Abstract
Introduction
Labor‐market participation is potentially very difficult for patients with refractory myasthenia gravis (MG). In this study, employment status and work absences are compared between refractory and nonrefractory MG.
Methods
Adults (aged 18–64 years, all diagnosed ≥2 years previously) were included if enrolled in the Myasthenia Gravis Foundation of America Patient Registry during July 2013 to February 2018.
Results
Seventy‐six patients (9.2%) had refractory and 749 (90.8%) had nonrefractory disease; demographic data did not differ between groups. Relative to the nonrefractory group, the refractory group patients were more than twice as likely to work fewer hours per week (odds ratio [95% confidence interval]: currently employed, 2.777 [1.640–4.704]; employed over previous 6 months, 2.643 [1.595–4.380]), but those employed were not more likely to be absent from work.
Discussion
Because absence from the labor market adversely affects quality of life and personal finances, these findings reaffirm the considerable disease burden associated with refractory MG.
Keywords: absence from work, employment, MGFA Registry, myasthenia gravis, patient survey, refractory disease
Abbreviations
- CI
confidence interval
- HRQoL
health‐related quality of life
- IVIg
intravenous immunoglobulin
- MG
myasthenia gravis
- MG‐ADL
Myasthenia Gravis—Activities of Daily Living
- MGFA
Myasthenia Gravis Foundation of America
- OR
odds ratio
1. INTRODUCTION
Myasthenia gravis (MG) is a chronic autoimmune disease characterized by impaired synaptic transmission and fatigable muscle weakness. Generalized MG may lead to debilitating symptoms, including slurred speech, difficulty swallowing (with an increased risk of choking), extreme fatigue, limb weakness, and impaired respiratory function (with episodes of respiratory failure requiring mechanical ventilation1).
Acetylcholinesterase inhibitors, corticosteroids, and/or immunosuppressant therapies are successfully employed to alleviate MG symptoms in most patients. However, it is estimated that symptoms persist in up to 15% of those diagnosed with MG despite concerted therapeutic efforts; these individuals are considered to have refractory disease.2, 3 The treatments used in patients with refractory disease, such as long‐term therapy with immunosuppressant treatments, intravenous immunoglobulin (IVIg), or plasmapheresis,1 may increase the burden of illness. Patients with refractory MG are also significantly more likely than those with nonrefractory disease to experience myasthenic exacerbations and crises, and to visit an emergency department and/or be admitted to a hospital.2, 4 Given the persistence of symptoms, patients with refractory MG may continue to experience problems with daily activities such as eating, personal grooming, reading, and driving. Collectively, these factors suggest that patients with refractory MG are highly likely to have difficulties meeting the demands of employment. This is compounded by the timing of the typical onset of refractory MG in middle age,3 a time that is generally otherwise of high productivity.
An adverse impact of MG on employment has been reported in various studies.5, 6, 7, 8 This may be apparent as reduced participation in the labor market,5, 7, 8 increased sick leave,5, 6 and higher levels of patient‐reported hardship in the workplace.7 Given inevitable socioeconomic differences across countries, it is unclear to what extent the findings from these previous non‐US studies may apply to the labor market in the USA. In addition, although there is some evidence that factors such as increased disease severity or a greater treatment burden exacerbate employment problems in MG,5, 8 there is a dearth of research pertaining specifically to refractory disease. The objective of this study was to examine the impact of refractory MG, compared with nonrefractory MG, on employment status and absences from work in the USA.
2. METHODS
In previous work, we have used patient‐reported data from the MG Patient Registry developed by the Myasthenia Gravis Foundation of America (MGFA) to compare health‐care resource utilization in patients with refractory MG to that in patients with nonrefractory MG.4 Here, we use the same data set to explore employment status and absences from work.
2.1. Data source
The MG Patient Registry is an active database of adults (≥18 years of age) in the USA who have MG. The Registry was developed for the purposes of research, treatment, and patient information. The enrollment survey comprises approximately 200 questions, encompassing information such as demographics, history of MG, comorbidities, previous and current therapies, family history of MG, functional status (assessed using the Myasthenia Gravis—Activities of Daily Living [MG‐ADL] questionnaire), lifestyle, and employment status. The MG‐ADL questionnaire is a validated, eight‐item, patient‐reported outcome measure developed to assess MG symptoms and their functional impact.9, 10 Participants report the level of functional disability (0 [normal] to 3 [most severe]) for each of the eight items (ocular [two items], bulbar [three items], respiratory [one item], and gross motor or limb impairment [two items]). Summing item scores provides an MG‐ADL questionnaire total score ranging from 0 to 24 points. In the enrollment survey, patients were asked to select the level of disability corresponding to their experience during the preceding 4 weeks.
The present study includes data from enrollment surveys completed between July 17, 2013 (the start of the Registry) and February 22, 2018. Although participants were asked to complete surveys about their condition twice a year after enrollment, data in the analyses presented here relate only to the enrollment survey. The study was approved by the institutional review board of the University of Alabama at Birmingham, in the USA. Data were de‐identified for research, and consent for participation was provided by participants electronically at registration, before completion of the survey.
2.2. Participants
The study population comprised participants between 18 and less than 65 years of age, representing the working population of the USA.
Participants in the data set had reported being diagnosed with MG by their doctor at least 2 years before completing the enrollment survey. This was to ensure that there was sufficient time between the diagnosis of MG and inclusion in the study for physicians to have explored a number of treatment approaches. A number of different definitions of refractory MG have been used in previous studies.11 The definition used in the present study combines some elements of those published previously,2, 3, 11, 12, 13 and is based on data reported about treatment and MG‐ADL scale scores. Specifically, participants had refractory MG if their past treatments included at least two immunosuppressant therapies (azathioprine, cyclophosphamide, cyclosporine, methotrexate, mycophenolate, prednisone, rituximab, and/or tacrolimus) for at least 6 months each, or at least one of the immunosuppressant therapies for any duration plus repeated use of IVIg or plasmapheresis (at least four rounds in the previous year). Participants with refractory disease were also required to have reported the following at enrollment: current use of at least one of the immunosuppressant therapies, IVIg, or plasmapheresis; and a total score for the MG‐ADL scale of at least 6. Participants who did not meet the past or current treatment criteria for refractory MG were considered nonrefractory, regardless of their MG‐ADL scale score. Participants meeting the treatment criteria but with an MG‐ADL scale total score of less than 6 were excluded from the analyses because they were difficult to categorize confidently into either cohort. Participants with incomplete MG‐ADL scale data or treatment data of insufficient duration to be classified as refractory or nonrefractory were also excluded.
2.3. Study measures
Participants reported their current employment status (ie, at the time of enrollment) and their employment status in the preceding 6 months by selecting from prespecified answers. These included full‐time (the number of hours per week defining “full‐time” was not specified in the question), part‐time, or not employed. Participants were also asked to indicate whether MG had caused them to miss workdays in the past 6 months. Participants who had missed workdays selected the periods of time missed from among 1 to 3 days, 4 to 7 days, 8 to 13 days, 2 to 4 weeks, 1 to 2 months, 3 to 4 months, 5 to 6 months, and unknown. Other participant‐reported demographic data, along with treatment and functional status (MG‐ADL scale) data, were extracted previously from enrollment surveys.4 Previously reported demographic data are included here because of their potential associations with employment. These data comprise age, gender, ethnicity (Hispanic/Latino/Spanish or not Hispanic/Latino/Spanish), marital status (six predefined options, which were simplified as married or unmarried), living arrangements (10 predefined options, which were simplified for analyses as living alone or with others), and level of education (six options, ranging from no high school degree up to postgraduate degree). Participants provided information regarding therapy prompted by a predefined list of individually named drugs (eg, prednisone), treatment types (eg, plasma exchange), and free‐text entries. They were asked to note the duration of treatment for medications received at enrollment and at any point in the past.
Demographic (including employment status and absences from work), treatment, and MG‐ADL scale data were compared between participants with refractory and nonrefractory MG (bivariate analyses). Adjusted regression analyses were undertaken with the demographic data to examine the impact of refractory MG, compared with nonrefractory MG, on: current and previous employment status; and, for those employed over the previous 6 months, absences from work.
2.4. Statistical analyses
Summary statistics were calculated for all study variables. Bivariate analyses, comparing refractory and nonrefractory MG groups, were undertaken using χ2 tests for categorical variables and t tests for continuous variables. Adjusted regression analyses were conducted using ordered multinomial logit models with proportional odds for employment status and absences from work. Ordinal categories for employment status comprised full‐time (category 1), part‐time (category 2), and not employed (category 3). Ordinal categories for absences from work comprised 1 to 3 days (category 1), 4 to 7 days (category 2), 8 days to 4 weeks (category 3), greater than 1 month (category 4), and unknown (data excluded). Missing and unknown data were excluded from all analyses. P < .05 was considered to indicate potential differences between refractory and nonrefractory MG cohorts. Odds ratios (ORs) with 95% confidence intervals (CIs) and P values are provided to summarize the results of modeling. Analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
3. RESULTS
3.1. Characteristics of study population
The data set comprised 825 participants, of whom 76 (9.2%) had refractory MG and 749 (90.8%) had nonrefractory MG.4
Demographic characteristics have been reported in a previous study.4 The mean (standard deviation [SD]) ages of the refractory and nonrefractory MG groups at enrollment into the Registry were 48.0 (11.4) and 49.3 (11.1) years, respectively. Each group had more females than males (86.8% of the refractory group and 78.8% of the nonrefractory group were female). In the bivariate analyses, no significant differences were apparent between refractory and nonrefractory MG groups for age, gender, ethnicity, marital status, living arrangements, or level of education (data for employment status are reported in what follows).
Current and previous treatments are summarized in Table S1 online. In the bivariate analyses, most of the previous treatments (received at any time before enrollment) were received by significantly greater proportions of participants in the refractory MG group than in the nonrefractory MG group. In addition, current treatment use tended to be lower than previous treatment use for the refractory MG group.
As expected, the mean MG‐ADL scale total scores were significantly higher (reflecting poorer functioning) for the refractory MG group than for the nonrefractory MG group (9.6 [SD, 2.7] and 6.7 [SD, 4.0], respectively; P < .01).4
3.2. Employment status—bivariate analyses
There was a significant association between disease status and previous employment status (Table 1). The proportion of individuals employed full‐time was smaller in the refractory MG group than the nonrefractory MG group, and the proportion of individuals who were not employed was greater for the refractory group. Similar findings were apparent for current employment status (Table 1).
Table 1.
Employment status and absences from work reported by the study population
| Characteristic | Refractory MG (n = 76) | Nonrefractory MG (n = 749) | P value* |
|---|---|---|---|
| Previous employment† | (n = 75) | (n = 744) | <.01 |
| Full‐time | 20 (26.7) | 340 (45.7) | |
| Part‐time | 7 (9.3) | 114 (15.3) | |
| Not employed | 48 (64.0) | 290 (39.0) | |
| Current employment | (n = 76) | (n = 746) | <.01 |
| Full‐time | 18 (23.7) | 332 (44.5) | |
| Part‐time | 6 (7.9) | 85 (11.4) | |
| Not employed | 52 (68.4) | 329 (44.1) | |
| Absences from work‡ | (n = 23) | (n = 275) | .41 |
| 1–3 days | 7 (30.4) | 76 (27.6) | |
| 4–7 days | 2 (8.7) | 64 (23.3) | |
| 8 days to 4 weeks | 9 (39.1) | 79 (28.7) | |
| >1 month | 5 (21.7) | 56 (20.4) |
Abbreviation: MG, myasthenia gravis.
Using the χ2 test (P < .05 statistically significant).
In the 6 months before enrollment.
In the 6 months before enrollment, for those participants who were employed during that time.
3.3. Employment status—adjusted regression analyses
After adjusting for other demographic variables, a significant association was still apparent in the overall study population between disease status (refractory or nonrefractory MG) and employment status (Figure 1). Relative to those with refractory MG, participants with nonrefractory MG were more likely to have a full‐time job rather than a part‐time job, or a part‐time job rather than no job (OR [95% CI]: previous employment status, 2.643 [1.595–4.380]; current employment status, 2.777 [1.640–4.704]). Age, gender, and aspects of the level of education were also significantly associated with employment status (Figure 1). Males were more likely than females to be employed and, in general, younger individuals were more likely to be employed than those aged 55 to 64 years. Similarly, relative to those who had not graduated from high school, participants with bachelor or postgraduate degrees were more likely to have a full‐ or part‐time job. For current (but not previous) employment, individuals with an associate degree were more likely than those who had not graduated from high school to have a full‐ or part‐time job.
Figure 1.
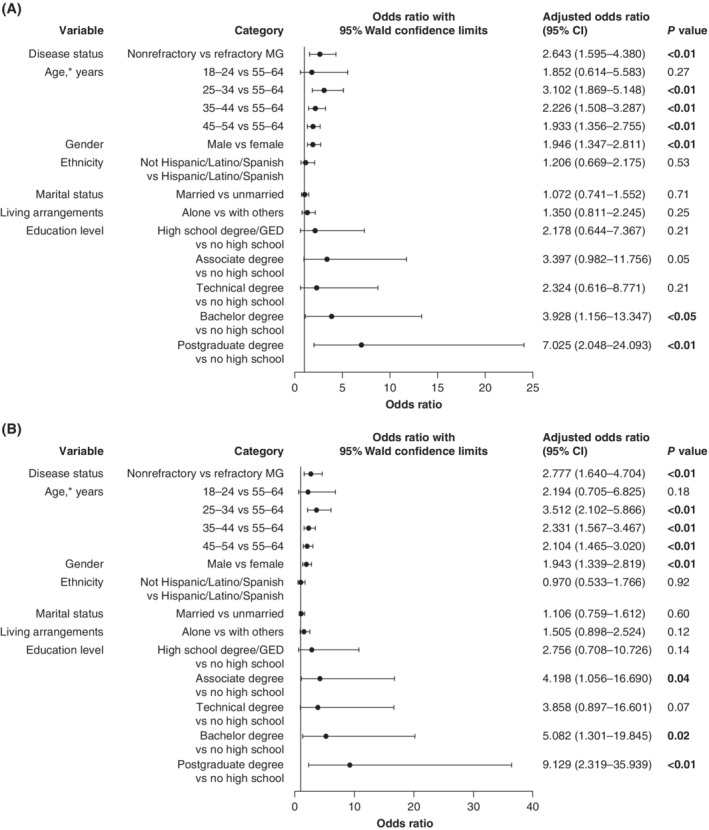
Adjusted regression analyses examining the impact of demographic characteristics on: A, previous employment status (in the 6 months before enrollment); and B, current employment status. CI, confidence interval; GED, general equivalency diploma; MG, myasthenia gravis. *Overall effect of age for previous and current employment status: P < 0.01
3.4. Absences from work—bivariate analyses
There was no significant association between disease status (refractory or nonrefractory MG) and absences from work among participants who were employed in the 6 months before enrollment (Table 1).
3.5. Absences from work—adjusted regression analyses
Among those who were employed in the 6 months before enrollment, disease status (refractory or nonrefractory MG) was not significant in the adjusted regression analysis (Figure 2). Married participants, however, were less likely than those who were unmarried (OR [95% CI], 0.490 [0.277–0.868]) to miss fewer working days.
Figure 2.
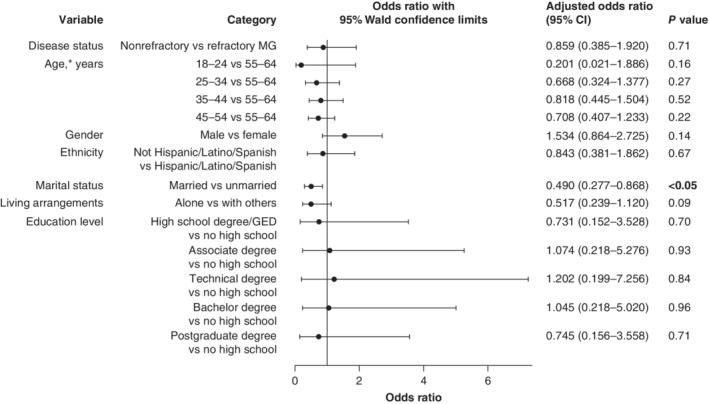
Adjusted regression analyses examining the impact of demographic characteristics on absences from work among participants employed in the 6 months before enrollment. CI, confidence interval; GED, general equivalency diploma; MG, myasthenia gravis. *Overall effect of age, P = 0.51
4. DISCUSSION
Adjusted regression analyses indicated that participants with refractory disease in the MGFA Patient Registry were more likely to work fewer hours per week than those with nonrefractory disease. This pattern was apparent for current employment but also for previous employment (over the preceding 6 months). There was no significant association, however, between disease status and absences from work (among patients in employment during the 6 months before enrollment). Importantly, there were no significant differences in demographic characteristics between the two groups of participants, other than those related to employment.
The criteria used to identify refractory disease in the present study were informed by the different definitions used in previous studies.11 The proportion of participants assigned to the refractory MG cohort in the present study (9%) accords with those reported from US medical and pharmacy claims (also 9%)2 and from a retrospective chart review in a US tertiary neuromuscular clinic (15%),3 despite any differences in the criteria for refractory disease. As acknowledged in a previous analysis conducted with the current study population,4 the proportion of women was higher (80%) when compared with other studies.2, 3 This is likely to be because participants aged over 65 years were excluded, and women have an earlier mean age of MG onset.14, 15
Previous studies have provided important insights into the impact of MG on employment status and absences from work. In a large study of German patients with MG, more than two thirds were unemployed, and more than one quarter reported being forced to retire early because of MG.7 More than one third of patients in an Australian study also reported having to stop working and, among those younger than 65 years of age, there was a 38% reduction in full‐time employment.6 Importantly, over half of the patients who were working reported taking MG‐related sick leave in the previous 12 months; in some cases, the sick leave was in excess of 2 months for the period studied.6 In a Danish study, the odds of employees taking long‐term sick leave (at least 9 weeks) were significantly higher among patients than the general population.5 The present study builds on these collective findings. The results showed a negative impact on hours worked per week in participants with refractory disease that was greater than that in participants with nonrefractory disease. Although refractory status was not a significant variable in analyses of absences from work due to MG in the 6 months before enrollment, this may be a direct consequence of participants with refractory disease generally participating less in the labor market than those with nonrefractory disease. The causes of reduced participation in the labor market were not characterized, but the persistent debilitating symptoms of MG despite concerted therapeutic attempts, and the added treatment burden of immunosuppressant therapy, IVIg and plasmapheresis required for some patients, may be contributing factors.
MG is a rare disease and is refractory in only a subgroup of patients.2, 3 The societal impact of reduced participation in the labor market due to refractory MG may thus not be large. The impact on patients and their families, however, is likely to be considerable in terms of financial security, access to associated benefits, and stimulation in the form of physical and mental activities and social contact.7 Such factors may contribute to the negative association between unemployment and aspects of health‐related quality of life (HRQoL) in MG.7
Limitations to the analyses reported here necessarily align with those associated with previous research conducted with this study population.4 The participant‐reported data are unconfirmed by health‐care professionals. Participants may have been classified as having refractory disease because they were not receiving adequate treatment to manage symptoms and the reasons for this may not have been limited to contraindications and tolerability problems, which reflect an inability to treat the disease. Some participants with uncontrolled MG may also not have fulfilled the strict criteria for refractory disease used in the study; however, their inclusion in the nonrefractory group would tend to ameliorate differences between refractory and nonrefractory MG cohorts. We acknowledge that the recall period for the MG‐ADL questionnaire in the survey is longer (previous 4 weeks) than that used in other studies (7 days),16, 17 and that the Registry participants might comprise individuals more inclined than the general patient population to seek medical information or with higher educational and socioeconomic status that facilitated participation. Participants with refractory disease, moreover, may be more motivated to participate in the Registry. Notwithstanding these limitations, the data in the present study provide important insight into the employment status of individuals with refractory MG. Further research is warranted to characterize the factors leading to MG‐related withdrawal from, and preventing a return to, paid employment for both those with refractory and those with nonrefractory disease. The associations between work status and treatment‐related side effects or comorbidities are likely to be of particular interest.
In conclusion, our study has shown that individuals with refractory MG were more likely to work fewer hours per week or to not have a job than those with nonrefractory disease, but they were not more likely to be absent from work if employed. Given that participation in the labor market influences an individual's financial circumstances and HRQoL, these findings reaffirm the considerable disease burden experienced by patients with refractory MG.
CONFLICT OF INTEREST
L.H. was formerly employed by and holds stocks in Alexion Pharmaceuticals, Inc; I.B.A., and G.C. are employed by the University of Alabama at Birmingham, which received financial support from Alexion for this study; H.X. was formerly employed by the University of Alabama at Birmingham. G.C. is also president of Pythagoras, Inc, a private consulting company located in Birmingham, Alabama, and Professor of Biostatistics at the School of Public Health at the University of Alabama at Birmingham. G.C. has served as a member of consulting or advisory boards (Argenix, Atara Biotherapeutics, Axon, Biogen, Brainstorm Cell Therapeutics, Charleston Laboratories, Click Therapeutics, Genentech, Genzyme, GW Pharmaceuticals, Klein Buendel, MedDay Pharmaceuticals, MedImmune, Novartis, Roche, Scifluor Life Sciences, Somahlution, Teva, TG Therapeutics, and UT Houston) and data and safety monitoring boards (AMO Pharma, BioLineRx, Hisun USA, Horizon Pharma, Merck, Merck/Pfizer, Neurim Pharmaceuticals, National Heart, Lung, and Blood Institute [protocol review committee], National Institute of Child Health and Human Development [OPRU oversight committee], Novartis, Orphazyme, OPKO Biologics, Reata Pharmaceuticals, Receptos/Celgene, Sanofi‐Aventis, Teva).
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
SUPPLEMENTARY TABLE 1 Previous (at any time before enrollment) and current treatment use reported by the study population
ACKNOWLEDGMENTS
The authors thank the Myasthenia Gravis Foundation of America for establishing the Myasthenia Gravis Patient Registry and for its continued support, the MGFA Patient Registry committee, and the coordinating center at the University of Alabama at Birmingham. We also thank Róisín Armstrong and Sivani Paskaradevan (Alexion Pharmaceuticals), and Kelley Capocelli and Kenji Fujita (formerly of Alexion Pharmaceuticals) for critical review of the manuscript. Editorial assistance was provided by Oxford PharmaGenesis, Oxford, UK, and funded by Alexion Pharmaceuticals, Inc.
Harris L, Aban IB, Xin H, Cutter G. Employment in refractory myasthenia gravis: A Myasthenia Gravis Foundation of America Registry analysis. Muscle Nerve. 2019;60:700–706. 10.1002/mus.26694
L.H. is now with Biohaven Pharmaceuticals, New Haven, Connecticut.
The content of this article was presented in preliminary form by A. Boscoe, G. Cutter, and H. Xin at the International Congress on Neuromuscular Diseases, July 2016, Toronto, Ontario, Canada. The methodology, including the date of extraction of data, was revised for the present analysis.
Funding information Alexion Pharmaceuticals
REFERENCES
- 1. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87:419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engel‐Nitz NM, Boscoe A, Wolbeck R, Johnson J, Silvestri NJ. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve. 2018;58:99‐105. [DOI] [PubMed] [Google Scholar]
- 3. Suh J, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med. 2013;86:255‐260. [PMC free article] [PubMed] [Google Scholar]
- 4. Xin H, Harris LA, Aban IB, Cutter G. Examining the impact of refractory myasthenia gravis on healthcare resource utilization in the United States: analysis of a Myasthenia Gravis Foundation of America Patient Registry sample. J Clin Neurol. 2019;15:376‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frost A, Svendsen ML, Rahbek J, Stapelfeldt CM, Nielsen CV, Lund T. Labour market participation and sick leave among patients diagnosed with myasthenia gravis in Denmark 1997‐2011: a Danish nationwide cohort study. BMC Neurol. 2016;16:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blum S, Lee D, Gillis D, McEniery DF, Reddel S, McCombe P. Clinical features and impact of myasthenia gravis disease in Australian patients. J Clin Neurosci. 2015;22:1164‐1169. [DOI] [PubMed] [Google Scholar]
- 7. Twork S, Wiesmeth S, Klewer J, Pöhlau D, Kugler J. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual Life Outcomes. 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagane Y, Murai H, Imai T, et al. Social disadvantages associated with myasthenia gravis and its treatment: a multicentre cross‐sectional study. BMJ Open. 2017;7:e013278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487‐1489. [DOI] [PubMed] [Google Scholar]
- 10. Muppidi S, Wolfe GI, Conaway M, Burns TM. MG Composite and MG‐QOL15 Study Group . MG‐ADL: still a relevant outcome measure. Muscle Nerve. 2011;44:727‐731. [DOI] [PubMed] [Google Scholar]
- 11. Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord. 2018;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buzzard KA, Meyer NJ, Hardy TA, Riminton DS, Reddel SW. Induction intravenous cyclophosphamide followed by maintenance oral immunosuppression in refractory myasthenia gravis. Muscle Nerve. 2015;52:204‐210. [DOI] [PubMed] [Google Scholar]
- 13. Drachman DB, Adams RN, Hu R, Jones RJ, Brodsky RA. Rebooting the immune system with high‐dose cyclophosphamide for treatment of refractory myasthenia gravis. Ann NY Acad Sci. 2008;1132:305‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141‐149. [DOI] [PubMed] [Google Scholar]
- 15. Lee I, Kaminski HJ, Xin H, Cutter G. Gender and quality of life in myasthenia gravis patients from the Myasthenia Gravis Foundation of America Registry. Muscle Nerve. 2018;58:90‐98. [DOI] [PubMed] [Google Scholar]
- 16. Howard JF Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti‐acetylcholine receptor antibody‐positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double‐blind, placebo‐controlled, multicentre study. Lancet Neurol. 2017;16:976‐986. [DOI] [PubMed] [Google Scholar]
- 17. Howard JF Jr, Barohn RJ, Cutter GR, et al. A randomized, double‐blind, placebo‐controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48:76‐84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY TABLE 1 Previous (at any time before enrollment) and current treatment use reported by the study population


