Abstract
Difficulty understanding speech-in-noise (SIN) is a pervasive problem faced by older adults particularly those with hearing loss. Previous studies have identified structural and functional changes in the brain that contribute to older adults’ speech perception difficulties. Yet, many of these studies use neuroimaging techniques that evaluate only gross activation in isolated brain regions. Neural oscillations may provide further insight into the processes underlying SIN perception as well as the interaction between auditory cortex and prefrontal linguistic brain regions that mediate complex behaviors. We examined frequency-specific neural oscillations and functional connectivity of the EEG in older adults with and without hearing loss during an active SIN perception task. Brain-behavior correlations revealed listeners who were more resistant to the detrimental effects of noise also demonstrated greater modulation of α phase coherence between clean and noise-degraded speech, suggesting α desynchronization reflects release from inhibition and more flexible allocation of neural resources. Additionally, we found top-down β connectivity between prefrontal and auditory cortices strengthened with poorer hearing thresholds despite minimal behavioral differences. This is consistent with the proposal that linguistic brain areas may be recruited to compensate for impoverished auditory inputs through increased top-down predictions to assist SIN perception. Overall, these results emphasize the importance of top-down signaling in low-frequency brain rhythms that help compensate for hearing-related declines and facilitate efficient SIN processing.
Keywords: Cognitive aging, EEG, functional connectivity, time-frequency analysis, neural oscillations, speech processing
INTRODUCTION
Difficulty understanding speech-in-noise (SIN) is highly prevalent among the aging population including individuals both with and without hearing loss. Older adults exhibit greater listening effort (Anderson Gosselin and Gagné, 2011) and more significant performance deficits in adverse listening conditions than younger adults (Helfer and Wilber, 1990; Wong et al., 2010). Age-related hearing loss further exacerbates SIN difficulties (Helfer and Wilber, 1990). Previous studies characterizing the underlying mechanisms contributing to SIN difficulties reveal both peripheral and central brain mechanisms play a role in accurate and efficient SIN processing (Frisina and Frisina, 1997; Humes, 1996; Wong et al., 2010).
Indeed, neuroimaging studies reveal that structural and functional neural changes associated with aging (Bidelman et al., 2019a; Bidelman et al., 2019b; Du et al., 2016; Grady, 2012; Park and McDonough, 2013) contribute to older adults’ SIN difficulties. Electrophysiological (EEG) studies often show exaggerated amplitudes and increased latencies of auditory cortical responses with aging, which has been taken as evidence for reduced inhibition (Alain and Woods, 1999; Bidelman et al., 2014; Caspary et al., 2008; Chao and Knight, 1997) and decreased temporal fidelity in the aging auditory system (Tremblay et al., 2003). The presence of hearing loss can amplify these changes due to the typical aging process (Lin et al., 2014; Pichora-Fuller and Levitt, 2012; Wayne and Johnsrude, 2015), resulting in even greater increases in response amplitude and latency when those with hearing loss are compared to their normal hearing peers (Alain et al., 2014; Campbell and Sharma, 2013; Cardin, 2016).
To date, EEG studies have primarily relied on event-related potentials (ERPs) to infer the neural processes contributing to SIN perception. However, evaluating changes in gross activation within isolated brain regions can lead to misleading or ambiguous conclusions regarding the neurobiology of aging (Morcom and Henson, 2018; Wong et al., 2010). For instance, increases in evoked response amplitude commonly observed in older adults may be due either to the recruitment of additional neural resources (Bidelman et al., 2014; Wong et al., 2010), disinhibition (Bidelman et al., 2014; Caspary et al., 2008), or inefficient neural coding (Fabiani et al., 2006). Evaluating ERPs alone prevents full understanding of the underlying mechanisms of aging, particularly how different brain regions might coordinate to orchestrate successful SIN perception. Alternative EEG analyses may better delineate the underlying neural mechanisms for speech processing that are not always apparent with traditional ERP approaches (Bidelman, 2015; Bidelman, 2017; Yellamsetty and Bidelman, 2018).
In this vein, neural oscillations have provided novel insight into functional neural networks underlying complex perceptual and cognitive functions. Therefore, evaluating oscillatory components of neural responses may provide a more sensitive measure and more thorough understanding of the neural correlates of speech processing. Different brain “rhythms” are thought to play unique roles in the hierarchy of speech processing. High frequency γ oscillations are thought to contribute to localized processing within sensory cortices (Fontolan et al., 2014; Giraud and Poeppel, 2012; von Stein and Sarnthein, 2000) and the extraction of acoustic features (Yellamsetty and Bidelman, 2018) while lower frequency α and β oscillations have been involved in global, distributed cognitive processing across brain regions (Fontolan et al., 2014; von Stein and Sarnthein, 2000) including attention (Klimesch, 2012), inhibition of irrelevant cues (Adrian and Matthews, 1934; Klimesch, 2012; Pfurtscheller, 2001), working memory (Shahin et al., 2009; Zarahn et al., 2007), and template matching (Bidelman, 2015; Bidelman, 2017; Shahin et al., 2009; Yellamsetty and Bidelman, 2018). Evaluating how neural oscillations within different frequency bands of the EEG contribute to speech processing could provide further insight into the underlying processes supporting SIN perception in older adults.
In our ongoing studies on aging and the brain, we recently documented subtle neurophysiological changes in older adults with normal hearing (NH) and mild hearing loss (HL) that may reflect deficits in speech representations (Bidelman et al., 2019b). Using source-resolved brainstem and cortical ERPs, we found somewhat spared region-specific responses to speech, at least in listeners with mild hearing impairment. More significant differences were identified in functional connectivity between the auditory brainstem and cortex, suggesting neural transmission within the early auditory pathway is critical for robust SIN processing in older adults. Additional full-brain, functional connectivity analysis revealed more widespread and less efficient connectivity patterns in HL compared to NH listeners suggesting more diffuse processing strategies are employed in those with hearing loss (Bidelman et al., 2019a). However, neither of these studies addressed the role of neural oscillations and how functionally distinct frequency channels of the EEG relate to senescent changes in SIN perception. Moreover, how the aging lemniscal hearing system (e.g., auditory cortex) interfaces with high-order brain regions that support linguistic decisions (e.g., prefrontal areas) is not well understood.
The current study aimed to examine contributions of neural oscillations and their role in neural signaling between auditory cortical and linguistic brain areas during SIN processing. In this reanalysis of our existing dataset (Bidelman et al., 2019b), we measured frequency-specific neural oscillations and functional connectivity via EEG in older adults with and without hearing loss during rapid SIN perception tasks. Based on previous studies on aging, the effects of hearing loss on SIN processing, and putative roles of neural oscillations, we hypothesized that differences in α activity would emerge in more difficult listening conditions and that HL listeners would demonstrate enhanced connectivity between auditory and prefrontal cortex to compensate for poorer signal transmission apparent in earlier stages of the speech hierarchy (e.g., diminished brainstem-cortical connectivity; Bidelman et al., 2019b). Our findings reveal that (1) modulations in α phase coherence between clean and noise-degraded speech predicts accuracy in SIN tasks; (2) changes in functional brain connectivity precede measurable behavioral deficits in SIN processing; (3) “top-down” β connectivity increases in strength with increasing severity of hearing loss suggesting that the transfer of information between auditory-linguistic brain regions may be more sensitive to hearing-related changes than localized activity within regions.
EXPERIMENTAL PROCEDURES
Analyses of the ERPs and behavioral responses associated with this dataset are reported in (Bidelman et al., 2019b). New time-frequency analyses (applied here) were used to evaluate the correspondence between rhythmic brain oscillations and SIN perception in older adults.
Participants
Thirty-two older adults ranging in age from 52 to 75 years were divided into groups based on their average hearing thresholds (Fig. 1A). Listeners with average thresholds better than 25 dB HL comprised the normal hearing (NH; n=13) group while average thresholds worse than 25 dB HL classified participants with hearing loss (HL; n=19). The level of 25 dB HL reflects the upper limit of the normal hearing range as specified by the clinical determination of hearing loss (Gelfand, 2009). The groups were otherwise matched for age (NH: 66.2±6.1 years, HL: 70.4±4.9 years; t22.2=−2.05, p = 0.052) and gender (NH: 5/8 male/female; HL: 11/8; Fisher’s exact test, p=0.47) (for complete demographic details, see Bidelman et al., 2019b).
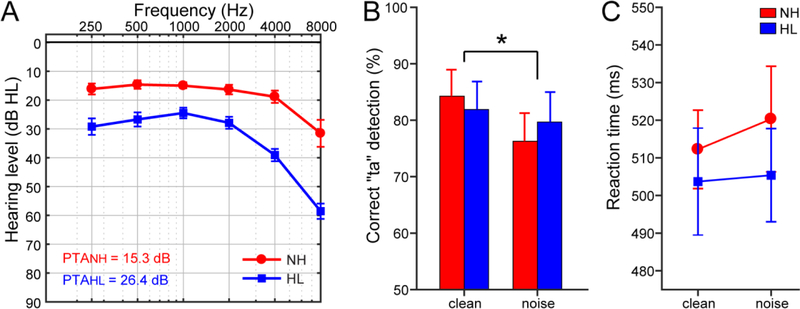
Figure 1: Audiometric and behavioral results.
Adapted from Bidelman et al. (2019b) with permission from Springer-Verlag. (A) Audiograms for listeners with normal hearing (NH) and hearing loss (HL). Hearing was ~10 dB better in NH vs. HL listeners. (B) Behavioral accuracy for detecting infrequent /ta/tokens in clean and noise-degraded conditions. Noise-related declines in behavioral performance were prominent but no group differences were observed. (C) Reaction times (RTs) for speech detection were similar between groups and speech SNRs. errorbars = ± s.e.m., *p< 0.05.
Stimuli and task
Electrophysiologic responses were recorded while participants performed an active SIN perception task in which they were directed to identify an infrequent speech token (i.e., /ta/) via button press. The stimuli included three naturally produced English consonant-vowel phonemes (/ba/, /pa/, and /ta/) spoken by a female talker. The stimuli were presented binaurally in clean (i.e., no background noise) and noise-degraded conditions [10 dB signal-to-noise ratio (SNR) using 8-talker babble noise, cf. Killion et al., 2004]. In each condition, the frequent tokens /ba/ and /pa/ were each presented 3000 times while the infrequent, target token /ta/ was presented 210 times. Between presentations, the interstimulus interval was randomly jittered between 55–155 ms. Both speech detection accuracy (%) and reaction times (RTs) were logged. See Bidelman et al., 2019b.
EEG time-frequency analysis on source waveforms
The EEG recording protocol and data pre-processing is described in our original report (Bidelman et al., 2019b). Briefly, cortical event-related potentials were recorded from 32 channels across the scalp. Ocular artifacts (saccades and blinks) were first corrected in the continuous EEG using a principal component analysis (PCA) (Picton et al., 2000). Cleaned EEGs were then epoched (−10–200 ms) and baseline corrected to the pre-stimulus period for each trial and stimulus condition per participant. The pre-stimulus interval was limited due to the pace of the perceptual task.1
To first reduce the dimensionality of the data and enable functional connectivity analysis between brain regions of interest (ROIs), full band (1–100 Hz), single trial scalp potentials were transformed to source space using the AEP virtual source montage in BESA (Scherg et al., 2002). This process applies a spatial filter to all electrodes and optimizes the relative weights of their contribution to the recorded scalp response to estimate the activity within each source while reducing overlapping activity from other brain regions (for details, see Scherg and Ebersole, 1994; Scherg et al., 2002). This allowed us to reduce each listener’s EEG (32-channels) to 15 source channels with regional dipoles in bilateral primary auditory cortex (PAC), left/right frontal cortex near inferior frontal gyrus (IFG) (i.e., Broca’s area), and left/right parietal cortex as well as sources along the mid-line (depicted in Fig. 1A of Zendel and Alain, 2014). From this model, we extracted the estimated neural current within single ROIs of the brain most relevant to our hypotheses including tangential and radial components of each auditory source as these orientations capture the majority of auditory cortical ERPs (Picton et al., 1999) and radial components of each frontal source (BESA default). Furthermore, the selection of these sources enabled us to assess the effects of hearing loss on band-specific connectivity between auditory (PAC) and linguistic (IFG) brain areas and potential recruitment of additional neural resources (e.g., compensatory processing) due to age-related hearing loss. Time-frequency analysis (TFA) was then performed on the single-trial epochs at the source level to improve spatial accuracy and reduce smearing due to volume conduction (Hoechstetter et al., 2004) using BESA® Research v7 (BESA, GmbH).
TFA assessed the frequency-specific contributions of time-locked neural oscillations to older adults’ SIN processing. Prior to TFA analysis, additional artifact correction was performed using a threshold of ±120 μV. Initial analysis revealed negligible induced activity likely due to the restricted baseline (10 ms); therefore, subsequent analyses focused on phase-locked oscillatory activity. The time-frequency transformation was achieved using a sliding window complex demodulation (for detailed description, see Papp and Ktonas, 1977) using 10 ms/5 Hz resolution step sizes. These settings permitted analysis of frequencies ≥10 Hz (i.e., α band and higher) across the entire epoch window. The resulting time-frequency displays, akin to neural spectrograms (see Fig. 2), were then produced by computing inter-trial phase-locking (ITPL) at each time-frequency point across single trials (Hoechstetter et al., 2004).
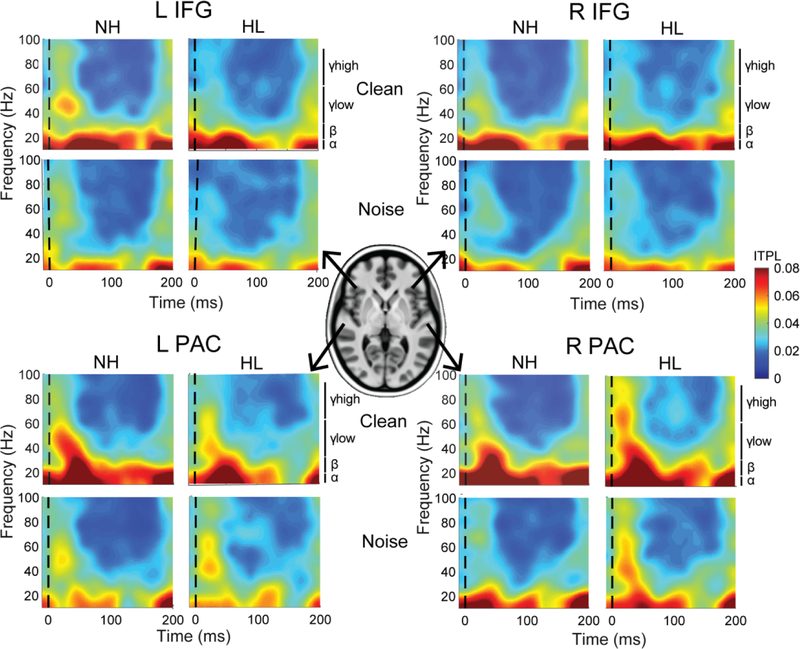
Figure 2: ITPL spectrograms for radial auditory and frontal sources by SNR and group.
Time-frequency analysis demonstrates phase synchrony (ITPL) within each neural source across frequency and time. Trending differences are observed when comparing synchronicity across sources (PAC > IFG), SNR (clean > noise), and group. Hotter colors denote stronger neural phase synchrony across trials.
ITPL measures the phase consistency (i.e., trial-to-trial synchrony) of neural activity within each frequency band across time (Tallon-Baudry et al., 1996). Values range from 0 to 1 indicating the degree of phase synchronicity across trials (i.e., 0 – random noise; 1 – perfect trial-to-trial repeatability). For each ROI, we extracted band-specific time courses from the ITPL spectrograms in the α (10–12 Hz), β (15–29 Hz), low γ (30–59 Hz), and high γ (60–90 Hz) frequency bands (e.g., Bidelman, 2017) (see Fig. 3). We then measured the peak maximum ITPL and associated latency from each band waveform using MATLAB. Latency windows were guided by visual inspection of the grand averaged traces [α: 25–100 ms; β: 25–75 ms; low/high γ:15–50 ms]. Peak responses were then used to assess the effects of SNR and hearing loss on neural oscillations involved in older adults’ SIN perception.
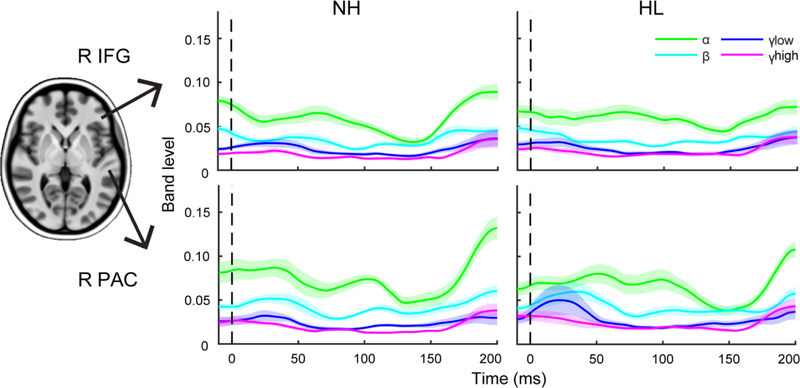
Figure 3: Band-specific time-course waveforms within auditory and frontal sources.
The time-course waveforms illustrate the degree of phase synchronicity across trials over time for each frequency band. Bands were extracted from ITPL maps (see Fig. 2). Waveforms reflect grand averaged traces for each group from the right frontal and auditory sources in the noise condition. Clean and left hemisphere responses not shown.
Functional connectivity
We measured band-specific functional connectivity between PAC and IFG sources (for each hemisphere) using phase transfer entropy (PTE) (e.g., Bidelman et al., 2018; Bidelman et al., 2019b; Lobier et al., 2014). PTE is a directional measure of signal dependence. Additionally, PTE can be implemented in a frequency-specific manner to assess connectivity in individual EEG bands (Lobier et al., 2014). We computed PTE between source signals in the PAC and IFG ROIs in both directions (i.e., X→Y and Y→X) to quantify differences in the strength of afferent/bottom-up (PAC→IFG) vs. efferent/top-down (IFG→PAC) connectivity within the auditory-linguistic pathway as a function of speech SNR and group.
Statistical Analysis
Mixed model ANOVAs were performed to assess all dependent variables of interest (GLIMMIX, SAS® 9.4, SAS Institute; Cary, NC) with participants serving as a random effect. Degrees of freedom were estimated using PROC GLIMMIX’s containment option2. Unless otherwise specified, Bonferroni adjustments controlled for Type I error inflation. The significance level for all statistical analyses was set at α = 0.05. Independent samples t-tests (un-pooled variance, two-tailed) were used to compare demographic variables between groups. Correlational analyses (Pearson’s-r) and robust regression (bisquare weighting - achieved using the ‘fitlm’ function in MATLAB) were used to evaluate relationships between neural and behavioral measures. Specifically, to evaluate the relationship between neural oscillations and behavioral SIN perception, we used robust regression. We first collapsed clean and noise responses by computing their difference (clean - noise). We then conducted correlational analyses between neural responses (i.e., phase coherence peak amplitude/latency within each frequency channel and source) and the behavioral measures [i.e., pure-tone average (PTA), RT, %]. This allowed us to assess the degree to which modulations in neural oscillations between clean and noise-degraded speech were related to changes in hearing thresholds and behavioral performance. False discovery rate (FDR) was used to correct for multiple correlations (Benjamini and Hochberg, 1995). One RT data point was identified as an outlier and was excluded from correlation analyses. All analyses and results were collapsed across the frequent tokens (i.e., /ba/ and /pa/) to further reduce the dimensionality of the data. Responses to infrequent /ta/ tokens were not included in analysis due to the limited number of trials andto avoid mismatch negativities.
RESULTS
Behavioral data
Behavioral responses, reproduced from (Bidelman et al., 2019b), are shown in Figure 1. Analyses of these results are reported in depth elsewhere (Bidelman et al., 2019b). In short, we found no differences between groups in accuracy (Fig. 1B) nor RT speed (Fig. 1C) for target speech detection. However, noise had an expected detrimental effect on perceptual accuracy for both groups (Fig. 1B).
Electrophysiological data
Time-frequency (ITPL) spectrograms for the PAC and IFG sources are shown for each SNR and group in Figure 2. Band time courses are shown in Figure 3. Diagnostics for amplitude analyses revealed a positive skew; thus, a cube-root transform was used. ANOVAs conducted on the transformed amplitude measures revealed significant effects of SNR for all frequency bands (all p < 0.03) but no main effect of group or SNR×group interaction. For latency, no significant group or SNR effects were observed for any frequency band. The lack of group effects might be anticipated given the relatively mild differences in hearing loss between groups and our previous study which did not observe differences in ERP responses (Bidelman et al., 2019b). This further motivates the examination of band-specific oscillations in these data.
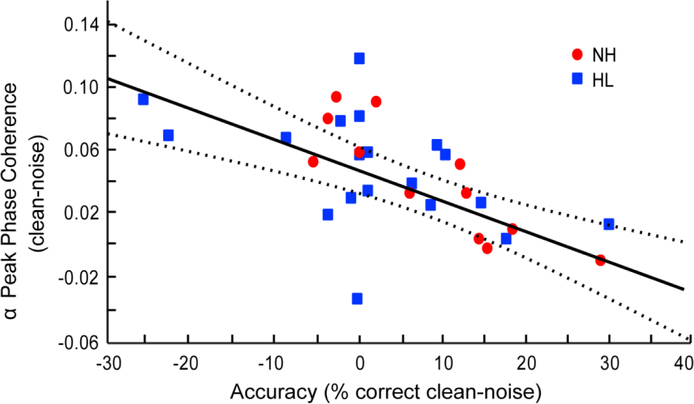
To determine whether neural activity within different frequency bands was associated with perceptual SIN measures, we used robust regression to assess brain-behavior relations. We found a significant negative correlation between α oscillations in the right frontal (IFG) source and speech detection accuracy [r30 = 0.41, pFDR = 0.007; Fig. 4; left IFG: r30 = 0.02, pFDR = 3.008; not shown]. This suggests that listeners who were more resistant to the detrimental effects of noise (i.e., performed equally as well or better in noise) also demonstrated less coherence of α activity while performing the SIN perception task. Little to no change in α phase coherence was observed in the listeners who performed more poorly in noise.
Figure 4: Phase coherence within right IFG α-band predicts accuracy of SIN performance.
Difference scores between clean and noise conditions are plotted for α phase coherence within right IFG and /ta/ detection accuracy for each participant. Greater modulations in α band are observed in listeners whose behavioral performance was more resistant to the detrimental effects of noise. Dotted lines represent the 95% confidence interval.
Auditory-frontal functional connectivity
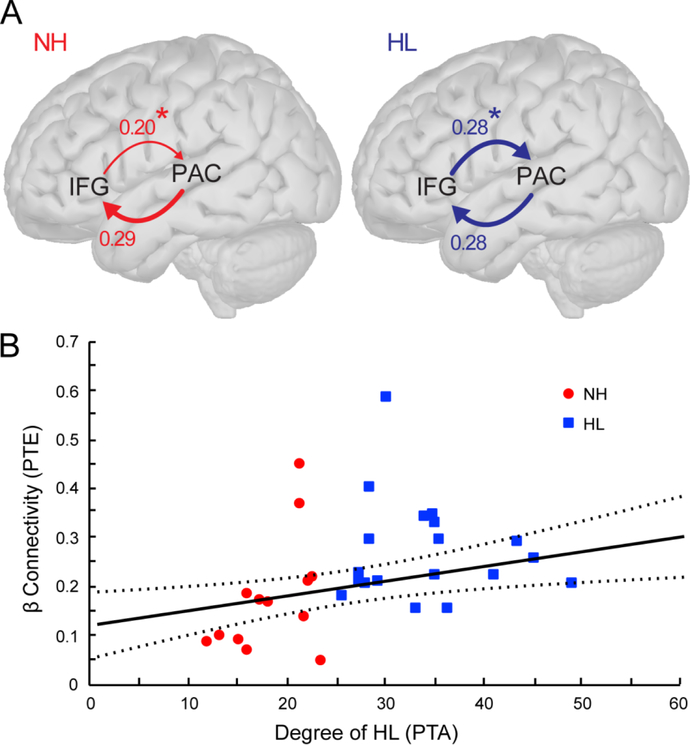
We next asked whether differences in neural transmission (i.e., feedforward or feedback connectivity) between PAC and IFG is altered in individuals with mild hearing loss. Because initial inspection of the data revealed minimal connectivity within the low and high γ frequency bands (data not shown), subsequent analyses focused on connectivity within the α- and β-band channels. Mixed model ANOVAs (subjects=random effect) were performed for both afferent (PAC→IFG) and efferent (IFG→PAC) connectivity to evaluate the effects of group, hemisphere, and condition as well as potential interactions. These analyses were conducted separately by frequency band and each dipole orientation (i.e., tangential and radial). These analyses revealed that HL listeners demonstrated stronger efferent β connectivity between IFG and the radial PAC component than NH listeners (mean ± SE; HL: 0.28 ± 0.02, NH: 0.20 ± 0.02; F1,30 = 7.14, p = 0.0121; Fig. 5A) regardless of SNR. In contrast, afferent (PAC→IFG) signaling did not differ between groups (HL: 0.27±0.03, NH: 0.29±0.03; F1,30 = 0.08, p = 0.78; Fig. 5A). None of the other comparisons or interaction effects remained statistically significant following correction for multiple comparisons.
Figure 5: Efferent functional connectivity (IFG→PAC) within β band varies with degree of hearing loss.
(A) Phase transfer entropy reflecting the directed (casual) afferent (PAC→IFG) and efferent (IFG→PAC) neural signaling between auditory and prefrontal cortex for the noise degraded speech condition. Efferent connectivity is stronger in listeners with hearing loss compared to those with normal hearing; afferent connectivity is similar between groups. (B) Efferent IFG→PAC connectivity increases in strength in listeners with poorer hearing (i.e., higher PTAs). Dotted lines represent the 95% confidence interval. *p < 0.05
Lastly, to relate neural connectivity effects to behavior, we conducted correlations between β connectivity (the only band showing group differences) and behavioral measures (i.e., PTA, RT, %). As in the previous correlation analyses, we used difference measures between clean and noise responses in these calculations. We found that efferent β connectivity (in noise) between IFG and radial PAC was positively correlated with PTA (r30 = 0.24, p = 0.0044; Fig. 5B) such that stronger efferent connectivity was associated with greater degrees of hearing loss. No other significant correlations were noted including those involving the clean speech responses.
DISCUSSION
By measuring neural oscillations in older adults during SIN perception, our data reveal three primary findings: (1) modulations in α phase coherence between clean and noise-degraded speech predicts accuracy in SIN perception; (2) changes in functional brain connectivity precede measurable behavioral deficits in SIN processing; (3) “top-down” β connectivity from IFG to PAC increases in strength with increasing severity of hearing loss.
α phase coherence predicts accuracy in SIN perception
We found that older adults who were more resistant to the detrimental effects of noise behaviorally demonstrated reduced α phase synchronicity in noise, particularly within right IFG. Previous studies suggest that α enhancement functions to inhibit task-irrelevant inputs (Adrian and Matthews, 1934; Pfurtscheller, 2001) while reductions in α facilitate task-relevant processing (Klimesch, 2012). Similar to our findings, greater event-related desynchronization (ERD) in α has been related to improved performance in semantic (Doppelmayr et al., 2005; Klimesch et al., 1997) and working memory (Bashivan et al., 2014) tasks. Klimesch et al. (2007) further suggest that ERD reflects “active information processing” related to excitatory rather than inhibitory processes in the brain and that this desynchronization is likely related to more generalized attentional demands required for the completion of a task. Furthermore, they posited that ERD may play a role in the release of inhibition related to spreading activation. Likewise, Proskovec et al. (2019) found greater decreases in α activation in high- compared to low-load conditions during a verbal working memory task. Attentional models further suggest that increasing task complexity, or cognitive load, leads to improved performance due to greater attentional focus (Kahneman, 1973) and requires higher levels of processing and attentional selection (Lavie, 1995; Lavie et al., 2004). Therefore, it is possible that our SIN detection task was less challenging, requiring less attentional and other neural resources, for listeners who showed greater α coherence during clean speech (those to left side of graph; Fig. 4) compared to listeners who were “low α modulators.” However, when greater cognitive resources are required during more difficult noise conditions, less synchrony within α band, reflecting a release from inhibition, may enable the brain to deploy attention more flexibly to aid syllable detection accuracy. While these outcomes are limited to phase-locked neural oscillations, changes in induced activity may reveal different underlying mechanisms of SIN processing (Bidelman, 2015; Petersen et al., 2015). Future studies could incorporate analyses of induced activity to provide a more thorough representation of event-related neural processes contributing to SIN tasks.
Paralleling our data, previous studies have also shown that age-related changes in α activity are localized to frontal and sensorimotor regions (Dushanova and Christov, 2016; Nobukawa et al., 2019). Activity within frontal cortical areas may serve as a compensatory mechanism for deficits in speech processing in older adults, particularly in more adverse listening conditions (Binder et al., 2004; Du et al., 2016; Zekveld et al., 2006). Specifically, IFG and superior temporal gyrus (STG) activation within the right hemisphere is particularly salient for difficult sound contrasts (cf. our noise condition) (Doeller et al., 2003). Furthermore, increased α activity within the right hemisphere, particularly IFG, has been associated with inhibitory processes (Garavan et al., 1999), which provides additional support to our conclusion that desynchronization in α activity within right IFG functions as a release from inhibition in older adults’ speech-in-noise processing. Additional evidence of right lateralized compensation in SIN processing has been observed in passive listening tasks in normal hearing, young adults which reveals altered neural response laterality from being leftward dominant to include greater right hemispheric contribution within both PAC and IFG with decreasing SNR (Bidelman and Howell, 2016). It is possible that the compensatory rightward shifts in response laterality observed by Bidelman & Howell were exaggerated in our sample due to increased age and the presence of hearing loss in some of our listeners (Bidelman et al., 2019b).
Changes in functional connectivity precede measurable behavioral deficits in SIN processing
While no behavioral differences were observed between groups in our SIN detection task (Fig. 1), HL listeners demonstrated enhanced efferent β connectivity when processing SIN (Fig. 5). Overall, these data suggest that central compensation through the recruitment of additional, non-canonical auditory brain areas help overcome peripheral deficits to assist older adults’ speech perception in noise (e.g., central gain compensation; Chambers et al., 2016). Numerous studies have described age-related changes in both brain structure and function, including inter-regional connectivity (Betzel et al., 2014; Bidelman et al., 2019a; Bidelman et al., 2019b; Grady, 2012; Sullivan and Pfefferbaum, 2006) and compensatory processing (Du et al., 2016; Grady, 2012; Park and McDonough, 2013). Hearing loss is thought to exacerbate the effects observed in typical aging (Lin et al., 2014; Pichora-Fuller and Levitt, 2012; Wayne and Johnsrude, 2015). In fact, studies have shown that increased recruitment of frontal cortical regions is associated with morphological changes particularly in auditory regions, and this additional recruitment has been further linked to behavioral performance (Tyler et al., 2010; Wong et al., 2009). Specifically, our results show enhanced connectivity directed from IFG to PAC in noise suggesting increased neural signaling from linguistic to auditory sensory areas in HL listeners. Previous studies have shown IFG contributes to “top-down” processing of speech in more adverse listening conditions (Binder et al., 2004; Zekveld et al., 2006), and it has also been associated with other cognitive functions like working memory (Crinion et al., 2003; Specht et al., 2000) and template matching of the input stimulus to an internal representation within auditory memory (Zekveld et al., 2006). These processes are critical for SIN perception and may account for the hearing-related changes we find in IFG→PAC signaling.
Our data suggest that functional connectivity may perhaps provide a more sensitive measure of changes induced by hearing loss than behavioral measures. The recruitment of frontal sources in aging adults and those with hearing loss may reflect broader alterations within functional networks and compensatory cortical reorganization (Campbell and Sharma, 2013; Cardin, 2016). The stronger efferent (IFG→PAC) connectivity we observed in HL listeners suggests that even mild degrees of hearing loss can alter functional communication between cortical regions subserving speech-language functions. Such changes in functional connectivity may provide a means by which older adults with HL could compensate for impoverished representations in auditory cortices.
Top-down β connectivity increases in strength with poorer hearing
Older adults with hearing loss demonstrated stronger β connectivity in noise between frontal and auditory regions (Fig. 5A) which also scaled with greater degrees of hearing impairment (Fig. 5B). Because no differences were observed in RTs between clean and noise-degraded conditions for either group (Fig. 1C), strengthened β connectivity is unlikely attributed to changes in general listening effort. Rather, we interpret these data to reflect alternative cognitive processing strategies that are utilized with impoverished auditory inputs. This notion aligns with previous studies that have related oscillatory β-band activity to cognitive processes associated with task demands including working memory (Shahin et al., 2009; Zarahn et al., 2007), encoding and integrating sensory information (Brovelli et al., 2004; von Stein and Sarnthein, 2000; Wang et al., 2017), speech template matching (Bidelman, 2015; Bidelman, 2017; Shahin et al., 2009; Yellamsetty and Bidelman, 2018), as well as predictive coding (Cope et al., 2017; Sedley et al., 2016).
Predictive coding utilizes prior knowledge and experience to form top-down predictions that assist in perception (Friston, 2005; Rao and Ballard, 1999) particularly when sensory inputs are degraded (Cope et al., 2017). Specifically, β-band activity has been related to the updating and precision of predictions (Cope et al., 2017; Sedley et al., 2016) and top-down signaling during speech processing (Fontolan et al., 2014; Wang, 2010). Under a predictive coding framework, fronto-temporal interactions would tend to increase in cases of degraded sensory information (Cope et al., 2017). Aging is associated with increased activation of frontal and motor cortex that helps compensate for impaired SIN perception in older adults (Bilodeau-Mercure et al., 2015; Du et al., 2016). Older listeners also show greater specificity of phoneme representations in frontal articulatory regions compared to auditory brain areas (Du et al., 2016). The increased IFG-PAC connectivity we find could reflect predictive coding that would naturally need to be stronger in listeners who have impoverished sensory encoding (i.e., HL listeners). The fact that this predictive inferencing is restricted to the β-band suggests the “top-down” mechanism observed here is not general attention or listening effort per se (which would be expected in α-band) but template matching and/or interactions between higher (IFG) and lower (PAC) order speech representations.
Additional studies have shown that older adults with hearing loss demonstrate reduced cognitive reserve which impacts higher order language processing as well as other complex processing and tasks that rely heavily on cognitive resources (i.e., SIN) (Cardin, 2016; Mishra et al., 2013; Mishra et al., 2014; Rudner et al., 2009). Furthermore, lower cognitive reserve has been related to higher functional connectivity (Lopez et al., 2014), which in turn is related to perceptual SIN abilities (Bidelman et al., 2019a; Bidelman et al., 2019b; Giordano et al., 2017). Because observed increases in functional connectivity only occurred in noise, it is likely that the increased task demands of more difficult listening conditions further reduced the spare capacity of available cognitive resources in HL listeners leading to the recruitment of frontal regions to overcome depleted sensory resources. The increased efferent connectivity within the β-band may reflect the online recruitment of these additional resources (e.g., IFG) to bolster the matching of sound to speech templates and facilitate SIN comprehension. Alternatively, increased prefrontal activity/connectivity in older, hearing impaired adults might instead reflect nonspecific neural responses (i.e., arousal, attention) rather than compensation via recruitment of specific complementary neural regions to benefit task performance (Morcom and Henson, 2018).
In summary, our findings suggest that α desynchronization in challenging listening conditions reflects a release from inhibition contributing to better SIN performance. This finding supports the notion that a decrease in α functions to assist in active cognitive processing of task-relevant inputs. Additionally, even mild degrees of hearing loss in older adults result in neurophysiological changes in connectivity between cortical auditory and linguistic areas during SIN processing despite negligible behavioral deficits. That this top-down connectivity is restricted to β band suggests hearing loss increases the need to make high-order inferences on noisier sensory representations. Collectively, our findings suggest that functional connectivity is more sensitive to hearing-related changes than region-specific activation and that neural signaling is altered prior to observable behavioral changes. These results emphasize the importance of compensatory, top-down signal transmission in impaired systems to aid SIN perception.
Highlights.
Increased α phase coherence predicts accuracy in SIN perception
Changes in functional connectivity precede behavioral deficits in SIN processing
Top-down β connectivity increases in strength with poorer hearing
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (MOP 106619) and the Natural Sciences and Engineering Research Council of Canada (NSERC, 194536) awarded to C.A, and the National Institutes of Health (NIDCD) R01DC016267 awarded to G.M.B. Requests for reprints and materials should be directed to G.M.B. [gmbdlman@memphis.edu].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
For the current study, the paradigm was designed to record frequency-following responses (FFRs) from the brainstem and cortical ERPs simultaneously. Because FFRs require many more trials (approximately 2000 per token) than traditional ERP measures, shortening the interstimulus interval was necessary to reduce the overall time required for data collection.
To satisfy model convergence and ensure estimable variance, it was necessary to remove the random term for the efferent (IFG-PAC) β connectivity variable. In this case, PROC GLIMMIX estimated degrees of freedom using the between-within approximation procedure (Schluchter & Elashoff, 1990), which divides the residual degrees of freedom into between-subject and within-subject portions.
References
- Adrian ED, Matthews BH (1934), The interpretation of potential waves in the cortex. J Physiol 81:440–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Roye A, Salloum C (2014), Effects of age-related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front Syst Neurosci 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Woods DL (1999), Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychol Aging 14:507–519. [DOI] [PubMed] [Google Scholar]
- Anderson Gosselin P, Gagné J-P (2011), Older Adults Expend More Listening Effort Than Young Adults Recognizing Speech in Noise. Journal of Speech, Language, and Hearing Research 54:944–958. [DOI] [PubMed] [Google Scholar]
- Bashivan P, Bidelman GM, Yeasin M (2014), Spectrotemporal dynamics of the EEG during working memory encoding and maintenance predicts individual behavioral capacity. Eur J Neurosci 40:3774–3784. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995), Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 57:289–300. [Google Scholar]
- Betzel RF, Byrge L, He Y, Goni J, Zuo XN, Sporns O (2014), Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 102 Pt 2:345–357. [DOI] [PubMed] [Google Scholar]
- Bidelman GM (2015), Induced neural beta oscillations predict categorical speech perception abilities. Brain Lang 141:62–69. [DOI] [PubMed] [Google Scholar]
- Bidelman GM (2017), Amplified induced neural oscillatory activity predicts musicians’ benefits in categorical speech perception. Neuroscience 348:107–113. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Davis MK, Pridgen MH (2018), Brainstem-cortical functional connectivity for speech is differentially challenged by noise and reverberation. Hear Res 367:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Howell M (2016), Functional changes in inter- and intra-hemispheric cortical processing underlying degraded speech perception. Neuroimage 124:581–590. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Mahmud MS, Yeasin M, Shen D, Arnott SR, Alain C (2019a), Age-related hearing loss increases full-brain connectivity while reversing directed signaling within the dorsal-ventral pathway for speech. Brain Struct Funct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Price CN, Shen D, Arnott SR, Alain C (2019b), Afferent-efferent connectivity between auditory brainstem and cortex accounts for poorer speech-in-noise comprehension in older adults. Hear Res 382:107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Villafuerte JW, Moreno S, Alain C (2014), Age-related changes in the subcortical-cortical encoding and categorical perception of speech. Neurobiol Aging 35:2526–2540. [DOI] [PubMed] [Google Scholar]
- Bilodeau-Mercure M, Lortie CL, Sato M, Guitton MJ, Tremblay P (2015), The neurobiology of speech perception decline in aging. Brain Struct Funct 220:979–997. [DOI] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD (2004), Neural correlates of sensory and decision processes in auditory object identification. Nat Neurosci 7:295–301. [DOI] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL (2004), Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A 101:9849–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Sharma A (2013), Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin V (2016), Effects of Aging and Adult-Onset Hearing Loss on Cortical Auditory Regions. Front Neurosci 10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF (2008), Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB (2016), Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 89:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT (1997), Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex 7:63–69. [DOI] [PubMed] [Google Scholar]
- Cope TE, Sohoglu E, Sedley W, Patterson K, Jones PS, Wiggins J, Dawson C, Grube M, et al. (2017), Evidence for causal top-down frontal contributions to predictive processes in speech perception. Nat Commun 8:2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJ (2003), Temporal lobe regions engaged during normal speech comprehension. Brain 126:1193–1201. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E (2003), Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuroimage 20:1270–1282. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Hodlmoser K, Sauseng P, Gruber W (2005), Intelligence related upper alpha desynchronization in a semantic memory task. Brain Res Bull 66:171–177. [DOI] [PubMed] [Google Scholar]
- Du Y, Buchsbaum BR, Grady CL, Alain C (2016), Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nat Commun 7:12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushanova J, Christov M (2016), Do the changes of event-related potentials and frequency band responses to sensory stimuli correlate to age cognitive decline? International Journal of Neurology Research 3:327–334. [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G (2006), Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J Cogn Neurosci 18:637–650. [DOI] [PubMed] [Google Scholar]
- Fontolan L, Morillon B, Liegeois-Chauvel C, Giraud AL (2014), The contribution of frequency-specific activity to hierarchical information processing in the human auditory cortex. Nat Commun 5:4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD (1997), Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res 106:95–104. [DOI] [PubMed] [Google Scholar]
- Friston K (2005), A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360:815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999), Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proc Natl Acad Sci U S A 96:8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand SA (2009) Essentials of Audiology. New York: Thieme. [Google Scholar]
- Giordano BL, Ince RAA, Gross J, Schyns PG, Panzeri S, Kayser C (2017), Contributions of local speech encoding and functional connectivity to audio-visual speech perception. eLife 6:e24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D (2012), Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci 15:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C (2012), The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer KS, Wilber LA (1990), Hearing loss, aging, and speech perception in reverberation and noise. J Speech Hear Res 33:149–155. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M (2004), BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr 16:233–238. [DOI] [PubMed] [Google Scholar]
- Humes LE (1996), Speech understanding in the elderly. J Am Acad Audiol 7:161–167. [PubMed] [Google Scholar]
- Kahneman D (1973) Attention and effort. Englewood Cliffs, N.J.,: Prentice-Hall. [Google Scholar]
- Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S (2004), Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am 116:2395–2405. [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012), Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B (1997), Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett 238:9–12. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007), EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev 53:63–88. [DOI] [PubMed] [Google Scholar]
- Lavie N (1995), Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform 21:451–468. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E (2004), Load theory of selective attention and cognitive control. J Exp Psychol Gen 133:339–354. [DOI] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Davatzikos C, Kraut MA, et al. (2014), Association of hearing impairment with brain volume changes in older adults. Neuroimage 90:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobier M, Siebenhuhner F, Palva S, Palva JM (2014), Phase transfer entropy: a novel phase-based measure for directed connectivity in networks coupled by oscillatory interactions. Neuroimage 85 Pt 2:853–872. [DOI] [PubMed] [Google Scholar]
- Lopez ME, Aurtenetxe S, Pereda E, Cuesta P, Castellanos NP, Bruna R, Niso G, Maestu F, et al. (2014), Cognitive reserve is associated with the functional organization of the brain in healthy aging: a MEG study. Front Aging Neurosci 6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Lunner T, Stenfelt S, Ronnberg J, Rudner M (2013), Visual information can hinder working memory processing of speech. J Speech Lang Hear Res 56:1120–1132. [DOI] [PubMed] [Google Scholar]
- Mishra S, Stenfelt S, Lunner T, Ronnberg J, Rudner M (2014), Cognitive spare capacity in older adults with hearing loss. Front Aging Neurosci 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Henson RNA (2018), Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. J Neurosci 38:7303–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobukawa S, Kikuchi M, Takahashi T (2019), Changes in functional connectivity dynamics with aging: A dynamical phase synchronization approach. Neuroimage 188:357–368. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P (1977), Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum 13:135–145. [PubMed] [Google Scholar]
- Park DC, McDonough IM (2013), The Dynamic Aging Mind: Revelations From Functional Neuroimaging Research. Perspect Psychol Sci 8:62–67. [DOI] [PubMed] [Google Scholar]
- Petersen EB, Wostmann M, Obleser J, Stenfelt S, Lunner T (2015), Hearing loss impacts neural alpha oscillations under adverse listening conditions. Front Psychol 6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G (2001), Functional brain imaging based on ERD/ERS. Vision Res 41:1257–1260. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Levitt H (2012), Speech comprehension training and auditory and cognitive processing in older adults. Am J Audiol 21:351–357. [DOI] [PubMed] [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, Bosch-Bayard J, Trujillo NJ (1999), Intracerebral sources of human auditory-evoked potentials. Audiol Neurootol 4:64–79. [DOI] [PubMed] [Google Scholar]
- Picton TW, van Roon P, Armilio ML, Berg P, Ille N, Scherg M (2000), The correction of ocular artifacts: a topographic perspective. Clin Neurophysiol 111:53–65. [DOI] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW (2019), Load modulates the alpha and beta oscillatory dynamics serving verbal working memory. Neuroimage 184:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Ballard DH (1999), Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 2:79–87. [DOI] [PubMed] [Google Scholar]
- Rudner M, Foo C, Ronnberg J, Lunner T (2009), Cognition and aided speech recognition in noise: specific role for cognitive factors following nine-week experience with adjusted compression settings in hearing aids. Scand J Psychol 50:405–418. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ebersole JS (1994), Brain source imaging of focal and multifocal epileptiform EEG activity. Neurophysiol Clin 24:51–60. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P (2002), Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. J Clin Neurophysiol 19:91–112. [DOI] [PubMed] [Google Scholar]
- Schluchter MD, Elashoff JD (1990), Small-sample adjustments to tests with unbalanced repeated measures assuming several covariance structures. Journal of Statistical Computation and Simulation 37. [Google Scholar]
- Sedley W, Gander PE, Kumar S, Kovach CK, Oya H, Kawasaki H, Howard MA, Griffiths TD (2016), Neural signatures of perceptual inference. Elife 5:e11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin AJ, Picton TW, Miller LM (2009), Brain oscillations during semantic evaluation of speech. Brain Cogn 70:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht K, Shah NJ, Jancke L (2000), Bilateral inferior frontal networks are involved in speech perception processes. Neuroimage 11:S292. [Google Scholar]
- Sullivan EV, Pfefferbaum A (2006), Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J (1996), Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci 16:4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P (2003), Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol 114:1332–1343. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA (2010), Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex 20:352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J (2000), Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38:301–313. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang W, Yan T, Song J, Yang W, Wang B, Go R, Huang Q, et al. (2017), Beta-Band Functional Connectivity Influences Audiovisual Integration in Older Age: An EEG Study. Front Aging Neurosci 9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ (2010), Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90:1195–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne RV, Johnsrude IS (2015), A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev 23:154–166. [DOI] [PubMed] [Google Scholar]
- Wong PC, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S (2010), Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear 31:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S (2009), Aging and cortical mechanisms of speech perception in noise. Neuropsychologia 47:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellamsetty A, Bidelman GM (2018), Low- and high-frequency cortical brain oscillations reflect dissociable mechanisms of concurrent speech segregation in noise. Hear Res. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y (2007), Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging 28:784–798. [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Heslenfeld DJ, Festen JM, Schoonhoven R (2006), Top-down and bottom-up processes in speech comprehension. Neuroimage 32:1826–1836. [DOI] [PubMed] [Google Scholar]
- Zendel BR, Alain C (2014), Enhanced attention-dependent activity in the auditory cortex of older musicians. Neurobiol Aging 35:55–63. [DOI] [PubMed] [Google Scholar]