Abstract
A 35-year-old woman was referred for a symptomatic liver mass. Diagnostic workup detected a septated cyst located centrally in the liver measuring 10 × 7 cm. The cyst had gradually increased in size from previous studies with new intrahepatic biliary dilation. Due to concern for malignancy and symptomatic presentation of the patient, a partial central hepatectomy was performed. Pathology revealed a smooth-walled, multiloculated cyst lined with mucinous epithelium and ovarian-type stroma. The diagnosis of low-grade mucinous cystic neoplasm of the liver (MCN-L) was made. Characteristics of MCN-L have not been elucidated due to its rarity.
INTRODUCTION
Mucinous cystic neoplasm of the liver (MCN-L), previously called hepatobiliary cystadenoma, is rare, benign hepatic tumors seen almost exclusively in women. The most recent World Health Organization (WHO) classification of MCN-L in 2010 defined it as cyst-forming epithelial neoplasm, associated with ovarian-type subepithelial stroma and usually demonstrating no connection with the bile ducts [1, 2]. Since classification, the characteristics of MCN-L have garnered great interest but remain poorly understood. We present a case of MCN-L that was treated by resection.
CASE PRESENTATION
A 35-year-old female with a medical history significant for acute biliary pancreatitis status post laparoscopic cholecystectomy, presented to the surgery clinic as a new consult for a large cystic liver mass with increasing abdominal pain for 2 weeks. Patient reported suffering from severe pain in right chest, shoulder and diffuse abdominally that worsened with standing. Patient complained of inability to inspire due to pain and a 10-pound weight gain over 2 months. She confirmed decreased appetite especially with solids, nausea and emesis, alternating constipation and diarrhea, bruising, and pruritus at night. Computed tomography (CT) scan demonstrated a peripherally septated 10 x 7 cm cystic mass in the liver with intrahepatic biliary dilation (Fig. 1). The patient was referred for endoscopic retrograde cholangiopancreatography (ERCP) to establish preoperative biliary anatomy and was found to have moderate compression of the common hepatic duct managed with a right hepatic biliary endoprosthesis (Fig. 2); no obvious communication of the biliary tree with the cystic lesion was seen. Patient symptoms persisted despite optimizing with a protein-rich liquid diet; thus, the decision was made to proceed with the surgical plan for an open partial central hepatectomy. The patient was taken to the operative theater. After induction of general anesthetic, an upper midline incision was made. Inspection of the abdomen and liver showed no metastatic lesions grossly or with ultrasonographic imaging. The cyst was visible upon entry into the abdomen with no solid component to the mass in proximity to the cystic neoplasm. The second portion of the duodenum was adherent to the cyst with inflammatory adhesions and was quite boggy. A partial central hepatectomy was performed; a 3 mm biliary duct was found communicating to the cyst only with no drainage to the minimal liver parenchyma that was removed (Fig. 3). The cyst was resected en-bloc and was sent for permanent section (Fig. 4), which diagnosed the tumor as a low-grade mucinous cystic neoplasm measuring 8.5 × 7.2 × 6.4 cm. Microscopy revealed a smooth-walled, multiloculated cyst filled with a yellow-golden, semi-transparent and mucinous fluid (Fig. 5a–c). The cyst was lined by a mucinous epithelium with ovarian-type stroma. No high-grade dysplasia or malignancy was identified. The postoperative course was uneventful, and the patient was discharged on postoperative day 6. At the 4-week postoperative visit, the patient was healing well with some incisional soreness; patient was seen for removal of her biliary endoprosthesis, and ERCP found left sided intrahepatic duct biliary dilatation including a small biliary fistula from the left system (Fig. 6) with the appearance of torque on the extrahepatic biliary tree. A biliary endoprosthesis was left in place, and the patient returned for repeat ERCP 4 weeks later with resolution of all findings (Fig. 7). At present, patient remains alive with no signs of recurrence.
Figure 1.
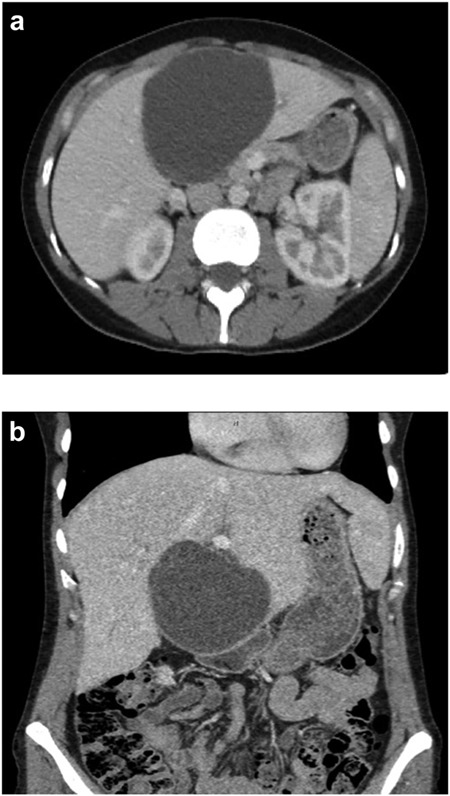
(a) Axial CT abdomen of a septated 10 × 7 cm cystic liver neoplasm. (b) Coronal CT abdomen of a duodenal obstruction secondary to a liver mass.
Figure 2.
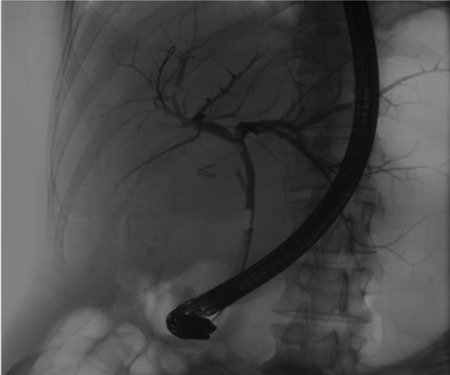
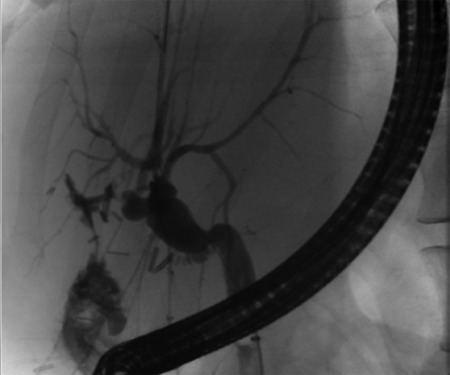
Pre-operative ERCP demonstrating compression of the common hepatic duct.
Figure 3.
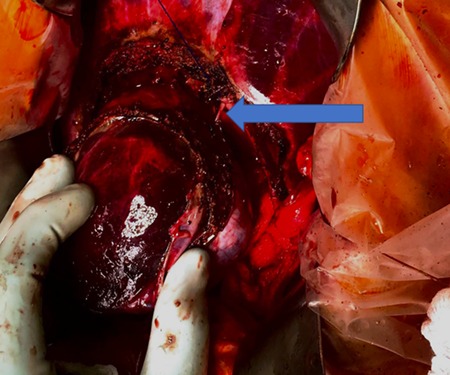
Independent biliary duct (blue arrow) draining to the cyst and not to the liver parenchyma.
Figure 4.
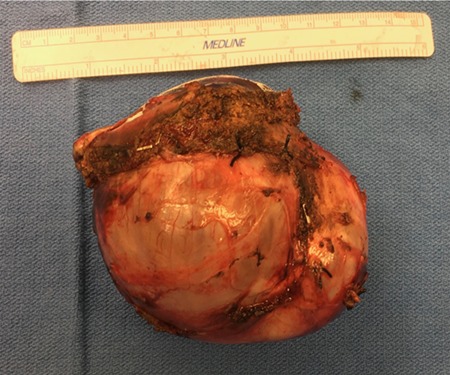
Cystic tumor following resection.
Figure 5.
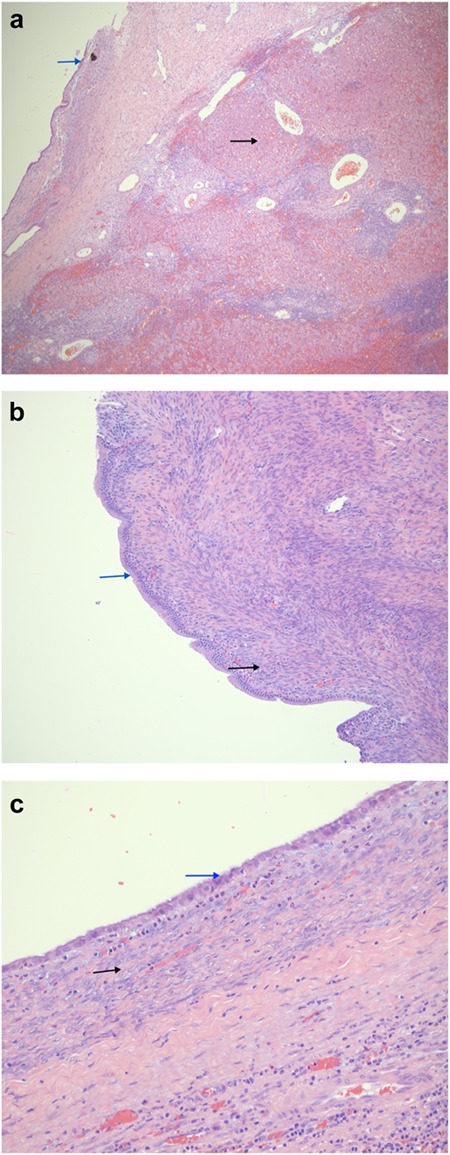
(a) A low power magnification showing a cystic lesion (blue arrow) in a congested and inflamed liver tissue (black arrow). (H&E, 40×). (b) A higher magnification showing the cystic lesion with a columnar mucinous epithelium lacking nuclear atypia (blue arrow); overlying a cellular stroma consisting of ovoid to spindle cell ovarian-type stroma (black arrow) (H&E, 100×). (c) The cystic portion of the lesion has a rather cuboidal lining (blue arrow). The black arrow points the characteristic ovarian storm.
Figure 6.
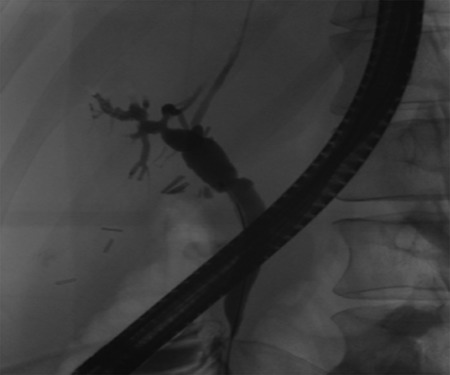
Post-operative ERCP displaying left sided intrahepatic duct biliary dilatation.
Figure 7.
Follow-up ERCP 4 weeks later displaying resolution of all abnormalities.
DISCUSSION
MCN-L is cystic tumor with benign (cystadenoma) or malignant (cystadenocarcinoma) features; they are lined by columnar, cuboidal or flattened mucus secreting epithelial cells with a mesenchymal stroma linked to ovarian stroma. Papillary projections may be present [2]. MCN-L is seen almost exclusively in females between the ages of 50–60 [2]. Patients present with symptoms of abdominal pain and/or mass effect resulting in symptoms such as gastric outlet obstruction or jaundice with elevated CA19-9 levels. The diagnosis of MCN-L preoperatively on cross-sectional imaging can be elusive, thus leading to the possibility of missed cases having gone untreated and an explanation for its rarity compared with counterpart neoplasms [2, 3]. The differential diagnosis of MCN-L includes intraductal papillary neoplasm of the bile duct (IPNB) and intrahepatic cholangiocarcinoma with cystic change. Characteristics shared between IPNB and MCN-L include communication with the bile ducts, bile duct dilatation and papillary projections in the bile ducts. However, the presence of ovarian-like stroma is required for a diagnosis of MCN-L and has clarified the distinction [4].The discrepancy of biliary duct involvement between the WHO criteria and this case appears to be a common occurrence throughout the literature. Shiono et al. reported an inconsistent presence of communication between hepatobiliary MCN and the biliary system in the cases they reviewed [5]. It remains unclear whether MCN-L with or without communication might demonstrate variation of the same tumor or criteria that should be used to distinguish between different ones [6].
According to Zen et al., 39 of the 54 cases (72%) of MCN-L they reviewed developed in the left lobe of the liver with malignant transformation being uncommon in these 54 tumors [3]. Besides a single case of malignancy, all other cases showed low-to-intermediate grade dysplasia. Patients with MCN-L rarely suffered from other liver diseases such as cirrhosis or viral hepatitis and have demonstrated a good overall prognosis following hepatectomy [3].
Similarities between this case and reported cases in the literature are MCN-L predominance in middle aged females and a low rate of malignant transformation. Noted difference was the bile duct communication seen in our case that was not apparent on preoperative diagnostics. With a large diameter, erosion of the expanding cyst wall into the biliary ducts has been described as the cause for communication [4]. Indications for resection have been described for cysts larger than 100 mm, growth over time, or the development of symptoms [3].
In summary, this case highlights the variability in characteristics of mucinous cystic neoplasms of the liver and the need for clarification of features that differentiate it from neoplasms of the biliary system including involvement of the extrahepatic biliary duct. Presently, hepatic resection should be considered in the presence of cystic neoplasms of the liver as the ability to distinguish benign versus malignant forms clinically has not yet been established [7].
ACKNOWLEDGEMENTS
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest statement
None.
References
- 1. Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study ad comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol 2011;24:1079–89. [DOI] [PubMed] [Google Scholar]
- 2. Hamilton SR, Aaltonen LA (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press, 2000, 182–3 [Google Scholar]
- 3. Zen Y, Jang KT, Ahn S, Kim DH, Choi DW, Choi SH, et al. Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: demographic differences between Asian and western populations, and comparison with pancreatic counterparts. Histopathology 2014;65:164–73. [DOI] [PubMed] [Google Scholar]
- 4. Nakayama Y, Kato Y, Okubo S, Takahashi D, Okada R, Nishida Y, et al. A case of mucinous cystic neoplasm of the liver: a case report. Surg Case Rep 2015;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiono S, Suda K, Nobukawa B, Arakawa A, Yamasaki S, Sasahara N, et al. Pancreatic, hepatic, splenic, and mesenteric mucinous cystic neoplasms (MCN) are lumped together as extra ovarian MCN. Pathol Int 2006;56:71–7. [DOI] [PubMed] [Google Scholar]
- 6. Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, et al. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol 2006;19:1243–54. [DOI] [PubMed] [Google Scholar]
- 7. Hai S, Hirohashi K, Uenishi T, Yamamoto T, Shuto T, Tanaka H, et al. Surgical management of cystic hepatic neoplasms. J Gastroenterol 2003;38:759–64. [DOI] [PubMed] [Google Scholar]


