Abstract
Myocardial free wall rupture (MFWR) refers to laceration of the heart ventricle or atria, which is a rare but fatal complication of acute myocardial infarction (AMI). In this study, we aim to identify the clinical characteristics and protective factors of free wall rupture after myocardial infarction. This is a single-center, retrospective observational analysis. The study screened all patients admitted to the cardiology department of the First Affiliated Hospital of Xi’an Jiaotong University between January 2013 and April 2018. The biochemical, clinical, angiographic and echocardiographic features of these patients were then collected and analyzed. Among the 5946 screened patients with AMI, 23 patients with a diagnosis of MFWR after AMI were enrolled in the present study. 18 (78.3%) patients were diagnosed with acute ST segment elevated myocardial infarction and the remaining 5 (21.7%) have acute non-ST segment elevated myocardial infarction. Early-phase MFWR happened in 12 (52.2%) and late-phase accounted for 8 (34.8%) in total. Late-phase MFWR had lower left ventricle ejection fraction value (45.8%±5.6% vs 63.0±3.8%, p<0.001) as compared with early-phase. Patients who survived from MFWR has higher ACE inhibitor/angiotensin II receptor blocker (ACEI/ARB) and β-blocker coverage in the in-hospital treatment of AMI (ACEI/ARB: 100.0% vs 35.3%, p=0.014; β-blocker: 100.0% vs 47.1%, p=0.048). The present study provides evidence for better understanding of the clinical characteristics and protective functions in MFWR after AMI. Reduced cardiac function is correlated with higher incidence of later phase free wall rupture. Higher ACEI/ARB and β-blocker coverage in the AMI treatment strategy is associated with lower MFWR incidence.
Keywords: myocardial free wall rupture, acute myocardial infarction, clinical characteristics, protective factors
Significance of this study.
What is already known about this subject?
Myocardial free wall rupture (MFWR) refers to laceration of the heart ventricle or atria, which is considered a serious and dreadful complication of acute myocardial infarction (AMI).
As a rare but fatal complication of AMI, the prognosis of MFWR is always poor, and it could lead to sudden and devastating death in patients after myocardial infarction.
Moreover, as the sample sizes are all relatively limited in the previous studies, there has also been lack of consensus in the prevention of MFWR.
What are the new findings?
In this study, we found that several clinical factors are related to the incidence and prognosis of MFWR after AMI.
Reduced cardiac function is correlated with higher incidence of later phase free wall rupture.
Higher ACE inhibitors/angiotensin II receptor blockers and β-blocker coverage in the AMI treatment strategy is associated with lower MFWR incidence.
How might these results change the focus of research or clinical practice?
The present study provides evidence for better understanding of cardiac rupture and gives a clue for its prevention.
Introduction
Myocardial free wall rupture (MFWR) refers to laceration of the heart ventricle or atria, which is considered a serious and dreadful complication of acute myocardial infarction (AMI).1 As a rare but fatal complication of AMI, the prognosis of MFWR is always poor, and it could lead to sudden and devastating death in patients after myocardial infarction (MI). However, due to the rare and unpredictable incidence of MFWR, there is lack of evidence for its clinical characteristics. Moreover, as the sample sizes are all relatively limited in the previous studies, there has also been lack of consensus in the prevention of MFWR.
The diagnosis of myocardial rupture is generally based on physical examination, changes in vital signs, clinical suspicion and can be confirmed with echocardiography.2 Generally speaking, myocardial rupture can be divided into three categories based on the site of the lesion: rupture of the ventricular free wall, of the interventricular septum and of the papillary muscles.3 Normally in patients with AMI, myocardial rupture occurs within the first 5 days after MI in about half of cases and within 2 weeks in over 90% of patients.4–6 As a result, a previous study7 has divided myocardial rupture into early phase (<72 hours) and late phase (>96 hours). The treatment for myocardial rupture is normally supportive in the immediate setting, and surgical correction of the rupture is recommended if feasible. Among the different mechanical complications of MI, including MFWR, interventricular septum rupture and acute mitral regurgitation, MFWR has the most devastating outcome and is one of the major causes of sudden death in patients with AMI. Therefore, it is essential to identify the clinical characteristics and explore the potential protective factors of MFWR for reducing mortality and morbidity in AMI.
With the advancement of AMI therapies, percutaneous coronary intervention (PCI) has become a more preferable therapy to previous fibrinolytic therapy in AMI, reducing one of the major risks for myocardial rupture.4 8 9 Nevertheless, it is necessary to study the predicting factors and clinical outcomes of myocardial rupture so as to improve the survival rate and overall outcome of AMI. Hence, in this retrospective analysis study, by investigating the clinical characteristics of MFWR in patients with AMI, we aim to identify the predictive and protective factors of MFWR in order to provide clinical evidence for better prevention and treatment of AMI.
Methods
Study design
This is a single-center, retrospective observational analysis. The study screened all patients with a diagnosis of AMI admitted to the cardiology department of the First Affiliated Hospital of Xi’an Jiaotong University between January 2013 and April 2018. AMI and MFWR were defined based on the universal definition criteria by the American College of Cardiology.10 The inclusion criteria were (1) confirmed admission diagnosis of AMI and (2) MFWR diagnosed by echocardiography during hospitalization.
Clinical data collection
Detailed medical histories were screened from the patients enrolled. Patient characteristics were collected, including age, sex, disease history, the correct time of MI (according to the resident admit note) and myocardial rupture, physical examination, and records of PCI. Biochemical results were evaluated immediately after the patients were admitted to the hospital, and cardiac enzymes including creatine kinase (CK)/creatine kinase isoenzyme-B (CKMB) were measured serially throughout the hospital stay. Echocardiography was performed before and after myocardial rupture for patients of whom MFWR happened during hospital treatment, and was performed after myocardial rupture for patients who were admitted to our hospital with both MI and MFWR. Ejection fraction (EF) was measured using the Simpson methods by an experienced echocardiography doctor.
Statistical analysis
All statistical analyses were performed by SPSS for Windows V.17.0. Data were presented as frequencies or percentages for categorical variables and mean±SD for continuous variables, unless otherwise indicated. Simple t-test was used to compare continuous variables which are in normal distribution. Mann-Whitney U test was used to compare continuous variables which do not conform to the normal distribution. χ2 test was used to compare categorical variables. Simple linear analysis was used to calculate the correlation between myocardial rupture time and cardiac function. A value of p<0.05 was considered statistically significant.
Results
Study population
From January 2013 to April 2018, 5946 patients admitted to the First Affiliated Hospital of Xi’an Jiaotong University with a diagnosis of AMI were screened, among them a total of 23 (0.4%) patients with an onset of in-hospital MFWR were enrolled in the present study. Baseline patient characteristics are shown in table 1. The mean age was 70.0±8.3 years and 34.8% of the overall was female. Among 23 patients with AMI and MFWR, 18 (78.3%) were diagnosed with acute ST segment elevated myocardial infarction (STEMI) by electrocardiograph, chest pain and elevated myocardial enzymes. Nine patients suffered from anterior, three patients from inferior posterior, three patients from high lateral, and three patients from inferior wall and right ventricle MI. The remaining five (21.7%) patients had acute non-ST segment elevated myocardial infarction (NSTEMI). In 10 (43.5%) patients, infarct-related arteries (IRAs) were opened after PCI, and the mean time to revascularization therapy after MI was 50.5 hours. Of 23 patients, 3 underwent surgery within an hour after myocardial rupture and all of them survived. Early-phase MFWR happened in 12 (52.2%) and late-phase accounts for 8 (34.8%) patients in total. Of 23 patients, 17 (73.9%) died due to myocardial rupture-related pericardial tamponade in the hospital, and the rest of the patients survived at the end.
Table 1.
Baseline patient characteristics
| Item | Value |
| Patients (n) | 23 |
| Age (years) | 69.7±8.3 |
| Female (%) | 34.8 |
| Systolic BP (mm Hg) | 107.5±25.9 |
| Diastolic BP (mm Hg) | 69.8±18.2 |
| Heart rate (beats per minute) | 83.6±20.1 |
| SPO2 (%) | 86.4±11.3 |
| Blood biochemistry | |
| CK (peak, u/L) | 2011.9±2418.3 |
| CKMB (peak, u/L) | 168.1±199.3 |
| RBC (×109/L) | 4.1±0.6 |
| PLT (×109/L) | 180.7±58.7 |
| Creatine (µmol/L) | 88.7±24.9 |
| Death (%) | 73.9 |
| Early rupture (%) | 52.2 |
| Late rupture (%) | 34.8 |
| Ultrasonic cardiogram | |
| LVEDD (mm) | 54.3±5.2 |
| LVESD (mm) | 39.3±6.3 |
| EF (%) | 52.4±9.6 |
| Hypertension (%) | 52.2 |
| Diabetes (%) | 17.4 |
| Cerebral infarction (%) | 17.4 |
| CKD (%) | 0.0 |
| COPD (%) | 4.2 |
| STEMI | 18 (78.3%) |
| Anterior | 9 |
| Inferior posterior | 3 |
| High lateral | 3 |
| Inferior and right ventricle | 3 |
| NSTEMI | 5 (21.7%) |
| Mean time to revascularization (hours) | 50.5 |
| Patency rate of IRA (%) | 43.5 |
| Surgery | 3 (13.0%) |
| IABP (%) | 8.3 |
| Aspirin (%) | 87.5 |
| Clopidogrel (%) | 82.6 |
| Ticagrelor (%) | 4.3 |
| β-blocker (%) | 60.9 |
| ACEI/ARB (%) | 52.2 |
| CCB (%) | 4.3 |
| Statin (%) | 95.7 |
Data are mean±SD and n (%).
ACEI/ARB, ACE inhibitors/angiotensin II receptor blockers; BP, blood pressure; CCB, calcium channel blocker; CK, creatine kinase; CKD, chronic kidney disease; CKMB, creatine kinase isoenzyme-B; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; IAPB, intra-aortic balloon pump; IRA, infarct-related artery; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; NSTEMI, non-ST segment elevated myocardial infarction; PLT, platelets; RBC, red blood cell; SPO2, oxyhemoglobin saturation by pulse oximetry; STEMI, ST segment elevated myocardial infarction.
Comparison between early-phase and late-phase MFWR
Because patients with early-phase or late-phase MFWR have different clinical features, we carried on to investigate the potential factors contributing to MFWR in different stages. After test of normality, we used simple t-test analysis to compare the cardiac function and Mann-Whitney U test to compare cardiac enzymes between early-phase and late-phase MFWR. Data are shown in table 2.
Table 2.
Comparison between early-phase and late-phase MFWR
| Item | Early rupture | Late rupture | P value |
| Patients (n) | 12 | 8 | |
| Age (years) | 72.3±8.5 | 66.7±7.4 | 0.346 |
| Female (%) | 41.7 | 25.0 | 0.642 |
| Systolic BP (mm Hg) | 110.0±29.2 | 108.0±25.0 | 0.876 |
| Diastolic BP (mm Hg) | 70.2±19.4 | 71.3±19.2 | 0.904 |
| Heart rate (beats per minute) | 92.3±16.9 | 76.6±19.4 | 0.070 |
| SPO2 (%) | 83.8±11.6 | 87.3±12.0 | 0.533 |
| Blood biochemistry | |||
| CK (peak, u/L) | 1018.4±1135.5 | 3430.8±3214.4 | 0.076 |
| CKMB (peak, u/L) | 75.9±74.5 | 305.3±262.1 | 0.044 |
| RBC (×109/L) | 4.0±0.7 | 4.2±0.5 | 0.567 |
| PLT (×109/L) | 202.8±47.4 | 171.0±70.6 | 0.270 |
| Creatine (µmol/L) | 78.9±23.5 | 102.6±26.1 | 0.077 |
| Death (%) | 66.7 | 87.5 | 0.603 |
| Patency rate of IRA | 33.3 | 62.5 | 0.362 |
| Ultrasonic cardiogram | |||
| LVEDD (mm) | 52.0±3.9 | 57.0±5.5 | 0.146 |
| LVESD (mm) | 35.5±6.7 | 42.3±6.2 | 0.121 |
| EF (%) | 63.0±3.8 | 45.8±5.6 | <0.001 |
| Hypertension (%) | 58.3 | 50.0 | 1.000 |
| Diabetes (%) | 16.7 | 12.5 | 1.000 |
| Cerebral infarction (%) | 8.3 | 25.0 | 0.537 |
| CKD (%) | 0.0 | 0.0 | |
| COPD (%) | 0.0 | 12.5 | 0.400 |
| STEMI (%) | 75.0 | 87.5 | 0.122 |
| Anterior (%) | 77.8 | 28.6 | |
| Inferior posterior (%) | 0.0 | 28.6 | |
| High lateral (%) | 11.1 | 28.6 | |
| Inferior and right ventricle (%) | 11.1 | 14.3 | |
| NSTEMI (%) | 25.0 | 12.5 | 0.619 |
| IABP (%) | 16.7 | 0.0 | 0.495 |
| Aspirin (%) | 83.3 | 100.0 | 0.495 |
| Clopidogrel (%) | 75.0 | 87.5 | 0.619 |
| Ticagrelor (%) | 0.0 | 12.5 | 0.400 |
| β-blocker (%) | 66.7 | 62.5 | 1.000 |
| ACEI/ARB (%) | 58.3 | 50.0 | 1.000 |
| CCB (%) | 8.3 | 0.0 | 1.000 |
| Statin (%) | 91.7 | 100.0 | 1.000 |
Data are mean±SD and n (%).
ACEI/ARB, ACE inhibitors/angiotensin II receptor blockers; BP, blood pressure; CCB, calcium channel blocker; CK, creatine kinase; CKD, chronic kidney disease; CKMB, creatine kinase isoenzyme-B; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; IAPB, intra-aortic balloon pump; IRA, infarct-related artery; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MFWR, myocardial free wall rupture; NSTEMI, non-ST segment elevated myocardial infarction; PLT, platelets; RBC, red blood cell; SPO2, oxyhemoglobin saturation by pulse oximetry; STEMI, ST segment elevated myocardial infarction.
There was a trend that patients with late-phase free wall rupture (FWR) exhibited worse cardiac function, which was reflected on echocardiography showing a larger left ventricle size of 57.0±5.5 mm for the left ventricular end-diastolic dimension (LVEDD) as compared with 52.0±3.9 mm (p=0.146), and 42.3±6.2 mm for the left ventricular end-systolic diameter (LVESD) as compared with 35.5±6.7 mm (p=0.121), in the early phase. The left ventricle EF value was also significantly lower in patients with late-phase MFWR (p<0.001): 45.8%±5.6% as compared with 63.0%±3.8% in early-phase MFWR. Besides, late-phase MFWR also showed significantly higher peak CK enzymes as compared with early-phase (305.3±262.1 vs 75.9±74.5 u/L, p=0.044), while the percentage of anterior wall MI is higher in early-phase FWR than in late-phase (77.8% vs 28.6%, p=0.057).
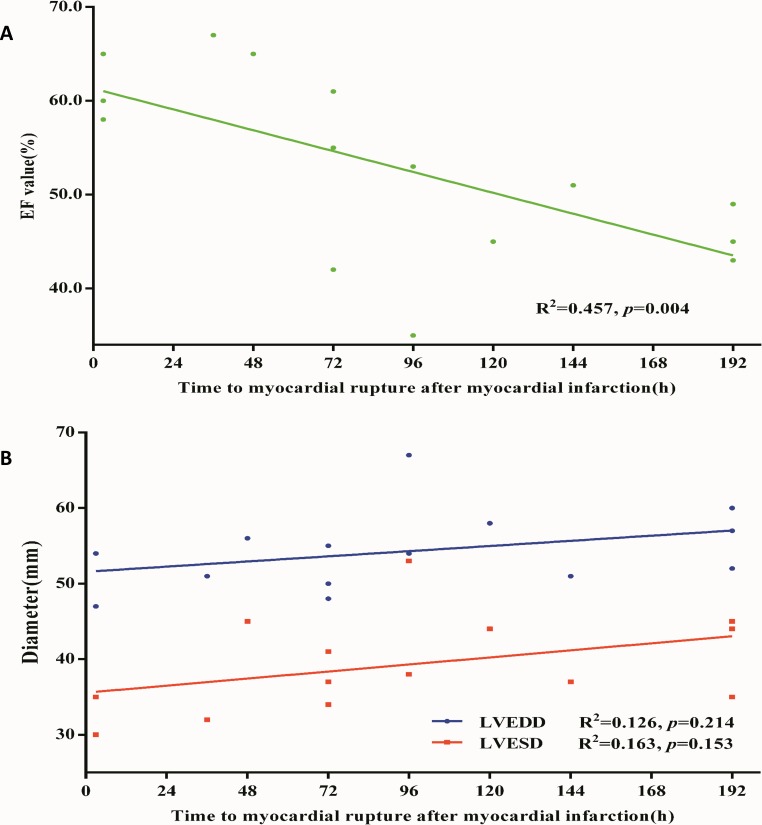
Thus to further investigate the relationship between pathologic phases and cardiac function, we calculated the time between myocardial rupture and MI in all patients and used simple linear regression analysis. The time to myocardial rupture after MI was found to be significantly positively correlated with EF value (R2=0.457, 95% CI of slope −0.151 to −0.035, p=0.004) but not to LVEDD (R2=0.126, 95% CI of slope −0.019 to 0.076, p=0.214) and LVESD (R2=0.163, 95% CI of slope −0.017 to 0.095, p=0.153) due to the small sample size (figure 1). The above results indicated that cardiac EF was correlated with the onset time of in-hospital MFWR in the current observational analysis.
Figure 1.
Simple linear analysis between time to myocardial rupture and cardiac function in patients with MI. (A) Simple linear regression model with time to myocardial rupture after MI in relation to the EF value. The y-axis represents the EF value (%) evaluated by echocardiography. The x-axis represents time to myocardial rupture. Green dots stand for EF value in each patient. 15 patients with MI were enrolled in the analysis (R2=0.457, 95% CI of slope −0.151 to −0.035, p=0.004). (B) Simple linear regression model with time to myocardial rupture after MI in relation to LVEDD/LVESD. The y-axis represents the diameter (mm) evaluated by echocardiography. The x-axis represents time to myocardial rupture. Green dots stand for EF value in each patient. 14 patients with MI were enrolled in the analysis (LVEDD: R2=0.126, 95% CI of slope −0.019 to 0.076, p=0.214; LVESD: R2=0.163, 95% CI of slope −0.017 to 0.095, p=0.153). EF, ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction.
Association between infarction sites and clinical characteristics of MFWR
We further sought to find the association between infarction area and the clinical characteristics of MFWR, including infraction phase, IRA, death and so on. However, due to limited sample size, no statistical difference was observed (table 3), whereas it was notified that early-phase MFWR was more likely to happen in patients with anterior wall infarction compared with other infarction sites. Besides, the survival rate is higher in patients with NSTEMI rather than in STEMI after MFWR.
Table 3.
Association between infarction sites and clinical manifestation of MFWR
| Item | Anterior | Inferior posterior | High lateral | Inferior and right ventricle | NSTEMI | P value |
| Patients (n) | 9 | 3 | 3 | 3 | 5 | |
| Rupture phase (%) | 0.319 | |||||
| Early | 77.8 | 0 | 33.3 | 33.3 | 60.0 | |
| Middle | 0 | 33.3 | 0 | 33.3 | 20.0 | |
| Late | 22.2 | 66.7 | 66.7 | 33.3 | 20.0 | |
| Patency rate of IRA (%) | 44.4 | 66.7 | 0 | 66.7 | 60.0 | 0.456 |
| Death (%) | 88.9 | 66.7 | 100 | 66.7 | 40.0 | 0.262 |
Data are n (%).
IRA, infarct-related artery; MFWR, myocardial free wall rupture; NSTEMI, non-ST segment elevated myocardial infarction.
Association between clinical factors and in-hospital mortality rate of MFWR
Lastly, we investigated the predictive factors for in-hospital mortality rate of MFWR. We divided patients into in-hospital death (78.3%) and alive (21.7%) groups (table 4). Small sample size as it is, the utilization percentage of ACE inhibitor/angiotensin II receptor blocker (ACEI/ARB) and β-blocker was significantly higher in the alive group than in the death group (ACEI/ARB: 100.0% vs 35.3%, p=0.014; β-blocker: 100% vs 47.1%, p=0.048). Apart from these, no other factors exhibited significant correlation with the in-hospital mortality of MFWR between the two groups. The result also complied with the previous study by blood biochemistry or ultrasonic cardiogram.11–13
Table 4.
Association between clinical factors and in-hospital mortality of MFWR
| Item | Death | Alive | P value |
| Patients (n) | 17 | 6 | |
| Age (years) | 70.7±7.3 | 66.8±11.0 | 0.346 |
| Female (%) | 35.3 | 33.3 | 1.000 |
| Systolic BP (mm Hg) | 109.4±27.6 | 102.2±21.6 | 0.572 |
| Diastolic BP (mm Hg) | 71.7±20.0 | 64.3±11.3 | 0.406 |
| Heart rate (beats per minute) | 82.6±20.5 | 86.3±20.5 | 0.704 |
| SPO2 (%) | 86.4±11.3 | 86.3±12.6 | 0.997 |
| Blood biochemistry | |||
| CK (peak, u/L) | 2271.0±2677.6 | 1364.2±1616.5 | 0.452 |
| CKMB (peak, u/L) | 201.0±217.6 | 85.7±122.2 | 0.240 |
| RBC (×109/L) | 4.2±0.6 | 3.8±0.6 | 0.212 |
| PLT (×109/L) | 178.9±68.9 | 185.3±21.0 | 0.826 |
| Creatine (µmol/L) | 86.8±25.8 | 92.8±24.4 | 0.638 |
| Early rupture (%) | 47.1 | 66.7 | 0.640 |
| Patency rate of IRA (%) | 47.1 | 33.3 | 0.660 |
| Ultrasonic cardiogram | |||
| LVEDD (mm) | 54.2±3.3 | 54.5±9.3 | 0.953 |
| LVESD (mm) | 38.8±4.5 | 40.5±10.5 | 0.772 |
| EF (%) | 51.0±8.4 | 55.6±12.3 | 0.392 |
| Hypertension (%) | 52.9 | 50.0 | 1.000 |
| Diabetes (%) | 17.6 | 16.7 | 1.000 |
| Cerebral infarction (%) | 23.5 | 0.0 | 0.539 |
| CKD (%) | 0.0 | 0.0 | |
| COPD (%) | 5.9 | 0.0 | 1.000 |
| STEMI | 0.572 | ||
| Anterior (%) | 53.3 | 33.3 | |
| Inferior posterior (%) | 13.3 | 33.3 | |
| High lateral (%) | 20.0 | 0.0 | |
| Inferior and right ventricle (%) | 13.3 | 33.3 | |
| NSTEMI (%) | 11.8 | 50.0 | 0.089 |
| IABP (%) | 5.9 | 16.7 | 0.462 |
| Aspirin (%) | 94.1 | 83.3 | 0.462 |
| Clopidogrel (%) | 88.2 | 66.7 | 0.270 |
| Ticagrelor (%) | 5.9 | 0.0 | 1.000 |
| β-blocker (%) | 47.1 | 100.0 | 0.048 |
| ACEI/ARB (%) | 35.3 | 100.0 | 0.014 |
| CCB (%) | 0.0 | 16.7 | 0.261 |
| Statin (%) | 94.1 | 100.0 | 1.000 |
Data are mean±SD and n (%).
ACEI/ARB, ACE inhibitors/angiotensin II receptor blockers; BP, blood pressure; CCB, calcium channel blocker; CK, creatine kinase; CKD, chronic kidney disease; CKMB, creatine kinase isoenzyme-B; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; IAPB, intra-aortic balloon pump; IRA, infarct-related artery; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MFWR, myocardial free wall rupture; NSTEMI, non-ST segment elevated myocardial infarction; PLT, platelets; RBC, red blood cell; SPO2, oxyhemoglobin saturation by pulse oximetry; STEMI, ST segment elevated myocardial infarction.
Discussion
In this study, we have enrolled patients with AMI complicated with MFWR and sought to identify the clinical characteristics of MFWR with regard to late or early phase, infarction sites, and clinical correlators. It is identified that worse cardiac function is correlated with late-phase MFWR; moreover, patients with higher ACEI/ARB and β-blocker coverage for preventing myocardial remodeling during the initial treatment of AMI showed higher survival rate from MFWR.
The incidence and death rate of MFWR after AMI declined progressively over the past decades. In this single-center, retrospective analysis, we enrolled 0.4% patients with in-hospital MFWR from patients with AMI. The MFWR incidence is 0.4%, which is relatively lower than previous investigations.14 15 It is also interesting that all patients with MFWR had not had fibrinolytic therapies before. The lower FWR incidence could be attributed to the representativeness of the patient population. The patients enrolled in the present study came from one of the best cardiovascular centers in Western China. With the prevalence of PCI and due to its clinical benefits as compared with fibrinolytic therapy, most of the patients with AMI admitted to our hospital received PCI except for economic concerns. Moreover, the construction of chest pain centers in China over the past decades has also given rise to more standardized therapies and decreasing door-to-balloon time in the AMI treatment,16 which might further reduced the overall incidence of FWR and overall AMI mortality. Lastly, since we evaluated only in-hospital MFWR incidents, late-phase MFWR happening after discharge have not been investigated.
The time to myocardial rupture could be divided into early phase and late phase.7 The early phase of myocardial rupture is often characterized by an abrupt, slit-like tear in the infarcted myocardium, which is more likely to occur in patients with acute anterior infarction as a result of further decreased apical wall thickness in systole and enhanced tensile stress after MI.7 On the other hand, the late phase of myocardial rupture is associated with cardiac fibrosis scar and early aneurysm formation,7 which is most likely to happen in patients with large MI area and heart dysfunction. In the present analysis, it is also found that 52.2% of MFWRs after AMI are in the early phase, 34.8% are in the late phase, and yet 13.0% in between. Moreover, EF and peak of myocardial enzymes differ between early-phase and late-phase MFWR, which could contribute to the possible different pathologic mechanisms. Patients with low EF tend to have smaller size of infarction and have a late onset of MFWR; however, further investigations based on larger cohorts are warranted.4
The prognosis of myocardial rupture is always poor due to its unpredictable nature and dreadful clinical outcomes.17 Some studies show that the prognosis of myocardial rupture is dependent on a number of factors,18 including how long after AMI myocardial rupture occurs, which portion of the myocardium is involved in the rupture and the treatment that patients receive. However, the present study has not identified statistical difference in the correlation between infarction site and clinical manifestations, although early MFWR tends to happen in patients with anterior wall infarction. The not statistically significant results might be attributed to limited sample size; hence, further, larger scale analysis is warranted in order to define the association between the prognosis of MFWR and clinical manifestations.
The treatment for MFWR is mainly supportive in the immediate setting. Emergency surgery is recommended or performed in most centers; however, a number of patients have sudden death and did not survive until surgical correction.19 Increased PCI rate, better control of blood pressure, and the use of β-blockers and ACEI/ARB have all contributed to lower incidence and death rate of FWR as previously reported.15 20 In the present analysis, we have also identified that the utilization of β-blockers and ACEI/ARB is correlated with higher in-hospital survival rate of FWR, although the sample size is relatively small. Therefore, it is suggested by the present analysis that early medication for preventing ventricular remodeling may improve the MFWR survival rate.
Limitations
This is a single-center-based, retrospective study. The sample size in this study is relatively small because of the low incidence of myocardial rupture. During the emergency condition, cardiac tamponade might prevent us from figuring out which specific part of the myocardial free wall was ruptured. In addition, the small number of patients enrolled could contribute to type 1 statistical error. As a result, although difference was notified with regard to mean CK, CKMB and age, no statistical difference was identified. Follow-up work based on bigger cohorts is warranted to further explore the prognosis and preventive factors of MFWR.
Conclusion
In sum, in this single-center, retrospective analysis, we have identified several clinical factors related to the incidence and prognosis of MFWR after AMI. Reduced cardiac function is correlated with higher incidence of later phase FWR, and higher ACEI/ARB and β-blocker coverage in the AMI treatment strategy is associated with less MFWR incidence. The present study provides evidence for better understanding of cardiac rupture and gives a clue for its prevention.
Footnotes
Contributors: JS and ZY designed the study. BL, YL, XH, LS, YD, MG and JL collect the data. JS and BZ analyzed the data. JS and BL wrote the paper.
Funding: This study was supported by the National Natural Science Foundation of China (81500219, 81400302, 81570406), the Natural Science Foundation of Shaanxi Province (2018KW067, 2017JM8016, 2016SF217), the Fundamental Research Funds for the Central Universities in China (1191329724, 191329849), and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (no XJTU1AF-CRF-2018-025).
Competing interests: None declared.
Ethics approval: The study was approved by the ethics committee of the hospital. Because of the retrospective nature of the study, informed consent was not deemed necessary.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Naeim F, De la Maza LM, Robbins SL. Cardiac rupture during myocardial infarction. A review of 44 cases. Circulation 1972;45:1231–9. 10.1161/01.cir.45.6.1231 [DOI] [PubMed] [Google Scholar]
- 2. Trindade ML, Tsutsui JM, Rodrigues AC, et al. Left ventricular free wall impeding rupture in post-myocardial infarction period diagnosed by myocardial contrast echocardiography: case report. Cardiovasc Ultrasound 2006;4:7 10.1186/1476-7120-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Figueras J, Cortadellas J, Calvo F, et al. Relevance of delayed hospital admission on development of cardiac rupture during acute myocardial infarction: study in 225 patients with free wall, septal or papillary muscle rupture. J Am Coll Cardiol 1998;32:135–9. 10.1016/S0735-1097(98)00180-6 [DOI] [PubMed] [Google Scholar]
- 4. Purcaro A, Costantini C, Ciampani N, et al. Diagnostic criteria and management of subacute ventricular free wall rupture complicating acute myocardial infarction. Am J Cardiol 1997;80:397–405. 10.1016/S0002-9149(97)00385-8 [DOI] [PubMed] [Google Scholar]
- 5. Oliva PB, Hammill SC, Edwards WD. Cardiac rupture, a clinically predictable complication of acute myocardial infarction: report of 70 cases with clinicopathologic correlations. J Am Coll Cardiol 1993;22:720–6. 10.1016/0735-1097(93)90182-Z [DOI] [PubMed] [Google Scholar]
- 6. López-Sendón J, González A, López de Sá E, et al. Diagnosis of subacute ventricular wall rupture after acute myocardial infarction: sensitivity and specificity of clinical, hemodynamic and echocardiographic criteria. J Am Coll Cardiol 1992;19:1145–53. 10.1016/0735-1097(92)90315-E [DOI] [PubMed] [Google Scholar]
- 7. Nakatsuchi Y, Minamino T, Fujii K, et al. Clinicopathological characterization of cardiac free wall rupture in patients with acute myocardial infarction: difference between early and late phase rupture. Int J Cardiol 1994;47(1 Suppl):S33–8. 10.1016/0167-5273(94)90324-7 [DOI] [PubMed] [Google Scholar]
- 8. Moreno R, López-Sendón J, García E, et al. Primary angioplasty reduces the risk of left ventricular free wall rupture compared with thrombolysis in patients with acute myocardial infarction. J Am Coll Cardiol 2002;39:598–603. 10.1016/S0735-1097(01)01796-X [DOI] [PubMed] [Google Scholar]
- 9. Leitman M, Tsatskin L, Hendler A, et al. Cardiac Rupture: New Features of the Old Disease. Cardiology 2016;133:257–61. 10.1159/000442815 [DOI] [PubMed] [Google Scholar]
- 10. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 20162016;134:e123–55. [DOI] [PubMed] [Google Scholar]
- 11. Little SH, Ramasubbu K, Zoghbi WA. Real-time 3-dimensional echocardiography demonstrates size and extent of acute left ventricular free wall rupture. J Am Soc Echocardiogr 2007;20:538.e1–3. 10.1016/j.echo.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 12. Liu S, Glavinovic T, Tam JW. Early diagnosis and management of myocardial rupture. Can J Cardiol 2015;31:88–90. 10.1016/j.cjca.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 13. Ipek G, Onuk T, Karatas MB, et al. Relationship between Neutrophil-to-Lymphocyte Ratio and Left Ventricular Free Wall Rupture in Acute Myocardial Infarction. Cardiology 2015;132:105–10. 10.1159/000431354 [DOI] [PubMed] [Google Scholar]
- 14. Patel MR, Meine TJ, Lindblad L, et al. Cardiac tamponade in the fibrinolytic era: analysis of >100,000 patients with ST-segment elevation myocardial infarction. Am Heart J 2006;151:316–22. 10.1016/j.ahj.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 15. Figueras J, Alcalde O, Barrabés JA, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008;118:2783–9. 10.1161/CIRCULATIONAHA.108.776690 [DOI] [PubMed] [Google Scholar]
- 16. Zheng W, Wang J, Xu F, et al. Evaluation and management of patients with acute chest pain in China (EMPACT): protocol for a prospective, multicentre registry study. BMJ Open 2018;8:e017872 10.1136/bmjopen-2017-017872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Honan MB, Harrell FE, Reimer KA, et al. Cardiac rupture, mortality and the timing of thrombolytic therapy: a meta-analysis. J Am Coll Cardiol 1990;16:359–67. 10.1016/0735-1097(90)90586-E [DOI] [PubMed] [Google Scholar]
- 18. Lemery R, Smith HC, Giuliani ER, et al. Prognosis in rupture of the ventricular septum after acute myocardial infarction and role of early surgical intervention. Am J Cardiol 1992;70:147–51. 10.1016/0002-9149(92)91266-7 [DOI] [PubMed] [Google Scholar]
- 19. Figueras J, Cortadellas J, Soler-Soler J. Left ventricular free wall rupture: clinical presentation and management. Heart 2000;83:499–504. 10.1136/heart.83.5.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sulzgruber P, El-Hamid F, Koller L, et al. Long-term outcome and risk prediction in patients suffering acute myocardial infarction complicated by post-infarction cardiac rupture. Int J Cardiol 2017;227:399–403. 10.1016/j.ijcard.2016.11.037 [DOI] [PubMed] [Google Scholar]