Abstract
The objective of this article was to provide an account of some of the developments related to saliva over the first 100 years of the Journal of Dental Research and to outline some of the many biomarkers identified in saliva in the last few years. The first section covers findings in salivary physiology, biochemistry, calcium phosphate chemistry related to saliva, microbiology, and the role of saliva in maintaining oral health. The second section highlights salivary diagnostics, salivaomics, and saliva exosomics in the context of the emerging theme of personalized and precision medicine.
Keywords: parotid, submandibular/sublingual, exosomics, proteomics, salivaomics, transcriptomics
Salivary Physiology
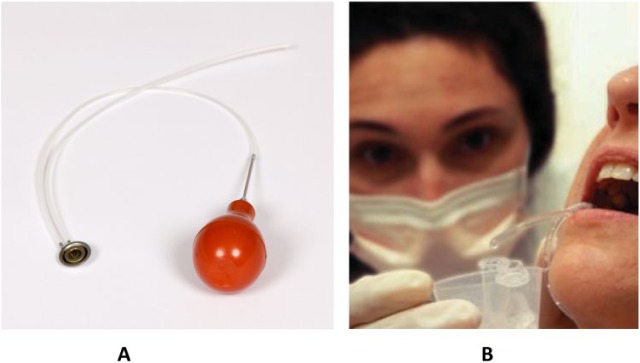
Prior to the 20th century, little was known about human saliva physiology. However, it was known that saliva contained amylase, and the parasympathetic and sympathetic nerve supplies to most of the salivary glands had been determined, primarily by studies on animals. A few years before the first issue of the Journal of Dental Research in 1919, Carlson and Crittenden (1910) developed a collection device for parotid saliva (Fig. 1), which allowed the study of secretion from an individual salivary gland. However, it was not until 1955 that Schneyer developed a device for collection of submandibular and sublingual saliva, and an improved version was described by Truelove et al. (1967). The composition of secretions from minor salivary glands of the lips was first described by Dawes and Wood (1973). Veerman et al. (1996) collected and compared the compositions of stimulated parotid, submandibular, sublingual, and palatine secretions. A major stimulus for salivary physiology research, although primarily in animals, was the monograph by Burgen and Emmelin (1961).
Figure 1.
Collection device for parotid saliva: (A) a modern version of the Carlson-Crittenden device, which can be used for collection of parotid saliva from the left or right gland; (B) clinical collection of parotid fluid (courtesy of the National Institute of Dental and Craniofacial Research).
Two pioneers who studied variations in flow rate and calcium and phosphate concentrations in human whole saliva were Becks and Wainwright, who published a series of articles in the Journal of Dental Research in the 1930s and 1940s. Their 1943 paper on the normal unstimulated flow rate of whole saliva is still widely quoted, and others have since confirmed their finding that about 10% of the population has an unstimulated salivary flow rate ≤0.1 mL/min, whereas the mean value in the population is 0.3 to 0.4 mL/min. However, flow rate is virtually zero during sleep (Schneyer et al. 1956).
An important study by Thaysen et al. (1954) on 3 young women showed that the concentrations of the main electrolytes (sodium, potassium, bicarbonate, and chloride) in parotid saliva elicited by beta-methyl-acetyl-choline were very dependent on flow rate. Since this is a key factor influencing saliva composition, development of a negative-feedback technique (Dawes 1967) for maintaining a constant stimulated flow rate, up to the physiologic limit of the gland, allowed study of the effects of other physiologic variables—such as flow rate itself, duration of stimulation, nature of the stimulus, circadian rhythms, previous stimulation, exercise, and stop-flow conditions—on human salivary composition.
Because of variation in nomenclature used in different branches of salivary research, a group of researchers in the field recommended a standard nomenclature (Atkinson et al. 1993), which seems to have been generally accepted.
The mechanisms by which salivary glands secrete electrolytes from plasma into saliva are rather complex, but a recent mathematical model (Vera-Sigűenza et al. 2018) appears to fit the theoretical processes and the experimental data quite well. Several neurotransmitters—including acetyl choline, norepinephrine, vasoactive intestinal peptide, substance P, and nitric oxide—act as transmitters in salivary secretion (Pedersen et al. 2018), but there is still much to be learned about the factors influencing the superior and inferior salivary nuclei in the pons and medulla. The mechanisms involved in secretion of protein by pancreatic cells (which also apply to salivary acini) were described by Jamieson and Palade (1967a, 1967b).
Salivary Biochemistry
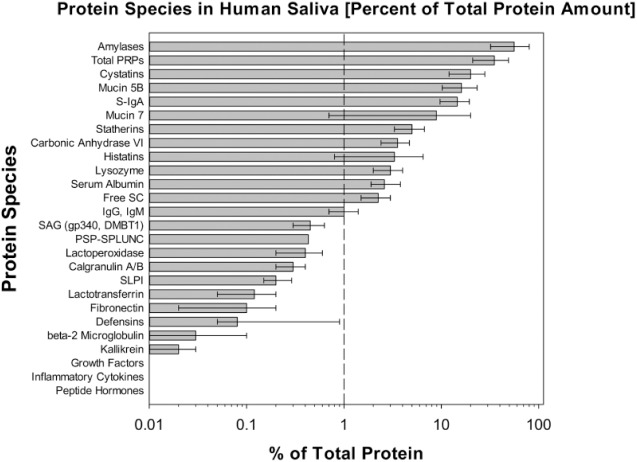
Studies on the structure, composition, and function of the different proteins and glycoproteins in saliva began later than those on salivary electrolytes and made little progress until the development of separating media such as Sephadex, polyacrylamide gel electrophoresis, and advances in molecular biology, mass spectrometry, and protein databases. Important contributors with respect to proteins include Mandel et al. (1965), Azen and Oppenheim (1973), Schlesinger and Hay (1977), Bennick (1987), Kauffman et al. (1986), Oppenheim et al. (1988), and Levine (1993) for individual salivary proteins and Tabak (1995) for studies on salivary mucins. The acquired enamel pellicle was initially thought to contain only salivary proteins but has now been found to contain 123 proteins, and, surprisingly, only 14% of these are of salivary origin (Siqueira et al. 2007). In contrast, using proteomics, Denny et al. (2008) detected 914 proteins in parotid saliva and 917 in submandibular/sublingual saliva, the majority being in very low concentrations. Ruhl (2012; Fig. 2) listed the estimated mean concentrations of the 23 most abundant proteins (0.001% to 60% of the total protein amount) in whole saliva. The 9 in highest concentration, forming >90% of total protein, are synthesized in the salivary glands. However, the number of proteins and glycoproteins in whole saliva, mostly at minute concentrations, is up to 3,000, as whole saliva also contains proteins derived from desquamated epithelial cells, crevicular fluid, and the oral microbiome.
Figure 2.
Relative abundancies of protein and peptide species in human saliva. Reproduced from Ruhl (2012) by kind permission.
Calcium Phosphate Studies
Although there are 6 types of calcium phosphate, which can form under different conditions, only 4 occur in dental calculus, and only 1, an impure hydroxyapatite, is found in enamel and dentine.
Important considerations are the factors influencing the solubility of enamel, which relates to both dental caries and enamel erosion. Some of the first quantitative studies were those of Ericsson (1949), when enamel was already known to have an apatite structure with a basic formula of Ca10(PO4)6(OH)2. However, enamel is not a pure hydroxyapatite but contains traces of other substances, such as magnesium, bicarbonate, and fluoride, the last of which tends to reduce its solubility. Larsen and Bruun (1986) discussed the complex interactions between enamel and saliva. An important clinical question is the value of the so-called critical pH, which is the saliva pH at which enamel starts to dissolve. This question relates primarily to enamel erosion, and a salivary pH below about 5.5 will initiate this process, depending on the calcium and phosphate concentrations in saliva. Above that pH, saliva is supersaturated with respect to tooth mineral. A new acquired enamel pellicle forms immediately after a tooth has been eroded with acid, such as that in a typical soft drink, which makes erosion irreversible. Since caries occurs only under a layer of dental plaque and as the fluid phase of plaque contains a higher phosphate concentration than does saliva (Tatevossian and Gould 1976), the critical pH for development of caries (about 5.2) is less than that for erosion. Plaque pH may fall to as low as 4.0 after consumption of carbohydrates such as glucose (Fig. 3; Stephan 1944) or sucrose. Saliva is normally supersaturated with respect to 4 of the 6 calcium phosphates: hydroxyapatite, brushite, β-tricalcium phosphate, and octacalcium phosphate. Thus, there is a tendency for these to precipitate out in dental plaque as calculus, as discussed by ten Cate (2012). Patients with renal disease often develop high levels of urea in their saliva, and this can be converted to ammonia in dental plaque. The resulting high pH in their plaque makes these people particularly susceptible to calculus formation because of the increased degree of saturation with the various calcium phosphates.
Figure 3.
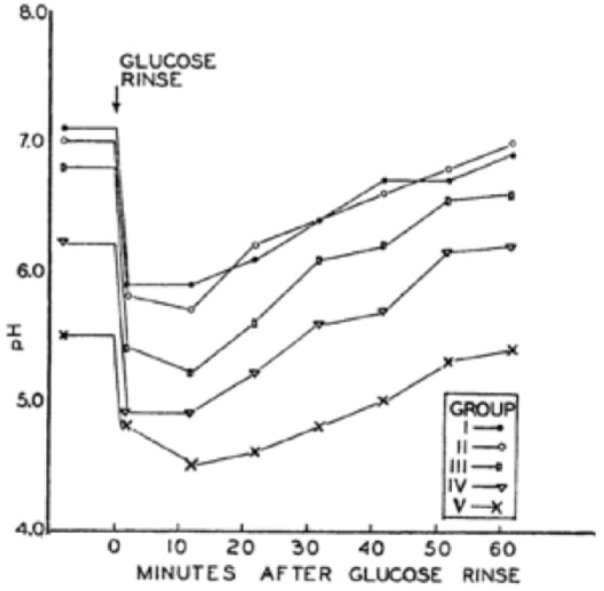
A copy of Figure 1 from the article by Stephan (1944) shows the decrease and subsequent increase in plaque pH after a glucose rinse (the famous Stephan curve). Permission from the Journal of Dental Research.
Salivary Microbiology
One of the most influential books on the importance of frequent removal of dental plaque (the dental biofilm) for prevention of caries and periodontal disease is probably that by W. D. Miller (1890), a fascinating book available online (https://archive.org/details/microorganismsof00mill). The essence of a biofilm is that the bacteria that it contains can attach to a surface and to one another. The bacteria in a biofilm exhibit quorum sensing and were found to share proteases and glycosidases, making the metabolism of the biofilm more efficient than in single microorganisms. Over most of the years since 1919, individual bacteria in the mouth could be studied only if they could be cultured. It was just a decade ago that genomic sequencing became available and the human oral microbiome database was described by Dewhirst et al. (2010). It is now known that the mouth contains nearly 700 prevalent taxa, a third of which cannot yet be cultured, in a range of 13 phyla. Useful updates on oral microbiology are those by Kilian et al. (2016) and Sanz et al. (2017).
Although Streptococcus mutans had been detected in the mouth as early as 1924 (Clarke), it was in the later years of the last century that it was thought to be the main cause of dental caries (Loesche 1979), but even today there is uncertainty about its importance (Banas and Drake 2018). Its advantage with respect to caries is that, not only can it convert sucrose to lactic acid to create a pH of 4.0 or slightly less, but it can also form intra- and extracellular polysaccharides, which can later be converted to acid; furthermore, it can metabolize sucrose at a much lower pH (4.0) than most other oral microorganisms. The many deleterious oral effects of sucrose in our diets were recently outlined by Sheiham and James (2015).
Dental plaque may also initiate periodontal disease as well as caries, and an important advance was made by Löe et al. (1965) showing that gingivitis could be produced experimentally by cessation of all oral hygiene measures for 4 wk (in dental students!), followed by recovery over a similar length of time after renewal of oral hygiene measures. The proteins and glycoproteins in saliva are the main sources of nutrition for most oral microorganisms, but the ones in deep periodontal pockets have access to gingival crevicular fluid, which facilitates the growth of proteolytic and obligately anaerobic bacteria. Their production of inflammatory factors causes exudation of blood into the gingival crevice, which promotes further growth of periodontopathic microorganisms. Theilade (1986) argued that different combinations of indigenous microorganisms, rather than a single species, cause the transition from gingivitis to periodontitis.
It was realized recently that a major function of the indigenous oral microflora is to occupy sites that would otherwise be available to pathogenic microorganisms. Xu et al. (2015) found that the mouth contains 8 sites with site-specific microflora—namely teeth, gingival sulcus, attached gingivae, tongue, cheek, lip, and hard and soft palate—perhaps because saliva in the mouth is present as a thin film (<0.1 mm; Collins and Dawes 1987) and secretions from the different glands do not mix well. Saliva was also found to contain many antibacterial components, such as secretory IgA, lactoferrin, lactoperoxidase, lysozyme, statherin, and histatins.
Saliva and Oral Health
Mandel (1979) and Tabak (2006) both discussed the roles of saliva in protecting the oral cavity. A low unstimulated flow rate not only makes a person susceptible to xerostomia but greatly delays clearance of food from the mouth. The main buffers in saliva are bicarbonate, the concentration of which is proportional to flow rate (Thaysen et al. 1954) and, to a lesser extent, phosphate. Thus, people with a low unstimulated salivary flow rate are particularly susceptible to dental caries because of the low buffering capacity of their saliva and the low clearance rate for food debris. According to an accepted model of salivary clearance (Dawes 1983; Lagerlöf and Dawes 1984), high values of 2 other factors—namely, Vmax (the volume of saliva in the mouth before swallowing is elicited) and Resid (the volume left in the mouth after swallowing)—delay clearance considerably.
Unfortunately, the roles of saliva in maintaining good oral health receive very little attention in most dental schools, particularly in the clinical years, possibly because in all areas of clinical dentistry, the presence of saliva is a great nuisance that can interfere with tooth assessment, preparation, or restoration, as well as impression taking, cementation (crowns, inlays, bridges, orthodontic bands), endodontics, and oral surgery. However, since dentists are encouraged to inform patients of their disease susceptibility, a group with expertise in oral medicine (Wolf et al. 2017) recommended that all dental schools teach their students how to measure a patient’s unstimulated salivary flow rate so that those with low values can receive greater emphasis on prevention of caries and periodontal disease. Since unstimulated flow rate does not change much with age, this measurement needs to be made only once, takes no more than 15 min, and, in a general dental practice, could be delegated to a dental assistant.
At present, only in Sweden are dental students taught to measure the unstimulated salivary flow rate, but the failure in other countries seems to us to be equivalent to a medical student never being taught to check a patient’s weight or blood pressure as a measure of disease susceptibility!
Translation of Saliva Research toward Personalized Precision Medicine/Dentistry
Saliva as a diagnostic tool is now of public interest, as it can be used 1) to detect at least 24 drugs, including cocaine, ethanol, and MDMA (i.e., ecstasy; Simonsen et al. 2012); 2) viruses, such as those for HIV, hepatitis C, and human papilloma virus infection; and 3) DNA for genetic analysis (Regalado, 2018).
The year 2004 marked a defining moment in saliva research. The National Institute of Dental and Craniofacial Research (NIDCR), under the leadership of Lawrence Tabak, issued 2 landmark initiatives to decipher the human salivary proteome and develop point-of-care microfluidic devices that can assay particular proteins for personalized precision medicine applications.
The Human Salivary Proteome initiative was launched to elucidate disease pathogenesis and evaluate the influence of medications on the different proteins of saliva. The 3 NIDCR-supported programs—Susan Fisher, University of California–San Francisco; John Yates, Scripps Research Institute; and David Wong, University of California–Los Angeles (UCLA)—formed a consortium to identify all of the peptides and proteins in salivary fluid and gain knowledge of their functions. A landmark work was published by this consortium in 2008 identifying, cataloging, and annotating 1,166 proteins in human saliva, constituting the first saliva diagnostic alphabet (Denny et al. 2008).
The NIDCR-catalyzed point-of-care initiatives had 2 cycles of funding to develop microfluidics and microelectrical mechanical devices for salivary diagnostics. Various innovative technologies were advocated by the final 4 groups: John McDervitt, University of Texas at Austin; Daniel Malamud, University of Pennsylvania and New York University; David Watt, Tufts University; and David Wong, UCLA (Wei et al. 2009; Chen et al. 2010; Miller et al. 2010; Nie et al. 2014). The anticipated outcome of this initiative is the commercialization of saliva-based diagnostic point-of-care technologies optimized for the detection of highly sensitive and specific salivary biomarkers for human diseases. These visionary NIDCR initiatives for saliva research, while still in progress, laid the foundation for personalized precision medicine that will affect clinical practice in the next decade.
Salivaomics
Salivary Genomics
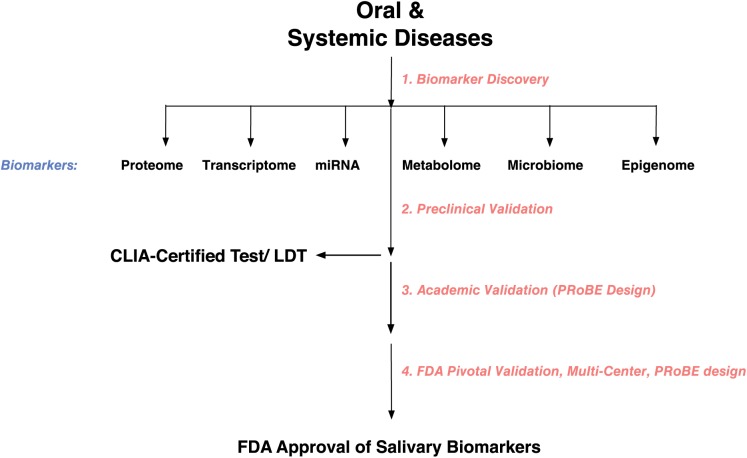
Salivaomics is the integrative study of saliva and its constituents, functions, and related techniques (Ai et al. 2010, 2012; Fig. 4). Saliva is not a homogeneous fluid: genomics, epigenomics, transcriptomics, proteomics, metabolomics, and microbiomics are the key components that make up salivaomics. The 3 major -omic groups include circulating DNA (genomics), RNA (transcriptomics), and protein (proteomics).
Figure 4.
Salivary biomarker development. CLIA, Clinical Laboratory Improvement Amendments; FDA, Food and Drug Administration; LDT, laboratory development test; PRoBE: prospective specimen collection and retrospective blinded evaluation.
Saliva contains cell-free DNA, of which 70% originates from the host and 30% from the oral microbiota (Rylander-Rudqvist et al. 2006). Salivary DNA is stable and the quality relatively high (Hansen et al. 2007; Bonne and Wong 2012; Looi et al. 2012), suggesting that salivary DNA is a useful target for the development of biomarkers.
The presence of the host genome in saliva has been exploited to access personal genomic information pertaining to ancestry, health, and wellness. Companies (23andMe, Ancestry Health, National Geographic) harness the host genomic content in saliva and next-generation sequencing (NGS) to reveal genomic variants (single-nucleotide polymorphisms) with risk of health conditions (cancer risks such as BRCA1 and BRCA2) and ancestry information (Regalado 2018).
Circulating tumor DNA (ctDNA) is cell-free DNA, 180 to 200 base pairs in length, that is shed from tumor cells into the circulatory system (Jahr et al. 2001; Diehl et al. 2005, 2008; Fan et al. 2008; Mouliere et al. 2011; Diaz and Bardelli 2014). ctDNA has been detected in a number of body fluids, including blood, urine, and saliva (Wei et al. 2014; Patel and Tsui 2015), and it can be distinguished from normal cell-free DNA by the presence of mutations. The clinical testing of ctDNA in body fluids is referred to as liquid biopsy (Overman et al. 2013; Haber and Velculescu 2014). The most common technologies for the detection of ctDNA are NGS and digital polymerase chain reaction (Chaudhuri et al. 2015; Ignatiadis et al. 2015). However, a simple, compact, and more cost-effective analysis with high levels of sensitivity and specificity is desirable to facilitate routine clinical care. An electrochemical technology developed by the UCLA group, termed electric field–induced release and measurement (EFIRM), found a unique entry point into liquid biopsy (Wei et al. 2014). EFIRM detects the 2 front-line actionable epidermal growth factor receptor (EGFR) mutations, exon 19 deletion and L858R mutations, with receiver operating characteristic curve analysis and area under the curve of 0.94 and 0.96, respectively, suggesting that saliva-based EFIRM liquid biopsy has superior performance than digital droplet polymerase chain reaction (ddPCR) and NGS to detect ctDNA for lung cancer (Wei et al. 2014; Pu et al. 2016).
Salivary Transcriptomics
The transcriptome is the complete set of RNA molecules, including mRNA, microRNA, piwi-interacting RNA, and other small RNAs, such as rRNA and tRNA. Salivary transcriptomics has emerged as a powerful approach for salivary biomarker development (Park et al. 2006; Kaczor-Urbanowicz et al. 2018). Although the genome is the same in all host cells, different cells and body fluids show different patterns of RNA composition; therefore, transcriptomic analysis can provide valuable information about disease states. In 2004, the human salivary transcriptome was first discovered at UCLA with microarray technology (Li, Zhou, et al. 2004). The salivary transcriptome consists of coding and noncoding gene transcripts derived from host and oral microbiota (Beard et al. 2006; Park et al. 2007; Spielmann et al. 2012). In 2009, microRNA was discovered in saliva and characterized as a biomarker for oral cancer detection (Park et al. 2009). More recently, piwi-interacting RNAs in saliva are emerging potential biomarkers for cancers (Bahn et al. 2015). High-throughput RNA sequencing of human saliva from healthy individuals showed expression patterns and levels comparable to those in other body fluids (Spielmann et al. 2012; Bahn et al. 2015).
In 2013, the NIH Common Fund established the Extracellular RNA Communication Consortium (ERCC) to independently validate the use of extracellular RNA. Once thought to exist only within cells, RNA is now known to be exported from cells and to play a role in newly discovered mechanisms of cell-to-cell communication. The ERCC aims 1) to establish fundamental biological principles of extracellular RNA secretion, delivery, and impact on recipient cells; 2) to describe exRNAs in human biofluids and the extent to which nonhuman exRNAs are present; 3) to test the clinical utility of exRNAs; and 4) to provide a data and resource repository for the community at large. The ERCC independently validated salivary exRNA toward the development of salivary exRNA biomarkers for gastric cancer detection. Salivary RNA is now validated and included in the ERCC’s Extracellular RNA Atlas (https://exrna-atlas.org).
Salivary Proteomics
The first attempt at cancer diagnosis with salivary protein was made by Hoerman (1959), who showed that patients with prostate cancer exhibited elevated acid phosphatase enzymatic activity in parotid saliva. In the past decades, there have been marked advances in protein analytic technologies that, combined with bioinformatics, created a new revolution in salivary proteomics.
Comprehensive analysis of the salivary proteome is critical for appreciating its full diagnostic potential. The data set from the study of Denny et al. (2008) has been deposited into the Saliva Proteome Knowledge Base (http://www.skb.ucla.edu/cgi-bin/spkbcgi-bin/main.cgi) and the NIDCR’s Human Saliva Proteome Wiki (https://salivaryproteome.nidcr.nih.gov) for public access (Ai et al. 2010). Bandhakavi et al. (2009) significantly expanded our understanding of the salivary proteome by using 3-dimensional peptide fractionation and generated the largest whole saliva proteome data set, including 2,340 proteins that are involved in a variety of biological functions in the oral cavity. Comparative analysis of the human saliva and plasma proteomes showed that distribution of salivary proteins is enhanced in 2 gene ontology categories (metabolic and catabolic processes) as compared with plasma, suggesting that saliva may be advantageous over plasma, especially for less abundant proteins involved in these biological processes (Loo et al. 2010). In comparison with serum proteins, salivary proteins appear to be more susceptible to degradation (Helmerhorst and Oppenheim 2007; Schulz et al. 2013). Esser et al. (2008) reported that salivary protein can be degraded rapidly even during saliva collection and handling, which may hamper the downstream experiments and application.
Saliva Exosomics
Extracellular vesicles are classified into 3 subgroups (exosomes, microvesicles, and apoptotic bodies) based on their size and biogenetic pathways (Kalra et al. 2012; Simpson et al. 2012). Exosomes are intraluminal vesicles that are formed within multivesicular bodies and released upon fusion of the multivesicular bodies with the cell membrane (Denzer et al. 2000). Exosomes in human saliva were first described by Ogawa et al. (2008). The stability of exosomes in the circulation and body fluids has made exosomes attractive as potential biomarkers. Typically, exosomes are defined as vesicles ranging from 30 to 100 nm in size and 1.13 to 1.19 g/mL in density and are isolated through density gradient or sucrose cushion by ultracentrifugation at 100,000 g (Thery et al. 2006). Exosome isolation from saliva has been optimized (Michael et al. 2010; Lasser et al. 2011), and the use of this small but highly informative fraction may simplify analysis of saliva, currently complicated as a result of contributions from local and systemic sources (Al-Tarawneh et al. 2011). For these reasons, the study of vesicles secreted by cancer cells into saliva will be an interesting approach for the development of discriminatory biomarkers in systemic diseases, including cancer.
Saliva Liquid Biopsy
Liquid biopsy is used to detect actionable mutations in biofluids. The most noted is in lung cancer, where 3 mutations (L858R, exon 19del, and T790M) in the epidermal growth factor receptor (EGFR) gene can be therapeutically targeted to have an impact on the survival of patients with non–small cell lung carcinoma (NSCLC; Wei et al. 2014).
Current clinical practices for detection of signature EGFR ctDNA for NSCLC are ddPCR and NGS, with performance ranges from 60% to 80% concordance with biopsy genotyping (Sholl et al. 2016). In 2 blinded clinical studies, the EFIRM technology detected signature oncogenic EGFR mutations in the plasma and saliva of patients with NSCLC, with near-perfect concordance with biopsy genotyping (96% to 100%; Wei et al. 2014; Pu et al. 2016). Salivary EFIRM liquid biopsy presents superior performance over current liquid biopsy technologies of ddPCR and NGS.
Future Perspectives
In the past decade, salivaomics studies have revealed the translation and clinical utilities of saliva for biomarker development. Note that while saliva biomarkers are considered to mirror those in blood, it is often the contrary. Disease discriminatory biomarkers are often present only in saliva. A good example is the discriminatory panel of exRNA biomarkers for oral cancer detection in saliva (IL-1β, OAZ1, SAT, and IL-8; Li, St John, et al. 2004), which is entirely different from the circulatory panel (ARHA, FTH1, H3F3A, COX4I1, FTL1; Li et al. 2006), with receiver operating characteristic values of 0.95 and 0.88, respectively. In addition, while some biomarkers are present in blood and saliva, the level is often higher in saliva than in blood. Last, when biomarkers are present in blood and saliva, the patient’s preference is usually to donate saliva rather than blood.
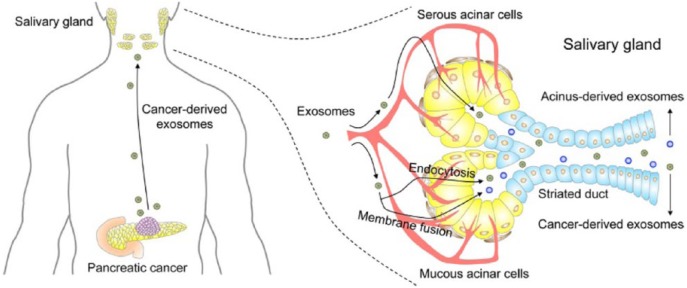
Much progress has been made in understanding characteristics of saliva, with advances in how the salivary constituents relate to biomarkers and functions. The unique properties of cancer-derived exosomes in saliva, which originate from organelles migrating into saliva, could be used as diagnostic biomarkers, potential surrogate markers for other physical conditions, or novel immune regulatory systems through the gastrointestinal tract (Fig. 5; Zhang et al. 2010; Lau et al. 2013). However, the utility of salivary exosomes as biomarkers of diseases and conditions requires much further investigation due to the current paucity of studies in this emerging area. Key future tasks will be to validate salivary exosome biomarkers and determine the molecular mechanisms of exosome interaction between distal tumors and salivary glands. Also, establishing rapid and sensitive technologies to purify and analyze the exosomes will represent important immediate and future challenges.
Figure 5.
The exosome-mediated transfer of cancer-derived products from distal tumor to salivary gland. Cancer-derived exosomes enter the circulation and reach salivary glands. Exosome uptake at salivary gland acinar cells occurs via endocytosis or membrane fusion. Two salivary exosomes are released into saliva. Cancer-derived exosomes are released through exocytosis, while acinus-derived exosomes are released through fusion of multivesicular bodies with the plasma membrane. Both types of salivary exosomes carry cargos that include cancer-derived products. Permission from Elsevier.
Saliva liquid biopsy may soon be the method of choice to detect actionable mutations in patients with lung cancer, as well as monitor treatment efficacy and recurrence in such patients. Other translational and clinical inroads will soon follow, such as screening for KRAS mutations, which occur in 90% of patients with pancreatic cancer.
The next hundred years of saliva and saliva diagnostic research will harness the scientific foundations and new horizons, with a promise toward translating and clinically maturing saliva to benefit oral and systemic health.
Author Contributions
C. Dawes, D.T.W. Wong, contributed to conception, design, data acquisition, analysis and interpretation, drafted, finalized and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We are grateful to Dr. Stefan Ruhl for sending us a top copy of Figure 2. C. Dawes is a consultant to the Wrigley Company. D.T.W. Wong is cofounder of RNAmeTRIX Inc., a molecular diagnostic company. He holds equity in RNAmeTRIX and serves as a company director and scientific advisor. The University of California also holds equity in RNAmeTRIX. Intellectual property that D.T.W. Wong invented and that was patented by the University of California has been licensed to RNAmeTRIX. D.T.W. Wong is consultant to GlaxoSmithKlein, Wrigley, and Colgate-Palmolive.
Footnotes
Funding support to D.T.W. Wong from National Institute of Health (NIH): U01 CA 233370, UH2/UH3 CA206126, UH2/UH3 TR000923, U01 DE17790, UO1 DE16275, U01 DE15018, U01 DE17593, RO1 DE17170, RO1 DE17593, R21 CA17790, TRDRP 21RT-0112 & P20PT-0032, DoD W92XWH-12-1-0330 & UCLA JCCC.
ORCID iD: D.T.W. Wong  https://orcid.org/0000-0002-0290-2206
https://orcid.org/0000-0002-0290-2206
References
- Ai J, Smith B, Wong DT. 2010. Saliva ontology: an ontology-based framework for a salivaomics knowledge base. BMC Bioinformatics. 11:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai JY, Smith B, Wong DT. 2012. Bioinformatics advances in saliva diagnostics. Int J Oral Sci. 4(2):85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tarawneh SK, Border MB, Dibble CF, Bencharit S. 2011. Defining salivary biomarkers using mass spectrometry-based proteomics: a systematic review. OMICS. 15(6):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JC, Dawes C, Ericson T, Fox PC, Gandara BK, Malamud D, Mandel ID, Navazesh M, Tabak LA. 1993. Guidelines for saliva nomenclature and collection. Ann NY Acad Sci. 694:xi–xii. [Google Scholar]
- Azen EA, Oppenheim FG. 1973. Genetic polymorphisms of proline-rich human salivary proteins. Science. 180(1490):1067–1069. [DOI] [PubMed] [Google Scholar]
- Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X. 2015. The landscape of microRNA, piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 61(1):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas JA, Drake DR. 2018. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health. 18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandhakavi S, Stone MD, Onsongo G, Van Riper SK, Griffin TJ. 2009. A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J Proteome Res. 8(12):5590–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Jones BC. 2006. Cellular iron concentrations directly affect the expression levels of norepinephrine transporter in PC12 cells and rat brain tissue. Brain Res. 1092(1):47–58. [DOI] [PubMed] [Google Scholar]
- Becks H, Wainwright WW. 1943. Human saliva: XIII. Rate of flow of resting saliva of healthy individuals. J Dent Res. 22(5):391–396. [Google Scholar]
- Bennick A. 1987. Structural and genetic aspects of proline-rich proteins. J Dent Res. 66(2):457–461. [DOI] [PubMed] [Google Scholar]
- Bonne NJ, Wong DT. 2012. Salivary biomarker development using genomic, proteomic and metabolomic approaches. Genome Med. 4(10):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgen ASV, Emmelin NG. 1961. Physiology of the salivary glands. London (UK): Edward Arnold. [Google Scholar]
- Carlson AJ, Crittenden AL. 1910. The relation of ptyalin concentration to the diet and to the rate of secretion of the saliva. Am J Physiol. 26(1):169–177. [Google Scholar]
- Chaudhuri AA, Binkley MS, Osmundson EC, Alizadeh AA, Diehn M. 2015. Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol. 25(4):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Mauk M, Qiu X, Liu C, Kim J, Ramprasad S, Ongagna S, Abrams WR, Malamud D, Corstjens PL, et al. 2010. An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomed Microdevices. 12(4):705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JK. 1924. On the bacterial factor in the aetiology of dental caries. Brit J Exp Pathol. 5(3):141–147. [Google Scholar]
- Collins LM, Dawes C. 1987. The surface area of the adult human mouth and the thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 66(8):1300–1302. [DOI] [PubMed] [Google Scholar]
- Dawes C. 1967. The effect of flow rate and length of stimulation on the protein concentration in human parotid saliva. Arch Oral Biol. 12(7):783–788. [DOI] [PubMed] [Google Scholar]
- Dawes C. 1983. A mathematical model of salivary clearance of sugar from the oral cavity. Caries Res. 17(4):321–334. [DOI] [PubMed] [Google Scholar]
- Dawes C, Wood CM. 1973. The composition of human lip mucous gland secretions. Arch Oral Biol. 18(3):343–350. [DOI] [PubMed] [Google Scholar]
- Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, Bassillian S, Bedi GS, Boontheung P, Coclorva D, et al. 2008. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 7(5):1994–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 113(19):3365–3374. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade W. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz LA, Jr, Bardelli A. 2014. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 32(6):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, Diaz LA, Jr, Goodman SN, David KA, Juhl H, et al. 2005. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Nat Acad Sci U S A. 102(45):16368–16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al. 2008. Circulating mutant DNA to assess tumor dynamics. Nat Med. 14(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser D, Alvarez-Llamas G, de Vries MP, Weening D, Vonk RJ, Roelofsen H. 2008. Sample stability and protein composition of saliva: implications for its use as a diagnostic fluid. Biomark Insights. 3:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson Y. 1949. Enamel apatite solubility: investigations into the calcium phosphate equilibrium between enamel and saliva and its relation to dental caries. Acta Odont Scand. 8 Suppl 3:1–139. [Google Scholar]
- Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. 2008. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Nat Acad Sci U S A. 105(42):16266–16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber DA, Velculescu VE. 2014. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 4(6):650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TV, Simonsen MK, Nielsen FC, Hundrup YA. 2007. Collection of blood, saliva, and buccal cell samples in a pilot study on the Danish nurse cohort: comparison of the response rate and quality of genomic DNA. Cancer Epidemiol Biomarkers Prev. 16(10):2072–2076. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Oppenheim FG. 2007. Saliva: a dynamic proteome. J Dent Res. 86(8):680–693. [DOI] [PubMed] [Google Scholar]
- Hoerman KC. 1959. On the zone electrophoresis of human parotid saliva in starch gels. J Lab Clin Med. 53(1):64–68. [PubMed] [Google Scholar]
- Ignatiadis M, Lee M, Jeffrey SS. 2015. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 21(21):4786–4800. [DOI] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. 2001. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 61(4):1659–1665. [PubMed] [Google Scholar]
- Jamieson JD, Palade GE. 1967. a. Intracellular transport of secretory proteins in the pancreatic exocrine cell: I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 34(2):577–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JD, Palade GE. 1967. b. Intracellular transport of secretory proteins in the pancreatic exocrine cell: II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 34(2):597–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor-Urbanowicz KE, Kim Y, Li F, Galeev T, Kitchen RR, Gerstein M, Koyano K, Jeong SH, Wang X, Elashoff D, et al. 2018. Novel approaches for bioinformatic analysis of salivary rna sequencing data for development. Bioinformatics. 34(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borras FE, Breakefield X, Budnik V, et al. 2012. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 10(12):e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman DL, Hoffman T, Bennick A, Keller P. 1986. Basic proline-rich proteins from human parotid saliva: complete covalent structure of proteins IB-1 and IB-6. Biochemistry. 25(9):2387–2392. [DOI] [PubMed] [Google Scholar]
- Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML, Tonetti MS, Wade WG, Zaura E. 2016. The oral microbiome—an update for oral healthcare professionals. Brit Dent J. 221(10):657–666. [DOI] [PubMed] [Google Scholar]
- Lagerlöf F, Dawes C. 1984. The volume of saliva in the mouth before and after swallowing. J Dent Res. 63(5):618–621. [DOI] [PubMed] [Google Scholar]
- Larsen MJ, Bruun C. 1986. Enamel/saliva—inorganic chemical reactions. In: Thylstrup A, Fejerskov O, editors. Textbook of cariology. Copenhagen (Denmark): Munksgaard; p. 181–203. [Google Scholar]
- Lasser C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. 2011. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Kim Y, Chia D, Spielmann N, Eibl G, Elashoff D, Wei F, Lin YL, Moro A, Grogan T, et al. 2013. Role of pancreatic cancer–derived exosomes in salivary biomarker development. J Biol Chem. 288(37):26888–26897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MJ. 1993. Development of artificial salivas. Crit Rev Oral Biol Med. 4(3–4):270–286. [DOI] [PubMed] [Google Scholar]
- Li Y, Elashoff D, Oh M, Sinha U, St John MA, Zhou X, Abemayor E, Wong DT. 2006. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol. 24(11):1754–1760. [DOI] [PubMed] [Google Scholar]
- Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC, Eisele D, Abemayor E, Elashoff D, Park NH, Wong DT. 2004. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 10(24):8442–8450. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou X, John MA, Wong DT. 2004. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 83(3):199–203. [DOI] [PubMed] [Google Scholar]
- Löe H, Theilade E, Jensen SB. 1965. Experimental gingivitis in man. J Periodontol. 36(3):177–187. [DOI] [PubMed] [Google Scholar]
- Loesche WJ. 1979. Clinical and microbiological aspects of chemotherapeutic agents used according to the specific plaque hypothesis. J Dent Res. 58(12):2404–2412. [DOI] [PubMed] [Google Scholar]
- Loo JA, Yan W, Ramachandran P, Wong DT. 2010. Comparative human salivary and plasma proteomes. J Dent Res. 89(10):1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi ML, Zakaria H, Osman J, Jamal R. 2012. Quantity and quality assessment of DNA extracted from saliva and blood. Clin Lab. 58(3–4):307–312. [PubMed] [Google Scholar]
- Mandel ID. 1979. In defense of the oral cavity. In: Kleinberg I, Ellison SA, Mandel ID, editors. Proceedings “saliva and dental caries.” New York (NY): Information Retrieval Inc; p. 473–491. [Google Scholar]
- Mandel ID, Thomson RH, Ellison SA. 1965. Studies on the mucoproteins of human parotid saliva. Arch Oral Biol. 10(3):499–507. [DOI] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. 2010. Exosomes from human saliva as a source of microrna biomarkers. Oral Dis. 16(1):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, et al. 2010. Current developments in salivary diagnostics. Biomark Med. 4(1):171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WD. 1890. Microorganisms of the human mouth: the local and general diseases which are caused by them. Philadelphia (PA): S. S. White Dental Mfg Co. [Google Scholar]
- Mouliere F, Robert B, Arnau Peyrotte E, Del Rio M, Ychou M, Molina F, Gongora C, Thierry AR. 2011. High fragmentation characterizes tumour-derived circulating DNA. PLoS One. 6(9):e23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie S, Henley WH, Miller SE, Zhang H, Mayer KM, Dennis PJ, Oblath EA, Alarie JP, Wu Y, Oppenheim FG, et al. 2014. An automated integrated platform for rapid and sensitive multiplexed protein profiling using human saliva samples. Lab Chip. 14(6):1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. 2008. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 31(6):1059–1062. [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion: isolation, characterization, primary structure, and fungistatic effects of Candida albicans. J Biol Chem. 263(16):7472–7477. [PubMed] [Google Scholar]
- Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, Abbruzzese JL, Tam AL. 2013. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol. 31(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Li Y, Yu T, Brinkman BM, Wong DT. 2006. Characterization of RNA in saliva. Clin Chem. 52(6):988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. 2009. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 15(17):5473–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Zhou X, Yu T, Brinkman BM, Zimmermann BG, Palanisamy V, Wong DT. 2007. Characterization of salivary RNA by cDNA library analysis. Arch Oral Biol. 52(1):30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KM, Tsui DW. 2015. The translational potential of circulating tumour DNA in oncology. Clin Biochem. 48(15):957–961. [DOI] [PubMed] [Google Scholar]
- Pedersen AML, Sørensen CE, Proctor GB, Carpenter, Ekström J. 2018. Salivary secretion in health and disease. J Oral Rehabil. 45(9):730–746. [DOI] [PubMed] [Google Scholar]
- Pu D, Liang H, Wei F, Akin D, Feng Z, Yan Q, Li Y, Zhen Y, Xu L, Dong G, et al. 2016. Evaluation of a novel saliva-based epidermal growth factor receptor mutation detection for lung cancer: a pilot study. Thorac Cancer. 7(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado A. 2018. 2017 was the year consumer testing blew up. MIT Technology Rev; [accessed 2018 Nov 13]. https://www.technologyreview.com/s/610233/2017-was-the-year-consumer-dna-testing-blew-up/.
- Ruhl S. 2012. The scientific exploration of saliva in the post-proteomic era: from database back to basic function. Expert Rev Proteomics. 9(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander-Rudqvist T, Håkansson N, Tybring G, Wolk A. 2006. Quality and quantity of saliva DNA obtained from the self-administrated oragene method—a pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 15(9):1742–1745. [DOI] [PubMed] [Google Scholar]
- Sanz M, Beighton D, Curtis MA, Cury J, Dige I, Dommisch H, Ellwood R, Giacaman RA, Herrera D, Herzberg MC, et al. 2017. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases: consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 44 Suppl 18:S5–S11. [DOI] [PubMed] [Google Scholar]
- Schlesinger DH, Hay DI. 1977. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from parotid saliva. J Biol Chem. 252(5):1689–1695. [PubMed] [Google Scholar]
- Schneyer LH. 1955. Method for the collection of separate submaxillary and sublingual salivas in man. J Dent Res. 34(2):257–261. [DOI] [PubMed] [Google Scholar]
- Schneyer LH, Pigman W, Hanahan L, Gilmore RW. 1956. Rate of flow of human parotid, sublingual, and submaxillary secretions during sleep. J Dent Res. 35(1):109–114. [DOI] [PubMed] [Google Scholar]
- Schulz BL, Cooper-White J, Punyadeera CK. 2013. Saliva proteome research: current status and future outlook. Crit Rev Biotechnol. 33(3):246–259. [DOI] [PubMed] [Google Scholar]
- Sheiham A, James WP. 2015. Diet and dental caries: the pivotal role of free sugars reemphasized. J Dent Res. 94(10):1341–1347. [DOI] [PubMed] [Google Scholar]
- Sholl LM, Aisner DL, Allen TC, Beasley MB, Cagle PT, Capelozzi VL, Dacic S, Hariri LP, Kerr KM, Lantuejoul S, et al. 2016. Liquid biopsy in lung cancer: a perspective from members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 140(8):825–829. [DOI] [PubMed] [Google Scholar]
- Simonsen KW, Steentoft A, Hels Y, Bernhoft IM, Rasmussen BS, Linnet K. 2012. Presence of psychoactive substances in oral fluid from randomly selected drivers in Denmark. Forensic Sci Int. 221(1–3):33–38. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Kalra H, Mathivanan S. 2012. Exocarta as a resource for exosomal research. J Extracell Vesicles. doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Zhang W, Helmerhorst EJ, Gygi SP, Oppenheim FG. 2007. Identification of protein components in in vivo human acquired enamel pellicle using LC-ESI-MS/MS. J Proteome Res. 6(6):2152–2160. [DOI] [PubMed] [Google Scholar]
- Spielmann N, Ilsley D, Gu J, Lea K, Brockman J, Heater S, Setterquist R, Wong DT. 2012. The human salivary RNA transcriptome revealed by massively parallel sequencing. Clin Chem. 58(9):1314–1321. [DOI] [PubMed] [Google Scholar]
- Stephan RM. 1944. Intraoral hydrogen-ion activity associated with dental caries activity. J Dent Res. 23(4):257–266. [Google Scholar]
- Tabak LA. 1995. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 57:547–564. [DOI] [PubMed] [Google Scholar]
- Tabak LA. 2006. In defence of the oral cavity: the protective role of the salivary secretions. Pediatr Dent. 28(2):110–117. [PubMed] [Google Scholar]
- Tatevossian A, Gould CT. 1976. The composition of the aqueous phase in human dental plaque. Arch Oral Biol. 21(5):319–323. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. 2012. The role of saliva in mineral equilibria: caries, erosion and calculus formation. In: Edgar M, Dawes C, O’Mullane D, editors. Saliva and oral health. 4th ed. London (UK): Stephen Hancocks Ltd; p. 135–150. [Google Scholar]
- Thaysen JH, Thorn NA, Schwartz IL. 1954. Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Am J Physiol. 178(1):155–159. [DOI] [PubMed] [Google Scholar]
- Theilade E. 1986. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol. 13(10):905–911. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3:unit 3.22. [DOI] [PubMed] [Google Scholar]
- Truelove EL, Bixler D, Merritt AD. 1967. Simplified method for collection of pure submandibular saliva in large volumes. J Dent Res. 46(6):1400–1403. [DOI] [PubMed] [Google Scholar]
- Veerman EC, van den Keybus PA, Vissink A, Nieuw Amerongen AV. 1996. Human glandular salivas: their separate collection and analysis. Eur J Oral Sci. 104(4, pt 1):346–352. [DOI] [PubMed] [Google Scholar]
- Vera-Sigűenza E, Catalân MA, Peña-Műnzenmayer G, Melvin JE, Sneyd J. 2018. A mathematical model supports a key role for Ae4 (Sic4a9) in salivary gland secretion. Bull Math Biol. 80(2):255–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Lin CC, Joon A, Feng Z, Troche G, Lira ME, Chia D, Mao M, Ho CL, Su WC, et al. 2014. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 190(10):1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Patel P, Liao W, Chaudhry K, Zhang L, Arellano-Garcia M, Hu S, Elashoff D, Zhou H, Shukla S, et al. 2009. Electrochemical sensor for multiplex biomarkers detection. Clin Cancer Res. 15(13):4446–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Joshi RK, Ekström J, Aframian D, Pedersen AML, Proctor G, Narayama N, Villa A, Sia YW, Aliko A, et al. 2017. A guide to medications inducing salivary gland dysfunction, xerostomia and subjective sialorrhea: a systematic review sponsored by the World Workshop on Oral Medicine VI. Drugs R D. 17(1):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, He J, Xue J, Wang Y, Li K, Zhang K, Guo G, Liu X, Zhou Y, Cheng L, et al. 2015. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 17(3):699–710. [DOI] [PubMed] [Google Scholar]
- Zhang L, Farrell JJ, Zhou H, Elashoff D, Akin D, Park NH, Chia D, Wong DT. 2010. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 138(3):949–57.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]