Abstract
Background
CD40 is a transmembrane protein mainly expressed on the antigen‐presenting cells surface. CD40 plays a crucial role in immunoglobulin class switching and antibodies production. Genetic polymorphisms in the CD40 gene have been associated with increased risk of systemic lupus erythematosus (SLE) in several populations. This study aimed to evaluate the association of CD40 polymorphisms (−1 C > T, rs1883832 and 6,048 G > T, rs4810485) with SLE susceptibility, as well as with mRNA expression and soluble CD40 (sCD40) levels.
Methods
The study included 293 patients with SLE and 294 control subjects (CS). Genotyping was performed by PCR‐RFLP method. CD40 mRNA expression was determined by quantitative real‐time PCR, and ELISA quantified sCD40 levels.
Results
The CD40 polymorphisms −1 C > T and 6,048 G > T were associated with SLE susceptibility. There was no difference between CD40 mRNA expression and CD40 polymorphisms. The sCD40 levels were lower in SLE patients with TT haplotype, whereas higher sCD40 levels were associated with damage and impaired renal function according to SLICC and KDIGO. The sCD40 levels were negatively correlated with eGFR.
Conclusion
The CD40 gene polymorphisms increase the risk of SLE in the western Mexican population. The sCD40 levels are associated with −1 C > T polymorphism and chronic kidney disease.
Keywords: CD40 polymorphisms; rs1883832; rs4810485 −1 C > T and 6,048 G > T polymorphisms; soluble CD40; systemic lupus erythematosus
The CD40 gene polymorphisms increase the risk of SLE in the western Mexican population. The sCD40 levels are associated with −1 C > T polymorphism and chronic kidney disease.
1. INTRODUCTION
The systemic lupus erythematosus (SLE) is an autoimmune disease characterized by increased auto‐antibody production, mainly directed to the cytoplasm and nuclear components that can lead to an inflammatory process promoted by immune complex deposition, affecting multiple organs and tissues (Rahman & Isenberg, 2008). The etiology of the disease remains elusive; however, the involvement of environmental and genetic factors is well known (Tsokos, 2011). CD40 is a type 1 transmembrane protein that belongs to the tumor necrosis factor receptor superfamily. CD40 is expressed on the surface of antigen‐presenting cells like dendritic cells, macrophages, and B cells, as a costimulatory molecule (Loskog & Eliopoulos, 2009). Its ligand, CD40L (CD154), is a type 2 transmembrane protein expressed mostly on activated T CD4 + cells. The interaction between CD40 and CD154 is essential in the regulation of B cell proliferation, germinal center formation, immunoglobulin class switching, antibody production, and generation of memory B cells (Elgueta et al., 2009; Toubi & Shoenfeld, 2004). Several studies have shown the importance of CD40‐CD154 interaction in autoimmune diseases, and higher levels of soluble CD40 (sCD40) have been observed in SLE patients compared with control subjects (Petrackova et al., 2017; Wagner et al., 2014). However, the role of sCD40 form in the SLE pathogenesis is still poorly understood.
Two single nucleotide polymorphisms (SNPs) in the CD40 gene (OMIM: *109,535), −1 C > T (rs1883832) and 6,048 G > T (rs4810485), have been associated with different autoimmune diseases in several populations (Chen et al., 2015; García‐Bermúdez et al., 2012; Sokolova et al., 2013). The −1 C > T polymorphism locates in the Kozak sequence, which has an essential role in mRNA translation. Moreover polymorphic −1T allele seems implicated with lower protein production compared with −1C allele (Jacobson, Concepcion, Oashi, & Tomer, 2005). Meanwhile, the 6,048 G > T polymorphism, localized in the second intron of the CD40 gene, has been associated with SLE in the Korean population (Joo et al., 2013). In the Greek population, a significantly reduced CD40 mRNA and protein expression have been observed in carriers of the GT and TT genotypes of 6,048 G > T polymorphism compared with carriers of CC genotype (Vazgiourakis et al., 2011). Both SNPs have been reported to be in strong linkage disequilibrium (r2 = 0.95) in the Asian population (Chen et al., 2015; Joo et al., 2013).
In Mexican population, the CD40 polymorphisms −1 C > T and 6,048 G > T did not found associated with rheumatoid arthritis; however, CD40 mRNA levels were 1.5‐fold higher in RA patients compared with control subjects (Román‐Fernández et al., 2016). This study aimed to evaluate the association between the −1 C > T and 6,048 G > T genetic variants with disease susceptibility, CD40 mRNA expression, and sCD40 levels in SLE patients.
2. MATERIALS AND METHODS
2.1. Editorial policies and ethical considerations
According to the ethical guidelines stated on the declaration of Helsinki (Brazil, 2013), the informed written consent was obtained from all patients and control subjects (CS) before their enrollment to the study. Investigation and Ethics committee of the Hospital General de Occidente approved the investigation (Number of approval 449/16).
2.2. Subjects
A total of 587 subjects were included in the study: 294 control subjects (CS) and 293 patients diagnosed with SLE according to the American College of Rheumatology criteria (Hochberg, 1997). The patients were recruited from the Department of Rheumatology of the Hospital General de Occidente, Guadalajara, Mexico. The Mexican version of the Systemic Lupus Disease Activity Index (MexSLEDAI) (Guzmán, Cardiel, Arce‐Salinas, Sánchez‐Guerrero, & Alarcón‐Segovia, 1992) and Systemic Lupus International Collaborating Clinics (SLICC) damage index (Gladman et al., 1996) were applied to all SLE patients at the moment of enrollment. Patients were stratified according to the Mex‐SLEDAI score as follows: inactive (0–1), mild‐moderate (2–6) and severe (≥7) disease. The CS group included healthy subjects with no history of autoimmune diseases. All the participants were unrelated Mexican mestizo individuals from western Mexico with at least three generations of Mexican ancestry.
2.3. CD40 polymorphisms genotyping
Blood samples were collected from all subjects, and genomic DNA was extracted following the modified Miller's method (Miller, Dykes, & Polesky, 1988). Genetic variants rs1883832 (c.‐1C > T) and rs4810485 (c.51 + 914G>T) were genotyping using polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP) technique. For −1 C > T SNP, the following primers were used: forward 5'‐CCC CGA TAG GTG GAC CGC GAT TGG T‐3' and reverse 5'‐CCC GCC CTC TGA ACC CCC TAC CAG T‐3'; whereas for 6,048 G > T SNP the primers used were: forward 5'‐TAT TTT TGT AGT TCC TCA TTC TGG A‐3' and reverse 5'‐GCC CCC CTT TAC CTC TTT CCA‐3'. The PCR conditions were as described by Román‐Fernández et al. (Román‐Fernández et al., 2016; Román‐Fernández, Muñoz‐Valle, & Palafox‐Sánchez, 2019). The amplified 505 bp PCR product containing the −1 C > T SNP was digested with 5 U of NcoI restriction enzyme (New England BioLabs®) at 37°C for 1 hr. The resulting restriction fragments were CC: 373 and 132 bp, CT: 505, 373, and 132 bp and TT: 505 bp. The 283 bp PCR product with the 6,048 G > T SNP was digested with 5 U of MspI restriction enzyme (New England BioLabs®) at 37°C for 2 hr. The restriction pattern for 6,048 G > T genotyping was GG: 180 and 103 bp, GT: 283, 180, and 103 bp and TT: 283 bp.
2.4. CD40 mRNA expression analysis
Relative quantification was performed by real‐time quantitative PCR (qPCR). RNA was extracted from peripheral blood leukocytes, using the Chomczynski and Sacchi method (Chomczynski & Sacchi, 1987), from 22 CS and 21 SLE patients carrying the CC/GG, CT/GT, and the TT/TT genotypes for both polymorphisms (−1C > T/6048G > T). The RNA purity was determined by spectrophotometry (NanoDrop lite, Thermo Fisher Scientific), samples with A260/280 ratio below 1.7 were discarded. Total RNA (1 μg) was reversed transcribed using Oligo‐dT and Moloney murine leukemia transcriptase (Promega Corp.,) following the manufacturer's protocol. CD40 (Hs01002915_g1) mRNA expression was determined using hydrolysis probes (TaqMan ®, Applied Biosystems, Thermo Fisher Scientific). GAPDH was used as the reference gene (Hs02786624_g1). qPCR was performed with 100 ng cDNA in a LightCycler 96 system (Roche Applied Science). All samples were run in triplicates. A validation experiment using serial dilutions of cDNA was conducted to verify amplification efficiencies for both genes. The data were analyzed with the relative expression software tool (REST) described by Pfaffl et al. (Pfaffl, Horgan, & Dempfle, 2002).
2.5. Soluble CD40 quantification
Serum samples were obtained from peripheral blood of SLE patients and CS and store at −20ºC until use. The sCD40 levels were determined using a commercial ELISA test (DCCD40 Quantikine ELISA kit [R&D Systems®]) according to manufacturer's conditions. The ELISA test sensitivity and detection limits are 5.56 and 19.5–1,250 pg/mL, respectively. All samples were run in duplicate and analyzed using a Multiskan™ Go Microplate Spectrophotometer (Thermo Fisher Scientific).
2.6. Statistical analysis
Statistical differences in the genotype and allele frequencies of CD40 were compared using the chi‐square test and Fisher's exact test, when appropriate. The dominant genetic model was also evaluated (Shi & He, 2005). Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated to assess the risk of SLE in association with the CD40 SNPs. Haplotypes inference was performed by EM algorithm using the SHEsis software (http://analysis.bio-x.cn/myAnalysis.php). The linkage disequilibrium was estimated using Lewontin's D’ measure (Z. Li et al., 2009). Groups were compared using the Mann‐Whitney U test and Kruskal‐Wallis test; Dunn's test was used to analyze differences between groups. Spearman's correlation coefficient was used to analyze the relationship between quantitative variables. P‐values < .05 were considered statistically significant. Graph Pad Prism version 6.0 (GraphPad Software) was used for statistical analysis.
3. RESULTS
3.1. Subjects demographic and clinical characteristics
The median age was 38 [Interquartile Range (IQR) 27–56] and 36.5 (IQR 28–47.7) years for CS and SLE patients, respectively. The female gender distribution was 96% in CS and 94% in SLE patients. For SLE patients, the median Mex‐SLEDAI score was 2 (IQR 0–4), and SLICC damage index was 0 (IQR 0–1), while the median of disease evolution was 4 (IQR 2–12) years. The main clinical manifestations were hematological cytopenias (75%, including lymphopenia, leukopenia, and thrombocytopenia), mucocutaneous (66.2%) and renal involvement (25.6%); from the latter active lupus nephritis and chronic kidney disease were observed in 15.9% and 9.7% of patients, respectively. The most common autoantibodies were anti‐dsDNA (57%), followed by anti‐RNP 33% and anti‐Ro 29%. SLE patients were under conventional treatment with prednisone (74.3%) with a median dosage of 10 (IQR 5–20) mg/day, azathioprine (54.9%) and antimalarials (52.1%).
3.2. Genotype and allele frequencies of CD40 gene polymorphisms
The genotype and allele frequencies of the −1 C > T and 6,048 G > T CD40 gene polymorphisms in the CS and SLE patients are shown in Table 1. The genotype distributions of both SNPs in the CS group were in Hardy‐Weinberg equilibrium (p > .05). There were significant differences in the genotype and allele frequencies of both polymorphisms between CS and SLE patients. The −1T allele and −1TT genotype from −1 C > T SNP were associated with a higher risk of SLE (OR = 1.424, 95% CI, 1.074–1.889, p = .014 and OR = 3.236, 95% CI, 1.247–8.397, p = .016, respectively). Likewise, the 6048T allele and 6048GT genotype were associated with a significant risk of SLE (OR = 1.442, 95% CI, 1.090–1.907, p = .010 and OR = 1.463, 95% CI, 1.038–2.061, p = .029, respectively).
Table 1.
Genotype, allele, and haplotype frequencies of CD40 SNPs (−1 C > T and 6,048 G > T) in CS and SLE patients
| SNP | CS n = 294 (%) | SLE n = 293 (%) | p value | OR (CI 95%) | p value |
|---|---|---|---|---|---|
| −1 C > T (rs1883832) | |||||
| CC | 193 (65.5) | 169 (57.7) | .027 | 1 | — |
| CT | 95 (32.3) | 107 (36.5) | 1.286 [0.911–1.816] | .152 | |
| TT | 6 (2) | 17 (5.8) | 3.236 [1.247–8.397] | .016 | |
| Allele | |||||
| C | 481 (81.8) | 445 (75.9) | .014 | 1 | — |
| T | 107 (18.2) | 141 (24.1) | 1.424 [1.074–1.889] | .014 | |
| Dominant | |||||
| CC | 193 (65.6) | 169 (57.7) | .047 | 1 | — |
| CT + TT | 101 (34.4) | 124 (42.3) | 1.402 [1.004–1.958] | .047 | |
| 6,048 G > T (rs4810485) | |||||
| GG | 192 (65.3) | 162 (55.3) | .03 | 1 | — |
| GT | 94 (32) | 116 (39.6) | 1.463 [1.038–2.061] | .029 | |
| TT | 8 (2.7) | 15 (5.1) | 2.222 [0.918–5.376] | .085 | |
| Allele | |||||
| G | 478 (81.3) | 440 (75.1) | .010 | 1 | — |
| T | 110 (18.7) | 146 (24.9) | 1.442 [1.090–1.907] | .010 | |
| Dominant | |||||
| GG | 192 (65.3) | 162 (553) | .013 | 1 | — |
| GT + TT | 102 (34.7) | 131 (44.7) | 1.522 [1.091–2.123] | .013 | |
| Haplotype* | 2n = 588 (%) | 2n = 586 (%) | |||
| C G | 475 (80.7) | 435 (74.2) | ‐ | 1 | — |
| T T | 104 (17.7) | 136 (23.2) | 1.428 [1.072–1.902] | .014 | |
Abbreviations: CI, Confidence interval; CS, control subjects; OR, odds ratio; SLE, systemic lupus erythematosus; SNP, single nucleotide polymorphism.
Haplotype analysis included the −1 C > T (rs1883832) and 6048G > T (rs4810485) polymorphisms of CD40 gene and all those with a frequency <0.03 were ignored in the analysis.
3.3. Haplotype analysis
The CD40 gene −1 C > T polymorphism was in strong linkage disequilibrium with the 6,048 G > T polymorphism (D´ = 0.95). Haplotype frequencies are shown in Table 1. The most frequent haplotypes were CG and TT (80.7% and 17.7% in CS and 74.2% and 23.2% in SLE patients, respectively). The TT haplotype was associated with a significantly increased risk of SLE compared with the CG haplotype (OR = 1.428, 95% CI, 1.072–1.902, p = .014).
3.4. CD40 mRNA and protein expression according to CD40 polymorphisms
The sCD40 levels stratified according to the genotypes of −1 C > T and 6,048 G > T CD40 polymorphisms in SLE patients are shown in Figure 1c, d, f. The SLE subjects carrying the homozygous polymorphic genotypes showed decreased sCD40 levels [−1TT/6048TT 257.2 (IQR 165.1–308.8) pg/mL] compared with SLE subjects carriers of homozygous wildtype genotypes [−1CC 392.3 (272.4–570.9) pg/mL) and 6048GG 366 (IQR 270.9–558.8) pg/mL], p < .05.
Figure 1.
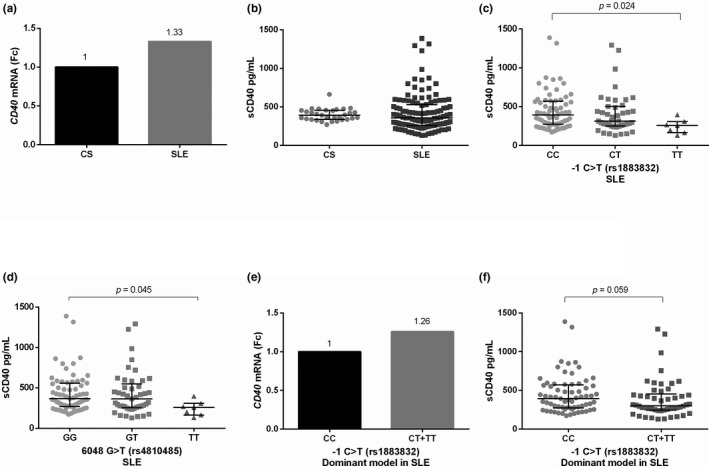
CD40 gene expression and sCD40 levels according to −1 C > T (rs1883832) and 6,048 G > T (rs4810485) polymorphisms. (a) CD40 gene expression in CS and SLE patients, (b) sCD40 levels in CS and SLE patients, (c) and (d) sCD40 levels according to −1 C > T (rs1883832) and 6,048 G > T (rs4810485) genotypes; (e) CD40 gene expression according to dominant model of −1 C > T (rs1883832) polymorphism in SLE patients, and (f) sCD40 levels according to dominant model of −1 C > T (rs1883832) polymorphism in SLE patients. The qualitative gene expression analysis was obtained through to Pfaffl's method. Mann‐Whitney U test, Kruskal‐Wallis test, and Dunn's post hoc test were used. Fc was obtained through the 2‐ΔΔCq method. Data are shown in median and IQR. CS: Control subjects, Fc: Fold change, IQR: Interquartile range, SLE: Systemic Lupus Erythematosus
The CD40 mRNA expression, according to the −1 C > T SNP is shown in Figure 1e. Following the dominant genetic model, the SLE patients carrying the −1T allele (CT + TT) displayed 1.26‐fold more CD40 mRNA expression compared with carriers of −1CC genotype.
3.5. CD40 mRNA and protein expression according to clinical disease
The SLE patients showed 1.33‐fold more CD40 mRNA expression compared with the CS group (Figure 1a). However, no significant differences regarding sCD40 levels were found in SLE in comparison with CS [355.8 (IQR 257.2–528.5) pg/mL vs. 391.6 (IQR 336.7–454.2) pg/mL; p = .253], Figure 1b. No differences were observed in CD40 mRNA expression according to disease activity, Figure 2a. The SLE patients with severe disease activity (n = 17) [576.9 (IQR 280–855) pg/mL] displayed higher sCD40 levels in comparison with mild‐moderate activity (n = 60) [345 (IQR 255.7–514.9) pg/mL] and inactive patients (n = 42) [338.1 (IQR 242.3–451.6) pg/mL], however this difference was not significant (p = .073, Figure 2b). In addition, no significant correlation between the sCD40 levels and the MexSLEDAI was observed (r = .073, p = .428). Nevertheless, the sCD40 levels were positively correlated with SLICC damage index (r = .272, p = .003, Figure 2c). Of note, sCD40 levels showed a negative correlation with the estimated glomerular filtration rate [eGFR, (r = −.313, p = .002, Figure 2d)] and sCD40 levels were higher in SLE patients with Kidney Disease Improving Global Outcomes (KDIGO) stage IV (p = .004, Figure 2e) and chronic kidney disease (p = .006, Figure 2f).
Figure 2.
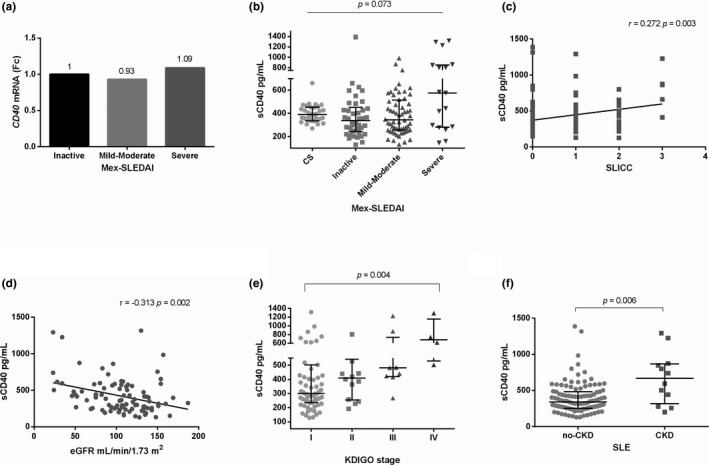
CD40 gene expression and sCD40 levels according to SLE activity. (a) CD40 gene expression according to disease activity (b) sCD40 levels according to disease activity, (c) sCD40 levels and SLICC damage index correlation, (d) sCD40 levels and eGFR correlation, (e) association between sCD40 levels and KDIGO stage, and (f) sCD40 levels and CKD (SLE patients with an eGFR < 50ml/min/1.73m2). The qualitative gene expression analysis was obtained through to Pfaffl's method. Mann‐Whitney U test, Kruskal‐Wallis test, Dunn's post hoc test, and Spearman rank correlation coefficient were used, Fc was obtained through the 2‐ΔΔCq method. Data are shown in median and IQR. CS: Control Subjects, CKD: Chronic Kidney Disease, eGFR: estimated Glomerular Filtration Rate, KDIGO: Kidney Disease Improving Global Outcomes, Fc: Fold change, IQR: Interquartile range, SLE: Systemic Lupus Erythematosus
4. DISCUSSION
CD40 is an important transmembrane protein that, together with its ligand CD154, plays a key role in T cell‐dependent humoral and adaptive cellular immunity (Toubi & Shoenfeld, 2004). Therefore, several studies have focused on CD40 genetic variants as susceptibility biomarkers for autoimmune diseases in diverse populations (Blanco‐Kelly et al., 2010; Chen et al., 2015; Joo et al., 2013; M. Li et al., 2012; Piotrowski, Lianeri, Wudarski, Olesińska, & Jagodziński, 2013; Sokolova et al., 2013; Teruel et al., 2012; Wang et al., 2017). However, in the Mexican population, the CD40 polymorphisms as susceptibility markers for SLE have not been assessed. Hence, in this study, the −1 C > T and 6,048 G > T CD40 polymorphisms, CD40 mRNA expression, and soluble levels of CD40 were evaluated in a group of SLE patients from western Mexico. Our results showed a clear association for both CD40 polymorphisms and SLE risk (p < .05), contrary to a previous study in the Mexican population, who found no association between the −1 C > T and 6,048 G > T CD40 genetic variants and rheumatoid arthritis (Román‐Fernández et al., 2016). In western Mexican population, the overall polymorphic allele frequencies for both polymorphisms were lower to those found in Spanish (−1 C > T: 27.7%, 6,048 G > T: 27.2%) and Chinese (−1 C > T: 41.1%, 6,048 G > T: 50.2%) populations (Chen et al., 2015; García‐Bermúdez et al., 2012). These differences could be explained by the heterogeneity of the genetic background between populations; the western Mexican mestizo is a population with markedly European ancestry (64.9%), followed by Amerindian (30.8%) and African (≈15%) (Martínez‐Cortés et al., 2012). We found an association for both CD40 polymorphisms; −1 C > T (OR = 1.424 [1.074–1.889], p = .014) and 6,048 G > T (OR = 1.442 [1.090–1.907], p = .010), with a significant increased risk to SLE development in our population, similar to the reported previously for Asian populations (Chen et al., 2015; Wu et al., 2016). Furthermore, the highest risk was found in the −1TT genotype carriers (OR = 3.236 [1.247–8.397], p = .016). The CD40 polymorphisms were in linkage disequilibrium, similar to that previously reported (Chen et al., 2015; Joo et al., 2013; Raychaudhuri et al., 2008). The more prevalent haplotypes were CG and TT in SLE patients and CS; a significantly increased risk for SLE was found in the TT haplotype compared with the CG haplotype (OR = 1.428 [1.072–1.902], p = .014), similar to the one seen in Asian populations (Chen et al., 2015; Wu et al., 2016) but not in European‐Caucasian populations (Chadha et al., 2005; Piotrowski et al., 2013; Vazgiourakis et al., 2011). It is worth to mention that the SLE studies for the Caucasians were carried out in regions of southeastern, central and western Europe and that the shared genetic background of the Mexican mestizo is with the Caucasian population belonging to southwest Europe; thus, the lack of concordance between our findings and those reported for European‐Caucasians could be due to differences in the genetic background between populations.
Given the association observed between the −1 C > T and 6,048 G > T CD40 polymorphisms and risk of SLE in our population, the CD40 expression was evaluated according to the genotypes. We found lower sCD40 levels in SLE patients carriers of the risk −1T allele (p = .033). These results are in agreement with the functional impact attributed to the −1 C > T SNP, localized within the Kozak sequence, on CD40 mRNA translation. The recognition of the Kozak sequence GCCRCCaugG by the ribosome subunit can be affected by nucleotide changes in −3 and + 4 positions (M Kozak, 1987). Mainly, polymorphisms in positions −1 to −3 of the mRNA are related to changes in protein yield (Marilyn Kozak, 2002). The above was confirmed for CD40 mRNA by Jacobson et al. (Jacobson et al., 2005), who found that the presence of the −1T allele resulted in up to 32% less CD40 protein expression in transfected fibroblasts and suggested that this could be due to a modification on ribosome binding affinity reducing the efficiency of translation. Besides, they observed less CD40 protein expression on the surface of B cells from healthy subjects carrying the −1CT and −1TT genotypes compared with −1CC genotype carriers (Jacobson et al., 2005). It is worth to mention that other authors have consistently made these observations on other cell populations (Field et al., 2015; Skibola et al., 2008; Vazgiourakis et al., 2011).
In contrast to the sCD40 levels, we found a mild increment of CD40 mRNA relative expression in the −1CT/‐1TT carriers in comparison with subjects carrying the −1CC genotype in CS and SLE. These findings suggest no dependence of CD40 mRNA transcription and protein translation efficiency. Jacobson et al. have also suggested the above, who found similar results on unstimulated human B cells (Jacobson et al., 2005). Altogether, our findings support the influence of the −1 C > T CD40 polymorphism on CD40 protein expression and suggest that the presence of the −1T allele also has an impact on protein translation efficiency for the transcripts encoding the CD40 soluble form.
Two main processes generate the sCD40 form: alternative splicing (Eshel et al., 2008; Tone, Tone, Fairchild, Wykes, & Waldmann, 2001) and proteolytic cleavage by the tumor necrosis factor‐α‐converting enzyme (TACE/ADAM‐17) (Contin, Pitard, Itai, et al., 2003). The cleavage and release of CD40 in its soluble form seem to be dependent on cell contact, which could be a mechanism for regulating B cell activation (van Kooten et al., 1994). Chatzigeorgiou et al. (Chatzigeorgiou et al., 2010) reported increased levels of sCD40 in plasma and urine of Type 1 Diabetes mellitus (T1DM) patients compared with controls. Also, the membrane CD40 expression in peripheral blood mononuclear cells was correlated with plasma sCD40 levels, suggesting a relation between sCD40 expression and the inflammatory process. It has been reported that the soluble form of CD40 can inhibit immunoglobulin production by CD154‐activated B lymphocytes in vitro, which suggests that the soluble form can alter CD40/154 interaction, whence sCD40 is considered a decoy receptor (Contin, Pitard, Delmas, et al., 2003).
In this study, the mRNA and protein CD40 expression in SLE patients showed no difference in comparison with CS, which is similar to what was observed in other studies (Chen et al., 2015; Wu et al., 2016). Since CD40 has an essential role in B cell activation and differentiation and thus in plasma cell production, CD40 expression was evaluated according to SLE disease activity. SLE patients with severe disease activity showed a trend toward increased sCD40 levels, which was not statistically significant. The treatment of the SLE patients could explain the above since most of them were under conventional treatment with prednisone and immunosuppressive drugs. Our findings suggest that despite treatment, there is a higher production of sCD40 in active lupus; considering the regulatory role attributed to sCD40, further studies addressing this concerning its production in active inflammatory diseases are needed.
The sCD40 levels were positively correlated with SLICC damage index; besides the type of damage was investigated in our SLE patients. A negative correlation of sCD40 levels with the eGFR was found, SLE patients with a KDIGO stage III and IV as well as those with chronic kidney disease showed higher sCD40 levels. Previously, it has been detected an increase of the soluble form of CD40 in serum from patients with uremic and impaired renal function, which was correlated with creatinine levels in uremic non‐hemodialyzed patients (Contin, Pitard, Delmas, et al., 2003; Schwabe, Engelmann, Hess, & Fricke, 1999). Similar to our findings, Xie et al. found a negative correlation of sCD40 form with declines in eGFR in a chronic kidney disease cohort (Xie et al., 2017). Since the expression of CD40 is not restricted to B cells, it could be important to determine if, in SLE patients with chronic kidney disease, other cell types can produce sCD40. In particular the expression of CD40 has been reported on renal parenchymal and tubular epithelial cells, also sCD40 levels has been associated with renal fibrosis and local inflammatory (Gaweco, Mitchell, Lucas, McClatchey, & Van Thiel, 1999; H. Li & Nord, 2009; Starke, Wüthrich, & Waeckerle‐Men, 2007). Majority of SLE patient develop renal involvement, and the autoantibodies play a key role in the pathogenesis. The release of soluble CD40 form could reflect a mechanism for regulating B cell activation and autoantibody production induced by T cell interaction or might be a control system to regulate damage in a target tissue. To the best of our knowledge, this is the first study that reported an association of sCD40 levels with damage, particularly impaired renal function in SLE patients, however additional studies in a prospective design should be considered to define the role of the soluble CD40 form in the course of SLE.
In conclusion, the CD40 polymorphisms have an important role as genetic markers to SLE susceptibility in the western Mexican population. Also, higher sCD40 levels were associated with damage in SLE, particularly with impaired renal function. Although further studies are still necessary to get a better understanding of the role in SLE, the soluble levels of CD40 receptor could be considered as a potential marker of chronic kidney disease.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by PRO‐SNI 2017‐2018 grant to CAPS from Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara.
Tapia‐Llanos R, Muñoz‐Valle JF, Román‐Fernández IV, et al. Association of soluble CD40 levels with ‐1 C > T CD40 polymorphism and chronic kidney disease in systemic lupus erythematosus. Mol Genet Genomic Med. 2019;7:e1014 10.1002/mgg3.1014
REFERENCES
- Blanco‐Kelly, F. , Matesanz, F. , Alcina, A. , Teruel, M. , Díaz‐Gallo, L. M. , Gómez‐García, M. , … Urcelay, E. (2010). CD40: Novel association with Crohn's disease and replication in multiple sclerosis susceptibility. PLoS ONE, 5(7), 12–15. 10.1371/journal.pone.0011520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha, S. , Miller, K. , Farwell, L. , Lightstone, L. B. , Daly, M. J. , Rioux, J. D. , & Vyse, T. J. (2005). Haplotype structure of TNFRSF5‐TNFSF5 (CD40‐CD40L) and association analysis in systemic lupus erythematosus. European Journal of Human Genetics, 13(5), 669–676. 10.1038/sj.ejhg.5201367 [DOI] [PubMed] [Google Scholar]
- Chatzigeorgiou, A. E. , Lembessis, P. E. , Mylona‐Karagianni, C. F. , Tsouvalas, E. A. , Diamanti‐Kandarakis, E. , & Kamper, E. F. (2010). CD40 expression and its association with low‐grade inflammation in a greek population of type 1 diabetic juveniles: Evidence for differences in CD40 mRNA isoforms expressed by peripheral blood mononuclear cells. Experimental and Clinical Endocrinology and Diabetes, 118(1), 38–46. 10.1055/s-0029-1224151 [DOI] [PubMed] [Google Scholar]
- Chen, J.‐M. , Guo, J. , Wei, C.‐D. , Wang, C.‐F. , Luo, H.‐C. , Wei, Y.‐S. , & Lan, Y. (2015). The association of CD40 polymorphisms with CD40 serum levels and risk of systemic lupus erythematosus. BMC Genetics, 16(1), 121 10.1186/s12863-015-0279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski, P. , & Sacchi, N. (1987). Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Analytical Biochemistry, 162(1), 156–159. 10.1006/abio.1987.9999 [DOI] [PubMed] [Google Scholar]
- Contin, C. , Pitard, V. , Delmas, Y. , Pelletier, N. , Defrance, T. , Moreau, J. F. , & Déchanet‐Merville, J. (2003). Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology, 110(1), 131–140. 10.1046/j.1365-2567.2003.01716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contin, C. , Pitard, V. , Itai, T. , Nagata, S. , Moreau, J. F. , & Déchanet‐Merville, J. (2003). Membrane‐anchored CD40 is processed by the tumor necrosis factor‐α‐converting enzyme: Implications for CD40 signaling. Journal of Biological Chemistry, 278(35), 32801–32809. 10.1074/jbc.M209993200 [DOI] [PubMed] [Google Scholar]
- Elgueta, R. , Benson, M. J. , de Vries, V. C. , Wasiuk, A. , Guo, Y. , & Noelle, R. J. (2009). Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunological Reviews, 229(1), 152–172. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel, D. , Toporik, A. , Efrati, T. , Nakav, S. , Chen, A. , & Douvdevani, A. (2008). Characterization of natural human antagonistic soluble CD40 isoforms produced through alternative splicing. Molecular Immunology, 46(2), 250–257. 10.1016/j.molimm.2008.08.280 [DOI] [PubMed] [Google Scholar]
- Field, J. , Shahijanian, F. , Schibeci, S. , Johnson, L. , Gresle, M. , Laverick, L. , … Booth, D. (2015). The MS Risk Allele of CD40 Is Associated with Reduced Cell‐Membrane Bound Expression in Antigen Presenting Cells: Implications for Gene Function. PLoS ONE, 10(6), e0127080 10.1371/journal.pone.0127080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Bermúdez, M. , González‐Juanatey, C. , López‐Mejías, R. , Teruel, M. , Corrales, A. , Miranda‐Filloy, J. A. , & González‐Gay, M. A. (2012). Study of Association of CD40‐CD154 Gene Polymorphisms with Disease Susceptibility and Cardiovascular Risk in Spanish Rheumatoid Arthritis Patients. PLoS ONE, 7(11), 10.1371/journal.pone.0049214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaweco, A. S. , Mitchell, B. L. , Lucas, B. A. , McClatchey, K. D. , & Van Thiel, D. H. (1999). CD40 expression on graft infiltrates and parenchymal CD154 (CD40L) induction in human chronic renal allograft rejection. Kidney International, 55(4), 1543–1552. 10.1046/j.1523-1755.1999.00379.x [DOI] [PubMed] [Google Scholar]
- Gladman, D. , Ginzler, E. , Goldsmith, C. , Fortin, P. , Liagn, M. , Sanchez‐Guerrero, J. , … Zoma, A. (1996). The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and Rheumatism, 39(3), 363–369. 10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- Guzmán, J. , Cardiel, M. H. , Arce‐Salinas, A. , Sánchez‐Guerrero, J. , & Alarcón‐Segovia, D. (1992). Measurement of disease activity in systemic lupus erythematosus. Prospective validation of 3 clinical indices. The Journal of Rheumatology, 19(10), 1551–1558. [PubMed] [Google Scholar]
- Hochberg, M. C. (1997). Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism, 40(9), 1725 [DOI] [PubMed] [Google Scholar]
- Jacobson, E. M. , Concepcion, E. , Oashi, T. , & Tomer, Y. (2005). A Graves’ disease‐associated Kozak sequence single‐nucleotide polymorphism enhances the efficiency of CD40 gene translation: A case for translational pathophysiology. Endocrinology, 146(6), 2684–2691. 10.1210/en.2004-1617 [DOI] [PubMed] [Google Scholar]
- Joo, Y. B. , Park, B. L. , Shin, H. D. , Park, S. Y. , Kim, I. , & Bae, S. C. (2013). Association of genetic polymorphisms in CD40 with susceptibility to SLE in the Korean population. Rheumatology, 52(4), 623–630. 10.1093/rheumatology/kes339 [DOI] [PubMed] [Google Scholar]
- Kozak, M. (1987). An analysis of 5’‐noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Research, 15(20), 8125–8148. 10.1093/nar/15.20.8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak, M. (2002). Emerging links between initiation of translation and human diseases. Mammalian Genome, 13(8), 401–410. 10.1007/s00335-002-4002-5 [DOI] [PubMed] [Google Scholar]
- Li, H. , & Nord, E. P. (2009). IL‐8 amplifies CD40/CD154‐mediated ICAM‐1 production via the CXCR‐1 receptor and p38‐MAPK pathway in human renal proximal tubule cells. American Journal of Physiology ‐ Renal Physiology, 296(2), F438–F445. 10.1152/ajprenal.90214.2008 [DOI] [PubMed] [Google Scholar]
- Li, M. , Sun, H. , Liu, S. , Yu, J. , Li, Q. , Liu, P. , & Sun, D. (2012). CD40 C/T‐1 polymorphism plays different roles in Graves' disease and Hashimoto's thyroiditis: A meta‐analysis. Endocrine Journal, 59(12), 1041–1050. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Zhang, Z. , He, Z. , Tang, W. , Li, T. , Zeng, Z. , & Shi, Y. (2009). A partition‐ligation‐combination‐subdivision EM algorithm for haplotype inference with multiallelic markers : Update of the SHEsis (http://analysis.bio‐x.cn). Cell Research, 19(4), 519–523. 10.1038/cr.2009.33. [DOI] [PubMed] [Google Scholar]
- Loskog, A. S. I. , & Eliopoulos, A. G. (2009). The Janus faces of CD40 in cancer. Seminars in Immunology, 21(5), 301–307. 10.1016/j.smim.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Martínez‐Cortés, G. , Salazar‐Flores, J. , Fernández‐Rodríguez, L. G. , Rubi‐Castellanos, R. , Rodríguez‐Loya, C. , Velarde‐Félix, J. S. , & Rangel‐Villalobos, H. (2012). Admixture and population structure in Mexican‐Mestizos based on paternal lineages. Journal of Human Genetics, 57(9), 568–574. 10.1038/jhg.2012.67 [DOI] [PubMed] [Google Scholar]
- Miller, S. , Dykes, D. , & Polesky, H. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research, 16(3), 1215 10.1093/nar/16.3.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrackova, A. , Smrzova, A. , Gajdos, P. , Schubertova, M. , Schneiderova, P. , Kromer, P. , & Kriegova, E. (2017). Serum protein pattern associated with organ damage and lupus nephritis in systemic lupus erythematosus revealed by PEA immunoassay. Clinical Proteomics, 14(1), 1–15. 10.1186/s12014-017-9167-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W. , Horgan, G. W. , & Dempfle, L. (2002). Relative expression software tool (REST) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Research, 30(9), e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski, P. , Lianeri, M. , Wudarski, M. , Olesińska, M. , & Jagodziński, P. P. (2013). Single nucleotide polymorphism of CD40 region and the risk of systemic lupus erythematosus. Lupus, 22(3), 233–237. 10.1177/0961203312470184 [DOI] [PubMed] [Google Scholar]
- Rahman, A. , & Isenberg, D. A. (2008). Systemic lupus erythematosus. The New England Journal of Medicine, 358(9), 929–939. 10.1056/NEJMra071297 [DOI] [PubMed] [Google Scholar]
- Raychaudhuri, S. , Remmers, E. F. , Lee, A. T. , Hackett, R. , Guiducci, C. , Burtt, N. P. , & Plenge, R. M. (2008). Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nature Genetics, 40(10), 1216–1223. 10.1038/ng.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román‐Fernández, I. V. , Ávila‐Castillo, D. F. , Cerpa‐Cruz, S. , Gutiérrez‐Ureña, S. , Hernández‐Bello, J. , Padilla‐Gutiérrez, J. R. , & Muñoz‐Valle, J. F. (2016). CD40 functional gene polymorphisms and mRNA expression in rheumatoid arthritis patients from western Mexico. Genetics and Molecular Research, 15(4), 10.4238/gmr15048775 [DOI] [PubMed] [Google Scholar]
- Román‐Fernández, I. V. , Muñoz‐Valle, J. F. , & Palafox‐Sánchez, C. A. (2019). Letter to the editor: “The association of CD40 polymorphism (rs1883832C/T) and soluble CD40 with the risk of systemic lupus erythematosus among Egyptian patients”. Clinical Rheumatology, 38(5), 1529–1530. 10.1007/s10067-019-04491-8 [DOI] [PubMed] [Google Scholar]
- Schwabe, R. F. , Engelmann, H. , Hess, S. , & Fricke, H. (1999). Soluble CD40 in the serum of healthy donors, patients with chronic renal failure, haemodialysis and chronic ambulatory peritoneal dialysis (CAPD) patients. Clinical and Experimental Immunology, 117(1), 153–158. 10.1046/j.1365-2249.1999.00935.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. Y. , & He, L. (2005). SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Research, 15(2), 97–98. 10.1038/sj.cr.7290272 [DOI] [PubMed] [Google Scholar]
- Skibola, C. F. , Nieters, A. , Bracci, P. M. , Curry, J. D. , Agana, L. , Skibola, D. R. , & Holly, E. A. (2008). A functional TNFRSF5 gene variant is associated with risk of lymphoma. Blood, 111(8), 4348–4354. 10.1182/blood-2007-09-112144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova, E. A. , Malkova, N. A. , Korobko, D. S. , Rozhdestvenskii, A. S. , Kakulya, A. V. , Khanokh, E. V. , & Filipenko, M. L. (2013). Association of SNPs of CD40 Gene with Multiple Sclerosis in Russians. PLoS ONE, 8(4), 1–8. 10.1371/journal.pone.0061032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke, A. , Wüthrich, R. P. , & Waeckerle‐Men, Y. (2007). TGF‐beta treatment modulates PD‐L1 and CD40 expression in proximal renal tubular epithelial cells and enhances CD8+ cytotoxic T‐cell responses. Nephron ‐ Experimental Nephrology, 107(1), 22–30. 10.1159/000106506 [DOI] [PubMed] [Google Scholar]
- Teruel, M. , Simeon, C. P. , Broen, J. , Vonk, M. C. , Carreira, P. , Camps, M. T. , & Román‐Ivorra, J. A. (2012). Analysis of the association between CD40 and CD40 ligand polymorphisms and systemic sclerosis. Arthritis Research and Therapy, 14(3), R154 10.1186/ar3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone, M. , Tone, Y. , Fairchild, P. J. , Wykes, M. , & Waldmann, H. (2001). Regulation of CD40 function by its isoforms generated through alternative splicing. Proceedings of the National Academy of Sciences, 98(4), 1751–1756. 10.1073/pnas.98.4.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubi, E. , & Shoenfeld, Y. (2004). The role of CD40‐CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity, 37(6–7), 457–464. 10.1080/08916930400002386 [DOI] [PubMed] [Google Scholar]
- Tsokos, G. C. (2011). Systemic Lupus Erythematosus. New England Journal of Medicine, 365(22), 2110–2121. 10.1056/NEJMra1100359 [DOI] [PubMed] [Google Scholar]
- van Kooten, C. , Gaillard, C. , Galizzi, J. P. , Hermann, P. , Fossiez, F. , Banchereau, J. , & Blanchard, D. (1994). B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. European Journal of Immunology, 24(4), 787–792. 10.1002/eji.1830240402 [DOI] [PubMed] [Google Scholar]
- Vazgiourakis, V. M. , Zervou, M. I. , Choulaki, C. , Bertsias, G. , Melissourgaki, M. , Yilmaz, N. , & Goulielmos, G. N. (2011). A common SNP in the CD40 region is associated with systemic lupus erythematosus and correlates with altered CD40 expression: Implications for the pathogenesis. Annals of the Rheumatic Diseases, 70(12), 2184–2190. 10.1136/ard.2010.146530 [DOI] [PubMed] [Google Scholar]
- Wagner, M. , Wisniewski, A. , Bilinska, M. , Pokryszko‐Dragan, A. , Cyrul, M. , Kusnierczyk, P. , & Jasek, M. (2014). Investigation of gene‐gene interactions between CD40 and CD40L in Polish multiple sclerosis patients. Human Immunology, 75(8), 796–801. 10.1016/j.humimm.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Wang, D. , Chen, J. , Zhang, H. , Zhang, F. , Yang, L. , & Mou, Y. (2017). Role of Different CD40 Polymorphisms in Graves' Disease and Hashimoto's Thyroiditis. Immunological Investigations, 46(6), 544–551. 10.1080/08820139.2017.1319382 [DOI] [PubMed] [Google Scholar]
- Wu, C. J. , Guo, J. , Luo, H. C. , Wei, C. D. , Wang, C. F. , Lan, Y. , & Wei, Y. S. (2016). Association of CD40 polymorphisms and haplotype with risk of systemic lupus erythematosus. Rheumatology International, 36(1), 45–52. 10.1007/s00296-015-3345-7 [DOI] [PubMed] [Google Scholar]
- Xie, J. X. , Alderson, H. , Ritchie, J. , Kalra, P. A. , Xie, Y. , Ren, K. , & Haller, S. T. (2017). Circulating CD40 and sCD40L Predict Changes in Renal Function in Subjects with Chronic Kidney Disease. Scientific Reports, 7(1), 10.1038/s41598-017-08426-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


