Abstract
Introduction
Gait and balance impairments are cardinal features of Parkinson’s disease (PD) that require cognitive input. However, the extent to which specific gait and balance characteristics relate to cognition in PD is unclear. In addition, independent models of gait and balance have not been developed from the same cohort. We aimed to i) develop models of gait and balance in a large PD cohort and ii) determine which gait and balance characteristics best related to cognition.
Methods
One hundred and ninety-nine people with PD were recruited to the Pacific Udall Center. Using six inertial sensors (APDM, Inc.), comprehensive gait measurements were collected over a two-minute continuous walk and comprehensive static balance measures were collected during a 60-second standing task. Six domains of cognition were assessed: global cognition, attention, executive function, language, memory, and visuospatial function. Correlations and hierarchical linear regression determined independent associations.
Results
Principal components analysis identified a gait model containing four domains accounting for 77.3% of total variance: pace/turning, rhythm, variability, and trunk. The balance model contained four independent domains accounting for 84.5% of total variance: sway area/jerkiness, sway velocity, sway frequency anteroposterior, and sway frequency mediolateral. Gait domains of pace/turning and variability were strongly associated with attention and executive function. Sway area and jerkiness of balance associated with attention and visuospatial function.
Conclusions
Gait and balance characteristics were associated with specific types of cognition. The specific relationships between gait or balance with cognitive functions suggests shared cerebral cortical circuitry for mobility and cognitive functions.
Keywords: Gait, Balance, neurological disease
Introduction
Balance and gait deficits are cardinal motor features of Parkinson’s disease (PD), leading to increased risk of falls and reduced quality of life [1]. It is increasingly recognized that balance and gait are not pure motor tasks, but that cognition is also essential for safe mobility. In addition, not all balance and gait impairments are alleviated with levodopa, suggesting multiple underlying mechanisms of disease, in addition to dopamine loss [2]. The complex nature of gait and balance has led to the development of comprehensive gait models to map individual measurements onto domains to eliminate redundancy and ease interpretation [3, 4]. One previous model demonstrated that balance measures are independent from gait measures, suggesting they are independent features of mobility [5].
Neural control of balance and gait are distinct, complex systems with deficits that are not correlated among people with PD [5]. Cortical control of balance and gait are thought to differ, with previous imaging work suggesting static balance is controlled by posterior cortical regions and gait controlled by anterior cortical regions [6, 7]. For example, gait and cognitive associations in PD demonstrate a key role of attention and executive function for pace and variability of gait [8]. However, cognitive associations with static balance in PD are less well understood. Furthermore, comprehensive static balance and gait measures have not been associated with cognition within the same cohort to allow for a valid comparison.
A further understanding of distinct neural correlates underlying balance and gait deficits is required to improve future medication and therapeutics, and to tailor medical intervention for individual patients. The aims of this study therefore were to: i) produce separate comprehensive models of gait and static balance to provide a framework for relating to cognition, and ii) explore associations between cognition and static balance and between cognition and gait in people with PD using objective static balance and gait measures from body-worn inertial sensors. We hypothesized that measures of static balance and gait in people with PD would demonstrate distinct associations with cognitive domains due to different underlying neural correlates.
Methods
Participants
Potential participants with PD were recruited and enrolled as part of the Pacific Udall Center Clinical Core which was comprised of three sites; 1) University of Washington and the Veterans Administration (VA) Puget Sound Health Care system in Seattle, Washington, 2) Oregon Health and Science University and the Portland VA Medical Center in Portland, Oregon and 3) Stanford University, CA. Participants with PD were included in the study if they: i) met the criteria for diagnosis of idiopathic PD using the United Kingdom Parkinson’s Disease Society Brain Bank (UKBB) criteria [9], ii) had no history of other neurological disorders that affected cognition, e.g., large-vessel stroke or severe traumatic brain injury, and iii) were able to stand unsupported for 30 seconds. Volunteers with PD were assessed ‘on’ medication both for cognitive and mobility assessments.
Clinical Assessment
Age, gender, height, and years of education were recorded for all participants. PD disease motor severity was assessed using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) [10] and the modified Hoehn and Yahr (H&Y) score [11]. Medication dose was calculated for each PD patient using the levodopa equivalent daily dose (LEDD) [12]. Participants were also assigned to a cognitive diagnostic category: no cognitive impairment (NCI), mild cognitive impairment (MCI) or Parkinson’s disease dementia (PDD) at a diagnostic consensus conference [13].
Mobility Assessment
Participants performed instrumented gait and static balance assessments wearing six Opal inertial sensors (APDM Inc., Portland, OR.). Inertial sensors were placed bilaterally on the wrists and feet as well as on the sternum and the fifth lumbar vertebrae and were attached by elastic Velcro straps. For the gait assessment, participants were asked to walk at a comfortable pace back and forth on a straight 7m walkway in a quiet hallway for two minutes, with 180° degree turns at each end of the walkway. For the static balance assessment, participants were asked to stand quietly focusing on an image ahead for 60 seconds. For both gait and balance assessments, a template was used prior to the start of the trial to achieve consistent foot placement with 10 cm between the left and right heel and a 30-degree outward rotation of the feet.
Cognitive Function
Six domains of cognition were assessed using a comprehensive battery of neuropsychological assessments. Global cognition was assessed using the Montreal Cognitive Assessment (MoCA), which is sensitive to cognitive impairment in people with PD[14]. Attention was assessed using i) Letter Number Sequencing Test (LNST), ii) Trail Making Test (TMT) part A and iii) Stroop reading score. Executive function was assessed using i) Semantic Fluency (Animals), ii) TMT B-A, iii) Stroop Interference score and iv) phonemic fluency (letter F). Language was assessed using the Boston naming test. Memory was assessed using the i) Hopkins Verbal Learning Test (HVLT) immediate and delayed recall and ii) Logical Memory I and II. Visuospatial function was assessed using the Judgement of Line Orientation (JoLO).
Data Analysis
Data were initially inspected using histograms and boxplots and assessed for normality and transformed where needed (transformed variables are highlighted in the tables). Analyses were divided into two stages.
I). Principal components analysis for domains of balance and gait
In order to provide a framework upon which to base our analysis, principal components analysis (PCA) was performed to create separate models containing domains of gait and balance. First, seventeen characteristics of gait were entered into a model using an eigenvalue of greater than one and a varimax rotation was applied. Separately, 13 characteristics of static balance were entered into a PCA model constructed in the same way. A correlation value of ≥0.6 was used for factor loadings for both models.
II). Associations of cognition with gait and balance measures
For the statistical analysis, gait and balance domains were first calculated from the PCA. Domain scores were calculated by summing the Z score of each gait and static balance characteristic for each domain and dividing by the number of characteristics per domain. Preliminary partial correlations (controlling for age, gender, years of education, disease severity [MDS-UPDRS III], disease duration and site) between gait domains and cognitive assessments and balance domains and cognitive assessments were first assessed as a data reduction technique. Following this, to identify independent associations between gait and cognition and balance and cognition, partial correlations which reached a significance level of p ≤.01 were entered into a hierarchical regression model using the backward method. Demographic characteristics were entered into the first block (age, gender, years of education, disease severity [MDS-UPDRS III], disease duration and site). Separate models were used for gait and balance; all domains were entered into the second block. Cognitive assessments which reached the significance threshold from partial correlations were entered as the independent variable. A stringent p value of ≤.01 determined significance throughout analysis in order to reduce type I error.
Results
Participants
A total of 199 participants with idiopathic PD were recruited to the study. Table 1 shows the demographic characteristics for participants who completed clinical, mobility, and cognitive assessments. The PD group contained 126 males and 73 females with a mean age of 67.7 ± 8.2 and total years of education of 16.4 ± 2.3. On average, participants with PD had a disease duration of 8.6 ± 5.4 years with an average MDS-UPDRS III of 25.0 ± 13.0, H & Y of 2.1 ± 0.5 and LEDD of 654.4 ± 465.9. The average total years of education was 16.4 ± 2.3. Performance on cognitive assessments is presented in Table 1.
Table 1.
Demographic, clinical and cognitive characteristics, Mean (SD) of PD participants.
| Measure | PD (n=199) Mean (SD) | |
|---|---|---|
| Demographics | Age (years) | 67.7(8.2) |
| Gender (M/F) | 126/73 | |
| Years of Education | 16.4 (2.3) | |
| MDS-UPDRS III | 25.0 (13.0) | |
| H & Y | 2.1 (0.5) | |
| LEDD (mg/day) | 654.4 (465.9) | |
| Disease Duration (years) | 8.6 (5.4) | |
| Cognitive Status | NCI (101) / MCI+ (74) / PDD (24) | |
| Global Cognition | MoCA | 25.0 (4.0) |
| Attention | Letter Number Sequencing | 9.53 (2.54) |
| Trail Making A | 37.0 (20.3) | |
| Stroop Reading | 84.6 (16.6) | |
| Executive Function | Semantic Fluency | 19.0 (6.0) |
| Trail Making B-A | 58.4 (46.7) | |
| Stroop Interference | 34.5 (10.2) | |
| Phonemic Fluency | 15.0 (5.0) | |
| Memory | HVLT-R Immediate | 23.6 (6.0) |
| HVLT-R Delayed | 8.08 (3.17) | |
| Logical Memory I | 12.5 (4.2) | |
| Logical Memory II | 11.5 (4.5) | |
| Language | Boston Naming Test | 28.6 (1.7) |
| Visuospatial | Judgement of Line Orientation | 12.0 (2.0) |
[Abbreviations: MDS-UPDRS (Movement Disorders Society Unified Parkinson’s disease rating scale), H & Y (Modified Hoehn & Yahr), LEDD (Levodopa Equivalent Daily Dose), NCI (no cognitive impairment), MCI (mild cognitive impairment), PDD (Parkinson’s disease dementia), MoCA (Montreal Cognitive Assessment), HVLT-R (Hopkins Verbal Learning Test-Revised)]
Independent models of balance and gait
Gait measures are presented in Table 2 for all participants. A total of 17 gait characteristics were entered into the PCA, yielding four factors (pace and turning, gait rhythm, gait variability, and trunk movement) accounting for 77.33% of total variance (Supplementary Figure 1A). Descriptive static balance data are presented in Table 2. A total of 13 static balance characteristics were entered into a separate PCA that contained four independent factors (sway area and jerkiness, sway velocity, sway frequency mediolateral, and sway frequency anteroposterior) accounting for 84.51% of total variance (Supplementary Figure 1B).
Table 2.
Gait and static balance characteristics, Mean (SD) for all participants with Parkinson’s disease.
| Gait and Balance Characteristics | PD (n=199) Mean (SD) | ||
|---|---|---|---|
| Gait | Pace and Turning | Gait Speed (m/sec) | 0.96 (0.20) |
| Stride Length (m) | 1.08 (0.20) | ||
| Foot Strike Angle (deg) | 18.76 (6.46) | ||
| Turn Duration (sec) | 2.50 (0.41) | ||
| Turn Velocity (deg/sec) | 151.51 (34.58) | ||
| Number of steps in Turn (#) | 4.14 (0.83) | ||
| Rhythm | Gait cycle duration (sec) | 1.14 (0.11) | |
| Stance Time (% GCT) | 61.15 (2.09) | ||
| Swing Time (% GCT) | 38.85 (2.09) | ||
| Variability | Stride Length SD (m) | 0.06 (0.02) | |
| Foot Strike Angle SD (deg) | 2.51 (0.92) | ||
| Gait Cycle Duration SD (sec) | 0.04 (0.02) | ||
| Stance Time SD (% GCT) | 1.12 (0.53) | ||
| Swing Time SD (% GCT) | 1.12 (0.53) | ||
| Trunk Movement | ROM trunk coronal (deg) | 4.94 ± 2.77 | |
| ROM Trunk sagittal plane (deg) | 4.35 ± 1.89 | ||
| ROM trunk transverse plane (deg) | 8.71 ± 4.86 | ||
| Balance | Sway Area & Jerk | Sway Area† | 1.86 (7.15) |
| Jerk AP† | 1.55 (10.84) | ||
| Jerk ML† | 1.06 (5.93) | ||
| RMS AP† (m/s2) | 0.11 (0.08) | ||
| RMS ML† (m/s2) | 0.05 (0.05) | ||
| Sway Velocity | Velocity AP† (m/s) | 0.39 (0.35) | |
| Velocity ML† (m/s) | 0.17 (0.16) | ||
| Sway Frequency ML | Frequency ML (Hz) | 0.79 (0.35) | |
| 95 Frequency ML (Hz) | 2.29 (0.54) | ||
| Centroidal Frequency ML (Hz) | 1.00 (0.29) | ||
| Sway Frequency AP | Frequency AP (Hz) | 0.55 (0.24) | |
| 95 Frequency AP (Hz) | 1.64 (0.46) | ||
| Centroidal Frequency AP (Hz) | 0.69 (0.20) | ||
[Abbreviations: AP (Anterior-posterior), ML (Mediolateral), GCT (Gait Cycle Time), SD (StandardDeviation)]
Values log transformed for statistical analysis.
Associations between gait and cognition
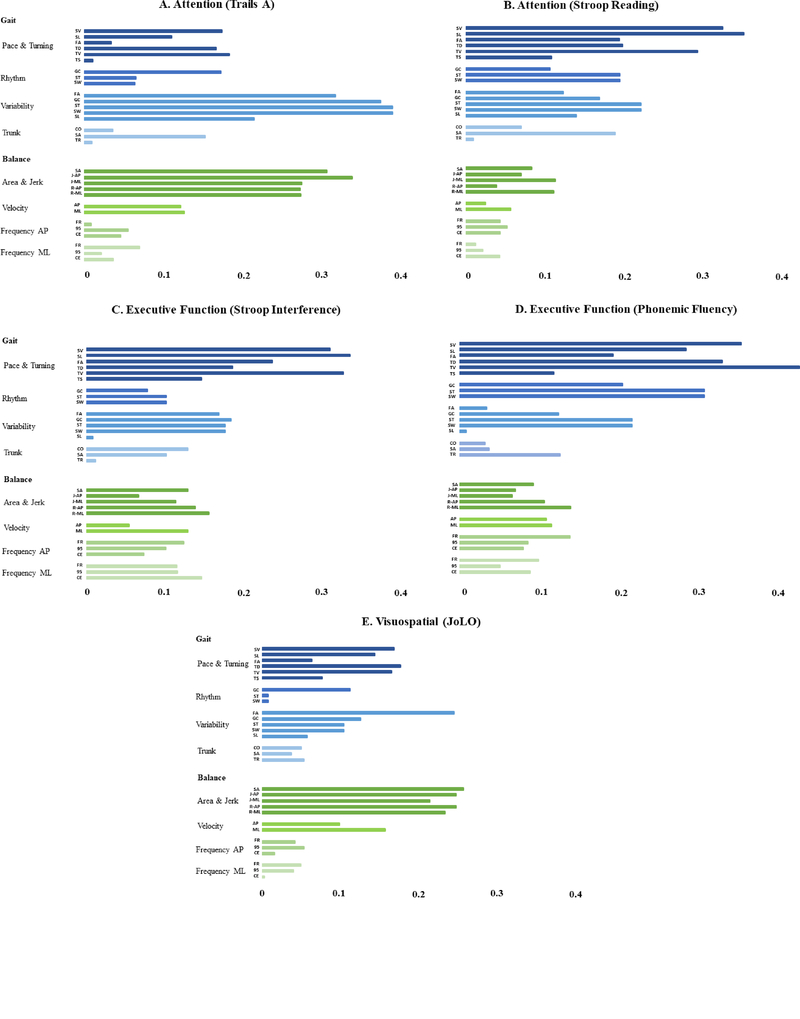
Hierarchical regression models revealed a number of independent predictors between gait and cognition (Table 3). Slower pace and turning domain of gait was associated with poorer attention (stroop reading score, β.314, P=<.001), poorer executive function (stroop interference, β.314, P=.000 and phonemic fluency, β.275, P=<.001). In contrast, increased variability domain of gait (i.e. reduced stability) was associated with poorer attention (Trails A, β.315, P=<.001). There were no associations for rhythm and trunk domains of gait. Associations (partial correlations) between individual gait metrics in each domain and cognition are summarized in Figure 1 to guide interpretation.
Table 3.
Hierarchical regression models of gait and static balance associations with cognition.
| Gait Domain/Balance Domain | Cognitive Domain | Cognitive Test | β Value | P Value | |
|---|---|---|---|---|---|
| Gait | |||||
| Pace and Turning | Attention | Stroop Reading | .314 | .000 | |
| Executive Function | Stroop Interference | .314 | .000 | ||
| Executive Function | Phonemic Fluency | .275 | .000 | ||
| Variability | Attention | Trails A | .315 | .000 | |
| Balance | |||||
| Sway and Jerk | Attention | Trails A | .185 | .007 | |
| Sway and Jerk | Visuospatial | JoLO | −.375 | .000 |
Figure 1:
Partial correlations between gait (blue) and balance (green) domains with measures of Attention; A) Trails A and B) Stroop Reading, Executive Function C) Stroop Interference and D) Phonemic Fluency, Visuospatial E) JoLO. Gait characteristics by domain; pace and turning; SV= step velocity, SL= stride length, FA= foot strike angle, TD= turn duration, TV= turn velocity, TS= number of steps in turn. Rhythm; GC= gait cycle duration, ST= stance time, SW= swing time. Variability as previous but SD. Trunk; CO=coronal ROM, SA= sagittal ROM, TR= transverse ROM. Balance characteristics by domain; area & jerk; SA= sway area, J-AP= jerk anteroposterior, J-ML= jerk mediolateral, R-AP= RMS Anteroposterior, R-ML= RMS mediolateral. Velocity; AP= anteroposterior, ML=mediolateral. Frequency AP; FR= frequency, 95= 95 frequency, CE= centroidal frequency. Frequency ML as previous.
Associations between balance and cognition
Hierarchical regression models revealed two independent associations between static balance and cognition (Table 3). Increased sway area and jerkiness was associated with poorer attention (trails A, β. 185, P=.007) as well as poorer visuospatial function (JoLO, β−.375, P=<.001). No other domains of static balance correlated with cognition. Associations (partial correlations) between specific balance metrics within each domain and cognition are summarized in Figure 1 to guide interpretation.
Discussion
This study aimed to identify models of gait and static balance to use as a framework to determine whether cognitive associations with gait and balance domains were overlapping or specific. We identified two independent models of gait and balance in 199 people with PD, both of which described four independent domains. Several domains of gait and balance were significantly associated with performance on cognitive tests but the patterns of association were distinct between the two mobility tests. Measures of pace, including turning, and variability related to executive function and attention. In contrast, measures of sway area and jerkiness of standing balance were associated with attention and visuospatial function. This confirms our hypothesis that associations with cognition differ between gait and balance, suggesting at least partially distinct involvement of underlying neural pathways.
Separate models of gait and balance have been identified
This is the first study to create independent models of gait and balance derived from the same cohort. A previous model that included both gait and static balance measures demonstrated that all gait metrics were independent domains from all balance metrics [5]. Similar to previous gait models in older adults and people with PD, we found measures of pace, rhythm and variability loaded onto separate gait domains, consistent with separate neural control mechanisms [3–5]. In contrast to previous models, however, our framework included characteristics of turning and trunk movement during gait. Indeed, turning is an essential component of gait with the majority of steps in the home and community environment occurring during turns [15]. Measures of turning loaded onto the domain that contained characteristics of pace, such as gait speed and stride length. This interdependence of pace and turning may signify the similar spatial aspects of these tasks or suggest a common neural or pathologic mechanism in PD for both pace and turning characteristics. Trunk movement during gait formed a separate domain, similar to a previous model [5]. Trunk movement represents dynamic postural stability which is particularly affected in people with PD due to rigidity and bradykinesia [16]. Our factor loading suggests these upper body measures are controlled independently from other gait variables and therefore should be assessed as part of a comprehensive gait measurement.
Cognitive associations between gait and balance differed
Associations between gait and cognition and between static balance and cognition were distinct, suggesting that the cognitive resources needed for the two tasks are different. Gait associations with cognition were primarily related to characteristics from the pace and turning domain with attention and executive function measures of cognition. These findings support previous work in PD that identified pace and variability measures of gait to be related to cognition more so than gait temporal measures, e.g., rhythm [8]. Cognitive associations of turning during gait are less well understood in PD but previous work in older adults suggests turning is highly associated with cognition [17]. The complexity of turning, a task that requires control of multiple components such as sensory integration, postural transitions and inter-limb coordination, is thought to be dependent on attention/executive function, as demonstrated in our results. Turning quality, such as slow velocity and excessive steps have been shown to be impaired very early in PD, even when gait speed is normal [18]. The close relationship between turning and attention/executive function is particularly important in PD due to their impact on falls risk [19] and therefore cognitive training to reduce falls may be critical for rehabilitation.
Interestingly, both attention and executive function were associated with pace and turning, but only attention associated with variability of gait. Although attention and executive function are both frontal cognitive functions, the domains are distinct and dependent on separate neurotransmitter systems; attention being dependent on the cholinergic network and executive function the dopaminergic network [20, 21]. This distinction has also been recognized in functional connectivity; those with faster gait speed have stronger functional connectivity within the frontoparietal control network which is heavily involved with executive function [22]. Comparatively, those with lower gait variability have stronger functional connectivity between the dorsal attention network and default network which has been linked to sustained attention [22]. In line with our findings, characteristics of pace and variability have been found to be sensitive predictors of cognitive decline in PD [23]. Our data suggests that as well as measures of gait speed and step length, turning characteristics were also highly correlated with attention and executive function (Figure 1). Therefore, we hypothesize that turning is also sensitive to cognitive decline and PD dementia. Thus, therapeutic targets should be tailored to gait components that are highly dependent on frontal cognitive function, including pace, turning and variability.
In contrast to gait and cognition, visuospatial function and attention were associated with measures of static balance. Consistent with our findings, previous work in a smaller cohort of people with PD demonstrated static measures of balance to be associated with visuospatial function [24]. This indicates that, unlike gait, balance measures during standing are associated with posterior rather than anterior cortical control, which is in agreement with previous imaging findings [7]. In addition, static balance may be highly dependent on vision, with visual deficits common in PD patients this further increases falls risk [25]. These findings suggest posterior cerebral cortical targets are needed to improve balance function via cortical control. Dopamine replacement therapy as well as deep brain stimulation (DBS) in the basal ganglia generally improve pace of gait but not balance, which can worsen and increase falls [26]. Studies using DBS of the pedunculopontine nucleus have aimed to improve balance impairments by increasing acetylcholine to the posterior cortex, but to date have proved difficult due to the intricate nature of the target and the multiple cell types that are positioned there [27]. A previous pharmacological study using cholinesterase inhibitors to increase acetylcholine showed improvements in static balance tasks but further work is needed to validate these findings with more comprehensive measures [28].
Clinical Implications
The findings from this study have clinical implications for treatment of balance and gait in PD. Gait and balance are independent behaviors and both are composed of multiple, independent domains that will not likely respond similarly to pharmacological, neurophysiological, or exercise/rehabilitation interventions. Recent evidence has suggested exercise therapy with a cognitive component may help improve gait and reduce falls [29]. Future studies should determine whether specific types of cognitive training can improve balance or gait and reduce falls. Our findings suggest that the type of cognitive task may enhance rehabilitation dependent on the specific mobility deficit.
Strengths and Limitations
This study had a number of strengths, including a large cohort of PD subjects and comprehensive measures of gait, static balance, and cognition. The study also had limitations. First, we did not compare our findings to an age-matched control cohort. However, cognitive and mobility differences between older adults and those with PD are well established, and our main aim was to further understand differences in associations within the same cohort. Second, our neuropsychological assessment battery contained a higher number of attention and executive function tests compared with language and visuospatial assessments and therefore our results may be slightly biased towards frontal cognitive function. Third, we characterized our cognitive domains largely in line with the Movement Disorders Task Force [30], however there are discrepancies across the literature regarding classification of neuropsychological assessments and therefore interpretation may be subjective. Finally, in order to account for multiple comparisons we associated domains of gait and balance rather than individual characteristics but in turn this may reduce specificity and therefore we used a correlation figure to guide interpretation.
Conclusions
This is the first study to identify separate models of gait and static balance from the same cohort of PD. Furthermore, cognitive associations with gait and balance were distinct indicating differing underlying mechanisms of disease. This may lead to different clinical targets for treatment of these two measures of mobility.
Supplementary Material
Highlights.
Gait and balance require higher cognitive control in PD
The extent to which cognitive associations differ between gait and balance is unclear
Measures of gait, static balance and cognition were assessed in 199 people with PD
Pace and variability of gait were associated with attention and executive function
Measures of static balance were associated with attention and visuospatial function
Acknowledgements
This work was supported by the Pacific Udall Center (P50 NS062684) and the Department of Veterans Affairs Northwest Parkinson’s Disease Research, Education and Clinical Care Center. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1TR002369 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Funding: This work was supported by the Pacific Udall Center (P50 NS062684) and the Department of Veterans Affairs Northwest Parkinson’s Disease Research, Education and Clinical Care Center.
Footnotes
Financial disclosures: Dr. Horak has a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ, Determinants of disability and quality of life in mild to moderate Parkinson disease, Neurology 70(23) (2008) 2241–2247. [DOI] [PubMed] [Google Scholar]
- [2].Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB, Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease, Mov Disord 30(10) (2015) 1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L, Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach, J Gerontol A Biol Sci Med Sci 68(7) (2013) 820–7. [DOI] [PubMed] [Google Scholar]
- [4].Verghese J, Wang C, Lipton RB, Holtzer R, Xue X, Quantitative gait dysfunction and risk of cognitive decline and dementia, J Neurol Neurosurg Psychiatry 78(9) (2007) 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Horak FB, Mancini M, Carlson-Kuhta P, Nutt JG, Salarian A, Balance and Gait Represent Independent Domains of Mobility in Parkinson Disease, Physical Therapy 96(9) (2016) 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJH, Albin RL, Müller MLTM, Gait speed in Parkinson disease correlates with cholinergic degeneration, Neurology 81(18) (2013)1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Müller MLTM, Albin RL, Kotagal V, Koeppe RA, Scott PJH, Frey KA, Bohnen NI, Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease, Brain 136(11) (2013) 3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morris R, Lord S, Bunce J, Burn D, Rochester L, Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease, Neurosci Biobehav Rev 64 (2016) 326–45. [DOI] [PubMed] [Google Scholar]
- [9].Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases, Journal of Neurology, Neurosurgery & Psychiatry 55(3) (1992) 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, U.R.T.F. Movement Disorder Society, Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results, Mov Disord 23(15) (2008) 2129–70. [DOI] [PubMed] [Google Scholar]
- [11].Hoehn MM, Yahr MD, Parkinsonism: onset, progression, and mortality, Neurology 50(2) (2001) 318–318. [DOI] [PubMed] [Google Scholar]
- [12].Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE, Systematic review of levodopa dose equivalency reporting in Parkinson’s disease, Mov Disord 25(15) (2010) 2649–53. [DOI] [PubMed] [Google Scholar]
- [13].Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu SC, Edwards KL, Montine TJ, Leverenz JB, Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics, J Parkinsons Dis 3(2) (2013) 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment, Journal of the American Geriatrics Society 53(4) (2005) 695–699. [DOI] [PubMed] [Google Scholar]
- [15].Mancini M, El-Gohary M, Pearson S, McNames J, Schlueter H, Nutt JG, King LA, Horak FB, Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential, NeuroRehabilitation 37(1) (2015) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Adkin AL, Bloem BR, Allum JHJ, Trunk sway measurements during stance and gait tasks in Parkinson’s disease, Gait & Posture 22(3) (2005) 240–249. [DOI] [PubMed] [Google Scholar]
- [17].Mancini M, Schlueter H, El-Gohary M, Mattek N, Duncan C, Kaye J, Horak FB, Continuous Monitoring of Turning Mobility and Its Association to Falls and Cognitive Function: A Pilot Study, J Gerontol A Biol Sci Med Sci 71(8) (2016) 1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El-Gohary M, Pearson S, McNames J, Mancini M, Horak F, Mellone S, Chiari L, Continuous Monitoring of Turning in Patients with Movement Disability, Sensors 14(1) (2014) 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goodwin VA, Pickering R, Ballinger C, Roberts H, McIntosh E, Lamb S, Nieuwboer A, Rochester L, Ashburn A, o.b.o.t.P.P.D. Group, A multi-centre, randomised controlled trial of the effectiveness of PDSAFE to prevent falls among people with Parkinson’s: study protocol, BMC Neurology 15(1) (2015) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kehagia AA, Barker RA, Robbins TW, Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease, The Lancet Neurology 9(12) (2010) 1200–1213. [DOI] [PubMed] [Google Scholar]
- [21].Svenningsson P, Westman E, Ballard C, Aarsland D, Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment, The Lancet Neurology 11(8) (2012) 697–707. [DOI] [PubMed] [Google Scholar]
- [22].Lo O-Y, Halko MA, Zhou J, Harrison R, Lipsitz LA, Manor B, Gait Speed and Gait Variability Are Associated with Different Functional Brain Networks, Frontiers in Aging Neuroscience 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ, Rochester L, Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease, The Journals of Gerontology: Series A (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill E, Stuart S, Lord S, Del Din S, Rochester L, Vision, visuo-cognition and postural control in Parkinson’s disease: An associative pilot study, Gait Posture 48 (2016) 74–76. [DOI] [PubMed] [Google Scholar]
- [25].Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR, Visual dysfunction in Parkinson’s disease, Brain 139(11) (2016) 2827–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].St George RJ, Carlson-Kuhta P, Nutt JG, Hogarth P, Burchiel KJ, Horak FB, The effect of deep brain stimulation randomized by site on balance in Parkinson’s disease, Movement Disorders 29(7) (2014) 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang J-W, Zhang Y-Q, Zhang X-H, Wang Y-P, Li J-P, Li Y-J, Deep Brain Stimulation of Pedunculopontine Nucleus for Postural Instability and Gait Disorder After Parkinson Disease: A Meta-Analysis of Individual Patient Data, World Neurosurgery 102 (2017) 72–78. [DOI] [PubMed] [Google Scholar]
- [28].Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JCT, Whone AL, Ben-Shlomo Y, Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial, The Lancet Neurology 15(3) (2016) 249–258. [DOI] [PubMed] [Google Scholar]
- [29].Mirelman A, Rochester L, Maidan I, Del Din S, Alcock L, Nieuwhof F, Rikkert MO, Bloem BR, Pelosin E, Avanzino L, Abbruzzese G, Dockx K, Bekkers E, Giladi N, Nieuwboer A, Hausdorff JM, Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial, The Lancet 388(10050) (2016) 1170–1182. [DOI] [PubMed] [Google Scholar]
- [30].Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M, Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines, Mov Disord 27(3) (2012) 349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.