Abstract
Background
Quality indicators are a commonly used improvement tool in health care. There is growing interest and activity in the use of quality indicators to improve community pharmacy practice.
Objectives
To conduct a scoping review of the use of quality indicators for community pharmacy practice, including their methods of development and evaluation.
Methods
Electronic databases (EMBASE and PubMed) were searched to identify papers published between January 2008 and April 2018. No limits were applied for language of publication or country of origin. Studies were included if they reported empirical data regarding the development or evaluation of quality indicators. All study designs were eligible for inclusion. Duplicate independent screening was undertaken of the search results. Data extraction was performed by one reviewer.
Results
Of the 988 records identified from the database search, 15 articles were included. The studies were conducted in 12 countries from six continents. Eleven studies described the development of quality indicators, eight of which included the evaluation of the psychometric properties of the indicators developed. Four studies examined the impact of quality indicators on practice all of which reported improvements in some aspects of quality, mainly with structure indicators rather than those relating to process and outcome.
Conclusions
Whilst there is a growing emphasis on promoting improvement in community pharmacy services, evidence is lacking of the effect of indicators on improving quality. Measurable process and outcome indicators are needed. The future development of quality indicators would also benefit from a multi‐stakeholder approach.
Keywords: healthcare quality indicators, pharmaceutical services, pharmacies, pharmacists, quality improvement, quality of health care
Introduction
Many healthcare systems suffer from poor quality leading to preventable deaths, reduced quality of life or serious adverse events, such as medication errors.1, 2 Reports on failures in the quality of health care have called for healthcare reform and quality improvement.2, 3, 4
Quality and quality improvement are multi‐dimensional concepts.5 Quality improvement was defined as ‘the combined and unceasing efforts of everyone – healthcare professionals, patients and their families, researchers, payers, planners and educators – to make the changes that will lead to better patient outcomes (health), better system performance (care) and better professional development (learning)’.6
With this definition in mind, five knowledge systems have been recognised as being involved in improvement, including: generalisable scientific evidence; particular context awareness; performance measurement; plans for change; and execution of planned changes.6 One of these systems is performance measurement which includes the use of balanced measures that can assess the effect of changes in quality over time. Quality indicators are required to measure performance and are ‘measurable elements of practice performance for which there is evidence or consensus that it can be used to assess the quality, and hence change in the quality, of care provided’.7 Quality indicators, like many healthcare instruments, are often subject to a psychometric validation (e.g. validity, reliability, feasibility, sensitivity to change) to ensure their suitability for quality assessment. Psychometric validation assesses each instrument through a series of defined tests on the population group for whom the instrument is intended.8
There is growing pressure to demonstrate and improve the quality of health care delivered in community pharmacies.9, 10, 11, 12 This demand is partly driven by the need to determine and evidence how the extended role of community pharmacy teams contributes towards health service delivery and the reduction of pressure on other health sectors.13, 14, 15
In 1999, the International Pharmaceutical Federation (FIP) and the World Health Organization (WHO) published a joint document on good pharmacy practice in community and hospital pharmacy settings.16 The document encouraged national pharmaceutical organisations to direct pharmacists to ensure service provision of appropriate quality to every patient. The FIP provided support to its member organisations in different countries for example Cambodia, Mongolia, Paraguay and Thailand, to develop their own national standards in line with the recommendations of FIP/WHO.17
Other countries have invested in developing quality indicators in community pharmacy. For example, in Australia, The Quality Care Pharmacy Program was established to assure and improve quality in community pharmacies.11 In the United States,18 the Pharmacy Quality Alliance, a voluntary, membership‐based collaborative comprising organisations from pharmacy, patient, employer and the health insurance plan communities, as well as state and federal government, committed to develop quality measure concepts in community pharmacies. Similarly, since 2008 in the Netherlands,19 the Dutch Health Care Transparency Programme has been working on developing quality indicators and enhancing their uptake in everyday practice. More recently in the UK in 2016,20 eight quality indicators were introduced into the Community Pharmacy Contractual Framework with revised elements introduced for 2019.21 The domains to which the payments apply included patient safety, public health, clinical effectiveness, digital/urgent care and the workforce. There is often limited information regarding how quality indicators have been developed, who participated in producing them and their impact on practice and patients outcomes.
A widely accepted conceptual framework to assess the quality of medical care or healthcare services was described by Donabedian.22, 23 The framework consists of three components in which quality indicators can be classified: structure, process and outcome. Structure indicators refer to the setting and the resources in which the care occurs, such as medical supplies, vehicles, personnel and organisational structure. Process indicators relate to interactions and what is actually done when giving and receiving care. Outcome indicators are associated with the consequences for the health status of patients or the population for example patient satisfaction. Standardised indicators and tools are needed to measure and improve the quality of community pharmacy services. A lack of appropriate indicators and tools may contribute to inconsistency between pharmacies and the quality of care delivered.24, 25 A recent study from the UK highlighted inconsistencies in community pharmacists’ attitudes towards, and beliefs about, quality in terms of how it is defined and measured.26
The aim of this study was to undertake a scoping review of studies which reported the development and/or evaluation of quality indicators for use in community pharmacies. The objectives were to explore how quality indicators were developed, by whom and the methods of evaluation used to assess their effect on practice.
Methods
A scoping review was undertaken to identify studies that had developed or evaluated quality indicators in community pharmacy settings. (The review protocol can be accessed on request from the authors.) This review was conducted to comply with the PRISMA‐ScR statement for scoping reviews.27
Search strategy
Electronic databases (EMBASE and PubMed) were searched from January 2008 to April 2018 using a combination of keywords, Emtree (EMBASE) and/or Medical Subject Headings (MeSH) (PubMed) (Appendix S1). Due to limited resources, the grey literature was not searched and search terms were limited to ‘major’ domains within the research databases. The concept of quality indicators is relatively new to community pharmacy; hence, the literature search was restricted to the period from January 2008. Additional studies were identified by searching the reference lists of retrieved articles and the authors’ reference collections.
Study selection process
The results from the electronic databases were imported into EndNote (version 8; Clarivate, Philadelphia, Pennsylvania USA), and duplicate records were removed. Duplicate independent screening of the titles and abstracts was undertaken to identify records which appeared to fulfil the inclusion criteria. The full‐text versions of potentially eligible records were retrieved and assessed independently in duplicate against the inclusion criteria.
Inclusion criteria
Studies were included if they reported the development of quality indicators for community pharmacy practice or evaluated the effect of quality indicators on practice. No limits were set on the language of publication or by the country of origin. Non‐English records were translated using Google Translate. When the accuracy of the translation was unclear, a bilingual person was consulted. Studies were included if they presented empirical data (qualitative and/or quantitative). All study designs were eligible for inclusion.
Exclusion criteria
Studies were excluded if they were as follows: poster abstracts, commentaries, literature reviews, assessed the quality of a single service (e.g. medicine use review), assessed only one element of quality (e.g. safety consumer satisfaction) and/or implemented or assessed the feasibility of using a quality data collection software (e.g. online data collection platform). We focused upon the development and testing of QIs using a holistic approach, that is whole sets of QIs, rather than reporting on one aspect of quality only. National quality indicator programmes were eligible for inclusion only if their development or evaluation was reported.
Data extraction
A bespoke data extraction form was developed, and data extraction was undertaken by one researcher (NA). Data extraction included: article characteristics (year of publication, country of origin and funders), study aims, participants, quality indicator characteristics, study design and methods, analysis and results, and strength and limitations (Appendix S2). Due to the heterogeneity of the studies included, the results are presented as a narrative synthesis.
Results
Literature search and studies characteristics
In total, 15 articles were identified as meeting the inclusion criteria. Figure 1 illustrates the study selection and the exclusion/inclusion processes. The studies were conducted in 12 countries, of which three were conducted in the Netherlands,28, 29, 30 two each in the UK,31, 32 USA,33, 34 and Thailand,35, 36 and one each in Argentina,37 Spain,38 Australia,39 Canada40 and Brazil.41 One study42 was conducted in three countries: Ethiopia, Uganda and Zimbabwe. Two studies were published in Spanish,37, 38 one in Portuguese41 and one in Thai.35 The remainder were published in English.
Figure 1.
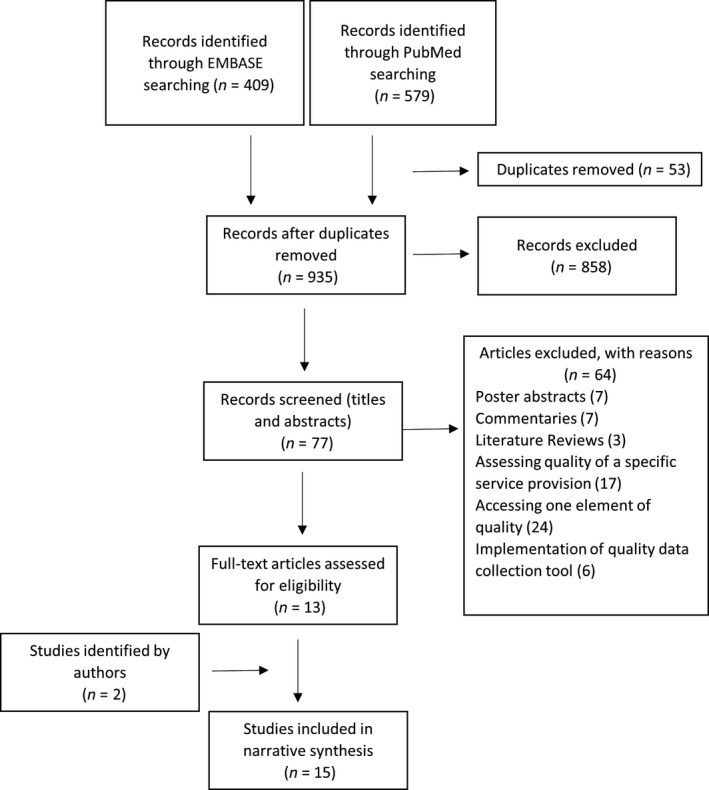
PRISMA flow chart of study selection process
The development and psychometric testing of quality indicators
Eleven studies reported the development of quality indicators for community pharmacies.28, 29, 31, 32, 33, 34, 35, 36, 37, 40, 42 Multiple methods were used to produce initial sets of indicators including literature review,28, 37, 42 focus groups,31, 33 surveys,32, 37 case studies32, 43 and interviews33 (Table 1). Initial sets of indicators were subjected to a selection exercise performed by expert or stakeholder consensus.28, 32, 33, 37 Testing of psychometric properties included validity,28, 29, 33, 37 reliability,29, 33, 35 feasibility28, 34, 36, 37, 40 and variability.33
Table 1.
Studies reporting the development of quality indicators
|
First author Country year |
Steps involved in developing quality indicators | Participants | Psychometric characteristics sought in the study |
|---|---|---|---|
|
Grey32 UK 2016 |
Delphi‐style survey (two rounds) |
22 participants completed both Delphi rounds Dispensing GPs (n = 2), community pharmacists (n = 8), pharmacy dispensing assistants (n = 2), pharmacy organisation board members (n = 1), large chain community pharmacy executives (n = 2), patients (n = 7) |
|
|
Wongpratat35 and Arkaravichien36 Thailand 2015, 2016 |
The tool was originally developed by the Community Pharmacy Association, with technical support from the FIP | Reliability | |
| Observation and interviews with pharmacists to collect data on the QI set | Accredited (n = 30) and non‐accredited pharmacies (n = 30), which were paired to the nearest setting of the accredited ones (500 m radius) in the north‐east region | ||
| Observation and interviews with pharmacists to collect data on the QI set | 81.1% (n = 60) of all accredited pharmacies in the north‐eastern part of Thailand | Feasibility | |
|
Schoenmakers29 Netherlands 2015 |
Online questionnaire provided data on the QI set collected in all Dutch community pharmacies | 91% (n = 1807) of all community pharmacies in the Netherlands in 2011 |
Content validity Absence of selection bias Absence of measurement bias Statistical reliability |
| Expert panel consensus | Expert panel of six pharmacists: practicing community pharmacists (n = 5) and a pharmacist/epidemiologist (project leader) | ||
|
Blalock33 USA 2012 |
Literature review |
Internal consistency Reliability Construct validity Variability |
|
| Focus groups | Four focus groups of consumers of pharmacy services (n = 30) | ||
| Interviews | Interviews with pharmacy patients (n = 12) | ||
| Stakeholders feedback and consensus | Pharmacy experts’ feedback (n > 50) | ||
| Survey/field test | Patients (n = 895) | ||
|
Halsall31 UK 2012 |
Focus group | 47 in total, patients and carers (n = 21), health managers (n = 16), and pharmacists and pharmacy staff (n = 10). | |
|
Winslade40 Canada 2011 |
Feasibility of routine medication‐related information of patients to screen the quality of care provided at community pharmacies. | Quebec's medication insurance programme provided data on prescriptions dispensed in 2002 by more than 5000 pharmacists in 1799 community pharmacies (n = 1.4 million patients) | Feasibility |
|
Bie28 Netherlands 2011 |
Literature and guideline search followed by two consensus rounds | Working group, pharmacy practice experts (round 1) (n = 14) and practising pharmacists in community (round 2) (n = 76) |
Face validity for quality of care or risk of harm to patients Clarity of wording Feasibility of data collection. |
|
Trap42 Ethiopia, Uganda and Zimbabwe 2010 |
Previous literature and different policies | ||
| Field test: direct observations, record reviews, interviews and simulated clients visits | 32 private and 39 public facilities in Ethiopia, 27 private and 33 public in Zimbabwe and 33 private in Uganda | ||
| Field test/ feasibility of data collection | Pharmacies (n = 30) | Feasibility | |
|
Pillittere‐Dugan34 USA 2009 |
Observational cohort study of pharmacy claim data of patients served by cross‐sectional of pharmacies from four health plans |
Plan A (n = 850, 461) Plan B (n = 867, 016) Plan C (n = 35, 369) Plan D (n = 1, 185) |
Feasibility of creating measures for each concept area of quality using only prescription drug claims data |
|
Sevilla37 Argentina 2008 |
Literature review of existing quality frameworks around the world |
Face and content validity, Feasibility |
|
| Interviews | Pharmaceutical professionals and relevant official | ||
| Consensus using a nominal group technique | Pharmacists with recognised professional experience and teachers with experience in the development of accreditation programmes |
In Argentina,37 a study was conducted to provide tools for accreditation of community pharmacies including evaluation components and quality indicators. The study used interviews with pharmaceutical professionals and official bodies, which explored quality criteria for health care in relation to international trends and recommendations, as well as Donabedian’s three dimensions (structure, process and outcome).22, 23 A nominal group technique30 was used to derive consensus on the criteria based on evaluating the face and content validity, the feasibility and the importance of the indicators. The process produced 24 quality indicators which included three structures (documentation n = 2, equipment/supplies n = 4 and human resources n = 4), two processes (patient care n = 8 and support needed n = 2) and one outcome (outcome of the care processes n = 3).
In Ethiopia, Uganda and Zimbabwe, 34 pharmacy practice indicators were developed using literature and policy reviews.42 The indicators included five domains: system (n = 5), storage (n = 7), services (n = 6), dispensing (n = 8) and rational drug use (n = 8). To test the functionality of the set in pharmacy practice settings, data from pharmacies were collected using direct observation, record reviews, interviews and simulated clients’ visits. Surveyors were trained on using a survey manual and a data collection sheet which included indicator definitions and sampling methodology. Results were presented using histograms and spider charts showing an assessed score against an ‘ideal’ Good Pharmacy Practice Score.
In the Netherlands,28 an initial set of 159 indicators was generated from a literature review, pharmacy practice guidelines, prescribing guidelines and pharmacy‐related indicators from other initiatives. Two consensus rounds followed: round one included pharmacy practice experts, and round two included practising pharmacists. During the consensus process, the main criteria for inclusion were relevance for pharmacy practice and validity for quality of care or risk of harm to patients. Clarity of wording, feasibility of data collection and qualitative comments were also examined. To further test the feasibility of data collection, a field test was conducted in which participating pharmacies were asked to provide data on the proposed indicators. The process resulted in a modified set of 42 indicators including: patient counselling (n = 6), clinical risk management (n = 10), compounding (n = 7), dispensing (n = 3), monitoring of medication use (n = 11), quality management (n = 5), structure (n = 13), process (n = 18) and outcome indicators (n = 11).
A second study in the Netherlands29 involved the development of a set of 52 quality indicators by the Dutch Health Care Transparency Programme. The majority of these indicators originated from the set of indicators generated in the previous study.28 The additional QIs related to patient counselling and safety, and health insurance companies. The indicators covered 10 domains: continuity of pharmaceutical care (n = 4), patient counselling (n = 3), clinical risk management (n = 12), compounding (n = 3), dispensing (n = 6), monitoring of medication use (n = 10), self‐care support and over‐the‐counter medications (n = 2), logistics (n = 5), quality management (n = 6) and professional development (n = 1). These indicators represented 21, 19 and 12 structure, process and outcome indicators, respectively. To assess the validity of the indicators, an expert panel applied a comprehensive ‘Indicator Assessment Framework’ to the data on the QI set collected from 1807 Dutch community pharmacies in 2011. The framework included examination of the indicators’ content validity, absence of selection bias, absence of measurement bias and statistical reliability. For the first three criteria, an expert panel rated the indicators as follows: meeting the requirements, partly meeting the requirements or not meeting the requirements. The expert panel considered pharmacists’ comments, questions and response rate. Statistical reliability was assessed for numerical indicators only. Of 52 quality indicators, 13 met all four criteria, 12 were structure indicators, and one was a process indicator.
In the UK,31 focus groups were conducted to develop a conceptual framework characterising healthcare quality in the community pharmacy setting. The study included patients and their carers, pharmacists and pharmacy staff, and National Health Service staff who commissioned pharmacy services. A constant comparative iterative analysis was used to interpret the data followed by identification of themes and domains that used to build the conceptual framework. Three dimensions emerged: accessibility, effectiveness and positive perceptions of the experience with each dimension associated with structure, process and outcome domains. The structure domains (n = 8) included premises, equipment, technology, information and data, patient information, medicines, services, pharmacy team (skills and numbers), communication systems, management, professionalism, internal quality systems and financial resources. Processes (n = 2) included providing standardised care and providing individualised care. Finally, outcome domains consisted of patient‐specific outcomes, pharmacy‐specific outcomes, societal outcomes and health status.
In the United States,33 a literature review and four focus groups with patients were conducted to identify quality attributes of pharmacy services and develop a consumer assessment survey. These attributes were developed into survey items. Patients’ ability to navigate the survey was evaluated using interviews with 12 pharmacy users. An additional evaluation was conducted with pharmacy professors, as well as Pharmacy Quality Alliance and practising pharmacists. The process produced a 50‐item pilot survey. The survey items were assessed based on confirmatory factor analysis, frequency of missing data and standard psychometric methods: variability, reliability and validity. The internal consistency was calculated using Cronbach's coefficient alpha, whereas the reliability within the pharmacy level was tested by using the analysis of variance. Construct validity was assessed by measuring the association between each survey item and its hypothesised composite measure. In terms of variability, the survey results were tested for ceiling effects (i.e. when the scale does not allow people to report higher levels of quality) and floor effects (when scale does not allow people to report lower levels of quality). The assessment resulted in three global indicators (overall pharmacy services, overall pharmacy staff and overall information about medication), and 15 items summarised in three multi‐items domains: general staff communication, health and medication‐focused communication and clarity of written information about medication.
In Thailand,35, 36 the reliability and feasibility of a 40‐item tool for quality assessment in community pharmacies were evaluated. The tool was originally developed by the Community Pharmacy Association with technical support from the FIP. The 40 items comprised five domains: premises and facilities (n = 7), personnel (n = 5), drug inventory and stocking (n = 7), dispensing and patient care (n = 17), and patient satisfaction and health promotion (n = 4). Data from interviews with pharmacists and observational methods were used to score quality indicators. The reliability of the quality indicator tool was tested by the Cronbach’s alpha coefficient and showed good reliability (0.87). Similarly, feasibility was evaluated by score analysis. The results were plotted by histograms of each domain’s assessable scores against its possible maximum score and were used to reflect how well the quality questions in that domain could be answered.
In a second study in the UK,43 multiple methods were used to derive quality indicators. A postal questionnaire was sent to community pharmacists and dispensing doctors to identify services provided and monitoring systems in place to record services. This was followed by in‐depth case studies in community pharmacies and dispensing doctor practices. The results were thematically analysed, and quality characteristics were identified that related to service provision. In the next stage,32 a two‐round Delphi‐type survey of the identified characteristics was sent to participants representing three stakeholder groups: dispensing doctors, community pharmacists and patients/lay members. Participants confirmed and ranked the importance of characteristics based on representing good quality criterion. The process produced 23 characteristics of good quality pharmaceutical service and covered four broad categories: patient safety and dispensing, patient–provider interaction, workplace culture and public health.
Pharmacy claim data for dispensed prescriptions have also been used to measure and monitor quality in community pharmacies. For example, in the United States, 22 pharmacies’ claim dispensing data were evaluated to test the performance of community pharmacies.34 The set included: proportion of days the patient was dispensed the medication of particular class (n = 7), gap in therapy (n = 7), diabetes medication dosing (n = 3), suboptimal asthma control (n = 1), absence of asthma controller (n = 1) and use of high‐risk medications in the elderly (n = 2). Each pharmacy was required to have a minimum of 30 patients for each measure to be included in the evaluation. Additionally, the measures were required to have sensitivity to variation between pharmacies. Less than 10% of pharmacies were evaluable for all measures except one measure associated with the use of high‐risk drugs in the elderly. This measure and another measure related to medication adherence showed potential for use as performance measures as they demonstrated room for improvement and variation among pharmacies.
In Canada,40 the feasibility of using pharmacy claim data to assess four quality indicators was also evaluated. The indicators included two safety indicators (dispensing of contraindicated benzodiazepines to seniors and dispensing of non‐selective beta‐blockers to patients with respiratory disease) and two effectiveness indicators (dispensing asthma or hypertension medications to non‐compliant patients). The proportion of community pharmacies where services were provided frequently (i.e. dispensed the relevant medication five or more times over the 1‐year period) was required to enable the reliable assessment of performance indicators. The study found that 86% of pharmacies provided sufficient services to assess performance on all four indicators. The study identified pharmacies that performed well, as well as pharmacies that needed to improve.
Evaluating the effects of quality indicators on improving the quality of pharmaceutical care
Four studies explored the effect of quality indicators on quality in community pharmacies over time (Table 2).
Table 2.
Studies reporting evaluating the effects of quality indicators on improving the quality of pharmaceutical care
|
First author Country Year |
Study design | Participants |
|---|---|---|
|
Teichert30 Netherlands 2016 |
Two evaluations: Online survey on the QI scores from April 2012 to May 2013; National survey during the whole study period of 5 years for indicators that remained unchanged during the study period |
88% (n = 1,739) of all community pharmacies in the Netherlands in 2013 |
|
Castro41 Brazil 2014 |
Retrospective, longitudinal survey | Workshops held in 2007/2008 and other two evaluations, 2002/2003 and 2010/2011 |
|
Pascual38 Spain 2010 |
Assessment of whether the implementation of a quality management system based on the ISO 9001: 2008 standard in the community pharmacy improved processes related to pharmaceutical management and care, as well as client/patient satisfaction. | Community pharmacies selected based upon their implementation of the quality management system |
|
Benrimoj39 Australia 2008 |
Randomly selected pharmacies were coached on the implementation of the standards. Pre‐ and post‐ measurements of the level of adherence to the standards were assessed using pseudo‐patron or simulated patient visits. | 50% (n = 2,706) of all Australian pharmacies |
In the Netherlands,30 the quality of pharmaceutical care in community pharmacies has been evaluated annually since 2008. In 2013, two evaluations were conducted. The first investigated improvement in areas assessed by 10 indicators that remained unchanged from 2008–2012 (management, patient experience, audit meetings, protocols for contraindications, medications reviews and five clinical QIs). The second evaluation was to assess changes in quality during the year 2012–2013 in community pharmacies using a set of 66 indicators. The set was developed by all major stakeholders including: community pharmacies, healthcare inspectorate, representatives of patient and consumer organisations and insurance companies. The 66 indicators contained 10 categories: ‘quality management’, ‘continuity of care’, ‘communication with the patient’, ‘clinical risk management’, ‘compounding’, ‘dispensing’, ‘follow up of pharmacotherapy guidelines’, ‘counselling’, ‘logistics’ and ‘training of pharmaceutical staff’. Scores were expressed either as categorical variables (yes/no) or as numerical variables (either a number or a proportion). Multi‐level analysis was used to assess the consistency of scores within each pharmacy for over 5 years. The results demonstrated that scores for structure indicators were higher compared with process and outcome indicators. Overall, scores improved from 2008 to 2013.
In Brazil,41 an evaluation of the effect of restructuring a pharmaceutical services system was conducted by applying the indicators of the Self‐Assessment Instrument for Pharmaceutical Services Planning. The instrument consisted of indicators in aspects of management, essential medicines selection, stock, storage, distribution, transport, prescription medication, dispensing, human resources and pharmacovigilance. The evaluation was conducted over three time periods (2002–2003, 2007–2008 and 2011–2012). For each indicator, quality was scored from one to three, with three is indicating the best quality. The results showed that the introduction of strategies to monitor quality led to improved management practice. Less satisfactory results were observed with prescribing and dispensing.
In Spain,38 a study was conducted to investigate the effects of the implementation of a quality management system (based on the international standard ISO 9001: 2008) in the community pharmacy. Sixteen process indicators were studied over time (the value of all pharmacies was averaged, and the time evolution of each indicator was adjusted to a straight line). The 16 indicators were related to internal management (n = 5), pharmaceutical care (n = 9) and customer/patient satisfaction (n = 2). Improvement was demonstrated in 10 of the 16 indicators including symptom improvement and patients who received health education.
In Australia,39 a quality improvement package was implemented in relation to the provision of non‐prescription medicines. The package included four standards with 20 criteria including: resource management (n = 3), customer care and advice (supply (n = 6), indirect supply (n = 2) and documentation (n = 3)), pharmacy design and environment (n = 3), and rights and needs of customers (n = 3). Half of all Australian pharmacies were randomly selected and included in the study. Each pharmacy was audited on the use of standards of practice for the provision of non‐prescription medicines. Three visits were conducted 7 weeks apart. During these visits, an assessment of the pharmacy's level of compliance and pseudo‐patron visits were used to monitor quality. After two visits, more than 80% of pharmacies had met most criteria. The lowest level of compliance was for indicators related to the documentation process. In visit three, there was a significant improvement compared with visits one and two. The results showed that pharmacies had low levels of compliance with written operating procedures but these improved over time.
Discussion
Main findings
Few studies have evaluated the effects of quality indicators on improving the quality of pharmaceutical care. This review identified 15 studies from 12 countries (and six continents) and reported a variety of methods for the development of quality indicators in the community pharmacy setting. Few studies included psychometric testing to assess the suitability of quality indicators.
Strengths and limitations
Duplicate independent screening of the search results minimised the risk of bias and omission. Bias was further reduced by having no language or country limitations, and this is also likely to have increased the generalisability of the results. Due to limited resources, duplicate data extraction was not undertaken. However, duplicate extraction is not an obligatory item in conducting a scoping review according to PRISMA‐ScR.27 The review focused on the methods involved in developing and evaluating quality indicators and not the specific indicators. Professional translators were not used for non‐English records.
General discussion
Quality indicators are often constructed using consensus methods combined with available published evidence or literature reviews. This is probably because scientific evidence in health care is limited or not methodologically rigorous (e.g. trial based).44
Stakeholder involvement varied substantially across the studies (Table 1). Patients, commissioners, general practitioners, public health organisations and insurance companies are all potential stakeholders of community pharmacy services. The involvement of commissioners or insurance companies is relevant especially in developing appropriate indicators that are included in pay‐for‐performance schemes.30 This scoping review showed little involvement of public or patient groups in the initial development of indicators. In one study, only pharmacists were included in an expert panel to evaluate the validity and the reliability of a set of quality indicators.29 This was justified by stating a qualified assessment was needed from experienced personnel involved in daily community pharmacy practice. In another study, professionals versus other stakeholders were involved in the development of QIs for cardiovascular risk management.45 The study reported that the professionals were ‘more qualified in assessing these QIs’ than other possible stakeholders. Patient values, preferences and characteristics in terms of quality should also be explored as these have been shown to have a positive impact on knowledge and medication adherence.46, 47
In the UK, over £1 billion is spent annually on the quality and outcome framework for general practices.48 This level of investment includes testing and piloting, and protocols have been developed for this purpose.48 The adoption of a protocol‐based approach to the development of quality indicators for community pharmacy could assist in the production of valid and reliable outputs.
The quality indicators identified in these studies broadly covered evaluating aspects of pharmacy design and environment, management, personnel, workplace culture, public health and promotion, medicine stock levels, delivery and refill, storage, patient care, patient counselling, over‐the‐counter medications, safety, compounding, dispensing, pharmacovigilance and professional development. Other aspects were less common in terms of quality management (e.g. errors and complaint management, patient experience and adverse drug reactions) and clinical risk management (e.g. the percentage of patients who concurrently use oral anticoagulants and co‐trimoxazole, percentage of patients with documented contraindication of heart failure who are dispensed NSAIDs). Few studies presented indicators to reflect the Donabedian framework (structure, process and outcome).22, 23 Process and outcome indicators were less likely to meet psychometric testing compared with structure indicators. For example, Bie et al. 28 found that developing a measurable indicator for process or outcome was not feasible due to differences in care organisations and the availability of data in community pharmacies. Schoenmakers et al. 29 reported a similar finding. Additionally, the difficulties of collecting clinical outcomes arising from the provision of pharmaceutical care to patients often led to use dispensing data as ‘outcome’ indicators (e.g. dispensing of contraindicated benzodiazepines to seniors and dispensing of non‐selective beta‐blockers to patients with respiratory disease).28, 29, 30, 34, 40 Evidence was lacking regarding the effect of quality indicators on patient outcome.
Quality improvement should reflect the context in which it is undertaken (i.e. context awareness).6 The studies included in this review were undertaken in 15 countries and six continents, demonstrating the interest and activity associated with the use of quality indicators at an international level. The context and target of the indicators in each of these studies differed substantially, and as such, the transferability of the results to other countries might be limited. However, this scoping review is the first to synthesise all identifiable data on this topic and provides an international perspective of the use of this approach for quality improvement in the community pharmacy setting.
Priorities change with time, and QIs should be revised to ensure that they reflect change. For example, in the Netherlands,29 the original set of QIs was developed for internal purposes to meet quality standards and to measure improvement. When these indicators were evaluated later for external use, that is, for patient awareness and health insurance companies, only 25% of them met all requirements.
Context also differs with the level of health care provided in different countries. For example, in Thailand,35 where pharmacies could be accredited and non‐accredited, QIs were developed to monitor quality in both settings and recommended the use of accredited pharmacies. Whereas, in Ethiopia, Uganda and Zimbabwe,42 efforts concentrated on developing QIs to assess structural elements including system, storage, services, dispensing and rational drug use.
The use of objective, reliable data is a challenge associated with the quality measurement of community pharmacy practice. Self‐report is common30, 49 and is likely to be associated with social desirability bias.50 Pharmacy dispensing or claims data have been investigated to derive quality indicators and as a method for avoiding self‐assessment.30, 34, 40 These methods are likely to be less expensive and more reliable compared with using on‐site inspections. However, not all aspects of quality can be evaluated using these data (e.g. over‐the‐counter consultations).
Implications on policy and research
This scoping review showed that there has been limited investigation of the effects of quality indicators on improving the quality of care in community pharmacies. Future research should seek to adopt a multi‐stakeholder approach to the development of QIs and should evaluate the effect of the introduction of QIs on patient outcome. The inclusion of QIs into policy and contractual arrangements should be evidence‐based and reviewed as an ongoing process to reflect the changing context of health care and concepts of quality.
Conclusions
Despite the growing emphasis on quality improvement in health care, there is limited reporting of the development and evaluation of QIs for community pharmacy practice. The future development of quality indicators should adopt a multi‐stakeholder approach and include testing of the quality indicators’ psychometric properties. Challenges exist with self‐assessment as well as the development of measurable process and outcome indicators. QIs should reflect the dynamic nature of health care and, as such, should be subject to periodic revision. The long‐term effects of QIs on improvement require further evaluation.
Declarations
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
Funding
This work was supported by MW’s Health Foundation Improvement Science Fellowship.
Supporting information
Appendix S1. Search strategy.
Appendix S2. Characteristics of included studies.
References
- 1. Nembhard IM et al Why does the quality of health care continue to lag? Insights from management research. Acad Manag Perspect 2009; 23: 24–42. [Google Scholar]
- 2. Berwick DM. The science of improvement. JAMA 2008; 299: 1182–1184. [DOI] [PubMed] [Google Scholar]
- 3. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press, 1999. [PubMed] [Google Scholar]
- 4. Hurtado et al, eds. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press, 2001. [PubMed] [Google Scholar]
- 5. Campbell SM, Roland MO, Buetow SA. Defining quality of care. Soc Sci Med 2000; 51: 1611–25. [DOI] [PubMed] [Google Scholar]
- 6. Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? BMJ Qual Saf 2007; 16: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawrence M, Olesen F. Indicators of quality in health care. Eur J Gen Pract 1997; 3: 103–108. [Google Scholar]
- 8. Bowling A. Research Methods in Health: Investigating Health and Health Services, 2nd edn Buckingham: Buckingham Open University Press, 2002. [Google Scholar]
- 9. Ross LA. Quality improvement in health care: opportunities and responsibilities for pharmacists. Ann Pharmacother 2013; 47: 1206–1209. [DOI] [PubMed] [Google Scholar]
- 10. Public Health England (PHE) publications gateway number 2017212 . Pharmacy: A way forward for public health, 2017. https://www.gov.uk/government/publications/community-pharmacy-public-health-interventions (accessed 06 December 2018).
- 11. Quality Care Pharmacy Program (QCPP) . The pharmacy guild of Australia. http://www.guild.org.au/guild-branches/nsw/professional-services/quality-care-pharmacy-program(accessed 06 December 2018).
- 12. Curtiss FR et al Framework for pharmacy services quality improvement – a bridge to cross the quality chasm. Part I. The opportunity and the tool. J Manag Care Pharm 2004; 10: 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Hooft CS et al Adverse drug reaction‐related hospitalisations: a nationwide study in The Netherlands. Drug Saf 2006; 29: 161–168. [DOI] [PubMed] [Google Scholar]
- 14. Leendertse AJ et al. Frequency of and risk factors for preventable medication‐related hospital admissions in the Netherlands. Arch Intern Med 2008; 168: 1890–1896. [DOI] [PubMed] [Google Scholar]
- 15. Howard RL et al Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007; 63: 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO) technical report series, No. 885, annex 7 (1999). Good pharmacy practice in community and hospital pharmacy settings. http://apps.who.int/medicinedocs/en/d/Jh1792e/22.html (accessed 06 December 2018).
- 17. World Health Organization (WHO) technical report series, No. 961, annex 8 (2011).Joint FIP/WHO guidelines on Good Pharmacy Practice: Standards for quality of pharmacy services. http://apps.who.int/medicinedocs/en/d/Js18676en/ (accessed 06 December 2018).
- 18. Pharmacy Quality Alliance (PQA) . https://www.pqaalliance.org/community-pharmacies (accessed 06 December 2018).
- 19. Zorginzicht . Insight into quality of care. https://www.zorginzicht.nl/Paginas/Home.aspx. (accessed 06 December 2018).
- 20. Pharmaceutical Services Negotiating Committee (PSNC) (2016). Quality payments scheme. http://psnc.org.uk/services-commissioning/essential-services/quality-payments/ (accessed 06 December 2018).
- 21. Pharmaceutical Services Negotiating Committee (PSNC) . 2018/2019. PSNC Briefing 051/18: A summary of the second Quality Payments Scheme 2018/19 (v2). https://psnc.org.uk/services-commissioning/psnc-briefings-services-and-commissioning/psnc-briefing-051-18-a-summary-of-the-second-quality-payments-scheme-2018-19-september-2018/ (accessed 31 January 2019).
- 22. Donabedian A. Evaluating the quality of medical care. Milbank Q 2005; 83: 691–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Health Service, UK (NHS) Improvement . Online library of quality, service improvement and redesign tools: A model for measuring quality care. https://improvement.nhs.uk/documents/2135/measuring-quality-care-model.pdf (accessed 06 December 2018).
- 24. Inch J et al It's not what you do it's the way that it's measured: Quality assessment of minor ailment management in community pharmacies. Int J Pharm Pract 2017; 25: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Eikenhorst L et al A systematic review in select countries of the role of the pharmacist in consultations and sales of non‐prescription medicines in community pharmacy. Res Social Adm Pharm 2017; 13: 17–38. [DOI] [PubMed] [Google Scholar]
- 26. Watson MC, Skea ZC. Jugglers and tightrope walkers: the challenge of delivering quality community pharmacy services. PLoS ONE 2018; 13: e0200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tricco AC et al Prisma extension for scoping reviews (PRISMA‐SCR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. [DOI] [PubMed] [Google Scholar]
- 28. De Bie J et al The development of quality indicators for community pharmacy care. BMJ Qual Saf 2011; 20: 666–671. [DOI] [PubMed] [Google Scholar]
- 29. Schoenmakers TW et al Evaluation of quality indicators for Dutch community pharmacies using a comprehensive assessment framework. J Manag Care Spec Pharm 2015; 21: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teichert M et al Quality indicators for pharmaceutical care: a comprehensive set with national scores for Dutch community pharmacies. Int J Clin Pharm 2016; 38: 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halsall D et al Characterizing healthcare quality in the community pharmacy setting: insights from a focus group study. Res Social Adm Pharm 2012; 8: 360–370. [DOI] [PubMed] [Google Scholar]
- 32. Grey E et al Characteristics of good quality pharmaceutical services common to community pharmacies and dispensing general practices. Int J Pharm Pract 2016; 24: 311–318. [DOI] [PubMed] [Google Scholar]
- 33. Blalock SJ et al Development of the Consumer Assessment of Pharmacy Services survey. J Am Pharm Assoc (2003) 2012; 52: 324–32. [DOI] [PubMed] [Google Scholar]
- 34. Pillittere‐Dugan D et al Development and testing of performance measures for pharmacy services. J Am Pharm Assoc (2003) 2009; 49: 212–219. [DOI] [PubMed] [Google Scholar]
- 35. Wongpratat A et al Quality of service determined by the community pharmacy association’s quality indicators between accredited and non‐accredited pharmacies in the north‐eastern part of Thailand. IJPS 2015; 11: 99–120. [Google Scholar]
- 36. Arkaravichien W et al Quality indicators to compare accredited independent pharmacies and accredited chain pharmacies in Thailand. Int J Clin Pharm 2016; 38: 899–907. [DOI] [PubMed] [Google Scholar]
- 37. Sevilla CN. Development of the accreditation of community pharmacies in the Province of Santa Fe, Argentina. Lat Am J Pharm 2008; 27: 110–117. [Google Scholar]
- 38. Arrebola Pascual I et al Results from the application of a quality management system in the community pharmacy. ARS Pharm 2010; 51(Suppl. 3): 277–283. [Google Scholar]
- 39. Benrimoj SI et al National implementation of standards of practice for non‐prescription medicines in Australia. Pharm World Sci 2009; 31: 230–237. [DOI] [PubMed] [Google Scholar]
- 40. Winslade N et al Monitoring community pharmacist's quality of care: a feasibility study of using pharmacy claims data to assess performance. BMC Health Serv Res 2011; 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Castro ÁV et al Assessment of the restructuring of pharmaceutical services in the city of Aracaju (Se, Brazil). RCFBA 2014; 35: 379–383. [Google Scholar]
- 42. Trap B et al A new indicator based tool for assessing and reporting on good pharmacy practice. South Med Rev 2010; 3: 4–11. [Google Scholar]
- 43. Weiss MC et al Dispensing doctor practices and community pharmacies: exploring the quality of pharmaceutical services. Prim Health Care Res Dev 2016; 17: 42–55. [DOI] [PubMed] [Google Scholar]
- 44. Campbell SM et al Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care 2002; 11: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Lieshout J et al Consistency of performance indicators for cardiovascular risk management across procedures and panels. Qual Saf Health Care 2010; 19: e31. [DOI] [PubMed] [Google Scholar]
- 46. Sackett DL et al Evidence based medicine: what it is and what it isn't. BMJ 1996; 312: 71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gourley GA, Duncan DV. Patient satisfaction and quality of life humanistic outcomes. Am J Manag Care 1998; 4: 746–752. [PubMed] [Google Scholar]
- 48. Campbell SM et al Framework and indicator testing protocol for developing and piloting quality indicators for the UK quality and outcomes framework. BMC Fam Pract 2011; 12: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doran T et al Pay‐for‐performance programs in family practices in the United Kingdom. N Engl J Med 2006; 355: 375–384. [DOI] [PubMed] [Google Scholar]
- 50. Latkin CA et al The relationship between social desirability bias and self‐reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addict Behav 2017; 73: 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.
Appendix S2. Characteristics of included studies.


