Abstract
Autologous hematopoietic cell transplantation (AHCT) is standard therapy for patients with chemosensitive relapsed diffuse large B cell lymphoma (DLBCL). We performed a retrospective cohort study to delineate subsequent (conditional) and relative survival in 371 adult patients with DLBCL who underwent AHCT between 2000 and 2014 and had survived for 1-, 2-, 3-, or 5-years after transplant. The probability of overall survival at 10-years after AHCT was 62%, 71%, 77%, and 86% for the four cohorts, while that of progression-free survival (PFS) was 55%, 65%, 72%, and 81%, respectively. The respective cumulative incidence of non-relapse mortality (NRM) at 10-years after transplantation was 13%, 12%, 11%, and 8%. In multivariable analysis, older age was associated with greater mortality risk among all but 5-year survivors; relapse within the landmark time was associated with greater mortality risk in all groups. Older age and relapse within the landmark time were associated with worse PFS in all groups. Standardized mortality ratio (SMR) was significantly higher than an age-, gender-, and race-matched general population, with the magnitude of SMR decreasing as the landmark time increases (4.0 for 1-year, 3.0 for 2-year, 2.4 for 3-year, and 1.8 for 5-year survivors). Our study provides information on long-term survival and prognosis that will assist in counseling patients with DLBCL who have received AHCT. Survival improves with longer time in remission post-transplantation, although patients continue to remain at risk for NRM, underscoring the need for continued vigilance and prevention of late complications.
Keywords: Diffuse large B-cell lymphoma, Survivorship, Conditional survival, Autologous hematopoietic cell transplantation
Introduction
Autologous hematopoietic stem cell transplantation (AHCT) is standard therapy for chemosensitive relapsed diffuse large B-cell lymphoma (DLBCL) and provides the potential for cure or prolonged remission [1, 2]. Relapse is the primary cause of treatment failure after AHCT and typically occurs early post-transplantation [3]. Many patients stay in remission, and survival after AHCT for DLBCL has been well described in large patient cohorts, although there is still a gap in life expectancy as compared to the general population [4]. However, a common question in clinical practice is the likelihood of subsequent survival for a given patient conditional to staying in remission for a certain time-period post-transplantation. This conditional survival is based on the principle that the mortality risk for a cohort of patients who are alive at a given time point changes in a measurable way as time evolves from the date of transplant [5, 6]. Therefore, we performed a retrospective cohort study to delineate conditional survival and prognostic factors for late mortality in patients with DLBCL who had survived 1-, 2-, 3-, and 5- years after AHCT. We also describe survival for these cohorts relative to their general population controls.
Methods
Using our institutional Blood and Marrow Transplant Program database, we identified 371 consecutive adult patients (≥18 years) with DLBCL who received AHCT from 2000–2014. Our database prospectively collects clinical and outcome information on transplant recipients at our program. The study was conducted under review of Cleveland Clinic’s Institutional Review Board. Written informed consent was obtained from all participants.
The primary objective of our analysis was to describe and identify prognostic factors for overall survival (OS) and progression-free survival (PFS) among patients who had survived for at least 1-year (N=272), 2-years (N=232), 3-years (N=203), or 5-years (N=157) after AHCT. All patients who were alive were included, irrespective of whether their lymphoma had relapsed. The event for OS was all-cause mortality. The events corresponding to PFS were progression, relapse, or all-cause mortality. Secondary endpoints were relapse mortality (RM) and non-relapse mortality (NRM). All outcomes were determined from time of AHCT.
Patient demographics were summarized using descriptive statistics. Outcomes were estimated with Kaplan-Meier (OS, PFS) or cumulative incidence (RM, NRM). Multivariable analyses using stepwise Cox regression were performed to evaluate prognostic factors for OS and PFS; variable entry criterion of P≤0.10 and a variable retention criterion of P≤0.05 was used to identify multivariable prognostic factors. Variables considered included year of AHCT, age at AHCT, sex, race, International Prognostic Index score at diagnosis and at AHCT, B-symptoms at diagnosis and at AHCT, history of transformation, stage, disease risk, time from diagnosis to transplant, performance status at transplant, number of prior chemotherapy regimens, prior radiation therapy, prior use of rituximab, days of apheresis, CD34+ and TNC doses, days to neutrophil and platelet engraftment, duration of transplant hospitalization, and relapse prior to inclusion in the cohort (e.g., relapse within 2-years of AHCT for the 2-year survivor cohort). Results are presented as hazard ratios (HR) with their corresponding 95% confidence intervals (CI). For relative survival, we estimated standardized mortality ratio (SMR) and its 95% CI within each survivor cohort as described by Finkelstein et al [7]. SMR is the ratio of the number of observed deaths among study patients to the expected number of deaths in an age-, gender-, and race-matched healthy population.
All P-values are two-sided and P<0.05 was considered to be significant. Unless otherwise noted, analyses were performed using SAS® software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient Characteristics
Table 1 summarizes patient demographics for 1-, 2-, 3-, and 5-year survivors. Across all cohorts, around 60% of the patients were male, more than 70% were stage III/IV, more than 70% had low/low-intermediate IPI at diagnosis and at transplant, and less than 5% had poor performance status (defined as ECOG > 1 or KPS < 80). All patients received peripheral blood stem cell grafts and had chemosensitive disease. Among the four cohorts of survivors, 77–82% underwent chemotherapy mobilization, 26–28% had received prior radiation, 79–82% had received prior rituximab, and busulfan/cyclophosphamide/etoposide (Bu/Cy/VP) was the conditioning regimen for 97% of patients.
Table 1.
Patient characteristics
| CHARACTERISTIC | 1-year survivors |
2-year survivors |
3-year survivors |
5-year survivors |
|---|---|---|---|---|
| N | 272 | 232 | 203 | 157 |
| Median age at AHCT (range), years | 56 (19–78) | 56 (19–78) | 55 (19–76) | 53 (19–75) |
| Sex, N (%) | ||||
| Female | 107 (39.3) | 92 (39.7) | 83 (40.9) | 61 (38.9) |
| Race, N (%) | ||||
| African-American | 14 (5.1) | 14 (6.0) | 13 (6.4) | 11 (7.0) |
| Other | 1 (0.4) | 1 (0.4) | 1 (0.5) | 1 (0.6) |
| IPI at diagnosis, N (%) | ||||
| Low/low intermediate | 191 (74.3) | 165 (74.7) | 146 (74.5) | 115 (73.7) |
| Unknown | 15 | 11 | 7 | 1 |
| IPI at AHCT, N (%) | ||||
| Low/low intermediate | 197 (73.2) | 164 (71.6) | 146 (72.3) | 113 (72.4) |
| Unknown | 3 | 3 | 1 | 1 |
| B symptoms at diagnosis, N (%) | 121 (45.1) | 106 (46.1) | 96 (47.5) | 78 (49.7) |
| B symptoms pre-AHCT, N (%) | 20 (7.4) | 19 (8.2) | 19 (9.4) | 15 (9.6) |
| Transformed DLBCL, N (%) | 59 (21.7) | 52 (22.4) | 46 (22.7) | 38 (24.2) |
| Stage at diagnosis, N (%) | ||||
| II | 37 (13.8) | 31 (13.5) | 28 (13.9) | 22 (14.1) |
| IV | 149 (55.4) | 131 (57.2) | 118 (58.4) | 91 (58.3) |
| Median time from diagnosis to AHCT (range), months | 18.5 (0.9–372.3) | 18.4 (0.9–372.3) | 18.5 (0.9–372.3) | 16.1 (0.9–372.3) |
| ≥ 3 chemotherapy regimens prior to AHCT | 73 (26.9) | 59 (25.4) | 50 (24.6) | 35 (22.3) |
| Prior rituximab, N (%) | 221 (81.2) | 192 (82.8) | 167 (82.3) | 124 (79.0) |
| Prior radiation, N (%) | 77 (28.3) | 61 (26.3) | 57 (28.1) | 42 (26.8) |
| Performance status at AHCT, N (%) | ||||
| Good (ECOG ≤1/KPS ≥80) | 249 (95.8) | 210 (95.5) | 185 (96.4) | 144 (98.0) |
| Unknown | 12 | 12 | 11 | 10 |
| Disease risk category, N (%)* | ||||
| High | 25 (9.2) | 20 (8.6) | 16 (7.9) | 11 (7.0) |
| Chemotherapy mobilization, N (%) | 210 (77.2) | 181 (78.0) | 161 (79.3) | 129 (82.2) |
| Mean CD34+ dose (standard deviation), ×106/kg | 8.99 (8.44) | 9.04 (8.52) | 9.49 (8.92) | 10.36 (9.56) |
| Conditioning regimen, N (%) | ||||
| Other | 8 (2.9) | 6 (2.6) | 6 (3.0) | 5 (3.2) |
| Mean duration of AHCT hospitalization (standard deviation), days | 22 (3) | 22 (2) | 22 (2) | 21 92) |
| Relapse after AHCT but prior to inclusion in the cohort, N (%) | 41 (15.1) | 36 (15.5) | 34 (16.7) | 23 (14.6) |
| Median follow-up of alive patients (range), months | 95.9 (15.8–198.4) | 96.9 (26.6–198.4) | 108.2 (37.2–198.4) | 119.0 (60.0–198.4) |
AHCT indicates autologous hematopoietic cell transplantation; IPI International Prognostic Index; ECOG, Eastern Cooperative Oncology Group; Bu/Cy/VP, Busulfan/Cyclophosphamide/Etoposide
Disease risk category includes: Low risk – first complete remission; Intermediate risk – second or greater complete remission or partial remission; high-risk – primary refractory or resistant relapse or never treated
Outcomes
Table 2 shows 6-, 8-, and 10-year estimates of OS, PFS, RM, and NRM after AHCT for all 4 cohorts. OS and PFS improved with increasing survival after transplantation, while RM and NRM decreased over time. Relapse was the primary cause of mortality in 1-year survivors (65% of deaths) and 2-year survivors (57% of deaths). Among 3-year survivors, however, patients were dying equally of relapse and non-relapse causes (50% due to relapse), while more patients died of non-relapse causes among 5-year survivors (39% of deaths due to relapse). Secondary malignancy was the most common cause of non-relapse death in all four time cohorts; it accounted for 16% of all deaths among 1-year survivors, 25% in 2-year survivors, 29% in 3-year survivors, and 26% in 5-year survivors.
Table 2.
6-, 8-, and 10-year outcomes in AHCT recipients with DLBCL conditional to surviving 1-, 2-, 3-, and 5-years after transplant.
| Outcomes | 1-year survivors |
2-year survivors |
3-year survivors |
5-year survivors |
|---|---|---|---|---|
| Overall survival | ||||
| At 6 years after AHCT | 71% (65–76%) | 81% (75–86%) | 88% (82–92%) | 98% (94–99%) |
| At 8 years after AHCT | 66% (59–72%) | 75% (68–81%) | 81% (74–87%) | 90% (84–94%) |
| At 10 years after AHCT | 62% (55–68%) | 71% (64–77%) | 77% (69–83%) | 86% (78–91%) |
| Progression-free survival | ||||
| At 6 years after AHCT | 63% (57–69%) | 75% (69–80%) | 83% (77–88%) | 94% (89–97%) |
| At 8 years after AHCT | 58% 51–64%) | 69% (62–75%) | 76% (68–82%) | 85% (77–90%) |
| At 10 years after AHCT | 55% (48–61%) | 65% (58–72%) | 72% (64–87%) | 81% (73–87%) |
| Relapse mortality | ||||
| At 6 years after AHCT | 21% (16–26%) | 13% (9–18%) | 8% (4–12%) | 1% (0.3–4%) |
| At 8 years after AHCT | 23% (18–29%) | 15% (11–21%) | 11% (6–16%) | 5% (2–10%) |
| At 10 years after AHCT | 25% (19–30%) | 17% (12–23%) | 12% (8–18%) | 7% (3–12%) |
| Non-relapse mortality | ||||
| At 6 years after AHCT | 8% (5–12%) | 6% (3–10%) | 4% (2–8%) | 0.7% (0.01–3%) |
| At 8 years after AHCT | 11% (7–16%) | 10% (6–14%) | 8% (4–13%) | 5% (2–10%) |
| At 10 years after AHCT | 13% (9–18%) | 12% (8–17%) | 11% (6–16%) | 8% (4–13%) |
Data presented are estimate (95% confidence intervals).
Appendix tables 1–4 provide detailed results of univariate and multivariable analyses. Briefly, among other patient and disease characteristics evaluated in risk factor analysis, age at transplantation and duration of hospitalization post-AHCT were the major factors that had a statistically significant impact on OS and PFS. Older age (in 10-year increments) was associated with greater mortality risk among 1-year survivors with a HR of 1.37 (95% CI, 1.13–1.65), 2-year survivors with a HR of 1.49 (95% CI, 1.16–1.91), and 3-year survivors with a HR of 1.47 (95% CI, 1.11–1.95), but not among 5-year survivors. Supplementary appendix provides conditional survival by age for 1-, 2-, 3- and 5-year survivors (Appendix table 5 and Appendix Figure 1). Older age at transplant was associated with worse PFS among all 4 cohorts (HRs 1.45, 1.48, 1.59, and 1.61, respectively). Prolonged hospital stay was used as a surrogate for early post-transplant complications. Hospital stay > 24 days was associated with lower OS for all 4 cohorts, and lower PFS for 2-, 3-, and 5- year survivors (results not shown).
Standardized Mortality Ratio
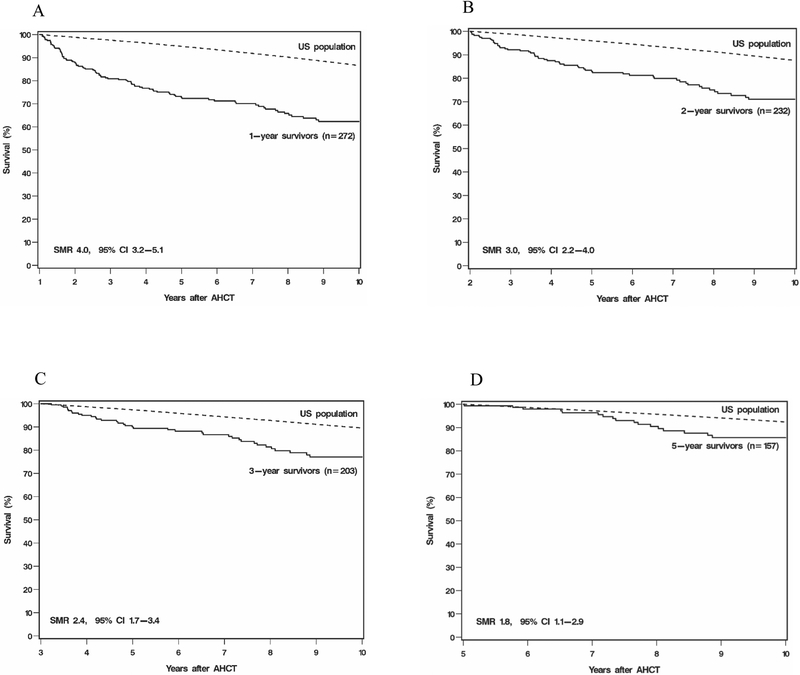
In each cohort, survival was lower when compared to the general age-, gender-, and race-matched healthy population. As shown in Figure 1, these numbers improved and approached that of healthy peers with increasing post-transplant survival. SMR was 4.0 (95% CI, 3.2–5.1) for 1-year survivors, 3.0 (95% CI, 2.2–4.0), for 2-year survivors, 2.4 (95% CI, 1.7–3.4) for 3-year survivors, and 1.8 (95% CI, 1.1–2.9) for 5-year survivors. Noticeably, the magnitude of SMR decreased as the landmark time increased.
Figure 1.
Relative mortality among survivors post-AHCT in patients with DLBCL compared to age-, gender-, and race-matched US healthy population. (A) SMR for 1-year survivors was 4.0 (95% CI, 3.2–5.1). (B) SMR for 2-year survivors was 3.0 (95% CI, 2.2–4.0). (C) SMR for 3-year survivors was 2.4 (95% CI, 1.7–3.4). (D) SMR for 5-year survivors was 1.8 (95% CI, 1.1–2.9).
Discussion
AHCT has become standard of care for a large number of otherwise life-threatening hematologic malignancies. Survivorship begins from day 0 of stem cell infusion, and is associated with variable complications [8]. Although other studies have not looked at conditional survival among lymphoma patients receiving AHCT, relative mortality among transplant survivors has been described [9, 10, 11]. Overall, findings have been mixed, with some studies showing survival approaching that of the general population while others have demonstrated continued elevation in SMR in this patient population. For instance, in a study by Bhatia et al of 854 patients with a variety of hematologic malignancies who had survived 2 years or more post-AHCT, mortality risk approached that of the healthy general population 10 years after transplantation [10]. On the other hand, in a retrospective study by Hill et al which included 309 patients with DLBCL who underwent AHCT, they concluded that although patients who do not relapse 5 years after AHCT are considered cured, their standardized mortality ratio does not return to baseline even 15 years after AHCT [12]. In a CIBMTR study of 1,367 lymphoma AHCT 2-year survivors, the SMR’s remained elevated compared to the general population at 10 years post-transplant [11]. A study in New Zealand and Australia found that mortality among 1461 allogeneic or autologous transplant recipients with different hematologic malignancies begins to approach that of the general healthy population 6 years after transplantation, but remains consistently higher even up to 10 years out [13]. Pond et al described that the mortality risk in 1386 Canadian patients 6 years after allogeneic transplantation is most likely not transplant-related, and proposed the definition of a long-term survivor as a patient who has survived 6 years after transplantation [14]. However, there is no pre-determined cut-off for when mortality in transplant recipients matches that of the healthy general population, owing to the differences in morbidity profiles each year survived post-transplantation.
Our study provides personalized survivorship information for patients with DLBCL who receive AHCT and identifies risk factors for poor conditional survival after transplantation. We show that the probability of surviving post-AHCT increases with longer follow-up post-transplantation. After surviving 1 year post-transplant, the likelihood of surviving to 10 years is approximately 62%, and after surviving the first 5 years the likelihood of surviving to 10 years increases to 86%. Relative mortality is shown to decrease with more time elapsed from transplantation, providing evidence that the survival of AHCT recipients approaches that of their healthy peers. In our analysis that has looked at conditional survival in a cohort of longer-term survivors, the relative mortality approaches that of the general population. Our data provide helpful prognostic information to counsel patients who are further out from AHCT.
An important finding to note in our study is the change in pattern of mortality as patients survive longer post-AHCT. More patients died of relapse earlier post-transplantation, however, non-relapse causes became the predominant driver of mortality in patients who had survived for 5 years. Hill et al found that relapse is the major cause of mortality within the first 2 years following AHCT, while non-relapse mortality is the major contributor of death 8 years following AHCT, with most common etiologies being respiratory failure and infection followed by secondary malignancies [12]. Majhail et al in 2009 provided evidence among a cohort of 407 Hodgkin Lymphoma patients and 960 Non-Hodgkin Lymphoma patients that the incidence of NRM exceeded 10% at the 10-year mark post-AHCT, mostly related to secondary malignancies and organ failure [11]. Taken together, our study highlights the importance of continued long-term follow-up and screening for late complications of transplantation [15, 16, 17].
In conclusion, the conditional survival of patients undergoing AHCT improves with time and approaches that of the general population after a certain period has been survived. Many factors contribute to survival, including patient or disease characteristics – whether modifiable or not -- and chemotherapy or transplant-related procedures. Our study also underscores the need for continued research on decreasing relapse risk and for surveillance for prevention and management of late complications in this population, ultimately providing evidence-based guidelines for long-term follow-up.
Supplementary Material
Highlights.
Survival improves with longer time in remission after autologous transplant for DLBCL
Mortality risk approaches that of general population with time
Older age at transplant and relapse are associated with overall mortality risk
Conflict of Interest Statement and Research Support:
None of the authors has a relevant conflict of interest to report in relationship to the work presented in this manuscript. Navneet Majhail is partially supported by a grant from the National Cancer Institute (R01-CA215134). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The results of this research were presented at the American Society of Hematology annual meeting in Atlanta, GA in December 2017.
References
- 1.Epperla N, Hamadani M Hematopoietic cell transplantation for diffuse large B-cell follicular lymphoma: current controversies and advances. Hematol Oncol Stem Cell Ther. 2017; 10(4): 277–284. DOI: 10.1016/j.hemonc.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 2.Modvig L, Vase M, d’Amore F Clinical and treatment-related features determining the risk of late relapse in patients with diffuse large B-cell lymphoma. Br J Haematol. 2017; 179(1): 75–82. DOI: 10.1111/bjh.14822 [DOI] [PubMed] [Google Scholar]
- 3.Smeland KB, Kiserud CE, Lauritzsen GF et al. A national study on conditional survival, excess mortality and second cancer after high dose therapy with autologous stem cell transplantation for non-Hodgkin lymphoma. Br J Haematol. 2016; 173(3): 432–443. DOI: 10.1111/bjh.13965 [DOI] [PubMed] [Google Scholar]
- 4.McCarthy PL Jr., Hahn T, Hassebroek A et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013; 19(7): 1116–11123. DOI: 10.1016/j.bbmt.2013.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers RM, Hill BT, Shaw BE et al. Long-term outcomes among 2-year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large B-cell lymphoma. Cancer. 2018; 124(4): 816–825. DOI: 10.1002/cncr.31114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanderWalde AM, Sun CL, Laddaran L et al. Conditional survival and cause-specific mortality after autologous hematopoietic cell transplantation for hematological malignancies. Leukemia. 2013; 27: 1139–1145. DOI: 10.1038/leu.2012.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein DM, Muzikansky A, Schoenfeld DA Comparing survival of a sample to that of a standard population. J Natl Cancer Inst. 2003; 95(19): 1434–1439. DOI: 10.1093/jnci/djg052 [DOI] [PubMed] [Google Scholar]
- 8.Epperla N, Fenske TS, Lazarus HM et al. Post-autologous transplant maintenance therapies in lymphoid malignancies: are we there yet? Bone Marrow Transplant. 2015; 50: 1393–1404. DOI: 10.1038/bmt.2015.184 [DOI] [PubMed] [Google Scholar]
- 9.Majhail NS, Rizzo JD Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013; 48: 1145–1151. DOI: 10.1038/bmt.2012.258 [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Robison LL, Francisco L et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the bone marrow transplant survivor study. Blood. 2005; 105: 4215–4222. DOI: 10.1182/blood-2005-01-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS, Bajorunaite R, Lazarus HM et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009; 147(1): 129–139. DOI: 10.1111/j.1365-2141.2009.07798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill BT, Rybicki L, Bolwell BJ et al. The non-relapse mortality rate for patients with B-cell lymphoma is greater than relapse mortality 8 years after autologous stem cell transplantation and is significantly higher than mortality rates of population controls. Br J Haematol. 2011; 152: 561–569. DOI: 10.1111/j.1365-2141.2010.08549.x [DOI] [PubMed] [Google Scholar]
- 13.Nivison-Smith I, Simpson JM, Dodds AJ et al. Relative survival of long-term hematopoietic cell transplant recipients approaches general population rates. Biol Blood Marrow Transplant. 2009; 15(10): 1323–1330. DOI: 10.1016/j.bbmt.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 14.Pond GR, Lipton JH, Messner HA Long-term survival after blood and marrow transplantation: comparison with an age- and gender- matched normative population. Biol Blood Marrow Transplant. 2006; 12(4): 422–429. DOI: 10.1016/j.bbmt.2005.11.518 [DOI] [PubMed] [Google Scholar]
- 15.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18(3): 348–371. DOI: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashmi S, Carpenter P, Khera N, et al. Lost in transition: the essential need for long-term follow-up clinic for blood and marrow transplantation survivors. Biol Blood Marrow Transplant. 2015; 21(2): 225–232. DOI: 10.1016/j.bbmt.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 17.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012; 47(3): 337–341. DOI: 10.1038/bmt.2012.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.