Abstract
Objective:
To explore whether patient-reported lymphedema-related symptoms, as measured by the Gynecologic Cancer Lymphedema Questionnaire (GCLQ), are associated with a patient-reported diagnosis of lymphedema of the lower extremity (LLE) and limb volume change (LVC) in patients who have undergone radical surgery, including lymphadenectomy, for endometrial, cervical, or vulvar cancer on Gynecologic Oncology Group (GOG) study 244.
Methods:
Patients completed the baseline and at least one post-surgery GCLQ and LVC assessment. The 20-item GCLQ measures seven symptom clusters—aching, heaviness, infection-related, numbness, physical functioning, general swelling, and limb swelling. LLE was defined as a patient self-reported LLE diagnosis on the GCLQ. LVC was measured by volume calculations based on circumferential measurements. A linear mixed model was fitted for change in symptom cluster scores and GCLQ total score and adjusted for disease sites and assessment time.
Results:
Of 987 eligible patients, 894 were evaluable (endometrial, 719; cervical, 136; vulvar, 39). Of these, 14% reported an LLE diagnosis (endometrial, 11%; cervical, 18%; vulvar, 38%). Significantly more patients diagnosed versus not diagnosed with LLE reported ≥4-point increase from baseline on the GCLQ total score (p < 0.001). Changes from baseline were significantly larger on all GCLQ symptom cluster scores in patients with LLE compared to those without LLE. An LVC increment of >10% was significantly associated with reported general swelling (p < 0.001), heaviness (p = 0.005), infection-related symptoms (p = 0.002), and physical function (p = 0.006).
Conclusions:
Patient-reported symptoms, as measured by the GCLQ, discerned those with and without a patient-reported LLE diagnosis and demonstrated predictive value. The GCLQ combined with LVC may enhance our ability to identify LLE.
Keywords: Endometrial cancer, Vulvar cancer, Cervical cancer, Lymphedema, Gynecologic cancer lymphedema, questionnaire, GCLQ
1. Introduction
Cancer treatment is a leading cause of secondary lymphedema [1], and as a result, many cancer survivors live with disfigurement, discomfort, and disruption of activities due to limb swelling [2,3]. Most lymphedema research within the field of oncology has focused on upper extremity lymphedema, mainly in patients treated for breast cancer. Investigations in gynecologic cancer are limited, are primarily retrospective in nature, and without validated patient-reported outcomes (PROs) [4–6], leaving unanswered questions as to the true incidence and impact of lymphedema of the lower extremity (LLE) during and after treatment. Investigations into the potential risk factors and associated psychomorbidity of LLE are needed to fully comprehend the implications of this condition on survivorship [7,8]. The best method to detect LLE has not been established. Limb circumferential measurement is often used [9] to detect LLE; however, this method can be labor intensive and challenging within the clinical setting. Although self-reported symptoms of upper extremity lymphedema have been associated with the development of lymphedema [10,11], simple screening mechanisms are needed to identify women at risk for or with early-stage lower limb lymphedema.
The primary aims of The LymphEdema and Gynecologic (LEG) Cancer Study were to prospectively estimate the incidence of LLE, and to identify risk factors associated with the development of LLE in patients undergoing surgery for gynecologic malignancy. Secondary aims were to explore the effect of LLE on quality of life (QOL), psychological adjustment, and physical disability and function, which will be examined in future analyses. This paper will explore whether patient self-reported lymphedema symptoms (as measured by the GCLQ) are associated with the development of lymphedema; investigate whether cluster symptoms are associated with the development of LLE, as documented in the literature of upper extremity lymphedema; and explore the predictive value of subjective PRO LLE symptom assessment in identifying patients at risk for early-onset LLE.
2. Methods
2.1. Study population
The LEG Cancer Study (Gynecologic Oncology Group [GOG] study 244) was a multi-institutional prospective study of women with newly diagnosed endometrial, cervical, or vulvar cancer who underwent surgery as primary intervention, with 2 years of follow-up. Eligible patients had to satisfy the following criteria: 1) planned for hysterectomy/bilateral salpingo-oophorectomy (BSO) and pelvic lymphadenectomy ± para-aortic node sampling via open or laparoscopic technique for clinical stage I-II uterine carcinoma; 2) planned for radical hysterectomy or trachelectomy and pelvic lymphadenectomy ± para-aortic node sampling via open or laparoscopic technique for clinical stage IA-IIA cervical carcinoma; or 3) planned for definitive surgery for primary stage I-IV vulvar cancer, consisting of radical vulvectomy or radical local excision with concurrent unilateral or bilateral inguinal or inguinal-femoral lymphadenectomy. Participants were allowed to receive therapy (radiation and/or chemotherapy) after primary surgical treatment. The accompanying, primary paper provides descriptions of the surgical procedures, including lymph node removal (Carlson et al.).
The study opened on June 4, 2012 and closed to accrual on November 17, 2014. Study participants completed the GCLQ and disability assessment with the Lower Extremity Function Scale (LEFS) at the time of lower limb volume measurement (baseline [within 14 days prior to surgery], 4—6 weeks, and 3-, 6-, 9-, 12-, 18-, and 24-months post-surgery). All patients signed written informed consent (GOG study 244). This study was funded by NCI GOG and NIH R01 CA162139.
2.2. Measures
Participant characteristics and medical and cancer treatment information were collected, as was information for known, suspected, and possible risk factors for the development of LLE. PRO surveys consisted of the self-report GCLQ to assess patients’ subjective impression of the presence of LLE symptoms, and other health-related quality of life measures (not reported herein).
2.3. The Gynecologic Cancer Lymphedema Questionnaire (GCLQ)
The GCLQ is a modification of the validated Lymphedema Breast Cancer Questionnaire (LBCQ) [10,11], initially adapted by Dr. Suzy Lockwood from the Lymphedema and Breast Cancer Questionnaire [12] to identify LLE symptoms in gynecologic cancer survivors (unpublished data). The GCLQ underwent further adaptation and validation for this national cooperative group study [13]. The GCLQ validation study demonstrated its ability to effectively distinguish between gynecologic cancer survivors with and without LLE, with good sensitivity and specificity [2]. The internal consistency reliability of the GCLQ total score is 0.95 [13].
The GCLQ assesses 20 symptoms associated with LLE, present within the past 4 weeks. Items are combined into seven symptom clusters of: heaviness (item 14), swelling (general) (items 8, 9, 20), swelling (limb) (items 18,19), infection-related (items 10 [redness], 11 [blistering], 13 [increased temperature in leg]), aching (item 17), numbness (items 7, 12, 15, 16), and physical functioning (items 1—6). Each question is scaled as either 1 or 0 (with or without symptom, respectively). A symptom cluster score was calculated by summing items if more than 50% of the questions were answered within the cluster. A total GCLQ score was the summation of the GCLQ item scores if at least 80% (or 16 questions) were answered. In the case of non-response, a score was prorated by multiplying the mean of responses by the number of questions). The clinical cut-off score of a 4-point increment from baseline was used based on the validation study [13], as this cut-off score yielded sensitivity and specificity greater than 60%, or optimal cut-off for identifying LLE. Exploratory supplemental items documented patients’ awareness of an LLE diagnosis and determined any utilization of lymphedema-specific treatment recommended by a health care professional to evaluate any actions taken to treat or reduce LLE (Appendix I).
2.4. Limb volume measurement
Limb volume change (LVC) was determined by circumferential measurements taken by trained professionals, as described in the accompanying, primary paper. In order to be classified as lymphedema, an LVC ≥10% is required [14]. Measurements were performed at the same time as the GCLQ and LEFS assessments.
2.5. Statistical analysis
Data analysis for this report was conducted for the total sample and disease sites. Descriptive statistics were performed on patient characteristics and medical and cancer treatment information. ‘Lymphedema’ in this paper is defined as patient-reported LLE and is treated as a time-dependent variable, which means the diagnosis of LLE might be reported at any time-point during the assessment time. The association between the change in GCLQ scores over time with the patient-reported diagnosis of lymphedema or LVC ≥10% was evaluated with a linear mixed model with adjustment for assessment time and disease sites. The association between the GCLQ total score increment ≥4 over time with patient-reported diagnosis of LLE or LVC ≥10% was evaluated with a generalized linear mixed model with adjustment for assessment time and disease sites. The assessment time points were treated as categorical due to unequal duration.
The predictive value of the GCLQ was explored by the association between the first patient-reported LLE diagnosis and the change in the previous GCLQ total score and was examined using a generalized linear mixed model adjusting for assessment time.
3. Results
Of 1054 women enrolled, 987 (158 with cervical, 787 with endometrial, and 42 with vulvar cancer) were eligible and had undergone lymphadenectomy. Seventy-three patients (20 with cervical and 53 with endometrial cancer) were deemed inevaluable for LVC due to a lack of baseline or valid follow-up leg volume measurement, and 20 (2 with cervical cancer, 15 with endometrial cancer, and 3 with vulvar cancer) were deemed inevaluable due to missing baseline or valid follow-up GCLQ scores. Evaluable participants for this data analysis consisted of 136 cervical cancer, 719 endometrial cancer, and 39 vulvar cancer patients with a valid baseline and at least one follow-up GCLQ assessment and leg measurement (Appendix II).
The compliance rate for the GCLQ was calculated based on 914 women who were evaluable for leg measurement and alive at the study time points. Ninety-eight percent of patients completed a baseline GCLQ assessment. The compliance rates were 93% at 6 weeks, 83% at 3 months, 81% at 6 months, 74% at 9 months, 73% at 12 months, 66% at 18 months, and 61% at 24 months. The major reasons for not completing study requirements were either the death of a patient or withdrawal from the study.
3.1. Characteristics and disease status for evaluable patients
The mean age was 46 years (range, 25—83 years) for patients with cervical cancer, 61 years (range, 28—91 years) for patients with endometrial cancer, and 60 years (range, 35—88 years) for patients with vulvar cancer. Approximately two-thirds of the women diagnosed with cervical cancer (63%, n = 86) were between 30 and 49 years of age; approximately three-quarters diagnosed with endometrial cancer (74%, n = 530) were between 50 and 69 years of age; and approximately three-quarters diagnosed with vulvar cancer (77%, n = 30) were between 50 and 79 years of age. Most patients (n = 723, 81%) identified themselves as White. Most patients had early-stage disease (n = 775, 87%), although in the vulvar group, 34% (n = 13) had stage III or IV disease (Table 1).
Table 1.
The Characteristics and Disease Status for Evaluable Patients.
|
|
Category | Cervix N = 136 |
Endometrium N = 719 |
Vulvar N = 39 |
All N = 894 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | (%) | N | (%) | N | (%) | N | (%) | |
| Age | <30 | 9 | (7) | 1 | (0) | – | – | 10 | (1) |
| 30–39 | 40 | (29) | 18 | (3) | 1 | (3) | 59 | (7) | |
| 40–49 | 46 | (34) | 46 | (6) | 6 | (15) | 98 | (11) | |
| 50–59 | 19 | (14) | 259 | (36) | 16 | (41) | 294 | (33) | |
| 60–69 | 16 | (12) | 271 | (38) | 8 | (21) | 295 | (33) | |
| 70–79 | 5 | (4) | 111 | (15) | 6 | (15) | 122 | (14) | |
| ≥80 | 1 | (1) | 13 | (2) | 2 | (5) | 16 | (2) | |
| Mean (range) | 46 yrs (25–83yrs) | 61 yrs (28–91yrs) | 60 yrs (35–88 yrs) | ||||||
| Race | Asian | 11 | (8) | 18 | (3) | – | – | 29 | (3) |
| Black | 6 | (4) | 67 | (9) | 3 | (8) | 76 | (9) | |
| Other/unspecified | 21 | (15) | 43 | (6) | 2 | (5) | 66 | (7) | |
| White | 98 | (72) | 591 | (82) | 34 | (87) | 723 | (81) | |
| Ethnicity | Hispanic | 20 | (15) | 37 | (5) | 2 | (5) | 59 | (7) |
| Non-Hispanic | 112 | (82) | 671 | (93) | 36 | (92) | 819 | (92) | |
| Other/Unspecified | 4 | (3) | 11 | (2) | 1 | (3) | 16 | (2) | |
| Performance Status | 0 | 129 | (95) | 665 | (92) | 29 | (74) | 823 | (92) |
| 1 | 6 | (4) | 53 | (7) | 9 | (23) | 68 | (8) | |
| 2 | 1 | (1) | 1 | (0) | 1 | (3) | 3 | (0) | |
| Stage of Disease | I | 133 | (98) | 577 | (80) | 23 | (59) | 733 | (82) |
| II | 2 | (1) | 37 | (5) | 3 | (8) | 42 | (5) | |
| III | 1 | (1) | 97 | (13) | 12 | (31) | 110 | (12) | |
| IV | – | – | 8 | (1) | 1 | (3) | 9 | (1) | |
3.2. Patient-reported diagnosis of LLE on the GCLQ
Among 894 evaluable patients, 121 (14%) self-reported receiving a formal diagnosis of LLE during the 2-year study (at any follow-up) as recorded on the GCLQ (items 21 & 22). By disease site, 18% (24/136) of cervical cancer, 11% (82/719) of endometrial cancer, and 38% (15/39) of vulvar cancer patients reported a diagnosis of LLE per the GCLQ.
3.3. Association between the change in GCLQ total score and patient-reported lymphedema
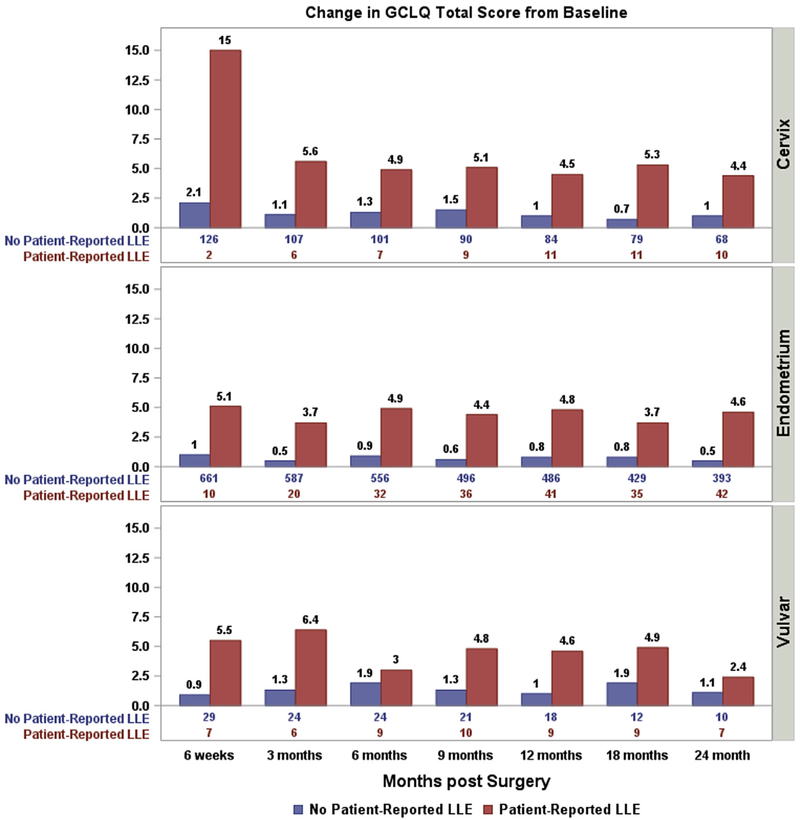
As displayed in Fig. 1, the incremental change in the GCLQ total score was significantly associated with an LLE diagnosis in patients with cervical cancer (p < 0.001), endometrial cancer (p < 0.001), and vulvar cancer (p = 0.015). Three hundred seventy-seven patients (42% of all evaluable patients) had a GCLQ total score incremental change ≥4 points from baseline during post-surgery assessments (66 with cervical cancer, 283 with endometrial cancer, and 28 with vulvar cancer). The percentage of patients whose GCLQ total score increased ≥4 from baseline was significantly associated with a patient-reported lymphedema diagnosis for the total sample (p < 0.001), and each of the three cancers independently.
Fig. 1.
The association between GCLQ score and GCLQ-reported lymphedema.
Change in GCLQ total score from baseline by patient-reported lymphedema diagnosis. The numbers at the bottom of the figure are the number of patients reporting diagnosed lymphedema (Yes/No) at each time points. (p < 0.001 for Endometrial cancer and Cervical cancer, respectively; p = 0.015 for Vulvar cancer).
3.4. GCLQ total symptom clusters
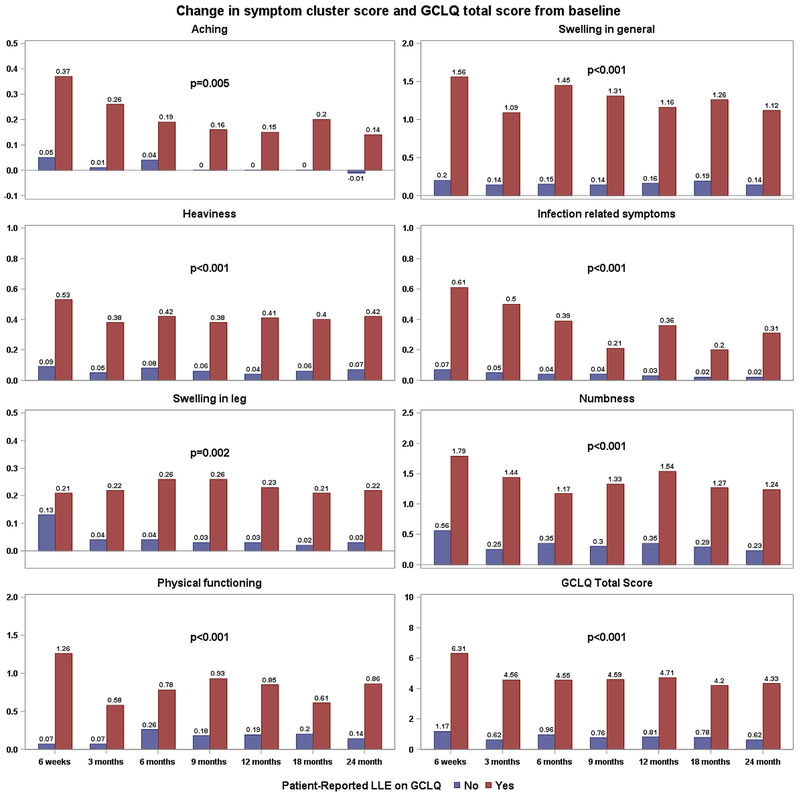
After adjustment for disease sites and assessment time points, the changes from baseline in all symptom cluster scores and GCLQ total score differed significantly for those with and without patient-reported LLE (Fig. 2). By disease site, in patients with endometrial cancer, symptoms of aching (p = 0.001), swelling (general) (p < 0.001), heaviness (p < 0.001), infection (p < 0.001), numbness (p < 0.001), and physical functioning (p < 0.001) were significantly different between those diagnosed with and without LLE; specifically, women with LLE were more symptomatic. The symptom cluster of swelling in the leg (p = 0.03) had a mild group difference. For the cervical cancer group, symptoms of swelling (general) (p < 0.001), heaviness (p = 0.006), swelling in the leg (p = 0.009), and numbness (p = 0.013) showed a strong significant difference, and a marginal difference for infection (p = 0.046). For the vulvar cancer group, symptoms of swelling (general) (p < 0.001), heaviness (p = 0.006), swelling in the leg (p = 0.01), and numbness (p < 0.001) were significantly different.
Fig. 2.
The association between GCLQ symptom cluster scores and patient-reported LLE on GCLQ for the total sample.
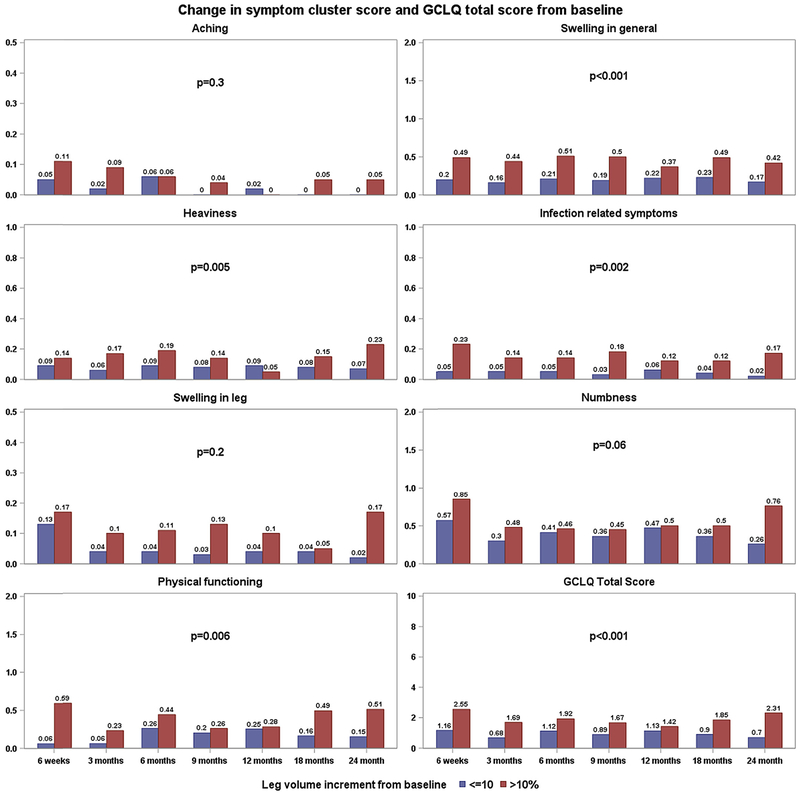
Fig. 3 demonstrates changes in the GCLQ symptom cluster and total score for those with and without an LVC ≥10% at the time leg volume was measured. After adjusting for assessment time and disease sites, the change in symptoms of swelling in general (p < 0.001), heaviness (p = 0.005), infection-related symptoms (p = 0.002), physical functioning (p = 0.006), and GCLQ total score (p < 0.001) were associated with an LVC ≥10%. By disease site, in the endometrial group, symptoms of swelling in general (p < 0.001), heaviness (p < 0.001), infection-related symptoms (p = 0.013), numbness (p = 0.01), and physical functioning (p = 0.002) were associated with an LVC ≥10%. An LVC ≥10% was not significantly associated with any of the cluster symptoms within the cervical cancer group.
Fig. 3.
The association between GCLQ and LVC ≥10% for total sample.
3.5. Predictive value of the GCLQ on patient-reported LLE on the GCLQ
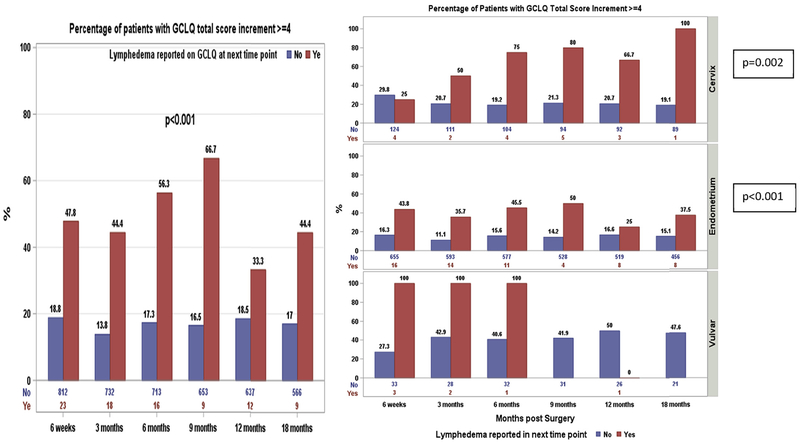
Among 377 patients with a GCLQ total score increment ≥4, 54 patients reported an LLE diagnosis after and 37 at the same time points when the increment was first observed. The incremental change of ≥4 in the GCLQ total score prior to the first LLE diagnosis was significantly associated with a patient-reported diagnosis of LLE at the next time point for the total sample (p < 0.001) (Fig. 4). By disease site, for example, of 16 endometrial cancer patients who reported LLE at 3 months, 43.8% had a GCLQ total score incremental change ≥4 at 6 weeks (prior to a patient-reported diagnosis of LLE), while of 655 endometrial cancer patients who did not report LLE at 3 months, only 16.3% had a GCLQ total score incremental change ≥4 at 6 weeks. The incremental change in the GCLQ total score prior to the patient-reported diagnosis of LLE was significantly associated with LLE at the next time point for patients with cervical cancer (p = 0.002) and endometrial cancer (p = 0.002). For the same 16 endometrial cancer patients mentioned above, the mean change in the GCLQ total score at 6 weeks (prior to receiving a diagnosis of lymphedema) was 2.3 points. In contrast, the mean change in the GCLQ total score was only 1.0 point at 6 weeks for the 655 patients with endometrial cancer who did not report LLE at 3 months.
Fig. 4.
Predictive of GCLQ on lymphedema diagnosis as reported by patient.
3.6. Reconceptualization of the definition of LLE
The objective estimate to define LLE was initially proposed as an LVC ≥10%. Among patients who were evaluable, 34% (n = 308/894) experienced a leg volume increase >10% from baseline. Of these patients, 20% (62/308) had patient-reported lymphedema on the GCLQ. Of the 121 patients with patient-reported lymphedema on the GCLQ, approximately half (62/121) experienced a leg volume increase ≥10% from baseline (Appendix III).
Due to concerns about possible measurement error and potential confounding factors, e.g., BMI, vascular insufficiency, and/or infection (as described in the accompanying, primary paper by Carlson et al.), in addition to the GCLQ’s ability as a PRO LLE symptom assessment to efficiently discern between those with and without a patient-reported diagnosis of LLE, the following steps were taken to enhance our ability to identify patients with possible undiagnosed LLE within this study: an LVC ≥10% was combined with an increase in PROs of LLE symptoms (GCLQ score of greater than 4 points from baseline), as well as any patient reporting a diagnosis of LLE during the study regardless of GCLQ score increment ≥4 or LVC ≥10%. In our sample, 83% (n = 100/121) of those who reported an LLE diagnosis were receiving some form of intervention that could influence symptoms and LVC (Appendix III).
Changes from Baseline in GCLQ Total Score (increment ≥4) and Leg Volume (increment ≥10%) and Patient-Reported LLE.
For the total cohort, 13.5% (n = 121/894) of patients indicated they were told they had a diagnosis of LLE within the 2-year study period. Based on the study parameters, LLE defined as a GCLQ total score increment ≥4 from baseline and LVC ≥10% from baseline, 17% (n = 153/894) were viewed to have LLE, with an additional 7% (n = 66/894) reporting a formal LLE diagnosis as reported by patients, for a total LLE rate of 24% (n = 219/894). Ninety-eight patients had a GCLQ total score increment ≥4 from baseline and LVC ≥10% from baseline but did not report a diagnosis of LLE on the GCLQ. It is probable that this represents undiagnosed lymphedema cases (Table 2).
Table 2.
GCLQ scores ≥4 points and LVC ≥10% from basement plus patient-reported LLE diagnosis.
| Leg volume change | GCLQ change | No lymphedema on GCLQ |
Lymphedema on GCLQ |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| Disease site | No Treatment | Treatment | No Treatment | Treatment | N | % | ||
| Cervix (n = 136) | <10% | <4 | 51 | . | . | . | 51 | 38 |
| ≥4 | 24 | . | 3 | 10 | 37 | 27 | ||
| ≥10% | <4 | 17 | . | . | 2 | 19 | 14 | |
| ≥4 | 20 | . | 2 | 7 | 29 | 21 | ||
| Endometrium (n = 719) | <10% | <4 | 289 | 6 | 1 | 8 | 304 | 42 |
| ≥4 | 138 | 3 | 7 | 23 | 171 | 24 | ||
| ≥10% | <4 | 123 | 4 | 1 | 4 | 132 | 18 | |
| ≥4 | 72 | 2 | 4 | 34 | 112 | 16 | ||
| Vulvar (n = 39) | <10% | <4 | 6 | . | . | 1 | 7 | 18 |
| ≥4 | 10 | . | 1 | 5 | 16 | 41 | ||
| ≥10% | <4 | 4 | . | . | . | 4 | 10 | |
| ≥4 | 4 | . | 2 | 6 | 12 | 31 | ||
| Total (n = 894) | <10% | <4 | 346 | 6 | 1 | 9 | 362 | 40 |
| ≥4 | 172 | 3 | 11 | 38 | 224 | 25 | ||
| ≥10% | <4 | 144 | 4 | 1 | 6 | 155 | 17 | |
| ≥4 | 96 | 2 | 8 | 47 | 153 | 17 | ||
Definition of LLE is a LV increase ≥ 10% and GCLQ score ≥ 4 change from baseline with any individual reporting a formal diagnosis of LLE.
Within the endometrial cohort, 11% (n = 82/719) of patients reported a diagnosis of LLE over the 2-year study. Applying the new reconceptualized definition of LLE (LVC ≥10% plus a GCLQ increment ≥4 from baseline, n = 112) and those with a patient-reported LLE diagnosis (n = 44), the number of patients with LLE increased to 22% (n = 156/719) within the endometrial cancer group. Within this group, 10% (N = 74/719) were viewed as undiagnosed LLE based on the combined definition criteria. For those with an LVC <10%, 41% (n = 295/719) had no significant GCLQ change from baseline (increment <4) and were viewed to not have LLE. Of the remaining 37%, 18% (n = 127/719) had significant LVC (≥10%) but no GCLQ LLE symptoms. It is unclear if this represented measurement error. Twenty percent (n = 141/719) of the endometrial group had no LVC but had more symptoms (GCLQ increment ≥4 from baseline), viewed as possibly developing LLE. Patients who reported a diagnosis of LLE, but did not have an LVC ≥10%, were difficult to analyze and may represent the transient nature of LLE or the impact of therapeutic intervention.
In the cervical and vulvar cancer groups, 18% (n = 24/136) and 38% (N = 15/39), respectively, were told they had LLE. Based on the LLE criteria (LVC ≥ 10% plus a GCLQ increment ≥4 from baseline, with formal diagnosis of LLE), 32% (n = 44/136) of the cervical and 49% (n = 19/39) of the vulvar cancer patients were noted to have LLE.
4. Discussion
This national cooperative group study sought to assess whether patient self-reported lymphedema symptoms (as measured by the GCLQ) were associated with the development of LLE. This PRO was able to distinguish between those with and without a formal LLE diagnosis (as reported by patients) and related LVC (estimate of LLE). Our results also support findings that cluster symptoms are associated with the development of LLE as measured by LVC and patient-reported LLE, confirming previous results observed with the LBCQ in identifying upper extremity lymphedema in breast cancer patients [12]. For the total sample, all GCLQ symptom clusters were significantly different between those with LLE by LVC and those without patient-reported LLE, as per GCLQ score change from baseline. In short, the PROs solidified the concept that patients’ reports of symptoms are indeed associated with developing LLE. While this is not surprising, it is important to note that the PROs are also predictive of LLE development. Moreover, noted differences of these symptoms—swelling (general), aching, heaviness, infection related, numbness and physical function—were also seen for those with LLE with LVC within the endometrial group. Smaller sample sizes in the cervical and vulvar samples limit determining implications of disease-specific speculations. It is worth noting that the accompanying, primary paper by Carlson et al. examines treatment-related factors.
In this study, the GCLQ was able to identify patients reporting a diagnosis of LLE and was also able to demonstrate a predictive value of PRO LLE symptom assessment in patients at risk for developing LLE by LVC assessment. In fact, a GCLQ score change was frequently noted in the visit prior to a formal diagnosis of LLE. Women were experiencing bothersome LLE symptoms before receiving a formal diagnosis. This important finding offers an intervention point that can be easily translated to the clinical setting to detect early-onset LLE. The GCLQ is a simple and feasible screening tool that could be offered to patients both pre-and post-treatment to monitor for potential changes that need further evaluation or referral. Establishing a mechanism for evidence-based triage and early intervention of LLE in gynecological cancer patients and survivors is clinically relevant and meaningful. Early management of symptoms (e.g., limb heaviness, aching, swelling) can positively impact the quality of these women’s lives.
Despite intensive training for each site, this study was presented with substantial challenges and limitations relating to measurement error with limb volume assessment, which are discussed in the accompanying paper. We believe, however, the innovative approach to combine LVC measurement (estimate of LLE) with PRO assessment of LLE symptoms (GCLQ) to identify undiagnosed LLE facilitated our ability to identify rates of LLE within our sample.
This large prospective trial showed that LVC is a surrogate for, but not equal to, LLE diagnosis. For the total sample, 14% reported an LLE diagnosis during the 2-year study and 34% had LVC ≥10%. Rates of LLE varied by disease site: 22% in the endometrial cancer group, 32% in the cervical cancer group, and 49% in the vulvar cancer group. Based on the existing literature, we expected the vulvar group to have the highest rate of LLE. The cervical cancer group had a higher LLE rate than the endometrial cancer group, a finding that deserves further examination.
This is the largest prospective trial examining LLE occurrence in women newly diagnosed and treated for gynecologic cancer over a 2-year period. We believe the predictive ability of the GCLQ, in combination with LVC assessment, enabled us to accurately detect those women with LLE, and to identify undiagnosed LLE. This highlights the importance and value of including PROs when examining medical outcomes and conditions.
We also undertook an exploratory analysis of the GCLQ in patients who had at least 5 valid GCLQ and LVC measurements, consistent with the accompanying, primary paper. Of the 894 evaluable patients, 650 had ≥5 valid leg volume measurements and ≥5 valid GCLQ assessments during the study (94 with cervical cancer, 528 with endometrial cancer, and 28 with vulvar cancer). That analysis demonstrated a slight improvement in p values that were already statistically significant and confirmed the overall findings in this targeted group with the best follow-up. The accompanying, primary paper noted the association of LLE with the number of lymph nodes removed. We examined the influence of a nodal count less than 8 with a GCLQ total score change ≥4 or patient-reported diagnosis of LLE or LVC ≥10%. There still was a significant risk of LLE (total sample 54%, n = 55/102; cervix 40%, n = 4/10; endometrial 49%, n = 39/80; and vulvar 83%, n = 10/12) for those with fewer than eight nodes sampled. These data may be helpful for hypothesis generation and power calculations for future studies that incorporate the GCLQ as a measurement tool (Appendix IV).
Future analyses will explore the effect that LLE has on QOL, psychological adjustment (distress and body image), physical disability, and physical function (including sexual function). We will also investigate potential protective mechanisms (e.g., social well-being, patient characteristics) that may have an effect on psychological adjustment and QOL in gynecologic cancer patients with lymphedema.
Supplementary Material
HIGHLIGHTS.
The Gynecologic Cancer Lymphedema Questionnaire (GCLQ) was able to distinguish between those with and without LLE.
The GCLQ demonstrated predictive value in patients at risk for or with early LLE.
The GCLQ could be easily translated to the clinical setting as a screening tool to detect early-onset LLE symptoms.
The GCLQ combined with an objective limb volume measurement may enhance our ability to identify LLE.
Acknowledgments
Funding
This study was supported by NCI grant R01CA162139, grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (U10 CA180822), and NRG Operations (U10CA180868). Drs. Carter, Zivanovic, and Barakat are supported in part by the NIH/NCI Memorial Sloan Kettering Cancer Center support grant P30 CA008748. Dr. Nolte is supported by NCI grant to Institution R01CA162139.
The following Gynecologic Oncology institutions participated in this study: University of Oklahoma, Women’s Cancer Center of Nevada, Cancer Research for the Ozarks NCORP, University of North Carolina at Chapel Hill, Women and Infants Hospital, Memorial Sloan Kettering Cancer Center, Georgia Center for Oncology Research and Education (CORE), Metro-Minnesota CCOP, Abington Memorial Hospital, University of California at Los Angeles Health System, Ohio State University Comprehensive Cancer Center, Froedhert and the Medical College of Wisconsin, University of New Mexico, Roswell Park Comprehensive Cancer Center, University of New Mexico, Mayo Clinic, The Hospital of Central Connecticut, University of Minnesota Medical Center-Fairview, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Delaware/Christiana Care CCOP, Virginia Commonwealth University, University of Alabama at Birmingham, Hartford Hospital, Emory University School of Medicine, Washington University School of Medicine, Gynecologic Oncology of West Michigan PLLC, Lewis Cancer and Research Pavilion at St. Joseph’s/Candler, Stony Brook University Medical Center, Saint Joseph’s Hospital and Medical Center, University of Arkansas for Medical Sciences, Cancer Research Consortium of West Michigan NCORP, Michigan Cancer Research Consortium Community Clinical Oncology Program, Main Medical Center – Scarborough Campus, William Beaumont Hospital, University of Iowa Hospitals and Clinics, University of California Medical Center at Irvine-Orange Campus, Case Western Reserve University, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Baystate Medical Center, Carle Cancer Center, Mainline Health CCOP, Southeast Cancer Control Consortium CCOP, University of Texas Southwestern Medical Center, MD Anderson Cancer Center, City of Hope, Wichita CCOP and Northside Hospital, Avera Cancer Institute, Upstate Carolina CCOP, Tulane University MBCCOP, New Hanover Regional Medical Center/Zimmer Cancer Center, and Cleveland Clinic Foundation.
Declaration of competing interest
Dr. Carter, Helen Huang, Dr. Carlson, Dr. Lockwood, Dr. Wenzel, Mr. Kauderer, Dr. Hutson, Dr. Walker, Dr. Fleury, Dr. Bonebrake, Dr. Soper, Dr. Mathews, Dr. Zivanovic, Dr. Richards, Dr. Tan, Dr. Alberts and Dr. Barakat report no conflicts of interest. Dr. Armer and Dr. Stewart report grant funding received from the NCI LEG study to their institution. Dr. Nolte reports salary support to institution from NCI R01CA162139 grant.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.09.027.
References
- [1].Cemal Y, Jewell S, Albornoz CR, et al. , Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity, Lymphatic Res. Biol 11 (2013) 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].International Society of Lymphology, The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology, Lymphology 49 (2016) 170–184. [PubMed] [Google Scholar]
- [3].Badger C, Preston N, Seers K, et al. , Physical therapies for reducing and controlling lymphedema of the limbs, Cochrane Database Syst. Rev 4 (2004) CD003141. [DOI] [PubMed] [Google Scholar]
- [4].Hayes SC, Janda M, Ward LC, et al. , Lymphedema following gynecologic cancer: results from a prospective, longitudinal cohort on prevalence, incidence and risk factors, Gynecol. Oncol 146 (2017) 623–629. [DOI] [PubMed] [Google Scholar]
- [5].Abu-Rustum NR, Alektiar K, Iasonos A, et al. , The Incidence of symptomatic lower extremity lymphedema following treatment of uterine corpus malignancies: 12-year experience at Memorial Sloan Kettering Cancer Center, Gynecol. Oncol 103 (2006) 714–718. [DOI] [PubMed] [Google Scholar]
- [6].Hareyana H, Hada K, Goto K, et al. , Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: a retrospective study, Int. J. Gynecol. Cancer 25 (2015) 751–757. [DOI] [PubMed] [Google Scholar]
- [7].Ryan M, Stainton MC, Jaconelli C, et al. , The experience of lower limb lymphedema for women after treatment for gynecologic cancer, Oncol. Nurs. Forum 30 (2003) 417–423. [DOI] [PubMed] [Google Scholar]
- [8].Beesley V, Janda M, Eakin E, et al. , Lymphedema after gynecologic cancer treatment: prevalence correlates, and supportive care needs, Cancer 109 (2007) 2607–2614. [DOI] [PubMed] [Google Scholar]
- [9].Biglia N, Zanfagnin V, Daniele A, et al. , Lower body lymphedema in patients with gynecologic cancer, Anticancer Res. 37 (2017) 4005–4015. [DOI] [PubMed] [Google Scholar]
- [10].Armer JM, Radina ME, Porock D, et al. , Predicting breast cancer-related lymphedema using self-reported symptoms, Nurs. Res 52 (2003) 370–379. [DOI] [PubMed] [Google Scholar]
- [11].Armer JM, Stewart BR, A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population, Lymphatic Res. Biol 3 (2005) 208–217. [DOI] [PubMed] [Google Scholar]
- [12].Armer MR, Fu J, Age differences in post-breast cancer lymphedema signs and symptoms, Cancer Nurs. 28 (2005) 200–207. [DOI] [PubMed] [Google Scholar]
- [13].Carter J, Raviv L, Appollo K, et al. , A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical tool to identify lower extremity lymphedema in gynecologic cancer survivors, Gynecol. Oncol 117 (2010) 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lymphoedema Framework, Best Practice for the Management of Lymphoedema. International Consensus, MEP Ltd, London, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.