methods. It is unclear if adverse events were measured as the paper does not report them. |
| Other bias | Unclear risk | The authors report Constant score over 100 points although the scale of the measure is 0 to 100. The reason is unclear and the authors did not respond to e‐mails. It is likely that authors used similar method of calculation with both group. |
Moosmayer 2010.
| Methods | Design: single‐centre, two‐arm, parallel randomised clinical trial Setting: Martina Hansen’s hospital, Norway Timing: September 2004 to October 2007 Interventions: rotator cuff repair, decompression and exercises versus exercises alone. Sample size: for sample size calculation, a between‐groups difference of 12 points on the Constant scale at 12 months was assumed to represent clinical relevance. On condition of a within‐group SD of 20 points on the Constant scale, 102 patients would be needed to obtain a power of 0.85 and a significance level of 0.05. Analysis: ITT‐analysis (for patients in the physiotherapy group who changed treatment, the score before secondary surgery was carried forward to the 6‐ and 12‐month analyses in primary report. True ITT‐data reported in 2014 paper. | |
| Participants |
Number of participants
281 patient screened
103 randomised (52 to surgery; 51 to exercises)
102 received allocated treatment (1 in surgery group did not receive treatment)
Data available for 99 (51 (98%) for surgery; 48 (100%) for exercises and 3 (100%) for exercises) at 6 months Data available for 102 (51 (98%) for surgery; 51 (100%)) for exercises at 12 months Inclusion criteria:
Exclusion criteria:
Baseline data Surgery group Mean (range) age: 59 (44 to 75) years Number (%) females: 15 (29%) Number (%) participants with right side affected: 31 (60) Number (%) participants with dominant side affected: 33 (63) Number (%) participants with duration of symptoms in months: 12.3 (18.7) Number (%) participants on work: 23 (44) Number (%) participants on sick leave: 15 (29) Number (%) participants on retirement: 11 (21) Number (%) participants with disability benefit: 3 (6) Number (%) participants with muscle atrophy (in volume) gr 0: 26 (50) Number (%) participants with muscle atrophy (in volume) gr 1: 12 (23) Number (%) participants with muscle atrophy (in volume) gr 2: 13 (25) Mean (SD) tear size short axis, mm: 15.6 (6.7) Mean (SD) tear size long axis:14.9 (5.7) Number (%) participants with tear in supraspinatus: 37 (71) Number (%) participants with tear in supra and infraspinatus: 14 (27) Number (%) participants withTear in supraspinatus and subscapularis: 1 (2) Mean (95% CI) pain: 5.6 (5.0 to 6.1) Mean (95% CI) Constant score: 35.3 (31.6 to 39.0) Mean (95% CI) SF‐36 physical health component score: 38.2(36.6 to 39.9) Mean (95% CI) SF‐36 mental health component score: 54.1 (50.9 to 57.3) Exercises group Mean (range) age: 61 (46 to 75) years Number (%) female: 15 (29) Number (%) participants with right side affected: 29 (57) Number (%) participants with dominant side affected: 31 (61) Mean (SD) duration of symptoms, months: 9.8 (9.8) Number (%) participants with on work: 24 (47) Number (%) participants on sick leave: 8 (15) Number (%) participants on retirement: 17 (33) Number (%) participants with on disability benefit: 2 (4) Number (%) participants with muscle atrophy (in volume) gr 0: 23 (44) Number (%) participants with muscle atrophy (in volume) gr 1: 18 (35) Number (%) participants with muscle atrophy (in volume) gr 2: 10 (19) Mean (SD) tear size short axis, mm: 14.3 (6.3) Mean (SD) tear size long axis, mm: 14.7 (6.9) Number (%) participants with tear in supraspinatus: 40 (78) Number (%) participants with tear in supra and infraspinatus:10 (20) Number (%) participants with tear in supraspinatus and subscapularis:1 (2) Mean (95% CI) Constant score: 38.4 (34.4 to 42.4) Mean (95% CI) pain: 5.3 (4.8 to 5.9) Mean (95% CI) SF‐36 physical health component score 38.6 (36.2 to 41.1) Mean (95% CI) SF‐36 mental health component score: 57.3 (54.7 to 59.9) |
|
| Interventions | Three surgeons performed the repairs. One of four physiotherapists experienced in conservative shoulder management supervised exercises.
Surgery group: Mini‐open (N = 9) or open (N = 42) cuff repair, following diagnostic arthroscopy, through a deltoid splitting approach, anteroinferior acromioplasty was performed. Rotator cuff mobilised until the tear was fully exposed. Footprint prepared to bleeding bone and tendon repair was performed with a combination of tendon‐to ‐tendon and tendon‐to‐bone techniques by passing sutures through bone tunnels in the greater tuberosity. The deltoid was repaired to the acromion through drill holes. Mini‐open tendon repair differed from open repair by a shorter incision and arthroscopic acromioplasty. Postoperative management, 6 weeks sling and passive ROM exercises, active assisted movements at 6 weeks postoperative and supplemented with strengthening exercises at 12 weeks. Exercise group: Physiotherapy, a rehabilitation programme describing treatment goals and methods was planned. Treatment was given on the basis of this programme in a non‐standardised manner according to clinical findings and progress. Treatment sessions of 40 minutes, on average twice weekly for 12 weeks, and with increasing intervals during the following 12 weeks. Local glenohumeral control was addressed by exercises to centre the humeral head in the glenoid fossa. Isometric exercises. When local glenohumeral control was achieved, exercises were given with increasing loads. During all exercises, scapular stability had to be maintained. |
|
| Outcomes | Outcomes were assessed at: baseline, 6 and 12 months and 5 years
Primary outcome:
Secondary outcomes:
Outcomes included in this review
|
|
| Source of funding | Sponsor: Martina Hansen's Hospital (According to the protocol) | |
| Notes |
Trial registration: NCT00852657 Data analysis: ITT‐data from 2014 report used in this review. For HRQoL analysis (Analysis 1.4), mental health component score used at 6‐month and 12‐month follow‐up. At two years, the mental health component score was not reported, and we used physical component score (Analysis 1.4) Withdrawals: one participant died in both groups before 5 years follow‐up point. One patient withdrew before treatment in surgery group Cross overs: in the exercise group, 3/51(6%) had surgery by 6 months and 9/51 (18%) by 12 months and 12/51(24%) by 5 years due to poor subjective improvement. Adverse events: no treatment related adverse events. 1 participant suffered humerus fracture in surgery group and 1 participant was diagnosed with polymyalgia rheumatica in exercise group. We did not consider them as treatment related events and did not include them in the Analysis 1.5. Serious adverse events: likely there were none as the authors reported that there were no treatment related adverse events. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation list (block length 20, ratio 1:1) was drawn up by our statistician." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, sealed envelopes were used to assign treatment according to the participants’ study number, given at baseline assessment. The randomisation sequence was concealed from the study’s collaborators until treatment was assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded except for a blinded outcome assessor. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were not blinded. |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | Low risk | Assessor was blinded to treatment allocation as participants wore a T‐shirt to cover the shoulder. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/52 (2%) withdrew before treatment in surgery group and this was the only participant lost by 1 year follow‐up. 1 (2%) participant in each group withdrew by 5‐year follow‐up. In exercise group, 9/51(17%) at 1 year and 12/52(23%) at 5 years dropped out and had surgery. . |
| Selective reporting (reporting bias) | Low risk | All results reported except for the SF‐36 mental health component missing at the 24‐month follow‐up point. |
| Other bias | High risk | 9/51 (18%) had surgery in the exercise group by 12 months and 12/51 (24%) by 5 years. This may mask the potential benefit of surgery and bias the result in favour of exercise therapy. Additionally, long head of biceps tenodesis was performed in 18 (35%) participants in the surgery group. It is unclear if the biceps side interventions would have resulted in bias. |
Shin 2012.
| Methods | Design: a single‐centre randomised controlled trial Setting: hospital in South Korea Timing: participants were recruited from March 2006 through April 2008 Interventions: rotator cuff repair and acromioplasty versus rotator cuff repair alone. Sample size: power analysis indicated that a total sample size of 120 patients (60 patients in each cohort) would provide 80% power (alfa 0.05) to detect significant differences in ASES scores, assuming an effect size of 0.5 (mean difference of five points and standard deviation of 10 points). The sample size was also estimated by use of an equation appropriate for comparing 2 independent group proportions and was based on a 2‐sided level of .05 having 80% statistical power to detect a 30% difference in University of California, Los Angeles shoulder score. Analysis: no flow chart and authors do not report any deviations from the allocated treatment. Thus, it appears that authors report data for ITT analysis. | |
| Participants |
Number of participants: number of screened or excluded not reported. 150 randomised (75 to both groups) Data available for 60 (80%) in both groups at mean follow‐up, 35 months (range 24 to 54 months) Inclusion criteria:
Exclusion criteria:
Baseline data: Rotator cuff repair with acromioplasty Mean (SD) age, years 57.8 (9.3) Number of females (%) 27 (45) Number (%) of dominant arm involved 42 (70) Mean (SD) duration of symptoms 13.9 (20.6) Number (%) of traumatic onset 21 (35) Shape of acromion flat 18 (30); curved 32 (53); hooked 10 (17) Mean (SD) thickness of acromion, mm 7.75 (1.26) Mean size (SD) of tear (anteroposterior) 14.6 (5.2) Mean size (SD) of tear (retraction) 14.0 (5.3) Mean (SD) Goutallier class 1.5 (0.6) Number (%) of concomitant subscapularis tear 21 (35) Number (%) of concomitant cartilage injury 4 (7) Number (%) of concomitant biceps procedures 12 (20) Mean (SD) pain on VAS 5.5 (2.4) Mean (SD) ASES 52.6 (18.5) Mean (SD) Constant score 58.3 (15.8) Mean (SD) UCLA score 18.9 (4.5) Rotator cuff repair without acromioplasty Mean (SD) age, years 55 (8.0) Number of females (%) 26 (43%) Number (%) of dominant arm involved 45 (75) Mean (SD) duration of symptoms 16.7 (22.7) Number (%) of traumatic onset 24 (40%) Shape of acromion flat 15 (25); curved 36 (60); hooked 9 (15) Mean (SD) thickness of acromion, mm 6.9 (1.98) Mean size (SD) of tear (anteroposterior) 15.3 (7.0) Mean size (SD) of tear (retraction) 14.2 (6.0) Mean (SD) Goutallier class 1.4 (0.6) Number (%) of concomitant subscapularis tear 23 (38) Number (%) of concomitant cartilage injury 6 (10) Number (%) of concomitant biceps procedures 17 (28) Mean (SD) pain on VAS 5.5 (2.4) Mean (SD) ASES 51.5 (16.9) Mean (SD) Constant score 56.8 (17.6) Mean (SD) UCLA score 19.7 (5.8) |
|
| Interventions |
Rotator cuff repair with acromioplasty After bursectomy, the surgeon performed subacromial decompression (acromioplasty and release of the coracoacromial ligament). Acromioplasty was confined to the anterolateral aspect of the acromion by use of the cutting‐block technique. The Coracoacromial ligament was completely released. With the arthroscope coming from the lateral portal the shaver was introduced from the posterior portal. The posterior aspect of the acromial undersurface served as a cutting block to guide the resection of the anterolateral edge of the acromion from posterior to anterior. Then, the shaver was switched to the lateral portal while the arthroscope was introduced from the posterior portal. The acromion was made flat from the medial to the lateral aspect of the acromion. Arthroscopic rotator cuff repair was performed by either a single‐row or double‐row technique according to the tear size and configuration. The single‐row repair technique was usually used for small‐sized rotator cuff tears and the double‐row technique for medium sized rotator cuff tears. When a partial‐thickness tear of the subscapularis was found, it was treated with arthroscopic debridement. Biceps tendon procedures were determined at the time of surgery depending on the patient’s age and the degeneration of the tendon. Biceps debridement alone was performed if the tendon tear was less than 50% of the thickness of the tendon or was partially frayed. Biceps tenodesis with a suture anchor was performed when the tendon tear involved more than 50% of the tendon thickness and the patient was aged less than 60 years. If patients were aged older than 60 years and the tendon tear involved more than 50% of the tendon thickness, biceps tenotomy was performed. Rotator cuff repair without acromioplasty Coracoacromial ligament was debrided without release. The authors do not explicitly report the extent of bursectomy in this group; it is likely that bursectomy was performed to facilitate visibility during repair. The repair was performed following same principles as in acromioplasty group. Both groups The same postoperative rehabilitation protocol was applied in all participants. A shoulder brace with 0° of external rotation and 30° of abduction was applied in all patients for four weeks (small‐sized tear) or five weeks (medium‐sized tear) postoperatively. Pendulum exercises and gentle passive shoulder range‐of‐motion exercises commenced three days after surgery. Active assisted shoulder range‐of‐motion exercises began after weaning from the brace, and resisted shoulder motion and strengthening exercises began at three months. |
|
| Outcomes | According to the methods section, the authors assessed outcomes 1, 3, 6, 12, and 24 months postoperatively and then annually (longest follow‐up at mean of 35 months, range 24 to 54 months) Primary outcome ASES score (American Shoulder and Elbow Surgeons score, 0 to 100, higher score indicates better function, used for sample size calculation) Secondary outcome UCLA score (0 to 35, higher score indicates better function) Constant score (0 to 100, higher score indicates better function) VAS for pain (0 to 10, higher indicates worse pain) VAS for satisfaction (0 to 10, higher score indicates likely – not explicitly reported – better satisfaction) Shoulder range of motion (degrees) Failure rate; number or proportion participants with re‐tear or non‐healing of repair (defined by MRI, CT or ultrasound) Postoperative shoulder stiffness Outcomes used in this review Mean pain; Pain in VAS Mean function; Constant score Adverse events Failure rate (participants with full‐thickness tear at six months diagnosed with MRI, 76 participants or CT, 42 participants or ultrasound, 2 participants) |
|
| Source of funding | Source of funding not reported. The authors report no conflicts of interest | |
| Notes |
Trial registration: N/A Data analysis: Withdrawals: 15/60 (25%) of participants dropped out or declined follow‐up in both groups. Re‐operations: in repair with subacromial decompression group, arthroscopic capsular release was performed in two patients who exhibited a contracted capsule due to inflammatory processes and arthroscopic bursectomy was performed in one patient who had decreased range of motion even after two months of intensive physical therapy. Adverse events: incompletely reported (Quote: "Postoperative complications including limitation of shoulder motion due to rotator cuff adhesion occurred in patients regardless of completion of an acromioplasty"). Serious adverse events: likely there were none as the authors reported. Not explicitly reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote: "Each patient was randomised into one of two groups." Not reported how the sequence was achieved |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Unclear risk | Not described, participants likely not blinded |
| Blinding of outcome assessment for incidence of full‐thickness tears at follow‐up (detection bias) | High risk | Not described, it is probably not possible to blind the radiologists reliably from allocation as the repair was performed using metal anchors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Similar rates (15/75 or 20%) lost in follow‐up due to drop out or declined to participate in follow‐up. The reasons not given. Unclear if the reasons were different. |
| Selective reporting (reporting bias) | Low risk | The authors reported collecting data at 1, at 3, 6, 12 and a mean of 35 months. One‐month data not reported although stated that they were collected. Patient satisfaction reported only at one time point. As one‐month data have little clinical significance, we assessed the risk of bias as low. |
| Other bias | Low risk | The extent of bursectomy in repair‐only group not described explicitly. It is likely that authors performed bursectomy and the only difference between the groups was removal of bone from undersurface of acromion and this is unlikely to cause bias. |
ASES: American Shoulder and Elbow Surgeons Score; CT: Computed Tomography; DASH: Disabilities for Arm, Shoulder and Hand; HRQoL: Health‐related quality of life; ITT: intention‐to‐treat; MID: Minimal Important Difference; MRI: Magnetic Resonance Image; SD: standard deviation; SF: Short Form; SST: Simple Shoulder Test; UCLA: University of California and Los Angeles; VAS: visual analogue scale;WORC: Western Ontario Rotator Cuff.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berth 2010 | Not an RCT ('matched‐pair study') |
| Flurin 2013 | Not a randomised study. Centres allocated participants in six months periods to repair and then subacromial decompression only. |
| Franceschi 2013 | Not relevant comparison (repair versus repair) |
| Franceschi 2015 | Not an RCT (cohort) |
| Heuberer 2016 | Not an RCT (cohort of consecutive patients) |
| Maillot 2018 | Not an RCT |
| Mardani‐Kivi 2016 | No randomisation. Comparison two consecutive cohorts with different operation |
| Shin 2012a | Not relevant comparison (repair versus repair) |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Lhee 2013.
| Methods | Design: Randomised, double‐blind single‐centre 1:1 parallel group trial Setting: Hospital in South‐Korea Timing: Recruitment from March 2009 to October 2010 Interventions: arthroscopic subscapularis tendon repair versus debridement Sample size: sample size calculation not provided; 256 participants recruited Analysis: not clearly reported |
| Participants |
Number of participants 394 assessed for eligibility 256 randomised (139 to repair and 117 to debridement) At minimum of 24 months data available for 101/139 (73%) in repair group and 90/117 (77%) debridement group Inclusion criteria
Exclusion criteria
surgery. Baseline data Rotator cuff repair group Mean (SD) Age, years 61 (5.3) Number (%) females 35 (35%) Tear size small to medium 46 (33%); medium to large 65 (47%); large to massive 28 (20%) Hidden partial tear (full thickness but not whole tendon; only visible in external rotation) 39 (28%) Debridement group Mean (SD) Age, years 62 (4.6) Number (%) females 58 (64%) Tear size small to medium 39 (33%); medium to large 55 (47%); large to massive 23 (20%) Hidden partial tear (full thickness but not the whole tendon; only visible in external rotation) 31 (27%) |
| Interventions | All surgeries
were performed by a single surgeon at a single hospital Rotator cuff repair group Lateral‐anterosuperior portal (“Miracle Portal”) was used to repair subscapularis tendon. Bursa anterior to the subscapularis tendon was usually removed for the accurate positioning of the suture‐hook. Subscapularis tendon was released, pulled and sutured with suture‐hook. One or two suture anchors of Modified Mason‐Allen technique was used to secure the tendon. If the subscapularis tendon was not sufficiently mobile, further anterior interval release between subscapularis and scapula was performed. LHB (long head of biceps tendon) was either treated with a biceps tenodesis or by tenotomy when there was tear or subluxation of it. The footprint area of the subscapularis tendon, which is trapezoidal in shape on the proximal part of the lesser tuberosity, was thoroughly cleaned of soft tissue and meticulous bone preparation was done prior to placement of anchor sutures. Debridement Anterosuperior portal was made for debridement (capsulectomy and anterior bursectomy). A systematic release of the glenohumeral ligaments and the overlying subscapularis bursa was also performed. The superior aspect of the tendon was freed. from the surrounding structures (the coracohumeral and superior glenohumeral ligaments). The middle glenohumeral ligament was always released to identify the upper border of the subscapularis tendon. Both groups Postoperative rehabilitation regimen not described. |
| Outcomes | The participants were assessed at 3 month‐intervals The primary outcome ASES score (0 to 100, higher score indicates better function) Outcomes used in this review Mean function; ASES score |
| Notes |
Trial registration: NCT01996904 Data analysis: the authors report ASES score at final follow‐up with a minimum of 24 months follow‐up. Withdrawals: reasons for loss in follow‐up not given. Cross‐overs: none reported Adverse events: no events in the groups (reported in ClinicalTrials.gov) Serious adverse events: no events in the groups(reported in ClinicalTrials.gov) |
ASES: American Shoulder and Elbow Surgeons; SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
NCT00695981.
| Trial name or title | Operative versus non‐operative management of rotator cuff tear |
| Methods | Randomised controlled trial |
| Participants | Estimated enrolment: 100 Inclusion Criteria
Exclusion Criteria
|
| Interventions | Arm 1: Rotator cuff repair surgery Rotator cuff repair + physical therapy according to a standardised protocol Arm 2: Non‐operative treatment Physiotherapy according to a standardised protocol |
| Outcomes |
Primary Outcome Change in pain (VAS) at 24 months Change function (Constant score) at 24 months Secondary Outcome Change in pain (VAS) at 3, 6, 12 months and 5 years Change in function (Constant score) at 3, 6, 12 months and 5 years |
| Starting date | June 2008 |
| Contact information | Juha Paloneva, MD, PhD 358‐14‐2691811 juha.paloneva@ksshp.fi Ilkka Kiviranta, MD, PhD 358‐50‐4271807 ilkka.kiviranta@helsinki.fi (Principle Investigator) |
| Notes | Estimated Study Completion Date: October 2017 Estimated primary completion date (final data collection for primary outcome): March 2017. Updated from Clinicaltrials registry 18th June, 2018 Active, not recruiting ‐ last verified 17th November, 2017 |
NCT01498198.
| Trial name or title | A randomized study of non‐operative management versus expedited surgery among WCB patients with small rotator cuff tears: affect upon time to claim closure in 2 Prairie provinces |
| Methods | Randomised trial |
| Participants | Planned sample size = 144 Inclusion Criteria
Exclusion Criteria
Age minimum:18 years Age maximum:N/A Gender: both |
| Interventions | Non‐operative treatment Rotator cuff repair surgery |
| Outcomes |
Primary outcome Time to return to final work status Secondary outcome Demographics Orebro Questionnaire Range of Motion Strength WORC Questionnaire |
| Starting date | January 2012 |
| Contact information | Jeff Leiter, PhD 204‐927‐2665 jleiter@panamclinic.com Peter B MacDonald, MD FRCSC ‐ University of Manitoba Faculty of Medicine |
| Notes | Recruitment status: not yet recruiting Above information copied from WHO ITCRP database, last refreshed on 19 February 2015 |
NCT02059473.
| Trial name or title | Treatment of small acute full‐thickness tears ‐ a prospective randomised controlled trial |
| Methods | Randomized Controlled Trial |
| Participants | Estimated enrolment: 50 Inclusion Criteria
Exclusion Criteria
|
| Interventions | Arm 1: physiotherapy and surgery (mini‐open rotator cuff repair) Arm 2: physiotherapy |
| Outcomes |
Primary Outcome Constant score at 12 months Secondary Outcome Western Ontario Rotator Cuff score Shoulder specific, patient reported outcome score MRI (measure of muscle atrophy, fatty infiltration, re‐rupture and enlargement of the tendon rupture) Euro‐Qol‐five‐Dimensions (EQ‐5D) Patient Global Impression of Change (PGIC) Patients Global impression of Change |
| Starting date | November 2013 |
| Contact information | Hanna Hallgren, MD +46 10 1037205 hanna.bjornsson.hallgren@lio.se Mats Ranebo, MD +46 480 81432 matsra@ltkalmar.se |
| Notes | Estimated study completion date: November 2017; Estimated primary completion date (final data collection date for primary outcome measure): December 2015 Recruitment Status : Unknown 18th June, 2018 |
NCT02885714.
| Trial name or title | ACCURATE (Operative treatment of acute rotator cuff tear related to trauma) |
| Methods | Design: multi‐centre, parallel group, two‐arm (1:1) double‐blind randomised placebo‐controlled trial |
| Participants | Planned sample size = 200 Inclusion Criteria
Exclusion Criteria
|
| Interventions | Group 1 (Placebo surgery and supervised specific exercises)
The arthroscope is introduced in the glenohumeral joint, and thereafter online randomisation is performed. The joint space is evaluated. Nothing is to be removed or excised and the use of any vapour or shaver device is not allowed. The presence of a full‐thickness rotator cuff tear is verified. Altogether 3 to 5 small stab wounds are made in typical locations resembling locations of typical rotator cuff repair. The time spent in the operating theatre with patients in placebo group should resemble the time spent with patients in the active treatment group and hence give an impression of a rotator cuff repair. Group 2 (Rotator cuff repair and supervised specific exercises) The arthroscope is introduced in the glenohumeral joint, and thereafter online randomisation is performed. The joint space is evaluated and the presence of a full‐thickness rotator cuff tear is verified. The cuff tear is repaired to its anatomic location using suture anchors according to surgeon preference. A biceps tenotomy or tenodesis may be performed according to surgeon preference if the biceps tendon is noted to be frayed, unstable or inflamed. An additional acromioplasty may be performed according to surgeon preference. |
| Outcomes |
Primary outcome Change in Western Ontario Rotator Cuff index (WORC) compared to baseline at two years. Secondary outcomes Constant Score Shoulder specific outcome measure combining subjective and objective variables Numeric rating scale of patients' shoulder pain during the last week at rest, during activity and at night (NRS) Scale 0 to 10. 0 = no pain and 10 = worst possible pain. 15D Generic health‐related quality of life instrument Subjective patient satisfaction Patient reported scale for treatment satisfaction Rotator cuff integrity in MRI investigation Development of osteoarthritic signs in radiographs Development of cuff tear arthropathy in radiographs |
| Starting date | December 2016 |
| Contact information | Contact: Ville Äärimaa, Adj.Prof.+35823130000 ville.aarimaa@tyks.fi |
| Notes | Updated 18th June 2018 from Clinicaltrials registry. Recruitment Status : Recruiting Last refreshed 17th October 2017 |
NCT03183466.
| Trial name or title | Prospective randomized clinical trial of arthroscopic repair versus debridement for subscapularis tendon tear more than 1/2 to entire 1st facet (representing 1/3 to more than 1/3 tear of subscapularis entire footprint) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions | Arthroscopic repair Arthroscopic debridement |
| Outcomes | Range of Motion (ROM) Pain Visual Analogue Scale (PVAS) American Shoulder and Elbow Surgeons' score Constant score Korean Shoulder Score (KSS) score Repair integrity analysis using postoperative MRI |
| Starting date | December 2011 |
| Contact information | Samsung Medical Center, South‐Korea |
| Notes | Last refreshed 6/2017. Status active, not recruiting. Publication sought but not found. |
NCT03295994.
| Trial name or title | Operative versus non‐operative treatment for atraumatic rotator cuff tears |
| Methods | Multi‐centre, randomised controlled trial. |
| Participants | Planned sample size = 700 Inclusion Criteria
Exclusion Criteria
|
| Interventions | Group 1: arthroscopic rotator cuff surgery followed by physical therapy. Group 2: non‐operative, physical therapy (without surgery). |
| Outcomes | Primary Outcome Shoulder Pain & Disability Index (SPADI) at 12 months Secondary Outcome American Shoulder and Elbow Surgeons (ASES) |
| Starting date | March 19, 2018 |
| Contact information | Helen M Koudelkova, MA615‐936‐8343 helen.koudelkova@vanderbilt.edu |
| Notes | Updated 18th June, 2018. Recruitment status: recruiting. Estimated primary completion date: December 30, 2022. Last refreshed June 5, 2018. |
MRI: magnetic resonance imaging; NRS: Numeric Rating Scale; RC: rotator cuff; VAS: visual analogue score; WCB: Workers' Compensation Board
Differences between protocol and review
We excluded the comparison of one repair technique versus another in this update as the benefit of repair has not yet been established.
We defined a hierarchy for functional outcomes.
Subgroup analyses: We excluded the subgroup analysis based on age (people older > 65 compared with those aged 65 or less), as there was no clinical reason for these subgroups and the trials did not allow separating the populations.
We added subgroup analysis exploring the impact of risk of selection bias and type of surgery and type of acromion in the second comparison, repair with acromioplasty versus repair only.
Contributions of authors
TK, RJ and NBJ were responsible for selecting trials, extracting and handling of data, interpreting results, and contributing to the writing of the updated review.
JH screened and selected the studies, extracted data and participated in writing of the review.
CP selected trials, analysed and interpreted results for the updated review, and contributed to the writing of the updated review.
RB was responsible for conceiving the review, supervising the updated review, interpreting the results of the updated review, and contributing to writing the updated review.
Sources of support
Internal sources
Department of Clinical Epidemiology at Cabrini Hospital, Australia.
Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia.
External sources
Australasian Cochrane Centre, Melbourne, Australia.
Cochrane Musculoskeletal Group, Canada.
-
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) 1K23AR059199 and 1U34AR069201., USA.
Nitin Jain is supported by funding from NIAMS.
-
The Finnish Medical Foundation and Finnish Centre of Evidence Based Orthopaedics (FICEBO), Finland.
The Finnish Medical Foundation and Finnish Centre of Evidence Based Orthopaedics (FICEBO) supported Teemu Karjalainen during the time this review was conducted.
-
National Health and Medical Research Council (NHMRC), Australia.
Rachelle Buchbinder is supported by an NHMRC Senior Principal Research Fellowship
Declarations of interest
Nitin Jain is performing a trial comparing surgery with non‐operative treatment (NCT03295994) and Rachelle Buchbinder is an external advisor on this project. Nitin Jain's contributions for this review are partially covered by grants from NIH and PCORI.
Renea Johnston is the Managing Editor of Cochrane Musculoskeletal, but is not involved in editorial decisions regarding this review. She is a recipient of an NHMRC (Australia) Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funding source did not participate in the conduct of this review.
Rachelle Buchbinder is the Co‐ordinating Editor of Cochrane Musculoskeletal but is not involved in editorial decisions regarding this review. She has received royalties from Wolters Kluwer Health for writing a chapter on plantar fasciitis in UpToDate. She is a recipient of a National Health and Medical Research Council (NHMRC) Cochrane Collaboration Round 7 Funding Program Grant, which supports the activities of Cochrane Musculoskeletal ‐ Australia and Cochrane Australia, but the funders do not participate in the conduct of reviews.
Teemu Karjalainen's time for this review is funded by the Finnish Medical Association through a grant from non‐profit foundation to support research work. He received money from Summed Finland (representative of Acumed surgical instruments) for travel expenses to participate in upper extremity trauma course organised by Acumed in June 2017.
Juuso Heikkinen and Cristina Page: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abrams 2014 {published data only}
- Abrams GD, Gupta AK, Hussey KE, Tetteh ES, Karas V, Bach BR Jr, et al. Arthroscopic repair of full‐thickness rotator cuff tears with and without acromioplasty: randomized prospective trial with 2‐year follow‐up. American Journal of Sports Medicine 2014;42(6):1296‐303. [PUBMED: 24733157] [DOI] [PubMed] [Google Scholar]
- Tetteh ES, Hussey EK, Abrams GD, Gupta AK, Dhawan A, Karas V, et al. A prospective randomized trial of functional outcomes following rotator cuff repair with and without acromioplasty. Orthopedic Journal of Sports Medicine 2013;1(4 suppl):PMCID: PMC4588967. [Google Scholar]
- Waterman BR, Newgren J, Gowd AK, Cabarcas BC, Bach BR, Cole BJ, et al. Randomized prospective trial of arthroscopic rotator cuff with or without acromioplasty: no difference in patient‐reported outcomes at long‐term follow‐up. Orthopaedic Journal of Sports Medicine 2018;6((7 suppl 4)):no pagination. [DOI] [PubMed] [Google Scholar]
Dezaly 2011 {published data only}
- Dezaly C, Sirveaux F, Philippe R, Wein‐Remy F, Sedaghatian J, Roche O, et al. Arthroscopic treatment of rotator cuff tear in the over‐60s: repair is preferable to isolated acromioplasty‐tenotomy in the short term. Orthopaedics & Traumatology, Surgery & Research : OTSR 2011;97(6 Suppl):S125‐30. [PUBMED: 21798838] [DOI] [PubMed] [Google Scholar]
- Jacquot A, Dezaly C, Goetzmann T, Roche O, Sirveaux F, Mole D. Is rotator cuff repair appropriate in patients older than 60 years of age? prospective, randomised trial in 103 patients with a mean four‐year follow‐up. Orthopaedics & Traumatology, Surgery & Research : OTSR 2014;100(6 Suppl):S333‐8. [PUBMED: 25155203] [DOI] [PubMed] [Google Scholar]
Gartsman 2004 {published data only}
- Gartsman GM, O'connor DP. Arthroscopic rotator cuff repair with and without arthroscopic subacromial decompression: a prospective, randomized study of one‐year outcomes. Journal of Shoulder and Elbow Surgery 2004;13(4):424‐6. [PUBMED: 15220883] [DOI] [PubMed] [Google Scholar]
Kukkonen 2014 {published data only}
- Kukkonen J, Joukainen A, Lehtinen J, Mattila KT, Tuominen EK, Kauko T, et al. Treatment of non‐traumatic rotator cuff tears ‐ A randomised controlled trial with one‐year clinical results. The Bone and Joint Journal 2014;96‐B:75–81. [DOI] [PubMed] [Google Scholar]
- Kukkonen J, Joukainen A, Lehtinen J, Mattila KT, Tuominen EK, Kauko T, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow‐up. Journal of Bone and Joint Surgery. American volume 2015;97(21):1729‐37. [PUBMED: 26537160] [DOI] [PubMed] [Google Scholar]
Lambers Heerspink 2015 {published data only}
- Lambers Heerspink FO, Raay JJ, Koorevaar RC, Eerden PJ, Westerbeek RE, ’t Roet E, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. Journal of Shoulder and Elbow Surgery 2015;24(8):1274‐81. [DOI] [PubMed] [Google Scholar]
MacDonald 2011 {published data only}
- MacDonald P, McRae S, Leiter J, Mascarenhas R, Lapner P. Arthroscopic rotator cuff repair with and without acromioplasty in the treatment of full‐thickness rotator cuff tears: a multicenter, randomized controlled trial. Journal of Bone and Joint Surgery. American volume 2011;93(21):1953‐60. [PUBMED: 22048089] [DOI] [PubMed] [Google Scholar]
- Mascaranhas R. Arthroscopic rotator cuff repair with and without acromiplasty in the treatment of full‐thickness rotator cuff tears: A multi‐centre, randomized controlled trial. Arthroscopy 2011;27(10 (Suppl)):e142–3. [DOI] [PubMed] [Google Scholar]
Milano 2007 {published data only}
- Milano G, Grasso A, Salvatore M, Zarelli D, Deriu L, Fabbriciani C. Arthroscopic rotator cuff repair with and without subacromial decompression: a prospective randomized study. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2007;23(1):81‐8. [PUBMED: 17210431] [DOI] [PubMed] [Google Scholar]
Moosmayer 2010 {published data only}
- Moosmayer S, Lund G, Seljom U, Svege I, Hennig T, Tariq R, et al. Comparison between surgery and physiotherapy in the treatment of small and medium‐sized tears of the rotator cuff: A randomised controlled study of 103 patients with one‐year follow‐up. Journal of Bone and Joint Surgery. British volume 2010;92(1):83‐91. [DOI] [PubMed] [Google Scholar]
- Moosmayer S, Lund G, Seljom US, Haldorsen B, Svege IC, Hennig T, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five‐year follow‐up. Journal of Bone and Joint Surgery: American volume 2014;96(18):1504‐14. [DOI] [PubMed] [Google Scholar]
Shin 2012 {published data only}
- Shin SJ, Oh JH, Chung SW, Song MH. The efficacy of acromioplasty in the arthroscopic repair of small‐ to medium‐sized rotator cuff tears without acromial spur: prospective comparative study. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2012;28(5):628‐35. [PUBMED: 22261136] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berth 2010 {published data only}
- Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. Journal of Orthopaedics and Traumatology : official journal of the Italian Society of Orthopaedics and Traumatology 2010;11(1):13‐20. [PUBMED: 20198404] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flurin 2013 {published data only}
- Flurin PH, Hardy P, Abadie P, Desmoineaux P, Essig J, Joudet T, et al. Rotator cuff tears after 70 years of age: a prospective, randomized, comparative study between decompression and arthroscopic repair in 154 patients. Orthopaedics & Traumatology, Surgery & Research : OTSR 2013;99(8 Suppl):S371‐8. [PUBMED: 24211128] [DOI] [PubMed] [Google Scholar]
Franceschi 2013 {published data only}
- Franceschi F, Papalia R, Buono A, Vasta S, Costa V, Maffulli N, et al. Articular‐sided rotator cuff tears: which is the best repair? A three‐year prospective randomised controlled trial. International Orthopaedics 2013;37(8):1487‐93. [PUBMED: 23580030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franceschi 2015 {published data only}
- Franceschi F, Papalia R, Vasta S, Leonardi F, Maffulli N, Denaro V. Surgical management of irreparable rotator cuff tears. Knee surgery, Sports Traumatology, Arthroscopy : official journal of the ESSKA 2015;23(2):494‐501. [PUBMED: 23212188] [DOI] [PubMed] [Google Scholar]
Heuberer 2016 {published data only}
- Heuberer PR, Kolblinger R, Buchleitner S, Pauzenberger L, Laky B, Auffarth A, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surgery, Sports Traumatology, Arthroscopy : official journal of the ESSKA 2016;24(12):3828‐37. [PUBMED: 26254089] [DOI] [PubMed] [Google Scholar]
Maillot 2018 {published data only}
- Maillot C, Harly E, Demezon H, Huec JC. Surgical repair of large‐to‐massive rotator cuff tears seems to be a better option than patch augmentation or debridement and biceps tenotomy: a prospective comparative study. Journal of Shoulder and Ebow Surgery 2018;27(9):1545‐52. [PUBMED: 29980338] [DOI] [PubMed] [Google Scholar]
Mardani‐Kivi 2016 {published data only}
- Mardani‐Kivi M, Karimi A, Keyhani S, Hashemi‐Motlagh K, Saheb‐Ekhtiari K. Rotator Cuff Repair: Is there any role for acromioplasty?. Physician and Sportsmedicine 2016;44(3):274‐7. [PUBMED: 27476731] [DOI] [PubMed] [Google Scholar]
Shin 2012a {published data only}
- Shin SJ. A comparison of 2 repair techniques for partial‐thickness articular‐sided rotator cuff tears. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2012;28(1):25‐33. [PUBMED: 22000411] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lhee 2013 {published data only}
- Lhee SH. Prospective randomized comparative study of 191 subscapularis tear: clinical and radiologic outcome – arthroscopic repair vs debridement. Journal of Elbow and Shoulder Surgery Vol. 22, issue 10:e24‐5.
- Lhee SH. Prospective randomized comparative study of outcome of subscapularis tear. https://clinicaltrials.gov/ct2/show/nct01996904 November 2013.
References to ongoing studies
NCT00695981 {published data only}
- Paloneva J. Operative versus non‐operative management of rotator cuff tear. https://clinicaltrials.gov/ct2/show/NCT00695981.
NCT01498198 {published data only}
- MacDonald P. Workers Compensation Board: rotator cuff tear management. https://clinicaltrials.gov/ct2/show/NCT01498198.
NCT02059473 {published data only}
- Hallgren H. Treatment of small acute cuff tears, a randomized study. https://clinicaltrials.gov/ct2/show/NCT02059473.
NCT02885714 {published data only}
- Äärimaa V, Ryösä A. ACCURATE Trial ‐ operative treatment of acute rotator cuff tear related to trauma. https://clinicaltrials.gov/ct2/show/NCT02885714.
NCT03183466 {published data only}
- Samsung Medical Center. Do you arthroscopic repair for subscapularis tendon tear, which accounts for more than half of the 1st facet? or do you perform debridement?. https://clinicaltrials.gov/ct2/show/NCT03183466.
NCT03295994 {published data only}
- Nitin J. Operative versus non‐operative treatment for atraumatic rotator cuff tears (ARC). https://clinicaltrials.gov/ct2/show/NCT03295994.
Additional references
Bennell 2007
- Bennell K, Coburn S, Wee E, Green S, Harris A, Forbes A, et al. Efficacy and cost‐effectiveness of a physiotherapy program for chronic rotator cuff pathology: a protocol for a randomised, double‐blind, placebo‐controlled trial. BMC Musculoskeletal Disorders 2007;8(86):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2003
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2011
- Buchbinder R, Johnston RV, Roos JF. Shock wave therapy for rotator cuff disease with or without calcification. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2017
- Buchbinder R, Page MJ, Huang H, Verhagen AP, Beaton D, Kopkow C, et al. for the Shoulder Core Outcomes Set Special Interest Group. A preliminary Core Domain Set for clinical trials of shoulder disorders: a report from the OMERACT 2016 Shoulder Core Outcome Set Special Interest Group. Journal of Rheumatology 2017;44(12):1880‐3. [DOI] [PubMed] [Google Scholar]
Cates 2008 [Computer program]
- Dr. Christopher Cates EBM web site. URL: http://www.nntonline.net. Visual Rx. Version 3. Dr. Christopher Cates EBM web site. URL: http://www.nntonline.net, 2008.
Chahal 2012
- Chahal J, Mall N, MacDonald PB, Thiel G, Cole BJ, Romeo AA, et al. The role of subacromial decompression in patients undergoing arthroscopic repair of full‐thickness tears of the rotator cuff: a systematic review and meta‐analysis. Arthroscopy : the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2012;28(5):720‐7. [PUBMED: 22305327] [DOI] [PubMed] [Google Scholar]
Chard 1991
- Chard MD, Hazleman R, Hazleman BL, King RH, Reiss BB. Shoulder disorders in the elderly: a community survey. Arthritis and Rheumatism 1991;34:766‐9. [DOI] [PubMed] [Google Scholar]
Chaudhury 2012
- Chaudhury S, Dines JS, Delos D, Warren RF, Voigt C, Rodeo SA. Role of fatty infiltration in the pathophysiology and outcomes of rotator cuff tears. Arthritis Care & Research 2012;64(1):76‐82. [PUBMED: 21770040] [DOI] [PubMed] [Google Scholar]
Chillemi 2016
- Chillemi C, Petrozza V, Franceschini V, Garro L, Pacchiarotti A, Porta N, et al. The role of tendon and subacromial bursa in rotator cuff tear pain: a clinical and histopathological study. Knee Surgery, Sports Traumatology, Arthroscopy : official journal of the ESSKA 2016;24(12):3779‐86. [PUBMED: 26003482] [DOI] [PubMed] [Google Scholar]
Coghlan 2008
- Coghlan JA, Buchbinder R, Green S, Johnston RV, Bell SN. Surgery for rotator cuff disease. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD005619.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cumpston 2009
- Cumpston M, Johnston RV, Wengier L, Buchbinder R. Topical glyceryl trinitrate for rotator cuff disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006355.pub2] [DOI] [PubMed] [Google Scholar]
Curry 2015
- Curry EJ, Matzkin EE, Dong Y, Higgins LD, Katz JN, Jain NB. Structural characteristics are not associated with pain and function in rotator cuff tears. Orthopaedic Journal of Sports Medicine 2015;3(5):2325967115584596. [PUBMED: 26675985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabija 2017
- Dabija DI, Gao C, Edwards TL, Kuhn JE, Jain NB. Genetic and familial predisposition to rotator cuff disease: a systematic review. Journal of Shoulder and Elbow Surgery 2017;26(6):1103‐12. [PUBMED: 28162885] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses.In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dinnes 2003
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technology Assessment 2003;7(29):1‐166. [DOI] [PubMed] [Google Scholar]
Dunn 2014
- Dunn WR, Kuhn JE, Sanders R, An Q, Baumgarten KM, Bishop JY, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross‐sectional study of 393 patients with a symptomatic atraumatic full‐thickness rotator cuff tear. Journal of Bone and Joint Surgery. American volume 2014;96(10):793‐800. [PUBMED: 24875019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engebretsen 2009
- Engebretsen K, Grotle M, Bautz‐Holter E, Sandvik L, Juel NG, Ekeberg OM, et al. Radial extracorporeal shockwave treatment compared with supervised exercises in patients with subacromial pain syndrome: single randomised study. BMJ 2009;339:b3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Familiari 2015
- Familiari F, Gonzalez‐Zapata A, Ianno B, Galasso O, Gasparini G, McFarland EG. Is acromioplasty necessary in the setting of full‐thickness rotator cuff tears? A systematic review. Journal of Orthopaedics and Traumatology : official journal of the Italian Society of Orthopaedics and Traumatology 2015;16(3):167‐74. [PUBMED: 26003837] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franceschi 2011
- Franceschi F, Papalia R, Buono A, Maffulli N, Denaro V. Repair of partial tears of the rotator cuff. Sports Medicine and Arthroscopy review 2011;19(4):401‐8. [PUBMED: 22089290] [DOI] [PubMed] [Google Scholar]
Gagnier 2018
- Gagnier JJ, Robbins C, Bedi A, Carpenter JE, Miller BS. Establishing minimally important differences for the American Shoulder and Elbow Surgeons score and the Western Ontario Rotator Cuff Index in patients with full‐thickness rotator cuff tears. Journal of Shoulder and Elbow Surgery 2018;27(5):e160‐6. [PUBMED: 29307675] [DOI] [PubMed] [Google Scholar]
Gialanella 2011
- Gialanella B, Prometti P. Effects of corticosteroids injection in rotator cuff tears. Pain Medicine 2011;12(10):1559‐65. [DOI] [PubMed] [Google Scholar]
Gombera 2014
- Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability : a systematic review. Clinical Orthopaedics and Related Research 2014;472(8):2448‐56. [PUBMED: 24043432] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomoll 2004
- Gomoll AH, Katz JN, Warner JJP, Millett PJ. Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis and Rheumatism 2004;50:3751‐61. [DOI] [PubMed] [Google Scholar]
Gotoh 1998
- Gotoh M, Hamada K, Yamakawa H, Inoue A, Fukuda H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. Journal of Orthopaedic Research : official publication of the Orthopaedic Research Society 1998;16(5):618‐21. [PUBMED: 9820287] [DOI] [PubMed] [Google Scholar]
Goutallier 1994
- Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre‐ and postoperative evaluation by CT scan. Clinical Orthopaedics and Related Research 1994;0(304):78‐83. [PUBMED: 8020238] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org. GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment, and efficacy. BMJ 1998;316:345‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2005
- Green S, Buchbinder R, Hetrick S. Acupunture for shoulder pain. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005319] [DOI] [PubMed] [Google Scholar]
Greenspoon 2015
- Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. Journal of Shoulder and Elbow Surgery 2015;24(9):1493‐505. [PUBMED: 26129871] [DOI] [PubMed] [Google Scholar]
Hao 2018
- Hao Q, Devji T, Zeraatkar D, Wang Y, Qasim A, Siemieniuk R, et al. Minimal important differences in shoulder condition patient‐reported outcomes: a systematic survey to inform a BMJ Rapid Recommendation. BMJ open 2019;9(2):e028777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hata 2001
- Hata Y, Saitoh S, Murakami N, Seki H, Nakatsuchi Y, Takaoka K. A less invasive surgery for rotator cuff tear: mini‐ open repair. Journal of Shoulder and Elbow Surgery 2001;10(1):11‐6. [DOI] [PubMed] [Google Scholar]
Hegedus 2010
- Hegedus EJ, Cook C, Brennan M, Wyland D, Garrison JC, Driesner D. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. British Journal of Sports Medicine 2010;44(12):838‐47. [PUBMED: 19293165] [DOI] [PubMed] [Google Scholar]
Hermans 2013
- Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma‐Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? The rational clinical examination systematic review. JAMA 2013;310(8):837‐47. [DOI] [PubMed] [Google Scholar]
Hertling 1990
- Hertling D, Kessler RM. The Shoulder and Shoulder Girdle. Management of Common Musculoskeletal Disorders: Physical Therapy Principles and Methods. Philadelphia: Lippincott. 3rd Edition. Vol. 1, Philadelphia: Lippincott Williams and Wilkins, 1990. [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2017
- Hill JR, McKnight B, Pannell WC, Heckmann N, Sivasundaram L, Mostofi A, et al. Risk factors for 30‐day readmission following shoulder arthroscopy. Arthroscopy 2017;33(1):55‐61. [DOI: 10.1016/j.arthro.2016.06.048] [DOI] [PubMed] [Google Scholar]
Huang 2016
- Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all‐arthroscopic versus mini‐openrRepair of rotator cuff tears: a meta‐analysis. Scientific Reports 2016;6:22857. [PUBMED: 26947557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2013
- Jain NB, Yamaguchi K. History and physical examination provide little guidance on diagnosis of rotator cuff tears. Evidence Based Medicine 2013;19(3):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2015
- Ji X, Bi C, Wang F, Wang Q. Arthroscopic versus mini‐open rotator cuff repair: an up‐to‐date meta‐analysis of randomized controlled trials. Arthroscopy: the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2015;31(1):118‐24. [PUBMED: 25442664] [DOI] [PubMed] [Google Scholar]
Johnson 2004
- Johnson MP, Crossley KL, O'Neil ME, Al‐Zakwani IS. Estimates of direct healthcare expenditures among individuals with shoulder dysfunction in the United States. American Society of Shoulder and Elbow Therapists. Available at: http://www.asset‐usa.org/Abstracts/Johnson_Crossley_Oneil_Al‐Kakwani.htm 2004.
Judge 2014
- Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone & Joint Journal 2014;96‐B(1):70‐4. [PUBMED: 24395314] [DOI] [PubMed] [Google Scholar]
Karjalainen 2019
- Karjalainen TV, Jain NB, Page CM, Lahdeoja TA, Johnston RV, Salamh P, et al. Subacromial decompression surgery for rotator cuff disease. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD005619.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kempf 1999
- Kempf JF, Gleyze P, Bonnomet F, Walch G, Mole D, Frank A, et al. A multicenter study of 210 rotator cuff tears treated by arthroscopic acromioplasty. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 1999;15(1):56‐66. [PUBMED: 10024034] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Altman DG, Williamson PR. Bias due to changes in specified outcomes during the systematic review process. PLOS One 2010;5(3):e9810. [PUBMED: 20339557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhn 2009
- Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence‐based rehabilitation protocol. Journal of Shoulder and Elbow Surgery 2009;18(1):138‐60. [DOI] [PubMed] [Google Scholar]
Lambers Heerspink 2014
- Lambers Heerspink FO, Dorrestijn O, Raay JJ, Diercks RL. Specific patient‐related prognostic factors for rotator cuff repair: a systematic review. Journal of Shoulder and Elbow Surgery 2014;23(7):1073‐80. [PUBMED: 24725900] [DOI] [PubMed] [Google Scholar]
Lenza 2013
- Lenza M, Buchbinder R, Takwoingi Y, Johnston RV, Hanchard NC, Faloppa F. Magnetic resonance imaging, magnetic resonance arthrography and ultrasonography for assessing rotator cuff tears in people with shoulder pain for whom surgery is being considered. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD009020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luime 2004
- Luime JJ, Koes BW, Hendriksen IJ, Burdorf A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scandinavian Journal of Rheumatology 2004;33(2):73‐81. [PUBMED: 15163107] [DOI] [PubMed] [Google Scholar]
Mall 2010
- Mall NA, Kim HM, Keener JD, Steger‐May K, Teefey SA, Middleton WD, et al. Symptomatic progression of asymptomatic rotator cuff tears: A prospective study of clinical and sonographic variables. Journal of Bone and Joint Surgery. American volume 2010;92:2623‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mall 2014
- Mall NA, Tanaka MJ, Choi LS, Paletta GA Jr. Factors affecting rotator cuff healing. Journal of Bone and Joint Surgery. American volume 2014;96(9):778‐88. [PUBMED: 24806015] [DOI] [PubMed] [Google Scholar]
Millett 2006
- Millett PJ, Wilcox RB, 3rd, O'Holleran JD, Warner JJ. Rehabilitation of the rotator cuff: an evaluation‐based approach. Journal of the American Academy of Orthopaedic Surgeons 2006;14(11):599‐609. [DOI] [PubMed] [Google Scholar]
Misamore 1995
- Misamore GW, Ziegler DW, Rushton JL, 2nd. Repair of the rotator cuff. A comparison of results in two populations of patients. Journal of Bone and Joint Surgery. American volume 1995;77(9):1335‐9. [DOI] [PubMed] [Google Scholar]
Moosmayer 2009
- Moosmayer S, Smith HJ, Tariq R, Larmo A. Prevalence and characteristics of asymptomatic tears of the rotator cuff: an ultrasonographic and clinical study. Journal of Bone and Joint Surgery. British volume 2009;91(2):196‐200. [PUBMED: 19190053] [DOI] [PubMed] [Google Scholar]
Moosmayer 2013
- Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three‐year follow‐up of fifty cases. Journal of Bone and Joint Surgery. American volume 2013;95(14):1249‐55. [PUBMED: 23864172] [DOI] [PubMed] [Google Scholar]
Morelli 2019
- Morelli KM, Martin BR, Charakla FH, Durmisevic A, Warren GL. Acromion morphology and prevalence of rotator cuff tear: A systematic review and meta‐analysis. Clinical anatomy (New York, N.Y.) 2019;32(1):122‐30. [PUBMED: 30362636] [DOI] [PubMed] [Google Scholar]
Nam 2012
- Nam D, Maak TG, Raphael BS, Kepler CK, Cross MB, Warren RF. Rotator cuff tear arthropathy: evaluation, diagnosis, and treatment: AAOS exhibit selection. Journal of Bone and Joint surgery. American volume 2012;94(6):e34. [PUBMED: 22438007] [DOI] [PubMed] [Google Scholar]
Neer 1983
- Neer CS. Impingement lesions. Clinical Orthopaedics and Related Research 1983;173:70‐7. [PubMed] [Google Scholar]
Nho 2007
- Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini‐open rotator cuff repair. Journal of Bone and Joint Surgery. American volume 2007;89(Suppl 3):127‐36. [DOI] [PubMed] [Google Scholar]
Nho 2009
- Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: Prognostic factors affecting clinical and ultrasound outcome. Journal of Shoulder and Elbow Surgery 2009;18(1):13‐20. [PUBMED: 18799326] [DOI] [PubMed] [Google Scholar]
Page 2015
- Page MJ, McKenzie JE, Green SE, Beaton DE, Jain NB, Lenza M, et al. Core domains and outcome measurement sets for shoulder pain trials are needed: systematic review of physical therapy trials. Journal of Clinical Epidemiology 2015;68:1270‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016a
- Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016b
- Page MJ, Green S, Mrocki MA, Surace SJ, Deitch J, McBain B, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012225] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedowitz 2012
- Pedowitz RA, Yamaguchi K, Ahmad CS, Burks RT, Flatow EL, Green A, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. Journal of Bone and Joint Surgery. American volume 2012;94(2):163‐7. [PubMed] [Google Scholar]
Piper 2018
- Piper CC, Hughes AJ, Ma Y, Wang H, Neviaser AS. Operative versus non‐perative treatment for the management of full‐thickness rotator cuff tears: a systematic review and meta‐analysis. Journal of Shoulder and Elbow Surgery 2018;27(3):572‐6. [PUBMED: 29169957] [DOI] [PubMed] [Google Scholar]
Reilly 2006
- Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don't lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Annals of the Royal College of Surgeons of England 2006;88(2):116‐21. [PUBMED: 16551396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rekola 1993
- Rekola KE, Keinanen‐Kiukaanniemi S, Takala J. Use of primary health services in sparsely populated country districts by patients with musculoskeletal symptoms: consultations with a physician. Journal of Epidemiology and Community Health 1993;47(2):153‐7. [PUBMED: 8326275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rugg 2018
- Rugg CM, Gallo RA, Craig EV, Feeley BT. The pathogenesis and management of cuff tear arthropathy. Journal of Shoulder and Elbow Surgery 2018;27(12):2271‐83. [PUBMED: 30268586] [DOI] [PubMed] [Google Scholar]
Ryosa 2017
- Ryosa A, Laimi K, Aarimaa V, Lehtimaki K, Kukkonen J, Saltychev M. Surgery or conservative treatment for rotator cuff tear: a meta‐analysis. Disability and Rehabilitation 2017;39(14):1357‐63. [PUBMED: 27385156] [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Sayampanathan 2017
- Sayampanathan AA, Andrew TH. Systematic review on risk factors of rotator cuff tears. Journal of Orthopaedic Surgery (Hong Kong) 2017;25(1):2309499016684318. [PUBMED: 28211286] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables.In Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane‐handbook.org.
Seida 2010
- Seida JC, LeBlanc C, Schouten JR, Mousavi SS, Hartling L, Vandermeer B, et al. Systematic review: non‐operative and operative treatments for rotator cuff tears. Annals of Internal Medicine 2010;153(4):246‐55. [DOI] [PubMed] [Google Scholar]
Shields 2015
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy : the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2015;31(5):807‐15. [PUBMED: 25661861] [DOI] [PubMed] [Google Scholar]
Song 2016
- Song L, Miao L, Zhang P, Wang WL. Does concomitant acromioplasty facilitate arthroscopic repair of full‐thickness rotator cuff tears? A meta‐analysis with trial sequential analysis of randomized controlled trials. SpringerPlus 2016;5(1):685. [PUBMED: 27350920] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical research ed.) 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Tashjian 2009
- Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. Journal of Shoulder and Elbow Surgery 2009;18(6):927‐32. [DOI] [PubMed] [Google Scholar]
Teunis 2014
- Teunis T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. Journal of Shoulder and Elbow Surgery 2014;23(12):1913‐21. [PUBMED: 25441568] [DOI] [PubMed] [Google Scholar]
van der Meijden 2012
- Meijden OA, Westgard P, Chandler Z, Gaskill TR, Kokmeyer D, Millett PJ. Rehabilitation after arthroscopic rotator cuff repair: current concepts review and evidence‐based guidelines. International Journal of Sports Physical Therapy 2012;7(2):197‐218. [PMC free article] [PubMed] [Google Scholar]
Vecchio 1995
- Vecchio P, Kavanagh R, Hazleman BL, King RH. Shoulder pain in a community‐based rheumatology clinic. British Journal of Rheumatology 1995;34:766‐9. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaguchi 2000
- Yamaguchi K, Sher JS, Andersen WK, Garretson R, Uribe JW, Hechtman K, et al. Glenohumeral motion in patients with rotator cuff tears: a comparison of asymptomatic and symptomatic shoulders. Journal of Shoulder and Elbow Surgery 2000;9(1):6‐11. [PUBMED: 10717855] [DOI] [PubMed] [Google Scholar]
Yamaguchi 2001
- Yamaguchi K, Tetro A, Blam O, Evanoff B, Teefey S, Middleton W. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. Journal of Shoulder and Elbow Surgery 2001;10:199‐203. [DOI] [PubMed] [Google Scholar]
Yamaguchi 2006
- Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. Journal of Bone and Joint surgery. American volume 2006;88(8):1699‐704. [PUBMED: 16882890] [DOI] [PubMed] [Google Scholar]
Yian 2005
- Yian EH, Ramappa AJ, Arneberg O, Gerber C. The Constant score in normal shoulders. Journal of Shoulder and Elbow Surgery 2005;14(2):128‐33. [PUBMED: 15789004] [DOI] [PubMed] [Google Scholar]
Östor 2005
- Ostor AJ, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology (Oxford, England) 2005;44(6):800‐5. [PUBMED: 15769790] [DOI] [PubMed] [Google Scholar]


