Summary
The Escherichia coli marRAB operon is a paradigm for chromosomally encoded antibiotic resistance. The operon exerts its effect via an encoded transcription factor called MarA that modulates efflux pump and porin expression. In this work, we show that MarA is also a regulator of biofilm formation. Control is mediated by binding of MarA to the intergenic region upstream of the ycgZ‐ymgABC operon. The operon, known to influence the formation of curli fibres and colanic acid, is usually expressed during periods of starvation. Hence, the ycgZ‐ymgABC promoter is recognised by σ38 (RpoS)‐associated RNA polymerase (RNAP). Surprisingly, MarA does not influence σ38‐dependent transcription. Instead, MarA drives transcription by the housekeeping σ70‐associated RNAP. The effects of MarA on ycgZ‐ymgABC expression are coupled with biofilm formation by the rcsCDB phosphorelay system, with YcgZ, YmgA and YmgB forming a complex that directly interacts with the histidine kinase domain of RcsC.
Expression of the multiple antibiotic resistance activator (MarA) protein can give rise to clinically relevant drug resistance in Escherichia coli. Counterintuitively, we showed that MarA production inhibits the formation of biofilms. Inhibition is mediated by the activation of the ycgZ‐ymgABC operon. To activate these genes, MarA acts selectively thus enhancing transcription by RNAP associated with the σ70 but not with the σ38 factor.
Introduction
The Escherichia coli multiple antibiotic resistance (mar) locus was discovered as a genetic element providing resistance to tetracycline (George and Levy, 1983). The region encodes an operon designated marRAB and also provides resistance to quinolones, β‐lactams and a range of phenolic compounds (George and Levy, 1983; Ariza et al., 1994; White et al., 1997). Usually transcribed stochastically, constitutive marRAB expression can result from mutation (Cohen et al., 1993; Ariza et al., 1994; El‐Meouche et al., 2016). Hence, clinical levels of drug resistance are associated with the inactivation of marR that encodes an auto repressor (Cohen et al., 1993; Ariza et al., 1994). Salicylic acid, and related phenolic molecules can also reduce repression by altering the conformation of MarR (Duval et al., 2013; Hao et al., 2014). The ability of the operon to provide resistance against antimicrobial compounds is dependent on marA that encodes a transcriptional activator (Ariza et al., 1994; Rhee et al., 1998). MarA plays an important role in drug resistance by activating the expression of the acrAB‐tolC encoded efflux pump (White et al., 1997; Zhang et al., 2008).
Many bacterial transcription factors act as dimers at palindromic DNA sequences (Robison et al., 1998; Aravind et al., 2005). In contrast, MarA binds to its DNA site, the marbox, as a monomer (Rhee et al., 1998). Hence, MarA–DNA complexes are asymmetrical with defined orientation (Martin et al., 1999). Promoters regulated by MarA can be divided into two classes. At class I promoters the marbox (5′‐GCAHWWWWTGYYAAA‐3′) is usually in the reverse orientation and located between ~50 and 70 base pairs (bp) upstream of the transcription start site (Martin et al., 1999). Consequently, MarA contacts the RNA polymerase (RNAP) α subunit C‐terminal domain (αCTD) to activate transcription (Martin et al., 1999). This interaction requires a surface of MarA comprising residues D18, W19, D22 and R36 (Dangi et al., 2004). At class II promoters, the marbox is in the forward orientation and overlaps the promoter −35 element (Martin et al., 1999). Hence, a contact with region 4 of the RNAP σ subunit may be involved (Zafar et al., 2011). In recent work, we identified more than 30 transcription units directly targeted by MarA (Sharma et al., 2017). A current aim is to understand the regulation and physiological functions of these targets.
Biofilms are populations of bacterial cells coalesced within a complex matrix of DNA, proteins and polysaccharides (Hall‐Stoodley et al., 2004; Flemming, et al., 2016). As well as being structural, the matrix helps to protect cells from damage (Hall‐Stoodley et al., 2004; Flemming, et al., 2016). Hence, biofilms may permit cell survival upon antibiotic treatment (Stewart and Costerton, 2001). In E. coli, the ability to form biofilms is regulated by the second messenger cyclic‐di‐GMP (Simm et al., 2004). The downstream signalling pathway enhances the expression of a transcriptional activator called CsgD (Hammar et al., 1995; Weber et al., 2006). Subsequently, curli fibres are produced (Hammar et al., 1995). These amyloid fibres facilitate surface adhesion, cell aggregation and are a major component of the biofilm matrix (Serra et al., 2013; Hobley et al., 2015). Curli expression can be inhibited by products of the ycgZ‐ymgABC operon. Briefly, these proteins induce the rcsCDB‐encoded phosphorelay system that reduces the levels of CsgD via the RprA sRNA (Tschowri et al., 2009; Mika et al., 2012; Tschowri et al., 2012). In this work, we showed that, in addition to controlling the expression of efflux pumps, MarA directly activates the ycgZ‐ymgABC operon and so represses the formation of curli fibres and biofilms. Activation of ycgZ‐ymgABC proceeds via a class I mechanism whereby MarA binds to the 62 bp upstream of the ycgZ‐ymgABC promoter. Unusually, for class I promoters, the marbox is in the forward orientation and this is essential for activation. Stimulation of ycgZ‐ymgABC by MarA is σ factor specific. Hence, MarA drives transcription by RNAP associated with σ70 but not σ38. Consistent with regulation via the RcsCDB system, we show that rcsB is required for the effects of MarA on biofilm production mediated by ycgZ‐ymgABC. We also show that YcgZ, YmgA and YmgB form a complex that directly interacts with the histidine kinase (HK) domain of RcsC, presumably altering its phosphorylation state.
Results
MarA binds to a specific target site at the ycgZ‐ymgABC promoter
Previously, we used chromatin immunoprecipitation (ChIP) coupled with sequencing (ChIP‐seq) to map MarA binding across the E. coli genome (Sharma et al., 2017). Locations bound by MarA included the intergenic region upstream of the ycgZ‐ymgABC operon. Figure 1A shows the ChIP‐seq data for MarA binding, the DNA sequence of the intergenic region and the predicted marbox. Our first aim was to determine if MarA bound at the proposed site. Hence, we generated a 119 bp DNA fragment corresponding to the sequence in Fig. 1A. This DNA fragment was named ycgZ.1. We also prepared mutated (ycgZ.1m) and truncated (ycgZ.2) derivatives. The mutations and site of truncation, both predicted to abolish MarA binding, are indicated alongside the ycgZ.1 DNA sequence in Fig. 1A. The ability of MarA to bind each of the DNA fragments was tested in vitro using electrophoretic mobility shift assays (Fig. 1B). As expected, MarA bound to the ycgZ.1 DNA fragment. However, MarA did not bind to ycgZ.1m or ycgZ.2. Hence, MarA binds to the predicted site 62 bp upstream of the ycgZ‐ymgABC transcription start site.
Figure 1.
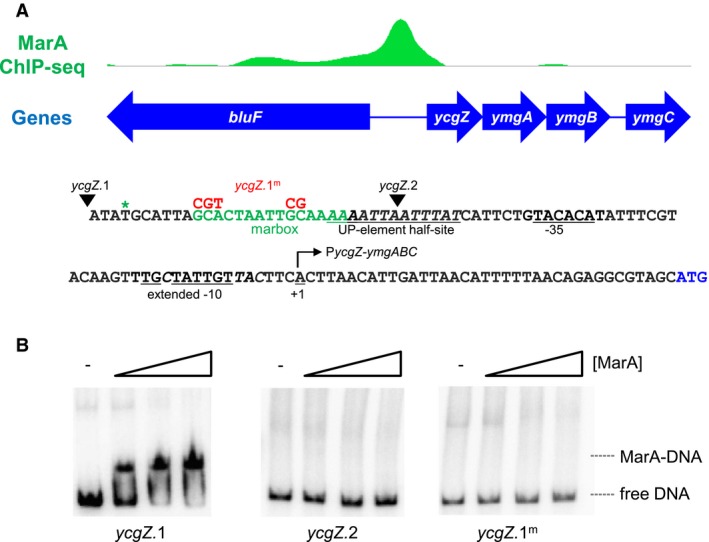
Binding of MarA to the ycgZ‐ymgABC intergenic region.
A. ChIP‐seq data for MarA binding at the ycgZ‐ymgABC locus. Genes are shown as blue arrows and the ChIP‐seq data for MarA binding is in green (ArrayExpress accession number E‐MTAB‐5521). The sequence of the intergenic region, corresponding to the ycgZ.1 DNA fragment, is shown below the ChIP‐seq profile. The sequence of the predicted marbox is in green and the centre of the ChIP‐seq peak for MarA is denoted by an asterisk. The ycgZ‐ymgABC transcription start site is indicated by a bent arrow and the promoter extended −10 and −35 elements are underlined. Bases in italic are important for conferring recognition by σ38. Mutations introduced in the ycgZ.1m DNA fragment are shown above the wild type DNA sequence in red. The 5' ends of the ycgZ.1 and ycgZ.2 DNA fragments are indicated by inverted triangles.
B. Binding of MarA to the ycgZ‐ymgABC intergenic region in vitro requires the predicted marbox. The results of electrophoretic mobility shift assays are shown for different derivatives of the ycgZ‐ymgABC intergenic region. Where present, MarA was used at concentrations of 0.4, 1.2, or 2.0 μM. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
The ycgZ‐ymgABC promoter is recognised by σ70‐ and σ38‐associated RNA polymerase in vitro
The ycgZ‐ymgABC operon is transcribed from a single promoter denoted PycgZ‐ymgABC (Tschowri et al., 2012) (Fig. 1A). Previous work noted reduced PycgZ‐ymgABC activity in cells lacking σ38, the alternative RNAP sigma factor from starved cells (Tschowri et al., 2009). Consistent with this, PycgZ‐ymgABC exhibits features that specifically enhance σ38‐mediated transcription (bases italicised in Fig. 1) (Typas et al., 2007a). We and others have previously shown that promoters recognised by σ38 can also be targets for the housekeeping σ70 factor (Typas et al., 2007b; Grainger et al., 2008; Singh et al., 2011). Furthermore, even when utilising the same promoter, the two σ factors may respond differently to adjacently bound regulatory proteins (Colland et al., 2000; Germer et al., 2001; Grainger et al., 2008; Singh et al., 2011). To understand the ability of each RNAP derivative to utilise the ycgZ‐ymgABC promoter we used in vitro transcription assays. To facilitate this, the 119 bp ycgZ.1 DNA fragment was cloned in plasmid pSR upstream of the λ oop transcription termination signal. Hence, transcripts generated from the ycgZ‐ymgABC promoter are 128 nt in length and can be detected following electrophoresis. The result of the experiment is shown in Fig. 2A. The smaller RNAI transcript originates from the plasmid replication origin and serves as an internal control. As expected, σ38‐associated RNAP stimulated transcription from the ycgZ‐ymgABC promoter (lane 1). An identically sized transcript was produced by the σ70‐associated RNAP but with 4‐fold lower efficiency (lane 8). Hence, the ycgZ‐ymgABC promoter can be recognised by both RNAP derivatives.
Figure 2.
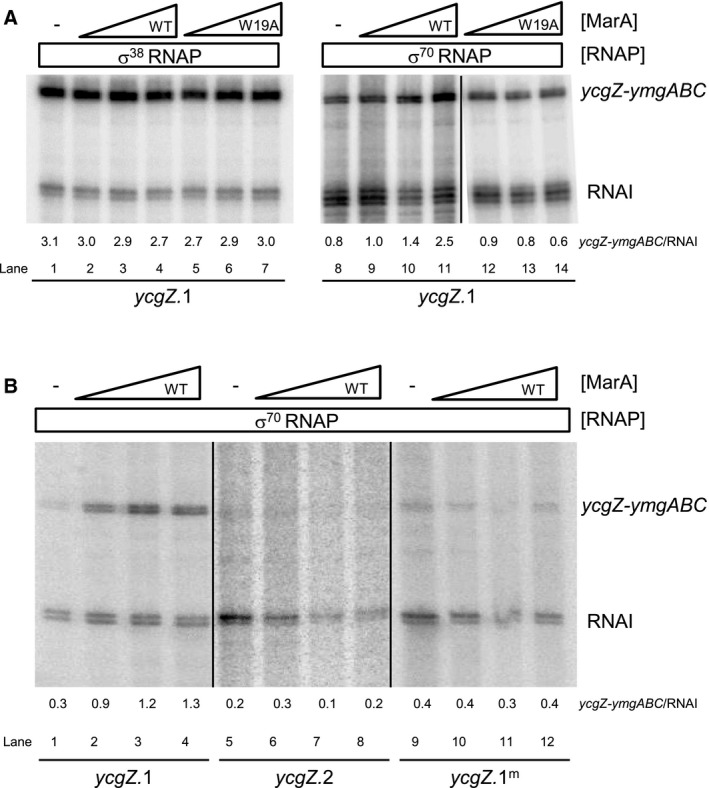
MarA activates transcription by σ70 but not σ38‐associated RNA polymerase at the ycgZ‐ymgABC promoter.
A. Activation of the ycgZ‐ymgABC promoter requires MarA side chain W19. The figure shows in vitro transcription assays using the ycgZ.1 DNA fragment cloned in plasmid pSR as a template. RNA polymerase was used at a concentration of 0.4 μM and MarA was used at concentrations of 0.4, 1.2, or 2.0 μM. Transcripts generated from the ycgZ‐ymgABC promoter are labelled and the RNAI transcript is generated from the plasmid replication origin.
B. Activation of the ycgZ‐ymgABC promoter requires the marbox. The figures show results of in vitro transcription assays. The DNA template was plasmid pSR carrying the ycgZ.1, ycgZ.1m or ycgZ.2 DNA fragments. RNA polymerase was used at a concentration of 0.4 μM and MarA was used at concentrations of 0.4, 1.2 or 2.0 μM.
Binding of MarA at the ycgZ‐ymgABC promoter stimulates transcription by σ70‐associated RNA polymerase in vitro
We next sought to understand if MarA could alter transcription from the ycgZ‐ymgABC promoter. Hence, we added increasing concentrations of MarA to our in vitro transcription incubations. For reactions with σ38, there was no detectable change in transcription at any of the MarA concentrations tested (Fig. 2B, lanes 1–4). Conversely, σ70‐dependent transcription increased 3‐fold in the presence of MarA (Fig. 2B, lanes 8–11). In equivalent experiments, using the ycgZ.1m or ycgZ.2 DNA sequences, the loss of the marbox prevented activation by MarA (Fig. 2B).
Stimulation of σ70‐dependent transcription at the ycgZ‐ymgABC promoter requires MarA side chain W19
The position of the ycgZ‐ymgABC marbox suggests activation by a contact with the RNAP αCTD. Previously, Dangi and co‐workers (2004) identified a surface of MarA, including key amino acid residue W19, which mediates αCTD interactions. Hence, we purified MarAW19A and tested its ability to stimulate transcription from PycgZ‐ymgABC. The data are shown in Fig. 2A. As expected, there was no effect on the MarA independent transcription driven by σ38‐associated RNAP (lanes 5–7). Conversely, stimulation of σ70‐dependent transcription by MarA required residue W19 (compare lanes 8–11 with 12–14). Taken together, the position of the MarA binding site, and role of residue W19, are consistent with PycgZ‐ymgABC stimulation involving a MarA contact with αCTD.
Marbox position is important for σ70‐dependent activation of the ycgZ‐ymgABC promoter
The forward orientation of the marbox at the ycgZ‐ymgABC regulatory region is unexpected; all other class I MarA activated promoters contain a marbox in the reverse orientation (Martin et al., 1999). The only exception is the zwf promoter where the marbox is positioned unusually close to the promoter −35 element (Martin et al., 1999). Hence, we next sought to understand the importance of marbox orientation and position upstream of PycgZ‐ymgABC. To do this, we created a series of ycgZ.1 derivatives in plasmid pSR. The full DNA sequences are shown in Fig. S1 and schematic illustrations are in Fig. 3A. In each case, either the position or orientation of the marbox was been altered (Fig. 3A). The consequences were measured using in vitro transcription assays (Fig. 3B). Whereas σ70‐dependent transcription from the starting ycgZ.1 fragment was enhanced by MarA (Fig. 3B, lanes 1–5), MarA could not stimulate transcription when the marbox was in the reverse orientation (lanes 6–10). Activation by MarA was also abolished when the marbox was moved upstream by 1 bp (lanes 11–15), 5 bp (lanes 16–20) or 10 bp (lanes 21–25). Positioning the marbox closer to the promoter was better tolerated. Thus, activation was observed when the marbox was −61 (lanes 26–30) or −52 (lanes 36–40) bp upstream of the transcription start site. Moving the marbox 5 bp closer to PycgZ‐ymgABC was deleterious to both basal promoter activity and activation by MarA (lanes 31–35).
Figure 3.
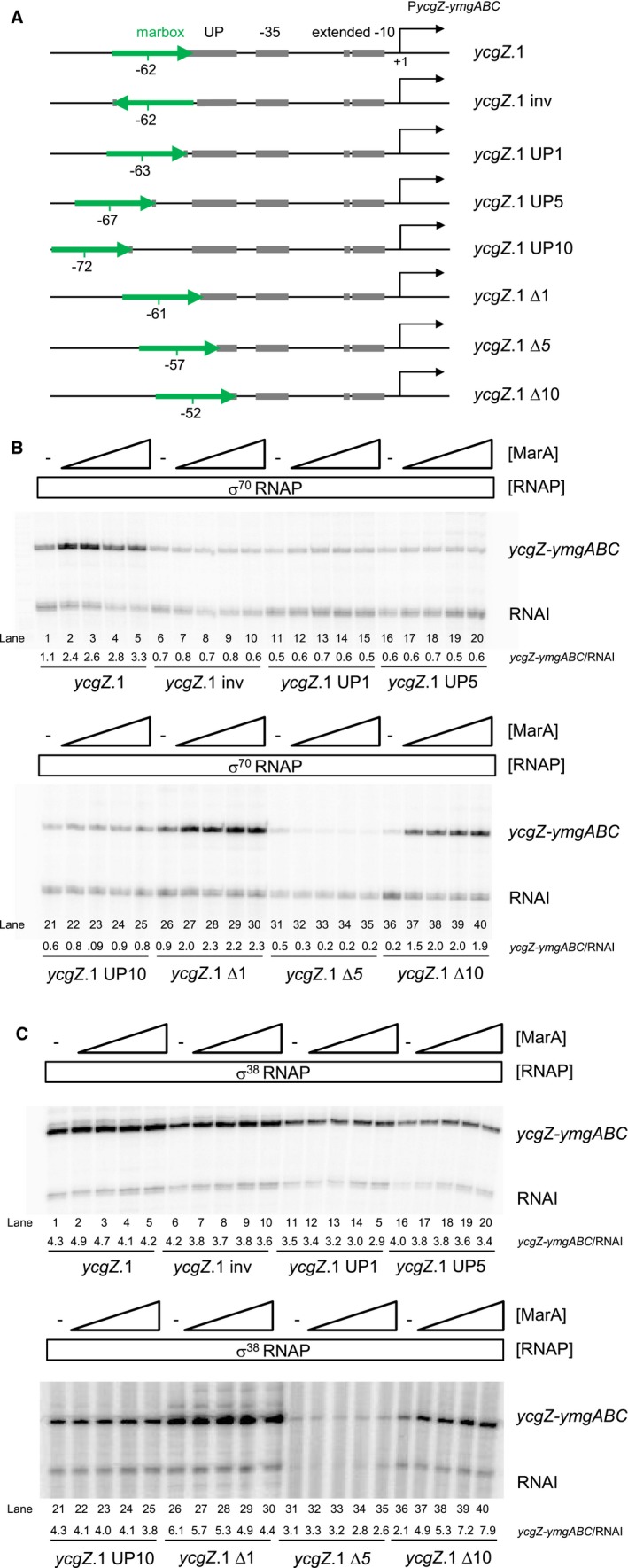
Spacing and orientation requirements for activation of the ycgZ‐ymgABC promoter by MarA.
A. The schematics show the ycgZ.1 DNA fragment and derivatives. The marbox is shown as a green arrow to depict orientation. The centre of the marbox with respect to the transcription start site (black bent arrow) is indicated. Promoter elements are in grey and labelled. Note that the UP‐element half‐site is labelled ‘UP’ for brevity.
B. Activation of transcription from different derivatives of ycgZ.1 by σ70‐associated RNA polymerase. The data are images of gels used to separate products from in vitro transcription assays. The DNA template was plasmid pSR carrying the different ycgZ.1 derivatives. RNA polymerase was used at a concentration of 0.4 μM and MarA was used at concentrations of 0.4, 0.8, 1.2, or 2.0 μM.
C. Activation of transcription from different derivatives of ycgZ.1 by σ38‐associated RNA polymerase. Data are otherwise as described for panel B. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
MarA activates σ38‐dependent transcription from a ycgZ‐ymgABC promoter derivative with a repositioned marbox
Interestingly, the ycgZ‐ymgABC promoter has a distal (i.e. not abutting the −35 hexamer) UP‐element half‐site (Fig. 1A). Previously, we showed that RNAP associated with σ38 is able to utilise such sequences. Conversely, σ70 containing holoenzyme is often defective at such promoters (Typas and Hengge, 2005). The PycgZ‐ymgABC promoter derivatives described above have different UP‐element configurations (Fig. 3A). Hence, we also measured the ability of σ 38 bound RNAP to utilise the variants (Fig. 3C). Inverting or moving the marbox further upstream left the UP‐element half‐site intact. These changes had little impact on σ38‐dependent transcription (Fig. 3C, lanes 1–30). Moving the marbox 5 bp closer to the −35 element simultaneously deleted 5 bp of UP‐element DNA. This promoter derivative was poorly able to drive transcription by σ38 holoenzyme (lanes 31–35). Strikingly, when the marbox was positioned 10 bp further downstream, replacing the UP‐element half‐site, σ38‐dependent transcription was reduced (lane 36) but could be stimulated ~4‐fold by MarA (lanes 36–40).
The ycgZ‐ymgABC promoter marbox is required for maximal activity in vivo
To understand the role that MarA might play in controlling ycgZ‐ymgABC transcription in vivo we fused various PycgZ‐ymgABC DNA fragments to lacZ in the reporter plasmid pRW50. The plasmid constructs were used to transform E. coli Δlac strain JCB387 and cells were grown to either mid‐log phase or stationary phase in Luria Broth. The cells were then lysed and β‐galactosidase activities determined using the lysates. The data are shown in Fig. 4A. In the presence of the marbox, β‐galactosidase activities were similar for growing and stationary phase cells (solid green bars). Deletion (open bars) or mutation (striped bars) of the marbox caused larger decreases in transcription for growing cells compared to starved cells (Fig. 4A). Recall that our in vitro transcription assays showed stimulation of σ70‐ but not σ38‐dependent transcription from PycgZ‐ymgABC by MarA (Fig. 2A). Hence, the reduced requirement for the marbox in starved cells is probably due to an increase in σ38‐dependent transcription.
Figure 4.
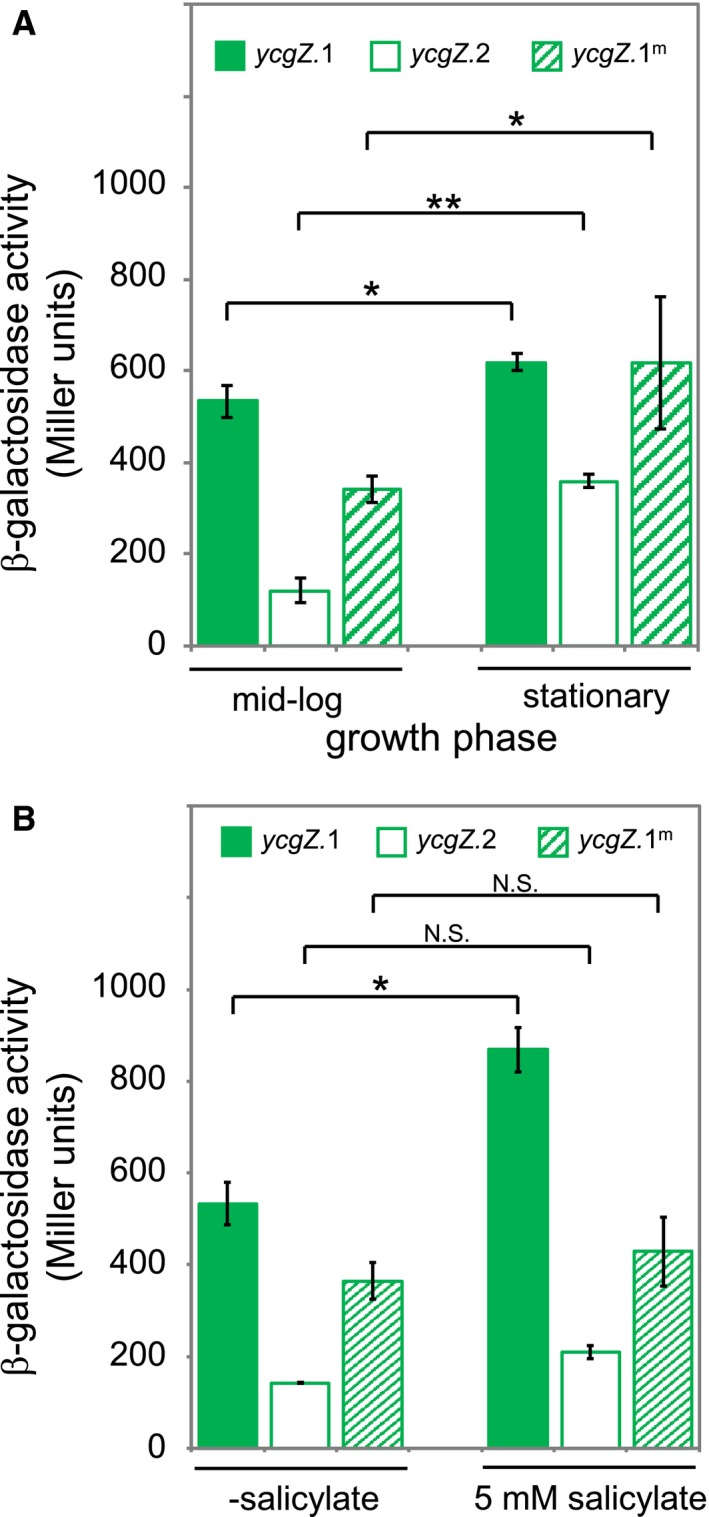
The ycgZ‐ymgABC promoter marbox is required for maximal activity in vivo during exponential growth and in the presence of salicylic acid.
A. The graphs show levels of β‐galactosidase activity measured in lysates of E. coli strain JCB387 carrying different ycgZ::lacZ fusions in plasmid pRW50. Cultures in M9 minimal media were grown to exponential phase or stationary phase as indicated. The value of P was calculated using a two‐tailed student's t‐test.
B. Levels of β‐galactosidase activity measured in lysates of cells grown in the presence of 5 mM sodium salicylate. The value of P was calculated using a two‐tailed student's t‐test. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
The ycgZ‐ymgABC promoter marbox is required for induction by salicylic acid in vivo
Promoters activated by MarA can be induced with sodium salicylate (Duval et al., 2013) because salicylic acid relieves repression of the marRAB operon by MarR (Duval et al., 2013). We reasoned that transcription from the ycgZ‐ymgABC promoter should increase in the presence of salicylic acid. Furthermore, any such increase should require the marbox. Hence, we repeated our measurements of ycgZ‐ymgABC promoter activity in growing cells with or without the addition of 5 mM sodium salicylate (Fig. 4B). As expected, ycgZ‐ymgABC transcription increased upon the addition of sodium salicylate (compare solid bars). Conversely, little or no increase was observed when the marbox was deleted (open bars) or changed by mutation (striped bars).
Curli fibre formation is inhibited by the ycgZ‐ymgABC operon in a marbox dependent manner
The ycgZ‐ymgABC operon inhibits the formation of biofilms by indirectly reducing the formation of curli fibres (Tschowri et al., 2009; Mika et al., 2012; Tschowri et al., 2012). Briefly, expression of ycgZ‐ymgABC ultimately reduces the abundance of CsgD; a positive regulator of curli production. To understand the role of MarA, we made derivatives of plasmid pBR322Δbla. These DNA constructs encoded ycgZ‐ymgABC under the control of its own promoter and the upstream marbox. We also made variants of the plasmid where the marbox was mutated or deleted as in Fig. 1A. The plasmids were used to transform E. coli JCB387 or a ΔycgZ‐ymgABC derivative. Production of curli was then monitored in macrocolonies grown on agar plates containing Congo red dye that binds the fibres (Reichhardt et al., 2015). Results are shown in Fig. 5. First, we compared macrocolonies formed by JCB387, or the ΔycgZ‐ymgABC derivative, carrying the control pBR322Δbla with no cloned insert. Wild type colonies had a pale pink appearance and a red ring at their periphery (panel A). Conversely, ΔycgZ‐ymgABC colonies were red with a narrow pink ring just inside the border of the colony (panel E). As expected, the introduction of the plasmid encoding ycgZ‐ymgABC, under the control of PycgZ‐ymgABC and the upstream marbox, reduced curli production. Hence, both wild type (panel B) and ΔycgZ‐ymgABC (panel F) colonies had the same pale pink appearance. Removal or mutation of the plasmid marbox triggered an increase in curli production. In wild type cells, this was evident as a solid red macrocolony (panels C and D). Similarly, cells lacking ΔycgZ‐ymgABC exhibited a red ring on the periphery of the colony and a deeper interior pink colour (panels G and H).
Figure 5.
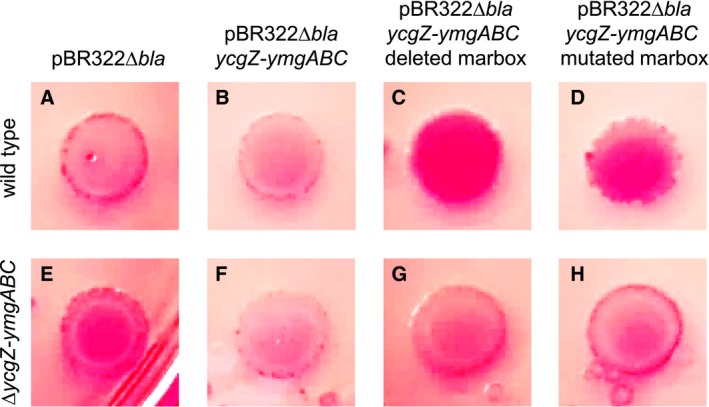
Expression of the ycgZ‐ymgABC operon reduces curli fibre production in a marbox dependent manner. Each of the panels (A) through (H) shows a macrocolony grown on Congo red agar plates. Images in panels A–D are of wildtype E. coli strain JCB387 transformed with empty plasmid vector (A), plasmid vector encoding ycgZ‐ymgABC under the control of its native promoter (B), or derivatives lacking (C) or having a mutated (D) ycgZ‐ymgABC marbox. Equivalent data are shown in panels E‐H for a JCB387 derived strain lacking the chromosomal ycgZ‐ymgABC operon. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Biofilm formation is inhibited by the ycgZ‐ymgABC operon in a marbox dependent manner
Biofilms are complex structures involving many extracellular components in addition to curli fibres (Tschowri et al., 2009; Hobley et al., 2015; Flemming et al., 2016). Hence, we next investigated the role of ycgZ‐ymgABC, and upstream marbox, in controlling biofilm formation in cell culture plates. As described above, we tested different combinations of wild type E. coli JCB387, and the ΔycgZ‐ymgABC derivative, carrying plasmid‐encoded ycgZ‐ymgABC with variants of the upstream regulatory DNA. Crystal violet dye was used to detect biofilms formed and the amount of dye bound by the biofilm was quantified by spectrophotometry. We first compared biofilms formed by JCB387, or the ΔycgZ‐ymgABC derivative, carrying the control pBR322Δbla with no cloned insert (Fig. 6A and 6B, grey bars). There was a small increase in the ΔycgZ‐ymgABC strain (P = 1.1e−5). As expected, the introduction of the plasmid encoding ycgZ‐ymgABC, under the control of PycgZ‐ymgABC and the upstream marbox, reduced biofilm formation (Fig. 6A and 6B, green bars). Removal or mutation of the marbox triggered an increase in biofilm production (Fig. 6A and 6B, open and striped bars). Surprisingly, differences were most pronounced for the wild type JCB387 strain (Fig. 6A). We speculate that deleting chromosomal ycgZ‐ymgABC may have additional uncharacterised downstream consequences.
Figure 6.
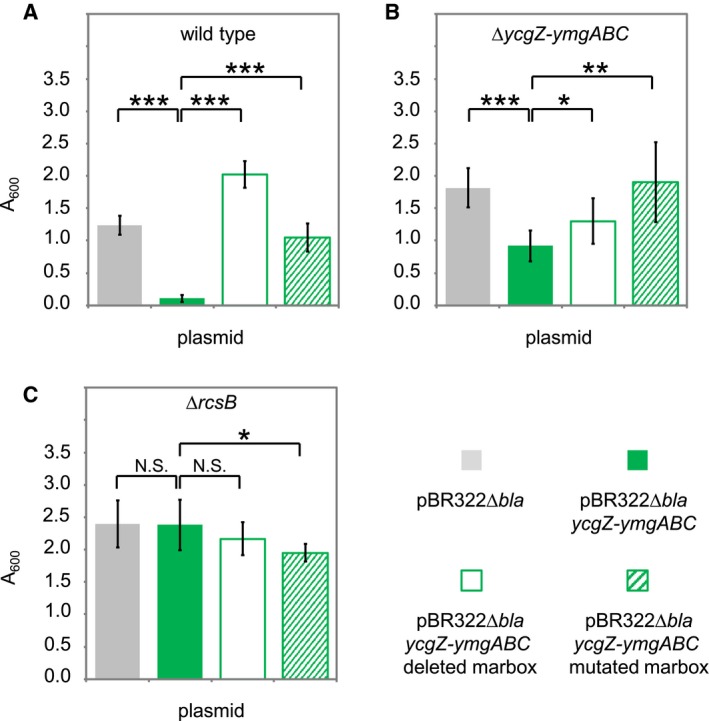
Expression of the ycgZ‐ymgABC operon reduces biofilm formation in a marbox and rcsB dependent manner. The figure shows results from assays of biofilm formation. Briefly, biofilms grown in culture plates were stained with crystal violet dye. After washing away excess dye, biofilms were dried and the dye solubilised. Spectrophotometry was then used to quantify the amount of dye bound by each biofilm. Data are shown for (A) wildtype E. coli strain JCB387 and derivatives lacking (B) ycgZ‐ymgABC or (C) rcsB. The strains were transformed with different plasmids. The plasmid derivatives are indicated by the key at the bottom of the figure. Each data point is the mean value from four independent biofilms and error bars indicate standard deviation. For each panel, the value of P was calculated using a two‐tailed student's t‐test. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Regulation of ycgZ‐ymgABC by MarA is uncoupled from biofilm formation in cells lacking rcsB
Recall that the ycgZ‐ymgABC operon exerts its effect on biofilms by activating the RcsCDB phosphorelay system (Tschowri et al., 2009; Mika et al., 2012; Tschowri et al., 2012). Briefly, RcsC is an inner membrane sensor kinase that can phosphorylate the phosphotransferase RcsD. In turn, RcsD phosphorylates the response regulator RcsB that activates the expression of a sRNA called RprA. The sRNA inhibits the translation of CsgD; a positive regulator of curli production and biofilm formation (Tschowri et al., 2009; Mika et al., 2012). Hence, we reasoned that effects of MarA on biofilms, mediated by ycgZ‐ymgABC, should be abolished in cells lacking RcsB. To test this prediction, we repeated our assays of biofilm production in derivatives of the E. coli JCB387 strain lacking rcsB. As expected, deletion of rcsB increased the production of biofilms twofold (compare grey bars in Fig. 6A and 6C). In this genetic background introducing the plasmid encoding ycgZ‐ymgABC, under the control of PycgZ‐ymgABC and the upstream marbox, had no effect (Fig. 6C, green, open and striped bars).
Putative interactions between ycgZ‐ymgABC and rcsCDB‐encoded proteins are revealed by two‐hybrid analysis
We next aimed to better understand how the ycgZ‐ymgABC gene products impact the RcsCDB phosphorelay system. In particular, we wondered if direct protein–protein interactions were involved. To test this, we utilised the BacterioMatch two‐hybrid system (Fig. 7A, Dove and Hochschild, 2004). The assay detects interactions between ‘bait’ and ‘target’ proteins fused to the λcI transcription factor and RNAP α subunit respectively. If fused proteins interact, the λcI derivative recruits modified RNAP to a semisynthetic promoter. This allows expression of the downstream yeast HIS3 gene, required for histidine biosynthesis. Hence, interactions between ‘bait’ and ‘target’ allow E. coli to grow on media containing 3‐amino‐1,2,4‐triazole (3‐AT), an inhibitor of histidine production (Dove and Hochschild, 2004). Figure 7B shows the growth of E. coli harbouring different combinations of YcgZ, YmgA, YmgB and YmgC, fused to λcI or RNAP αNTD, in plasmids pBT and pTRG respectively. To check reproducibility, five individual colonies of each strain were ‘patched’ on both selective (with 3‐AT) and nonselective (no 3‐AT) media. As expected, cells were able to grow on nonselective media regardless of the plasmid combination used (Fig. 7B, i–v). Conversely, growth on selective media was only permitted by certain combinations of the various fusions. (Fig. 7B, vi–x). Specifically, all combinations of fusions containing YcgZ, YmgA and YmgB allowed growth on selective plates (Fig. 7B, vi–vii, top two rows). Hence, these three factors can all interact and might form a complex. In contrast, fusions with YmgC did not reproducibly stimulate growth in any combination (Fig. 7B, viii).
Figure 7.
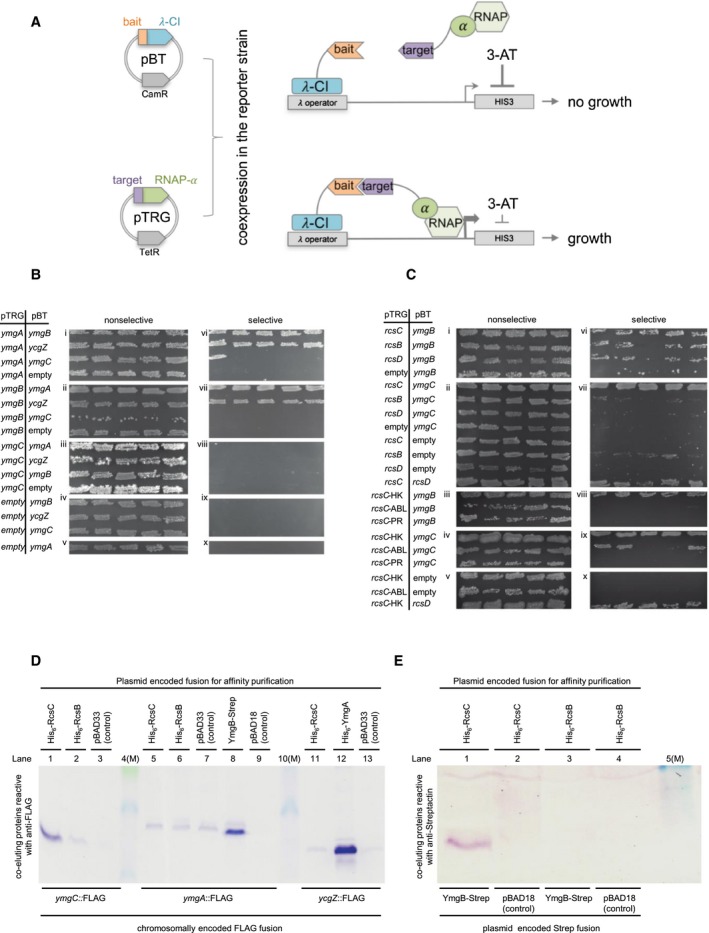
A complex formed by YmgA, YmgB and YcgZ interacts with RcsC.
A. Schematic representation of the BacterioMatch two‐hybrid system. Plasmids pBT and pTRG are used to fuse prey proteins to λCI or replace the RNA polymerase α C‐terminal domain with target proteins. Upon expression in vivo, interactions between λCI‐prey and α‐bait fusions allow sufficient expression of His3 for growth in the presence of the His3 inhibitor 3‐AT.
B. BacterioMatch two‐hybrid system reporter cells were cotransformed with derivatives of the pBT and pTRG plasmids and vector‐only controls. YcgZ, YmgA, YmgB and YmgC were expressed either as hybrid proteins fused to cI‐NTD from pBT or as fusions to RNAP alpha‐NTD from pTRG. For experiments with RcsC or RcsD we cloned the entire cytosolic part of the protein or the indicated histidine kinase (RcsC‐HK), alpha‐beta‐loop (RcsC‐ABL) and phosphoreceiver domain (RcsC‐PR). Full‐size RcsB was expressed from pTRG. Interactions were detected by growth in the presence of the His3 inhibitor 3‐AT (selective) at 37°C for 24 h following incubation at 28°C for 48 h. Each row on the plates shows patches of five independent cotransformants.
C. The E. coli strain MC4100 encoding chromosomal ymgC::Flag, ymgA::Flag or ycgZ::Flag was transformed with pBAD33‐derivatives expressing His6‐RcsC, His6‐RcsB, or His6‐YmgA. YmgB‐Strep was expressed from pBAD18 and the empty vectors served as negative controls. Flag‐tagged proteins coeluting in Ni‐NTA or Strep‐Tactin Sepharose based chromatography of cellular lysates were detected using immunoblot analysis and the monoclonal anti‐Flag antibody.
D. Wild‐type MC4100 was cotransformed with pBAD18 or pBAD18‐ymgB‐Strep and either pBAD33‐His‐rcsC (cytosolic part) or pBAD33‐His‐rcsB. YmgB‐Strep coeluting with His‐RcsC or His‐RcsB from extracts subject to Ni‐NTA chromatography was detected using the anti‐Strep‐Tactin antibody. Lanes labelled (M) contain size markers. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Next, we examined interactions between ycgZ‐ymgABC‐ and rcsCDB‐encoded proteins. We did not detect any interactions involving YcgZ or YmgA (data not shown). Conversely, a reproducible interaction was detected between YmgB and RcsC (Fig. 7C, vi, top row). For YmgB in combination with RcsB or RcsD, the data were erratic; growth was sparse and inconsistent (Fig. 7C, vi, middle two rows). Such ambiguities are not unusual and suggest a weak or artefactual interaction barely sufficient to permit survival (Tschowri et al., 2012). A reproducible interaction was detected between YmgC and RcsC (Fig. 7C, vii, top row). We were also able to detect interactions between RcsC and RcsD (Fig. 7C, vii, bottom row). To better define interactions, we tested the ability of YmgB or YmgC to interact with individual RcsC domains. Hence, we cloned the HK, alpha‐beta‐loop (ABL) or phosphoreceiver (PR) domains of RcsC in pTRG. The data show that both YmgB and YmgC can contact the cytoplasmic HK domain of RcsC (Fig. 7C, top row of panels viii and ix). We were unable to obtain a reproducible result for the interaction of YmgC and RcsC‐ABL (Fig. 7C, ix, second row).
Validation of protein–protein interactions by affinity purification and coelution
The two‐hybrid analysis suggests that YmgA, YmgB and YcgZ can all interact with each other (Fig. 7B). Furthermore, both YmgB and YmgC interact with RcsC (Fig. 7C). To independently validate these interactions we used in vivo coelution assays. Hence, we constructed plasmids encoding RcsC, RcsB, YmgA or YmgB with either a His6‐ or Strep‐tag. The plasmids were used to transform strains expressing Flag‐tagged YmgC, YmgA or YcgZ. After cell lysis, His6‐ or Strep‐tagged proteins were purified by affinity chromatography. Copurification of FLAG‐tagged proteins was probed by western blotting. The data show copurification of YmgC‐FLAG with His6‐RcsC (Fig. 7D, lane 1), YmgA‐FLAG with YmgB‐Strep (lane 8) and YcgZ‐Flag with His6‐YmgA (lane 12). To check interactions between YmgB and RcsB or RcsC we coexpressed YmgB‐Strep with His6‐ RcsB or RcsC. The His6 proteins were purified from cell lysates and the presence of YmgB‐Strep probed by western blotting. The YmgB‐Strep copurified with His‐RcsC (Fig. 7E, lane 1) but not RcsB (lane 3).
Discussion
In this work, we show that MarA is a positive regulator of the ycgZ‐ymgABC promoter in E. coli (Figs 2 and 3). We also demonstrate that activation of ycgZ‐ymgABC by MarA reduces biofilm production in a manner requiring rcsB (Fig. 6). Hence, the simplest explanation is that MarA exerts its effect via the known ability of ycgZ‐ymgABC to stimulate the RcsCDB phosphorelay system. We show that ycgZ‐ymgABC targets RcsCDB directly; YmgB forms a complex with YcgZ and YmgA that contacts the HK domain of RcsC (Fig. 7). Since activation of the RcsCDB system triggers the production of the RprA sRNA, which inhibits CsgD expression, the production of curli fibres is reduced (Fig. 5) (Tschowri et al., 2009; Mika et al., 2012). Our model is summarised in Fig. 8. Note that the regulation of ycgZ‐ymgABC likely impacts other aspects of biofilm formation beyond curli production. For instance, the bdm (biofilm‐dependent modulation) gene is also subjected to regulation by the RcsCDB cascade (Francez‐Charlot et al., 2005). It is initially counterintuitive that increased MarA production should inhibit biofilm formation; the biofilm mode of life is considered favourable for surviving treatment with antibiotics (Stewart and Costerton, 2001; Hall‐Stoodley et al., 2004; Hobley et al., 2015; Flemming et al., 2016). However, for growing planktonic cells, 24 h are required to establish a biofilm (Elvers et al., 2002; Adamus‐Białek et al., 2015). Clearly, a biofilm must already exist to provide protection (Stewart and Costerton, 2001; Hall‐Stoodley et al., 2004; Hobley et al., 2015; Flemming et al., 2016). Hence, nascent biofilm formation seems to be a poor strategy for surviving immediate threats. We suggest that, during planktonic growth, induction of the mar response inhibits biofilm formation and favours short term survival strategies including drug efflux, altered outer membrane permeability and DNA repair (White et al., 1997; Sharma et al., 2017).
Figure 8.
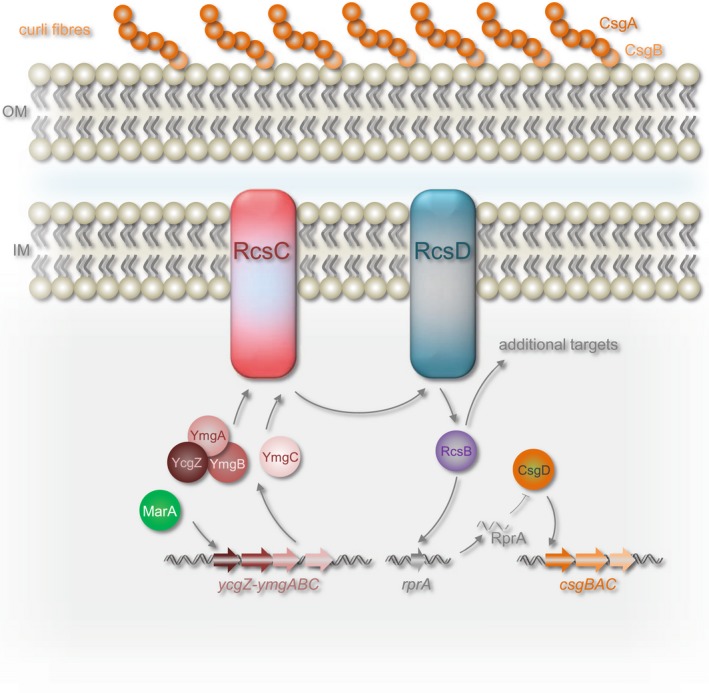
Model for repression of biofilm formation by MarA. Genes are shown as block arrows and proteins are shown as spheres or ovals. Stimulatory and inhibitory interactions are shown by arrows and bar‐headed lines respectively. Nucleic acids are shown as double (DNA) or single (sRNA) wavy lines. The inner membrane (IM), outer membrane (OM) and curli fibres are labelled. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Activation of the ycgZ‐ymgABC promoter requires MarA residue W19 (Fig. 2). Furthermore, MarA can exert its effect from different positions (Fig. 3). This suggests a contact with the RNAP αCTD (Gaston et al., 1990; Wing et al., 1995; Dangi et al., 2004). Surprisingly, activation only occurs when RNAP is associated with the σ70 subunit; σ38‐dependent transcription from PycgZ‐ymgABC is not stimulated by MarA. Hence, activation by MarA is sigma factor specific but does not require a direct interaction with σ70. It is well established that σ70 and σ38 have vastly different DNA bending capacities (Shin et al., 2005). Furthermore, we have shown previously that the holoenzyme variants interact with UP‐elements differently; complexes with σ38 preferentially utilise promoter distal UP‐element half‐sites. At PycgZ‐ymgABC, one such element is amongst several sequence characteristics that favour basal transcription initiation involving σ38 (Fig. 1A). The −13C base is directly contacted by σ38 K173 whilst nonoptimal spacers and AT‐rich discriminators are better tolerated by σ38 (Becker and Hengge‐Aronis, 2001; Typas and Hengge, 2006; Typas et al., 2007a). The different interactions that αCTD makes in the context of σ38 holoenzyme also explain selective activation by MarA. Hence, the σ38 derivative preferentially uses the distal UP‐element half‐site whilst MarA provides a point of contact for αCTD in the context of σ70 holoenzyme. Consistent with this, moving the marbox 10 bp downstream, to replace the UP‐element, negates basal preference of the promoter for σ38 and permits MarA to activate σ38‐dependent transcription. To our knowledge, selective regulation has not previously been demonstrated for AraC family regulators. However, other transcription factors are known to behave in this way (Colland et al., 2000; Germer et al., 2001; Typa et al., 2007b; Grainger et al., 2008; Singh et al., 2011). For example, OxyR, Fis and H‐NS regulate σ70 but not σ38‐dependent transcription at the dps locus (Grainger et al., 2008). Similarly, at the proP2 promoter, Fis activates σ38‐ but not σ70‐dependent transcription (Typas et al., 2007b). Hence, PycgZ‐ymgABC fits the general rule that σ38 can act more autonomously than σ70 (Typas et al., 2007a).
Interestingly, Vila and Soto previously noted that MarA could inhibit biofilm formation in uropathogenic E. coli (UPEC) (Vila and Soto, 2012). It was suggested that the reduced production of type I fimbriae was responsible and that this was mediated via repression of fimB by MarA (Vila and Soto, 2012) We suggest that any such repression must be indirect since the fimB promoter does not contain a marbox (Sharma et al., 2017). Conversely, UPEC strains do encode the ycgZ‐ymgABC operon and the position and sequence of the MarA binding site are conserved. Hence, an alternative explanation is that MarA repression of biofilm formation in UPEC involves the mechanism outlined here. We speculate that production of type I fimbriae may be modulated in response to expression of the ycgZ‐ymgABC operon (Fig. 8). We also note that Duval and coworkers reported the accumulation of ycgZ‐ymgABC‐encoded proteins in strains lacking the Lon protease (Duval et al., 2017). This is due to Lon targeting MarA for degradation and levels of MarA thus increasing substantially in Lon deficient cells (Martin et al., 2008). Hence, our work is concordant with, and provides an explanation for, several previous observations linking MarA, biofilm formation and control of ycgZ‐ymgABC.
Experimental procedures
Strains, plasmids and oligonucleotides
Strains and plasmids are listed in Table 1 and oligonucleotides in Table S1. To construct pET28a‐MarA, a DNA fragment encoding marA was generated by PCR using the MarA‐OE‐F and Mar‐OE‐R oligonucleotides. Following digestion with BamHI and NdeI, the DNA was ligated downstream of the T7‐lac promoter in pET28a. The pBR322Δbla plasmid was made by the digestion of pBR322 with AatII and VspI to excise the β‐lactamase gene. A small linker with terminal AatII and VspI sites was used to recircularise the plasmid. Fragments encoding ycgZ‐ymgABC operon, with variants of the upstream DNA, were then cloned in pBR322Δbla via the EcoRI and AatII restriction sites. The ΔycgZ‐ymgABC derivative of E. coli strain JCB387 was created using the gene doctoring method described by Lee et al. (2009). We transferred rcsB::kan by P1 transduction from a derivative of strain BW25113 (Baba et al., 2006). C‐terminally 3x Flag‐tagged chromosomally encoded ymgC::Flag, ymgA::Flag and ycgZ::Flag were constructed in E. coli K‐12 strain MC4100 using pSUB11 (Uzzau et al., 2001) as a template for PCR and oligonucleotides listed in Table S1 following λRED‐based recombination procedure (Uzzau et al., 2001). For in vivo interaction assays, BacterioMatch II Two‐Hybrid System vectors pBT and pTRG were used (Stratagene, Agilent Technologies). The relevant genes were cloned using primers listed in Table S1 to generate C‐terminal fusions either to the lambda cI repressor from pBT or to the N‐terminal domain of the α subunit of E. coli RNAP from pTRG and fusion proteins were tested for interaction in histidine auxotrophic XL1‐Blue MRF’‐derivative E. coli strain (Stratagene, Agilent Technologies). For in vivo coelution experiments, the soluble cytosolic parts of RcsC, RcsB and YmgA, respectively were N‐terminally fused to a 6xHis‐tag and cloned into pBAD33. YmgB carrying a Strep‐tag at the C‐terminus was cloned into pBAD18. pBAD33‐derived RcsC (cytosolic moiety only), RcsB and YmgA were either expressed in strains containing ymgC::Flag, ymgA::Flag or ycgZ::Flag, respectively, or together with cotransformed pBAD18‐ymgB‐Strep in MC4100. Coelution experiments were done with cell lysates as described further below.
Table 1.
Strains and plasmids.
| Name | Description | Source |
|---|---|---|
| Bacterial strains | ||
| JCB387 | ΔnirB, Δlac | Page et al. (1990) |
| JCB387ΔycgZ‐ymgABC ΔrcsB | ΔnirB, Δlac, ΔycgZ‐ymgABC, ΔrcsB | This work |
| JCB387ΔycgZ‐ymgABC ΔrcsB | ΔnirB, Δlac, ΔycgZ‐ymgABC, ΔrcsB | This work |
| BL21 DE3 | T7 RNApol + F‐ ompT rb‐ma‐ fhuA2 | Studier (1991) |
| T7 Express | lacZ::T7 gene1 [lon] ompT gal sulA11 | NEB |
| R(mcr‐73::miniTn10‐‐TetS)2 [dcm] | ||
| R(zgb‐210::Tn10‐‐TetS) endA1 | ||
| Δ(mcrC‐mrr)114::IS10 | ||
| MC4100 | E.coli K12 F‐ araD139 O(argF‐lac)U169 | Casadaban (1976) |
| deoC flbB5301 relA1 rpsL150 ptsF25 rbsR | ||
| NAT239 | MC4100 ycgZ::Flag | This work |
| NAT240 | MC4100 ymgA::Flag | This work |
| NAT242 | MC4100 ymgC::Flag | This work |
| XL1‐Blue MRF | Δ(mcrA)183 Δ(mcrCB‐hsdSMR‐mrr)173 | Agilent Technol. |
| endA1 supE44 thi‐1 recA1 gyrA96 | ||
| relA1 lac [F´ proAB lacIqZΔM15 Tn5 (Kanr)] | ||
| Plasmids | ||
| pRW50 | Low copy number 16 kb plasmid for making lacZ fusions. Contains the RK2 origin of replication and encodes TetR | Lodge et al. (1992) |
| pSR | 4 kb pBR322 derivative that encodes AmpR. Contains an EcoRI‐HindIII cloning site upstream of the λoop transcription terminator | Kolb et al. (1995) |
| pBR322 | 4.4kb, encodes TetR and AmpR. Contains the pMB1 origin of replication and rop for restriction of plasmid copy number | Bolivar et al. (1977) |
| pBR322Δbla | pBR322 lacking the bla gene | This work |
| pET‐28a | 5.4kb, encodes KanR. Contains the T7 lac promoter for high‐level IPTG‐inducible expression of recombinant proteins with N‐ or C‐terminal His tags and a thrombin cleavage site | Novagen |
| pDOC‐C | 5.8kb, encodes AmpR, derived from pEX100T. Used as a cloning vector for gene doctoring. Features a cloning region flanked by two I‐SceI recognition sites | Lee et al. (2009) |
| pDOC‐K | Derived from pEX100T and contains a kanamycin resistance cassette between two Flp recombinase recognition sites | Lee et al. (2009) |
| pACBSR | 7.3kb, encodes CamR. Recombination plasmid for gene doctoring; carries arabinose inducible λ‐Red and I‐SceI endonuclease genes | Herring et al. (2003) |
| pCP20 | 9.4kb, encodes CamR and AmpR. Encodes yeast FLP recombinase gene. Used to remove the kanamycin cassette in gene doctoring | Cherepanov and Wackernagel (1995) |
| pSUB11 | 3.5kb, encodes KanR. Used to amplify Flp recombinant target (FRT)‐flanked kanamycin resistance cassette with 3xFlag | Uzzau et al., 2001 |
| pKD46 | 6.3kb, encodes AmpR. Used to express λRed recombinase | Uzzau et al., 2001 |
| pBT | 3.2kb, encodes CamR. BacterioMatch II Two‐Hybrid System vector. Encodes λ phage cI protein | Agilent Technol. |
| pTRG | 4.4kb, encodes TetR. BacterioMatch II Two‐Hybrid System vector. Encodes RNAP αNTD | Agilent Technol. |
| pBAD18 | 4.6kb, encodes AmpR. Carries arabinose‐inducible araBAD promoter, pBR322 origin | Guzman et al. (1995) |
| pBAD33 | 5.3kb, encodes CamR. Carries arabinose‐inducible araBAD promoter, pACYC184 origin | Guzman et al. (1995) |
Protein purification
Preparations of σ70 and σ38 were made by the overexpression of the cloned rpoD and rpoS genes in BL21 DE3 cells, and subsequent purification by liquid chromatography, as described by Grainger et al. (2008). The RNAP core enzyme was purchased from NEB. The RNAP holoenzyme was generated by incubating the core enzyme with a 4‐fold excess of σ factor at room temperature for 20 minutes prior to use. MarA purification was based on that described by Jair et al. (1995). T7 Express cells containing pET28a‐MarA were grown to an OD600 of 0.8 and expression of MarA was induced with 0.4mM IPTG for 3 h. Cells were harvested by centrifugation and washed with a buffer containing 50mM Tris–HCl (pH 7.5), 1mM EDTA, 1M NaCl before lysis using an AVESTIN EmulsiFlex C3 high pressure motorised homogeniser. Inclusion bodies were collected by centrifugation at 75,000× g for 30 minutes and washed with a buffer containing 4M urea, 50mM Tris–HCl (pH 8.5). After recentrifugation, the pellet was solubilised with 50mM Tris–HCl (pH 8.5), 6M guanidinium–HCl. Material remaining in the suspension was removed by centrifugation and His‐tagged MarA was purified from the supernatant by immobilised nickel ion affinity chromatography. The 1ml HisTrap™ FF (GE Healthcare) column was equilibrated with Buffer A (1M NaCl, 5mM Tris–HCl (pH 8.5)) and loaded with cell lysate using an ÄKTAprime system (GE Healthcare). Bound protein was eluted using a linear gradient of Buffer B (Buffer A + 1M imidazole). Purified MarA was then transferred into a buffer containing 1M NaCl, 5mM HEPES, 1mM dithiothreitol, 5mM EDTA and 0.1mM Triton X‐100 by dialysis. After concentrating the sample to 1mg/ml with 5,000 MWCO Vivaspin® 20 columns (Sartorius) the peptide bond linking the His‐tag to MarA was cleaved using thrombin sepharose beads (BioVision) for 5 h at room temperature. Beads were removed by centrifugation and the His‐tag was removed by a second round of affinity chromatography. Untagged MarA that did not bind the HisTrap™ column was transferred into 1M NaCl, 5mM HEPES, 1mM dithiothreitol, 5mM EDTA, 0.1mM Triton X‐100 and 20% (v/v) glycerol by dialysis. Samples were stored at −80 °C until use.
Electrophoretic mobility shift assays
Assays were performed as described previously (Cosgriff et al., 2010). Briefly, DNA fragments were generated by PCR amplification from an E. coli genomic DNA template. Following purification, PCR products were cut with HindIII and EcoRI prior to being end‐labelled with [γ‐32P]‐ATP and polynucleotide kinase. The DNA fragments were incubated with MarA in buffer containing 20 mM Tris pH 7, 10 mM MgCl2, 100 mM EDTA and 120 mM KCl. Reactions were analysed by electrophoresis through a 5% polyacrylamide gel. Raw gel images are shown in Fig. S2.
Assays of in vitro transcription
The procedure for in vitro transcription was similar to that described (Haycocks et al., 2015) and used the system of Kolb et al. (1995). Briefly, a Qiagen Maxiprep kit was utilised to purify pSR plasmid with the required promoter insert. Sixteen microgram per millilitre of DNA template was preincubated with purified MarA in buffer containing 20 mM Tris pH 7.9, 5 mM MgCl2, 500 µM DTT, 50 mM KCl, 100 µg ml‐1 BSA, 200 µM ATP, 200 µM GTP, 200 µM CTP and 10 µM UTP with 5 µCi [α‐32P]‐UTP. The reaction was started by adding RNAP holoenzyme. Labelled RNA products were analysed on a denaturing polyacrylamide gel. Raw gel images are shown in Fig. S2.
β‐galactosidase assays
DNA fragments containing the desired derivative of the ycgZ‐ymgABC regulatory region were cloned in plasmid pRW50 to generate promoter::lacZ fusions. The β‐galactosidase levels in lysates of cells carrying these recombinants were measured by the Miller method (Miller, 1972). Activities are the average of three or more independent experiments.
Congo red binding assays
Bacterial strains were cultured overnight in lysogeny broth (LB) lacking salt (10 g/L of tryptone and 5 g/L of yeast extract). Curli fibres were detected by spotting 5 μl of overnight culture onto LB agar lacking salt and supplemented with 40 µg/ml of Congo red. The agar plates were then incubated at 37°C overnight. The morphology and colour of colonies were recorded by digital photography. The experiments were done multiple times to check that colony phenotypes were reproducible and images shown are representative. The raw image is shown in Fig. S2.
Crystal violet binding assays
The crystal violet assay described in Baugh et al. (2014) was used to quantify biofilm production between bacterial strains. Two independent overnight cultures per strain were diluted in LB to an OD600 of 0.1. A 200 μl aliquot was added to a flat‐bottomed 96‐well microtitre plate, with four replicate wells per culture. The plate was incubated at 30°C for 48 h. Wells were washed with water to remove unattached cells and 200 μl of 0.1% w/v crystal violet was added for 15 minutes. Wells were then washed with water again to remove unbound crystal violet and 200 μl of 70% ethanol was added to solubilise the retained crystal violet. The A600 was then measured using a CLARIOstar plate reader (BMG Labtech) to give a quantitative measure of biofilm formation.
Bacterial two‐hybrid assays
In vivo protein–protein interactions were detected using BacterioMatch II Two‐Hybrid System (Dove and Hochschild, 2004). Interaction of coexpressed hybrid proteins linked to the NTD of lambda cI (from pBT) and to the bacterial RNAP alpha‐NTD (from pTRG) activates HIS3 gene expression suppressing histidine auxotrophy of the reporter strain (E. coli XL1‐Blue MRF’ derivative). The assay was performed according to the instruction manual (Stratagene, Agilent technologies). Cotransformants were obtained on nonselective plates and five independent clones were patched on both, nonselective and selective medium respectively. Growth on selective medium containing the His3 inhibitor 3‐amino‐1,2,3‐triazole (3‐AT) indicates the interaction of the tested hybrid proteins leading to increased expression of HIS3 gene. Plates were incubated for 24 h at 37°C and for an additional 48 h at 28°C.
In vivo coelution and immunoblot analysis
In vivo protein–protein interactions were also analysed using coelution (‘pull‐down’) assays. pBAD33‐6xHis‐rcsC (cytosolic part only), pBAD33‐6xHis‐rcsB, pBAD‐6xHis‐ymgA and pBAD18‐ymgB‐Strep were transformed into E. coli K‐12 MC4100 containing chromosomally Flag‐tagged ymgC, ymgA and ycgZ respectively. Expression of pBAD‐encoded genes was induced with 0.1% arabinose at OD600 of 0.8. Cells were grown overnight at 28°C and cell pellets were lysed using a French press. Cells expressing His‐tagged genes were lysed in ‘His‐lysis buffer’: 50 mM NaH2PO4, 150 mM NaCl, 20 mM imidazole, pH 8. Strep lysis buffer (100 mM Tris–HCl pH8, 150 mM NaCl, 1 mM EDTA) was used for lysis of cells expressing ymgB‐Strep. Ni‐NTA agarose (Qiagen) was used for affinity chromatography of His‐tagged proteins. Strep‐Tactin Sepharose (IBA, Gottingen) was served for affinity purification of YmgB‐Strep. Chromatography columns were washed with the respective lysis buffer and elution was performed using 50 mM NaH2PO4, 150 mM NaCl, 250 mM imidazole, pH 8 for His‐tagged proteins and buffer E (IBA) for YmgB‐Strep. Copurified Flag‐tagged proteins were detected using monoclonal anti‐FLAG antibody (Sigma) following SDS polyacrylamide gel electrophoresis. To detect protein–protein interactions between YmgB‐Strep and either 6xHis‐RcsC (cytosolic part) or 6xHis‐RcsB, respectively, pBAD33‐6xHis‐rcsC or pBAD33‐6xHis‐rcsB were cotransformed and expressed with pBAD18‐ymgB‐Strep in MC4100. The cells were grown and treated as described above. His‐tagged proteins were purified using Ni‐NTA. Coeluted YmgB‐Strep was detected using anti‐Strep‐Tactin antibody (IBA).
Supporting information
Acknowledgements
Work in the Grainger lab was supported by BBSRC grant BB/N014200/1 awarded to D.C.G., a BBSRC MIBTP studentship awarded to R.A.K. and a Wellcome Trust studentship awarded to KL. MAW is supported by the BBSRC Institute Strategic Programme Microbes in the Food Chain BB/R012504/1 and its constituent project BBS/E/F/000PR10349. Work in the Hengge lab was supported by the Deutsche Forschungsgemeinschaft (DFG grant He1556/13‐2, awarded to R.H.). We thank Joseph Wade for helpful discussions.
Data availability statement
All data are available within the manuscript figures or within the cited references.
References
- Adamus‐Białek, W. , Kubiak, A. and Czerwonka, G. (2015) Analysis of uropathogenic Escherichia coli biofilm formation under different growth conditions. Acta Biochimica Polonica, 62, 765–771. [DOI] [PubMed] [Google Scholar]
- Aravind, L. , Anantharaman, V. , Balaji, S. , Babu, M.M. and Iyer, L.M. (2005) The many faces of the helix‐turn‐helix domain: transcription regulation and beyond. FEMS Microbiology Reviews, 29, 231–262. [DOI] [PubMed] [Google Scholar]
- Ariza, R.R. , Cohen, S.P. , Bachhawat, N. , Levy, S.B. and Demple, B. (1994) Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli . Journal of Bacteriology, 176, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, T. , Ara, T. , Hasegawa, M. , Takai, Y. , Okumura, Y. , Baba, M. , et al (2006) Construction of Escherichia coli K‐12 in‐frame, single‐gene knockout mutants: the Keio collection. Molecular Systems Biology, 2, 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh, S. , Phillips, C.R. , Ekanayaka, A.S. , Piddock, L.J. and Webber, M.A. (2014) Inhibition of multidrug efflux as a strategy to prevent biofilm formation. Journal of Antimicrobial Chemotherapy, 69, 673–681. [DOI] [PubMed] [Google Scholar]
- Becker, G. and Hengge‐Aronis, R. (2001) What makes an Escherichia coli promoter σS‐dependent? Role of the ‐13/‐14 nucleotide promoter positions and region 2.5 of σS. Molecular Microbiology, 39, 1153–1165. [DOI] [PubMed] [Google Scholar]
- Bolivar, F. , Rodriguez, R.L. , Greene, P.J. , Betlach, M.C. , Heyneker, H.L. , Boyer, H.W. , et al (1977) Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene, 2, 95–113. [PubMed] [Google Scholar]
- Casadaban, M.J. (1976) Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. Journal of Molecular Biology, 104, 541–555. [DOI] [PubMed] [Google Scholar]
- Cherepanov, P.P. and Wackernagel, W. (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp‐catalyzed excision of the antibiotic‐resistance determinant. Gene, 158, 9–14. [DOI] [PubMed] [Google Scholar]
- Cohen, S.P. , Hächler, H. and Levy, S.B. (1993) Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli . Journal of Bacteriology, 175, 1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colland, F. , Barth, M. , Hengge‐Aronis, R. and Kolb, A. (2000) Sigma factor selectivity of Escherichia coli RNA polymerase: a role for CRP, IHF and Lrp transcription factors. EMBO Journal, 19, 3028–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgriff, S. , Chintakayala, K. , Chim, Y.T. , Chen, X. , Allen, S. , Lovering, A.L. and Grainger, D.C. (2010) Dimerization and DNA‐dependent aggregation of the Escherichia coli nucleoid protein and chaperone CbpA. Molecular Microbiology, 77, 1289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi, B. , Gronenborn, A.M. , Rosner, J.L. and Martin, R.G. (2004) Versatility of the carboxy‐terminal domain of the alpha subunit of RNA polymerase in transcriptional activation: use of the DNA contact site as a protein contact site for MarA. Molecular Microbiology, 54, 45–59. [DOI] [PubMed] [Google Scholar]
- Dove, S.L. and Hochschild, A. (2004) A bacterial two‐hybrid system based on transcription activation. Methods in Molecular Biology, 261, 231–246. [DOI] [PubMed] [Google Scholar]
- Duval, V. , McMurry, L.M. , Foster, K. , Head, J.F. and Levy, S.B. (2013) Mutational analysis of the multiple‐antibiotic resistance regulator MarR reveals a ligand binding pocket at the interface between the dimerization and DNA binding domains. Journal of Bacteriology, 195, 3341–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval, V. , Foster, K. , Brewster, J. and Levy, S.B. (2017) A novel regulatory cascade involving BluR, YcgZ, and Lon controls the expression of Escherichia coli OmpF Porin. Frontiers in Microbiology, 8, 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Meouche, I. , Siu, Y. and Dunlop, M.J. (2016) Stochastic expression of a multiple antibiotic resistance activator confers transient resistance in single cells. Scientific Reports, 6, 19538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvers, K.T. , Leeming, K. and Lappin‐Scott, H.M. (2002) Binary and mixed population biofilms: time‐lapse image analysis and disinfection with biocides. Journal of Industrial Microbiology and Biotechnology, 29, 331–338. [DOI] [PubMed] [Google Scholar]
- Flemming, H.C. , Wingender, J. , Szewzyk, U. , Steinberg, P. , Rice, S.A. and Kjelleberg, S. (2016) Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology, 14, 563–575. [DOI] [PubMed] [Google Scholar]
- Francez‐Charlot, A. , Castanié‐Cornet, M.P. , Gutierrez, C. and Cam, K. (2005) Osmotic regulation of the Escherichia coli bdm (biofilm‐dependent modulation) gene by the RcsCDB His‐Asp phosphorelay. Journal of Bacteriology, 187, 3873–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston, K. , Bell, A. , Kolb, A. , Buc, H. and Busby, S. (1990) Stringent spacing requirements for transcription activation by CRP. Cell, 62, 733–43. [DOI] [PubMed] [Google Scholar]
- George, A.M. and Levy, S.B. (1983) Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non‐plasmid‐determined efflux of tetracycline. Journal of Bacteriology, 155, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germer, J. , Becker, G. , Metzner, M. and Hengge‐Aronis, R. (2001) Role of activator site position and a distal UP‐element half‐site for sigma factor selectivity at a CRP/H‐NS activated σS‐dependent promoter in Escherichia coli . Molecular Microbiology, 41, 705–716. [DOI] [PubMed] [Google Scholar]
- Grainger, D.C. , Goldberg, M.D. , Lee, D.J. and Busby, S.J. (2008) Selective repression by Fis and H‐NS at the Escherichia coli dps promoter. Molecular Microbiology, 68, 1366–1377. [DOI] [PubMed] [Google Scholar]
- Guzman, L.M. , Belin, D. , Carson, M.J. and Beckwith, J. (1995) Tight regulation, modulation, and high‐level expression by vectors containing the arabinose PBAD promoter. Journal of Bacteriology, 177, 4121–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall‐Stoodley, L. , Costerton, J.W. and Stoodley, P. (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology, 2, 95–108. [DOI] [PubMed] [Google Scholar]
- Hammar, M. , Arnqvist, A. , Bian, Z. , Olsén, A. and Normark, S. (1995) Expression of two csg operons is required for production of fibronectin‐ and congo red‐binding curli polymers in Escherichia coli K‐12. Molecular Microbiology, 18, 661–670. [DOI] [PubMed] [Google Scholar]
- Hao, Z. , Lou, H. , Zhu, R. , Zhu, J. , Zhang, D. , Zhao, B.S. , et al (2014) The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli . Nature Chemical Biology, 10, 21–28. [DOI] [PubMed] [Google Scholar]
- Haycocks, J.R.J. , Sharma, P. , Stringer, A.M. , Wade, J.T. and Grainger, D.C. (2015) The molecular basis for control of ETEC enterotoxin expression in response to environment and host. PLoS Path, 11, e1004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring, C.D. , Glasner, J.D. and Blattner, F.R. (2003) Gene replacement without selection: regulated suppression of amber mutations in Escherichia coli . Gene, 311, 153–163. [DOI] [PubMed] [Google Scholar]
- Hobley, L. , Harkins, C. , MacPhee, C.E. and Stanley‐Wall, N.R. (2015) Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiology Reviews, 39, 649–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jair, K.W. , Martin, R.G. , Rosner, J.L. , Fujita, N. , Ishihama, A. and Wolf, R.E. Jr . (1995) Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. Journal of Bacteriology, 177, 7100–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, A. , Kotlarz, D. , Kusano, S. and Ishihama, A. (1995) Selectivity of the Escherichia coli RNA polymerase E sigma 38 for overlapping promoters and ability to support CRP activation. Nucleic Acids Research, 23, 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.J. , Bingle, L.E. , Heurlier, K. , Pallen, M.J. , Penn, C.W. , Busby, S.J. and Hobman, J.L. (2009) Gene doctoring: a method for recombineering in laboratory and pathogenic Escherichia coli strains. BMC Microbiology, 9, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge, J. , Fear, J. , Busby, S. , Gunasekaran, P. and Kamini, N.R. (1992) Broad host range plasmids carrying the Escherichia coli lactose and galactose operons. FEMS Microbiology Letters, 74, 271–276. [DOI] [PubMed] [Google Scholar]
- Martin, R.G. , Gillette, W.K. , Rhee, S. and Rosner, J.L. (1999) Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Molecular Microbiology, 34, 431–441. [DOI] [PubMed] [Google Scholar]
- Martin, R.G. , Bartlett, E.S. , Rosner, J.L. and Wall, M.E. (2008) Activation of the Escherichia coli marA/soxS/rob regulon in response to transcriptional activator concentration. Journal of Molecular Biology, 380, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika, F. , Busse, S. , Possling, A. , Berkholz, J. , Tschowri, N. , Sommerfeldt, N. , et al (2012) Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli . Molecular Microbiology, 84, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. (1972) Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Page, L. , Griffiths, L. and Cole, J.A. (1990) Different physiological roles of two independent pathways for nitrite reduction to ammonia by enteric bacteria. Archives of Microbiology, 154, 349–354. [DOI] [PubMed] [Google Scholar]
- Reichhardt, C. , Jacobson, A.N. , Maher, M.C. , Uang, J. , McCrate, O.A. , Eckart, M. and Cegelski, L. (2015) Congo red interactions with curli‐producing E. coli and native curli amyloid. Fibers. PLoS One, 10, e0140388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, S. , Martin, R.G. , Rosner, J.L. and Davies, D.R. (1998) A novel DNA‐binding motif in MarA: the first structure for an AraC family transcriptional activator. Proceedings of the National Academy of Sciences of the United States of America, 95, 10413–10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison, K. , McGuire, A.M. and Church, G.M. (1998) A comprehensive library of DNA‐binding site matrices for 55 proteins applied to the complete Escherichia coli K‐12 genome. Journal of Molecular Biology, 284, 241–254. [DOI] [PubMed] [Google Scholar]
- Serra, D.O. , Richter, A.M. , Klauck, G. , Mika, F. and Hengge, R. (2013) Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. mBio, 4, e00103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P. , Haycocks, J.R.J. , Middlemiss, A.D. , Kettles, R.A. , Sellars, L.E. , Ricci, V. , et al (2017) The multiple antibiotic resistance operon of enteric bacteria controls DNA repair and outer membrane integrity. Nature Communications, 8, 1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, M. , Song, M. , Rhee, J.H. , Hong, Y. , Kim, Y.J. , Seok, Y.J. , et al (2005) DNA looping‐mediated repression by histone‐like protein H‐NS: specific requirement of Esigma70 as a cofactor for looping. Genes & Development, 19, 2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm, R. , Morr, M. , Kader, A. , Nimtz, M. and Romling, U. (2004) GGDEF and EAL domains inversely regulate cyclic di‐GMP levels and transition from sessility to motility. Molecular Microbiology, 53, 1123–1134. [DOI] [PubMed] [Google Scholar]
- Singh, S.S. , Typas, A. , Hengge, R. and Grainger, D.C. (2011) Escherichia coli σ⁷⁰ senses sequence and conformation of the promoter spacer region. Nucleic Acids Research, 39, 5109–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, P.S. and Costerton, J.W. (2001) Antibiotic resistance of bacteria in biofilms. Lancet, 358, 135–138. [DOI] [PubMed] [Google Scholar]
- Studier, F.W. (1991) Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. Journal of Molecular Biology, 219, 37–44. [DOI] [PubMed] [Google Scholar]
- Tschowri, N. , Busse, S. and Hengge, R. (2009) The BLUF‐EAL protein YcgF acts as a direct anti‐repressor in a blue‐light response of Escherichia coli . Genes & Development, 23, 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschowri, N. , Lindenberg, S. and Hengge, R. (2012) Molecular function and potential evolution of the biofilm‐modulating blue light‐signalling pathway of Escherichia coli . Molecular Microbiology, 85, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typas, A. and Hengge, R. (2005) Differential ability of sigma(s) and sigma70 of Escherichia coli to utilize promoters containing half or full UP‐element sites. Molecular Microbiology, 55, 250–260. [DOI] [PubMed] [Google Scholar]
- Typas, A. and Hengge, R. (2006) Role of the spacer between the ‐35 and ‐10 region in σS promoter selectivity in Escherichia coli . Molecular Microbiology, 59, 1037–1051. [DOI] [PubMed] [Google Scholar]
- Typas, A. , Becker, G. and Hengge, R. (2007a) The molecular basis of selective promoter activation by the σS subunit of RNA polymerase. Molecular Microbiology, 63, 1296–1306. [DOI] [PubMed] [Google Scholar]
- Typas, A. , Stella, S. , Johnson, R.C. and Hengge, R. (2007b) The ‐35 sequence location and the Fis‐sigma factor interface determine sigmas selectivity of the proP (P2) promoter in Escherichia coli . Molecular Microbiology, 63, 780–796. [DOI] [PubMed] [Google Scholar]
- Uzzau, S. , Figueroa‐Bossi, N. , Rubino, S. and Bossi, L. (2001) Epitope tagging of chromosomal genes in Salmonella . Proceedings of the National Academy of Sciences, 98, 15264–15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila, J. and Soto, S.M. (2012) Salicylate increases the expression of marA and reduces in vitro biofilm formation in uropathogenic Escherichia coli by decreasing type 1 fimbriae expression. Virulence, 3, 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, H. , Pesavento, C. , Possling, A. , Tischendorf, G. and Hengge, R. (2006) Cyclic‐di‐GMP‐mediated signalling within the sigma network of Escherichia coli . Molecular Microbiology, 62, 1014–1034. [DOI] [PubMed] [Google Scholar]
- White, D.G. , Goldman, J.D. , Demple, B. and Levy, S.B. (1997) Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli . Journal of Bacteriology, 179, 6122–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing, H.J. , Williams, S.M. and Busby, S.J. (1995) Spacing requirements for transcription activation by Escherichia coli FNR protein. Journal of Bacteriology, 177, 6704–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar, M.A. , Sanchez‐Alberola, N. and Wolf, R.E. Jr (2011) Genetic evidence for a novel interaction between transcriptional activator SoxS and region 4 of the sigma(70) subunit of RNA polymerase at class II SoxS‐dependent promoters in Escherichia coli . Journal of Molecular Biology, 407, 333–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, A. , Rosner, J.L. and Martin, R.G. (2008) Transcriptional activation by MarA, SoxS and Rob of two tolC promoters using one binding site: a complex promoter configuration for tolC in Escherichia coli . Molecular Microbiology, 69, 1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within the manuscript figures or within the cited references.


