Abstract
Background:
Chronic rhinosinusitis (CRS) is a heterogeneous chronic inflammatory disease subdivided based on presence or absence of nasal polyps (NPs). Histological features of CRS with NPs (CRSwNP) include inflammatory cell infiltration and excessive fibrin deposition in nasal polyps. Thrombin activatable fibrinolysis inhibitor (TAFI) is an enzyme that plays an antifibrinolytic role in the body. The significance of TAFI has been documented in chronic inflammatory diseases including chronic lung disease; however, it has not been evaluated in the pathogenesis of NPs.
Objective:
The objective of this study was to evaluate the potential role of TAFI in the pathogenesis of NPs.
Methods:
Nasal lavage fluids were collected from control subjects and patients with CRS. We measured levels of thrombin/anti-thrombin complex (TATc) and TAFI protein by ELISA.
Results:
TATc levels in nasal lavage fluids were significantly increased in patients with CRSwNP and CRS without NPs (CRSsNP) compared with control subjects, and levels of TAFI in nasal lavage fluids were also significantly elevated in CRSwNP subjects compared with control subjects and CRSsNP subjects. There was a significant correlation between levels of TATc and TAFI in nasal lavage fluids. Interestingly, patients with CRS and asthma showed increased TATc and TAFI in nasal lavage fluids compared to CRS without asthma, especially in patients with CRSwNP.
Conclusions:
Elevated TATc and TAFI in nasal passages of patients with CRSwNP might participate in fibrin deposition in NPs and may play a role in the pathogenesis of CRSwNP and asthma.
Keywords: CRS, fibrin, fibrinolysis, coagulation, thrombin, asthma and thrombin-activatable fibrinolysis inhibitor
Capsule Summary:
Patients with CRSwNP and asthma show high levels of TATc and TAFI in the nose and sinuses which may contribute to the fibrin deposition in nasal polyps.
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the upper airways and paranasal sinuses of at least 12 weeks’ duration. CRS is one of the most common chronic diseases in the United States, affects more than 10 million Americans and greatly impairs quality of life in affected patients (1–4). Progress is being made in advancing the understanding of the complex pathogenic mechanisms (5,6). CRS is a heterogeneous disease and is frequently divided into two groups based on the presence or absence of nasal polyps (NPs): CRS with NPs (CRSwNP) and CRS without NPs (CRSsNP). Each type of CRS shows distinct and varied inflammatory patterns and heterogeneity (7,8). Pathological features of CRSwNP tissue often include intense eosinophilic infiltration and a Th2-biased cytokine profile, especially in western countries (9). Clinical features of CRSwNP usually include nasal obstruction and olfactory loss, and all forms of CRS are frequently linked to comorbidities such as asthma. However, it is still unclear why severe asthma shows a particularly high comorbidity with CRSwNP (10,11).
Nasal polyps (NPs) usually present in the vicinity of the middle nasal meatus or paranasal sinuses as edematous masses. Histological features of NPs include an intense edematous stroma filled with plasma proteins, accompanied by infiltration of inflammatory cells (12). Another pathological feature of NPs is excessive deposition of fibrin (13). Fibrin deposition occurs by the activation of the coagulation cascade, which ordinarily mediates thrombosis, host defense and tissue repair (14). Although the activation of the coagulation cascade and fibrin deposition are well controlled in homeostasis, dysregulation of the coagulation cascade is involved in the etiology of many diseases including rheumatoid arthritis, adult respiratory distress syndrome and severe asthma (15–17). A previous report from our laboratory demonstrated low levels of d-dimer, a fibrin degradation product, in NPs, suggesting that fibrinolysis is impaired in NPs (13). Plasmin that is generated through cleavage of plasminogen either by urokinase plasminogen activator (u-PA) or tissue plasminogen activator (t-PA) degrades fibrin in the fibrinolysis cascade. Our previous study demonstrated profound down regulation of t-PA in NPs, which also may contribute to the fibrinolytic impairment and accumulation of cross-linked fibrin in NP tissue (13).
Thrombin is a serine protease generated through proteolytic cleavage of its inactive precursor, prothrombin (18). Thrombin has vital roles in the coagulation cascade that include the conversion of fibrinogen to fibrin and activation of coagulation factors FV, FVIII and FXIII that participate in the coagulation cascade by promoting fibrin formation in the tissue. Importantly, FXIIIA, a transglutaminase that plays a role in the final stage of the coagulation cascade, is elevated in NPs, accompanied by increased levels of albumin and other plasma proteins in nasal tissue (19). Clinically, the thrombin/anti-thrombin complex (TATc) is an evanescent marker of thrombin generation because thrombin itself can be degraded immediately. Elevated concentrations of TATc have been reported in sputum of patients with asthma (20). It has also been documented that TATc levels in bronchoalveolar lavage (BAL) fluids are elevated after allergen challenge in asthmatic patients (21), which implies the existence of local activation of coagulation in asthma.
In the fibrinolysis cascade, initial cleavage of fibrin by plasmin generates C-terminal lysine residues on the fibrin that are capable of binding both plasminogen and plasminogen activator. Generation of C-terminal lysine residues stimulates plasminogen activator-mediated plasminogen activation and accelerates fibrinolysis. Thrombin activatable fibrinolysis inhibitor (TAFI) regulates this feedback by cleaving the newly formed C-terminal lysine residues from the fibrin surface, and decreasing cofactor activity (22). TAFI is a carboxypeptidase zymogen originally identified in plasma, which is synthesized mainly in the liver and by megakaryocytes (23–25). Clinically, elevated plasma levels of TAFI are associated with venous thrombosis (26), and some TAFI gene polymorphisms are considered to be risk factors for venous thrombosis (27,28). It is also known that circulating TAFI levels are enhanced in several diseases including rheumatoid arthritis and inflammatory bowel disease (29,30), reflect the degree of inflammation and can be decreased by treatment (30,31). Thrombomodulin (TM) is a transmembrane glycoprotein expressed on the surface of all vascular endothelial cells and many other types of cells, which contributes to maintenance of homeostasis during the inflammatory response (32). Thrombin and thrombin/TM complexes can activate TAFI. Thus, thrombin, as a component of the coagulation cascade, also regulates the fibrinolysis cascade.
In this study, we evaluated known mechanisms that down regulate the fibrinolysis cascade in order to better understand the reduced fibrinolytic activity in NPs. We specifically tested TAFI and found that TAFI is elevated in the nasal lavage fluids from CRSwNP subjects, especially patients with CRSwNP and comorbid asthma. Our results provide insight into the mechanisms of fibrin accumulation in NP and also have uncovered a heretofore-unknown pathophysiological connection between CRSwNP and asthma.
Materials and Methods
Patient Recruitment and clinical sample collection
Patients with CRS were recruited from the Allergy-Immunology and Otolaryngology Clinics of the Northwestern Medical Group (NMG) and the Northwestern Sinus Center at NMG. All subjects gave informed consent, and the protocol and consent forms governing procedures for the study have been approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine. All subjects met the criteria for CRS as defined by the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force (33,34). Sinonasal and NP tissues were obtained during routine functional endoscopic sinus surgery in patients with CRS. Details of the subjects’ characteristics are included in Table 1. Further details are provided in the online supplement.
Table 1.
Characteristics of the subjects
| nasal lavage fluids | nasal tissue extracts | ||||||
|---|---|---|---|---|---|---|---|
| Control | CRSsNP | CRSwNP | Control | CRSsNP | CRSwNP | ||
| (n=15) | (n=15) | (n=21) | UT (n=8) | UT (n=7) | UT (n=7) | NP (n=15) | |
| Gender, male/female | 6/9 | 4/11 | 14/7* | 1/7 | 5/2 | 5/2 | 13/2* |
| Ethnicity, White/non-White/unknown | 6/4/5 | 13/1/1* | 14/5/2 | 7/0/1 | 6/1/0 | 5/2/0 | 8/5/2 |
| Age, years | 36.1±10.4 | 45.2±15.4 | 47.8±13.0** | 58.1±9.4 | 36.3±12.9* | 43.4±13.0* | 49.1±11.2# |
| Comorbidity, yes/no/unknown | |||||||
| Asthma | 0/15/0 | 6/9/0 | 9/12/0 | 1/7/0 | 3/4/0 | 4/3/0 | 6/9/0 |
| Atopy | 4/6/5 | 6/8/1 | 11/9/1 | 3/0/5 | 4/3/0 | 4/1/2 | 12/1/2 |
| Steroid use, yes/no | |||||||
| Oral | 0/15 | 0/15 | 3/18 | 1/7 | 1/6 | 2/5 | 2/13 |
| Inhaled | 0/15 | 2/13 | 3/18 | 0/8 | 1/6 | 2/5 | 1/14 |
| Intranasal | 2/13 | 2/13 | 2/19 | 0/8 | 2/5 | 2/5 | 4/11 |
p<0.05, vs. Control
p<0.01, vs. Control
p<0.05, vs. CRSsNP
Nasal lavage fluid sampling
Nasal lavage fluids were obtained according to a previously described protocol (35). The lavage fluids were centrifuged for 10 minutes at 3000 rpm, and the supernatants were then concentrated 2-fold in Amicon Ultra-4 10K Centrifugal Filter Units (EMD Millipore, Billerica, MA) at 3,000 rpm for 10 minutes at 4°C. The retentates were stored at −80°C.
Extraction of total protein from nasal tissue
Protein was extracted and measured from surgical uncinate tissue (UT) or NP samples as previously described, with additional details provided in the online supplement (35,36).
Measurement of total protein from nasal tissue and nasal lavage
For normalization purposes, total protein concentration was measured with a Bicinchoninic acid Protein Assay Kit per the manufacturer’s instructions (Thermo Fisher, Waltham, MA).
ELISA
Concentrations of TATc, TAFI, Eosinophil cationic protein (ECP), and albumin were determined with commercially available enzyme-linked immunosorbent assay (ELISA) kits using manufacturer’s protocols (TATc; Assaypro, St. Charles, MO, TAFI; Life Span Bioscience, Seattle, WA, ECP; MBL International, Woburn, MA, albumin; Bethyl Laboratories, Montogomery, TX). The absorbance was measured using a Bio-Rad Spectrophotometer Model 680 Microplate Reader (Bio-Rad, Hercules, CA) with associated software applied to the sandwich enzyme immunoassay technique.
Statistical analysis
Differences between the groups were analyzed with the Kruskal-Wallis ANOVA with Dunnett post hoc testing and Mann-Whitney U test. Correlations were assessed using the Spearman rank correlation. In all cases, P < 0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) software.
Results
Characteristics of the subjects
Table 1 shows the characteristics of the control, CRSsNP, and CRSwNP subjects providing nasal lavage fluids in the study and nasal tissue extract. As to nasal lavage fluids samples, and as reported previously, the CRSwNP group included more male patients compared to control subjects; this group was also of a slightly higher average age (gender; p < 0.05, age; p < 0.01). As for ethnicity, the CRSsNP group included more white patients compared to the control group (p < 0.05). The subjects providing UT samples in CRSsNP included younger aged patients compared to control subjects and subjects of NP samples from CRSwNP (p < 0.05). Subjects from whom UT samples were derived in the CRSwNP group were also younger compared to control subjects. However, there were no significant differences in the distribution of comorbid atopy, asthma, and steroid use among CRSsNP and CRSwNP subjects in both nasal lavage fluids and nasal tissue samples.
Levels of TATc in nasal lavage fluids and nasal tissue
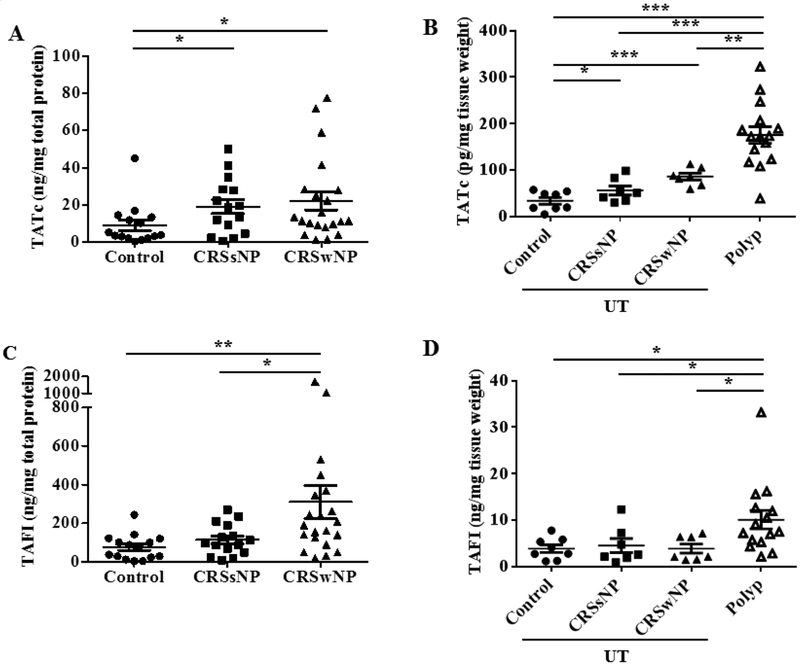
The first aim of this study was to determine the degree of the procoagulatory state in CRS. Nasal lavage fluids provide a convenient way to study CRS disease pathogenesis, and can represent tissue inflammation associated with elevated mediators (35,36). We measured the levels of TATc, a marker of thrombin, in nasal lavage fluids to determine whether it reflects the increased coagulation state previously described in the paranasal cavity (13,19). TATc levels of nasal lavage fluids could be detected and we found significantly elevated TATc levels in nasal lavage fluids from both CRSsNP and CRSwNP subjects compared to control subjects (p < 0.05 vs CRSsNP, p < 0.05 vs CRSwNP, Fig 1A). We also measured the levels of TATc in extracts of sinonasal tissue from a distinct group of subjects (i.e. not matched with patients providing nasal lavage fluid samples). Details of the characteristics of this group are also shown in Table 1. The levels of TATc were elevated in extracts of UT tissue from CRS patients compared to extracts of control UT (p < 0.05 vs CRSsNP, p < 0.001 vs CRSwNP, Fig. 1B). Furthermore, TATc levels in NP extracts were also significantly elevated compared to extracts of UT tissue (p < 0.001 vs control, p < 0.001 vs CRSsNP, p < 0.01 vs CRSwNP, Fig. 1B).
Figure 1.
Levels of TATc and TAFI in nasal lavage fluids and nasal tissue. (A) Increased TATc in nasal lavage fluids in patients with CRS compared to controls (●; control, ■; CRSsNP, ▲; CRSwNP in nasal lavage fluids, n = 15–21). (B) The level of TATc in nasal polyp tissue is increased compared to all UT (●; control UT, ■; CRSsNP UT, ▲; CRSwNP UT, △; CRSwNP NP tissue, n = 7 − 15). (C)) Increased TAFI in nasal lavage fluids in CRSwNP (n = 15–21). (D) Increased TAFI levels were observed in nasal polyp tissue compared to all UT tissue (n = 7 − 15). The levels of TATc and TAFI protein in nasal lavage fluids and nasal tissue extract were measured by ELISA. The concentrations of TATc and TAFI were normalized to the concentration of total protein. *P < .05, **P < .01, and ***P < .001.
Levels of TAFI in nasal lavage fluids and nasal tissue
We next evaluated the presence of the fibrinolytic inhibitor TAFI. The results showed that TAFI levels in nasal lavage fluids were significantly higher in CRSwNP subjects than control and CRSsNP subjects (p < 0.01 vs control, p < 0.05 vs CRSsNP, Fig 1C). We also measured the levels of TAFI in extracts of sinonasal tissue. The levels of TAFI were elevated in extracts of NPs compared to extracts of all UT samples (p < 0.05 vs control, p < 0.05 vs CRSsNP, p < 0.05 vs CRSwNP, Fig. 1D). To test whether the coagulation cascade and the fibrinolysis cascade are coordinately regulated, we assessed the correlation between levels of TATc and TAFI in nasal lavage fluids. There was a modest but significant correlation between TATc and TAFI in nasal lavage fluids (r = 0.4542, p < 0.001).
Levels of TAFI in serum
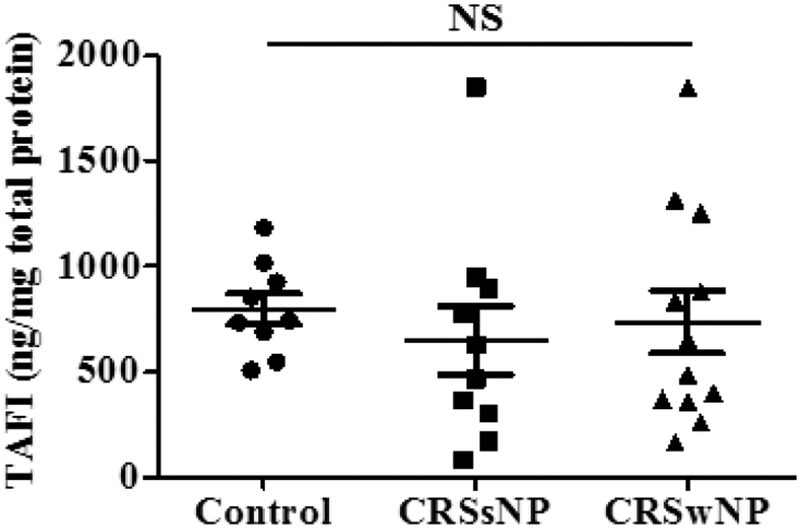
It is known that TAFI is produced mainly in the liver and circulates in the body. As the levels of TAFI are elevated in nasal lavage fluids from CRSwNP subjects and in NP tissue extracts, we measured levels of TAFI in serum samples matched with nasal lavage samples; there were no significant differences between the three groups in serum levels of TAFI, suggesting that alterations in the sinonasal cavity were due to local rather than systemic elevations (Fig. 2).
Figure 2.
Levels of TAFI in serum. The levels of TAFI protein in serum were measured by ELISA and normalized to total protein in serum (●; control, ■; CRSsNP, ▲; CRSwNP, n = 912). NS, not significant.
Levels of albumin in nasal lavage fluids
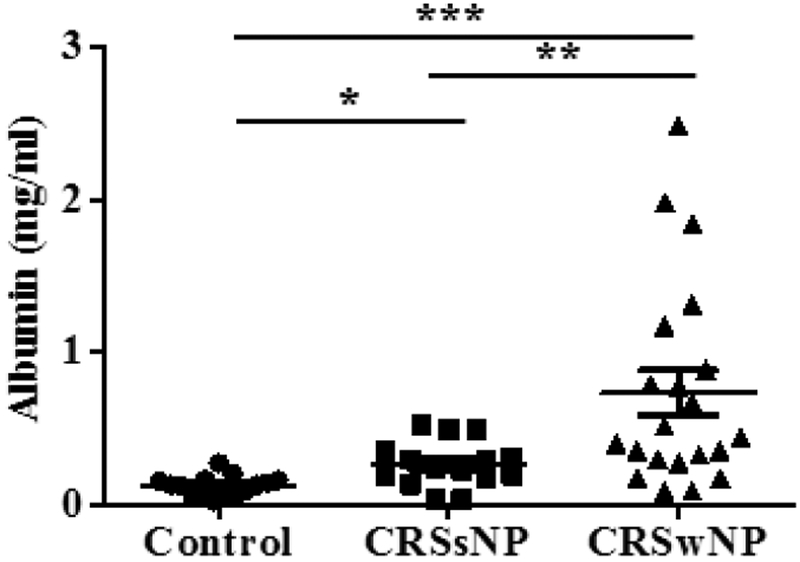
We next evaluated the potential role of plasma leak in elevating TAFI. Albumin is a major component of plasma, and leaks from vessels into tissues along with plasma during inflammation. Elevation of albumin is one feature of NPs (13), and increased albumin leakage has been proposed to be a result of eosinophil-driven inflammation in NPs (12). The concentrations of albumin in nasal lavage fluids from both CRS groups were significantly elevated compared to control subjects (p < 0.05 vs CRSsNP, p < 0.001 vs CRSwNP, Fig. 3). Furthermore, the levels of albumin in patients with CRSwNP were significantly higher than in patients with CRSsNP (p < 0.01), which is consistent with the levels of TAFI in nasal lavage fluids. To correct for plasma leakage, we normalized TAFI levels to albumin concentration of nasal lavage fluids. After normalization, we still found that the levels of TAFI were higher in CRSwNP compared to control subjects (p < 0.05, Supplementary Fig. 1A). There was a significant correlation of TAFI levels normalized to total protein and TAFI levels normalized to albumin (r = 0.7885, p < 0.0001, Supplementary Fig. 1B). These results support the hypothesis that TAFI found in nasal lavage fluids is derived from leakage of circulating TAFI into the tissue during inflammation.
Figure 3.
Levels of albumin in nasal lavage fluids. Increased albumin in nasal lavage fluids among CRSsNP and CRSwNP subjects (n = 15–21). The levels of albumin in nasal lavage fluids were measured by ELISA. *P < .05, **P < .01, and ***P < .001.
Elevated TATc and TAFI in nasal lavage fluids from CRSwNP subjects with asthma
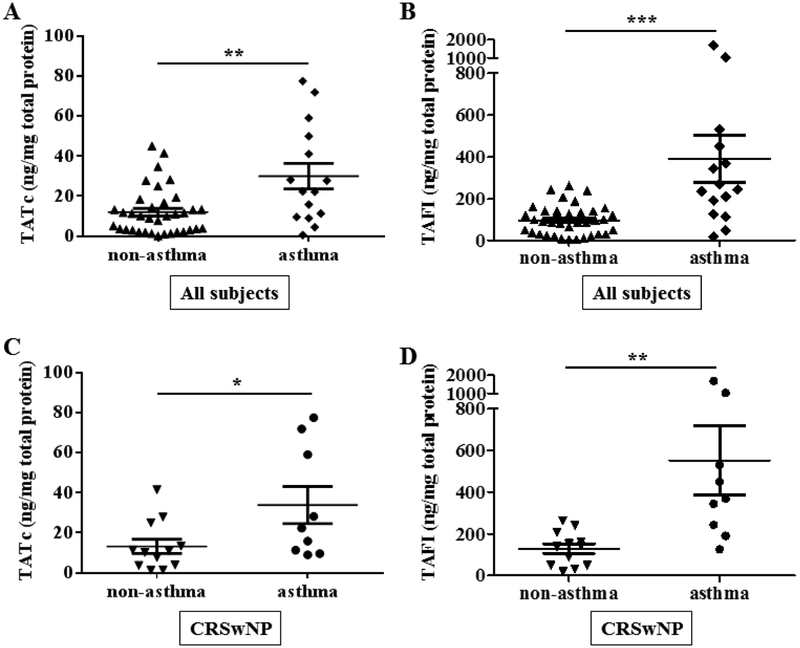
It is well established that patients with CRS have an increased prevalence of asthma compared to the general population (37), especially women with CRSwNP who have a more severe disease state and high comorbidity of asthma (38). We assessed the levels of TATc and TAFI in asthmatics by segregating our patient group data based on the diagnosis of asthma. The levels of TATc and TAFI in all subjects with CRS were significantly elevated in asthmatic subjects compared to those without asthma (p < 0.01; TATc, Fig. 4A, p < 0.001; TAFI, Fig. 4B). Furthermore, evaluation only of CRSwNP subjects also demonstrated that those with asthma showed high levels of TATc and TAFI compared to those without asthma (p < 0.05; TATc, Fig. 4C, p < 0.01; TAFI, Fig. 4D).
Figure 4.
Evaluation of TATc and TAFI levels in nasal lavage fluids according to clinical background. (A) TATc levels subdivided by comorbidity of asthma in all subjects including control subjects, (B) TAFI levels subdivided by comorbidity of asthma in all subjects including control subjects (▲; non-asthmatic subjects, ♦; asthmatic subjects, n = 15–36), (C) TATc levels subdivided by comorbidity of asthma in CRSwNP subjects, (D) TAFI levels subdivided by comorbidity of asthma in CRSwNP subjects (▼; non-asthmatic subjects, ●; asthmatic subjects, n = 9–12). *P < .05, **P < .01, and ***P < .001.
Discussion
Cross-linked fibrin mesh is a central end product of the coagulation cascade and the fibrin in the mesh is initially generated by cleavage of fibrinogen by thrombin (39). Fibrin mesh is typically formed at sites of vascular injury; however, it can also be generated in the airways (17). In addition to playing a key role in clotting, the function of thrombin to generate fibrin is also critical for tissue repair at sites of epithelial injury (40). When the coagulation cascade is set in motion, both coagulation and fibrinolytic cascades are activated so that the extent of thrombosis is well controlled systemically and restricted to a local site. Although fibrin will be degraded and inflammation will decrease under normal conditions, in some chronic disease states, an imbalance of coagulation and fibrinolysis cascades induces excessive fibrin deposition. Increased fibrin production is seen in the intra-alveolar space in asthmatic subjects (17) and in NPs (13). Excessive fibrin deposition accelerates inflammation by stimulating the expression of leukocyte adhesion molecules, chemokines and proinflammatory cytokines (41). Thus, fibrin production is not only a consequence of inflammation, but it may also promote inflammation by recruiting a variety of inflammatory cells into NPs (36,42,43). ECP is used as a marker of the presence of eosinophils in nasal tissue and elevated in NPs (35). To test whether elevated TATc and TAFI were related to eosinophilic inflammation, we measured ECP levels in the nasal tissue extract. There was a significant correlation between the levels of TATc and ECP (r = 0.4900, p < 0.01, Supplementary Fig. 2A). Furthermore, we also found a significant correlation between the levels of TAFI and ECP (r = 0.4566, p < 0.01, Supplementary Fig. 2B). These results indicate that elevated TATc and TAFI are related to eosinophilic inflammation in the tissue. Thrombin also has diverse functions in inflammatory responses, which can increase airway epithelial permeability (44). Shimizu et al. (45) first demonstrated the presence of thrombin and TATc in nasal secretions and found that allergic rhinitis patients and CRSwNP patients with asthma had elevated thrombin and TATc levels compared to control subjects. Our present study confirms and significantly extends Shimizu’s original findings, showing elevated TATc in both nasal lavage fluids and nasal polyp tissue, and further supports the concept of the presence of a procoagulant state in NPs (45). Our results also show elevated levels of TATc in CRS subjects. One important mechanism of NP formation may reflect enhanced levels of FXIIIA and downregulation of tPA in NPs (13,19). Furthermore, our data suggest that thrombin may also attenuate fibrinolysis by activating TAFI at the local site. Previous reports have shown a significant role of TAFI in interstitial lung disease (46). Fujimoto et al. reported attenuated bleomycin induced fibrin deposition in the lung of TAFI deficient mice (47). In the present study, we measured TAFI in nasal lavage fluids, and, to our knowledge, this is the first report that TAFI was elevated in CRSwNP patients. We also found that there was a significant correlation between the levels of TATc and TAFI in nasal lavage fluids, suggesting that acceleration of the coagulation cascade and suppression of the fibrinolytic cascade are coordinately linked in the formation of NPs.
As the nose is the first line of defense of the airways, the concept of unified airway disease (UAD), suggesting that upper airway diseases are closely related to lower airway disease, has been widely discussed (48). Tokunaga et al. (49) demonstrated that asthma is one of the risk factors for recurrence of NPs in eosinophilic CRS (ECRS). Asthma is a chronic inflammatory airway disease that is characterized by reversible airway obstruction and bronchial hyperresponsiveness. Growing evidence has highlighted the procoagulant state of the lower airway space in asthmatics (50), which is probably induced by leakage of plasma proteins into the bronchoalveolar lumen. When we evaluated data from nasal lavage fluids of all subjects, we found that the levels of TATc and TAFI were elevated to the greatest extent in the nasal cavity of asthmatic patients (Fig. 4A, B). This was also the case when we restricted the analysis to data from CRSwNP patients; i.e. the levels of TATc and TAFI were most highly elevated in subjects with asthma, compared to subjects without asthma (Fig. 4C, D). TAFI levels in sputum have been reported to be elevated in severe asthmatic subjects compared to healthy subjects and moderate asthmatics (51). Asthmatic subjects often require use of inhaled and or oral steroids, which also has been associated with altered hemostasis (52). Sneeboer et al. (53) showed the presence of a prothrombotic state in patients with severe asthma and prednisolone-dependent asthma by comparing the levels of hemostasis markers in the peripheral blood. We evaluated whether steroid use was associated with the elevation of TATc or TAFI in nasal lavage fluids. The results showed no significant differences in either TATc or TAFI levels in nasal lavage fluids of those on or off glucocorticoids (Supplementary Fig. 3). Insofar as patients on steroids may have more disease, it is hard to discern whether the treatment has any effect on the fibrinogenic response. Our data do indicate that patients with CRSwNP and comorbid asthma had the greatest prothrombotic phenotype (elevations of both TATc and TAFI) at the local site, perhaps reflecting the more severe and refractory disease state compared with CRS without asthma (54). We evaluated the degree of radiographic severity quantified by preoperative computed tomography (CT) score using the Lund-Mackay (LM) staging system to test the relationship between changes in fibrinolytic pathways and tissue structural changes. We correlated the LM score and the levels of TATc and TAFI in the nasal tissue extract. Interestingly, we found a strong and significant correlation between TATc and LM score (r = 0.7062, p < 0.0001, Supplementary Fig. 4A), as well as a correlation between TAFI and LM score (r = 0.3856, p < 0.05, Supplementary Fig. 4B). These results may indicate that elevated TATc and TAFI are related to structural changes that occur in CRS as measured by CT. Our data suggest that the primary mechanism of elevation of TAFI is by plasma leak, based upon correlation with albumin levels at the local site along with absence of systemic elevations of TAFI in the plasma. However, the levels of albumin in nasal lavage fluids were not significantly different in CRSwNP subjects with or without asthma (Supplementary Fig. 5), suggesting that other mechanism(s) may also affect the local elevation of TAFI.
TAFI is stored in two pools; a plasma pool and a pool in α-granules of platelets (24,25,55). Platelets are anuclear cell fragments that are released from megakaryocytes. In addition to their hemostatic role, recent reports showed that platelets take part in the allergic inflammatory response (56–60). Platelets store inflammatory mediators in their granules that can be released upon activation by stimuli such as thrombin (57–59,61), and have been found to actively participate in many manifestations of asthma (62,63). Platelets are found in BAL fluids and in bronchial biopsies of patients with asthma (63–65). Other reports also indicate that platelets exist in extravascular compartments and also in fibrous material in the airway lumen, suggesting that platelets have the ability to migrate or leak into inflamed tissue (66,67). It is considered likely that platelets also play a significant role in the pathogenesis in CRSwNP, especially in Aspirin-Exacerbated Respiratory Disease (AERD) (57,68,69). As thrombin is a strong activator for platelets, elevated TATc in CRSwNP can potentially activate platelets within the vasculature or in the tissue, potentially enhancing TAFI release from platelets in NPs. Furthermore, it has been demonstrated that activated platelets increase thrombin generation (70,71). Platelets may thus promote TAFI activation and vice versa. Figure 5 shows a hypothetical model to put the present findings in context. Altogether, our results indicate that activation of thrombin and generation of TAFI in CRSwNP, especially in patients with asthma, might be a result of plasma leakage result from activated platelets in sinonasal tissues. Further studies are required to clarify the precise mechanism of local TAFI elevations in CRSwNP.
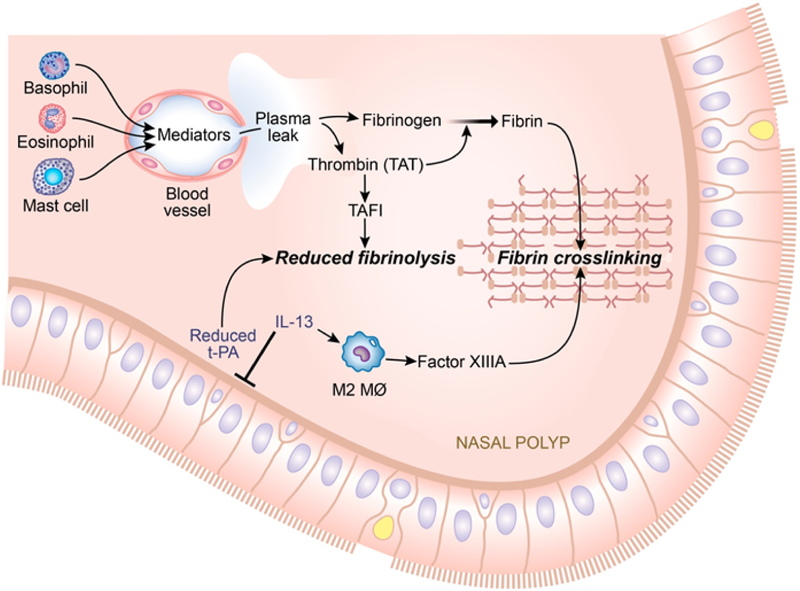
Figure 5.
Proposed hypothetical model for elevated TAFI and its role in the pathogenesis of NPs. In NPs, down regulation of tPA and elevated FXIIIA enhance fibrin formation in the presence of a Th2 milieu. Inflammatory cells produce inflammatory mediators that lead to vascular leakage, which also increases TAFI levels in NPs. In addition, thrombin (TATc) activates TAFI, which contributes to the down regulation of fibrinolysis further by attenuating the fibrinolysis cascade.
In conclusion, we report the presence of an elevated coagulant state in the nasal cavity of patients with CRSwNP as indicated by elevations of TATc in both nasal lavage fluids and nasal polyp tissue. We also demonstrate elevated levels of TAFI in nasal lavage fluids from patients with CRSwNP, a change which would diminish fibrinolysis, further enhancing fibrin deposition. These findings add further support to previous studies suggesting that the formation of NPs is related to profound deposition of fibrin in sinonasal tissues (13,19,45).
Supplementary Material
Key messages.
Fibrin deposition is one of the features of NPs and is in part caused by down regulation of fibrinolysis.
Levels of TATc in nasal lavage fluids and tissue extracts from CRSwNP patients were elevated, indicative of a procoagulant state in NPs.
Elevated TAFI in nasal lavage fluids from CRSwNP patients and tissue extracts from NPs may contribute to the fibrin deposition by attenuating fibrinolysis.
High levels of TATc and TAFI in the nose and sinuses of patients with CRSwNP and asthma may be an indicator of common pathology consistent with unified airways disease.
Funding:
This study was supported in part by Grants R37HL068546 and U19AI106683 (Chronic Rhinosinusitis Integrative Studies Program (CRISP)) from the NIH, and by the Ernest S. Bazley Foundation
Abbreviations
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- NPs
Nasal polyps
- TATc
thrombin/anti-thrombin complex
- TAFI
thrombin-activatable fibrinolysis inhibitor
- t-PA
tissue plasminogen activator
Footnotes
Conflict of Interest
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol 2011;120:423–427. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 2004;114:155–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol;22:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc 2009;6:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulse KE. Immune Mechanisms of Chronic Rhinosinusitis. Curr Allergy Asthma Rep 2016;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol 2017;12:331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomassen P, Vandeplas G, Van Zele T, Cardell L-O, Arebro J, Olze H et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 2016;137:1449–1456.e4. [DOI] [PubMed] [Google Scholar]
- 8.Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol 2017;139:699–703.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006;61:1280–1289. [DOI] [PubMed] [Google Scholar]
- 10.Lin DC, Chandra RK, Tan BK, Zirkle W, Conley DB, Grammer LC et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy;25:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam K, Hirsch AG, Tan BK. The association of premorbid diseases with chronic rhinosinusitis with and without polyps. Curr Opin Otolaryngol Head Neck Surg 2014;22:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol;14:279–290. [DOI] [PubMed] [Google Scholar]
- 13.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med 2013;187:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med; 17:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabazza EC, Osamu T, Yamakami T, Ibata H, Sato T, Sato Y et al. Correlation between clotting and collagen metabolism markers in rheumatoid arthritis. Thromb Haemost 1994;71:199–202. [PubMed] [Google Scholar]
- 16.Idell S Adult respiratory distress syndrome: do selective anticoagulants help? Am J Respir Med 2002;1:383–391. [DOI] [PubMed] [Google Scholar]
- 17.Wagers SS, Norton RJ, Rinaldi LM, Bates JHT, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004; 114:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258–264. [DOI] [PubMed] [Google Scholar]
- 19.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2013;132:584–592.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H et al. Thrombin in the airways of asthmatic patients. Lung 1999;177:253–262. [DOI] [PubMed] [Google Scholar]
- 21.Schouten M, VAN DE Pol MA, Levi M, VAN DER Poll T, VAN DER Zee JS. Early activation of coagulation after allergen challenge in patients with allergic asthma. J Thromb Haemost 2009;7:1592–1594. [DOI] [PubMed] [Google Scholar]
- 22.Bajzar L, Manuel R, Nesheim ME. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem 1995;270:14477–14484. [DOI] [PubMed] [Google Scholar]
- 23.Eaton DL, Malloy BE, Tsai SP, Henzel W, Drayna D. Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma. J Biol Chem 1991;266:21833–21838. [PubMed] [Google Scholar]
- 24.Mosnier LO, Buijtenhuijs P, Marx PF, Meijers JCM, Bouma BN. Identification of thrombin activatable fibrinolysis inhibitor (TAFI) in human platelets. Blood 2003;101:4844–4846. [DOI] [PubMed] [Google Scholar]
- 25.Schadinger SL, Lin JHH, Garand M, Boffa MB. Secretion and antifibrinolytic function of thrombin-activatable fibrinolysis inhibitor from human platelets. J Thromb Haemost 2010;8:2523–2529. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer ME, Lisman T, de Groot PG, Meijers JCM, le Cessie S, Doggen CJM et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 2010;116:113–121. [DOI] [PubMed] [Google Scholar]
- 27.Orikaza CM, Morelli VM, Matos MF, Lourenço DM. Haplotypes of TAFI gene and the risk of cerebral venous thrombosis--a case-control study. Thromb Res 2014;133:120–124. [DOI] [PubMed] [Google Scholar]
- 28.de Bruijne ELE, Darwish Murad S, de Maat MPM, Tanck MWT, Haagsma EB, van Hoek B et al. Genetic variation in thrombin-activatable fibrinolysis inhibitor (TAFI) is associated with the risk of splanchnic vein thrombosis. Thromb Haemost 2007;97:181–185. [PubMed] [Google Scholar]
- 29.Peters MJL, Nurmohamed MT, van Eijk IC, Verkleij CJN, Marx PF. Thrombin-activatable fibrinolysis inhibitor and its relation with inflammation in rheumatoid arthritis. Ann Rheum Dis 2009;68:1232–1233. [DOI] [PubMed] [Google Scholar]
- 30.Owczarek D, Undas A, Foley JH, Nesheim ME, Jabłonski K, Mach T. Activated thrombin activatable fibrinolysis inhibitor (TAFIa) is associated with inflammatory markers in inflammatory bowel diseases TAFIa level in patients with IBD. J Crohns Colitis 2012;6:13–20. [DOI] [PubMed] [Google Scholar]
- 31.Beyazit Y, Sayilir A, Tanoglu A, Kekilli M, Kocak E, Ekiz F et al. Plasma Thrombin-activatable Fibrinolysis Inhibitor Levels Correlate with the Disease Activity of Ulcerative Colitis. Intern Med 2016;55:1831–1836. [DOI] [PubMed] [Google Scholar]
- 32.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol 2012;34:107–125. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg 2004;131:S1–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearlman AN, Conley DB. Review of current guidelines related to the diagnosis and treatment of rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 2008;16:226–230. [DOI] [PubMed] [Google Scholar]
- 35.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med 2015;192:682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2008;121:1385–1392, 1392–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012;:3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 38.Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, Conley DB et al. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immunity, Inflamm Dis 2015;3:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezaie AR, Cooper ST, Church FC, Esmon CT. Protein C inhibitor is a potent inhibitor of the thrombin-thrombomodulin complex. J Biol Chem 1995;270:25336–25339. [DOI] [PubMed] [Google Scholar]
- 40.Perrio MJ, Ewen D, Trevethick MA, Salmon GP, Shute JK. Fibrin formation by wounded bronchial epithelial cell layers in vitro is essential for normal epithelial repair and independent of plasma proteins. Clin Exp Allergy 2007;37:1688–1700. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Piela-Smith TH. Fibrin(ogen)-induced expression of ICAM-1 and chemokines in human synovial fibroblasts. J Immunol 2000;165:5255–5261. [DOI] [PubMed] [Google Scholar]
- 42.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2012; 130:410–20.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol 2014;133:1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi S, Takeuchi K, Suzuki S, Tsunoda T, Tanaka C, Majima Y. Effect of thrombin on permeability of human epithelial cell monolayers. Pharmacology 2006;76:46–52. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu S, Gabazza EC, Ogawa T, Tojima I, Hoshi E, Kouzaki H et al. Role of thrombin in chronic rhinosinusitis-associated tissue remodeling. Am J Rhinol Allergy;25:7–11. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto H, Gabazza EC, Hataji O, Yuda H, D’Alessandro-Gabazza CN, Nakano M et al. Thrombin-activatable fibrinolysis inhibitor and protein C inhibitor in interstitial lung disease. Am J Respir Crit Care Med 2003; 167:1687–1694. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto H, Gabazza EC, Taguchi O, Nishii Y, Nakahara H, Bruno NE et al. Thrombin-activatable fibrinolysis inhibitor deficiency attenuates bleomycin-induced lung fibrosis. Am J Pathol 2006;168:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy 2012;67:91–98. [DOI] [PubMed] [Google Scholar]
- 49.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Boer JD, Majoor CJ, van ‘t Veer C, Bel EHD, van der Poll T. Asthma and coagulation. Blood 2012;119:3236–3244. [DOI] [PubMed] [Google Scholar]
- 51.Brims FJH, Chauhan AJ, Higgins B, Shute JK. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax 2009;64:1037–1043. [DOI] [PubMed] [Google Scholar]
- 52.van Zaane B, Nur E, Squizzato A, Gerdes VEA, Büller HR, Dekkers OM et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost 2010;8:2483–2493. [DOI] [PubMed] [Google Scholar]
- 53.Sneeboer MMS, Majoor CJ, de Kievit A, Meijers JCM, van der Poll T, Kamphuisen PW et al. Prothrombotic state in patients with severe and prednisolone-dependent asthma. J Allergy Clin Immunol 2016; 137:1727–1732. [DOI] [PubMed] [Google Scholar]
- 54.Haruna S, Shimada C, Ozawa M, Fukami S, Moriyama H. A study of poor responders for long-term, low-dose macrolide administration for chronic sinusitis. Rhinology 2009;47:66–71. [PubMed] [Google Scholar]
- 55.Vilahur G, Ben-Aicha S, Badimon L. New insights into the role of adipose tissue in thrombosis. Cardiovasc Res 2017;113:1046–1054. [DOI] [PubMed] [Google Scholar]
- 56.Idzko M, Pitchford S, Page C. Role of platelets in allergic airway inflammation. J Allergy Clin Immunol 2015;135:1416–1423. [DOI] [PubMed] [Google Scholar]
- 57.Laidlaw TM, Boyce JA. Platelets in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2015;135:1407–14; quiz 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda T, Unno H, Morita H, Futamura K, Emi-Sugie M, Arae K et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. J Allergy Clin Immunol 2016; 138:1395–1403.e6. [DOI] [PubMed] [Google Scholar]
- 59.Shah SA, Page CP, Pitchford SC. Platelet-Eosinophil Interactions As a Potential Therapeutic Target in Allergic Inflammation and Asthma. Front Med 2017;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda T, Morita H, Saito H, Matsumoto K, Matsuda A. Recent advances in understanding the roles of blood platelets in the pathogenesis of allergic inflammation and bronchial asthma. Allergol Int 2018;7:326–333. [DOI] [PubMed] [Google Scholar]
- 61.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001;12:261–273. [DOI] [PubMed] [Google Scholar]
- 62.Kornerup KN, Page CP. The role of platelets in the pathophysiology of asthma. Platelets 2007;18:319–328. [DOI] [PubMed] [Google Scholar]
- 63.Kowal K, Pampuch A, Kowal-Bielecka O, DuBuske LM, Bodzenta-Łukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy 2006;36:426–432. [DOI] [PubMed] [Google Scholar]
- 64.Maestrelli P, Boschetto P, Zocca E, Crescioli S, Baroldi P, Mapp C et al. Venous blood platelets decrease during allergen-induced asthmatic reactions. Clin Exp Allergy 1990;20:367–372. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan PJ, Jafar ZH, Harbinson PL, Restrick LJ, Costello JF, Page CP. Platelet dynamics following allergen challenge in allergic asthmatics. Respiration 2000;67:514–517. [DOI] [PubMed] [Google Scholar]
- 66.Metzger WJ, Sjoerdsma K, Richerson HB, Moseley P, Zavala D, Monick M et al. Platelets in bronchoalveolar lavage from asthmatic patients and allergic rabbits with allergen-induced late phase responses. Agents Actions Suppl 1987;21:151–159. [DOI] [PubMed] [Google Scholar]
- 67.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis 1989;140:1745–1753. [DOI] [PubMed] [Google Scholar]
- 68.Mitsui C, Kajiwara K, Hayashi H, Ito J, Mita H, Ono E et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016; 137:400–411. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi T, Kato A, Berdnikovs S, Stevens WW, Suh LA, Norton JE et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: Potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2017;140:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hemker HC, Al Dieri R, Béguin S. Thrombin generation assays: accruing clinical relevance. Curr Opin Hematol 2004;11:170–175. [DOI] [PubMed] [Google Scholar]
- 71.Carrieri C, Galasso R, Semeraro F, Ammollo CT, Semeraro N, Colucci M. The role of thrombin activatable fibrinolysis inhibitor and factor XI in platelet-mediated fibrinolysis resistance: a thromboelastographic study in whole blood. J Thromb Haemost 2011;9:154–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.