Abstract
Background:
Knee osteoarthritis (KOA) is a common degenerative articular disease that causes disability and poor quality of life (QoL) of the individuals. Electrotherapeutic agents such as therapeutic ultrasound (US), interferential current (IFC), and infrared radiation are used in the treatment. It is not clear which of these agents is the best in improving these variables.
Objective:
The study aimed to compare the effects of the combined application of US and IFC therapies and infrared radiation on pain, functional activities, and QoL in people with KOA.
Methods:
In a randomized controlled study, 60 participants were randomized into two groups, the combination therapy group (CTG) and the infrared radiation group (IRG). Each group received 15-min treatment three times per week for 12 weeks. The visual analog scale (VAS) was used to assess the pain, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) for functional activities and the Short Form Health Survey questionnaire for QoL.
Results:
Participants in the CTG had a significant reduction in pain and significant improvement in functional activities and QoL compared to the IRG.
Conclusion:
The results of this study support the use of the combination of IFC and US therapies to reduce pain and improve function and QoL for KOA patients.
Keywords: Combination therapy, interferential current therapy, infrared radiation, knee osteoarthritis, therapeutic ultrasound
Introduction
Osteoarthritis (OA) is a progressive degenerative articular disease characterized by marginal osteophyte formation, destruction of joint cartilage, and subchondral bone changes.1,2 Clinical symptomology includes joint pain, loss of joint functions, and limitation of joint range of motions.3 OA mostly affects weight-bearing joints such as knee and hip. The disease rate increases with increase in age and obesity, with arthritis pains and dysfunction affecting patient’s quality of life (QoL).4 OA is one of the commonest causes of disability among elderly individuals.5 It has been shown that 50% of people over the age of 65 years have radiological features of OA, with roughly 10% of men and 18% of women suffering symptomatic OA.6
The aims of knee OA treatment are to reduce pain and improve function or quality of life based on interferential current (IFC) approach.7 Moreover, drug treatments for the elderly are often limited, producing suboptimal benefits because of comorbidities, polypharmacy, and the associated high risk of side effects of drugs.7,8 No pharmacological treatments are recommended for the treatment of OA, such as exercises and physical therapy modalities to treat patients with knee OA, in an attempt to limit the side effects of medication. In addition to the use of heat and cold, therapeutic ultrasound (US) and interferential current were also used.9
IFC approach is characterized by superimposing of two slightly different medium-frequency currents (4,000 Hz) to form a new medium-frequency current with an amplitude modulation at low frequency (0–250 Hz).10,11 It has been stated that amplitude-modulated frequency (AMF) is the main electro-analgesic component of IFC.12 IFC therapy achieves its pain modulation by stimulating afferent large-diameter fibers. Studies have reported IFC therapy’s effectiveness in the treatment of painful musculoskeletal problems such as sports injuries; bruising and swelling, low back pain, osteoarthritis, rheumatoid arthritis, and muscular pain.13-15
Therapeutic US is one of the most frequently applied electrotherapeutic modalities in orthopedics physiotherapy.16 It produces thermal effects which increase tissue metabolism, collagen elasticity, and capillary blood flow and reduce skeletal muscle spasm.17 Therapeutic ultrasound is often used in the management of knee osteoarthritis and it is believed to be effective in enhancing inflammatory response, tissue repair, and is absorbed especially in tissues with high collagen contents.18 Besides the individual therapeutic effects of ultrasound and interferential current therapies, their combination [i.e., combination therapy (CT)] is more effective than each of them applied separately in eliciting localized analgesia on previously detected painful areas.19
Infrared radiation with wavelength range from 750 nm to 1 mm can stimulate the production of nitric oxide (NO), enhancing inflammatory response, tissue repair, and is absorbed especially in tissues with high collagen contents.29,34 Clinical investigations of the efficacy of OA therapies should include symptoms (such as pain), function, disability, and health-related quality of life (HRQoL).8,20 Further intensive research focusing on the therapeutic effects of ultrasound, interferential current, and infrared on patients with knee OA is required.7,20,21 To our knowledge, there have been no reports to date that evaluated the effects of combination therapy and infrared on pain, functional activity, and HRQoL of elderly patients with knee OA. We hypothesized there would be significant difference in the administrations of combination therapy and infrared radiation to improve HRQoL, relieve pain, and improve functional activities in patients with knee osteoarthritis. This study, therefore, is aimed at investigating the differences between the combined application of therapeutic ultrasound and interferential current therapies (combination therapy) and infrared lamp on pain, functional activities, and HRQoL of elderly patients with knee osteoarthritis.
Methods
Participants
Sixty outpatients with knee OA, diagnosed according to the American College of Rheumatology criteria, were recruited.22 Patients were excluded from the study if they had any knee diseases other than OA. Patients with serious concomitant systemic diseases, patients who had corticosteroid or hyaluronic acid injection in the last one month, and patients with previous history of any electrotherapy contraindications were excluded from the study. Subsequently, patients were made to understand the research protocols, before they were randomly allocated into two groups [combination therapy group (CTG) and infrared radiation group (IRG)].
Design
A prospective randomized controlled clinical trial was used.
Randomization
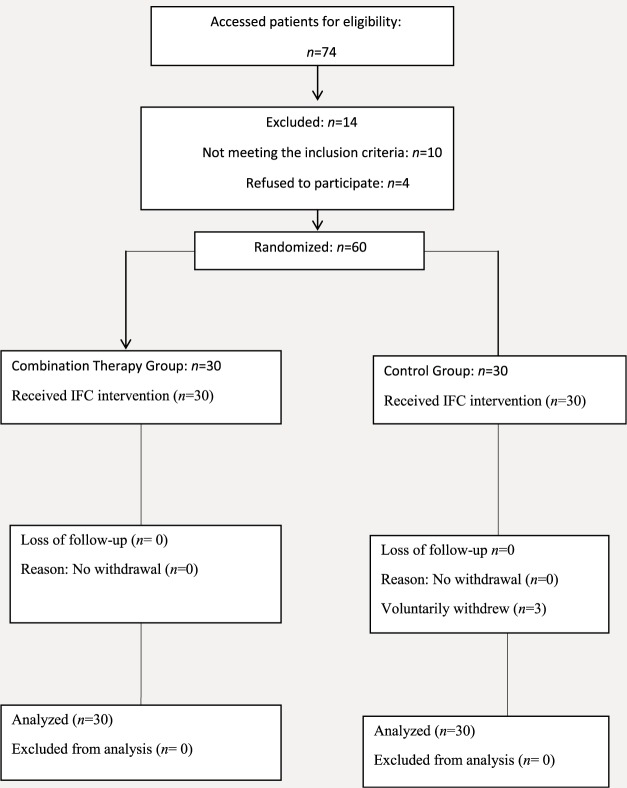
Patients were allocated to either CTG or IRG. The principle of block randomization was used to assign the patients to the groups, with a block size of four. Participants were allocated to their groups by sealed envelope containing their group assignment, which they opened when they were recruited into the study. One physiotherapist enrolled all the participants, and the other physiotherapist generated the allocation sequence and assigned participants to their groups as shown in the flowchart in Fig. 1.
Fig. 1.
Consort flowchart depicting the participants from enrolment to analysis.
Measurement
Pain
Pain intensity was assessed on full weight bearing using the visual analog scale (VAS). Participants were asked to indicate the level of their pain between 0 (no pain) and 10 (severe pain), and were instructed not to under- or over-estimate it. The VAS is a single-item numerical scale normally in a straight horizontal or vertical line of fixed length, usually 10 cm (i.e., 100 mm).23 The ends are defined as the extreme limits of the parameter to be measured with anchor points 0 (no pain) and 10 (maximum pain). It is a highly reliable instrument for measuring pain,24 with high psychometric values.25-28
Functional ability
The Western Ontario and McMaster University Osteoarthritis Index (WOMAC) was used to evaluate the functional ability of the participants, at the baseline and after 12 weeks of treatments. The instrument is an OA-specific outcome measure and self-administered questionnaire with three domains consisting of 24 items. The Likert-scale version of WOMAC was used for the purpose of this study. This scale allows patients to indicate their responses on a five-point scale , , , , and . The higher the response indicated, the lower the level of perceived health and physical function. Studies have shown high psychometric value for the WOMAC questionnaire. The instrument has been shown to be reliable, valid, and sensitive to changes in clinical symptoms of individuals with knee and hip OA.29,30
HRQoL
Participants’ health-related quality of life was assessed and recorded using the 36-item Short Form Health Survey (SF-36) questionnaire at baseline and post-treatment. This is a generic HRQoL measurement tool, self-administered, and user-friendly which has been reported as valid and reliable with high internal and external consistencies.31
Procedures
The study was conducted at the Outpatient Units of Physiotherapy Departments of Rasheed Shekoni Specialist Hospital, Dutse, Nigeria, and the Federal Medical Centre Birnin Kudu, Jigawa State, Nigeria. The study was approved by the Biomedical Research and Ethics Committee (BREC) of the University of KwaZulu-Natal, Durban, South Africa, and the Ethical Research Committee of the Ministry of Health, Jigawa State, Nigeria. Patients were briefed on the study protocol and signed informed consent to participate in the study which commenced on 1 June 2015 and ended on 31 May 2016.
Participants’ height and weight were measured and recorded. Body mass index (BMI) was calculated by dividing weight (kg) by height (m) and recorded. All assessments were conducted at baseline and at the end of 12 weeks of treatment. The primary outcome measures used to assess patients’ response to the treatment were WOMAC, SF-36 questionnaire, and the VAS.
Intervention
The CTG
Participants in the combination group underwent electro-diagnosis of the most painful knee area with continuous US (1 MHz; 0.5 W/cm2) and the IFC ( Hz) at tactile threshold intensity. Treatments were conducted at the intensity of continuous US (1 MHz; 1.5 W/cm2) applied with 5-cm transducer for 10 min using Sonoplus 920 (Sonicator Plus 920; Mettler Electronics, CA, USA). Participants were comfortably positioned in supine lying with pillow supported under the treated knee. Ultrasound Transmission Gel (Aqueous gel) was used as the contact medium. Two adhesive electrodes ( cm2) were placed opposite to each other (medial and lateral) for deeper penetrations. The US was first turned on, followed by turning of the IFC parameters as mentioned above. Participants were informed that they would experience tingling sensations which should not be unpleasant. Treatments were administered for 10 min three times a week for 12 weeks.
The IRG
Participants in this group were treated with luminous infrared lamp (IRR, Infraphil 150 W; Philips Electronics, Amsterdam, the Netherlands). The source of the radiation was placed at 60 cm from the patient’s skin for 15 min of a treatment session and the patient was treated three times a week for 12 weeks. Participants were positioned comfortably with knee flexed 20–30∘ supported with a pillow. Participants were warned that they were expected to feel comfortable “mild warmth” as too much heat could lead to skin burns.
All participants received quadriceps isometric exercises of both knees for 10 min, and were asked to refrain from taking non-steroidal anti-inflammatory drug (NSAIDS) and anti-depressants throughout the study period. However, they also were advised to take acetaminophen in case of unbearable pain and other comorbid medications throughout the study period.
Statistical Analyses
Statistical analyses were conducted with version 21.0 of Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA). The effect size for the sample size calculation was obtained from the previous studies conducted on knee osteoarthritis.20,32 Based on the data from these studies, it was estimated that a sample size of 30 patients in each study group would achieve a power of 80% to detect an effect size of 0.8 in the outcome measures of interest, assuming a type-I error of 0.05. Preliminary analysis was performed to check for normality, linearity, and homogeneity of variance, covariance, and multicollinearity with no serious violations noted with MAHAL. Descriptive statistics of mean, percentage, and standard deviation were used to describe the data. A one-way between-groups multivariate analysis of variance (MANOVA) was performed to investigate the differences between the combination therapy group and infrared group, four dependent variables were used for pain and physical function, and 10 dependent variables for quality of life. A -value equal to or less than 0.05 was considered as statistically significant. Furthermore, standardized effect sizes (Cohen’s ) with 95% confidence interval (CI) were included.
Results
A total of 63 patients with knee osteoarthritis participated in the study, and were randomized into CTG and IRG. During the study, three patients (one from CTG and two from IRG group) failed to follow up and were not included in the analyses (Fig. 1). Of the 60 participants who completed the study, 42 (70%) were female and 18 (30%) were male, with a mean age of years. Table 1 shows participants’ demographic characteristics at the baseline. There was no statistically significant difference in gender, age, and BMI between CTG and IRG at the baseline .
Table 1.
Patients’ demographic features between CTG and IRG.
| CTG | IRG | ||
|---|---|---|---|
| ( = 30) | |||
| Variables | M SD | M SD | -valuea |
| Age (years) | 65.8 9.21 | 66.8 8.61 | 0.153 |
| Weight (kg) | 69.29 10.88 | 70.04 9.66 | 0.985 |
| Height (m) | 1.66 0.08 | 1.67 0.76 | 0.780 |
| BMI (kg/m2) | 25.43 3.8 | 25.54 3.20 | 0.621 |
| Gender M/F, (%) | 20%/80% | 40%/60% | 0.146b |
| Gender ratio | 4:1 | 1.5:1 | 0.145 |
Note: BMI: Body mass index; M: male; F: female; M: Mean; SD: standard deviation. ; ND: No data; and , .
Pain and functional activity scores
At baseline, there was no statistically significant difference between the two groups in terms of pain and functional activities: ; ; Wilk’s ; and partial eta-. The -value for each dependent variable for the pain and functional activity scores is shown in Table 2.
Table 2.
Baseline comparison of VAS and WOMAC scores between CTG and IRG.
| CTG | IRG | ||||
|---|---|---|---|---|---|
| Pre-treatment | Pre-treatment | ||||
| Variable | M SD | M SD | Partial eta-squared | -value | |
| VAS | 7.07 1.74 | 6.24 3.12 | 0.692 | 0.612 | 0.409 |
| WOMAC | |||||
| Pain | 18.77 2.78 | 20.17 13.38 | 0.02 | 0.001 | 0.960 |
| Stiffness | 5.77 1.00 | 5.13 2.06 | 0.645 | 0.001 | 0.832 |
| PF | 56.10 7.35 | 14.83 16.22 | 0.086 | 0.001 | 0.770 |
Note: WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; M: mean; SD: standard deviation; “*” denotes the significance level, . The -values are for parametric test and independent sample -test for comparison groups.
There was a statistically significant difference between the two groups in terms of pain and functional activities after 12 weeks of intervention: ; ; Wilk’s ; and partial eta-. When the results for the dependent variables were considered separately, using a Bonferroni-adjusted alpha level of 0.012, all the variables were statistically significant as shown in Table 3.
Table 3.
Post-treatment changes between CTG and IRG following 12 weeks of treatment.
| CTG | IRG | ||||
|---|---|---|---|---|---|
| ( = 30) | ( = 30) | ||||
| Variable | M SD | M SD | Partial eta-squared | -value | |
| VAS | 2.23 4.34 | 6.24 3.12 | 43.6 | 0.983 | 0.000* |
| WOMAC (%) | |||||
| Pain | 16.97 3.38 | 20.17 13.38 | 246.08 | 0.809 | 0.000* |
| Stiffness | 7.13 2.06 | 10.33 0.80 | 7.66 | 0.116 | 0.008* |
| PF | 45.79 9.08 | 14.83 16.22 | 266.99 | 0.973 | 0.000* |
Note: WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; M: mean; SD: standard deviation; “*” denotes the significance level, .
HRQoL
At baseline, there was no statistically significant difference between the two groups in terms of quality of life using SF-36: ; ; Wilk’s ; and partial eta-. The -value for each dependent variable for the quality of life is shown in Table 4.
Table 4.
Baseline comparison of participants’ quality of life between CTG and IRG.
| CTG | IRG | ||||
|---|---|---|---|---|---|
| Pre-treatment | Pre-treatment | ||||
| Variable | M SD | M SD | Partial eta-squared | -value | |
| PF | 54.57 4.76 | 52.70 4.88 | 2.160 | 0.036 | 0.147 |
| RLPH | 52.38 5.62 | 52.42 7.35 | 2.625 | 0.016 | 6.808 |
| RLEP | 67.09 10.11 | 66.76 5.57 | 0.463 | 0.080 | 0.499 |
| E/F | 60.98 7.64 | 62.64 6.70 | 1.510 | 0.508 | 0.414 |
| EWB | 65.67 10.93 | 65.55 6.52 | 0.463 | 0.025 | 0.223 |
| SF | 57.65 8.57 | 57.95 5.71 | 1.646 | 0.023 | 0.205 |
| Pain | 51.54 7.67 | 50.92 5.49 | 1.369 | 0.023 | 0.247 |
| GH | 50.82 6.94 | 51.57 4.17 | 0.652 | 0.011 | 0.423 |
| PCS | 52.37 1.66 | 51.62 2.56 | 4.210 | 0.054 | 0.540 |
| MCS | 63.10 2.42 | 63.29 1.62 | 1.095 | 0.019 | 0.300 |
Notes: PCS: Physical component summary; MCS: mental component summary; PF: physical function; RLPH: role of limitation due to physical health; RLEP: role of limitation due to emotional problems; E/F: energy/fatigue; EWB: emotional well-being; SF: social functioning; GH: general health; M: mean; SD: standard deviation; and “*” denotes the significance level, . “*” indicates the statistical significance.
There was a statistically significant difference in quality of life between the two groups after 12 weeks of intervention: ; ; Wilk’s ; and partial eta-. When the results for the dependent variables were considered separately, the only difference to reach statistical significance, using a Bonferroni-adjusted alpha level of 0.005, was general health (GH): , , and partial eta-. An inspection of mean scores indicated combination therapy group reported higher levels of quality of life as shown in Table 5.
Table 5.
Post-treatment changes in QoL between the two groups (CTG and IRG).
| CTG | IRG | ||||
|---|---|---|---|---|---|
| Variable | M SD | M SD | Partial eta-squared | -value | |
| PF | 80.07 07 | 75.52 52 | 0.56 | 0.010 | 0.456 |
| RLPH | 79.82 7.87 | 74.05 8.13 | 1.08 | 0.018 | 0.302 |
| RLEP | 83.70 12.66 | 78.60 5.99 | 0.01 | 0.000 | 0.920 |
| E/F | 65.14 16.37 | 63.93 9.05 | 0.05 | 0.001 | 0.001 |
| WB | 78.37 11.68 | 71.63 11.46 | 0.03 | 0.001 | 0.850 |
| SF | 75.24 10.40 | 68.18 10.25 | 1.02 | 0.017 | 0.316 |
| Pain | 72.42 8.88 | 67.33 6.49 | 3.97 | 0.064 | 0.051 |
| GH | 80.13 11.69 | 52.70 11.69 | 14.6 | 0.202 | 0.000∗ |
| PCS | 78.27 4.93 | 65.49 3.49 | 7.84 | 0.119 | 0.007 |
| MCS | 72.90 14.08 | 68.96 5.60 | 1.64 | 0.028 | 0.205 |
Notes: PCS: Physical component summary; MCS: mental component summary; PF: physical function; RLPH: role of limitation due to physical health; RLEP: role of limitation due to emotional problems; E/F: energy/fatigue; EWB: emotional well-being; SF: social functioning; GH: general health; M: mean; SD: standard deviation; “*” denotes the significance level, .
Discussion
This was a randomized controlled trial, aimed at evaluating the efficacy of CTG when compared with ILG in terms of pain severity, functional activities, and HRQoL, in patients with knee osteoarthritis. The limitation of this study is that the long-term effects of combination therapy and infrared radiation cannot be obtained because the study only assesses the 12-week effects, therefore the results of this study should be interpreted with caution. The general applicability is limited as it can only be applied to the population of patients with knee osteoarthritis. Other limitation of the study includes the inability to blind the research assistant who delivered the intervention because in standard RCT both the participants and those who delivered the interventions are blinded but in physiotherapy it may sound so difficult. Self-reported outcomes such as VAS, WOMAC, and SF-36 scales are also a limitation as they may be influenced by placebo effects and outcome expectation. Moreover, some participants might have been taking other analgesics which might be a limitation to the intervention; this aspect is beyond the control of the researchers.
According to the study findings, patients with knee OA treated with CTG had better improvement in pain, physical function, and particularly the GH component of HRQoL compared with patients in the IRG, over a period of 12 weeks. This study clearly indicated that combination therapy is an electrotherapeutic modality that reduces pain and improves functional activities and HRQoL of elderly people with knee osteoarthritis.
In patients with OA, pain is the primary, most important, and frequent clinical symptom that leads to limited functional activities and poor quality of life.33,34 The primary goal of OA management is to alleviate the pain as well as improve functional activities and the quality of life of the individuals.35
In the current study, significant pain improvement reported by the CTG might be attributed to the combined effects of the electro-analgesia of IFC11 therapy and thermal analgesic effects of continuous US therapy.36 Several studies have shown that CT is an effective modality in the management of musculoskeletal disorders.37,38
Our findings were also supported by a study conducted by Švarcova et al.,39 who studied the combined effects of therapeutic ultrasound, galvanic current, and shortwave diathermy in patients with knee osteoarthritis. They reported significant improvement in pain level.
In spite of the fact that the mechanisms by which CT relieves pain are not properly understood, studies have shown that IFC therapy achieves its electro-analgesic effects through the activation of large diameter nerve fibres to inhibit the nociceptive impulses from the small-diameter fibres at the posterior horn of the spinal cord to modulate pain.11,40 OA pain is believed to be originating from both nociceptive and neuropathic pains as well as from unusual excitability in the nociceptive pathways of both peripheral nervous system and central nervous system (CNS).41 The pain is proven to be associated with central sensitization as a result of continued nociceptive activities from the affected knee that leads to prolonged hyper-excitability of pain in the CNS.42,43 IFC therapy may limit this prolonged abnormal hyper-excitation associated with pain observed in patients with knee OA. IFC therapy also achieves its electro-analgesic effects by blocking nociceptive impulses as explained by Melzack and Wall.44
Studies have shown that the application of continuous US therapy produces thermal effects.45,46 Thermal therapies are physiologically known to increase tissue metabolism, collagen elasticity, capillary blood flow, and reduce muscle spasm.47,48
Yeğin et al.36 reported that the US therapy is an effective treatment modality that reduces pain and improves physical function in the short term. In another study, Zeng et al.49 reported that the continuous US therapy could be used for effective pain relief in the management of knee osteoarthritis. Studies have shown that US therapy is an effective modality in reducing pain and improving functional activities and quality of life in the management of patients with knee OA.50,51
Unlike our study and the above-reported findings, Welch et al.52 conducted a systematic review aimed at studying the effectiveness of US therapy for45 patients with knee OA. They reported US therapy to have no beneficial effects when compared with placebo and shortwave diathermy on pain and function in the management of patients with osteoarthritis. In addition, some controlled clinical studies have reported that US therapy had no benefits in improving pain and functional activities in the management of patients with knee osteoarthritis.45,53
There is no literature that reports CT is unsafe. In all the available clinical studies on the use of CT on musculoskeletal disorders, no single study reported the side effects, either in CTG or in ILG.37,38,54 Likewise, in this current study no side effects had occurred during or after the CT treatment. Thus, the use of combination therapy was not associated with any negative or adverse effects in the management of knee OA.
The present study shows good improvement in pain relief, functional activities, and quality of life, but specifically the GH component of SF-36 quality of life showed improvement in patients treated with US and IFC therapies concurrently. The findings of this study add to the clinical evidence with regard to the use of CT in patients with knee OA.
Conclusion
Combination therapy was found to be an effective electrotherapeutic modality that can be used to relieve pain as well as improve functional activities and HRQoL in patients with knee osteoarthritis.
Conflict of Interest
The authors declare no competing interests.
Funding/Support
The authors received financial support from the College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
Author Contributions
Zubair Usman and Sonill Sooknunan Maharaj contributed to the study concept and design. Zubair Usman and Bashir Kaka helped in data acquisition and prepared the first draft of the paper. Sonill Sooknunan Maharaj revised the manuscript. All authors read and approved the final manuscripts.
Acknowledgments
The authors wish to thank all the patients who participated in this study and the staff members of the physiotherapy outpatient clinic in the two hospitals for assistance before and during the course of the study.
References
- 1. Laxafoss E, et al. Case definitions of knee osteoarthritis in 4,151 unselected subjects: Relevance for epidemiological studies. Skeletal Radiol 2010;39(9):859–66. [DOI] [PubMed] [Google Scholar]
- 2. McKinnis LN. Fundamentals of Musculoskeletal Imaging. Philadelphia: F.A. Davis Company, 2013. [Google Scholar]
- 3. Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med 2011;2(2):205–12. [PMC free article] [PubMed] [Google Scholar]
- 4. Felson DT, et al. Osteoarthritis: New insights — Part 1: The disease and its risk factors. Ann Intern Med 2000;133(8):635–46. [DOI] [PubMed] [Google Scholar]
- 5. Michael JW-P, Schlüter-Brust KU, Eysel P.. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int 2010;107(9):152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 7. Fitzcharles MA, Lussier D, Shir Y. Management of chronic arthritis pain in the elderly. Drugs Aging 2010;27(6):471–90. [DOI] [PubMed] [Google Scholar]
- 8. Walker UA, et al. Analgesic and disease modifying effects of interferential current in psoriatic arthritis. Rheumatol Int 2006;26(10):904–7. [DOI] [PubMed] [Google Scholar]
- 9. Thomas A, et al. Recommendations for the treatment of knee osteoarthritis, using various therapy techniques, based on categorizations of a literature review. J Geriatr Phys Ther 2009;32(1):33–8. [DOI] [PubMed] [Google Scholar]
- 10. Gadsby JG, Flowerdew M. Transcutaneous electrical nerve stimulation and acupuncture-like transcutaneous electrical nerve stimulation for chronic low back pain (withdrawn). Cochrane Database Syst Rev 1997; (1):CD000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gundog M, et al. Interferential current therapy in patients with knee osteoarthritis: Comparison of the effectiveness of different amplitude-modulated frequencies. Am J Phys Med Rehabil 2012;91(2):107–13. [DOI] [PubMed] [Google Scholar]
- 12. Noble GJ, Lowe AS, Walsh DM. Interferential therapy review — Part 1: Mechanism of analgesic action and clinical usage. Phys Ther Rev 2000;5(4):239–45. [Google Scholar]
- 13. Jarit GJ, et al. The effects of home interferential therapy on post-operative pain, edema, and range of motion of the knee. Clin J Sport Med 2003;13(1):16–20. [DOI] [PubMed] [Google Scholar]
- 14. Eftekharsadat B, et al. Efficacy of action potential simulation and interferential therapy in the rehabilitation of patients with knee osteoarthritis. Ther Adv Musculoskelet Dis 2015;7(3):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lara-Palomo IC, et al. Short-term effects of interferential current electro-massage in adults with chronic non-specific low back pain: A randomized controlled trial. Clin Rehabil 2013;27(5):439–49. [DOI] [PubMed] [Google Scholar]
- 16. Wong RA, et al. A survey of therapeutic ultrasound use by physical therapists who are orthopaedic certified specialists. Phys Ther 2007;87(8):986–94. [DOI] [PubMed] [Google Scholar]
- 17. Kapidzic S. Measurement of therapeutic effect of ultrasound on knee osteoarthritis; double blind study. Ann Phys Rehabil Med 2011;54:e181. [Google Scholar]
- 18. Atamaz FC, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: A double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil 2012;93(5):748–56. [DOI] [PubMed] [Google Scholar]
- 19. Jones A, et al. Concurrent validity and reliability of the Simple Goniometer iPhone app compared with the Universal Goniometer. Physiother Theory Pract 2014;30(7):512–6. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh R-L, et al. Therapeutic effects of short-term monochromatic infrared energy therapy on patients with knee osteoarthritis: A double-blind, randomized, placebo-controlled study. J Orthop Sports Phys Ther 2012;42(11):947–56. [DOI] [PubMed] [Google Scholar]
- 21. Hancock CM, Riegger-Krugh C. Modulation of pain in osteoarthritis: The role of nitric oxide. Clin J Pain 2008;24(4):353–65. [DOI] [PubMed] [Google Scholar]
- 22. Singh AK, et al. Prevalence of osteoarthritis of knee among elderly persons in urban slums using American College of Rheumatology (ACR) criteria. J Clin Diagn Res 2014;8(9):JC09–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hawker GA, et al. Measures of adult pain: Visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63(S11):S240–52. [DOI] [PubMed] [Google Scholar]
- 24. Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 2001;8(12):1153–7. [DOI] [PubMed] [Google Scholar]
- 25. Todd KH, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27(4):485–9. [DOI] [PubMed] [Google Scholar]
- 26. Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med 2001;38(6):633–8. [DOI] [PubMed] [Google Scholar]
- 27. Phan NQ, et al. Assessment of pruritus intensity: Prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012;92(5):502–7. [DOI] [PubMed] [Google Scholar]
- 28. Pedersen CB, et al. Reliability and validity of the Psoriasis Itch Visual Analog Scale in psoriasis vulgaris. J Dermatolog Treat 2016;28(3):213–20. [DOI] [PubMed] [Google Scholar]
- 29. Bellamy N. WOMAC Osteoarthritis Index: User Guide IX. Brisbane: Nicholas Bellamy, 2008. [Google Scholar]
- 30. McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Care Res 2001;45(5):453–61. [DOI] [PubMed] [Google Scholar]
- 31. Zhou K, et al. Reliability, validity and sensitivity of the Chinese (simple) short form 36 health survey version 2 (SF-36v2) in patients with chronic hepatitis B. J Viral Hepat 2013;20(4):e47–55. [DOI] [PubMed] [Google Scholar]
- 32. Yu A, et al. Pain management among Dominican patients with advanced osteoarthritis: A qualitative study. BMC Musculoskelet Disord 2016;17(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rutjes AW, et al. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2010; (1):CD003132. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26(3):355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang W, et al. EULAR evidence based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 2009;69(3):483–9. [DOI] [PubMed] [Google Scholar]
- 36. Yeğin T, Altan L, Aksoy MK. The effect of therapeutic ultrasound on pain and physical function in patients with knee osteoarthritis. Ultrasound Med Biol 2017;43(1):187–94. [DOI] [PubMed] [Google Scholar]
- 37. Almeida TF, et al. The effect of combined therapy (ultrasound and interferential current) on pain and sleep in fibromyalgia. Pain 2003;104(3):665–72. [DOI] [PubMed] [Google Scholar]
- 38. Çıtak-Karakaya İ, et al. Short and long-term results of connective tissue manipulation and combined ultrasound therapy in patients with fibromyalgia. J Manipulative Physiol Ther 2006;29(7):524–8. [DOI] [PubMed] [Google Scholar]
- 39. Švarcova J, Trnavský K, Zvarova J.. The influence of ultrasound, galvanic currents and shortwave diathermy on pain intensity in patients with osteoarthritis. Scand J Rheumatol 1988;17(Sup67):83–5. [DOI] [PubMed] [Google Scholar]
- 40. Samuel SR, Maiya GA. Application of low frequency and medium frequency currents in the management of acute and chronic pain-A narrative review. Indian J Palliat Care 2015;21(1):116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dray A, Read SJ. Arthritis and pain. Future targets to control osteoarthritis pain. Arthritis Res Ther 2007;9(3):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011;152(3):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mease PJ, et al. Pain mechanisms in osteoarthritis: Understanding the role of central pain and current approaches to its treatment. J Rheumatol 2011;38(8):1546–51. [DOI] [PubMed] [Google Scholar]
- 44. Melzack R, Wall PD. Pain mechanisms: A new theory. Surv Anesthesiol 1967;11(2):89–90. [Google Scholar]
- 45. Ulus Y, et al. Therapeutic ultrasound versus sham ultrasound for the management of patients with knee osteoarthritis: A randomized double-blind controlled clinical study. Int J Rheum Dis 2012;15(2):197–206. [DOI] [PubMed] [Google Scholar]
- 46. Johns LD. Nonthermal effects of therapeutic ultrasound: The frequency resonance hypothesis. J Athl Train 2002;37(3):293–9. [PMC free article] [PubMed] [Google Scholar]
- 47. Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: Biophysical effects. Phys Ther 2001;81(7):1351–8. [PubMed] [Google Scholar]
- 48. Benjaboonyanupap D, Paungmali A, Pirunsan U. Effect of therapeutic sequence of hot pack and ultrasound on physiological response over trigger point of upper trapezius. Asian J Sports Med 2015;6(3):e23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeng C, et al. Effectiveness of continuous and pulsed ultrasound for the management of knee osteoarthritis: A systematic review and network meta-analysis. Osteoarthritis Cartilage 2014;22(8):1090–9. [DOI] [PubMed] [Google Scholar]
- 50. Loyola-Sánchez A, Richardson J, MacIntyre N. Efficacy of ultrasound therapy for the management of knee osteoarthritis: A systematic review with meta-analysis. Osteoarthritis Cartilage 2010;18(9):1117–26. [DOI] [PubMed] [Google Scholar]
- 51. Jia L, et al. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: A randomized, double blind, placebo-controlled trial. Sci Rep 2016;6:35453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Welch V, et al. Therapeutic ultrasound for osteoarthritis of the knee. Cochrane Database Syst Rev 2001; (3):CD003132. [DOI] [PubMed] [Google Scholar]
- 53. Cakir S, et al. Efficacy of therapeutic ultrasound for the management of knee osteoarthritis: A randomized, controlled, and double-blind study. Am J Phys Med Rehabil 2014;93(5):405–12. [DOI] [PubMed] [Google Scholar]
- 54. Moretti FA, et al. Combined therapy (ultrasound and interferential current) in patients with fibromyalgia: Once or twice in a week? Physiother Res Int 2012;17(3):142–9. [DOI] [PubMed] [Google Scholar]