Abstract
Background
HVEM is a co-inhibitory molecule which can both stimulate and inhibit host immune responses. Altered expression of HVEM and its ligands is associated with increased nosocomial infections in septic patients. We hypothesize critically ill trauma patients will display increased lymphocyte HVEM expression and that such alteration is predictive of infectious events.
Materials and Methods
Trauma patients prospectively enrolled from the ICU were compared with healthy controls. Leukocytes were isolated from whole blood, stained for CD3 (lymphocytes) and HVEM, and evaluated by flow cytometry. Charts were reviewed for injuries sustained, APACHE II score, hospital course, and secondary infections.
Results
Trauma patients (N=31) were older (46.7 +/−2.4 vs 36.8+/− 2.1 years; p=0.03) than healthy controls (N=10), but matched for male sex (74% vs 60%; p=0.4). Trauma patients had higher presenting WBC (13.9 +/− 1.3 vs 5.6 +/−0.5 ×106/ml; p=0.002), lower percentage of CD3+ lymphocytes (7.5% +/−0.8 vs 22.5% +/−0.9; p<0.001), but significantly greater expression of HVEM+/CD3+ lymphocytes (89.6% +/−1.46 vs 67.3% +/−1.7; p<0.001). Among trauma patients, secondary infection during the hospitalization was associated with higher APACHE II scores (20.6 +/− 1.6 vs 13.6 +/−1.4; p=0.03) and markedly lower CD3+ lymphocyte HVEM expression (75% +/−2.6 vs 93% +/−0.7; p<0.01).
Conclusion
HVEM expression on CD3+cells increases after trauma. Patients developing secondary infections have less circulating HVEM+CD3+. This implies HVEM signaling in lymphocytes plays a role in maintaining host defense to infection in after trauma. HVEM expression may represent a marker of infectious risk as well as a potential therapeutic target, modulating immune responses to trauma.
Keywords: HVEM, Trauma, Immunosuppression, Lymphocytes
Background
Despite significant advances in trauma systems and care, trauma remains a leading cause of death and long-term functional disability. Trauma related deaths continue to demonstrate a trimodal distribution with the delayed third phase driven by secondary infections and end-organ dysfunction. Traumatic injuries induce significant disruptions in both the immune and inflammatory systems1. The CD3+ lymphocyte component of the immune system does not merely contribute to delayed adaptive functions, but rather lymphocytes play key central regulatory roles in the early initial immune response to surgical critical illness and traumatic injuries. It is now recognized that trauma induced immune-paralysis, specifically lymphocyte dysfunction, has been associated with secondary infection, long term organ dysfunction and an increased risk of death.2 Severe critical illness induced immune dysfunction can persist for considerable duration of time. Pelligrini et al noted trauma induced lymphocyte dysfunction over a month from the initial traumatic injury, and this persisted well after clinical resolution of the initial event.3 The early immune and inflammatory events can set in motion a perpetual cycle of inflammatory dysfunction leading to long term clinical effects.4 Despite the recognition of this pattern of trauma induced immune dysfunction, many of the mechanisms are poorly understood, with few studies addressing whether these findings are translatable to patients. Some of the mechanisms that lead to this trauma/injury and critical illness induced immune-paralysis in patients involve the role of check point proteins, specifically showing a central role for lymphocytes and co-inhibitory molecules upon lymphocytes.2, 5
HVEM (Herpes Virus Entry Mediator) is a TNF family transmembrane receptor expressed across a spectrum of immune cells including lymphocytes6–8 and dendritic cells, as well as epithelial cells.9 Much of the early work delineating HVEM’s signaling cascade was completed using T-cells, demonstrating this regulator’s interesting and powerful role as a bidirectional switch on these essential adaptive immune cells.10–13 HVEM interacts with a variety of ligands from both the TNF-related cytokine family and the immunoglobulin superfamily and its ultimate signaling effect is dependent on which ligand it binds, as well as its own confirmation within the membrane.14 Ligation with immunoglobulin superfamily member ligands CD160 and B sand T Lymphocyte Attenuator (BTLA) induces immune inhibition via Shp-1/2 phosphorylation while TNF-related cytokine family member ligands LIGHT and Lymphotoxin alpha (LTα) binding resulting in activation through an NFƙB mediated mechanism.11, 15 HVEM has multiple binding domains, allowing it to bind more than one ligand at once, even forming trimeric complexes with stimulatory and inhibitory ligands all at once. This allows a level of environmental specificity to HVEM signaling not possible with other immune regulators.
HVEM has emerged as an important coregulatory molecule in critical illness.5, 16 Altered expression of HVEM and one of its ligands, BTLA, are associated with poor outcomes and increased nosocomial infections in critically ill septic patients. The HVEM/BTLA axis has been studied in the murine and human septic populations revealing BTLA and HVEM expression is increased in the setting of these extreme stresses, and that increased BTLA expression is associated with poor outcomes and increased risk of nosocomial infection.17, 18 We have previously demonstrated a role for the BTLA axis in trauma induced immunosuppression.17 Furthermore, alterations in HVEM signaling have been shown to play a significant role in the immune response to sterile inflammation.19–22 To date, the role of HVEM in sterile trauma induced immunosuppression has not been translated to critically ill trauma patients.
Given that infections are a driving force in poor long-term trauma outcomes, we hypothesize critically ill trauma patients will induce an early alteration in HVEM expression on CD3+ lymphocytes and that such an alteration would be predictive of secondary infectious events.
Materials and Methods
Patients
Critically ill trauma patients, ages 18 years and older, who were admitted to the Trauma ICU at a single level I trauma center were prospectively consented and enrolled within 24 hours of presentation. Patients were included in the study if they had a trauma related critical illness requiring ICU admission. Exclusion criteria included death within 48 hours of arrival, pregnancy, or any patient with a known history of lymphoma or leukemia. Age and sex matched healthy controls were enrolled as the control group. This study was approved by the institutional review board of Rhode Island Hospital. For clinical information, charts were reviewed for demographics and patient characteristics, as well as comorbidities and all injuries sustained. Hematologic profiles from the day of lab draw, including White Cell Count, as well as all clinical features needed to calculate Acute Physiology of Chronic Health Evaluation II (APACHE II score). ICU and hospital courses were reviewed for all complications, including development of a secondary trauma related infections. Determination of infection as well as all clinical care was at the discretion of the treating team.
Specimen preparation and flow cytometry
Whole blood was obtained in heparin containing tubes. A portion of each sample was separated to collect serum which was then stored at −80°C for later cytokine analysis. The remaining whole blood was layered with Ficoll Histopaque −1077 and centrifuged. The layer containing leukocytes was aspirated and washed with phosphate buffered saline then centrifuged. Cells were stained using monoclonal antibodies for CD3 (BD Pharmingen, FITC Mouse Anti-Human CD3, Cat#555332; Beckman Coulter, APC Anti-Human CD3, PN#IM2467U), CD4 (Biolegend, PE-anti-human CD4, Cat# 318806) or CD8 (Biolegend, PE-anti-human CD8, Cat#344706) to identify lymphocytes, as well as for HVEM (Biolegend, PE-anti-human CD270, Clone 122, Cat#318806). Cellular populations were analyzed using flow cytometry. Initially, distinct forward and side scatter patterns were used to identify lymphocytes. Gating based on isotype controls was then used to identify HVEM+CD3+ lymphocytes. Flow cytometry data analysis was completed using FlowJo software.
For cytokine analysis we chose to examine patient plasma IL-6, IL-10, IL-2 and TNF-α levels using a Cytometric Bead Array (CBA) (BD Biosciences) according to the manufacture’s specifications and as we have previously described23.
Statistical Analysis
When data was normally distributed a t-test was used, however, if data was not normally distributed a Mann-Whitney U test was applied. Results are presented as means +/− standard error of the mean. Linear regression was used to correlate HVEM expression and APACHE II Score, and R2 was calculated. Statistical analysis was undertaken using SigmaPlot version 12 (Systat Software, Inc, Chicago, IL). Alpha was set to 0.05.
Results
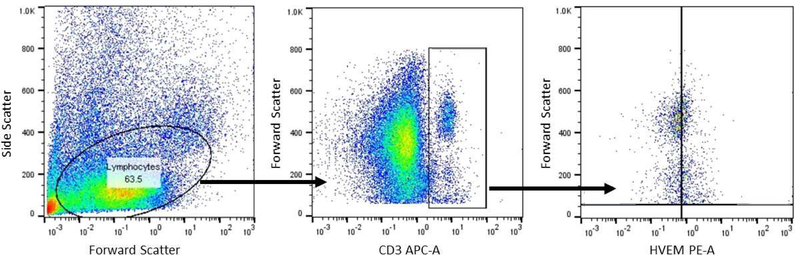
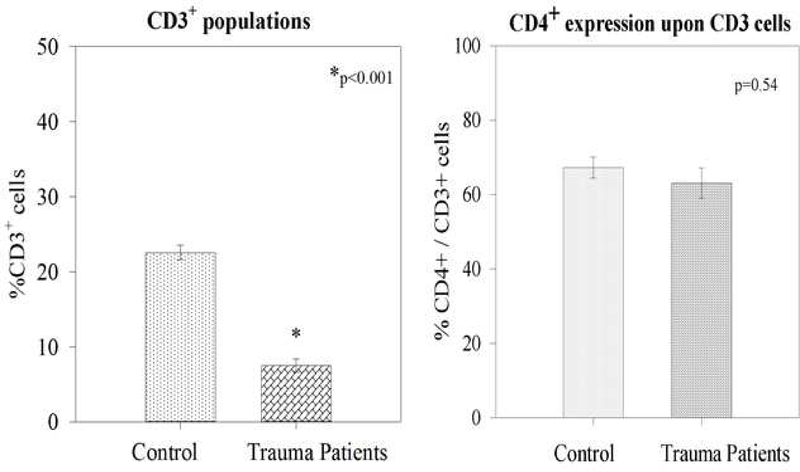
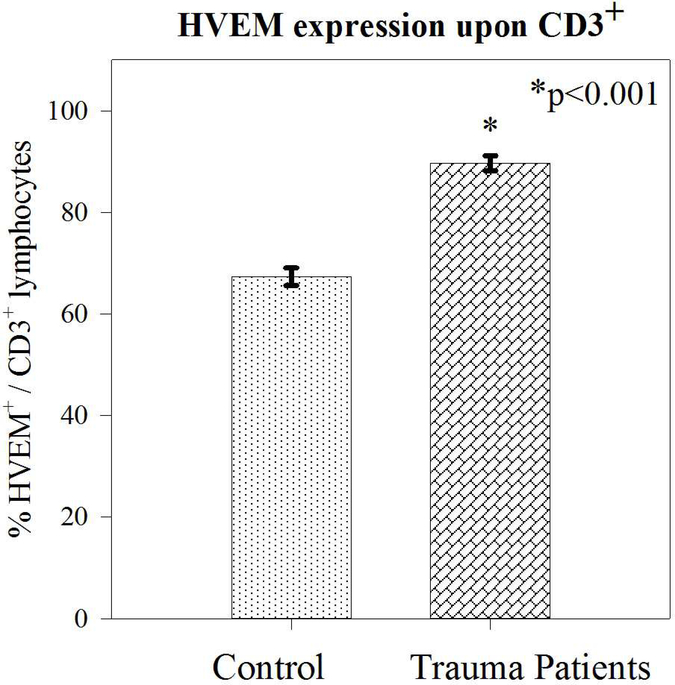
Thirty-one trauma patients and 10 healthy controls were enrolled for this study. Trauma patients were slightly older (46.7 +/−2.4 versus 36.8 +/−2.1 years; p=0.03) but matched for male sex (74% versus 60%; p=0.4). Among the trauma group as a whole, the average APACHE II score was 14.7+/−1.3). The overall mortality of the trauma group was 19% (6 patients) (Table 1). Trauma patients had higher presenting White Cell Count (13.9 +/− 1.3 versus 5.6 +/−0.5 ×106/ml; p=0.002). Blood samples were processed, and samples were gated and stained for CD3+, CD4+, and HVEM+ expression. A representative image is depicted in Figure 1. Although trauma patients, compared to controls displayed a lower percentage of CD3+ lymphocytes (7.5% +/−0.8 versus 22.5% +/−0.9; p<0.001), there was no difference in the expression of either CD4+CD3+ lymphocytes (approximately 65%), or the CD8+CD3+ lymphocytes (approximately 30%) (Table 1 & Figure 2). Among the CD3+ population, trauma patients did display a significantly greater frequency of HVEM+CD3+ lymphocytes (89.6% +/−1.5 versus 67.3% +/−1.7; p<0.001) (Figure 3). With respect to cytokine levels, it was noted that all measured cytokine levels (IL-6, IL-10, IL-2 and TNF-α) were significantly elevated among trauma patients when compared with healthy controls.
Table 1:
Patient clinical characteristics: Trauma patients had significantly lowered CD3+ lymphocytes, but no difference in CD4+/CD3+ cells or CD8+/CD3+ cells.
| Variable | Control N=10 (%) | Trauma Patients N=31 (%) | p Value |
|---|---|---|---|
| Age, mean (years) [SEM] | 36.8 [2.1] | 46.7 [2.4] | 0.03 |
| Male sex | 60 (6) | 74 (23) | 0.4 |
| APACHE II Score, mean [SEM] | 14.7 [1.3] | ||
| Mortality | 19 (6) | ||
| Presenting WCC (x106/mL) | 5.6 [0.5] | 13.9 [1.3] | 0.002 |
| CD3+ lymphocytes | 22.5 [0.9] | 7.5 [0.8] | <0.001 |
| CD4+CD3+ lymphocytes | 67.3 [2.7] | 63.1 [4.1] | 0.54 |
| CD8+CD3+ lymphocytes | 29.1 [1.1] | 32.2 [1.2] | 0.78 |
| HVEM+CD3+ lymphocytes | 67.3 [1.7] | 89.6 [1.5] | <0.001 |
SEM= Standard error of the mean: WCC=White Cell Count
Figure 1:
Representative flow cytometry plots depicting gating strategy as well as significant difference in CD3+ and HVEM+ expression between healthy controls and trauma patients
Figure 2–
Trauma patients displayed a lowered CD3+-lymphocyte population. However, there was no difference in CD4+ expression upon CD3+-lymphocytes.
Figure 3–
Trauma patients displayed a marked increase in HVEM expression upon CD3+-lymphocytes.
Among the 31 trauma patients, 22.5% developed a secondary infection. The most common source of infection was ventilator associated pneumonia (n=5), one of whom also developed a urinary tract infection. Two patients developed complex wound / soft tissue infections, one of whom also developed a urinary tract infection (Table 2). Comparing those who did versus did not develop a secondary infection, there was no difference in age (48.4 +/− 2.5 versus 39.7 +/− 6.7; p=0.15) or male sex (57% versus 79%; p=0.33) between the groups. Although there was no statistical difference, many of those who developed a secondary infection presented with pre-trauma medical co-morbidities (43% versus 21%; p=0.35). Among all patients, hypertension was the most common medical co-morbidity. With respect to the presenting lymphocyte populations, there was no difference in either presenting %CD3+ lymphocytes, %CD4+CD3+ cells or %CD8+CD3+ cells. Patients who developed a secondary infection during the hospitalization had a higher early APACHE II score (20.6 +/− 1.6 vs 13.6 +/− 1.4; p=0.03) (Table 3).
Table 2:
Types of infection among trauma patients. Pneumonia was the most frequent infection. Two patients had multiple sources of infection. Both UTIs occurred in patients with other infections.
| Source of Infection | Number of patients |
|---|---|
| Pneumonia | 5 |
| Complex wound / soft tissue infection | 2 |
| Urinary tract infections | 2 |
| Multiple sites of infection | 2 |
Table 3:
Patient clinical characteristics of those who did versus did not develop an infection: Trauma patients had significantly lowered CD3+ lymphocytes, but no difference in CD4+/CD3+ cells
| Variable | No Infection N=24 (%) | Infection N=7 (%) | p Value |
|---|---|---|---|
| Age, mean (years) [SEM] | 39.7 [6.7] | 48.4 [2.5] | 0.15 |
| Male sex | 79 (19) | 57 (4) | 0.33 |
| APACHE II Score, mean [SEM] | 13.6 [1.5] | 20.7 [1.6] | 0.03 |
| Medical comorbidities (mean) | 5 (20.8%) | 3 (42%) | 0.35 |
| CD3+ lymphocytes | 7.2 [0.9] | 8.5 [1.9] | 0.5 |
| CD4+CD3+ lymphocytes | 65.5 [4.9] | 55.4 [6.9] | 0.3 |
| CD8+CD3+ lymphocytes | 27.7 [3.5] | 35.2 [4.2] | 0.28 |
| HVEM+CD3+ lymphocytes | 75 [2.6] | 93 [0.7] | <0.001 |
SEM = Standard error of the mean
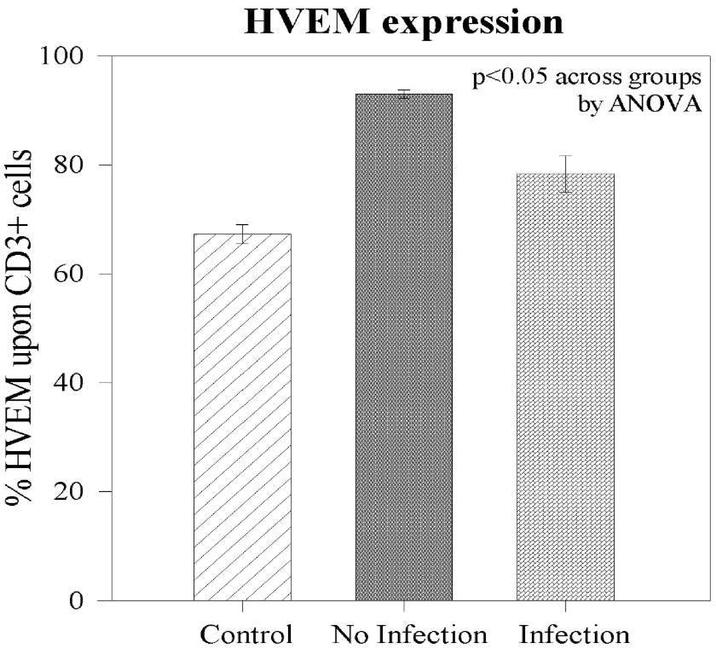
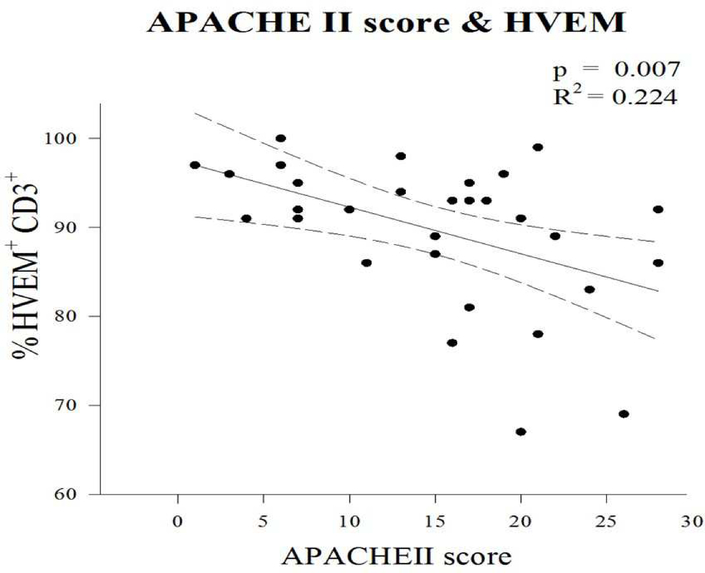
As noted, among the group as a whole, post-traumatically patients demonstrate an elevation of HVEM expression upon CD3+ cells. However, among patients who subsequently developed a secondary infection, the early expression of HVEM upon CD3+ lymphocytes following the traumatic injury was noted to be markedly reduced compared to patients who did not develop a secondary infection (75% +/−2.6 vs 93% +/−0.7; p<0.01) (Figure 4). Given the association between APAHCE II score and infection as well as the association between HVEM expression and infection, a linear regression was performed to assess the association between the frequency of HVEM+CD3+ cells and clinical degree of illness (APACHE II score). An association was noted, showing that increasing APACHE II Score correlated with decreasing HVEM expression upon CD3+ lymphocyte (R2=0.2; p=0.007) (Figure 5)
Figure 4 –
HVEM expression upon CD3+ lymphocytes. HVEM expression increased following trauma. Failure to increase HVEM expression was noted in patients who developed an infection.
Figure 5 –
Increasing APACHE II score was correlated with decreasing expression of HVEM upon CD3+ lymphocytes.
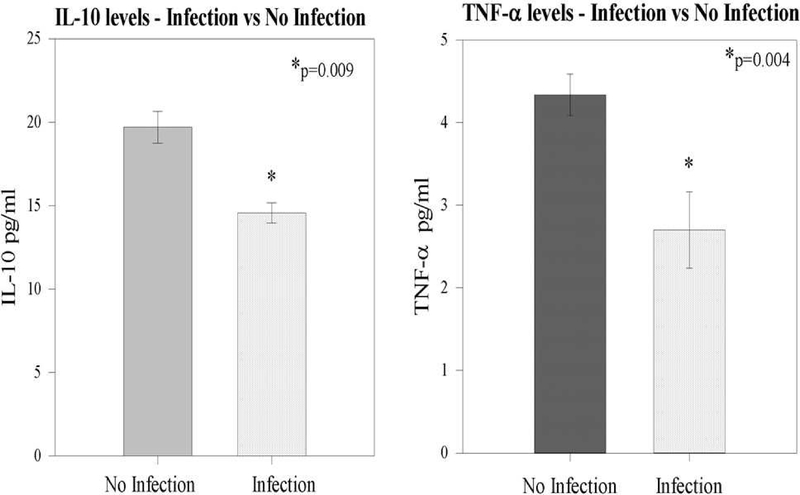
With respect to cytokine levels, there was no association between initial IL-6 or IL-2 levels with either HVEM expression or development of a subsequent infection. However, it was noted that among trauma patients who later developed a subsequent infection (patients with lowered HVEM expression upon CD3+ lymphocytes), both IL-10 (19.7 +/− 0.9 versus 14.6 +/− 0.6 pg/ml; p=0.009) and TNF-α (2.7 +/−0.4 vs. 4.3 +/− 0.3 pg/ml; p=0.04) levels were lower when compared with patients who did not develop a subsequent infection (Figure 6).
Figure 6 –
Both initial IL-10 and TNF-α levels were noted to be lower among patients who subsequently developed a trauma related infection.
Discussion
Trauma remains a leading cause of both death and long-term disability. Traumatic injuries induce a significant and dramatic state of immune suppression and immune dysfunction, persistent inflammation, secondary infections, end organ failure, and ultimately death. Despite significant advances in trauma care, trauma related complications, including infections, continue to show predictable patterns.24, 25 The trimodal distribution, first described in 1983, relates to immediate, early and late causes of death. The late causes of death occurred weeks to months after the initial traumatic injury, and up to 80% of these late deaths were attributed to infection and organ failure.26 Despite a flattening of the curve of the later stages of the traditional trimodal distribution27, later deaths still occur, accounting for 10–30% of trauma-related deaths, and are most commonly infection related.28–31 Many of these later secondary infectious complications may not lead to mortality but do impose a substantial financial burden and contribute significantly to prolonged hospital length of stay and a greater need for long term rehabilitation.31, 32
Trauma and traumatic injuries induce a significant early systemic effect, mediated by immune dysfunction. It is now well recognized that CD3+ lymphocytes, despite constituting an element of the adaptive immune system, play a critical role in the response to acute illness and injuries. In keeping with this observation, we have previously demonstrated that the normal lymphocyte response to trauma involves the development of a lymphopenia.2 We do not believe that our data reflects patients with an underlying history of immune dysfunction, but rather that our data supports the concept that is now well recognized that the traumatic injury itself induces a state of immunosuppression.33, 34 In one of the earlier studies that identified trauma induced immunosuppression among patients, Pellegrini et al demonstrated that trauma induces a systemic effect and is not just a localized effect around the injured tissues; noting systemic lymphocyte dysfunction that may persist for a long period following the injury.3 They demonstrated a persistence of T-cell anergy, marked by a significant and prolonged suppression of lymphocyte production of IL-2. The cytokine data in our study is in keeping with this observation that trauma patients display a reduced ability to generate an appropriate immune response. Our data expands upon this observation demonstrating an association with HVEM expression as a potential mechanism for these cytokine observations. Furthermore, Bandyopadhyay et al noted that negative signaling receptors among all CD3+ lymphocytes appeared to be capable of contributing to T-cell anergy seen in trauma patients.35 In a murine model, Chung et al demonstrated that survival from acute critical illness can be improved through blockage of lymphocyte anergy and apoptosis pathways including Fas signaling.36 Furthermore, Murao et al demonstrated that one of the immune mediated benefits of hypertonic saline resuscitation for hemorrhagic shock is driven in part through prevention of dysfunction of regulatory lymphocyte populations.37
Blocking individual cytokines in response to trauma, sepsis and surgical critical illness has failed across multiple clinical trials38. Many of the mechanisms driving the development of trauma induced immunosuppression remain to be properly elucidated and therefore immune modulating therapy lacks direct targeted intervention. However, while the role of checkpoint proteins as regulators of the immune system has begun to be better defined, including among surgical patients, much of the work to date has involved animal models with little correlation to trauma patients. Further a number of the clinical studies aimed at reducing trauma related inflammation have been crude attempts to dampen the entire immune cascade25 without regard to the underlying mechanistic drivers of inflammation. Many of these studies also include the use of agents such as steroids or free radical scavenging anti-oxidants. It is speculated that it is the non-specific nature of these therapeutic strategies that have failed to produce the desired clinical outcomes, and many have called for a more focused, pathway based approach to modulating immune effects of trauma.28 However, immune checkpoint directed therapy has shown tremendous potential in a range of medical conditions, most notably in mediating the immune response to malignancies.39, 40 Checkpoint proteins such as HVEM, BTLA, PD-1 are capable of regulating either an immunosuppressive or immunostimulatory role depending of the specific stressor. As such, these receptors can be neutralized or stimulated by clinically available therapeutic agents, and as such offer potential short-term therapeutic targets. In this study, we have begun to define HVEM as a potential mediator of trauma induced immunosuppression. It has been shown that HVEM, along with one its ligands BTLA, act as key regulators of the immune balance in both murine and human septic critical illness.17, 18 We describe a novel finding among critically ill trauma patients that traumatic injuries induce an upregulation of HVEM expression and that a failure to upregulate HVEM expression upon CD3+ lymphocytes was associated with an increased risk of secondary infections.
To date much of our understanding of the mechanisms of action of HVEM has been derived from the sepsis or oncology literature, however, there is very limited understanding of the role of the HVEM axis in traumatic injuries among patients14. Although HVEM is expressed on many tissue types and immune cell subsets, HVEM was originally described on T-cells and therefore much of our understanding of the downstream signaling generated by HVEM was elucidated using T-cells10, 11. As a result, many studies have sought to understand the role of HVEM in the context of T-cell signaling. For example, HVEM mediates effector T-cell survival and function at mucosal barriers10. HVEM interacts with a variety of ligands from both the TNF-related cytokine family and the immunoglobulin superfamily and its ultimate signal effect is dependent on which ligand it binds, as well as its own cis or trans confirmation within the membrane.14
HVEM and its ligands are all highly expressed on T-cells, and, as noted above, the ultimate signal generated from ligation is complex. While ligation of HVEM with CD160 or BTLA induces immune inhibition via Shp-1/2 phosphorylation, the ligation of LIGHT and LTα, alternatively, results in activation through NFκB mediated mechanisms.11, 12, 15 With its multiple cysteine rich binding domains, HVEM can bind more than one ligand simultaneously, often forming 3:3:3 trimeric complexes with LIGHT and BTLA or CD160 simultaneously. In addition to these variations of complexes, it is also noted that HVEM and BTLA can be co-expressed upon the same single cell, forming a stable complex when both proteins are expressed in the cis confirmation41. Traumatic injuries, ischemia-reperfusion, and tissue injury leads to release of Danger-Associated Molecular Patterns (DAMPs). DAMPs are endogenous mediators capable of signaling and activating the immune system inducing ongoing inflammation.42 In the compensatory anti-inflammatory response to trauma negative regulators such as HVEM are essential to preventing an overly exuberant immune and inflammatory response. It is the duality of mechanism of HVEM, capable of either immune suppression, or activation, that makes HVEM an intriguing target for immune modulation.
HVEM’s ability to target a dampening of the immune and inflammatory responses to a critical illness may also come at a consequence. While negative regulation may prevent excessive tissue damage, it also incurs an increased risk of secondary infections. The benefit of HVEM mediated inhibition has been shown to be prevention of the development or progression of intestinal inflammation. Steinberg et al demonstrated that HVEM, through binding with BTLA, triggers inhibitory signaling preventing intestinal inflammation.22 However, HVEM deficient mice displayed a higher susceptibility to experimental encephalomyelitis, hepatic necrosis and subsequently increased mortality.43 Other investigators have noted that HVEM mediates effector T-cell mediated immunity across both CD4+ and CD8+ lymphocytes, but demonstrate that this may come at the cost of excessive tissue destruction.44 Cheung et al demonstrated that when BTLA interactions with HVEM in a cis-complex, naïve T-cells are inhibited from activation, thus maintaining T-cells in a naïve state despite activating conditions in the surrounding environment.45 This inhibition and prevention of long term autoreactivity and autoimmunity has been shown to be beneficial in models of colitis.
In septic patients and sepsis models, it has been demonstrated that an absence of the ability to signal via the HVEM pathway increases septic mortality in mucosal gastrointestinal and respiratory infection models.19, 46, 47 Altered expression of HVEM, as well as its ligand BLTA, have previously been associated with poor outcomes and increased secondary nosocomial infections in critically ill septic patients.17, 18 In keeping with these prior observations, we demonstrate that a dampened HVEM expression upon CD3+ lymphocytes was associated with an increased risk of secondary infection in this patient cohort, most commonly pneumonia which is a mucosal associated infection. This implies that a robust HVEM signaling in lymphocytes is required to maintain host defenses to infection in critically injured trauma patients.
The single time point draw of this study is a limitation, as it did not allow us to track patient HVEM expression changes over time or relative to the actual infection resolution. Although a single time point may not fully reflect the entire profile of trauma induced immunosuppression, our work is in keeping with several authors who have demonstrated that the initial/early blood draws in patients are highly reflective of later events and/or complications among trauma patients.48, 49 For example, Wang et al demonstrated that initial trauma induced cytokine alterations within 24 hours correlated with trauma related infectious complications50. Furthermore, while single time point blood draws may not be ideal at explaining the continuum of activity within a patient, many clinical decisions are made based on single time point blood draws, especially in the care of critically ill trauma patients within the first 24 hours of their care. However, we feel that the strength of this single early time point study is the potential to identify patients at risk of developing a trauma related complication and thereby potentially benefit from checkpoint modulation. The other major limitation of a study of this nature is the inability to assess lymphocyte function or checkpoint protein expression prior to the traumatic injury. APACHE II score and risk of infection following trauma are influenced both by age as well as chronic pre-trauma medical co-morbidities. However, the additive cumulative effect of these factors upon the calculation of APACHE II score cannot be ignored. However, our findings offer insight into the immune mechanisms underlying infection risk in sicker trauma patients.
Conclusion
Lymphocytes play a central role in an appropriate immune and inflammatory response to acute surgical critical illness. Understanding the role of specific regulators of trauma responses will offer the potential for a more focused approach in potential pharmaceutical interventions aimed at regulating the lymphocytic response. Failure to increase frequency of HVEM was associated with an increased risk of secondary infection. Given that known ligands can differentially up or down regulate HVEM, this offers a modifiable mediator of the immune response to traumatic critical illness for potential biomarker/therapeutic consideration.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health [R35 GM118097 (AA), R25 GM083270 (CG), P20GM103652-Pilot (SFM), K08-GM110495 (DSH)] and Armand D. Versaci Research Scholar in Surgical Sciences Fellowship (MEW)].
Footnotes
Disclosures: No author has any proprietary, financial, intellectual or other disclosure
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lord J, Midwinter M, Chen Y, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;382(9952):1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffernan D, Monaghan S, Thakkar R, et al. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Critical Care. 2012;16(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegrini J, De A, Kodys K, et al. Relationship between T lymphocyte apoptosis and anergy following trauma. Journal of Surgical Research. 2000;88(2):200–206. [DOI] [PubMed] [Google Scholar]
- 4.Horiguchi H, Loftus T, HAwkins R, et al. Innate immunity in the persistent inflammation, immunosuppression and catabolism syndrome and its implications for therapy. Frontiers in Immunology. 2018;595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An M, Fan K, Cao Y, et al. Lymphtoxin beta receptor-Ig protectes from T-cell-mediated liver injury in mice through blocking LIGHT/HVEM signaling. Biological and Pharmaceutical Bulletin. 2006;29(10):2025–2030. [DOI] [PubMed] [Google Scholar]
- 6.Hsu H, Solowey I, Colombero A, et al. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. Journal of Biological Chemistry. 1997;272(21):13471–13474. [DOI] [PubMed] [Google Scholar]
- 7.Cody J, Bekiaris V, Ware C. Tumor necrosis factor superfamily in innate immunity and inflammation. Cold Spring Harbor Perspectives in Biology. 2014;7(4):e016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Far M, Pellerin C, Pilote L, et al. CD160 isoforms and regulation of CD4 and CD8 T-cell responses. Journal of Translational Medicine. 2014;12(217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy K, Nelson C, Sedy J. Balancing co-stimulation and inhibition with BTLA and HVEM. Nature Reviews Immunology. 2006;6(9):671–681. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery R, Warner M, Lum B, et al. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87(3):427–436. [DOI] [PubMed] [Google Scholar]
- 11.Marsters S, Ayres T, M S, et al. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. Journal of Biological Chemistry. 1997;272(22):14029–14032. [DOI] [PubMed] [Google Scholar]
- 12.Mauri D, Ebner R, Montgomery R, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. [DOI] [PubMed] [Google Scholar]
- 13.Filardy A, Pires D, Nunes M, et al. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulator phenotype in macrophages. Journal of Immunology. 2010;185(4):2044–2050. [DOI] [PubMed] [Google Scholar]
- 14.Ware C, Sedy J. TNF superfamily networks: bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14). Current Opinion in Immunology. 2011;23(5):627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai G, Freeman G. The CD160, BTLA, Light/HVEM pathway: a bidirectioanl switch regulating T-cell activation. Immunological reviews. 2009;229(1):244–258. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli M, Sedy J, Nelson C, et al. BTLA and HVEM cross talk regulates inhibition and costimulation. Advances in Immunology. 2006;92:157–185. [DOI] [PubMed] [Google Scholar]
- 17.Shubin N, Chung C, Heffernan D, et al. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. Journal of Leukocyte Biology. 2012;92(3):593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shubin N, Monaghan S, Heffernan D, et al. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections. Critical Care. 2013;17(6):R276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng T, Bai J, Chung C, et al. Herpes Virus Entry Mediator (HVEM) expression promotes inflammation / organ injury in response to experimental indirect actue lung injury. Shock. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Yang H, Liu X, et al. Role of B and T Lymphocyte Attenuator in renal transplant recipients with biopsy proven acute rejection. Medical Scinece Monitor. 2018;24:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Rio M, Schneider P, Fernandez-Renedo C, et al. LIGHT/HVEM/LTβR interaction as a target for the modulation of the allogenic immune response in transplantation. American Journal of Transplantation. 2013;13(3):541–551. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg M, Turovskaya O, Shaikh R, et al. A crucical role for HVEM and BTLA in preventing intestinal inflammation. Journal of Experimental Medicine. 2008;205(6):1463–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ottinger M, Monaghan S, Gravenstein S, et al. The geriatric cytokine response to trauma: time to consider a new threshold. Surgical Infections. 2014;15(6):800–805. [DOI] [PubMed] [Google Scholar]
- 24.Weintrob A, Murray C, Xu J, et al. Early infections complicating the care of combat casualties from Iraq and Afghanistan. Surgical Infections. 2018;19(3):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Tian L, Liang H. Early prevention of trauma-related infection/sepsis. Military Medical Research. 2016;3(33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trauma Trunkey D.. Accidental and intentional injuries account for more years of life lost in the US than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Scientific America. 1983;249(2):28–35. [PubMed] [Google Scholar]
- 27.Bardes J, Inaba K, Schellenberg M, et al. The contemporary timing of trauma deaths. Journal of Trauma and Acute Care Surgery. 2018;84(6):893–899. [DOI] [PubMed] [Google Scholar]
- 28.Spruijt N, Visser T, Leenen L. A systematic review of randomized controlled trials exploring the effect of immunomodulative interventions on infection, organ failure, and mortality in trauma patients. Critical Care. 2010;14(4):R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caplan E, Hoyt N. Identification and treatment of infections in multiply traumatized patients. American Journal of Medicine. 1985;79(1A):68–76. [DOI] [PubMed] [Google Scholar]
- 30.Mathyr P Infections in traumatised patients: a growing medico-surgical concern. Indian Journal of Medical Microbiology. 2008;26(3):212–216. [DOI] [PubMed] [Google Scholar]
- 31.Joseph B, Zangbar B, Khalil M, et al. Factors associated with failure to rescue in patients undergoing trauma laparotomy. Surgery. 2015;158(2):393–398. [DOI] [PubMed] [Google Scholar]
- 32.Gunst M, Ghaemmaghami V, Gruszecki A, et al. Changing epidemiology of trauma deaths leads to a bimodal distribution. Proceedings (Baylor University, Medical Center). 2010;23(4):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller S, Miller C, Trunkey D. The immune consequences of trauma. Surgical Clinics of North America. 1982;62(1):167–181. [DOI] [PubMed] [Google Scholar]
- 34.Manolakaki D, Velmahos G, Kourkoumpetis T, et al. Candida infection and colonization among trauma patients. Virulence. 2010;1(5):367–375. [DOI] [PubMed] [Google Scholar]
- 35.Bandyopadhyay G, De A, Laudanski K, et al. Negative signaling contributes to T-cell anergy in trauma patients. Critical Care Medicine. 2007;35(3):794–801. [DOI] [PubMed] [Google Scholar]
- 36.Chung C, Yang S, Song G, et al. Inhibition of Fas signaling prevents hepatic injury and improved organ blood flow during sepsis. Surgery. 2001;130(2):339–345. [DOI] [PubMed] [Google Scholar]
- 37.Murao Y, Isayama K, Saito F, et al. Effect of hypertonic saline resuscitation on CD4+CD25+ regulatory T cells and gammadelta T cells after hemorrhagic shock and resuscitation in relation to apoptosis and iNOS. Journal of Trauma. 2009;67(5):975–982. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss R, Monneret G, Payen D. Sepsis induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature Reviews Immunology. 2013;13(12):862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasero C, Speiser D, Derre L, et al. The HVEM network: new directions in targeting novel costimulatory/coinhibitory molecules for cancer therapy. Current Opinion in Pharmacology. 2012;12(4):478–485. [DOI] [PubMed] [Google Scholar]
- 40.Ren S, Tian Q, Amar N, et al. The immune checkpoint HVEM may contribute to immune escape in non-small cell lung cancer lacking PD-L1 expression. Lung Cancer. 2018;125:115–120. [DOI] [PubMed] [Google Scholar]
- 41.Cheung T, Osborne L, Steinberg M, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding environment. Journal of Immunology. 2009;183(11):7286–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang P, Porterfield N, Pannell D, et al. Trauma is danger. Journal of Translational Medicine. 2011;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Subudhi S, Anders R, et al. The role of herpesvirus entry mediator as a negative regulator of T-cell mediated responses. Journal of Clinical Investigation. 2005;115(3):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaikh R, Santee S, Granger S, et al. Constitutive expression of LIGHT on T-cells leads to lymphocyte activation, inflammation and tissue destruction. Journal of Immunology. 2001;167(11):6330–6337. [DOI] [PubMed] [Google Scholar]
- 45.Cheung A, Oborne L, Steinberg M, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding environment. Journal of Immunology. 2009;183(11):7286–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai H, Chen S, Xu S, et al. Deficiency of LIGHT signaling pathway exacerbates Chlamydia psittaci respiratory tract infection in mice. Microbial pathogenesis. 2016;100:250–256. [DOI] [PubMed] [Google Scholar]
- 47.Shui J, Kronenberg M. HVEM is a TNF receptor with multiple regulatory roles in the mucosal immune system. Immune Network. 2014;14(2):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girardot T, Rimmele T, Venet F, et al. Apoptosis induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22(2):295–305. [DOI] [PubMed] [Google Scholar]
- 49.Loomis W, Namiki S, Hoyt D, et al. Hypertonicity rescues T cells from suppression by trauma-induced anti-inflammatory mediators. American Journal of Physiology Cell Physiology. 2001;281(3):C840–848. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Liu Q, Liu T, et al. Early plasma monocyte chemoattractant protein 1 predicts the development of sepsis in trauma patients: A prospective observational study. Medicine (Baltimore). 2018;97(14):e0356. [DOI] [PMC free article] [PubMed] [Google Scholar]