Abstract
Purpose
Clinically evaluate intraocular pressure (IOP) measurements taken with a Goldmann applanation tonometer (GAT) prism and a modified surface Goldmann prism examining measurement differences correlated to central corneal thickness (CCT) and corneal hysteresis (CH) values.
Design
Prospective, open-label, randomised, controlled, multicentre reference device accuracy analysis.
Methods
A GAT and a modified surface GAT prism measured IOP on 243 unique eyes. The study design and methodology complied with International Standard Organization (ISO) tonometer evaluation guidelines, except the inclusion of thin (<500 µm) and thick (>600 µm) corneas. All eyes were randomised to IOP measurement by one of five standard Goldmann prisms and five modified prisms. Pressures were measured by six investigators, two times with each prism for a total of 1936 IOP measurements. Analysis included a multiple linear regression including CCT and CH correlation.
Results
The difference in IOP measurements of the standard and modified Goldmann prisms correlated well to CCT particularly in thin (<500 µm) and thick (>600 µm) corneas (R2=0.404, p=0.007). Corneal hysteresis (CH) also significantly correlated to the difference in prism measurements (R2=0.125, p=0.039). There was no significant overall mean IOP bias between the two prisms (+0.43 mm Hg in modified, p=0.19).
Discussion
The paired IOP measurement difference between GAT and a modified surface Goldmann replacement prism indicated a statistically significant correlation to CCT and CH. A simple modified replacement prism for any Goldmann-type tonometer may significantly improve IOP measurement accuracy by minimising corneal biomechanical errors associated with CCT and CH.
Trial registration number
NCT02990169 and NCT02989909.
Keywords: Glaucoma, Intraocular pressure, Cornea, corneal hysteresis, central corneal thickness, tonometry, corneal biomechanics
Introduction
The standard for measurement of intraocular pressure (IOP) remains Goldmann applanation tonometry (GAT). Intraocular pressure is the clinical indicator in the diagnosis of ocular hypertension, which may lead to glaucoma. Furthermore, it is the only modifiable parameter in the treatment of glaucoma.1 Errors in IOP measurement may result in potentially sight-threatening conditions.2 Individual patient variability in corneal thickness, rigidity, curvature and corneal tear film has led to significant errors in GAT IOP measurement.3 In addition, the corneal biomechanical differences incurred after LASIK surgery and present in children have also led to inaccuracies in GAT IOP measurement.4–7
IOP readings have been demonstrated to be underestimated in thin corneas and overestimated in thick corneas, respectively leading to a potential failure of diagnosis or over-diagnosis of glaucoma.8 The preferred practice among eye care professionals has changed to consider low central corneal thickness (CCT) values as a risk factor for glaucoma comparison and progression.9–11 Standardisation corrections for the various other GAT IOP errors have been attempted in order to yield a comparable IOP between patients.3 12 The correction process in practice involves additional data collection and calculation leading to minimal clinical adoption. The IOP corrections by CCT nomograms alone are shown to be ineffective by themselves as a predictive model for glaucoma.13 14
A correcting applanation tonometry surface (CATS) tonometer prism is a Goldmann applanation tonometer (GAT) prism with a modified applanating surface (figure 1). The United States Food and Drug Administration (U.S. FDA) recently cleared the CATS tonometer prism for IOP measurement in existing Goldmann-type applanation tonometers. Alterations to the original GAT prism design include a sinusoidal curved modification of applanating surface with a compensatory increase in prism length.15 Lengthening of the prism was required to maintain a zero average IOP bias between the GAT and CATS prisms over a large standard population. A zero average bias maintains long established GAT IOP bench marks (ie, borderline high at 21 mm Hg) and was required for clinical adoption as well as tonometer retrofitting without recalibration. The modified Goldmann (CATS) prism significantly decreases individual patient IOP error due to corneal biomechanical and tear-film properties. Improved IOP accuracy with the modified Goldmann (CATS) prism has been demonstrated in direct clinical GAT prism comparison and by surgically placed intracameral transducer pressure comparison.15–19
Figure 1.

Applanating surface of the centrally concave and annularly convex (CATS) prism.
Any existing Goldmann measurement armature may be retrofitted with the modified (CATS) prism without recalibration. A clinician measures IOP using the same protocol and techniques as those currently employed in GAT measurement. A reduction in corneal biomechanical errors is achieved by partially matching the curvature of the tonometer surface to curvature of the cornea minimising the intracorneal stress during applanation.15–19 This process minimises the contribution of total force on the prism face due to corneal deformation, measuring predominantly the IOP force.15 The annular curvature away from the cornea simultaneously minimises the tear-film error.15 19
The present study was completed to evaluate the modified Goldmann (CATS) prism IOP accuracy compared with the reference GAT prism with known glaucoma risk factors such as CCT and CH in healthy subjects. Study design complies with Guidance for Industry and FDA Staff Tonometers—Premarket Notification [510(k)] Submissions as well as International Standards Organization (ISO) 8612:2009 standards.
Methods
Enrolment included healthy subjects undergoing a series of IOP measurements with the modified (CATS) and standard GAT prisms. The design was a randomised, controlled, prospective, open-labelled, multicentre device comparison. Eligible subjects were screened, enrolled and evaluated according to the study protocol and were recruited from two sites in Arizona, USA. Participation in the study included subjects 18 years or older, meeting the protocol criteria, who provided written informed consent.
Description of study population
Healthy adult subjects were enrolled into the study to include high-astigmatism and refractive errors, as well as high IOP. Specifically, both thin and thick corneas with a CCT >600 µm and <500 µm were included. The sample size was calculated at 70 eyes (of those in the thin and thick CCT range) based on statistically significant correlation probability from previous studies.16–18 20
The following conditions were excluded from participation in the study: corneal scarring, lid, corneal or ocular conditions, disease, disorders or infection that may have confounded the study results. Pregnant or nursing women and contact lens wearers were also excluded as well as ocular surgery within 3 months of enrollment.
Protocol
Each enrolled subject received a standard ophthalmic examination from one of six investigators. CCT was measured by an assistant investigator with a Zeiss HD-OCT-5000 spectral domain ocular coherence tomographer (Zeiss, Jena, Germany). Corneal topography was completed with a Zeiss Atlas model 9000 (Jena, Germany) using the central 3 mm diameter of the cornea for analysis in accordance with ANSI Z80.23. An Ocular Response Analyzer was used to measure corneal hysteresis (CH) (Reichert, Depew, New York, USA) according to its instruction for use. Each investigator was masked to the results of the assistant investigator’s results and were also masked to the randomised and alternated use of the CATS and reference GAT prism devices, chosen by a random number generator. Topical anaesthetic drops were applied prior to each measurement with fluorescein (fluorescein sodium and benoxinate hydrochloride ophthalmic solution 0.25%/0.4%; Bausch & Lomb, Tampa, Florida, USA). A calibrated Haag-Streit model 900 applanation tonometer armature (Mason, Ohio, USA) was used to measure IOP with one of five cleaned and disinfected Haag-Streit reference GAT prisms and one of five CATS prisms. Pressure measurements were made two times with each GAT and CATS prism (one measurement consisted of averaged measurements at 180 and 90 degrees for astigmatism correction). Sequential measurements more than 2 mm Hg different required a third measurement. All three measurements were then averaged, if the third was within the range of the first two, otherwise the measurements were discarded. Four measurements were taken (two with each prism, four total).
Test product
Five modified surface Correcting Applanation Tonometry Surface (CATS) Tonometer prisms.
Control product
Five flat surface Haag-Streit Goldmann Applanation Tonometer (GAT) prisms.
Objectives
The clinical study’s primary performance objective was to examine improved IOP accuracy in accordance with expected standard Dresdner CCT correction for GAT IOP measurement. A secondary objective was to examine IOP measurement correlation with corneal hysteresis.
Endpoints
The primary endpoint was CCT corrected accuracy of IOP measurement readings, as measured by applanation tonometry. The criteria for success was the difference in IOP readings between modified (CATS) and GAT demonstrating a statistically significant correlation to CCT in accordance with standard published Dresdner CCT correction. Using the modified (CATS) tonometer prism and the Goldmann tonometer (GAT) prism to measure IOP enrolment continued until a minimum of 70 eligible eyes were identified equally in the high CCT (>600 µm) and low CCT (<500 µm) cornea categories. The sample population from a previous sister study of 172 eyes, which was limited to corneas with nominal CCTs (between 500 µm and 600 µm), was included in the CCT correlation to ensure continuity across the spectrum of probable CCT values.20 The study set of 173 eyes was run concurrently with the present study (70 eyes) using the identical protocol and clinical investigators. The only difference between the two patient populations was the range of CCT values.
Statistical methods
The Full Analysis Set (FAS) was used for the analysis of the primary endpoint. All eligible eyes were included in the FAS.
Continuous variables were used for descriptive statistics including mean, SD, median and range. An assessment of change from GAT to CATS IOP measurements baseline was provided using two-sided CIs and α=0.05 or one-sided CIs and α=0.025. The primary endpoint was analysed using a homoscedastic, two-tailed t-test (α=0.05) separately in both the high and low CCT value categories. A linear regression analysis of the difference in paired GAT and CATS IOP measurements was correlated to CCT. Linear regression analysis included the present set of 70 eyes and the previously published set of 173 eyes for a total of 243 unique eyes. Both data sets were collected concurrently using the same protocol in which CCT measurement determined the subject’s enrollment into each study.20
The secondary endpoint was evaluated using a homoscedastic, two-tailed t-test (α=0.05) separately in both the high and low CH value categories. High was defined as higher than mean and low was defined as lower than mean. A linear regression analysis of the difference in paired GAT and CATS IOP measurements was correlated to CH using the full set of 243 unique eyes. A multiple linear regression analysis was completed on the FAS using a general linear mixed effects model examining CCT, CH, IOP, age, gender and astigmatism.
Results
Two hundred forty-three (243) unique eyes were measured. There were 173 eyes in the nominal CCT category (between 500 µm and 600 µm) and 33 eyes in the high CCT category (>600 µm) and 37 eyes in the low CCT category (<500 µm) (table 1). Measurements within all categories were completed in accordance with the ANSI Z80.10–2014 guidelines. There were 91 (37%) men and 154 (63%) women. who completed the study with an average age of 59.9±19.0 years. Forty-five (45) eyes had high astigmatism (>3.0 dioptres) and 198 eyes had low astigmatism (≤3.0 dioptres). Fifty-nine (59) eyes were measured in the high IOP category (>23 mm Hg), 112 eyes were measured in the moderate IOP category (16 mm Hg to 23 mm Hg) and 72 eyes were measured in the low IOP category (<16 mm Hg).
Table 1.
Table of quantities of individual measurements in each CCT and CH category
| Individual measurements | Low CCT (<500 µm) | Moderate CCT (>500 µm to <600 µm) | High CCT (>600 µm) | Low CH (<9.86 mm Hg): | High CH (>9.86 mm Hg) | Total |
| Unique eyes | 37 | 173 | 33 | 124 | 119 | 243 |
CCT, central corneal thickness; CH, corneal hysteresis.
Accuracy of IOP
Figure 2 shows the modified (CATS) versus GAT prism scatter-plot of all data according to FDA Tonometer Guidelines. The scatterplot indicates excellent correlation between the CATS and GAT IOP measurements throughout the useful range of measurement IOPs with a correlation coefficient of R2=0.91, slope=+0.93 and y-intercept of +2.15. Bland-Altman paired difference plots were previously demonstrated as substantially equivalent (±5 mm Hg) with ANSI Z80.10–2014 tonometer testing using limited CCTs (500 µm to 600 µm).20 Overall, the mean bias in paired IOP measurements was +0.43 mm Hg higher with the CATS prism, which was not statistically significant (p=0.19).
Figure 2.
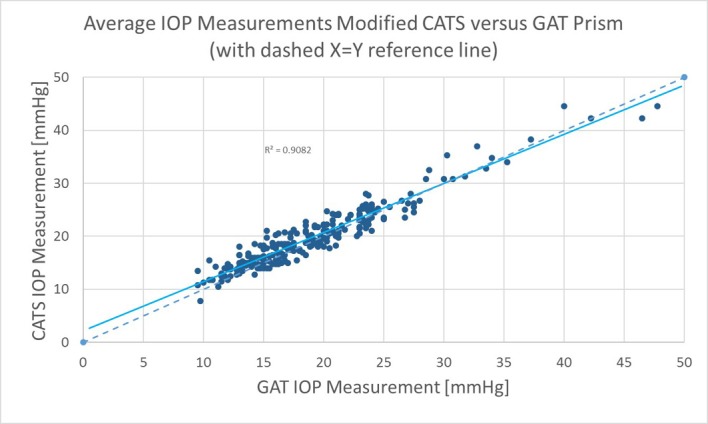
Scatter plot of the average intraocular pressure (IOP) values for GAT and modified (CATS) prisms from each of the 243 measurements along with a reference x=y line.
Central corneal thickness correlation analysis
The paired IOP measurement difference in modified (CATS) and GAT prisms was correlated to CCT. The subject’s average CCT was 545±40 µm with a range of between 401 and 652 µm. The results shown in figure 3 indicate a CATS/GAT difference slope of −0.0277 mm Hg/µm for CCT. The modified (CATS) prism reduced the IOP sloped sensitivity due to CCT by up to +4.7 mm Hg to −3.6 mm Hg over the GAT prism, which compares well with the published Dresdner GAT error over this same range of CCT values.21 The CCT correlation was statistically significant (p=0.007) indicating good correlation in the paired difference of IOP readings between the modified (CATS) and GAT prisms over the corresponding range of CCT values (R2=0.404). The multiple linear regression analysis also indicated a statistically significant CCT correlation (p=0.012).
Figure 3.
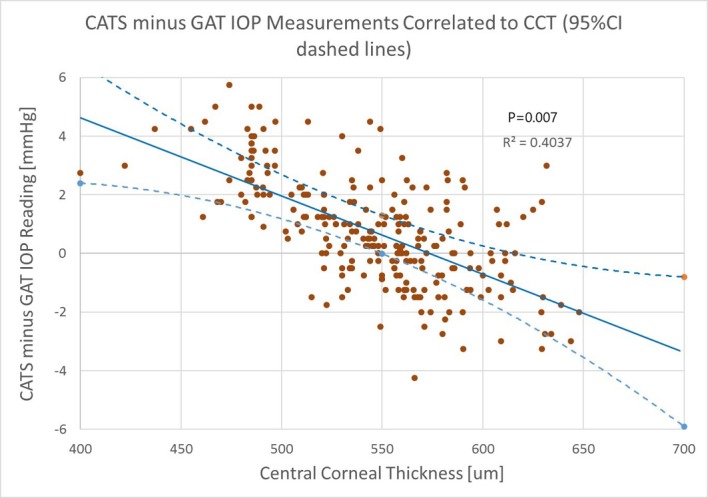
Measured intraocular pressure (IOP) difference between modified (CATS) minus GAT correlated to central corneal thickness, 95% CI on average mean difference and slope indicated by dashed lines.
Figure 4 is a statistical comparison between the thin (<500 µm) and thick (>600 µm) cornea groups using a two-sample Student’s t-test on the means (p<0.00001).
Figure 4.
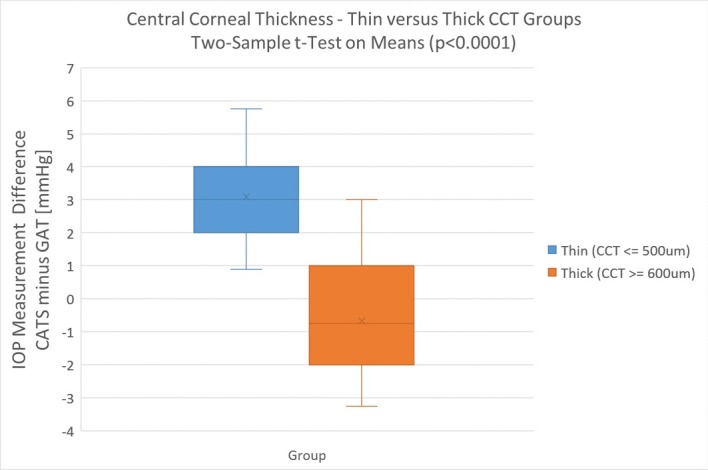
Paired differences (CATS–GAT) between thin (<500 µm) and thick (>600 µm) cornea groups compared using a two-sample Student’s t-test on the means. CCT, central corneal thickness; IOP, intraocular pressure.
Corneal hysteresis correlation analysis
The paired IOP measurement difference in modified (CATS) and GAT prisms was correlated to corneal hysteresis (CH). The subject’s average CH was 9.86±2.13 mm Hg with a range between 4.1 and 16.1 mm Hg. The results shown in figure 5 indicate a CATS/GAT difference slope of −0.3025 mm Hg/mm Hg for CH. The associated CH correlation coefficient was statistically significant (p=0.039) indicating good correlation in the paired difference of IOP readings between the modified (CATS) and GAT prisms over the corresponding range of CH values (R2=0.125). The multiple linear regression analysis also indicated a statistically significant CH correlation (p=0.042). Astigmatism correlation was statistically significant in the multiple linear regression (p=0.033); IOP, age and gender were not correlated.
Figure 5.
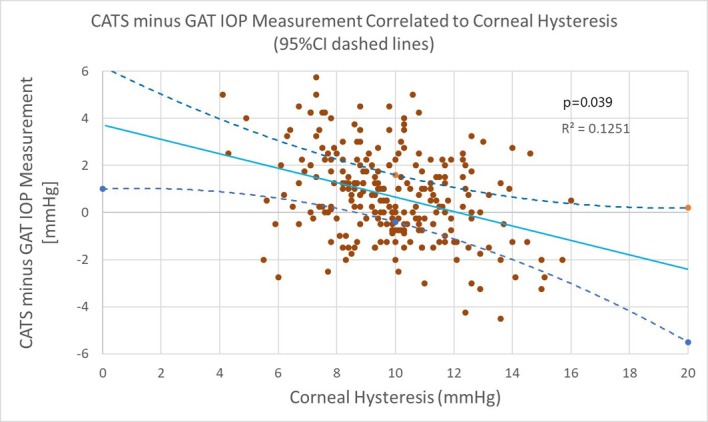
Measured intraocular pressure (IOP) difference between modified (CATS) minus GAT correlated to corneal hysteresis, 95% CI on average mean difference and slope indicated by dashed lines.
Conclusions
A significant reduction in modified Goldmann (CATS) prism’s sensitivity to CCT and CH was demonstrated in the clinical study when compared with the standard GAT prism. The results extend the narrow CCT correlation findings in the previously published, concurrently run study with limited CCT values between 500 and 600 µm.20Additionally, the results verify published modified (CATS)/standard GAT prism comparisons including intracameral pressure differences between modified and standard GAT prism measurements correlated to CCT and CH.15–19 There were no device failures nor adverse events. The modified (CATS) and GAT prisms showed no differences in measurement based on age, gender or IOP, but indicated a statistically significant correlation with the degree of astigmatism. The CATS prism has been shown to have a decreased sensitivity to corneal curvature which may be associated with the astigmatism correlation.15 16
Major sources of Goldmann IOP measurement error include individual patient variation in the rigidity of the cornea which is a function of both its CCT and intrinsic modulus of elasticity.3 11 12 17 21 Additionally, patient variability in the corneal curvature and tear film contribute significantly to the error.11 12 19 The CATS prism’s concave-convex applanation surface is a modelled iterative parametric solution to simultaneously minimise all four sources of error.12
Both CCT and CH are recognised corneal biomechanical metrics associated with error in GAT IOP measurement.3 8–12 22 23 Corneal hysteresis (CH) is a dynamic measurement of corneal energy absorption from an impulse force which has an indirect relationship to global corneal elasticity produced by CCT and the cornea’s modulus of elasticity.24 The present study demonstrates a decreased sensitivity to variations in CH with the modified CATS compared with the GAT, which is likely reflective of CATS design minimising errors due to corneal biomechanical variability. Many studies have demonstrated in vitro and in vivo the correlation between CCT and CH to Goldmann IOP measurement error.11 12 17 21 24 With respect to CCT, studies have produced GAT CCT correction algorithms which produce a more accurate GAT IOP when compared with true intracameral pressure.11 12 17 21 However, these CCT corrections have not demonstrated a more accurate predictive clinical model for primary open-angle glaucoma.13 14 Presently, clinical evidence only indicates that low CCT and low CH values are risk factors for glaucoma progression.13 14 24 25 CCT is only one source of error and its correction alone is likely insufficient to correct GAT IOP producing a clinically effective predictive model for glaucoma.3 24 26
The present study presents evidence that a simple modified (CATS) replacement prism on any Goldmann-type tonometer may significantly improve the IOP measurement accuracy of a Goldmann tonometer. This advancement in Goldmann technology may further its use as the standard of care in the diagnosis and treatment of pressure-related diseases.
Future studies will examine the modified (CATS) prism and standard GAT prism IOP measurements in problematic patient populations. For the same reasons that the modified (CATS) prism demonstrates decreased corneal biomechanical sensitivity, it will likely afford improved IOP accuracy in both paediatric and post-refractive surgery patients. Planned studies will be conducted on patients before and after LASIK refractive procedures as well as on paediatric patients comparing with intracameral pressure. These two populations comprise nearly 30% of the population.4 23 Additional studies are underway examining the difference in CATS and GAT IOP measurement differences in patients with keratoconus before and after corneal cross-linking as well as examining paired IOP differences with the introduction of topical prostaglandin analogues for glaucoma. Changes in the difference in CATS and GAT IOP measurements may reflect changes in overall corneal elasticity.20
Acknowledgments
Arizona Eye Consultants, and Cornea Associates, Tucson, AZ for extensive facilities use.
Footnotes
Contributors: SJM: design, drafting, data acquisition, analysis and interpretation. KT, AM, WC, MM: design, data acquisition, analysis. JL: design, drafting, data acquisition, analysis.
Funding: This study was supported in part by NIH SBIR Grant R43 EY026821-01 and Arizona Eye Consultants, Tucson, AZ. SJM has an interest in Intuor Technologies (Tucson, AZ) which owns the technology being examined in this clinical trial. Additional grant support unrelated to this study has been provided by Abbott Medical Optics (Santa Ana, CA) and Alcon, Inc. (Ft. Worth, TX). JL has unrelated study grant support from InnFocus, Inc.
Competing interests: The submitted manuscript was completed by referencing studies funded by an NIH/NEI SBIR grant, 1R43 EY026821-01. Requirements of this grant are commercialisation of potentially beneficial ophthalmic/optometric medical devices/products. The commercialisation necessitates intellectual property and a company to produce the product. Commonly in new technology start-up companies, the corresponding author is also part owner in the intellectual property and the associated company (Intuor Technologies) producing the medical device. This is a conflict of interest. However, the authors attest to the stringent efforts made to provide unbiased information provided in this manuscript. We believe, as does the NIH and ASCRS, that the tonometer device has a potential to bring considerable value to the ophthalmic medical community and their patients.
Patient consent for publication: Not required.
Ethics approval: This clinical study was conducted in accordance with the ethical principles contained within Declaration of Helsinki, Protection of Human Volunteers (21 CFR 50), Institutional Review Boards (21 CFR 56) and Obligations of Clinical Investigators (21 CFR 812).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Chan MPY, Broadway DC, Khawaja AP, et al. Glaucoma and intraocular pressure in EPIC-Norfolk eye study: cross sectional study. BMJ 2017;358 10.1136/bmj.j3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Susanna R, De Moraes CG, Cioffi GA, et al. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol 2015;4:1–10. 10.1167/tvst.4.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 2005;31:146–55. 10.1016/j.jcrs.2004.09.031 [DOI] [PubMed] [Google Scholar]
- 4. Schallhorn JM, Schallhorn SC, Ou Y. Factors that influence intraocular pressure changes after myopic and hyperopic LASIK and photorefractive keratectomy: a large population study. Ophthalmology 2015;122:471–9. 10.1016/j.ophtha.2014.09.033 [DOI] [PubMed] [Google Scholar]
- 5. Tsai ASH, Loon SC. Intraocular pressure assessment after laser in situ keratomileusis: a review. Clin Exp Ophthalmol 2012;40:295–304. Vol. 10.1111/j.1442-9071.2011.02641.x [DOI] [PubMed] [Google Scholar]
- 6. Giangiacomo A, Beck A. Pediatric glaucoma: review of recent literature. Curr Opin Ophthalmol 2017;28:199–203. 10.1097/ICU.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng CS, Jin KW, Yi K, et al. Comparison of intraocular pressure measurements obtained by rebound, noncontact, and Goldmann applanation tonometry in children. Am J Ophthalmol 2015;160:937–43. 10.1016/j.ajo.2015.07.029 [DOI] [PubMed] [Google Scholar]
- 8. Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13. [DOI] [PubMed] [Google Scholar]
- 9. Iester M, Mete M, Figus M, et al. Incorporating corneal pachymetry into the management of glaucoma. J Cataract Refract Surg 2009;35:1623–8. 10.1016/j.jcrs.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 10. Kotecha A, Elsheikh A, Roberts CR, et al. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci 2006;47:5337–47. 10.1167/iovs.06-0557 [DOI] [PubMed] [Google Scholar]
- 11. Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol 1993;38:1–30. 10.1016/0039-6257(93)90053-A [DOI] [PubMed] [Google Scholar]
- 12. Damji KF, Muni RH, Munger RM. Influence of corneal variables on accuracy of intraocular pressure measurement. J Glaucoma 2003;12:69–80. 10.1097/00061198-200302000-00015 [DOI] [PubMed] [Google Scholar]
- 13. Park SJK, Ang GS, Nicholas S, et al. The effect of thin, thick, and normal corneas on Goldmann intraocular pressure measurements and correction formulae in individual eyes. Ophthalmology 2012;119:443–9. 10.1016/j.ophtha.2011.07.058 [DOI] [PubMed] [Google Scholar]
- 14. Brandt JD, Gordon MO, Gao F, et al. Adjusting intraocular pressure for central corneal thickness does not improve prediction models for primary open-angle glaucoma. Ophthalmology 2012;119:437–42. 10.1016/j.ophtha.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCafferty S, Lim G, Duncan W, et al. Goldmann tonometer prism with an optimized error correcting applanation surface. Transl Vis Sci Technol 2016;5:4–5. 10.1167/tvst.5.5.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCafferty S, Lim G, Duncan W, et al. Goldmann tonometer error correcting prism: clinical evaluation. Clin Ophthalmol 2017;11:835–40. 10.2147/OPTH.S135272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCafferty S, Levine J, Schwiegerling J, et al. Goldmann applanation tonometry error relative to true intracameral intraocular pressure in vitro and in vivo. BMC Ophthalmol 2017;17 10.1186/s12886-017-0608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCafferty S, Levine J, Schwiegerling J, et al. Goldmann and error correcting tonometry prisms compared to intracameral pressure. BMC Ophthalmol 2018;18 10.1186/s12886-017-0668-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCafferty S, Enikov E, Schwiegerling J, et al. Goldmann tonometry tear-film error and partial correction with a shaped applanation surface. Clin Ophthal 2018;12:71–8. 10.2147/OPTH.S152492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCafferty S, Tetrault K, McColgin A, et al. Intraocular pressure measurement accuracy and repeatability of a modified Goldmann prism: multicenter randomized clinical trial. Am J Ophthalmol 2018;196:145–53. 10.1016/j.ajo.2018.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohlhaas M, Boehm AG, Spoerl E. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol 2006;124:471–6. 10.1001/archopht.124.4.471 [DOI] [PubMed] [Google Scholar]
- 22. American Academy of Ophthalmology, preferred practice pattern. Primary Open-Angle Glaucoma 2015. [Google Scholar]
- 23. Eisenberg D, Sherman B, Mckeown C, et al. Tonometry in adults and children: a manometric evaluation of pneumotonometry, applanation, and TonoPen in vitro and in vivo. Ophthalmol 1998;105:1173–81. [DOI] [PubMed] [Google Scholar]
- 24. Medeiros FA, Meira-Freitas D, Lisboa R, et al. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology 2013;120:1533–40. 10.1016/j.ophtha.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Medeiros FA, Weinreb RN. Is corneal thickness an independent risk factor for glaucoma? Ophthalmology 2012;119:435–6. 10.1016/j.ophtha.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Touboul D, Roberts C, Kérautret J, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg 2008;34:616–22. 10.1016/j.jcrs.2007.11.051 [DOI] [PubMed] [Google Scholar]


